Abstract
Background
The aim of the study was to assess the effect of circRNA CDYL on myocardial angiogenesis after acute myocardial infarction (AMI).
Material/Methods
We compared changes in circRNA CDYL and myocardial angiogenesis in myocardial infarction tissue and normal heart tissue by establishing a myocardial infarction mouse model to clarify the relationship between circRNA CDYL and changes in myocardial infarction and myocardial angiogenesis. Secondly, we used the RegRNA website to predict downstream miRNA, and we performed gain-of-function and loss-of-function experiments.
Results
CircCDYL was downregulated in myocardial tissues and hypoxia myocardial cells, and overexpression and downregulation of circCDYL improved and aggravated, respectively, heart function after AMI. CircCDYL overexpression and downregulation can promote and inhibit, respectively, proliferation of cardiomyocytes in vitro. Finally, we found that circCDYL can sponge miR-4793-5p and regulate its expression, and then miR-4793-5p regulates APP expression.
Conclusions
CircCDYL can promote the proliferation of cardiomyocytes through the miR-4793-5p/APP pathway.
MeSH Keywords: MicroRNAs, Myocardial Infarction, Regeneration
Background
According to the latest world health statistics report released by the World Health Organization, cardiovascular diseases (CVD) are one of the leading causes of death worldwide [1], with an annual mortality rate of 17.9 million and a predicted mortality rate of 23.6 million by 2030, resulting in a direct economic loss of about $860 billion per year. According to the Chinese cardiovascular report 2017, the prevalence and mortality rates of cardiovascular diseases in China are still on the rise. CVD is the leading cause of death in urban and rural residents, overshadowing cancer and other diseases. The increasing burden of CVD has become a major public health problem. CVD is estimated to affect 290 million people, including 11 million with coronary heart disease, among which, myocardial infarction (MI) is the most important component of cardiovascular mortality.
MI is an acute and severe disease that is a major cause of death. Myocardial cells can restore blood supply quickly after ischemia, and their morphology and function can return to normal. However, after long-term ischemia, myocardial cells die through various mechanisms and lose basic functions. Even with the great progress made in drug and interventional therapy, the mortality rate of this disease has not been significantly reduced, largely due to myocardial cell loss and loss of heart function after MI. Irreversible myocardial cells necrosis leads to a decrease in the number of myocardial cells and a decrease in cardiac function, which is one of the key causes of this disease’s high mortality. It was previously thought that human myocardial cells lose their ability to regenerate and multiply after birth. However, as research has progressed, it has been shown that about half of heart myocardial cells are replaced during a person’s lifetime [2]. In adult rat hearts, myocardial cell renewal has also been demonstrated, mainly through activation of resident cells, at a rate of about 1.3–4% per year, especially in damaged MI border areas [3]. Studies on myocarditis and primary cardiomyopathy [4,5] have demonstrated that endogenous cardiac regeneration is a very effective method to compensate for myocardial cells loss. Endogenous cardiac regeneration research has shown that cardiac-related factors (e.g., transcription factors, growth factors, microRNAs, lncRNAs transferred to the ischemic myocardium, and pre-existing cardiac muscle cells) can be induced to differentiate and proliferate, thus reducing the scar area [6], healing the lesion site, and improving cardiac function [5,7].
CircRNA and lncRNA are both non-coding RNAs, and their functions and mechanisms have many similarities [8,9]. For example, both can regulate downstream mRNA through a molecular sponge mechanism, both are widely involved in the occurrence and development of various diseases, and both play a wide range of biological roles through promotion and inhibition [10]. However, circRNAs have a covalent closed loop structure, and thus have good resistance to RNA nucleic acid exonuclease [11]. CircRNAs have better stability and longer duration of action. Therefore, CircRNAs have gradually become the focus of research in recent years. More and more studies have suggested that circRNAs play important roles in the pathophysiology of cardiovascular diseases. For example, studies have shown that circRNA ACR in cardiomyocytes can increase the expression of Pink1, which is the pathway for reducing autophagy after MI [12]. Combined with the above analysis, the study of circRNAs promoting the proliferation of myocardial cells after MI has great advantages and possibilities. We performed in vitro and in vivo functional verification along with bioinformatics analysis and step-by-step verification to explore the molecular mechanism.
Material and Methods
Isolation and culture of suckling mouse cardiac muscle cells
This study was approved by the Animal Ethics Committee of Qingdao University Animal Center. We obtained C57BL/6 1-day-old suckling mice from the Experimental Animal Center of Qingdao University and injected 4% pentobarbital into the chest at 0.01 mL/g and tore the hearts apart with an eye tweezer. We added 400 uL 0.25% trypsin and placed the tissue in a 4°C refrigerator for digestion for about 12 h. After digestion, trypsin was replaced and then samples were placed on a multi-function magnetic agitator (Olympus, Tokyo, Japan) for stirring and mixing at a speed of 1000 rpm for 5 min. Then, the supernatant was discarded and the sample was repeatedly rinsed with fresh medium to mix the cells. Finally, the cells were incubated for 70–90 min. The cells were counted on a scale using a counter plate and replanted in a new cell culture dish.
Isolation and culture of adult mouse cardiomyocytes
C57BL/6 28-day-old adult mice from the Experimental Animal Center of Qingdao University were injected with 4% pentobarbital at 0.05 mL/g. We removed the exposed heart with sterile scissors and tweezers, quickly squeezed the heart to expose it, then cut it off with sterile scissors and quickly placed it in a calcium-free solution (Thermo Fisher Scientific, Waltham, MA, USA). Then, the vascular walls of both sides of the aorta were carefully clipped with ophthalmic tweezers and suspended at the head end of aseptic gavage. Then, the assistant quickly ligated the aortic root with aseptic wires to ensure that the head end of the gavage did not protrude into the left ventricle. The calcium-free solution and the pre-prepared trypsin (Thermo Fisher Scientific, Waltham, MA, USA) digest were then alternately gavaged. The digested tissue was then placed in KB solution (Thermo Fisher Scientific, Waltham, MA, USA) to allow for adequate digestion and degradation of the heart tissue. The heart tissue was cut into 0.5-cm3 pieces and then gently blown and mixed with sterile LML solution, the tissue fluid was filtered with nylon mesh, and the filtered tissue fluid was settled statically. After settling for about 25–30 min, the supernatant of the filtrate was discarded and the previously added liquid was added for settling. The resulting cells were isolated adult mouse cardiomyocytes.
Echocardiography
The mice were attached to a conventional connected oxygen tube and anesthesia system. The mice were placed in the induction box for anesthesia induction, and 6–10 cardiac cycles were measured continuously. Two-dimensional echocardiography (VINNO, Beijing, China) was used to detect the parameters of left ventricular end-systolic diameter (LVSD), left ventricular end-diastolic diameter (LVED), left ventricular fractional shortening (LVFS), and left ventricular ejection fraction (LVEF) in mice.
Immunofluorescence staining
The cells were removed from the cell incubator, and the state of the cells was checked. The cells were eluted 3 times with phosphate-buffered saline (PBS) solution (Thermo Fisher Scientific, Waltham, MA, USA) for 5 min each time. Then, we added 4% paraformaldehyde (Regal, Shanghai, China) for 30 min. The cells were then eluted 3 times in PBS for 5 min each time. Then, the pre-configured triton solution (Jiancheng, Nanjing, China) was used to break the membrane for 20 min, after which staining was performed using a 5-Ethynyl-2′-deoxyuridine (EdU) kit, and 4′,6-diamidino-2-phenylindole (DAPI) nuclear dye solution (Regal, Shanghai, China) was then used for dyeing. Finally, confocal microscopy (Olympus, Tokyo, Japan) was used to assess the glycerine-sealed tablets.
RT-PCR (quantitative reverse transcription polymerase chain reaction)
The expressions of circCDYL and miRNA-4793-5p were detected by TRIzol method (Invitrogen, Carlsbad, CA, USA). We extracted RNA from tissues and reverse transcription RNA was used to obtain cDNA (according to the instruction manual). The reaction system was prepared according to the instructions and pre-denatured at 94°C for 30 s. We performed denaturation at 94°C for 5 s, annealing at 60°C for 15 s, extension at 72°C for 10 s, and amplification for 45 cycles. We used the threshold method of quantitative analysis. Using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) or U6 as internal references, the corresponding ΔΔCt values in each group of cells were calculated, and the quantitative analysis was performed based on the quantity of the target factor (2−ΔΔCt). All primers are listed in Table 1.
Table 1.
Real time PCR primers.
| Gene name | Forward (5′>3′) | Reverse (5′>3′) |
|---|---|---|
| MiR-4793-5p | ACATCCTGCTCCACAGGG | AAACCAACCAACCACTACCA |
| circCDYL | CTTAGCTGTTAACGGGAAA | CTGTTGAAGTCGTGGATGT |
| CDYL | GTGATGTGAGGCGTTGG | TCTGGTTTGGGGTATGCT |
| U6 | GCTTCGGCAGCACATATACTAAAAT | CGCTTCACGAATTTGCGTGTCAT |
| GAPDH | ACAACTTTGGTATCGTGGAAGG | GCCATCACGCCACAGTTTC |
RT-PCR – quantitative reverse-transcription polymerase chain reaction.
Western blot analysis
Cardiomyocytes were seeded in 6-well plates (Thermo Fisher Scientific, Waltham, MA, USA). After the cells were treated, the culture medium was aspirated and washed with cold PBS, and cell lysate (Thermo Fisher Scientific, Waltham, MA, USA) was added. The cells were lysed on ice for 20 min at 4°C and centrifuged at 14 000 rpm for 10 min. The supernatant was used for protein quantification. Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis was performed for separation, transfer membrane, and blocked with blocking solution at room temperature with primary antibody (APP, Abcam, 1: 1000, Cambridge, MA, USA) and GAPDH primary antibody 1: 2000, Proteintech, Rosemont, IL, USA) and incubated at 4°C overnight. Anti-rabbit or mouse IgG secondary antibody (1: 1 000 dilution) was applied for 2 h, and then exposed the chemiluminescence.
Luciferase reporter analysis
CircCDYL overexpression was co-transfected with reporter gene plasmids and 293T (Thermo Fisher Scientific, Waltham, MA, USA). Culture was performed on 24-well plates, and 48 h later lentivirus (GeneChem, Shanghai, China) containing circCDYL was infected [4×105 cells with 20 μL virus (109 TU/mL) and polybrene (final concentration 5 mg/mL)]. Luciferase activity was detected on day 5.
Statistical analysis
Statistical Product and Service Solutions (SPSS) 21.0 (IBM, Armonk, NY, USA) was used for statistical analysis. Measurement data are expressed as mean and standard deviation, and the independent-samples t test was used for comparisons between 2 groups. Comparisons between multiple groups was done using one-way ANOVA followed by LSD post hoc analysis. The Welch test was performed if the variances of data in each group were not uniform. P<0.05 was considered statistically significant.
Results
Identification of circRNA-circCDYL
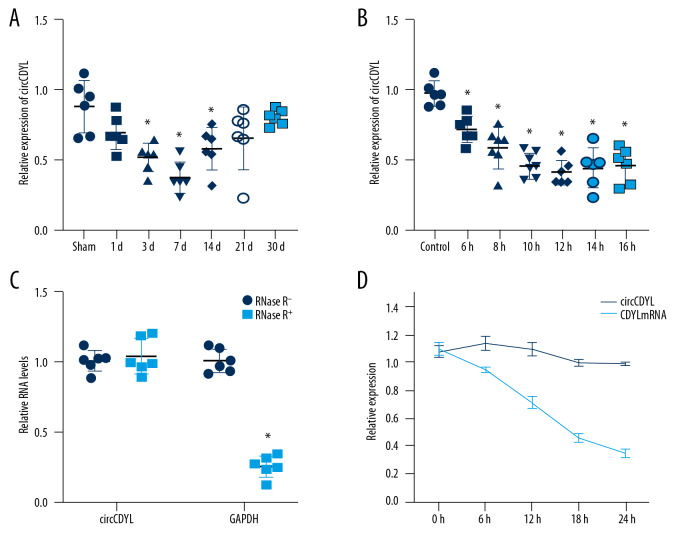
RT-PCR was performed to detect circCDYL expression in mouse heart tissue and cardiomyocytes, showing that the expression was significantly decreased during myocardial infarction. CircCDYL expression was detected in myocardial tissue 1, 3, 7, 14, 21, and 30 days after myocardial infarction, and was found to be lowest at 7 days after myocardial infarction, then gradually increased but still was less than in normal mouse myocardial tissue (Figure 1A). In the model of suckling mouse cardiomyocytes hypoxia, the expression levels of circCDYL at 6 h, 8 h, 10 h, 12 h, 14 h, and 16 h were detected, and the expression levels were mostly unchanged after 12 h (Figure 1B). The stability of circCDYL was confirmed by digestion and degradation by RNA enzymes, and the tolerance of circCDYL to nucleic acid exonuclease was measured (Figure 1C). By measuring the half-life of circCDYL, it was confirmed that circCDYL has a long half-life (Figure 1D).
Figure 1.
Identification of circRNA-circCDYL. (A) CircCDYL expression was detected in myocardial tissue at 1, 3, 7, 14, 21, and 30 days after myocardial infarction. (B) The expression levels of circCDYL at 6 h, 8 h, 10 h, 12 h, 14 h, and 16 h in cardiomyocytes. (* Indicates significant difference from the control group P<0.05). (C) Effects of RNase on Circular Hipk3 and GAPDH. (* Indicates compared with the non-RNase enzyme group. P<0.05). (D) Comparison of half-life between Circular Hipk3 and GAPDH. (* Represents the relative expression between circCDYL and linear CDYL at each time point. P<0.05).
Overexpression and downregulation of circCDYL promotes and inhibits proliferation of cardiomyocytes in vitro
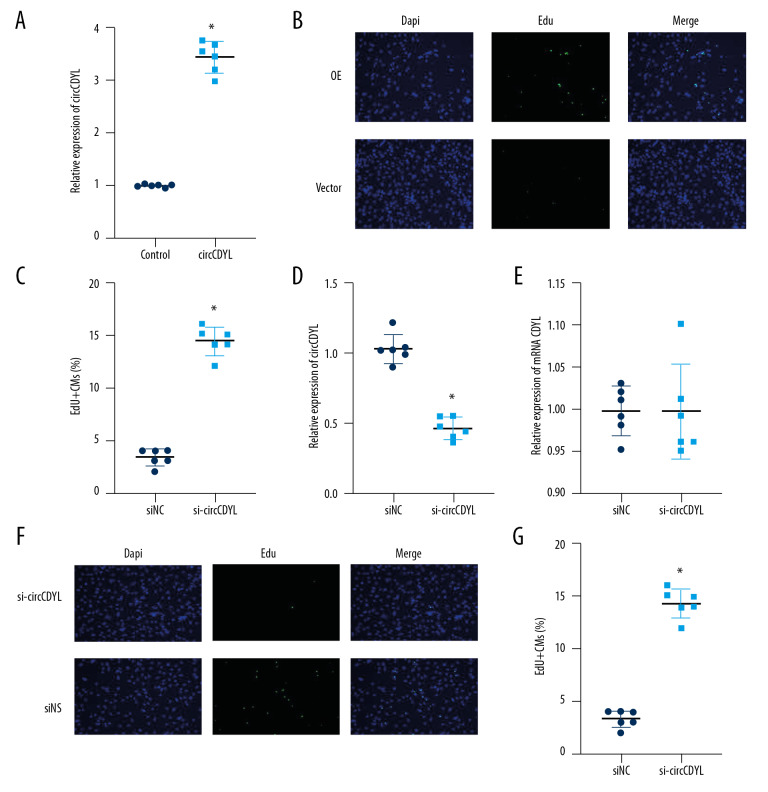
To investigate the role of circCDYL in the proliferation of cardiomyocytes, we transfected 28-day postnatal cardiomyocytes with circCDYL to increase CircCDYL expression (Figure 2A). The results showed that circCDYL overexpression significantly increased the level of cell proliferation indicator EdU (Figure 2B, 2C). To design the interference fragment, we used the small interfering RNA (siRNA) si-circCDYL to target the reverse splicing region of circCDYL. We found that si-circCDYL downregulated the circular transcript but not the linear transcript (Figure 2D, 2E). We transfected l-day-old mouse cardiomyocytes with si-circCDYL and used EdU to detect proliferation. The results showed that deletion of circCDYL significantly reduced the percentage of myocardium in 1-day-old mice expressing EdU, providing further evidence that circCDYL plays a role in regulating proliferation of cardiomyocytes (Figure 2F, 2G). The results of this section indicate that overexpression of circCDYL in vitro can promote the proliferation of cardiomyocytes, and knocking down the expression of circCDYL can inhibit the proliferation of cardiomyocytes, which proves that circCDYL is an important factor regulating the proliferation of cardiomyocytes in vitro.
Figure 2.
Overexpression and downregulation of circCDYL promote and inhibit proliferation of cardiomyocytes in vitro. (A) RT-PCR detects the relative expression of circCDYL. (B) Immunofluorescence staining of EdU. (C) Proportion of EdU-positive CMs in vector and overexpression groups (magnification: 200×). (D) RT-PCR detected the relative expression of circCDYL. (E) Immunofluorescence staining of EdU. (F, G) Proportion of EdU-positive CMs (magnification: 200×) (* Indicates statistical difference from the control group P<0.05).
Overexpression and downregulation of circCDYL in vivo can promote or inhibit, respectively, myocardial regeneration and repair after MI in adult mice
AAV9-circCDYL virus was injected into the marginal area of myocardial infarction in multiple points, and ultrasonic measurements were performed on the day of injection and at 2 weeks and 4 weeks later, indicating that the FS and EF in the overexpression group at corresponding time points were higher than that in the vector group but lower than that in the sham operation group (Figure 3A, 3B). Multipoint injection of ADV-shcircCDYL virus into the margin of myocardial infarction was performed. The results showed that the number of EF in the shcircCDYL group at the corresponding time point was lower than in the vector group and the sham operation group (Figure 3C, 3D). The results of this part of the experiment indicate that overexpression or downregulation of circCDYL can promote or inhibit, respectively, cardiac regeneration and repair after MI in newborn mice. This suggests that circCDYL plays a role in regulating myocardial proliferation in vivo.
Figure 3.
Overexpression and downregulation of circCDYL in vivo can promote or inhibit myocardial regeneration and repair after myocardial infarction in adult mice. (A, B) The FS and EF in the overexpression and control groups at corresponding time points. (C, D) The FS and EF in the shcircCDYL and control groups at the corresponding time point (* Indicates significant difference from the control group. P<0.05).
CircCDYL regulates cell proliferation by inhibiting the activity of miR-4793-5p through a “molecular sponge” mechanism
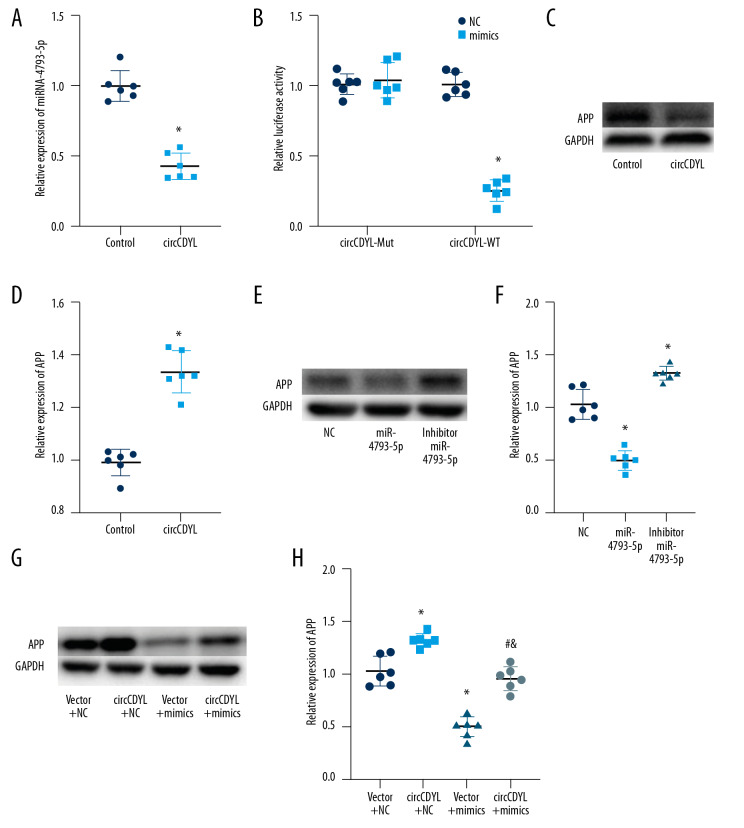
To investigate whether circCDYL acts as a “miRNA sponge” to regulate gene expression, we made bioinformatics predictions using the RegRNA website, suggesting that miR-4793-5p interacts with circCDL. We further found that increased circCDYL expression significantly inhibited the expression of miR-4793-5p (Figure 4A). Previous studies have found that miR-4793-5p is associated with delayed cerebral infarction, so we hypothesized that miR-4793-5p is a downstream gene of circCDYL. We then inserted the entire circCDYL sequence into the Pgl3 luciferase gene to construct a luciferase (luc-circCDYL) vector. Luc-circCDYL mutants without miR-4793-5p binding sites were also constructed. Transfection of miR-4793-5p mimics significantly reduced the activity of luc-circCDYL, but had no effect on the activity of luc-circCDYL mutants (Figure 4B). Next, we used TargetScan to predict the downstream target gene of mir-4793-5p, and verified that it was APP. We then stimulated them under different conditions to observe the changes in APP protein levels. In the circCDYL group, the APP protein level was higher than in the control group (Figure 4C, 4D), and in the miR-4793-5p mimics group the APP level was lower than in the control or inhibitor group (Figure 4E, 4F). In the rescue research, we formed 4 groups – vector+NC, circCDYL+NC, vector+mimics, and circCDYL+mimics – showing that circCDYL inhibits the activity of miR-4793-5p through a molecular sponge mechanism (Figure 4G, 4H).
Figure 4.
CircCDYL regulates cell proliferation by inhibiting the activity of miR-4793-5p through a “molecular sponge” mechanism. (A) RT-PCR detected the relative expression of miR-4793-5p. (B) The luciferase report assay. (C, D) Western blot bands of APP. (* Indicates significant difference from the control group. P<0.05). (E, F) Western blot bands of APP. (* Indicates significant difference from the NC group. P<0.05). (G, H) Western blot bands of APP (* Indicates significant difference from the vector+NC group. P<0.05; # indicates significant difference from the circCDYL+NC group. P<0.05; & indicates significant difference from the vector+mimics group. P<0.05).
Discussion
Promoting endogenous myocardial regeneration is a promising and important research direction. Due to its unique physiological characteristics, circRNA has a unique advantage in promoting endogenous myocardial regeneration, and it is an ideal target to find a circRNA that can promote myocardial proliferation. After verification, our study suggests that circCDYL is a potential candidate target due to the above characteristics. RNase digestion showed that circCDYL was more stable than the corresponding linear transcripts, suggesting that circCDYL may have a longer functional duration. Our study shows that circCDYL is a new regulator of myocardial generation during myocardial regeneration, which can improve cardiac function [13,14]. This suggests that circCDYL may be a valuable therapeutic target for inducing cardiac regeneration and improving prognosis after myocardial infarction. Regarding the mechanism involved, previous studies on circCDYL have shown its functions through sponge-related miRNAs, and bioinformatics analysis also showed that circCDYL enriched the seed sequence of miR-4793-5p [15]. miR-4793-5p was proved to be associated with delayed cerebral infarction after aneurysmal subarachnoid hemorrhage [16].
Our study has certain limitations. First, although we showed that circCDYL overexpression triggers CM proliferation during cardiac regeneration, further research is needed to better understand the role of circCDYL, and the upstream mechanism regulating circCDYL expression in cardiac myocytes needs to be clarified. Furthermore, CM-specific knockout mice would be more helpful in elucidating the potential molecular mechanisms for circCDYL overexpression to reduced infarct size and improved survival. Finally, we did not explore the upstream gene APP.
Conclusions
CircCDYL can promote the proliferation of cardiomyocytes through the miR-4793-5p/APP pathway.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics – 2016 update: A report from the American Heart Association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Zhou M, Zou YG, Xue YZ, et al. Long non-coding RNA H19 protects acute myocardial infarction through activating autophagy in mice. Eur Rev Med Pharmacol Sci. 2018;22:5647–51. doi: 10.26355/eurrev_201809_15831. [DOI] [PubMed] [Google Scholar]
- 3.Malliaras K, Zhang Y, Seinfeld J, et al. Cardiomyocyte proliferation and progenitor cell recruitment underlie therapeutic regeneration after myocardial infarction in the adult mouse heart. Embo Mol Med. 2013;5:191–209. doi: 10.1002/emmm.201201737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koitabashi N, Kass DA. Reverse remodeling in heart failure – mechanisms and therapeutic opportunities. Nat Rev Cardiol. 2011;9:147–57. doi: 10.1038/nrcardio.2011.172. [DOI] [PubMed] [Google Scholar]
- 5.Ghiroldi A, Piccoli M, Ciconte G, et al. Regenerating the human heart: Direct reprogramming strategies and their current limitations. Basic Res Cardiol. 2017;112:68. doi: 10.1007/s00395-017-0655-9. [DOI] [PubMed] [Google Scholar]
- 6.Srivastava D, Heidersbach AJ. Small solutions to big problems: microRNAs for cardiac regeneration. Circ Res. 2013;112:1412–14. doi: 10.1161/CIRCRESAHA.113.301409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milasinovic D, Mohl W. Contemporary perspective on endogenous myocardial regeneration. World J Stem Cells. 2015;7:793–805. doi: 10.4252/wjsc.v7.i5.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sallam T, Sandhu J, Tontonoz P. Long noncoding RNA discovery in cardiovascular disease: Decoding form to function. Circ Res. 2018;122:155–66. doi: 10.1161/CIRCRESAHA.117.311802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z, Huang C, Bao C, et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol. 2015;22:256–64. doi: 10.1038/nsmb.2959. [DOI] [PubMed] [Google Scholar]
- 10.Szabo L, Morey R, Palpant NJ, et al. Statistically based splicing detection reveals neural enrichment and tissue-specific induction of circular RNA during human fetal development. Genome Biol. 2015;16:126. doi: 10.1186/s13059-015-0690-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vausort M, Salgado-Somoza A, Zhang L, et al. Myocardial infarction-associated circular RNA predicting left ventricular dysfunction. J Am Coll Cardiol. 2016;68:1247–48. doi: 10.1016/j.jacc.2016.06.040. [DOI] [PubMed] [Google Scholar]
- 12.Zhou LY, Zhai M, Huang Y, et al. The circular RNA ACR attenuates myocardial ischemia/reperfusion injury by suppressing autophagy via modulation of the Pink1/FAM65B pathway. Cell Death Differ. 2019;26:1299–315. doi: 10.1038/s41418-018-0206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cui W, Dai J, Ma J, Gu H. circCDYL/microRNA-105-5p participates in modulating growth and migration of colon cancer cells. Gen Physiol Biophys. 2019;38:485–95. doi: 10.4149/gpb2019037. [DOI] [PubMed] [Google Scholar]
- 14.Mei M, Wang Y, Wang Q, et al. CircCDYL serves as a new biomarker in mantle cell lymphoma and promotes cell proliferation. Cancer Manag Res. 2019;11:10215–21. doi: 10.2147/CMAR.S232075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei Y, Chen X, Liang C, et al. A noncoding regulatory RNAs network driven by circ-CDYL acts specifically in the early stages hepatocellular carcinoma. Hepatology. 2019;71:130–47. doi: 10.1002/hep.30795. [DOI] [PubMed] [Google Scholar]
- 16.Lu G, Wong MS, Xiong M, et al. Circulating microRNAs in delayed cerebral infarction after aneurysmal subarachnoid hemorrhage. J Am Heart Assoc. 2017;6:e005363. doi: 10.1161/JAHA.116.005363. [DOI] [PMC free article] [PubMed] [Google Scholar]