Burgos-Moron et al, in their letter to the Editor1 entitled “The dark side of curcumin” suggest that curcumin can be cytotoxic and can induce DNA damage. The authors of this paper cite several lines of evidence in support of their contention including a 1976 paper by Goodpasture and Arrighi2 that shows interference with chromosomal condensation, signs of chromosome banding, breakage, fragmentation and disintegration, mitotic arrest and decrease in nucleic acid synthesis when turmeric (solubilized in absolute ethanol) was incubated with cultured Don cells (cells of the Chinese hamster, Cricetulus griseus) or Indian muntjac cells (Muntiacus muntjac). Goodpasture and Arrighi2 point out that several DNA-intercalating agents induce chromosomal banding patterns in vitro. They also point out that compounds binding to DNA at specific places during G2 or prophase could interfere with the binding of chromosomal proteins necessary for chromosomal condensation as they prepare for mitosis.3 We investigated the ability of heat-solubilized curcumin4,5 and curcumin in 0.5N sodium hydroxide6 or ethanol to intercalate into DNA. Since curcumin fluoresces at 446-549 nm when irradiated with ultra violet light (excitation 355 nm), it is possible to visualize its binding or intercalation into DNA.
Here, we first comment on the observation made by Burgos-Moron et al1 that curcumin has a dark side to it, in spite of the numerous papers that have been published regarding its therapeutic effects.4-11 Secondly, we present data that shows curcumin, solubilized in water by heat, 0.5N sodium hydroxide or ethanol do not intercalate into DNA. Even though turmeric was soluble in 100% DMSO,12 the curcuminoids precipitated out when diluted in water. Hence, it was not possible to use DMSO-solubilized curcumin in this experiment.
Curcumin, a naturally occurring “nutraceutical”, is the most active component in the curry spice turmeric (Curcuma longa). This polyphenolic antioxidant has a long history of use in the traditional diet of Asian countries, especially in Indian herbal medicine. It has been shown to interact with multiple targets to regress diseases safely and inexpensively. Curcumin has been reported to lower cholesterol, suppress diabetes, enhance wound healing, modulate multiple sclerosis and Alzheimer’s disease and block HIV replication. In addition, curcumin has been shown to inhibit tumorigenesis, metastasis, platelet aggregation, inflammatory cytokine production, cataract formation, inflammatory bowel disease and myocardial infarction.4-11 Burgos-Moron et al point out that; (a) most therapeutic studies with curcumin have been carried out using micromolar levels in in vitro studies; (b) plasma concentrations of people consuming high oral doses (8-12 g/day) of curcumin are just in the nanomolar range; (c) in in vitro studies, cancer cells do not die unless the cells are exposed to 5-50 μM for several hours and such levels are not achieved in vivo; (d) proper studies of long term side effects of curcumin have not been undertaken; (e) just because curcumin is a common dietary constituent does not make it harmless; (f) increasing the solubility of curcumin will increase its cytotoxicity; and, (g) curcumin induces several toxic effects, of which the prime one is the induction of DNA damage.
We mostly agree with Burgos-Moron et al.1 However, we would like to comment on the point that curcumin induces DNA damage, since DNA alterations are important events in carcinogensis. Goodpasture and Arrighi’s work2 shows dramatic DNA damage and disintegration when 10 to 50 μg/ml of turmeric was incubated with cultured cells for periods up to 24 h. Goodpasture and Arrighi2 used mammalian cell cultures to study the effect of various food seasonings, especially turmeric, on chromosome morphology and cell cycle progression. They showed that turmeric arrested mitosis, changed chromosome morphology and interfered in synthesis of nucleic acid.
A turmeric fraction, solubilized in absolute ethanol, was added to cells. The authors show dramatic results, including chromosome breakage when Muntjac cells were treated with turmeric (10 μg/ml) for 4 h and shattered chromosomes of Don cells incubated with turmeric (50 μg/ml) for 24 h. Even though the authors state that absolute ethanol was added to control cells, in amounts equal to that added for turmeric, no results are provided for controls. No mention is made in the text either, regarding the effect of ethanol on cells. Figure 1 in the paper shows metaphase chromosomes of normal Don cells, without specifying the solvent added to it. This is important because it is the only control shown for all seasonings (curry powder, onion juice, paprika, cayenne pepper and turmeric) that were solubilized with water, Hanks’ balanced salt solution or 95% ethanol. We believe, from the results shown, that the cells have undergone cell death, as described in the next paragraph. Whether this is the effect of curcumin alone, curcumin plus ethanol or ethanol alone is not clear. Also, as suggested by Burgos-Moron et al1 it has to be borne in mind that levels used in in vitro experiments have never been achieved in vivo.
Figure 1:
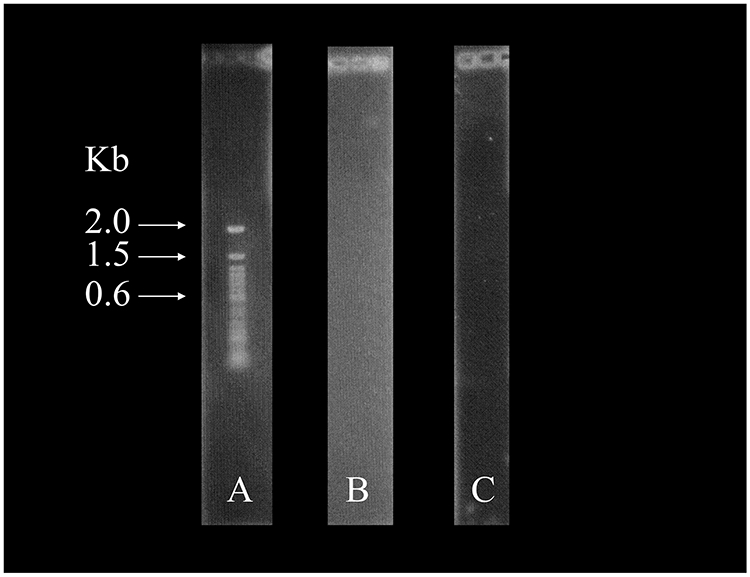
Staining of 100 bp ladder with ethidium bromide and curcumin. (A) Stained with ethidium bromide (B) Stained with curcumin in water and (C) Stained with curcumin in 0.5N sodium hydroxide.
Low levels of ethanol (43-86 mM; similar to blood levels during alcohol consumption) has been shown to also induce apoptosis (increased caspase 3 activity) and bring about cell death in human and rodent mast cells, while 860 and 1720 mM levels (50 and 100% ethanol levels respectively) appear to be toxic to cells (causes decreased caspase activity).13 Ethanol has been shown to also induce apoptosis in some other cell types including liver (Hep G2) cells, macrophages, and neural crest cells in embryo.13 Moreover, ethanol has been shown to bring about hepatic cell cycle arrest.14 Therefore, it is hard to conclude from Goodpasture and Arrighi’s paper that turmeric is the sole factor responsible for the DNA damage observed in the in vitro experiments.
References #22-30 cited by Burgos-Moron et al1for DNA damage, either use metals such as copper to induce DNA damage or use non-physiological solvents to solubilize curcumin. Therefore it is hard to reconcile whether curcumin alone is responsible for the DNA damage observed.
Insolubility of curcumin or turmeric in water has been the prime reason for the use of solvents like ethanol, DMSO12 and alkali.6 Recently turmeric and curcumin has been solublized in water with the use of heat,4 resulting in a curcumin yield of about 7.4 μg/ml water. This heat treatment procedure still left the bulk of curcumin (98.5%) insoluble.4 It would be of interest to use this heat solubilized turmeric/curcumin to investigate the effects of curcumin on apoptosis and DNA damage in various cell lines. Curcumin/turmeric has been solubilized with dilute alkali.6 Both, heat-solubilized and dilute alkali solubilized inhibited 4-hydroxy-2-nonenal (HNE)-mediated protein modification significantly, using an enzyme-linked immunosorbent assay that used HNE-modification of a solid-phase multiple antigen peptide substrate.15
Very recently, curcumin has been shown to modulate BRCA1 protein and induce apoptosis in triple negative breast cancer cell lines. Triple negative breast cancers lack expression of progesterone and estrogen receptors as well as do not over-express human epidermal growth factor receptor 2.16 However, curcumin did not bring about apoptosis and BRCA1 modulation in non-transformed mammary epithelial cells. Thus, the results of these authors suggest that curcumin may have limited non-specific toxicity. Curcumin used in these experiments was dissolved in ethanol and diluted two-fold when added to cells.
Furthermore, in vivo mice studies, Mukhopadhyay et al in 199810 have shown that turmeric or curcumin (dissolved in absolute ethanol at first and diluted 1:10 with water prior to administration) given by oral gavage at 8 mg/kg body weight did not show clastogenic activity (chromosome aberrations or cell death) even after 7 days of priming. However, neither curcumin or turmeric was able to prevent cyclophosphamide or mitomycin-induced clastogenicity.10
Additionally, studies carried out by Shukla et al in 200211 evaluated the anti-mutagenic activity of curcumin using an in vivo chromosomal aberration assay in Wistar rats. Curcumin (100 to 200 mg/kg body weight) was administered through gastric intubation for a period of 7 days prior to treatment with cyclophosphamide. Chromosomal aberration was observed in cyclophosphamide-only treated animals while no such change was observed in curcumin supplemented animals.11
Just as Burgos-Moron et al1 suggest, we feel that it is important for investigators to balance the beneficial effects with the deleterious effects seen with curcumin. All efforts need to be taken to show that curcumin is harmless. Since the publication of the article by Burgos-Moron et al1 a case of transient complete atrioventricular block associated with curcumin intake has been reported.17 The patient had ingested 40-60 tablets per day for one month, instead of 10-20 suggested by a doctor of Korean medicine (ingredients/tablet- turmeric 50% (about 75 mg), black soybean 20%, mulberry leaves 10%, garlic 10%, and arrowroot starch 10%). In addition, Kurien et al show that curcumin binds to proteins in a non-specific manner on SDS-PAGE gel, and also to autoantibodies in in vitro experiments to inhibit antigen antibody interaction.18 While this property could be made use of in treating autoimmune disorders, it may be important not to take curcumin in times when acquired immunity needs to kick in, especially during bacterial or viral infections.
As Burgos-Moron et al suggest in the latter part of their letter, there is ample experimental evidence suggesting that curcumin has beneficial properties.4-11,16 However, a lingering issue is the lack of solubility of curcumin in aqueous solutions. Burgos-Moron et al suggest that cytotoxicity of curcumin would increase if its solubility is increased. The in vitro assays that have been employed so far had to rely on solubilizing curcumin in various organic solvents. Therefore, direct effect of turmeric/curcumin alone solubilized in aqueous solutions either in in vitro or in vivo have not been carried out. It appears, therefore, there is a lot of grey area in this regard. Consequently, studies with curcumin solubilized in water using heat have been suggested.4,5,12,19
We therefore, investigated the ability of heat-solubilized curcumin4 and curcumin solubilized in 0.5N sodium hydroxide or ethanol to intercalate into DNA and induce damage. DNA-intercalating agents have been shown to induce chromosomal banding patterns in vitro. Compounds binding to DNA during G2 or prophase have the ability to interfere with the binding of chromosomal proteins that is necessary for chromosomal condensation as they prepare for mitosis.2 For this purpose turmeric was solubilized as mentioned below and incubated with DNA electrophoresed on an agarose gel.
Hot water (~90 °C) was added to a 50-ml centrifuge tube containing turmeric (purchased from local grocery), to obtain a solution of 5 mg turmeric/ml distilled water. The tube was mixed vigorously and heated for 10 minutes in a boiling water-bath (even though turmeric was dissolved at 5 mg/ml, the bulk of it remained insoluble). The tube was centrifuged at 800 x g for 15 minutes. The supernatant was transferred to a fresh centrifuge tube and centrifuged as before. The supernatant from this step constituted the heat-solubilized curcumin. Turmeric was dissolved at 5 mg/ml in 0.5N sodium hydroxide or absolute ethanol (Fisher Scientific Co, Dallas, TX). Even though turmeric was soluble in 100% DMSO, the curcuminoids precipitated out when diluted in water. Hence, it was not possible to use DMSO-solubilized turmeric in this experiment.
DNA 100 bp standard (1.5 μg total) (Invitrogen, Carlsabad, CA) was electrophoresed on a 1% agarose gel and stained with either ethidium bromide or curcumin solubilized in water using heat, 0.5N sodium hydroxide or absolute ethanol. The gels were stained with ethidium bromide or curcumin solutions for 30 minutes as well as overnight. The ethidium bromide or curcumin-stained gels were visualized by ultraviolet light using an UVP BioDoc-It™ system.
As shown in Figure 1A, ethidium bromide stains the 100 bp DNA nicely. However, curcumin in water (Figure 1B) or 0.5N sodium hydroxide (Figure 1C) did not stain the DNA. The gels were also incubated overnight with the curcumin solutions, resulting in the same outcome. The electrophoresed DNA was also incubated with curcumin in ethanol similarly. Curcumin solubilized in ethanol also did not stain the DNA (data not shown).
Curcumin solubilized in water by heat does not undergo heat-mediated disintegration4 and has been shown to be functional, inhibiting 4-hydroxy-2-nonenal mediated oxidative modification of a multiple antigenic peptide substrate.15 The results clearly show that heat- solubilized curcumin or curcumin solubilized by organic solvents does not intercalate between DNA bases. However, in the case of proteins the results obtained with curcumin are interesting. We found that heat-solubilized curcumin binds proteins in a non-specific manner on SDS-PAGE gels.18 Binding of curcumin to human serum albumin and to the CDRs of Fab of intravenous Ig have been reported.18
According to a preliminary survey, the daily intake of turmeric was found to be 0.6 g/person/day in rural parts of India and 0.2 g/person/day in urban areas.10 In such a scenario, the incidence of cancers should have been higher in India and other Asian countries compared to countries like United States, where turmeric in not a part of daily diet. Significantly, epidemiological studies show that cancers of colon20 and prostate cancer,9 are lower in India and other Asian countries compared to the United States.
The DNA damage described by Goodpasture and Arrighi2 and others,1 therefore, cannot be possibly due to the binding of curcumin to DNA or its intercalation into DNA. It would be of interest to see the effect of turmeric/curcumin solubilized in water using heat on DNA damage in cell culture experiments rather than using turmeric/curcumin solubilized in organic solvents.
References
- 1.Burgos-Moron E, Calderón-Montaño JM, Salvador J, Robles A, López-Lázaro M. The dark side of curcumin. Int J Cancer. 2009. October 14. [DOI] [PubMed]
- 2.Goodpasture CE, Arrighi FE. Effects of food seasonings on the cell cycle and chromosome morphology of mammalian cells in vitro with special reference to turmeric. Food Cosmet Toxicol 1976; 14:9–14. [DOI] [PubMed] [Google Scholar]
- 3.Hsu TC, Pathak S, Shafer DA. Induction of chromosome crossbanding by treating cells with chemical agents before fixation. Exp Cell Res 1973; 79:484–7. [DOI] [PubMed] [Google Scholar]
- 4.Kurien BT, Singh A, Matsumoto H, Scofield RH. Improving the solubility and pharmacological efficacy of curcumin by heat treatment. Assay Drug Dev Technol 2007; 5:567–76. [DOI] [PubMed] [Google Scholar]
- 5.Kurien BT, Scofield RH. Oral administration of heat-solubilized curcumin for potentially increasing curcumin bioavailability in experimental animals. Int J Cancer 2009; 125:1992–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurien BT, Scofield RH. Curcumin/turmeric solubilized in sodium hydroxide inhibits HNE protein modification--an in vitro study. J Ethnopharmacol 2007; 110:368–73. [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci 2009; 30:85–94. [DOI] [PubMed] [Google Scholar]
- 8.Bright JJ. Curcumin and autoimmune disease. Adv Exp Med Biology 2007; 595:425–51. Review. [DOI] [PubMed] [Google Scholar]
- 9.Kurien BT, Scofield RH. Curry spice curcumin and prostate cancer. Mol Nutr Food Res 2009;53: 939–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mukhopadhyay MJ, Saha A, Mukherjee A. Studies on the anticlastogenic effect of turmeric and curcumin on cyclophosphamide and mitomycin C in vivo. Food Chem Toxicol 1998; 36:73–6. [DOI] [PubMed] [Google Scholar]
- 11.Shukla Y, Arora A, Taneja P. Antimutagenic potential of curcumin on chromosomal aberrations in Wistar rats. Mutat Res 2002; 515:197–202. [DOI] [PubMed] [Google Scholar]
- 12.Kurien BT. Comment on Curcumin attenuates acrylamide-induced cytotoxicity and genotoxicity in HepG2 cells by ROS scavenging. J Agric Food Chem 2009; 57:5644–6. [DOI] [PubMed] [Google Scholar]
- 13.Nurmi K, Methuen T, Mäki T, Lindstedt KA, Kovanen PT, Sandler C, Eklund KK. Ethanol induces apoptosis in human mast cells. Life Sci 2009; 85:678–84. [DOI] [PubMed] [Google Scholar]
- 14.Clemens DL. Effects of ethanol on hepatic cellular replication and cell cycle progression. World J Gastroenterol 2007;13:4955–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurien BT, Scofield RH. In vitro modification of solid phase multiple antigenic peptides/autoantigens with 4-hydroxy-2-nonenal (HNE) provide ideal substrates for detection of anti-HNE antibodies and peptide antioxidants. J Immunol Methods 2005; 303:66–75. [DOI] [PubMed] [Google Scholar]
- 16.Rowe DL, Ozbay T, O’Regan RM, Nahta R. Modulation of the BRCA1 protein and induction of apoptosis in triple negative breast cancer cell lines by the polyphenolic. Breast Cancer 2009; 3:61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SW, Nah SS, Byon JS, Ko HJ, Park SH, Lee SJ, Shin WY, Jin DK. Transient complete atrioventricular block associated with curcumin intake. Int J Cardiol 2009. November 3. [Epub ahead of print]. [DOI] [PubMed]
- 18.Kurien BT, D’Souza A, Scofield RH. Heat-solubilized curry spice curcumin inhibits antibody-antigen interaction in in vitro studies: A possible therapy to alleviate autoimmune disorders. Mol Nutr Food Res 2010. February 9. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 19.Kurien BT, Scofield RH. Bubbling hookah smoke through heat-solubilized curcumin/turmeric and incorporation of the curry spice as an additive or filter in cigarettes to minimize tobacco smoke-related toxicants. Med Hypotheses 2009; 73:462–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chauhan DP. Chemotherapeutic potential of curcumin for colorectal cancer. Curr Pharm Des 2002; 8:1695–706. [DOI] [PubMed] [Google Scholar]


