Abstract
Background:
Across sub-Saharan Africa, men who have sex with men (MSM) and transgender women (TGW) have disproportionately poor HIV treatment outcomes. Stigma and criminalization create barriers to healthcare engagement and adherence to antiretroviral therapy (ART), potentially promoting the development of HIV drug resistance (HIVDR). We evaluated transmitted, pre-treatment, and acquired HIVDR among MSM and TGW in Lagos and Abuja, Nigeria.
Methods:
Adults with HIV RNA ≥1000 copies/mL in the TRUST/RV368 cohort, including incident cases diagnosed via 3-monthly screening, underwent HIVDR testing using the Sanger sequencing method. Major mutations conferring resistance to nucleoside reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), and protease inhibitors (PIs) were identified from the 2017 IAS-USA list. World Health Organization surveillance drug resistance mutations (SDRMs) were identified in ART-naïve participants.
Results:
From March 2013 to June 2017, 415 participants with median age 24 (interquartile range [IQR] 21-27) years, CD4 370 (IQR 272-502) cells/mm3, and HIV RNA 4.73 (IQR 4.26-5.15) log10copies/mL underwent HIVDR testing. SDRMs were observed in 36 of 373 ART-naïve participants (9.7%, 95% confidence interval [95%CI 6.8-13.1%]), including 8 of 39 incident cases (20.5%, [95%CI] 9.3-36.5%). Among 42 ART-experienced participants, NNRTI resistance was detected in 18 (42.9%, 95%CI 27.7-59.0%) and NRTI resistance in 10 (23.8%, 95%CI 12.0-39.4%). No PI resistance was detected.
Conclusions:
The high prevalence of transmitted and acquired drug resistance among Nigerian MSM and TGW living with HIV suggests the need for programmatic solutions to improve uninterrupted access to ART and timely switch to second-line regimens in cases of viral failure.
Keywords: Africa, Transgender Persons, Sexual and Gender Minorities, Acquired Immunodeficiency Syndrome, Drug Resistance, Public Health Surveillance
INTRODUCTION
Genotypic testing for HIV drug resistance (HIVDR) is routine in resource-rich settings prior to antiretroviral therapy (ART) initiation or re-initiation in persons living with HIV (PLWH)[1] but is rarely available in resource-limited settings such as sub-Saharan Africa[2]. Empiric ART selection based on nationally standardized regimens is common, but is driven by population-level data on HIVDR prevalence that may not reflect the epidemiology of sub-epidemics affecting marginalized populations such as men who have sex with men (MSM) and transgender women (TGW). Failing empiric therapies can promote development and transmission of resistant viruses within these populations[3]. As access to ART improves across sub-Saharan Africa, routine surveillance for HIVDR is essential to inform national HIV treatment strategies[4].
In Nigeria, sub-Saharan Africa’s most populous country, HIV prevalence among reproductive-age males is about 1% but as high as 44-66% among MSM and TGW[5, 6]. Criminalization and stigma associated with same-sex sexual practices impede healthcare engagement and ART adherence by MSM and TGW[7, 8]. Resource constraints can further complicate ART access for programmatic reasons such as drug stock-outs[9], potentially accelerating the development of HIVDR.
We report the prevalence of transmitted, pre-treatment, and acquired HIVDR among MSM and TGW receiving HIV care in Lagos and Abuja, Nigeria.
METHODS
The TRUST/RV368 cohort used respondent-driven sampling to recruit MSM and TGW (aged ≥16 years in Abuja; ≥18 years in Lagos) who reported anal intercourse with a male partner in the preceding year[10]. Participants were provided HIV testing, treatment, and prevention services at three-monthly visits. All participants provided written informed consent. Institutional review board approval was received from all participating institutions.
HIV was diagnosed using a parallel algorithm of rapid tests[11]. HIV RNA was measured via nucleic acid amplification using the COBAS® AmpliPrep/COBAS® TaqMan® HIV-1 Test, v2.0 (Roche Molecular Diagnostics, Pleasanton, CA). Plasma samples with HIV RNA ≥1000 copies/mL at either enrollment or the first visit after HIV seroconversion underwent retrospective viral RNA extraction, cDNA generation, and near-endpoint dilution prior to PCR amplification, purification, and sequencing with an ABI 3730XL capillary sequencer[12]. HIV subtype was determined by evaluating Pol sequences and achieving a consensus assignment[12]. Pol sequences were evaluated for major mutations conferring resistance to nucleoside reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs) and protease inhibitors (PIs) using the IAS-USA 2017 Update of the Drug Resistance Mutations in HIV[13]. Prior ART exposure was ascertained by medical record review. ART-naïve participants were evaluated for World Health Organization (WHO) surveillance drug resistance mutations (SDRMs)[14].
Demographic and other characteristics were compared between ART-naïve and ART-experienced participants using Wilcoxon rank-sum and Pearson’s Chi-squared tests, as appropriate. Prevalence of HIVDR mutations was calculated by dividing the number of participants with ≥1 detectable mutation by the total number of participants genotyped. Ninety-five percent confidence intervals (95%CIs) were calculated using the Clopper-Pearson interval. Fisher’s exact test was used to compare HIVDR prevalence across sites and other groups of interest. Analyses were performed using Stata 15.0 (StataCorp LP, College Station, TX).
RESULTS
From March 2013 to June 2017, 1539 participants underwent HIV screening, 785 (51.0%) had prevalent infection at enrollment, and 58 (3.8%) seroconverted during follow-up. HIV RNA ≥1000 copies/mL was observed in 553 PLWH, of whom 415 (74.7%) underwent retrospective HIV sequencing, including 376 prevalent and 39 incident cases. Participants with sequencing data had median age 24 (interquartile range [IQR] 21-27) years, CD4 370 (IQR 272-502) cells/mm3, and HIV RNA 4.73 (IQR 4.26-5.15) log10copies/mL. HIV subtype CRF02_AG and CRF02_AG-containing recombinants were observed in 319 (76.9%) participants (Table 1).
Table 1.
Characteristics of ART-Naïve and ART-Experienced Nigerian Men who have Sex with Men and Transgender Women at the Time of Genotypic Testing for Drug Resistance Mutations
| Characteristics | ART-Naïve (n=373) | ART-Experienced (n=42) | p-value |
|---|---|---|---|
| Age | |||
| <25 years | 205 (55.0) | 23 (54.8) | 0.98 |
| ≥25 years | 168 (45.0) | 19 (45.2) | |
| Gender Identity | |||
| Cisgender Man | 290 (77.7) | 33 (78.6) | 0.31 |
| Transgender Woman | 50 (13.4) | 3 (7.1) | |
| Other/Unknown | 33 (8.8) | 6 (14.3) | |
| Sexual Orientation | |||
| Gay/Homosexual | 137 (36.7) | 17 (40.5) | 0.85 |
| Bisexual | 235 (63.0) | 25 (59.5) | |
| Other/Unknown | 1 (0.3) | 0 (0) | |
| Education Level | |||
| Junior Secondary or Less | 30 (8.0) | 4 (9.5) | 0.93 |
| Senior Secondary | 214 (57.4) | 24 (57.1) | |
| Higher than Senior Secondary | 124 (33.2) | 13 (31.0) | |
| Unknown | 5 (1.3) | 1 (2.4) | |
| Occupation | |||
| Unemployed | 79 (21.2) | 9 (21.4) | 0.17 |
| Student | 96 (25.7) | 6 (14.3) | |
| Professional/Self-Employed | 77 (20.6) | 10 (23.8) | |
| Entertainment/Hospitality | 42 (11.3) | 2 (4.8) | |
| Driver/Laborer | 11 (2.9) | 3 (7.1) | |
| Other/Unknown | 68 (18.2) | 12 (28.6) | |
| Marital Status | |||
| Single/Never Married | 325 (87.1) | 36 (85.7) | 0.43 |
| Married/Living with a Woman | 25 (6.7) | 5 (11.9) | |
| Living with a Man | 11 (2.9) | 0 (0) | |
| Divorced/Widowed/Separated/Other | 12 (3.2) | 1 (2.4) | |
| Site | |||
| Abuja | 195 (52.3) | 25 (59.5) | 0.37 |
| Lagos | 178 (47.7) | 17 (40.5) | |
| CD4 Count (cells/mm3) | |||
| >500 | 72 (19.3) | 7 (16.7) | 0.65 |
| 351-500 | 88 (23.6) | 10 (23.8) | |
| 201-350 | 81 (21.7) | 10 (23.8) | |
| ≤200 | 37 (9.9) | 7 (16.7) | |
| Unknown/Missing | 95 (25.5) | 8 (19.0) | |
| HIV RNA (log10copies/mL) | |||
| Median (IQR) | 4.75 (4.30-5.17) | 4.44 (3.64-4.80) | 0.002 |
| HIV Subtypea | |||
| CRF02_AG | 222 (59.5) | 21 (50.0) | 0.23 |
| CRF02_AG-containing Recombinants | 69 (18.5) | 7 (16.7) | |
| G | 42 (11.3) | 10 (23.8) | |
| A1 and A1-containing Recombinants | 25 (6.7) | 2 (4.8) | |
| Other | 15 (4.0) | 2 (4.8) | |
| Year of Sample Collection | |||
| 2013 | 86 (23.1) | 6 (14.3) | 0.17 |
| 2014 | 121 (32.4) | 17 (40.5) | |
| 2015 | 131 (35.1) | 16 (38.1) | |
| 2016 | 34 (9.1) | 2 (4.8) | |
| 2017 | 1 (0.3) | 1 (2.4) | |
| Previous ART Exposure | |||
| EFV/TDF/XTC | - | 28 (66.7) | N/A |
| NVP/ZDV/XTC | - | 10 (23.8) | |
| NVP/TDF/XTC | - | 2 (4.8) | |
| LPV/r/TDF/FTC | - | 1 (2.4) | |
| Unknown | - | 1 (2.4) |
Abbreviations: ART, antiretroviral therapy; IQR, interquartile range; EFV, efavirenz; TDF, tenofovir disproxyl fumarate; XTC, emtricitabine or lamivudine; NVP, nevirapine; ZDV, zidovudine; LPV/r, ritonavir-boosted lopinavir. All data are presented as n (%) unless otherwise specified. P-values were calculated using Student’s t-test for HIV RNA as a continuous variable and Pearson’s Chi-squared test for all other variables. Statistically significant p-values (<0.05) are in bold.
HIV subtype categories are mutually-exclusive; recombinant viruses containing both CRF02_AG and A1 are included in the CRF02_AG-containing recombinants category.
Among the 42 ART-experienced participants, 28 (66.7%) had been treated with a combination of efavirenz, tenofovir disoproxyl fumarate, and either lamivudine or emtricitabine. Twelve (28.6%) had been previously exposed to nevirapine (36.0% in Abuja and 17.6% in Lagos, p=0.20).
Retrospective genotyping was performed on 39 incident HIV cases at a median of 130 (IQR 84-202) days after their last negative HIV test. SDRMs were observed in 8 (21% [95%CI 9.3-36.5%]) with no statistically significant differences between participants in Abuja and Lagos (Figure 1A).
Figure 1. Prevalence of Drug Resistance Mutations by Site.
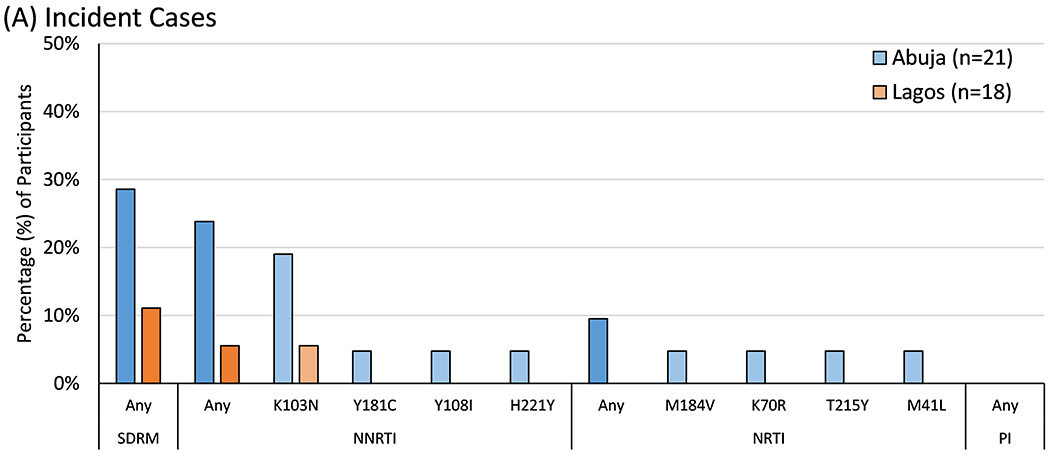
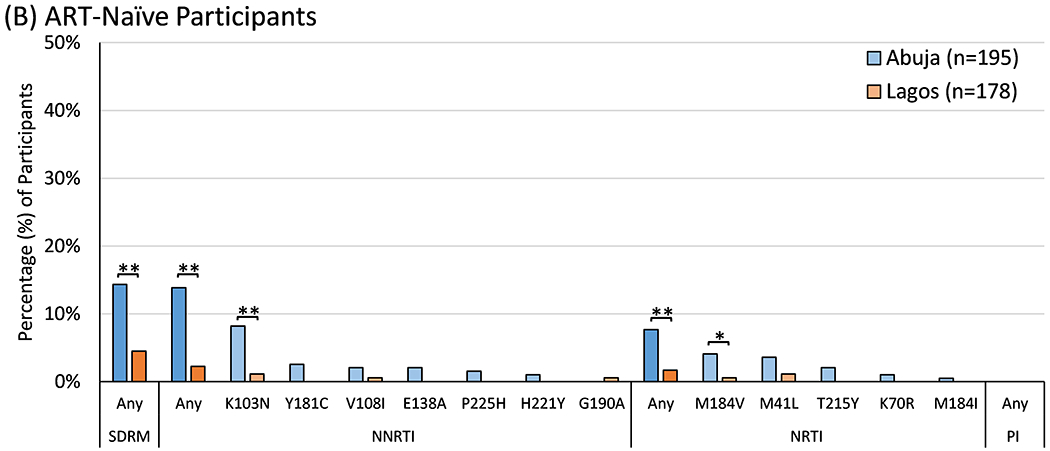
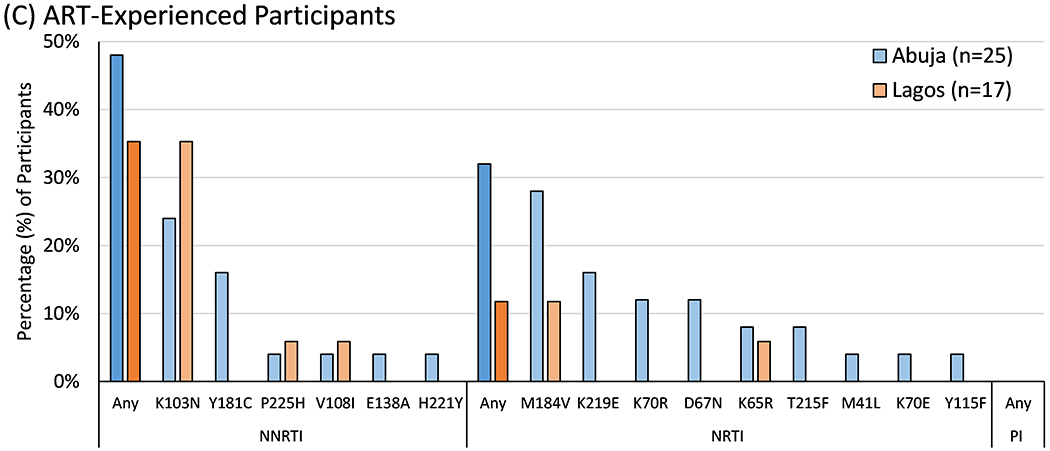
The prevalence of World Health Organization surveillance drug resistance mutations (SDRMs) and IAS-USA drug resistance mutations to non-nucleoside reverse transcriptase inhibitors (NNRTIs), nucleoside reverse transcriptase inhibitors (NRTIs), and protease inhibitors (PIs) were tallied separately by site and compared using Fisher’s exact test. Statistically significant differences between sites are marked with either one asterisk (*, p<0.05) or two (**, p<0.01).
The 39 incident cases were combined with 334 other ART-naïve participants to assess pre-treatment HIVDR. SDRMs were observed in 36 (9.7% [95%CI 6.8-13.1%]), including 28 (14.4% [9.8-20.1%]) in Abuja and 8 (4.5% [2.0-8.7%]) in Lagos (p=0.001). This included resistance to both NNRTIs and NRTIs (Figure 1B). K103N was the most common mutation overall, with higher prevalence in Abuja than Lagos (8.2% vs. 1.1%, p=0.001). SDRM prevalence did not vary significantly by year of sample collection (14.0% [95%CI 7.4-23.1%] in 2013 vs. 5.7% [0.7-19.2%] in 2016/2017, p=0.38).
Forty-two ART-experienced participants underwent genotyping at a median of 0.99 (IQR 0.36-2.19) years after ART initiation. Major resistance mutations to NNRTIs were detected in 18 (42.9% [95%CI 27.7-59.0%]) and NRTIs in 10 (23.8% [12.0-39.4%]). The most common mutations in each class were K103N and M184V, respectively (Figure 1C).
No major PI resistance mutations were detected in any participants.
DISCUSSION
We observed a high prevalence of transmitted HIVDR among MSM and TGW with incident HIV infection in Abuja and a moderate prevalence in Lagos, driven largely by NNRTI resistance mutations such as K103N. This mutation causes high-level resistance to most available NNRTIs and is selected by both efavirenz and nevirapine, longstanding components of first-line ART and strategies to prevent mother-to-child transmission of HIV, respectively. The prevalence of transmitted HIVDR in both cities suggests the need for evaluation of early indicators at clinics, such as on-time pill pick-up and viral suppression rates, as well as assessment of HIV screening program coverage to identify gaps that promote transmission of resistant viruses.
HIVDR mutations were less common among all ART-naïve participants as compared to just the incident HIV cases. This could partially reflect overgrowth of more fit and drug-sensitive viral quasi-species[15, 16]. However, much of the difference in HIVDR prevalence was driven by a lower prevalence of K103N mutations in ART-naïve participants, which minimally impacts HIV replication and may be slower to revert than other HIVDR mutations, such as M184V[17]. Some data suggest that M184V reversion and wild-type overgrowth may occur more quickly when M184V is transmitted as a single substitution[16], but more slowly when linked to other mutations, including K103N[18, 19]. The mechanisms underlying HIVDR reversion without selective pressure from ART in HIV subtypes CRF02_AG and G are poorly defined.
Pre-treatment HIVDR was more common in Abuja than Lagos. Developed in the 1980s for the purpose of becoming the nation’s capital, Abuja is a relatively new city that has ethnically diverse and transient populations. It was also one of the first regions in Nigeria to undergo a massive scale-up of infrastructure to support ART delivery in 2004.
Understanding the magnitude of transmitted and pre-treatment HIVDR in MSM and TGW populations is critical for sub-Saharan Africa, where resources to prevent the development and transmission of HIVDR may inconsistently reach these marginalized populations. In similar resource-limited settings, data suggest that transmitted resistance is more common among MSM than other HIV risk groups[20]. While a recent meta-analysis described NNRTI resistance in under 3% of ART-naïve patients in Western and Central Africa [21], we found a prevalence of about 10% in ART-naïve participants and twice that in newly-seroconverted cases within our highly-marginalized population of MSM and TGW. WHO guidelines recommend changing empiric first-line NNRTI-based ART to an integrase inhibitor-based regimen when the prevalence of resistance exceeds 10%[22]. It is fortuitous that dolutegravir-based first-line ART is becoming the standard of care in Nigeria, one of the first countries to implement the planned continent-wide transition promoted by the U.S. President’s Emergency Plan for AIDS Relief.
The high prevalence of HIVDR among ART-experienced Nigerian MSM and TGW highlights that adherence is not the only factor driving viral failure among PLWH. Guidelines that emphasize adherence counseling and delay confirmation of viral failure for months after initial detection of viremia may delay ART regimen switches, thereby promoting the development and transmission of drug resistant viruses[23]. The resistance pattern in our study was consistent with failure of first-line therapy with NNRTI and NRTI components, including the K65R mutation that confers high-level resistance to tenofovir. Over 70% of ART-experienced participants were prescribed tenofovir-containing regimens, some of which had compromised efficacy. No major PI resistance mutations were detected, supporting continued empiric use of PI-based regimens as second-line therapy.
Our study used respondent-driven sampling to recruit a highly-marginalized population for longitudinal HIV screening that enabled evaluation for transmitted HIVDR within months of HIV acquisition in incident cases. However, for technical and logistical reasons, not all eligible samples underwent HIVDR testing. Under-reporting of ART use may have led to some misclassification of ART status, as is common in resource-limited settings, although extensive review of medical records likely minimized this. Our study did not include assessment of integrase inhibitor resistance, but this will be important for future research as these agents become more common in sub-Saharan Africa.
Our findings support the programmatic shift to integrase inhibitor-based first-line ART and the continued use of PI-based second-line ART. They also underscore the urgency of ensuring uninterrupted access to ART and timely switch to second-line regimens upon viral failure among marginalized communities of PLWH to prevent the development and transmission of resistance to emerging empiric therapies.
ACKNOWLEDGMENTS
The study team would like to thank the study participants for their valuable contributions to this research. The TRUST/RV368 Study Group includes Principal Investigators: Manhattan Charurat (IHV, University of Maryland, Baltimore, MD, USA), Julie Ake (MHRP, Walter Reed Army Institute of Research, Silver Spring, MD, USA); Co-Investigators: Sylvia Adebajo, Stefan Baral, Erik Billings, Trevor Crowell, George Eluwa, Charlotte Gaydos, Afoke Kokogho, Hongjie Liu, Jennifer Malia, Olumide Makanjuola, Nelson Michael, Nicaise Ndembi, Jean Njab, Rebecca Nowak, Oluwasolape Olawore, Zahra Parker, Sheila Peel, Habib Ramadhani, Merlin Robb, Cristina Rodriguez-Hart, Eric Sanders-Buell, Sodsai Tovanabutra; Institutions: Institute of Human Virology at the University of Maryland School of Medicine (IHV-UMB), University of Maryland School of Public Health (UMD SPH), Johns Hopkins Bloomberg School of Public Health (JHSPH), Johns Hopkins University School of Medicine (JHUSOM), U.S. Military HIV Research Program (MHRP), Walter Reed Army Institute of Research (WRAIR), Henry M. Jackson Foundation for the Advancement of Military Medicine (HJF), Henry M. Jackson Foundation Medical Research International (HJFMRI), Institute of Human Virology Nigeria (IHVN), International Centre for Advocacy for the Right to Health (ICARH), The Initiative for Equal Rights (TIERs), Population Council Nigeria, Imperial College London.
Funding
This work was supported by cooperative agreements between the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., and the U.S. Department of Defense [W81XWH-11-2-0174, W81XWH-18-2-0040]; the National Institutes of Health [R01 MH099001, R01 AI120913, R01 MH110358]; Fogarty Epidemiology Research Training for Public Health Impact in Nigeria program [D43TW010051]; and the President’s Emergency Plan for AIDS Relief through a cooperative agreement between the Department of Health and Human Services/Centers for Disease Control and Prevention, Global AIDS Program, and the Institute for Human Virology-Nigeria [NU2GGH002099].
Footnotes
Prior Presentation: This work was presented, in part, at the 22nd International AIDS Conference in Amsterdam, Netherlands, 23-27 July 2018.
Publisher's Disclaimer: Disclaimer: The views expressed are those of the authors and should not be construed to represent the positions of the U.S. Army, Department of Defense, Henry M. Jackson Foundation for the Advancement of Military Medicine, or the Department of Health and Human Services. The investigators have adhered to the policies for protection of human subjects as prescribed in AR-70.
Conflicts of Interest
The authors declare no relevant conflicts of interest. Gustavo Kijak is currently employed by GSK Vaccines but his participation in this study preceded the current employment.
REFERENCES
- 1.Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2018 Recommendations of the International Antiviral Society-USA Panel. JAMA 2018; 320:379–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lessells RJ, Avalos A, de Oliveira T. Implementing HIV-1 genotypic resistance testing in antiretroviral therapy programs in Africa: needs, opportunities, and challenges. AIDS Rev 2013; 15:221–229. [PMC free article] [PubMed] [Google Scholar]
- 3.Hosseinipour MC, Gupta RK, Van Zyl G, Eron JJ, Nachega JB. Emergence of HIV drug resistance during first- and second-line antiretroviral therapy in resource-limited settings. J Infect Dis 2013; 207 Suppl 2:S49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gunthard HF, Calvez V, Paredes R, et al. Human Immunodeficiency Virus Drug Resistance: 2018 Recommendations of the International Antiviral Society-USA Panel. Clin Infect Dis 2019; 68:177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keshinro B, Crowell TA, Nowak RG, et al. High prevalence of HIV, chlamydia and gonorrhoea among men who have sex with men and transgender women attending trusted community centres in Abuja and Lagos, Nigeria. J Int AIDS Soc 2016; 19:21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nigeria HIV/AIDS Indicator and Impact Survey. Nigeria HIV/AIDS Indicator and Impact Survey: National Summary Sheet. Federal Ministry of Health; (Accessed 13 November 2019) Available from https://www.naiis.ng/Fact_Sheet [Google Scholar]
- 7.Duvall S, Irani L, Compaore C, et al. Assessment of policy and access to HIV prevention, care, and treatment services for men who have sex with men and for sex workers in Burkina Faso and Togo. J Acquir Immune Defic Syndr 2015; 68 Suppl 2:S189–197. [DOI] [PubMed] [Google Scholar]
- 8.Ramadhani HO, Ndembi N, Nowak RG, et al. Individual and Network Factors Associated With HIV Care Continuum Outcomes Among Nigerian MSM Accessing Health Care Services. J Acquir Immune Defic Syndr 2018; 79:e7–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meloni ST, Chaplin B, Idoko J, et al. Drug resistance patterns following pharmacy stock shortage in Nigerian Antiretroviral Treatment Program. AIDS Res Ther 2017; 14:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baral SD, Ketende S, Schwartz S, et al. Evaluating Respondent-Driven Sampling as an Implementation Tool for Universal Coverage of Antiretroviral Studies Among Men Who Have Sex With Men Living With HIV. J Acquir Immune Defic Syndr 2015; 68:107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nowak RG, Mitchell A, Crowell TA, et al. Individual and Sexual Network Predictors of HIV Incidence Among Men Who Have Sex With Men in Nigeria. J Acquir Immune Defic Syndr 2019; 80:444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Billings E, Kijak GH, Sanders-Buell E, et al. New Subtype B Containing HIV-1 Circulating Recombinant of sub-Saharan Africa Origin in Nigerian Men who have Sex with Men. J Acquir Immune Defic Syndr 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wensing AM, Calvez V, Gunthard HF, et al. 2017 Update of the Drug Resistance Mutations in HIV-1. Top Antivir Med 2017; 24:132–133. [PMC free article] [PubMed] [Google Scholar]
- 14.Bennett DE, Camacho RJ, Otelea D, et al. Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PLoS One 2009; 4:e4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yanik EL, Napravnik S, Hurt CB, et al. Prevalence of transmitted antiretroviral drug resistance differs between acutely and chronically HIV-infected patients. J Acquir Immune Defic Syndr 2012; 61:258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang WL, Kouyos RD, Boni J, et al. Persistence of transmitted HIV-1 drug resistance mutations associated with fitness costs and viral genetic backgrounds. PLoS Pathog 2015; 11:e1004722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wertheim JO, Oster AM, Johnson JA, et al. Transmission fitness of drug-resistant HIV revealed in a surveillance system transmission network. Virus Evol 2017; 3:vex008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wainberg MA, Moisi D, Oliveira M, Toni TD, Brenner BG. Transmission dynamics of the M184V drug resistance mutation in primary HIV infection. J Antimicrob Chemother 2011; 66:2346–2349. [DOI] [PubMed] [Google Scholar]
- 19.Kayondo JK, Ndembi N, Parry CM, et al. Intrapatient Evolutionary Dynamics of Human Immunodeficiency Virus Type 1 in Individuals Undergoing Alternative Treatment Strategies with Reverse Transcriptase Inhibitors. AIDS Res Hum Retroviruses 2015; 31:749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pham QD, Wilson DP, Law MG, Kelleher AD, Zhang L. Global burden of transmitted HIV drug resistance and HIV-exposure categories: a systematic review and meta-analysis. AIDS 2014; 28:2751–2762. [DOI] [PubMed] [Google Scholar]
- 21.Gupta RK, Gregson J, Parkin N, et al. HIV-1 drug resistance before initiation or re-initiation of first-line antiretroviral therapy in low-income and middle-income countries: a systematic review and meta-regression analysis. Lancet Infect Dis 2018; 18:346–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. Guidelines on the public health response to pretreatment HIV drug resistance. 2017.
- 23.Shroufi A, Van Cutsem G, Cambiano V, et al. Simplifying switch to second-line antiretroviral therapy in sub Saharan Africa: predicted effect of using a single viral load to define efavirenz-based first-line failure. AIDS 2019; 33:1635–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]


