Abstract
Given the essential role of the blood-brain barrier (BBB) in the central nervous system (CNS), cumulative investigations have been performed to elucidate how modulation of BBB structural and functional integrity affects the pathogenesis of CNS diseases such as stroke, traumatic brain injuries, dementia, and cerebral infection. Recent studies have demonstrated that microRNAs (miRNAs) contribute to the maintenance of the BBB and thereby mediate CNS homeostasis. This review summarizes emerging studies that demonstrate cerebral miRNAs regulate BBB function in CNS disorders, emphasizing the direct role of miRNAs in BBB molecular composition. Evidence presented in this review will encourage a deeper understanding of the mechanisms by which miRNAs regulate BBB function, and facilitate the development of new miRNAs-based therapies in patients with CNS diseases.
Keywords: MicroRNAs, Central nervous system diseases, Blood-brain barrier, Vascular integrity
1. Introduction
1.1. Blood-brain barrier
The central nervous system (CNS) is one of the most complex and highly organized systems in the human body and the blood-brain barrier (BBB) is a highly sealed cell-to-cell contacted barrier that regulates the transportation of proteins, nutrients, and molecules from blood to brain, and metabolic waste products from the brain into circulation (Ballabh et al., 2004; Maiuolo et al., 2018; Zhao et al., 2015d). The BBB is centrally positioned within the neurovascular unit and several factors contribute to the physical and functional BBB. Cerebral endothelial cells (ECs) are the major component of the brain microvasculature, playing an essential role in the maintenance of BBB integrity and cerebral homeostasis (Daneman, 2012; Sweeney et al., 2019). Junction proteins mainly include tight junctions (TJs) and adherens junctions (AJs) (Hartsock and Nelson, 2008). TJs are responsible for connecting neighboring endothelial cells to reduce intercellular distance, thus, preventing free exchange between the CNS and vasculature. Many transmembrane proteins are required to form a functional BBB such as claudins, occludin, zonula occludens (ZO), and junctional adhesion molecules (JAMs). Claudins, the major transmembrane molecules, have been well demonstrated to play critical barrier functions in both human cell lines and rodent knockout models as summarized in a previous publication (Daneman, 2012). AJs initiate and stabilize cell-cell adhesion, thereby regulating intracellular signaling and the transcription of important transmembrane components. The core of the AJs includes certain cadherin and catenin family members. Vascular endothelial (VE)-cadherin is exclusively responsible for endothelial AJs’ assembly and barrier architecture. E-cadherin is the core transmembrane protein and N-cadherin forms the pericyte-endothelial junction (Wolburg and Lippoldt, 2002). Other components such as pericytes, astrocytes, and the basement membrane (BM) may also contribute to BBB function and structure. Pericytes maintain BBB integrity by covering the capillaries, and astrocytes participate in BBB maintenance by interacting with pericytes and ECs through their endfeet (Wong et al., 2013). The BM is a unique form of the extracellular matrix. Although the molecular and cellular mechanisms are not clear, an increasing number of studies suggest that the BM also actively participates in vascular integrity (Xu et al., 2019).
The complicated structural and functional integrity of the BBB separates the circulating blood from the brain and controls the entry of most blood-derived molecules into the brain (Keaney and Campbell, 2015). Therefore, maintaining BBB integrity and function is critical to regulating the microenvironment of the CNS.
1.2. BBB disruption in CNS disorders
As revealed in numerous studies, BBB breakdown is significantly enhanced under pathophysiological conditions and disruption of the BBB has been observed in many pathologies of CNS diseases, ranging from stroke to inflammatory responses (Daneman, 2012; Jiang et al., 2018; Kamphuis et al., 2015; Liebner et al., 2018; Yang and Rosenberg, 2011). Although it has been well accepted that BBB leakage is a secondary event that occurs in ischemic stroke and traumatic brain injury (TBI), it remains unclear whether BBB leakage occurs in other CNS disorders such as Alzheimer’s disease (AD). BBB breakdown may play an important role in facilitating the entry of immune components to remove existing debris. It may also be harmful by causing brain edema, myelin sheath damage, and further neuronal dysfunction. Both ischemic and hemorrhagic stroke cause BBB breakdown, which could have resulted from increased MMP activity and free reactive oxygen species (ROS) as illustrated in previous reviews (Haley and Lawrence, 2017; Keep et al., 2018). As shown in Figure 1, several molecular events lead to brain endothelial barrier disruption, including alterations in junctions, endothelial cell death, and increased endothelial cell transcytosis (Maiuolo et al., 2018). Structural and functional abnormality of endothelial cell-to-cell junctions, mainly the TJs, is the most notable feature during stroke-induced BBB opening. In addition, increased vascular permeability and loss of TJ-related proteins in brain tumors have been reported (Groothuis et al., 1991; Liebner et al., 2000). Other neuroinflammation-related diseases such as TBI, vascular cognitive impairment and the human immunodeficiency virus (HIV) infection can also cause inflammatory responses in many cell types in the periphery and within the CNS, all leading to an altered BBB integrity (McRae, 2016; Price et al., 2016; Toyama et al., 2019). AD, the most common type of dementia, is considered to be the accumulation of blood-derived products including the amyloid beta peptide (Aβ) and tau protein in brain parenchyma (Sweeney et al., 2018). BBB disruption may allow influx of these products into the brain, which consequently initiates multiple pathways of neurodegeneration. In the course of HIV infection, HIV-1 Tat, a viral protein produced from the HIV-affected cell, has been observed to decrease the expression of TJs and involves multiple signaling pathways (McRae, 2016).
Figure 1. BBB disruption in CNS diseases.
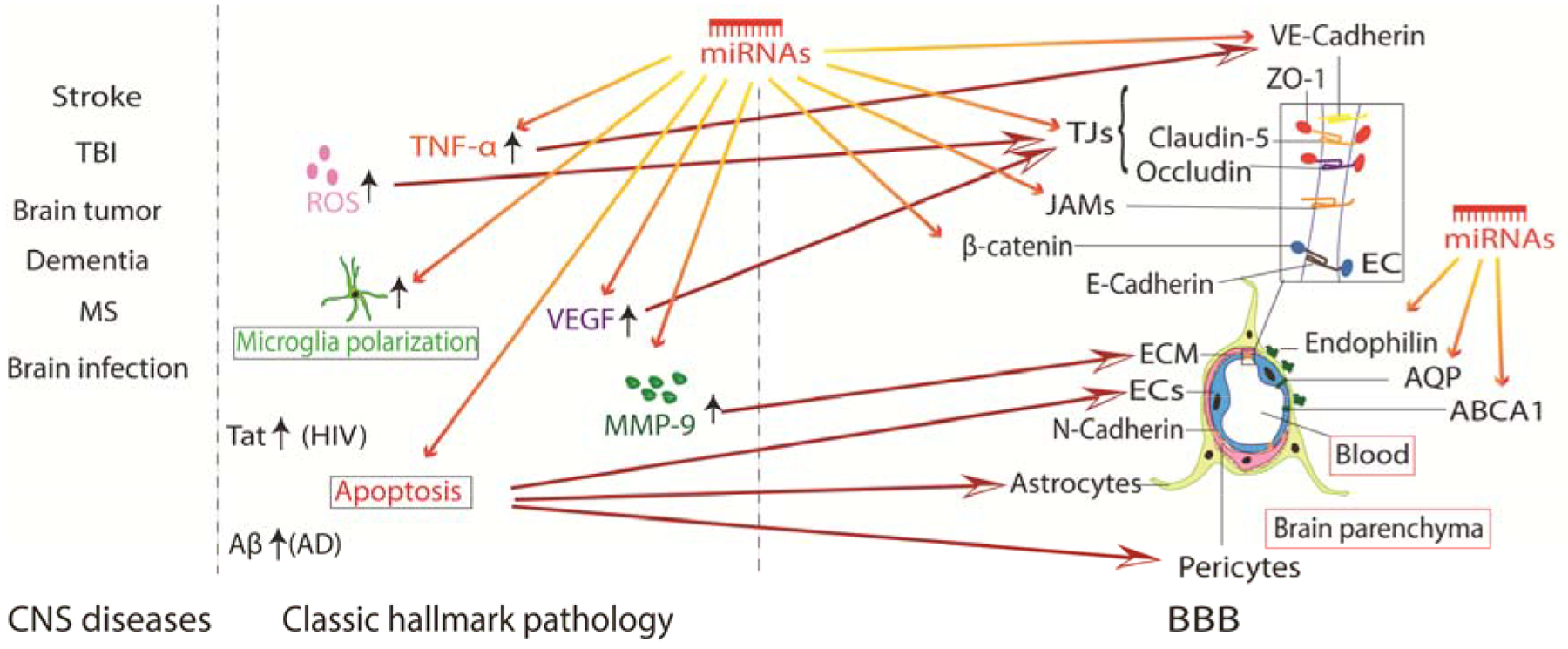
Simplified pathologies of common CNS diseases and the most relevant alterations in the BBB. CNS disorders cause a series of molecular events including apoptosis and activation of ROS, proinflammatory/anti-inflammatory factors, MMPs and immune cells. All of these pathological events contribute to BBB damage by altering BBB composition.
1.3. MicroRNAs
MicroRNAs (miRNAs) are small endogenous non-coding RNAs that modulate gene expression by directly targeting the 3’-untranslated regions (3’-UTR) of messenger RNAs (mRNAs). In humans, at least 98% of the total genome does not contain protein-coding DNA sequences but can be transcribed into non-coding RNAs (International Human Genome Sequencing, 2004). The binding of miRNAs leads to either destabilization or degradation of targeted mRNAs, thereby inhibiting mRNA translation (Inui et al., 2010). Although functional miRNAs (named functional non-coding RNAs at that time) were first reported in the early 1990s, which were studied as functional regulators of RNA-RNA interaction, the involvement of miRNAs in diseases was not reported until 2005 (Lee et al., 1993; Lu et al., 2005; Wightman et al., 1993). Since then, miRNAs have been shown to be important post-transcriptional regulators of gene expression and play vital roles in the cellular process of numerous diseases including cerebrovascular diseases (Blandford et al., 2018; Yin et al., 2014). Vascular endothelial cells are enriched with various miRNAs that are known to be involved in endothelial biology and physiological function.
2. MiRNAs in CNS disorders
To date, studies clearly depict a crucial role of miRNA in brain development and function (Im and Kenny, 2012; Kamphuis et al., 2015; Reijerkerk et al., 2013; Saba and Schratt, 2010; Sun and Shi, 2015; Sun et al., 2018). As important post-transcriptional regulators of gene expression, miRNAs constitute an essential regulatory control of immune cell development and function by turning on several processes triggered by immune cells delicately, and regulating apoptosis in various cell types (Baltimore et al., 2008). In addition, some miRNAs can also modulate BBB integrity by binding to the 3’-UTR of mRNAs encoding several essential junctional proteins. MiR-21, miR-150 and miR-181 are the most studied miRNAs in the regulation of cell development and pathology during CNS diseases. However, most studies were conducted in males only and sex differences were not taken into account. Developmental changes in the expression of miRNAs has been found in rat cortex, and sex differences in ischemic sensitivity may cause different regulation of miRNA responses (Lusardi et al., 2014; Murphy et al., 2014). Sohranji et al. summarized this difference in miRNA expression recently, finding that sex-specific effects exist in the expression of at least two miRNAs: Let7f and miR363–3p (Sohrabji and Selvamani, 2019). In addition, a small cohort of miRNAs were found to be highly expressed in adult females compared with middle-aged females (Selvamani et al., 2014). In this regard, a variety of models that account for sex differences and age are still needed.
2.1. MiRNAs in Stroke
Stroke is a severe CNS disorder that results in vascular and neuronal damage, severe brain tissue injury, and neurological deficits. The most common type of stroke is ischemic stroke, which occurs when the blood supply to oxygen- and glucose-enriched vessels is rapidly interrupted in specific brain regions. Accumulating evidence has shown that ischemic stroke induces rapid alterations of endothelial integrity and function (Haley and Lawrence, 2017; Yang and Rosenberg, 2011). Several mechanisms, including the production of ROS and the activation of MMPs or inflammatory cytokines contribute to the vascular disruption, which ultimately leads to BBB dysfunction (Yin et al., 2014). Some miRNAs have been reported to play a role in post-stroke endothelial pathophysiology and BBB regulation (Table 1).
Table 1.
MiRNAs modulate BBB function in stroke
| Disorders | miRNAs | Study subject | Proposed contribution to BBB | Target gene/Mechanism | Refs |
|---|---|---|---|---|---|
| Ischemic stroke | Negative | ||||
| miR-150 | Rat | Increases BBB permeability | Reducing the claudin-5 expression by binding to angiopoietin receptor Tie-2 | (Fang et al., 2016) | |
| miR-210 | Rat | MiR-210 disrupts BBB integrity | Suppressing the expression of junction proteins by directly binding to occludin and β-catenin | (Ma et al., 2017) | |
| miR-143 | Mouse | The inhibited miR-143 activity maintains BBB integrity | Regulating TJs expression by binding to Hectd 1 | (Bai et al., 2018) | |
| miR-130a | Rat | MiR-130a increases BBB permeability | Down-regulating the expression of occludin by binding to homeobox A5 | (Wang et al., 2018) | |
| miR-15a/16–1 | mouse | MiR-15a/16–1 impairs BBB integrity | Directly binding to claudin-5 | (Zuo et al., 2019) | |
| miR-34a | bEnd.3 cell line | MiR-34a enhances BBB permeability | Impairing mitochondrial function in ECs by binding to CYCS | (Bukeirat et al., 2016) | |
| miR-155 | HBMECs | MiR-155 impairs endothelial monolayer integrity | Reducing the expression of ZO-1 by directly binding to claudin-1 | (Pena-Philippides et al., 2018) | |
| Positive | |||||
| miR-149–5p | Rat | MiR-149–5p reduces BBB permeability | Enhancing N-cadherin expression by binding to S1PR2 | (Song et al., 2018) | |
| miR-539 | Rat | MiR-539 preserves BBB integrity | Mediating BBB structure by binding to MMP-9 | (Fan et al., 2018) | |
| miR-21 | Rat | MiR-21 alleviates I/R-induced BBB disruption | Blocking the MAPK signaling pathway by binding to MAPK2K3 | (Yao et al., 2018) | |
| miR-132 | Mouse | MiR-132 preserves BBB integrity | Regulating the degradation of VE-cadherin and β-catenin by directly binding to MMP-9 | (Zuo et al., 2019) | |
| Hemorrhagic stroke | Positive | ||||
| miR-126–3p | Rat | MiR-126–3p preserves BBB integrity and function | Inhibiting neural inflammation by targeting PIK3/Akt pathway | (Xi et al., 2017) | |
| miR-132 | Mouse | MiR-132 mitigates BBB permeability | Inhibiting the expression of ZO-1 and claudin-5 | (Zhang et al., 2017) | |
| miR-27a-3p | Rat | MiR-27a-3p attenuates brain edema and BBB leakage | Protecting against post-ICH complications by directly binding to AQP11 | (Xi et al., 2018) |
2.1.1. MiRNAs modulate BBB function in ischemic stroke
Early studies have suggested that miRNAs manipulate BBB function in rodent models of ischemic stroke. As shown in Table 1, most miRNAs negatively regulate BBB permeability, such as miR-155, miR-150, miR-210, miR-143, miR-130a, and miR-15a/16–1 (Bai et al., 2018; Caballero-Garrido et al., 2015; Fang et al., 2016; Ma et al., 2017; Yao et al., 2018; Zuo et al., 2019). These miRNAs can enhance ischemia-induced BBB leakage by directly or indirectly targeting TJs including ZO-1, claudin-5, occludin, cadherin, and β-catenin. For example, in a model of distal middle cerebral artery occlusion (dMCAo), improved microvascular integrity in the peri-infarct area was found in miR-155 inhibitor-treated mice (Caballero-Garrido et al., 2015). Further studies revealed that improved TJ integrity in the inhibitor-treated mice could be associated with stabilization of ZO-1, and it was mediated by the miR-155-targeted protein, Ras homolog enriched in brain. In line with the in vivo finding, miR-155 inhibition improved monolayer integrity in human primary cerebral microvascular endothelial cells (hCMEC/D3) after oxygen-glucose deprivation (OGD) exposure and significantly increased the expression of claudin-1 and ZO-1 (Pena-Philippides et al., 2018). Luciferases reporter assay revealed that claudin-1 is directly targeted by miR-155. Inhibition of miR-155 also enhanced the interaction between claudin-1 and ZO-1 and indirectly upregulated the expression of ZO-1. Claudin-5 is considered as the most abundantly expressed claudin in endothelial cell-to-cell junctions, and claudin-5-deficient mice die at birth due to BBB leakage of small molecules (Nitta et al., 2003). MiR-15a/16–1 has been recently reported to impair BBB integrity by directly binding to the 3’-UTR of claudin-5, and endothelial cell-specific deletion of the miR-15a/16–1 cluster in mice presented only minor BBB leakage compared with wild-type controls (Zuo et al., 2019). Moreover, miR-210 is identified as being complementary to the 3’-UTR of mRNA transcripts of occludin and β-catenin, which constitute the TJs and AJs respectively (Ma et al., 2017). When miR-143 activity was inhibited by circular RNA DLGAP4, BBB integrity was maintained in a mouse stroke model (Bai et al., 2018). In this study, miR-143, as a positive modulator of endothelial-mesenchymal transition, inhibited the expression of TJ proteins by targeting homologous to the E6-AP C-terminal domain E3 ubiquitin protein ligase 1. Next, overexpression of miR-34a increased BBB permeability in murine cerebrovascular endothelial cells and inhibited the expression of ZO-1 (Bukeirat et al., 2016). Bioinformatics analysis indicated that miR-34a targeted several mitochondria-associated genes but did not directly target any TJ-related genes. Together with other findings, miR-34a is suggested to enhance BBB permeability through impairing mitochondrial function by binding to cytochrome c gene (CYCS).
On the contrary, miR-149–5p, miR-539, miR-21, and miR-132 are proven to preserve BBB integrity after ischemic stroke via various mechanisms including enhancing TJ expression, regulating MMP-9, and blocking the mitogen-activated protein kinase (MAPK) signaling pathway (Fan et al., 2018; Song et al., 2018; Yao et al., 2018; Zuo et al., 2019). MiR-21, originally identified as an oncogene involved in the pathological process of many diseases, is the most well-studied miRNA during CNS diseases-induced alteration of BBB permeability (Ge et al., 2015; Ji et al., 2018; Yao et al., 2018). The upregulated miR-21 decreases BBB permeability in a rat model of cerebral ischemia-reperfusion (IR) injury and represses the expression of MAPK signaling pathway-related proteins by directly targeting the 3’-UTR of mitogen-activated protein kinase kinase 3 (MAP2K3) (Yao et al., 2018). Besides, MMP-9 has been shown to be a critical effector of BBB breakdown in many CNS disorders, including ischemic stroke. And MMP-9 can be upregulated by a variety of signals such as vascular endothelial growth factor (VEGF), ROS and inflammatory cytokines (Lakhan et al., 2013). MiR-132 and miR-539 were recently reported to preserve post-stroke BBB integrity by directly binding to MMP-9 (Fan et al., 2018; Zuo et al., 2019). MiR-132 is a neuron-enriched miRNA that plays important roles in the developmental plasticity of the neural system and maintains brain vascular integrity (Xu et al., 2017b). Evidence in intact zebrafish suggested that miR-132 regulates the expression of VE-cadherin by directly targeting eukaryotic elongation factor 2 kinase (Xu et al., 2017a). Mice treated with exogenous miR-132 present reduced brain edema compared with the control group; moreover, agomir-132 significantly suppresses the expression of MMP-9 and inhibits the degradation of VE-cadherin and β-catenin in ischemic stroke mice (Zuo et al., 2019).
2.1.2. MiRNAs regulate BBB function in hemorrhagic stroke
Although hemorrhage-induced BBB disruption was less studied than ischemic-induced BBB dysfunction, cerebral hemorrhage can also cause increased BBB permeability, brain edema, and inflammation. There is growing interest in assessing miRNA levels and function in hemorrhage-induced BBB disability. MiR-126, as the only identified miRNA to show an EC-specific expression pattern, has been well studied in vascular development and inflammation (Fish et al., 2008; Harris et al., 2008; Wang et al., 2008). Endothelial miR-126 inhibits the expression of vascular adhesion molecule-1 (VCAM-1), which mediates leukocyte adherence to endothelial cells and limits immune cell infiltration into the brain parenchyma (Harris et al., 2008). In a rat model of collagenase-induced intracerebral hemorrhage (ICH), miR-126–3p was found to attenuate ICH-induced BBB disruption at 24h after ICH, and silencing of miR-126–3p resulted in impaired barrier integrity in rat brain microvascular endothelial cells (BMECs) (Xi et al., 2017). MiR-132 was initially studied for its anti-inflammatory role in several diseases, such as Kaposi’s sarcoma-associated herpesvirus infection and human inflammatory bowel disease (Lagos et al., 2010; Maharshak et al., 2013; Shaked et al., 2009). In the lentiviral miR-132-treated mouse brain, both edema and Evans Blue (EB) leakage were decreased at day 3 after ICH compared with the controls, and the expression levels of classic TJs, such as claudin-5 and ZO-1, were significantly increased (Zhang et al., 2017). These data suggested that miR-132 provided a protective effect on the BBB. Furthermore, in line with the reduced serum levels of miR-27a-3p in ICH patients, rats administrated the miR-27a-3p mimic had attenuated brain edema, EB leakage, and leukocyte infiltration to the perihematomal zone at 24 h after ICH (Xi et al., 2018; Zhu et al., 2015).
The mechanisms involved in miRNA-modulated BBB permeability after ICH can be variable. MiR-126–3p reduces the expression of the phosphoinositide-3-kinase regulatory subunit 2 in the perihematomal region and regulates the PI3K/Akt pathway that is responsible for BBB leakage (Kilic et al., 2006; Xi et al., 2017). Whereas, miR-27a-3p regulates endothelial water channel proteins in rat BMECs by directly binding to the 3’-UTR of aquaporin (AQP)-11, which is highly expressed in the endothelium of brain capillaries and is positively associated with increased BBB permeability (Koike et al., 2016).
2.2. MiRNAs manipulate BBB function in other CNS disorders
A summary of miRNAs involved in BBB function in other CNS disorders was presented in Table 2. Studies from both animal and in vitro models were included.
Table 2.
MiRNAs modulate BBB function in non-stroke CNS diseases
| Diseases | miRNAs | Study subject | Proposed contribution to BBB | Target gene/Mechanism | Refs |
|---|---|---|---|---|---|
| TBI | Negative | ||||
| miR-21–3p | Mouse | MiR-21–3p aggravates BBB damage | Regulating TJ protein expression by binding to MAT2B | (Ge et al., 2019) | |
| Positive | |||||
| miR-21 | Rat | MiR-21 alleviates brain edema and BBB leakage | Reducing the loss of TJ proteins by promoting the expression of Ang-1/Tie-2 axis in BMECs | (Ge et al., 2015; Navi et al., 2014) | |
| miR-212/132 | Mouse | MiR212/132 maintains the BBB integrity | Directly targeting claudin-1, JAM3, and Tjap1 | (Burek et al., 2019) | |
| miR-21–5p | Rat BMVECs | MiR-21–5p alleviates BBB leakage in vitro | Suppressing inflammation and apoptosis | (Ge et al., 2016) | |
| Spinal cord injury | Positive | ||||
| miR-320a | Rat | mir-320a protects BSCB integrity | Binding to AQP1 | (Li et al., 2016) | |
| miR-129–5p | Mouse | miR-129–5p prevents BSCB leakage | Targeting HMGB1 | (Li et al., 2017) | |
| AD | Negative | ||||
| miR-33 | Mouse | MiR-33 impairs cellular cholesterol efflux and increases the accumulation of Aβ in neural cells | Regulating Aβ metabolism by directly targeting ABCA1, promoting Aβ secretion and impairing Aβ clearance | (Kim et al., 2015) | |
| Positive | |||||
| miR-107 | hCMEC/D3 | MiR-107 prevents Aβ-induced BBB disruption | Targeting endophilin-1 | (Liu et al., 2016) | |
| miR-224–5p(miR497–5p) | hCMEC/D3 | MiR-224–5p decreases the BBB permeability | Increasing the expression of ZO-1, occludin, and claudin-5 by binding to endophilin-1 | (Zhu et al., 2019) | |
| VD | Positive | ||||
| miR-501–3p | Mouse | MiR-501–3p decreases the BBB permeability | Directly binding to ZO-1 | (Toyama et al., 2018) | |
| Brain tumor | Negative | ||||
| miR-181c | Mouse | Breast cancer cell-derived EVs contained miR-181 triggers the BBB disruption | Controlling the reorganization of actin filament by suppressing PDPK-1 | (Tominaga et al., 2015) | |
| miR-181a | hCMEC/D3 | MiR-181a impairs BTB integrity | Regulating the expression of ZO-1, occludin, and claudin-5 by binding to KLF6 | (Ma et al., 2014) | |
| miR-18a | hCMEC/D3 | MiR-18a increases the permeability of BTB | Down-regulating the expressions of ZO-1, claudin-5, and occludin by binding to MEF2D | (Zhao et al., 2015c) | |
| miR-34c | hBMEC/D3 | MiR-34c impairs BTB integrity | Regulating the BBB integrity by directly binding to MAZ gene | (Zhao et al., 2015a) | |
| miR-34a | Glioma ECs | MiR-34a impairs BTB integrity | Reducing the expression of TJ-related proteins by directly binding to PKCε gene | (Zhao et al., 2015b) | |
| miR-144 | hCMEC/D3 | MiR-144 increases BTB permeability | Regulating the expression of ZO-1, occludin, and claudin-5 by binding to HSF2 | (Cai et al., 2015) | |
| miR-140 | hCMEC/D3 | MiR-140 increases BTB permeability | Regulating the expression of ZO-1, occludin, and claudin-5 by targeting NFYA | (Ma et al., 2016) | |
| miR-181d-5d | hCMEC/D3 | MiR-181d-5d increases the BTB permeability | Regulating the expression of ZO-1, occludin, and claudin-5 by binding to SOX5 | (Guo et al., 2017) | |
| miR-137 | hCMEC/D3 | MiR-137 increases BTB permeability | Regulating the expression of ZO-1, occludin, and claudin-5 by binding to USF1 | (Yu et al., 2017) | |
| miR-148b-3p | hCMEC/D3 | MiR-148b-3p increases BTB permeability | Regulating the expression of ZO-1, occludin, and CXCR7 by binding to ZO-2 and FOXC1 | (Sa et al., 2017) | |
| piR-DQ590027/miR-17HG | hCMEC/D3 | piR-DQ590027/miR-17HG increases the BBB permeability | Decreasing the expression of ZO-1, occludin, and claudin-5 by binding to miR-153 (miR-377) which binds to FOXR2 | (Leng et al., 2018) | |
| miR-429 | hCMEC/D3 | MiR-429 increases the BTB permeability | Regulating the expression of tight junction proteins by binding to ZO-1 and occludin genes | (Dai et al., 2018) | |
| Positive | |||||
| miR-509 | Mouse | MiR-509 decreases the BBB permeability | Promoting the secretion of TNF-α and inhibiting the expression of MMP-9 by binding to RhoC | (Xing et al., 2015) | |
| miR-497 | Mouse | MiR-497 reduces the permeability of blood vessels | Targeting CHEK1, AKT3, and VEGF-A | (Soriano et al., 2016) | |
| miR-200b | hCMEC/D3 | MiR-200b restores the BTB integrity | Regulating the expression of occludin and claudin-5 by binding to RhoA and ROCKII genes | (Ma and Xue, 2016) | |
| miR-330–3p | hCMEC/D3 | MiR-330–3p decreases the BTB permeability | Increasing the expression of TJs such as ZO-1, occludin, and claudin-5 by binding to PKCα | (Liu et al., 2017) | |
| MS | Negative | ||||
| miR-155 | Mouse | MiR-155 impairs BBB integrity | Reorganizing the structures of vinculin and ZO-1 by binding to DOCK-1, syntenin-1, claudin-1, and annexin-2 genes | Lopez-Ramirez et al. 2014 | |
| miR-125a-5p | hCMEC/D3 | MiR-125a-5p increases brain endothelial cell barrier function and the expression level of 107 miRNAs | Regulating claudin-5 and ZO-1 | Reijerkerk et al. 2013 | |
| miR-126/miR-126* | hCMEC/D3 | MiR-126/miR-126* reduces leukocyte adhesion to endothelial cells | Reducing monocytic and T cell shear-resistant adhesion by binding to VCAM1, CCL2, E selection, and CCL7 | Cerutti et al. 2017 | |
| Positive | |||||
| miR-98 and let-7g* | Mouse | MiR-98 and let-7g* diminish BBB permeability | Reducing pro-inflammation by directly binding to CCL5 gene | Rom et al. 2015 | |
| Brain infection | Negative | ||||
| miR-101 | hBMVECs | MiR-101 impairs BBB integrity and permeability | Inhibiting claudin-5 expression by directly binding to VE-cadherin | Mishra et al. 2013 | |
| miR-155 | Mouse | MiR-155 impairs BBB integrity | Targeting Ang/Tie2 axis | Barker et al. 2017 | |
| miR-1303 | Human umbilical vein ECs | MiR-1303 increases the BBB permeability | Degrading junction proteins claudin-4, claudin-5, VE-cadherin, and ZO-1 by binding to MMP-9 | Song et al. 2018 | |
| Others | Negative | ||||
| miR-29b | Mouse | MiR-29a mediates the opening of the BBB | Directly binding to DNMT3β and subsequently regulating MMP-9 | Kalani et al. 2014 | |
| miR-143 | Mouse | MiR-137 increases BBB permeability | Inducing the activation of NF-κB and p53 transcription factors by binding to PUMA, consequently regulating the expression of TJ-related proteins | Bai et al. 2016 | |
| Positive | |||||
| miR-203 | Z310 cells (immortalized murine choroidal epithelial cells) | MiR-203 preserves the lead-induced BCB integrity | Directly binding to tricellulin | Su et al. 2015 |
2.2. Traumatic brain injury and spinal cord injury
TBI is a complex process that occurs when an external force traumatically injures the brain. The role of miR-21 on BBB integrity in CNS diseases was first studied in TBI. MiR-21 was upregulated in the rat cerebral cortex after moderate TBI and also observed in vessel-like structures in the brain (Redell et al., 2011). Ge et al. demonstrated that up-regulation of miR-21 in rat brains alleviated brain edema and BBB leakage of EB after TBI. The protective effects of miR-21 are achieved by inhibiting apoptosis and promoting the expression of angiopoietin-1 and Tie-2 (Ang-1/Tie-2 axis) in BMECs (Ge et al., 2015; Navi et al., 2014). In addition, miR-21–3p and miR-21–5p were also found to be involved in BBB damage after TBI. MiR-21–5p alleviates scratch injury-induced BBB leakage in rat BMECs through suppressing inflammation and apoptosis, which could be related to its down-regulation of nuclear factor of kappa light polypeptide gene enhancer in B cells (NF-κB) and up-regulation of Akt and Ang-1/Tie-2 activities (Ge et al., 2016; Ge et al., 2019). In contrast, miR-21–3p aggravates TBI-induced BBB damage in mice and regulates OGD-induced cellular apoptosis and inflammation in the bEnd.3 endothelial cell line by targeting methionine adenosyltransferase 2B (MAT2B) (Ge et al., 2019). Moreover, miR-212 and miR-132 are highly expressed in the CNS and participate in synaptic plasticity, neurite outgrowth and endothelial vasodilatory function (Ucar et al., 2012; Wanet et al., 2012). Burek et al. revealed that the miR-212/132 cluster helps maintain BBB integrity by directly targeting the 3’-UTR of claudin-1, JAM 3, occluding, and tight junction-associated protein 1 (Burek et al., 2019).
In a rat model of spinal cord edema, IR resulted in the dysfunction of the blood-spinal cord barrier (BSCB) at 48 h after surgery, and microarray analysis showed that ten miRNAs were upregulated, and seven were downregulated (Li et al., 2016). Further genomic screening and luciferase assays identified that miR-320a protects BSCB integrity by binding to the 3’-UTR of the water-channel protein AQP1, a small integral membrane protein of epithelial and/or glial cells. Whereas, miR-129–5p was found to prevent BSCB leakage by directly targeting the 3’-UTR of high-mobility group box-1 (HMGB1) in a mouse model of spinal cord IR injury (Li et al., 2017).
2.3. MiRNAs in dementia
Dementia is a class of brain vascular diseases contributing to any degree of cognitive impairment or dementia, and it is one of the severest public health concerns for the modern aging world. The two most common forms are AD and vascular dementia (VD) (Goodman et al., 2017). Increasing reports indicated that BBB damage is an important feature in the pathogenesis of dementia and abnormal BBB permeability correlated with enlarged regions of cerebral damage in the white matter of patients with dementia (Toyama et al., 2019; Wong et al., 2019). A meta-analysis revealed that patients with dementia present increased BBB permeability and that BBB impairment mainly occurs in the hippocampus (Farrall and Wardlaw, 2009; Montagne et al., 2015).
As described above, BBB integrity is strictly controlled under physiological condition, whereas AD patients present increased cerebrospinal fluid/serum or cerebrospinal fluid/plasma ratio of albumin and reduced expression of TJ-related proteins (Algotsson and Winblad, 2007; Yamazaki and Kanekiyo, 2017). Moreover, the accumulation of Aβ initiates BBB dysregulation by affecting multiple components of the neurovascular unit. MiR-33 regulates the Aβ level in mouse brains by regulating the ATP-binding cassette transporter A1 (ABCA1), a key regulator of apolipoprotein E lipidation (Kim et al., 2015). Overexpression of miR-33 impairs cellular cholesterol efflux and dramatically increases the extracellular Aβ level by suppressing ABCA1. MiR-107 was found to be significantly downregulated in endothelial cells pre-incubated with Aβ and overexpression of miR-107 largely abrogated Aβ-induced BBB disruption and endothelial cell dysfunction (Liu et al., 2016). This protective role of miR-107 is achieved by miR-107-induced down-regulation of endophilin-1, which is involved in the regulation of BBB permeability and expression of TJ-related proteins (Chen et al., 2015). Moreover, in the human cerebral microvascular endothelial cell line hCMEC/D3, overexpression of miR-224–5p or miR-497–5p decreased BBB permeability in the AD microenvironment (Zhu et al., 2019). Dual-luciferase assay revealed that miR-224–5p or miR-497–5p directly binds to the 3’-UTR of endophilin-1 mRNA, and increases the expression of ZO-1, occluding, and claudin-5 via a miR-224–5p/Endophilin-1 or miR-497–5p/Endophilin-1 signaling cascade.
There are several lines of mechanisms involved in VD, including oxidative stress, neuroinflammation, endothelial cell damage, and astrocyte/pericyte alteration (Parfenov et al., 2019). BBB leakiness is likely due to hypoxia and neuroinflammation-triggered vascular deterioration and apoptosis, resulting in the redistribution of TJs (Biron et al., 2011). Changes in miRNA levels contribute to altered expression of dementia-related proteins, consequently impacting the development of this disease (Viegas et al., 2017). Toyama et al. observed that miR-501–3p was upregulated after bilateral common carotid artery stenosis surgery using a mouse model of vascular cognitive impairment (Toyama et al., 2018). What’s more, miR-501–3p directly binds to the 3’-UTR of ZO-1 mRNA and downregulated transendothelial electric resistance. Manipulating target genes that regulate BBB or TJ integrity could be effective therapy for dementia.
2.4. MiRNAs in brain tumors
Glioma is one of the most common types of primary brain tumors, including astrocytoma, ependymoma, glioblastoma, and oligodendroglioma. Oncogenic or tumor-suppressive miRNAs have been discovered in malignant glioma in recent years (Nie et al., 2019; Petrescu et al., 2019). As shown in Table 2, numerous miRNAs can modulate the permeability and integrity of the BBB in an in vivo or in vitro model of glioma. In a mouse model of brain metastasis, several miRNAs, including miR-181c, were found in cancer-derived extracellular vesicles (EVs). MiR-181c triggers the breakdown of the BBB and promotes the extravasation of cancer cells through the BBB (Tominaga et al., 2015). Although the contribution of miR-181c in this process is not clear, it was found that breast cancer patients presented an increased level of circulating miR-181c, suggesting a major role of miR-181c in the destruction of the BBB. MiR-509 was found to be significantly decreased in brain metastatic lesions, and miR-509 contributes to decreased BBB permeability through promoting the secretion of TNF-α and inhibiting MMP-9 expression by binding to RhoC (Xing et al., 2015). In a mouse model of neuroblastoma, it was reported that miR-497 reduces the proliferation of chemoresistant neuroblastoma cells and inhibited vascular permeability by targeting key genes such as vascular endothelial growth factor A (VEGF-A), which causes a decrease in transendothelial electric resistance of cultured endothelial cells (Argaw et al., 2009; Soriano et al., 2016).
Growing evidence has revealed that other miRNAs are also involved in the manipulation of blood-tumor barrier (BTB) in human endothelial cells as summarized in Table 2. Overexpression of miR-181a, miR-18a, miR-34c, miR-34a, miR-144, miR-140, miR-181d-5d, miR-137, miR-148b-3p, piR-DQ590027/miR17HG, and miR-429 significantly increases the permeability of BTB and disrupts or downregulates the expression of junction-related proteins (Cai et al., 2015; Dai et al., 2018; Guo et al., 2017; Leng et al., 2018; Ma et al., 2016; Ma et al., 2014; Sa et al., 2017; Yu et al., 2017; Zhao et al., 2015a; Zhao et al., 2015b; Zhao et al., 2015c). Knockdown of miR-200b and miR-330–3p restores BTB integrity in vitro (Liu et al., 2017; Ma and Xue, 2016).
2.5. MiRNAs in autoimmune disorders
Multiple Sclerosis (MS) is a chronic immune-mediated disease that attacks the CNS and affects the brain, spinal cord, and optic nerves. In active MS lesions, large numbers of leukocytes infiltrate into brain parenchyma through the immune-activated BBB, which is tightly regulated by various cell adhesion molecules, integrins, cytokines, and chemokines (Aube et al., 2014). Traditional treatments are not available to halt the disease progression and approaches aiming to relieve symptoms and reduce disease progression have been limited to date (Kamphuis et al., 2015). MiR-155 is one of the most highly expressed miRNAs in acute MS lesions and was found to be rapidly upregulated under inflammatory conditions (Lopez-Ramirez et al., 2014). MiR-155 impairs BBB integrity and reorganizes the structure of vinculin and ZO-1 by binding to two focal adhesion components: dedicator of cytokinesis 1 (DOCK-1) and syntenin-1, two interendothelial junctional complex molecules: claudin-1 and annexin-2. Evidence from the in vitro studies suggested that more miRNAs, such as miR-125a-5p and miR-126, are involved. MiR-125a-5p, known as a tumor suppressor miRNA, is reduced in ECs derived from MS lesions and was reported to regulate barrier function-related proteins features such as claudin-5 and ZO-1 under a currently unexplored mechanism (Reijerkerk et al., 2013). MiR-126 and its complement miR-126* originating from the same precursor are amongst the most abundant miRNAs expressed in resting endothelium (Harris et al., 2008). In a human BMEC based in vitro BBB model, down-regulation of endothelial miR-126 and miR-126* enhances monocyte and T cell adhesion to human brain ECs, and increases the expression of E-selectin and VCAM1 respectively, while overexpression of miR-126 reduced VCAM1 and CCL2 levels (Cerutti et al., 2017).
In addition, miR-98 and let-7g* were also identified under neuroinflammatory conditions (Rom et al., 2015). Both miR-98 and let-7g* belong to highly conserved miRNAs, the let-7 group. They are capable of decreasing leukocyte adhesion and migration across the BBB by targeting the inflammatory chemokine ligands, CCL2 and CCL5.
2.6. MiRNAs in cerebral infections
The HIV-1 Tat protein has been shown to affect BBB integrity and permeability in HIV neuropathogenesis (Hui et al., 2012). It was found that miR-101 overexpression effectively suppresses the expression of VE-cadherin and claudin-5 in human BMECs (Mishra and Singh, 2013). MiR-101 has been previously summarized as an important mediator of tumorigenesis in a subset of cancer cells, glioblastoma multiforme, acute lymphocytic leukemia and plays a significant role in cerebral infection (Gui and Shen, 2012). In human BMECs, miR-101 directly binds to mutated VE-cadherin 3’-UTR and consequently influences the expression level of claudin-5. Brain endothelial miR-155 also negatively regulates BBB function during neuroinflammation (Caballero-Garrido et al., 2015; Pena-Philippides et al., 2018). It was demonstrated in a mouse model of severe cerebral malaria that miR-155 enhances BBB permeability through interaction with the Ang/Tie2 axis (Barker et al., 2017). In addition, coxsackievirus A16 is another highly neurotropic virus that is commonly seen in children’s brain enterovirus infection disease. Recently, Song et al. found that miR-1303 increases BBB permeability in human umbilical vein ECs and this effect is associated with degradation of claudin-4, claudin-5, VE-cadherin, and ZO-1 (Song et al., 2018).
2.7. MiRNAs in other CNS disorders
MiRNAs are also found to be involved in other CNS disorders such as hyperhomocysteinemia, lead poisoning, and methamphetamine abuse. Hyperhomocysteinema causes the BBB damage, and miR-29 mimics significantly increases the BBB permeability in hyperhomocysteinemic mouse brain endothelial cells by elevating the expression of MMP-9. Further work has demonstrated that miR-29b regulated BBB dysfunction through DNA methyltransferase 3 beta (DNMT3β) and MMP-9. Lead exposure significantly increases the leakage of the blood-cerebrospinal fluid barrier (BCB) in immortalized murine choroidal epithelial cells, and treatment of a miR-203 inhibitor attenuates lead-induced BCB leakage by binding to the 3’-UTR of tricellulin (Su et al., 2015). Methamphetamine exposure disrupts the BBB structure and Silencing miR-143 ameliorates the increased permeability induced by methamphetamine by targeting p53 up-regulated modulator of apoptosis (PUMA), consequently regulating the expression of TJ related protedownins (Bai et al., 2016).
3. MiRNAs as the target of BBB-based therapies
The BBB is clearly very important for both physiological and pathological conditions of CNS function and miRNAs are important regulators of protein expression that are involved in many cellular processes (Sweeney et al., 2019). Some miRNAs, such as miR-30c, miR-143/miR-145, miR-15a/16–1, and miRNA let-7b, have been identified as candidate biomarkers to aid diagnosis in clinical trials (Baraniskin et al., 2018; Derkow et al., 2018; Sun et al., 2018; Wei et al., 2016). The approval of patisiran, the first RNA interference-based drug, heralds a new era for in this field. Several RNA-based therapies are moving through more mature clinical research and has been summarized in a recent publication (Setten et al., 2019). However, most studies focusing on the therapeutic usage of miRNAs are still at the phase of animal experiments. MiR-494 has been shown to alleviate I/R injury in mouse models via various signal pathways (Lan et al., 2012; Wang et al., 2010; Xiong et al., 2014) and is the only ongoing clinical trial designed to identify its role in the recovery of ischemic stroke (Clinicaltrail.gov. Identifier: NCT03577093). In this regard, more clinical studies will be needed to bridge the gap between preclinical and clinical studies.
In animal studies, intravenous injection of miRNA agomir/mimic or miRNA hairpin inhibitor/antagomir is the most common way of miRNA delivery (Sun et al., 2018). However, the injured brain is not the only target of these special drugs, because miRNA inhibitors or antagomirs may affect other organs or systems as well. The use of exosomes is a possible way to overcome this side effect. Exosomes are 30–100 nm extracellular lipid membrane vesicles secreted by most cell types. Exosomes that contain miRNAs and other small or large molecules can travel to distant tissues to directly influence various aspects of cell behavior (Zhao and Zlokovic, 2017). In some neurological diseases including neurodegenerative disorders, exosome miRNAs can pass through the BBB or transport across the synapse, thereby affecting communication between the blood and brain or neural activities (Blandford et al., 2018). Results from recent studies suggested that exosomes can be efficient delivery tools and their usage in the application of miRNA therapeutic strategies for CNS disorders is under active investigation (Tominaga et al., 2015). However, several issues should be further addressed before using exosomes in clinical trials: the optimization of methods to obtain the most proficient and pure exosomes is critical to ensure therapeutic safety (Lener et al., 2015). In addition, for an effective and particularly safe translation of exosome-based therapies into clinical practice, a higher level of cooperation between researchers, clinicians, and other medical professionals is essential. In addition, miRNA sponges can be another gene therapy strategy that employ artificial non-coding RNA with multiple binding sites for a specific miRNA. Some circle RNAs may act as endogenous sponges that interact with miRNAs and regulate the expression of miRNA-targeted genes or serve as protein decoys to directly control cellular function (Bai et al., 2018; Yu and Kuo, 2019). However, whether the accumulation of circle RNAs is deleterious for nervous system function is still unexplored.
Although these above-described therapeutic molecules are designed to only bind to their targets, blocking or enhancing a single miRNA could probably produce multifaceted effects due to its ability to facilitate multiple compensatory pathways. Moreover, miRNAs at non-physiological concentrations likely have unknown targets that lead to adverse effects by targeting normal cell homeostasis (Rupaimoole and Slack, 2017). It is important to carefully and comprehensively investigate the mRNA “targetome” before proceeding to clinical therapeutics.
Furthermore, the timing of miRNA regulation must also be considered according to their cellular development due to possible developmental changes in miRNA expression.
4. Summary and perspective
MiRNAs are versatile regulatory molecules that influence almost every aspect of BBB structure and function. First, the translational dysfunction of individual proteins that constitute the BBB structure can impair BBB physiological function. Second, miRNAs are involved in regulating biological processes such as apoptosis and inflammation, which can exacerbate BBB dysfunction. Although much progress has been made, the role of miRNAs in BBB function remains poorly characterized. Understanding the mechanisms of miRNAs in regulating BBB structure and function may lead us to discover novel pharmaceutical targets for the development of effective microRNA-based therapies against CNS disorders.
Other miRNAs are also found to regulate endothelial cell barrier permeability in many different peripheral organs or tissues. For instance, decreased expression of miR-30 family members was found to be associated with the loss of cell adhesion by targeting snail-1, which is shown to repress the expression of claudins, occludin, and E-cadherin in pancreatic cells (Cano et al., 2000; Ikenouchi et al., 2003; Joglekar et al., 2009). Rho-activated protein kinase (ROCK1) was found to reduce vascular integrity by preceding cell-cell junction delocalization and miR-146 suppressed the expression of ROCK1 in prostate carcinoma cells (Huang et al., 2008; Lu et al., 2014). MiR-874 promoted intestinal barrier permeability and regulated the expression of occludin and claudin-1 by binding to aquaporins, whereas let-7b preserves the intestinal barrier and promotes the expression of occludin, ZO-1, and protein phosphatase 2A by regulating p38 MAPK molecular pathways (Liu et al., 2018; Zhi et al., 2014). Besides, miR-142–3p/5p, miR-26a, and miR-98 were also widely reported to regulate endothelial barrier integrity in testes, heart, thyroid, lung, and skin (Good et al., 2018; Hu et al., 2015; Hu et al., 2018; Xu et al., 2018; Zhu et al., 2016). Several of these miRNAs may play an essential role in the BBB modulation in CNS disorders as well, and further studies are warranted.
Highlights:
MiRNAs play a crucial role in the regulation of BBB structure and function.
MiRNAs directly regulate the expression of junctional proteins in CNS diseases.
MiRNAs mediate BBB structure and function via multiple mechanisms in CNS diseases.
Acknowledgment
This work was supported by the National Institutes of Health Grants: NS091175, NS094930, NS086820.
Abbreviations:
- ABCA1
ATP-binding cassette transporter A1
- AD
Alzheimer’s disease
- Aβ
Amyloid beta peptide
- AJs
Adherens junction proteins
- Ang
Angiopoietin
- AQP
Aquaporin
- BBB
Blood-brain barrier
- BCB
Blood cerebrospinal fluid barrier
- BMEC
Brain microvascular endothelial cell
- BSCB
blood-spinal cord barrier
- BTB
Blood tumor barrier
- CNS
Central nervous system
- CYCS
Cytochrome c
- dMCAo
Distal middle cerebral artery occlusion
- DNMT3β
DNA methyltransferase 3 beta
- DOCK-1
Dedicator of cytokinesis 1
- EB
Evans Blue
- ECs
Endothelial cells
- EVs
Extracellular vesicles
- FOXC1
Forkhead box C1
- HIV
Human immunodeficiency viruses
- HMGB1
High-mobility group box-1
- HSF2
Heat shock transcription factor 2
- ICH
Intracerebral hemorrhage
- IR
Ischemia-reperfusion
- JAM
Junctional adhesion molecule
- KLF6
Krüppel-like factor 6
- MAT2B
Methionine adenosyltransferase 2B
- MAPK
Mitogen-activated protein kinase
- MEF2D
Myocyte enhancer factor 2D
- MiRNAs
MicroRNAs
- MMPs
Matrix metalloproteases
- mRNAs
Messenger RNAs
- MS
Multiple sclerosis
- MAZ
Myc-associated zinc-finger protein
- NFYA
Nuclear factor YA
- NF-κB
Nuclear factor of kappa light polypeptide gene enhancer in B cells
- OGD
Oxygen-glucose deprivation
- PDPK-1
Phosphoinositide-dependent protein kinase-1
- PKC
Protein kinase C
- PUMA
P53 unregulated modulator of apoptosis
- ROCK1
Rho-activated protein kinase 1
- ROS
Reactive oxygen species
- S1PR2
Sphingosine-1-phosphate receptor 2
- SOX5
Sex determining region Y-box protein 5
- TBI
Traumatic brain injury
- TJs
Tight junction proteins
- TNF-α
Tumor necrosis factor alpha
- USF1
Upstream stimulatory factor 1
- VCAM1
Vascular adhesion molecule 1
- VD
Vascular dementia
- VE-cadherin
Vascular endothelial-cadherin
- VEGF-A
Vascular endothelial growth factor A
- ZO
Zonula occludens
- 3’-UTR
3’- untranslated regions
References
- Algotsson A, Winblad B, 2007. The integrity of the blood-brain barrier in Alzheimer’s disease. Acta neurologica Scandinavica 115, 403–408. [DOI] [PubMed] [Google Scholar]
- Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR, 2009. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proceedings of the National Academy of Sciences of the United States of America 106, 1977–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aube B, Levesque SA, Pare A, Chamma E, Kebir H, Gorina R, Lecuyer MA, Alvarez JI, De Koninck Y, Engelhardt B, Prat A, Cote D, Lacroix S, 2014. Neutrophils mediate blood-spinal cord barrier disruption in demyelinating neuroinflammatory diseases. Journal of immunology 193, 2438–2454. [DOI] [PubMed] [Google Scholar]
- Bai Y, Zhang Y, Han B, Yang L, Chen X, Huang R, Wu F, Chao J, Liu P, Hu G, Zhang JH, Yao H, 2018. Circular RNA DLGAP4 Ameliorates Ischemic Stroke Outcomes by Targeting miR-143 to Regulate Endothelial-Mesenchymal Transition Associated with Blood-Brain Barrier Integrity. The Journal of neuroscience : the official journal of the Society for Neuroscience 38, 32–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, Zhang Y, Hua J, Yang X, Zhang X, Duan M, Zhu X, Huang W, Chao J, Zhou R, Hu G, Yao H, 2016. Silencing microRNA-143 protects the integrity of the blood-brain barrier: implications for methamphetamine abuse. Scientific reports 6, 35642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M, 2004. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiology of disease 16, 1–13. [DOI] [PubMed] [Google Scholar]
- Baltimore D, Boldin MP, O’Connell RM, Rao DS, Taganov KD, 2008. MicroRNAs: new regulators of immune cell development and function. Nature immunology 9, 839–845. [DOI] [PubMed] [Google Scholar]
- Baraniskin A, Chomiak M, Ahle G, Gress T, Buchholz M, Turewicz M, Eisenacher M, Margold M, Schlegel U, Schmiegel W, Hahn S, Schroers R, 2018. MicroRNA-30c as a novel diagnostic biomarker for primary and secondary B-cell lymphoma of the CNS. Journal of neuro-oncology 137, 463–468. [DOI] [PubMed] [Google Scholar]
- Barker KR, Lu Z, Kim H, Zheng Y, Chen J, Conroy AL, Hawkes M, Cheng HS, Njock MS, Fish JE, Harlan JM, Lopez JA, Liles WC, Kain KC, 2017. miR-155 Modifies Inflammation, Endothelial Activation and Blood-Brain Barrier Dysfunction in Cerebral Malaria. Molecular medicine 23, 24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard RS Jr., Reynolds JJ, Bearden SE, 2011. Hyperhomocysteinemia increases permeability of the blood-brain barrier by NMDA receptor-dependent regulation of adherens and tight junctions. Blood 118, 2007–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron KE, Dickstein DL, Gopaul R, Jefferies WA, 2011. Amyloid triggers extensive cerebral angiogenesis causing blood brain barrier permeability and hypervascularity in Alzheimer’s disease. PloS one 6, e23789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandford SN, Galloway DA, Moore CS, 2018. The roles of extracellular vesicle microRNAs in the central nervous system. Glia 66, 2267–2278. [DOI] [PubMed] [Google Scholar]
- Bukeirat M, Sarkar SN, Hu H, Quintana DD, Simpkins JW, Ren X, 2016. MiR-34a regulates blood-brain barrier permeability and mitochondrial function by targeting cytochrome c. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 36, 387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burek M, Konig A, Lang M, Fiedler J, Oerter S, Roewer N, Bohnert M, Thal SC, Blecharz-Lang KG, Woitzik J, Thum T, Forster CY, 2019. Hypoxia-Induced MicroRNA-212/132 Alter Blood-Brain Barrier Integrity Through Inhibition of Tight Junction-Associated Proteins in Human and Mouse Brain Microvascular Endothelial Cells. Transl Stroke Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero-Garrido E, Pena-Philippides JC, Lordkipanidze T, Bragin D, Yang Y, Erhardt EB, Roitbak T, 2015. In Vivo Inhibition of miR-155 Promotes Recovery after Experimental Mouse Stroke. The Journal of neuroscience : the official journal of the Society for Neuroscience 35, 12446–12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Xue Y, Wang P, Wang Z, Li Z, Hu Y, Li Z, Shang X, Liu Y, 2015. The long noncoding RNA TUG1 regulates blood-tumor barrier permeability by targeting miR-144. Oncotarget 6, 19759–19779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA, 2000. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2, 76–83. [DOI] [PubMed] [Google Scholar]
- Cerutti C, Edwards LJ, de Vries HE, Sharrack B, Male DK, Romero IA, 2017. MiR-126 and miR-126* regulate shear-resistant firm leukocyte adhesion to human brain endothelium. Scientific reports 7, 45284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Liu W, Wang P, Xue Y, Su Q, Zeng C, Shang X, 2015. Endophilin-1 regulates blood-brain barrier permeability via EGFR-JNK signaling pathway. Brain research 1606, 44–53. [DOI] [PubMed] [Google Scholar]
- Dai B, Li H, Fan J, Zhao Y, Yin Z, Nie X, Wang DW, Chen C, 2018. MiR-21 protected against diabetic cardiomyopathy induced diastolic dysfunction by targeting gelsolin. Cardiovascular diabetology 17, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, 2012. The blood-brain barrier in health and disease. Ann Neurol 72, 648–672. [DOI] [PubMed] [Google Scholar]
- Derkow K, Rossling R, Schipke C, Kruger C, Bauer J, Fahling M, Stroux A, Schott E, Ruprecht K, Peters O, Lehnardt S, 2018. Distinct expression of the neurotoxic microRNA family let-7 in the cerebrospinal fluid of patients with Alzheimer’s disease. PloS one 13, e0200602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan F, Yang J, Xu Y, Guan S, 2018. MiR-539 Targets MMP-9 to Regulate the Permeability of Blood-Brain Barrier in Ischemia/Reperfusion Injury of Brain. Neurochemical research 43, 2260–2267. [DOI] [PubMed] [Google Scholar]
- Fang Z, He QW, Li Q, Chen XL, Baral S, Jin HJ, Zhu YY, Li M, Xia YP, Mao L, Hu B, 2016. MicroRNA-150 regulates blood-brain barrier permeability via Tie-2 after permanent middle cerebral artery occlusion in rats. FASEB J 30, 2097–2107. [DOI] [PubMed] [Google Scholar]
- Farrall AJ, Wardlaw JM, 2009. Blood-brain barrier: ageing and microvascular disease--systematic review and meta-analysis. Neurobiology of aging 30, 337–352. [DOI] [PubMed] [Google Scholar]
- Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D, 2008. miR-126 regulates angiogenic signaling and vascular integrity. Developmental cell 15, 272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Han Z, Chen F, Wang H, Zhang B, Jiang R, Lei P, Zhang J, 2015. MiR-21 alleviates secondary blood-brain barrier damage after traumatic brain injury in rats. Brain research 1603, 150–157. [DOI] [PubMed] [Google Scholar]
- Ge X, Huang S, Gao H, Han Z, Chen F, Zhang S, Wang Z, Kang C, Jiang R, Yue S, Lei P, Zhang J, 2016. miR-21–5p alleviates leakage of injured brain microvascular endothelial barrier in vitro through suppressing inflammation and apoptosis. Brain research 1650, 31–40. [DOI] [PubMed] [Google Scholar]
- Ge X, Li W, Huang S, Yin Z, Yang M, Han Z, Han Z, Chen F, Wang H, Lei P, Zhang J, 2019. Increased miR-21–3p in Injured Brain Microvascular Endothelial Cells after Traumatic Brain Injury Aggravates Blood-Brain Barrier Damage by Promoting Cellular Apoptosis and Inflammation through Targeting MAT2B. Journal of neurotrauma 36, 1291–1305. [DOI] [PubMed] [Google Scholar]
- Good RJ, Hernandez-Lagunas L, Allawzi A, Maltzahn JK, Vohwinkel CU, Upadhyay AK, Kompella UB, Birukov KG, Carpenter TC, Sucharov CC, Nozik-Grayck E, 2018. MicroRNA dysregulation in lung injury: the role of the miR-26a/EphA2 axis in regulation of endothelial permeability. American journal of physiology. Lung cellular and molecular physiology 315, L584–L594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RA, Lochner KA, Thambisetty M, Wingo TS, Posner SF, Ling SM, 2017. Prevalence of dementia subtypes in United States Medicare fee-for-service beneficiaries, 2011–2013. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 13, 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groothuis DR, Vriesendorp FJ, Kupfer B, Warnke PC, Lapin GD, Kuruvilla A, Vick NA, Mikhael MA, Patlak CS, 1991. Quantitative measurements of capillary transport in human brain tumors by computed tomography. Ann Neurol 30, 581–588. [DOI] [PubMed] [Google Scholar]
- Gui T, Shen K, 2012. miRNA-101: a potential target for tumor therapy. Cancer epidemiology 36, 537–540. [DOI] [PubMed] [Google Scholar]
- Guo J, Cai H, Zheng J, Liu X, Liu Y, Ma J, Que Z, Gong W, Gao Y, Tao W, Xue Y, 2017. Long non-coding RNA NEAT1 regulates permeability of the blood-tumor barrier via miR-181d-5p-mediated expression changes in ZO-1, occludin, and claudin-5. Biochimica et biophysica acta. Molecular basis of disease 1863, 2240–2254. [DOI] [PubMed] [Google Scholar]
- Haley MJ, Lawrence CB, 2017. The blood-brain barrier after stroke: Structural studies and the role of transcytotic vesicles. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 37, 456–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ, 2008. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proceedings of the National Academy of Sciences of the United States of America 105, 1516–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartsock A, Nelson WJ, 2008. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochimica et biophysica acta 1778, 660–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Yu Y, Wang C, Li D, Tai Y, Fang L, 2015. microRNA-98 mediated microvascular hyperpermeability during burn shock phase via inhibiting FIH-1. European journal of medical research 20, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Song J, Liu L, Zhang Y, Wang L, Li Q, 2018. microRNA-4516 Contributes to Different Functions of Epithelial Permeability Barrier by Targeting Poliovirus Receptor Related Protein 1 in Enterovirus 71 and Coxsackievirus A16 Infections. Frontiers in cellular and infection microbiology 8, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WH, Chen KH, Hsu CW, Chen YC, Hung CC, Huang JY, Lin JL, Yang CW, 2008. Residual renal function - one of the factors associated with arterial stiffness in peritoneal dialysis patients. Insight from a retrospective study in 146 peritoneal dialysis patients. Blood purification 26, 133–137. [DOI] [PubMed] [Google Scholar]
- Hui L, Chen X, Bhatt D, Geiger NH, Rosenberger TA, Haughey NJ, Masino SA, Geiger JD, 2012. Ketone bodies protection against HIV-1 Tat-induced neurotoxicity. Journal of neurochemistry 122, 382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikenouchi J, Matsuda M, Furuse M, Tsukita S, 2003. Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. Journal of cell science 116, 1959–1967. [DOI] [PubMed] [Google Scholar]
- Im HI, Kenny PJ, 2012. MicroRNAs in neuronal function and dysfunction. Trends in neurosciences 35, 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Human Genome Sequencing, C., 2004. Finishing the euchromatic sequence of the human genome. Nature 431, 931–945. [DOI] [PubMed] [Google Scholar]
- Inui M, Martello G, Piccolo S, 2010. MicroRNA control of signal transduction. Nature reviews. Molecular cell biology 11, 252–263. [DOI] [PubMed] [Google Scholar]
- Ji W, Jiao J, Cheng C, Shao J, 2018. MicroRNA-21 in the Pathogenesis of Traumatic Brain Injury. Neurochemical research 43, 1863–1868. [DOI] [PubMed] [Google Scholar]
- Jiang X, Andjelkovic AV, Zhu L, Yang T, Bennett MVL, Chen J, Keep RF, Shi Y, 2018. Blood-brain barrier dysfunction and recovery after ischemic stroke. Progress in neurobiology 163-164, 144–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joglekar MV, Patil D, Joglekar VM, Rao GV, Reddy DN, Mitnala S, Shouche Y, Hardikar AA, 2009. The miR-30 family microRNAs confer epithelial phenotype to human pancreatic cells. Islets 1, 137–147. [DOI] [PubMed] [Google Scholar]
- Kalani A, Kamat PK, Familtseva A, Chaturvedi P, Muradashvili N, Narayanan N, Tyagi SC, Tyagi N, 2014. Role of microRNA29b in blood-brain barrier dysfunction during hyperhomocysteinemia: an epigenetic mechanism. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 34, 1212–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphuis WW, Derada Troletti C, Reijerkerk A, Romero IA, de Vries HE, 2015. The blood-brain barrier in multiple sclerosis: microRNAs as key regulators. CNS & neurological disorders drug targets 14, 157–167. [DOI] [PubMed] [Google Scholar]
- Keaney J, Campbell M, 2015. The dynamic blood-brain barrier. The FEBS journal 282, 4067–4079. [DOI] [PubMed] [Google Scholar]
- Keep RF, Andjelkovic AV, Xiang J, Stamatovic SM, Antonetti DA, Hua Y, Xi G, 2018. Brain endothelial cell junctions after cerebral hemorrhage: Changes, mechanisms and therapeutic targets. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 38, 1255–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic E, Kilic U, Wang Y, Bassetti CL, Marti HH, Hermann DM, 2006. The phosphatidylinositol-3 kinase/Akt pathway mediates VEGF’s neuroprotective activity and induces blood brain barrier permeability after focal cerebral ischemia. FASEB J 20, 1185–1187. [DOI] [PubMed] [Google Scholar]
- Kim J, Yoon H, Horie T, Burchett JM, Restivo JL, Rotllan N, Ramirez CM, Verghese PB, Ihara M, Hoe HS, Esau C, Fernandez-Hernando C, Holtzman DM, Cirrito JR, Ono K, Kim J, 2015. microRNA-33 Regulates ApoE Lipidation and Amyloid-beta Metabolism in the Brain. The Journal of neuroscience : the official journal of the Society for Neuroscience 35, 14717–14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike S, Tanaka Y, Matsuzaki T, Morishita Y, Ishibashi K, 2016. Aquaporin-11 (AQP11) Expression in the Mouse Brain. International journal of molecular sciences 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagos D, Pollara G, Henderson S, Gratrix F, Fabani M, Milne RSB, Gotch F, Boshoff C, 2010. miR-132 regulates antiviral innate immunity through suppression of the p300 transcriptional co-activator. Nature Cell Biology 12, 513. [DOI] [PubMed] [Google Scholar]
- Lakhan SE, Kirchgessner A, Tepper D, Leonard A, 2013. Matrix metalloproteinases and blood-brain barrier disruption in acute ischemic stroke. Frontiers in neurology 4, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan YF, Chen HH, Lai PF, Cheng CF, Huang YT, Lee YC, Chen TW, Lin H, 2012. MicroRNA-494 reduces ATF3 expression and promotes AKI. Journal of the American Society of Nephrology : JASN 23, 2012–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RC, Feinbaum RL, Ambros V, 1993. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75, 843–854. [DOI] [PubMed] [Google Scholar]
- Lener T, Gimona M, Aigner L, Borger V, Buzas E, Camussi G, Chaput N, Chatterjee D, Court FA, Del Portillo HA, O’Driscoll L, Fais S, Falcon-Perez JM, Felderhoff-Mueser U, Fraile L, Gho YS, Gorgens A, Gupta RC, Hendrix A, Hermann DM, Hill AF, Hochberg F, Horn PA, de Kleijn D, Kordelas L, Kramer BW, Kramer-Albers EM, Laner-Plamberger S, Laitinen S, Leonardi T, Lorenowicz MJ, Lim SK, Lotvall J, Maguire CA, Marcilla A, Nazarenko I, Ochiya T, Patel T, Pedersen S, Pocsfalvi G, Pluchino S, Quesenberry P, Reischl IG, Rivera FJ, Sanzenbacher R, Schallmoser K, Slaper-Cortenbach I, Strunk D, Tonn T, Vader P, van Balkom BW, Wauben M, Andaloussi SE, Thery C, Rohde E, Giebel B, 2015. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. Journal of extracellular vesicles 4, 30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng X, Ma J, Liu Y, Shen S, Yu H, Zheng J, Liu X, Liu L, Chen J, Zhao L, Ruan X, Xue Y, 2018. Mechanism of piR-DQ590027/MIR17HG regulating the permeability of glioma conditioned normal BBB. Journal of experimental & clinical cancer research : CR 37, 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XQ, Chen FS, Tan WF, Fang B, Zhang ZL, Ma H, 2017. Elevated microRNA-129–5p level ameliorates neuroinflammation and blood-spinal cord barrier damage after ischemia-reperfusion by inhibiting HMGB1 and the TLR3-cytokine pathway. Journal of neuroinflammation 14, 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XQ, Fang B, Tan WF, Wang ZL, Sun XJ, Zhang ZL, Ma H, 2016. miR-320a affects spinal cord edema through negatively regulating aquaporin-1 of blood-spinal cord barrier during bimodal stage after ischemia reperfusion injury in rats. BMC neuroscience 17, 10. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Liebner S, Dijkhuizen RM, Reiss Y, Plate KH, Agalliu D, Constantin G, 2018. Functional morphology of the blood-brain barrier in health and disease. Acta neuropathologica 135, 311–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebner S, Fischmann A, Rascher G, Duffner F, Grote EH, Kalbacher H, Wolburg H, 2000. Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme. Acta neuropathologica 100, 323–331. [DOI] [PubMed] [Google Scholar]
- Liu J, Cai G, Li M, Fan S, Yao B, Ping W, Huang Z, Cai H, Dai Y, Wang L, Huang X, 2018. Fibroblast growth factor 21 attenuates hypoxia-induced pulmonary hypertension by upregulating PPARgamma expression and suppressing inflammatory cytokine levels. Biochemical and biophysical research communications 504, 478–484. [DOI] [PubMed] [Google Scholar]
- Liu J, Liu L, Chao S, Liu Y, Liu X, Zheng J, Chen J, Gong W, Teng H, Li Z, Wang P, Xue Y, 2017. The Role of miR-330–3p/PKC-alpha Signaling Pathway in Low-Dose Endothelial-Monocyte Activating Polypeptide-II Increasing the Permeability of Blood-Tumor Barrier. Frontiers in cellular neuroscience 11, 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Cai H, Lin M, Zhu L, Gao L, Zhong R, Bi S, Xue Y, Shang X, 2016. MicroRNA-107 prevents amyloid-beta induced blood-brain barrier disruption and endothelial cell dysfunction by targeting Endophilin-1. Experimental cell research 343, 248–257. [DOI] [PubMed] [Google Scholar]
- Lopez-Ramirez MA, Wu D, Pryce G, Simpson JE, Reijerkerk A, King-Robson J, Kay O, de Vries HE, Hirst MC, Sharrack B, Baker D, Male DK, Michael GJ, Romero IA, 2014. MicroRNA-155 negatively affects blood-brain barrier function during neuroinflammation. FASEB J 28, 2551–2565. [DOI] [PubMed] [Google Scholar]
- Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR, 2005. MicroRNA expression profiles classify human cancers. Nature 435, 834–838. [DOI] [PubMed] [Google Scholar]
- Lu QY, Chen W, Lu L, Zheng Z, Xu X, 2014. Involvement of RhoA/ROCK1 signaling pathway in hyperglycemia-induced microvascular endothelial dysfunction in diabetic retinopathy. International journal of clinical and experimental pathology 7, 7268–7277. [PMC free article] [PubMed] [Google Scholar]
- Lusardi TA, Murphy SJ, Phillips JI, Chen Y, Davis CM, Young JM, Thompson SJ, Saugstad JA, 2014. MicroRNA responses to focal cerebral ischemia in male and female mouse brain. Frontiers in molecular neuroscience 7, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Wang P, Yao Y, Liu Y, Li Z, Liu X, Li Z, Zhao X, Xi Z, Teng H, Liu J, Xue Y, 2016. Knockdown of long non-coding RNA MALAT1 increases the blood-tumor barrier permeability by up-regulating miR-140. Biochimica et biophysica acta 1859, 324–338. [DOI] [PubMed] [Google Scholar]
- Ma J, Yao Y, Wang P, Liu Y, Zhao L, Li Z, Li Z, Xue Y, 2014. MiR-181a regulates blood-tumor barrier permeability by targeting Kruppel-like factor 6. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 34, 1826–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Dasgupta C, Li Y, Huang L, Zhang L, 2017. MicroRNA-210 Suppresses Junction Proteins and Disrupts Blood-Brain Barrier Integrity in Neonatal Rat Hypoxic-Ischemic Brain Injury. International journal of molecular sciences 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Xue YX, 2016. MiRNA-200b Regulates RMP7-Induced Increases in Blood-Tumor Barrier Permeability by Targeting RhoA and ROCKII. Frontiers in molecular neuroscience 9, 9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Maharshak N, Shenhar-Tsarfaty S, Aroyo N, Orpaz N, Guberman I, Canaani J, Halpern Z, Dotan I, Berliner S, Soreq H, 2013. MicroRNA-132 modulates cholinergic signaling and inflammation in human inflammatory bowel disease. Inflammatory bowel diseases 19, 1346–1353. [DOI] [PubMed] [Google Scholar]
- Maiuolo J, Gliozzi M, Musolino V, Scicchitano M, Carresi C, Scarano F, Bosco F, Nucera S, Ruga S, Zito MC, Mollace R, Palma E, Fini M, Muscoli C, Mollace V, 2018. The “Frail” Brain Blood Barrier in Neurodegenerative Diseases: Role of Early Disruption of Endothelial Cell-to-Cell Connections. International journal of molecular sciences 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae M, 2016. HIV and viral protein effects on the blood brain barrier. Tissue barriers 4, e1143543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra R, Singh SK, 2013. HIV-1 Tat C modulates expression of miRNA-101 to suppress VE-cadherin in human brain microvascular endothelial cells. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 5992–6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV, 2015. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 85, 296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SJ, Lusardi TA, Phillips JI, Saugstad JA, 2014. Sex differences in microRNA expression during development in rat cortex. Neurochemistry international 77, 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navi BB, Singer S, Merkler AE, Cheng NT, Stone JB, Kamel H, Iadecola C, Elkind MS, DeAngelis LM, 2014. Recurrent thromboembolic events after ischemic stroke in patients with cancer. Neurology 83, 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie JH, Li TX, Zhang XQ, Liu J, 2019. Roles of Non-Coding RNAs in Normal Human Brain Development, Brain Tumor, and Neuropsychiatric Disorders. Non-coding RNA 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S, 2003. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol 161, 653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfenov VA, Ostroumova OD, Ostroumova TM, Kochetkov AI, Fateeva VV, Khacheva KK, Khakimova GR, Epstein OI, 2019. Vascular cognitive impairment: pathophysiological mechanisms, insights into structural basis, and perspectives in specific treatments. Neuropsychiatric disease and treatment 15, 1381–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena-Philippides JC, Gardiner AS, Caballero-Garrido E, Pan R, Zhu Y, Roitbak T, 2018. Inhibition of MicroRNA-155 Supports Endothelial Tight Junction Integrity Following Oxygen-Glucose Deprivation. J Am Heart Assoc 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrescu GED, Sabo AA, Torsin LI, Calin GA, Dragomir MP, 2019. MicroRNA based theranostics for brain cancer: basic principles. Journal of experimental & clinical cancer research : CR 38, 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price L, Wilson C, Grant G, 2016. Blood-Brain Barrier Pathophysiology following Traumatic Brain Injury, in: Laskowitz D, Grant G (Eds.), Translational Research in Traumatic Brain Injury, Boca Raton (FL). [PubMed] [Google Scholar]
- Redell JB, Zhao J, Dash PK, 2011. Altered expression of miRNA-21 and its targets in the hippocampus after traumatic brain injury. Journal of neuroscience research 89, 212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijerkerk A, Lopez-Ramirez MA, van Het Hof B, Drexhage JA, Kamphuis WW, Kooij G, Vos JB, van der Pouw Kraan TC, van Zonneveld AJ, Horrevoets AJ, Prat A, Romero IA, de Vries HE, 2013. MicroRNAs regulate human brain endothelial cell-barrier function in inflammation: implications for multiple sclerosis. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 6857–6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rom S, Dykstra H, Zuluaga-Ramirez V, Reichenbach NL, Persidsky Y, 2015. miR-98 and let-7g* protect the blood-brain barrier under neuroinflammatory conditions. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 35, 1957–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupaimoole R, Slack FJ, 2017. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nature reviews. Drug discovery 16, 203–222. [DOI] [PubMed] [Google Scholar]
- Sa L, Li Y, Zhao L, Liu Y, Wang P, Liu L, Li Z, Ma J, Cai H, Xue Y, 2017. The Role of HOTAIR/miR-148b-3p/USF1 on Regulating the Permeability of BTB. Frontiers in molecular neuroscience 10, 194. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Saba R, Schratt GM, 2010. MicroRNAs in neuronal development, function and dysfunction. Brain research 1338, 3–13. [DOI] [PubMed] [Google Scholar]
- Selvamani A, Williams MH, Miranda RC, Sohrabji F, 2014. Circulating miRNA profiles provide a biomarker for severity of stroke outcomes associated with age and sex in a rat model. Clinical science 127, 77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setten RL, Rossi JJ, Han SP, 2019. The current state and future directions of RNAi-based therapeutics. Nature reviews. Drug discovery 18, 421–446. [DOI] [PubMed] [Google Scholar]
- Shaked I, Meerson A, Wolf Y, Avni R, Greenberg D, Gilboa-Geffen A, Soreq H, 2009. MicroRNA-132 potentiates cholinergic anti-inflammatory signaling by targeting acetylcholinesterase. Immunity 31, 965–973. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Selvamani A, 2019. Sex differences in miRNA as therapies for ischemic stroke. Neurochemistry international 127, 56–63. [DOI] [PubMed] [Google Scholar]
- Song J, Hu Y, Li H, Huang X, Zheng H, Hu Y, Wang J, Jiang X, Li J, Yang Z, Fan H, Guo L, Shi H, He Z, Yang F, Wang X, Dong S, Li Q, Liu L, 2018. miR-1303 regulates BBB permeability and promotes CNS lesions following CA16 infections by directly targeting MMP9. Emerging microbes & infections 7, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano A, Paris-Coderch L, Jubierre L, Martinez A, Zhou X, Piskareva O, Bray I, Vidal I, Almazan-Moga A, Molist C, Roma J, Bayascas JR, Casanovas O, Stallings RL, Sanchez de Toledo J, Gallego S, Segura MF, 2016. MicroRNA-497 impairs the growth of chemoresistant neuroblastoma cells by targeting cell cycle, survival and vascular permeability genes. Oncotarget 7, 9271–9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su P, Zhao F, Cao Z, Zhang J, Aschner M, Luo W, 2015. Mir-203-mediated tricellulin mediates lead-induced in vitro loss of blood-cerebrospinal fluid barrier (BCB) function. Toxicology in vitro : an international journal published in association with BIBRA 29, 1185–1194. [DOI] [PubMed] [Google Scholar]
- Sun E, Shi Y, 2015. MicroRNAs: Small molecules with big roles in neurodevelopment and diseases. Experimental neurology 268, 46–53. [DOI] [PubMed] [Google Scholar]
- Sun P, Liu DZ, Jickling GC, Sharp FR, Yin KJ, 2018. MicroRNA-based therapeutics in central nervous system injuries. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 38, 1125–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MD, Sagare AP, Zlokovic BV, 2018. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nature reviews. Neurology 14, 133–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MD, Zhao Z, Montagne A, Nelson AR, Zlokovic BV, 2019. Blood-Brain Barrier: From Physiology to Disease and Back. Physiological reviews 99, 21–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga N, Kosaka N, Ono M, Katsuda T, Yoshioka Y, Tamura K, Lotvall J, Nakagama H, Ochiya T, 2015. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nature communications 6, 6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama K, Spin JM, Deng AC, Huang TT, Wei K, Wagenhauser MU, Yoshino T, Nguyen H, Mulorz J, Kundu S, Raaz U, Adam M, Schellinger IN, Jagger A, Tsao PS, 2018. MicroRNA-Mediated Therapy Modulating Blood-Brain Barrier Disruption Improves Vascular Cognitive Impairment. Arteriosclerosis, thrombosis, and vascular biology 38, 1392–1406. [DOI] [PubMed] [Google Scholar]
- Toyama K, Spin JM, Mogi M, Tsao PS, 2019. Therapeutic perspective on vascular cognitive impairment. Pharmacological research 146, 104266. [DOI] [PubMed] [Google Scholar]
- Ucar A, Gupta SK, Fiedler J, Erikci E, Kardasinski M, Batkai S, Dangwal S, Kumarswamy R, Bang C, Holzmann A, Remke J, Caprio M, Jentzsch C, Engelhardt S, Geisendorf S, Glas C, Hofmann TG, Nessling M, Richter K, Schiffer M, Carrier L, Napp LC, Bauersachs J, Chowdhury K, Thum T, 2012. The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy. Nature communications 3, 1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viegas ATB, Guedes JR, Oliveira AR, Cardoso AMS, Cardoso ALC, 2017. miRNAs: New Biomarkers and Therapeutic Targets in Dementia. Current pharmaceutical design 23, 669–692. [DOI] [PubMed] [Google Scholar]
- Wanet A, Tacheny A, Arnould T, Renard P, 2012. miR-212/132 expression and functions: within and beyond the neuronal compartment. Nucleic acids research 40, 4742–4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN, 2008. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Developmental cell 15, 261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang X, Ren XP, Chen J, Liu H, Yang J, Medvedovic M, Hu Z, Fan GC, 2010. MicroRNA-494 targeting both proapoptotic and antiapoptotic proteins protects against ischemia/reperfusion-induced cardiac injury. Circulation 122, 1308–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang MD, Xia YP, Gao Y, Zhu YY, Chen SC, Mao L, He QW, Yue ZY, Hu B, 2018. MicroRNA-130a regulates cerebral ischemia-induced blood-brain barrier permeability by targeting Homeobox A5. FASEB J 32, 935–944. [DOI] [PubMed] [Google Scholar]
- Wei YS, Xiang Y, Liao PH, Wang JL, Peng YF, 2016. An rs4705342 T>C polymorphism in the promoter of miR-143/145 is associated with a decreased risk of ischemic stroke. Scientific reports 6, 34620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman B, Ha I, Ruvkun G, 1993. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 75, 855–862. [DOI] [PubMed] [Google Scholar]
- Wolburg H, Lippoldt A, 2002. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascular pharmacology 38, 323–337. [DOI] [PubMed] [Google Scholar]
- Wong AD, Ye M, Levy AF, Rothstein JD, Bergles DE, Searson PC, 2013. The blood-brain barrier: an engineering perspective. Frontiers in neuroengineering 6, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SM, Jansen JFA, Zhang CE, Hoff EI, Staals J, van Oostenbrugge RJ, Backes WH, 2019. Blood-brain barrier impairment and hypoperfusion are linked in cerebral small vessel disease. Neurology 92, e1669–e1677. [DOI] [PubMed] [Google Scholar]
- Xi T, Jin F, Zhu Y, Wang J, Tang L, Wang Y, Liebeskind DS, He Z, 2017. MicroRNA-126–3p attenuates blood-brain barrier disruption, cerebral edema and neuronal injury following intracerebral hemorrhage by regulating PIK3R2 and Akt. Biochemical and biophysical research communications 494, 144–151. [DOI] [PubMed] [Google Scholar]
- Xi T, Jin F, Zhu Y, Wang J, Tang L, Wang Y, Liebeskind DS, Scalzo F, He Z, 2018. miR-27a-3p protects against blood-brain barrier disruption and brain injury after intracerebral hemorrhage by targeting endothelial aquaporin-11. The Journal of biological chemistry 293, 20041–20050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing F, Sharma S, Liu Y, Mo YY, Wu K, Zhang YY, Pochampally R, Martinez LA, Lo HW, Watabe K, 2015. miR-509 suppresses brain metastasis of breast cancer cells by modulating RhoC and TNF-alpha. Oncogene 34, 4890–4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong R, Wang Z, Zhao Z, Li H, Chen W, Zhang B, Wang L, Wu L, Li W, Ding J, Chen S, 2014. MicroRNA-494 reduces DJ-1 expression and exacerbates neurodegeneration. Neurobiology of aging 35, 705–714. [DOI] [PubMed] [Google Scholar]
- Xu B, Zhang Y, Du X-F, Li J, Zi H-X, Bu J-W, Yan Y, Han H, Du J-L, 2017a. Neurons secrete miR-132-containing exosomes to regulate brain vascular integrity. Cell research 27, 882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Zhang Y, Du XF, Li J, Zi HX, Bu JW, Yan Y, Han H, Du JL, 2017b. Neurons secrete miR-132-containing exosomes to regulate brain vascular integrity. Cell research 27, 882–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Nirwane A, Yao Y, 2019. Basement membrane and blood-brain barrier. Stroke Vasc Neurol 4, 78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Wu W, Fan Y, Jiang S, Jia X, Su W, 2018. MiR-142–3p Inhibits TGF-beta3-Induced Blood-Testis Barrier Impairment by Targeting Lethal Giant Larvae Homolog 2. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology 46, 253–268. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Kanekiyo T, 2017. Blood-Brain Barrier Dysfunction and the Pathogenesis of Alzheimer’s Disease. International journal of molecular sciences 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Rosenberg GA, 2011. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke 42, 3323–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X, Wang Y, Zhang D, 2018. microRNA-21 Confers Neuroprotection Against Cerebral Ischemia-Reperfusion Injury and Alleviates Blood-Brain Barrier Disruption in Rats via the MAPK Signaling Pathway. J Mol Neurosci 65, 43–53. [DOI] [PubMed] [Google Scholar]
- Yin KJ, Hamblin M, Chen YE, 2014. Non-coding RNAs in cerebral endothelial pathophysiology: emerging roles in stroke. Neurochemistry international 77, 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CY, Kuo h.c., 2019. The emerging roles and functions of circular RNAs and their generation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Xue Y, Wang P, Liu X, Ma J, Zheng J, Li Z, Li Z, Cai H, Liu Y, 2017. Knockdown of long non-coding RNA XIST increases blood-tumor barrier permeability and inhibits glioma angiogenesis by targeting miR-137. Oncogenesis 6, e303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Han B, He Y, Li D, Ma X, Liu Q, Hao J, 2017. MicroRNA-132 attenuates neurobehavioral and neuropathological changes associated with intracerebral hemorrhage in mice. Neurochemistry international 107, 182–190. [DOI] [PubMed] [Google Scholar]
- Zhao L, Wang P, Liu Y, Ma J, Xue Y, 2015a. miR-34c regulates the permeability of blood-tumor barrier via MAZ-mediated expression changes of ZO-1, occludin, and claudin-5. Journal of cellular physiology 230, 716–731. [DOI] [PubMed] [Google Scholar]
- Zhao W, Wang P, Ma J, Liu YH, Li Z, Li ZQ, Wang ZH, Chen LY, Xue YX, 2015b. MiR-34a regulates blood-tumor barrier function by targeting protein kinase Cepsilon. Molecular biology of the cell 26, 1786–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YY, Zhao LN, Wang P, Miao YS, Liu YH, Wang ZH, Ma J, Li Z, Li ZQ, Xue YX, 2015c. Overexpression of miR-18a negatively regulates myocyte enhancer factor 2D to increase the permeability of the blood-tumor barrier via Kruppel-like factor 4-mediated downregulation of zonula occluden-1, claudin-5, and occludin. Journal of neuroscience research 93, 1891–1902. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV, 2015d. Establishment and Dysfunction of the Blood-Brain Barrier. Cell 163, 1064–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Zlokovic BV, 2017. Remote control of BBB: A tale of exosomes and microRNA. Cell research 27, 849–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi X, Tao J, Li Z, Jiang B, Feng J, Yang L, Xu H, Xu Z, 2014. MiR-874 promotes intestinal barrier dysfunction through targeting AQP3 following intestinal ischemic injury. FEBS letters 588, 757–763. [DOI] [PubMed] [Google Scholar]
- Zhu J, Zhang Y, Zhang W, Zhang W, Fan L, Wang L, Liu Y, Liu S, Guo Y, Wang Y, Yi J, Yan Q, Wang Z, Huang G, 2016. MicroRNA-142–5p contributes to Hashimoto’s thyroiditis by targeting CLDN1. Journal of translational medicine 14, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Lin M, Ma J, Liu W, Gao L, Wei S, Xue Y, Shang X, 2019. The role of LINC00094/miR-224–5p (miR-497–5p)/Endophilin-1 axis in Memantine mediated protective effects on blood-brain barrier in AD microenvironment. Journal of cellular and molecular medicine 23, 3280–3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Wang JL, He ZY, Jin F, Tang L, 2015. Association of Altered Serum MicroRNAs with Perihematomal Edema after Acute Intracerebral Hemorrhage. PloS one 10, e0133783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X, Lu J, Manaenko A, Qi X, Tang J, Mei Q, Xia Y, Hu Q, 2019. MicroRNA-132 attenuates cerebral injury by protecting blood-brain-barrier in MCAO mice. Experimental neurology 316, 12–19. [DOI] [PubMed] [Google Scholar]


