Abstract
Background
Both the anterior cruciate ligament (ACL) and medial collateral ligament (MCL) bear load during athletic tasks of landing, cutting, pivoting, and twisting. As dynamic knee valgus is a purported mechanism for ACL injury, the MCL should bear significant strain load.
Hypothesis
The intact MCL will demonstrate a significant increase in strain upon failure of the ACL at 25° knee flexion.
Study Design
Controlled Laboratory Study
Methods
In vivo kinetics/kinematics of 42 healthy, athletic subjects were measured to determine stratification of injury risk (i.e. low, medium, and high) in three degrees of knee forces/moments (knee abduction moment, anterior tibial shear, and internal tibial rotation). These stratified kinetic values were input into a cadaveric impact simulator to assess ligamentous strain during a simulated landing task. Uniaxial and multiaxial load cells and differential variable reluctance transducer strain sensors were utilized to collect mechanical data for analysis. Conditions of external loads applied to the cadaveric limbs were varied and randomized.
Results
ACL strain increased with increased dynamic knee abduction moment (χ2(5)=14.123; p=0.0148). The most extreme dynamic knee abduction moment condition demonstrated significantly higher ACL strain to lower loaded trials (p≤0.0203). Similarly, MCL strain increased with dynamic knee abduction moment (χ2(5)=36.578; p<0.0001). Matched pairs analysis compared ACL strain to MCL strain (Max. ACL strain – Max. MCL strain) and demonstrated high strain for the ACL vs the MCL (S(177)=6,223.5; p<0.0001).
Conclusion
Although significant, MCL strain had minimal increase with increased dynamic knee abduction moment and the event of ACL failure did not significantly increase MCL strain compared to high dynamic knee abduction moment conditions in the cadaver model. The ACL bears more strain than the MCL at increasing amounts of dynamic knee abduction moment at 25° knee flexion, which may explain the limited concomitant MCL injury rate that can occur during a dynamic valgus collapse of the knee.
Clinical Relevance
These characteristics of ACL and MCL strain are important to understand the mechanisms that drive these injuries at the knee and will improve rehabilitation and injury prevention techniques.
Keywords: anterior cruciate ligament (ACL), cadaveric, simulation, medial collateral ligament (MCL), strain
INTRODUCTION
Both the anterior cruciate ligament (ACL) and medial collateral ligament (MCL) bear loads along with the lower extremity musculature during athletic tasks of landing, cutting, pivoting, and twisting.44 When considered in a single plane, the ACL is known as the primary restraint to anterior translation of the tibia with respect to the femur; the MCL is known as the main restraint of valgus movement (i.e. tibial abduction with respect to the femur).2,28,37 However, knee injuries in the athletic environment occur in a multiplanar fashion6,20,21,37 and ACL injury is generally non-contact in nature, with estimates of the non-contact mechanism accounting for approximately 75% of all ACL injuries.15,19,43
The non-contact injury mechanism for the ACL indicates that ACL tears are likely influenced by deficits in neuromuscular control and risky movement strategies that include dynamic knee abduction (valgus), hip adduction, and internal rotation of the tibia and hip.21,24,36,37 Biomechanical and epidemiological evidence supports dynamic knee valgus as a mechanism of ACL injuries during landing tasks,17,18,34,42 yet controversy and debate continue14,46 as the MCL is a likely candidate for restraint of knee valgus (abduction) and would reasonably injure first.27,29,36,39,50 Interestingly, it has been shown that the ACL provides greater contributions to knee restraint than the MCL with dynamic knee valgus loading5,6 and concomitant MCL injury only occurs in a minority of ACL injuries (20–40%).2,25,37,49 Even if the MCL is the primary restraint to a dynamic knee valgus load, suboptimal fiber orientation and collagen property differences from the ACL could account for lower MCL strain characteristics.36 There is currently little in the literature on how MCL loading changes upon ACL failure,27 especially with dynamic, multiplanar loading. Observation of MCL loading characteristics would further elucidate the contributions of the MCL to dynamic knee valgus before and after ACL failure.
Consequently, this study was designed to investigate the loading of the MCL in a dynamic, multiplanar fashion with increased levels of abduction loading. During these conditions, both MCL and ACL strains were observed, with specific interest in the biomechanical behavior of MCL strain during trials in which ACL failure occurred. We have previously demonstrated that with increased levels of knee abduction moments (KAM) during landing, MCL strain did not significantly increase from baseline conditions in both sexes.42 In this study, the hypothesis tested was that cadaveric specimens with intact MCLs would demonstrate a significant increase in MCL strain upon failure of the ACL at 25° knee flexion.
METHODS
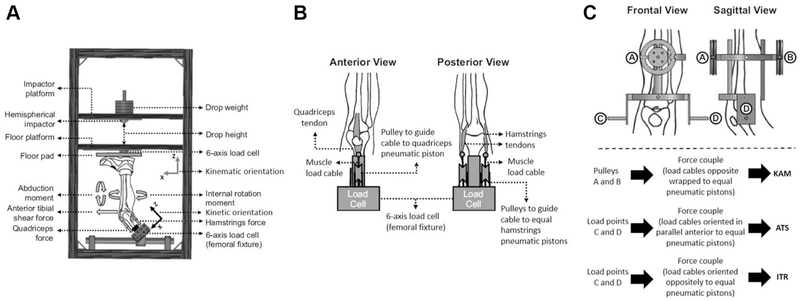
A custom-designed mechanical impact simulator (Figure 1) that reproduced clinically-relevant ACL and MCL injury patterns, which has been used to answer a variety of hypotheses,4,7,8,31,41,42 was utilized to simulate athletic drop landings in 45 cadaveric lower extremity specimens. Lower extremity cadaveric specimens (n=45; 24 unique donors) were obtained (Anatomy Gifts Registry, Hanover, MD, USA) according to predetermined inclusion criteria: age 18 to ≤ 50, no evidence of significant trauma or surgery to lower extremity, no extensive bed rest (defined as > 1 month), and no evidence of extended chemotherapy (defined as > 4 weeks). Specimens were dissected and prepared according to specifications outlined in a recent methodology manuscript.8 Of the 45 available specimens (22M:23F), 6 were excluded from final analysis due to poor data quality from equipment failure, mechanical weakness due to specimen preparation, or a pre-evaluation non-functional ACL determined by a board-certified orthopedic surgeon (AJK). Data analyses were therefore performed on 39 specimens [age 41.4 (8.3) years; 86.2 (23.7) kg; 19M:20F]. Of these 39 specimens, 12 (31%) sustained MCL failure with 8 (21%) concomitant ACL failures and 4 (10%) MCL failures prior to ACL failure (MCL failure prior to ACL failure was not noted until post-orthopedic examination and data post-processing).
Figure 1:
(A) Meta-view of custom designed mechanical impact simulator for creation of ACL ruptures,9 (B) Cable pulley system used to deliver pneumatically actuated loads to the quadriceps and hamstrings tendons, (C) External fixation frame attached to the tibia and used to deliver pneumatically actuated knee abduction moment (KAM), anterior tibial shear (ATS), and internal tibial rotation (ITR) loads to each specimen. This figure has been reproduced from Bates, et al. 2018 Am J Sports Med. 7,8,21.
In vivo kinetics/kinematics of 44 healthy athletic subjects [age = 23.3 (4.1) years; mass = 72.6 (13.9) kg; height = 172 (10) cm] were measured to determine stratification of injury risk (i.e. low, medium, and high) in three degrees of knee forces/moments [i.e. KAM, anterior tibial shear (ATS), and internal tibial rotation (ITR).41,42 These 44 subjects were selected from a cohort of 67 healthy controls based on data quality. Pneumatic cylinders applied the designated knee forces/moments at the tibia determined from the in vivo subjects in a randomized order (Table 1) and then a gravity-driven drop sled of 0.5 body weight (34 kg) was released from a height of 31 cm with quadriceps and hamstrings co-contracted with a 1:1 ratio. All limbs were oriented in the impactor at 25° to the vertical sagittal plane. A knee flexion angle of 25° was used as it is representative of initial contact (IC) knee orientation in young athletes landing from a drop height of 31 cm.3 The long axis of the tibia was then aligned with the vertical axis of impact delivery in both the frontal and sagittal planes.
Table 1:
KAM, ATS, and ITR load magnitudes based on in vivo population percentage.
| KAM | ATS | ITR | ||||
|---|---|---|---|---|---|---|
| Risk Classification | Percentile of in vivo Population Cohort | Load (Nm) | Percentile of in vivo Population Cohort | Load (N) | Percentile of in vivo Population Cohort | Load (N) |
| Baseline | 2% | 2.4 | 0% | 40.0 | 0% | 1.0 |
| Low | – | – | – | – | 33% | 9.7 |
| Moderate | 68% | 27.0 | – | – | 67% | 18.6 |
| High | 99% | 53.6 | 90% | 98.0 | 100% | 53.7 |
| Very High | 200% | 114.6 | – | – | – | – |
Two differential variable reluctance transducers (DVRTs; LORD MicroStrain, Inc., Williston, VT, USA) were implanted into the MCL midsubstance and anteromedial bundle of the ACL, respectively, to measure ligament strains during landing simulations with increasing magnitudes of KAM.10,26,37 Each DVRT was placed into the respective ligament with real-time voltage data display to ensure mid-range placement of the DVRT sensor. Ligament strain data were collected from DVRTs at 10 kHz with custom-designed LabVIEW software (LabVIEW 2015, National Instruments, Austin, TX, USA). During data collection, the software triggered the pneumatic cylinders for external loading approximately one second prior to the electromagnet release of the drop sled to deliver the impact; the pneumatic cylinders released the external loads approximately one second after impact. All data was filtered with a 12 Hz low pass fourth-order Butterworth filter and then post-processed with LabVIEW (Version 2016, Austin, TX, USA) and MATLAB (version 2015b, Natick, MA, USA). All concomitant MCL injuries were removed from the analysis to accurately depict intact MCL strain with ACL failure.
As ligament strain is a percentage calculated by displacement over initial length,13
normalization to body height and/or weight was not possible. The DVRTs were initially calibrated by an posterior-anterior force (for ACL DVRT) and a varus-valgus moment (for MCL DVRT) that allowed each respective ligament to pass through its taut to lax inflection point (Supplemental Figure). This process was repeated twice for each ligament and the average of these values was used. This allowed for the determination of the initial length of the ligament for strain calculation as the initial length is defined as the inflection point where the ligament transfers from a lax to a taut state.
Statistical analyses were performed with JMP 13 (SAS Institute Inc., Cary, NC, USA) with utilization of Kruskal-Wallis test and Wilcoxon Signed Rank matched pairs test for ligament strain (ACL or MCL) across conditions. Six experimental conditions were sampled for this study with increasing levels of KAM: 1) 2% KAM, 0% ATS, 33% ITR (Baseline), 2) 68% KAM, 0% ATS, 33% ITR (Moderate), 3) 99% KAM, 0% ATS, 33% ITR (High), 4) 200% KAM, 0% ATS, 33% ITR (Very High), 5) the trial prior to ACL failure (ACL Pre-Failure), and 6) the trial of ACL failure (ACL Failure). With the latter two conditions, the amount of KAM, ATS, and ITR vary across each specimen. These six trials were selected for consistency across ATS and ITR with increasing KAM (to provide a multiplanar dynamic valgus). The larger valgus load, in theory, should load the MCL with additional strain.5,6 The trial prior to ACL failure provided accurate demonstrations of strain for the specimens and provides a reference for the trial of ACL failure; the actual ACL failure trial exhibited high noise for ACL strain as the sensor has the potential to completely separate, dislodge from the tissue, or not measure strain as the ligament may separate from the bone and no longer displace the sensor. Accordingly, ACL failure statistical comparisons were affected by high variability and should be critically interpreted. If there was prior or concomitant MCL damage to ACL failure of a specimen, the invalid trials were excluded from analysis.
RESULTS
Of the 39 specimens included in this study, there were 34 ACL failures (17M:17F; 25 femoral avulsions, 7 mid-substance failures, and 2 tibial avulsions) of 39 specimens (87.2%). Although randomization was applied to the external loads to avert effects of fatigue from impact, the median number of impacts of each specimen prior to ACL rupture was 34 (29.8, 51). By sex, prior to ACL rupture males had a median number of 49 (42.5, 52) impacts and females had a median of 29.5 (25.5, 38.8).
A Kruskal-Wallis test demonstrated significance of ACL strain across conditions (χ2(5)=14.123; p=0.0148). Post-hoc analysis demonstrated significance of ACL strain of ‘ACL Pre-Failure’ against the lower loading KAM loading conditions: Baseline, Moderate, High, and Very High (p=0.0027; p=0.0030; p=0.0203; p=0.0120, respectively; Table 2). All other comparisons were p>0.05.
Table 2: Maximum Ligament Strain [%; median (IQR)] with increased KAM load.
Except in the ‘ACL Pre-Failure’ and ‘ACL Failure’ conditions, ATS and ITR were held constant. It should be noted that at the ‘Very High’ load condition, the n-value decreased due to onset of specimen failure under greater loading. Consequently, specimens with higher baseline ACL strains were not available for inclusion as they had ruptured, and the resultant data demonstrates a lack of linearity in strain increase relative to loading increase. For this reason, the ‘Pre-Failure’ condition for each specimen is also presented, which clearly illustrates heightened ACL strain.
| Baseline (n=34) | Moderate (n=34) | High (n=33) | Very High (n=18) | ACL Pre-Failure (n=33) | ACL Failure (n=26) | |
|---|---|---|---|---|---|---|
| ACL Strain | 6.8 (3.3, 11.5) | 6.6 (4.1, 10.5) | 8.1 (5.2, 11.2) | 6.9 (5.1, 11.2) | 11.2 (8.3, 17.2) | 9.9 (5.5, 17.9)* |
| MCL Strain | 1.1 (0.6, 2.3) | 1.6 (0.7, 2.6) | 2.6 (1.2, 3.7) | 4.7 (1.8, 6.8) | 4.3 (2.0, 7.2) | 4.6 (2.4, 8.1) |
Strain values at ACL failure are affected by high variability as the sensor has the potential to completely separate, dislodge from the tissue, or not measure strain as the ligament may separate from the bone and no longer displace. Data should be assessed cautiously.
A Kruskal-Wallis test demonstrated significance of MCL strain across conditions (χ2(5)=36.578; p<0.0001). Post-hoc analysis demonstrated significance of MCL strain during the ‘ACL Failure’ condition versus ‘Baseline’, ‘Moderate’, and ‘High’ loading conditions (p<0.0001; p=0.0004; p=0.0137, respectively) (Table 2). Similarly, MCL strain was significant across conditions for ‘ACL Pre-Failure’ versus ‘Baseline’, ‘Moderate’, and ‘High’ loading conditions (p<0.0001; p=0.0005, p=0.0217, respectively) and for the ‘Very High’ conditions versus ‘Baseline’, ‘Moderate’, and ‘High’ loading conditions (p=0.0004; p=0.0061, p=0.0413, respectively). ‘High’ loading was significantly different from ‘Baseline’ and ‘Moderate’ loading (p=0.0086; p=0.0482, respectively); all other comparisons were p>0.05.
Wilcoxon Signed Rank matched pairs analysis compared ACL strain to MCL strain (Max. ACL strain – Max. MCL strain) across conditions. The results demonstrated high strain for the ACL vs the MCL (S(177)=6,223.5; p<0.0001). In addition, there was no significant difference between sex across all load conditions (p>0.1199);42 both males and females demonstrated significantly higher ACL strain than MCL strain for all matched trial conditions (p≤0.0159). Differences of medians and respective p-values are provided in Table 3 for specific trials.
Table 3: Matched Pair Analysis Median Differences of Maximum Strain (ACL – MCL) by loading condition.
These data demonstrate higher ACL strain than MCL strain with increased KAM loading. In addition, there is limited evidence of MCL strain increase with ACL failure. As in Table 2, it should be noted that at the ‘Very High’ load condition, the n-value decreases due to onset of specimen failure under greater loading. Consequently, specimens with higher baseline ACL strains were not available for inclusion as they had ruptured, and the resultant data demonstrates a decrease at ‘Very High’ load. For this reason, the ‘Pre-Failure’ condition for each specimen is also presented, which clearly illustrates heightened ACL strain.
| Loading Condition | Median Difference of % strain (IQR) | p-value |
|---|---|---|
| Baseline (n=34) | 5.5 (1.0, 10.9) | <0.0001 |
| Moderate (n=34) | 4.5 (0.6, 10.0) | <0.0001 |
| High (n=33) | 5.3 (1.2, 11.0) | <0.0001 |
| Very High (n=18) | 2.4 (−0.3, 7.1) | 0.0159 |
| ACL Pre-Failure (n=33) | 6.3 (0.6, 14.3) | <0.0001 |
| ACL Failure (n=26) | 4.9 (−0.7, 9.6)* | 0.0044* |
Bold text indicates significance p<0.05.
Strain values at ACL failure are affected by high variability as the sensor has the potential to completely separate, dislodge from the tissue, or not measure strain as the ligament may separate from the bone and no longer displace. Data should be assessed cautiously.
DISCUSSION
This study demonstrates that although both ACL and MCL strain significantly increase from baseline loads with higher KAM loading, the overall MCL strain remains less when compared to ACL strain values, which is consistent with previous findings.5,6,21,37,42 Previous literature demonstrates that the ACL fails at approximately 15% strain and that the MCL fails at approximately 17% strain.11,35 With observation of the specific trials of ‘ACL Pre-Failure’ and ‘ACL Failure,’ the strain values of the MCL did not increase above that with ‘Very High’ loading (Table 2) and remained far from failure strain whereas the ACL approximated failure strain.
The initial length of the ACL and MCL was determined by a calibration prior to all testing. From the continuous voltage data from the DVRT sensor, an inflection point could be determined where the ligament transitioned from lax to taut. Thus, with the initial length determined, the strains of the ligaments could be compared to one another under the dynamic load conditions. When a matched analysis was performed, all loading conditions demonstrated significance between ACL and MCL strains (Table 3), with ACL strain greater than MCL strain across all conditions. Of interest, the ‘Very High’ loading condition decreased in statistical significance, an indication that the MCL bore more load and approximated the load of the ACL, but this is likely due to the loss of specimens that had higher baseline ACL strain and were removed from analysis due to ACL rupture prior to this load condition. Consequently, the lower baseline ACL strain specimens would still be included and the difference between ACL and MCL strain would be decreased. Of interest, the ‘Very High’ load did not specifically cause an MCL injury in these cadaveric impact simulations. The ‘ACL Pre-Failure’ and ‘ACL Failure’ conditions were not constrained to similar ATS and ITR loads and thus these coupled motions with KAM likely further loaded the ACL, whereas the MCL is not effective for either anterior tibial translation or internal rotation of the knee. Due to these dynamic differences, there is more variability in the measured strain between the ACL and MCL in these conditions.
In order to address the hypothesis that intact MCLs would demonstrate an increase strain upon failure of the ACL, all previous MCL injuries or concomitant MCL injuries were removed from analysis. It is possible that removal of specimens with concomitant injury from analysis may have eliminated the higher strains of the MCL as these specimens may have had less ligamentous elasticity. However, all trials that occurred prior to concomitant injuries were included in the analysis to observe functional characteristics of the two ligaments when intact (Table 2). Thus, the higher strained MCLs are included in the lower strain trials and are included for statistical analysis prior to ligament failure for inference of MCL strain characteristics. Furthermore, the exclusion of concomitant ACL/MCL injury is warranted due to the nature of DVRT data during ligament failure; MCL strain data would be unreliable due to the failure of the ligament, especially in comparison to unreliable data from a DVRT during ACL failure. The removal of these specimens from analysis is consistent with the reported quantity of concomitant ACL/MCL injuries (20–40%).8,25,37,38 Of the 12 (31%) excluded specimens that experienced both MCL and ACL injury, only three (8%) had MCL damage prior to ACL injury. These three injuries were not noted until post-processing of data and the observed data during testing did not signify reason to discontinue impact testing during the outlined protocol. Overall, this study observed the characteristics of MCL strain with increased KAM and the MCL strain that was present concurrent with ACL failure.
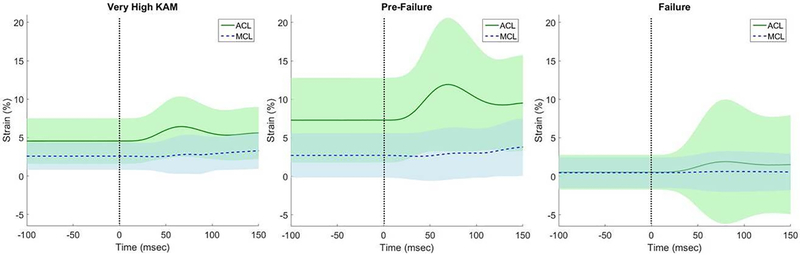
Aside from calculation of maximum peak strain values, the mean overall strain across all specimens can be observed across time (Fig. 2). The strain produced from the impact on the specimen is largely borne by the ACL with increase of KAM, with minimal MCL contribution. Even upon failure of the ACL, the MCL increases in overall variability – an indicator that some specimens bear additional load in the MCL while others do not. This difference could also be caused by the addition of ATS and ITR coupled motion to the KAM as this was not controlled in the ‘ACL Pre-Failure’ and ‘ACL Failure conditions’. Overall, there is no significant increase in MCL ligament strain after ACL failure. Thus, contrary to the hypothesis, the MCL does not bear additional strain concurrent or subsequent to ACL failure. As the additional load must go elsewhere, we speculate that the load transfers to the cartilage and underlying bone, potentially the cause of meniscal tears and bone contusions that also occur concomitantly with ACL injury.
Figure 2. ACL / MCL Strain During ‘Very High’, ‘ACL Pre-Failure’, and ‘ACL Failure’ Conditions.
Impact occurs at time point zero (vertical dashed line). There is limited increase of MCL strain with ‘Very High KAM’ and also ‘ACL Pre-Failure’. With an intact MCL at ACL failure, there is virtually no increase of MCL strain from the trial previous to ACL failure. For progressive MCL strain characteristics, the ‘ACL Failure’ condition was included; however, due to high variability of the sensor (low and high values) that occurs with ligament failure, cautious assessment is warranted. Shaded areas are +/− 1 SD. For n-values of each condition, please refer to Table 2 or 3.
The novel, mechanical impact simulator utilized in this study can capably produce clinically-relevant ACL failures with a high rate of success.7,8 In this study, there were 34 ACL failures (17M:17F; 25 femoral avulsions, 7 mid-substance failures, and 2 tibial avulsions) of 39 specimens (87.2%) that were included in the analysis as verified by a board certified orthopedic surgeon (AJK). Five ACLs survived all testing procedures without failure (2M:3F), but four of the five specimens experienced other soft or hard tissue failure. Consequently, the impactor induced physiologically relevant forces and moments encountered by athletes in typical sport maneuvers of landing; and thus, relevant ACL and MCL strains. This is important as many previous impactors have failed to reproduce clinically-relevant failures in a multi-planar system in a physiologically relevant gravity-driven environment.1,9,16,32,33,37,40,48 Consequently, with a high degree of certainty, this cadaveric impactor utilized a dynamic, multi-planar knee injury simulation that is highly relevant to 6 degree of freedom (DOF) loading seen in typical sport maneuvers of landing, cutting, and pivoting.7,30,40 The current reported data indicate that even with high levels of KAM loading, the ACL bears more load than the MCL during functional movement of the DVJ.6 This is of high interest as it has been disputed that KAM would cause simultaneous loading of both the ACL and MCL and lead to concomitant injuries.37,50 The current data demonstrate that even with high level KAM loading at 25° knee flexion, the ACL bears significantly more strain than the MCL and thus can be strained to produce ligamentous failure without injury to the MCL (Table 3). Our results largely coincide with cadaveric results of Lujan et al. in which they report with their 4 DOF system that MCL strain does not increase with valgus loading from the ACL intact to the ACL deficient conditions (4 – 5% strain; compare with Table 2). Our study adds to the current literature as we demonstrate similar strain values by addressing the limitations outlined by Lujan et al: addition of joint compressive forces, stabilization of muscle forces, an intact patella, high-speed, dynamic motions in a combined loading configuration analogous to injury mechanisms, and a non-artificially induced ACL deficiency.
Although this study specifically observed ligamentous strain with primary changes in KAM, ACL injury is also hypothesized to occur with other mechanisms, including ATS, ITR, and greater knee extension than that simulated in this study.23 Some studies negate that knee valgus contributes to ACL strain based on bone bruising patterns22,34 or static 3D knee models,46 but there is heterogeneity to the bone bruise data47 based on the sampled population and inclusion/exclusion criteria.12,18 In addition, static46 and quasi-static models14 will differ from the dynamic model presented here due to the timing of the impact.45 Our other impact simulator manuscripts analyze the other hypothesized contributors to ACL injury (i.e. KAM, ATS, and ITR) in a random fashion, with the exception of knee extension as the knee angle was kept constant.4,7,8,31,41,42
A limitation of this study is the potentially large variability that DVRTs are known to exhibit when implanted in soft tissue structures.6,10 However, even with this variability, significant values were able to be reported. In addition, the DVRT variability occurs especially at ligament failure as this event can cause the sensor to give inaccurate data. For example, the ligament could slacken with complete failure at either the tibial or femoral side, the sensor could dislodge from the lack of ligamentous integrity, or the sensor could completely separate and not able to provide usable data. Consequently, we have outlined in the manuscript when data should be critically evaluated by the reader. Furthermore, all concomitant MCL injuries were excluded from analysis as the data from both sensors would be highly variable and ineffective to interpret. However, the trials prior to concomitant injury were included in the analysis, thus the MCL behavioral characteristics have been observed. Given these limitations, the data provided allows for adequate assessment of the behavior of the MCL concurrent with ACL failure.
Future work should analyze all events of MCL and ACL failure (concomitant or not) with kinematics and kinetics to determine whether knee joint forces, torques, and angles can predict failure of one or both ligaments. As a continuation of these results, this information will further define the risks of ligamentous injury of the knee.
CONCLUSIONS
The hypothesis tested was not supported, as MCL strain had minimal increase with increased dynamic KAM loads and the event of ACL failure did not significantly increase MCL strain compared to high dynamic KAM load conditions. The ACL bears more strain than the MCL at increasing amounts of dynamic KAM at 25° knee flexion, which explains the limited concomitant MCL injury rate that can occur during a dynamic valgus collapse of the knee. These characteristics of ACL and MCL strain are important to understand the mechanisms that drive these injuries at the knee and will improve rehabilitation and injury prevention techniques.
Supplementary Material
What is known about the subject
Strong biomechanical and epidemiological evidence supports dynamic knee valgus as a primary mechanism of ACL injuries during landing tasks, yet controversy and debate continue as the MCL is the likely candidate for restraint of knee valgus (abduction) and would reasonably injure first. It has been shown that the ACL provides greater contributions to knee restraint than the MCL with dynamic valgus loading and concomitant MCL injury occurs in a minority of ACL injuries (20–40%).
What this study adds to existing knowledge
It is currently unreported in the literature how the MCL loading changes upon ACL failure. The observation of MCL loading characteristics at ACL failure further elucidate the contributions of the MCL to the dynamic knee valgus injury mechanism.
ACKNOWLEDGEMENTS
NIH funding include: K12HD065987 and L30AR070273 to NDS, and R01AR055563 and R01AR056259 to TEH.
REFERENCES
- 1.Bakker R, Tomescu S, Brenneman E, Hangalur G, Laing A, Chandrashekar N. Effect of sagittal plane mechanics on ACL strain during jump landing. J Orthop Res. 2016;34(9):1636–1644. doi: 10.1002/jor.23164. [DOI] [PubMed] [Google Scholar]
- 2.Bates NA, Ford KR, Myer GD, Hewett TE. Impact differences in ground reaction force and center of mass between the first and second landing phases of a drop vertical jump and their implications for injury risk assessment. J Biomech. 2013;46(7):1237–1241. doi: 10.1016/j.jbiomech.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bates NA, Ford KR, Myer GD, Hewett TE. Kinetic and kinematic differences between first and second landings of a drop vertical jump task: Implications for injury risk assessments? Clin Biomech 2013;28(4):459–466. doi: 10.1016/j.clinbiomech.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bates NA, Mejia Jaramillo MC, Vargas M, et al. External loads associated with anterior cruciate ligament injuries increase the correlation between tibial slope and ligament strain during in vitro simulations of in vivo landings. Clin Biomech. 2018;61:84–94. doi: 10.1016/J.CLINBIOMECH.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bates NA, Nesbitt RJ, Shearn JT, Myer GD, Hewett TE. Knee Abduction Affects Greater Magnitude of Change in ACL and MCL Strains Than Matched Internal Tibial Rotation In Vitro. Clin Orthop Relat Res. 2017;475(10):2385–2396. doi: 10.1007/s11999-017-5367-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bates NA, Nesbitt RJ, Shearn JT, Myer GD, Hewett TE. Relative Strain in the Anterior Cruciate Ligament and Medial Collateral Ligament During Simulated Jump Landing and Sidestep Cutting Tasks: Implications for Injury Risk. Am J Sports Med. 2015;43(9):2259–2269. doi: 10.1177/0363546515589165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bates NA, Schilaty ND, Nagelli C V., Krych AJ, Hewett TE. Novel mechanical impact simulator designed to generate clinically relevant anterior cruciate ligament ruptures. Clin Biomech. 2017;44:36–44. doi: 10.1016/j.clinbiomech.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bates NA, Schilaty ND, Nagelli CV, Krych AJ, Hewett TE. Validation of Noncontact Anterior Cruciate Ligament Tears Produced by a Mechanical Impact Simulator Against the Clinical Presentation of Injury. Am J Sports Med. 2018;46(9):2113–2121. doi: 10.1177/0363546518776621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beaulieu ML, Wojtys EM, Ashton-Miller JA. Risk of Anterior Cruciate Ligament Fatigue Failure Is Increased by Limited Internal Femoral Rotation During In Vitro Repeated Pivot Landings. Am J Sports Med. 2015;43(9):2233–2241. doi: 10.1177/0363546515589164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beynnon B, Howe JG, Pope MH, Johnson RJ, Fleming BC. The measurement of anterior cruciate ligament strain in vivo. Int Orthop. 1992;16(1):1–12. http://www.ncbi.nlm.nih.gov/pubmed/1572761. Accessed May 17, 2016. [DOI] [PubMed] [Google Scholar]
- 11.Butler David L., Kay DCS Mathew D.. Fascicle-Bone Units From Human Patellar Tendon and Knee Ligaments. J Biomech. 1986;19(6):425–432. doi: 10.1016/0021-9290(86)90019-9. [DOI] [PubMed] [Google Scholar]
- 12.Filardo G, Andriolo L, Di Laura Frattura G, Napoli F, Zaffagnini S, Candrian C. Bone bruise in anterior cruciate ligament rupture entails a more severe joint damage affecting joint degenerative progression. Knee Surgery, Sport Traumatol Arthrosc. 2018;27(1):44–59. doi: 10.1007/s00167-018-4993-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleming BC, Beynnon BD, Tohyama H, et al. Determination of a Zero Strain Reference for the Anteromedial Band of the Anterior Cruciate Ligament. J Orthop Res. 1994;12:789–795. [DOI] [PubMed] [Google Scholar]
- 14.Fleming BC, Fleming BC, Renstrom PA, et al. The effect of weightbearing and external loading on anterior cruciate ligament strain. J Biomech. 2001;34:163–170. https://ac.els-cdn.com/S0021929000001548/1-s2.0-S0021929000001548-main.pdf?_tid=71207dbc-de98-4a37-ba5c-0043ea3a4065&acdnat=1527108957_0cb6119306789b2bcfbbfb2090e41f5b. Accessed May 23, 2018. [DOI] [PubMed] [Google Scholar]
- 15.Griffin LY, Agel J, Albohm MJ, et al. Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies. J Am Acad Orthop Surg. 2008;8(MAY):141–150. [DOI] [PubMed] [Google Scholar]
- 16.Hashemi J, Chandrashekar N, Jang T, Karpat F, Oseto M, Ekwaro-Osire S. An alternative mechanism of non-contact anterior cruciate ligament injury during jump-landing: In-vitro simulation. Exp Mech. 2007;47(3):347–354. doi: 10.1007/s11340-007-9043-y. [DOI] [Google Scholar]
- 17.Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sport Med. 2005;33(4):492–501. doi: 10.1177/0363546504269591. [DOI] [PubMed] [Google Scholar]
- 18.Hewett TE, Schilaty ND. Determination of the Position of the Knee at the Time of an Anterior Cruciate Ligament Rupture for Male Versus Female Patients by an Analysis of Bone Bruises. Am J Sports Med. 2018;46(7):1559–1565. doi: 10.1177/0363546518764681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnston JT, Mandelbaum BR, Schub D, et al. Video Analysis of Anterior Cruciate Ligament Tears in Professional American Football Athletes. Am J Sports Med. 2018:1–7. doi: 10.1177/0363546518756328. [DOI] [PubMed] [Google Scholar]
- 20.Kiapour A, Kiapour AM, Kaul V, et al. Finite element model of the knee for investigation of injury mechanisms: development and validation. J Biomech Eng. 2014;136(1):11002. doi: 10.1115/1.4025692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiapour AM, Demetropoulos CK, Kiapour A, et al. Strain Response of the Anterior Cruciate Ligament to Uniplanar and Multiplanar Loads During Simulated Landings: Implications for Injury Mechanism. Am J Sport Med. 2016;44(8):2087–2096. doi: 10.1177/0363546516640499. [DOI] [PubMed] [Google Scholar]
- 22.Kim SY, Spritzer CE, Utturkar GM, Toth AP, Garrett WE, Defrate LE. Knee Kinematics during Noncontact Anterior Cruciate Ligament Injury as Determined from Bone Bruise Location. Am J Sports Med. 2015;43(10):2515–2521. doi: 10.1177/0363546515594446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koga H, Nakamae A, Shima Y, et al. Mechanisms for Noncontact Anterior Cruciate Ligament Injuries: Knee Joint Kinematics in 10 Injury Situations from Female Team Handball and Basketball. Am J Sport Med. 2010;38(11):2218–2225. doi: 10.1177/0363546510373570. [DOI] [PubMed] [Google Scholar]
- 24.Krosshaug T, Nakamae A, Boden BP, et al. Mechanisms of anterior cruciate ligament injury in basketball: video analysis of 39 cases. Am J Sports Med. 2007;35(3):359–367. doi: 10.1177/0363546506293899. [DOI] [PubMed] [Google Scholar]
- 25.LaPrade RF, Wentorf FA, Fritts H, Gundry C, Hightower CD. A Prospective Magnetic Resonance Imaging Study of the Incidence of Posterolateral and Multiple Ligament Injuries in Acute Knee Injuries Presenting With a Hemarthrosis. Arthrosc - J Arthrosc Relat Surg. 2007;23(12):1341–1347. doi: 10.1016/j.arthro.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 26.Levine JW, Kiapour AM, Quatman CE, et al. Clinically relevant injury patterns after an anterior cruciate ligament injury provide insight into injury mechanisms. Am J Sports Med. 2012;41(2):385–395. doi: 10.1177/0363546512465167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lujan TJ, Dalton MS, Thompson BM, Ellis BJ, Weiss JA. Effect of ACL Deficiency on MCL Strains and Joint Kinematics. J Biomech Eng. 2007;129(3):386. doi: 10.1115/1.2720915. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto H, Suda Y, Otani T, Niki Y, Seedhom B, Fujikawa K. Roles of the anterior cruciate ligament and the medial collateral ligament in preventing valgus instability. J Orthop Sci. 2001;6(1):28–32. [DOI] [PubMed] [Google Scholar]
- 29.Mazzocca A, Missen C, Geary M, Adams D. Valgus medial collateral ligament rupture causes concomitant loading and damage of the anterior cruciate ligament. J Knee Surg. 2003;16(3):148–151. [PubMed] [Google Scholar]
- 30.McLean SG, Huang X, Su A, Van Den Bogert AJ. Sagittal plane biomechanics cannot injure the ACL during sidestep cutting. Clin Biomech. 2004;19(8):828–838. doi: 10.1016/j.clinbiomech.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 31.McPherson AL, Bates NA, Schilaty ND, Nagelli CV, Krych AJ, Hewett TE. Ligament Strain Response Between Lower Extremity Contralateral Pairs During In Vitro Landing Simulation. Orthop J Sport Med. 2018;6(4):1–7. doi: 10.1177/2325967118765978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer EG, Haut RC. Anterior cruciate ligament injury induced by internal tibial torsion or tibiofemoral compression. J Biomech. 2008;41(16):3377–3383. doi: 10.1016/j.jbiomech.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 33.Oh YK, Kreinbrink JL, Wojtys EM, Ashton-Miller JA. Effect of axial tibial torque direction on ACL relative strain and strain rate in an in vitro simulated pivot landing. J Orthop Res. 2012;30(4):528–534. doi: 10.1002/jor.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Owusu-Akyaw KA, Kim SY, Spritzer CE, et al. Determination of the Position of the Knee at the Time of an Anterior Cruciate Ligament Rupture for Male Versus Female Patients by an Analysis of Bone Bruises. Am J Sports Med. 2018:036354651876468. doi: 10.1177/0363546518764681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quapp KM, Weiss JA. Material Characterization of Human Medial Collateral Ligament. J Biomech Eng. 1998;120(6):757. doi: 10.1115/1.2834890. [DOI] [PubMed] [Google Scholar]
- 36.Quatman CE, Hewett TE. The anterior cruciate ligament injury controversy: is “valgus collapse” a sex-specific mechanism? Br J Sport Med. 2009;43(5):328–335. doi: 10.1016/j.surg.2006.10.010.Use. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quatman CE, Kiapour AM, Demetropoulos CK, et al. Preferential loading of the ACL compared with the MCL during landing: a novel in sim approach yields the multiplanar mechanism of dynamic valgus during ACL injuries. Am J Sport Med. 2014;42(1):177–186. doi: 10.1177/0363546513506558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quatman CE, Quatman-Yates CC, Hewett TE. A “plane” explanation of anterior cruciate ligament injury mechanisms: a systematic review. Sport Med. 2010;40(9):729–746. doi: 10.2165/11534950-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 39.Quatman CE, Quatman CC, Hewett TE. Prediction and prevention of musculoskeletal injury: a paradigm shift in methodology. Br J Sports Med. 2009;43(14):1100–1107. doi: 10.1136/bjsm.2009.065482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schilaty ND, Bates NA, Hewett TE. Letter to the Editor: Effect of sagittal plane mechanics on ACL strain during jump landing. J Orthop Res. 2017;35(6):1171–1172. doi: 10.1002/jor.23523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schilaty ND, Bates NA, Nagelli CV, Krych AJ, Hewett TE. Sex-Based Differences of Knee Kinetics that Occur with Anterior Cruciate Ligament Strain on Cadaveric Impact Simulations. Orthop J Sport Med. 2018;6(3):1–7. doi: 10.1177/2325967118761037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schilaty ND, Bates NA, Nagelli CV, Krych AJ, Hewett TE. Sex-Based Differences of Medial Collateral and Anterior Cruciate Ligament Strains with Cadaveric Impact Simulations. Orthop J Sport Med. 2018;6(4):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schilaty ND, Nagelli CV, Bates NA, et al. Incidence of Second Anterior Cruciate Ligament Tears and Identification of Associated Risk Factors From 2001 to 2010 Using a Geographic Database. Orthop J Sport Med. 2017;5(8):1–8. doi: 10.1177/0363546517694026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shelbourne KD, Gray T, Haro M. Incidence of subsequent injury to either knee within 5 years after anterior cruciate ligament reconstruction with patellar tendon autograft. Am J Sports Med. 2009;37(2):246–251. doi: 10.1177/0363546508325665. [DOI] [PubMed] [Google Scholar]
- 45.Sigurðsson HB, Sveinsson Þ, Briem K. Timing, not magnitude, of force may explain sex-dependent risk of ACL injury. Knee Surgery, Sport Traumatol Arthrosc. 2018:1–6. doi: 10.1007/s00167-018-4859-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Utturkar GM, Irribarra LA, Taylor KA, et al. The Effects of a Valgus Collapse Knee Position on In Vivo ACL Elongation. Ann Biomed Eng. 2013;41(1):123–130. doi: 10.1007/s10439-012-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Viskontas DG, Giuffre BM, Duggal N, Graham D, Parker D, Coolican M. Bone bruises associated with ACL rupture: Correlation with injury mechanism. Am J Sports Med. 2008;36(5):927–933. doi: 10.1177/0363546508314791. [DOI] [PubMed] [Google Scholar]
- 48.Withrow TJ, Huston LJ, Wojtys EM, Ashton-Miller JA. The effect of an impulsive knee valgus moment on in vitro relative ACL strain during a simulated jump landing. Clin Biomech. 2006;21(9):977–983. doi: 10.1016/j.clinbiomech.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 49.Yoon KH, Yoo JH, Kim K-I. Bone Contusion and Associated Meniscal and Medial Collateral Ligament Injury in Patients with Anterior Cruciate Ligament Rupture. J Bone Jt Surgery-American Vol. 2011;93(16):1510–1518. doi: 10.2106/JBJS.J.01320. [DOI] [PubMed] [Google Scholar]
- 50.Yu B, Garrett WE. Mechanisms of non-contact ACL injuries. Br J Sports Med. 2007;41 Suppl 1(suppl_1):i47–51. doi: 10.1136/bjsm.2007.037192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.