Abstract
Malignancy relapse is the most common cause of treatment failure among recipients of hematopoietic cell transplantation (HCT). Conditioning dose intensity can reduce disease relapse, but it is offset by toxicities. Improvements in radiotherapy techniques and supportive care may translate to better outcomes with higher irradiation doses in the modern era. This study compares outcomes of recipients of increasing doses of high dose total body irradiation (TBI) divided into intermediate high dose (IH 13–13.75 Gy) and high dose (HD 14 Gy) to standard dose (SD 12Gy) with cyclophosphamide (Cy). A total of 2,721 patients ages of 18 to 60 with hematologic malignancies receiving HCT from 2001 to 2013 were included. Cumulative incidence of non-relapse mortality (NRM) at 5 years was 28% (95% Cumulative Incidence [CI] 25–30%), 32% (95%CI 29–36%) and 34% (95%CI 28–39%) for SD, IH and HD, respectively (p=0.02). Patients receiving IH-TBI had a 25% higher risk of NRM compared to SD-TBI (12 Gy) (p=0.007). Corresponding cumulative incidence of relapse was 36% (95%CI 34–38%), 32% (95%CI 29–36%) and 26% (95%CI 21–31%) (p=0.001). Hazard ratio for mortality compared to SD were 1.06 (95% 0.94–1.19, p=0.36) for IH and 0.89 (95% CI 0.76–1.05, p=0.17) for HD. The study demonstrates that despite improvements in supportive care, myeloablative conditioning using higher doses of TBI (with Cy) leads to worse non-relapse mortality and offers no survival benefit over SD, despite reducing disease relapse.
Keywords: total body irradiation, allogeneic hematopoietic cell transplantation, myeloablative conditioning, hematologic malignancies
INTRODUCTION
Relapse of the underlying disease is the most frequent cause of treatment failure after allogeneic HCT for hematologic malignancies1. Non-relapse mortality (NRM) accounts for the bulk of the remainder of deaths (20–30%)2–5. One of the strategies to reduce relapse risk is to intensify the pre-transplant conditioning regimen. Several studies have demonstrated that increasing the intensity of the conditioning regimen can reduce relapse risk 6–9. Indeed, a prospective randomized trial of myeloablative (MAC) versus reduced intensity conditioning (RIC) for adults with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) in first remission confirmed that greater conditioning intensity resulted in significantly lower relapse risk and improved relapse-free survival10. The outcomes after MAC regimens of cyclophosphamide (Cy) with total body irradiation (Cy/TBI) and busulfan with Cy (Bu/Cy) after allogeneic HCT for acute and chronic leukemia have been compared in a prospective study11 which demonstrated that the adjusted 3-year overall survival (OS) was higher with Cy/TBI (vs. Bu/Cy; oral busulfan), although there was no difference in relapse-free survival between the cohorts. More recently, however, a few observational studies have reported that Bu/Cy (using intravenous busulfan) may offer a survival advantage over Cy/TBI in patients with AML12–14, whereas for acute lymphoblastic leukemia (ALL) patients, TBI was associated with a lower relapse rate and favorable event-free survival, compared to oral busulfan when combined with Cy15. Attempts to further optimize conditioning regimens have not improved outcomes except in small, single-center studies16–20.
Radiation is highly lethal to leukemic cells in a dose-dependent fashion21,22. This observation led investigators, over three decades ago, to attempt to escalate radiation doses given as conditioning prior to transplant. The use of higher doses of TBI (>12 Grays [Gy]), in combination with chemotherapy, has been reported in small, single-institution studies23–26. The upper limit of TBI dose of 16 Gy was established in combination with Cy and of 14.4 Gy when used in combination with etoposide. A study comparing 12 Gy to 15.75 Gy established that the maximum tolerable dose of TBI with Cy was fractionated TBI at a dose of 12 Gy27. Although higher doses of radiation were indeed associated with lower relapse risk, this benefit was negated by increased NRM, and there was no difference in OS. However, in the last two decades, advances in the delivery of radiation therapy as well as substantial improvements in supportive care raise the question of whether, in the current era, higher doses of TBI (>12 Gy) result in improved non-relapse mortality and lower relapse rate and therefore, improved OS outcomes following allogeneic HCT, respectively28–30. We queried the Center for International Blood and Marrow Transplant Research (CIBMTR)’s registry to understand whether high dose TBI would translate into improved survival outcomes; we hypothesized that advances in supportive care and radiation delivery would reduce toxicity and NRM, thus yielding an OS advantage to higher doses of TBI.
METHODS
Data Source
The CIBMTR is a research collaboration between the Medical College of Wisconsin and the National Marrow Donor Program. The CIBMTR comprises a network of more than 450 transplantation centers worldwide that contribute data on allogeneic and autologous HCTs to a centralized statistical center for observational studies31. Health information is collected and maintained in the CIBMTR’s capacity as a public health authority under the Health Insurance Portability and Accountability Act privacy rules.
Patients
The study included 2721 adults with AML, ALL, MDS, and chronic myeloid leukemia (CML) receiving Cy/TBI, with TBI at varying doses, as conditioning in anticipation of a first allogeneic HCT from a well-matched sibling or unrelated donor between 2001–2013. Either matched siblings or well- or partially- (7/8-) matched unrelated donors were included. Patients with inherited syndromes predisposing to acute leukemia, those with central nervous system involvement with disease, and those who received prior radiation for any reason were excluded. We defined three TBI dose groups: patients receiving standard dose (12 Gy) (SD-TBI), intermediate high dose (13–13.75 Gy) (IH-TBI), and high dose (14 Gy) (HD-TBI).
Study Endpoints
The primary endpoint of the study was non-relapse mortality (NRM). NRM was defined as death from any cause in continuous remission or death within the first 28 days of transplant from any cause and was summarized by cumulative incidence estimate with relapse as competing risk. Secondary endpoints included OS, defined as time from transplant to death from any cause, with surviving patients censored at time of last contact, and disease-free survival (DFS), in which events were defined as death or relapse. Relapse was summarized by cumulative incidence estimate with NRM as the competing risk. We also sought to evaluate the incidence of other forms of toxicity and morbidity after allogeneic HCT including acute and chronic graft-versus-host disease (GVHD), veno-occlusive disease (VOD)/sinusoidal obstruction syndrome (SOS) of the liver32,33, and idiopathic pneumonia syndrome (IPS)34, all diagnosed on the basis of established criteria. Grading of acute and chronic GVHD was based on previously defined consensus criteria35,36.
Statistical Analysis
Patient-, disease-, and transplant-related characteristics were compared among the TBI dose groups using the chi-square test for categorical variables and the Kruskal-Wallis test for continuous variables. Outcomes of three TBI dose groups were compared using log-rank and Gray’s test. NRM was described using the cumulative incidence function, with relapse as a competing risk, according to the method of Fine and Gray37. Cumulative incidence of GVHD, VOD/SOS, and IPS were evaluated by Fine and Gray’s method of competing risks as well, with death as competing risk. Disease relapse was also reported using the cumulative incidence function, with NRM as the competing risk. Survival probabilities of OS and DFS were calculated using the Kaplan-Meier estimator and compared using the log-rank test. Multivariate analysis was performed using Cox proportional hazards regression models for OS, DFS, acute GVHD, chronic GVHD, VOD/SOS, and IPS; while Fine and Gray subdistribution hazards models37 were used for relapse and NRM. The following variables were included in the analysis: recipient age, disease, disease status at HCT, donor type, in vivo T cell depletion, GVHD prophylaxis, Karnofsky performance score, donor-recipient sex match, year of transplant. All clinical variables were tested first for the affirmation of the proportional hazards assumption. Factors violating the proportional hazards assumption were adjusted through stratification. Then a stepwise, forward-backward procedure was performed to select the adjusted clinical variables (with a threshold of 0.05 for both entry and stay in the model) and to build the multivariate models. To account for multiple comparisons, p<0.01 was used as the significance level for the main effect. Analysis was also conducted to evaluate center-effect. All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
Patient and transplant characteristics
The characteristics of the patients across the three TBI groups in the study cohort (n=2721) are shown in Table 1. The patients in the three groups received TBI dose of 12 Gy (SD-TBI; n=1745), 13–13.75 Gy (IH-TBI; n=648), and 14 Gy (HD-TBI; n=328). The completeness index at 5 years post-alloHCT was excellent (92–96%). The HD-TBI group were older and had lower Karnofsky performance scores (KPS). AML was the most common indication for allogeneic HCT across the cohort and was more frequent indication in the HD-TBI group compared to the SD-TBI patients (60% vs 48%, respectively). ALL, in contrast, was less common in the HD-TBI versus SD-TBI group (15 vs. 37%, respectively). HD-TBI-based HCTs were reported from 13 centers, compared to SD-TBI recipients from 155 centers and IH-TBI recipients from 49 centers. Median follow-up of survivors was similar across the groups at 67–73 months post-alloHCT.
Table 1.
Patient characteristics in the observational study of allogeneic transplant patients receiving myeloablative conditioning regimen of cyclophosphamide and total body radiation (TBI) with different doses of TBI between 2001 and 2013.
| Characteristics | 12 Gy (n=1745) n (%) |
13–13.75 Gy (n=648) n (%) |
14 Gy (n=328) n (%) |
P-value |
|---|---|---|---|---|
| Number of patients | 1745 | 648 | 328 | |
| Number of centers | 155 | 49 | 13 | |
| Age, median (range), years | 39 (18–60) | 39 (18–60) | 43 (18–60) | <0.001 |
| Male sex | 959 (55) | 344 (53) | 169 (52) | 0.436 |
| KPS 90–100% | 1197 (69) | 405 (63) | 190 (58) | <0.001 |
| Disease | <0.001 | |||
| Acute myelogenous leukemia | 836 (48) | 352 (54) | 198 (60) | |
| Acute lymphoblastic leukemia | 647 (37) | 175 (27) | 49 (15) | |
| Chronic myelogenous leukemia | 202 (12) | 89 (14) | 50 (15) | |
| Myelodysplastic syndrome | 60 (3) | 32 (5) | 31 (9) | |
| Disease status prior to transplant | 0.50 | |||
| Early | 912 (52) | 331 (51) | 157 (48) | |
| Intermediate | 421 (24) | 164 (25) | 78 (24) | |
| Advanced | 407 (23) | 153 (24) | 90 (27) | |
| Not reported | 5 (<1) | 0 | 3 (<1) | |
| BMI, median (range), kg/m^2 | 25 (16 – 49) | 24 (17 – 49) | 27 (17 – 49) | <0.001 |
Transplant characteristics are presented in Table 2. Fractionated TBI was administered to patients in all three groups: IH-TBI group received a median of 8 fractions with a median dose of 165 cGy per fraction, compared to a median of 6 and 7 fractions (with median doses of 200 cGy per fraction) in SD-TBI and HD-TBI groups, respectively (p<0.001). The HD-TBI group received a lower dose of Cy (median, 90 mg/kg vs 120 mg/kg in the other two groups, p<0.001). SD-TBI groups had a higher proportion of patients with a matched sibling donor (43% vs. 33% in the other two groups, p<0.001). Approximately 17% of patients in all three groups received allogeneic HCT using 7/8-matched unrelated donor. With respect to GVHD prophylaxis, most patients (>98%) received a calcineurin inhibitor (CNI), and most did not receive in vivo T-cell depletion. Peripheral blood grafts were used more commonly in HD-TBI group (76%) compared to both SD-TBI (71%) and IH-TBI (65%) groups.
Table 2.
Transplant characteristics in the observational study of allogeneic transplant patients receiving myeloablative conditioning regimen of cyclophosphamide and total body radiation (TBI) at different doses between 2001 and 2013.
| Characteristics | 12 Gy (n=1745) n (%) |
13–13.75 Gy (n=648) n (%) |
14 Gy (n=328) n (%) |
P-value |
|---|---|---|---|---|
| Time from diagnosis to transplant, median (range), months | 7 (1–252) | 7 (2–310) | 6 (1–222) | 0.44 |
| Number of fractions, median (range) | 6 (2–12) | 8 (3–12) | 7 (2–8) | <0.001 |
| TBI dose per fraction, median (range), cGy | 200 (100–600) | 165 (108–440) | 200 (175–700) | <0.001 |
| Cy dose, median (range), mg/kg | 120 (34–240) | 120 (36–239) | 90 (33–206) | <0.001 |
| Donor type | <0.001 | |||
| HLA-identical sibling | 746 (43) | 216 (33) | 109 (33) | |
| Matched unrelated (8/8) | 694 (40) | 313 (48) | 164 (50) | |
| Partially matched unrelated (7/8) | 305 (17) | 119 (18) | 55 (17) | |
| Graft source | <0.001 | |||
| Bone marrow | 504 (29) | 228 (35) | 79 (24) | |
| Peripheral blood | 1241 (71) | 420 (65) | 249 (76) | |
| Donor–Recipient sex match | 0.37 | |||
| Male–Male | 606 (35) | 218 (34) | 114 (35) | |
| Male–Female | 446 (26) | 176 (27) | 78 (24) | |
| Female–Male | 347 (20) | 125 (19) | 55 (17) | |
| Female–Female | 338 (19) | 128 (20) | 81 (25) | |
| Not reported | 8 (<1) | 1 (<1) | 0 | |
| Donor–Recipient cytomegalovirus status | 0.02 | |||
| −/− | 510 (29) | 157 (24) | 99 (30) | |
| −/+ | 429 (25) | 183 (28) | 96 (29) | |
| +/− | 193 (11) | 63 (10) | 38 (12) | |
| +/+ | 527 (30) | 207 (32) | 76 (23) | |
| Not reported | 86 (5) | 38 (6) | 19 (6) | |
| Unrelated donor age, median (range), years | 33 (19–61) | 33 (18–58) | 32 (19–60) | 0.95 |
| Year of transplant | 0.005 | |||
| 2001–2005 | 809 (46) | 267 (41) | 149 (45) | |
| 2006–2010 | 749 (43) | 308 (48) | 161 (49) | |
| 2011–2013 | 187 (11) | 73 (11) | 18 (5) | |
| Inpatient days, median (range) | 29 (<1–123) | 32 (<1–175) | 26 (<1–100) | |
| Median follow-up of survivors, range, months | 72 (3 – 167) | 67 (4 – 148) | 72 (5 – 144) |
Abbreviations: Cy, cyclophosphamide; HLA, human leukocyte antigen; TBI, total-body irradiation
Impact of conditioning TBI dose on post-transplant outcomes
Non-relapse mortality
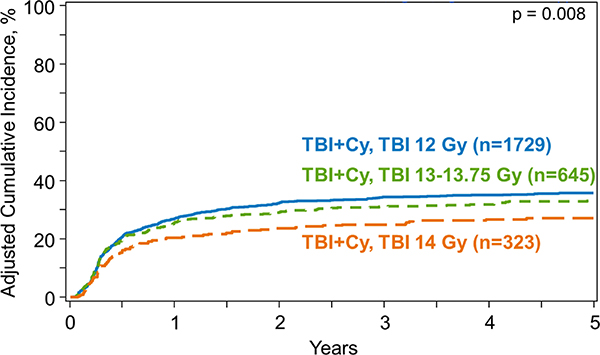
Univariate analysis revealed that at 5 years post-HCT, NRM was 28% (95% Confidence Interval [CI], 25–30%) for the SD-TBI group, 32% (95%CI, 29–36%) for the IH-TBI group and 34% (95%CI, 28–39%) for the HD-TBI group (p=0.02) (Table 3). Multivariate modelling using Fine and Gray’s method accounting for competing risks confirmed that TBI dose was statistically significantly associated with NRM (p=0.009) (Table 4) (Figure 1). Patients receiving IH-TBI (13–13.75 Gy) had a 25% higher risk of NRM compared to SD-TBI (12 Gy) (p=0.007). HD-TBI group (14 Gy), however, did not have a significantly increased NRM risk compared to SD-TBI (p=0.03) or IH-TBI (p=0.96) groups. Multivariate analysis also showed older patients (>30 years vs. <20 years), those with MDS and ALL (vs. AML), with unrelated donor (vs. matched sibling donor) and those who received CNI/MMF (vs. CNI/MTX) had a higher NRM risk (Supplemental Table S3). In addition, NRM risk improved with each time period (2011–2013 vs. 2001–2003; HR 0.46, p<0.0001) over the years.
Table 3.
Unadjusted clinical outcomes after myeloablative conditioning allogeneic transplant using matched sibling and unrelated donor by dose of total body irradiation (TBI) (2001–2013)
| Outcomes | 12 Gy (n=1745) Probability (95% CI) |
13–13.75 Gy (n=648) Probability (95% CI) |
14 Gy (n=328) Probability (95% CI) |
P-value |
|---|---|---|---|---|
| Veno-occlusive disease/sinusoidal obstruction syndrome | ||||
| 100-day | 5% (4–6) | 6% (4–7) | 9% (6–12) | 0.09 |
| Idiopathic pneumonia syndrome | ||||
| 2-year | 8% (6–9) | 8% (6–11) | 9% (6–13) | 0.57 |
| Grade II-IV acute graft-vs-host disease | ||||
| 1-year | 43% (40–45) | 49% (45–53) | 42% (37–47) | 0.02 |
| Grade III-IV acute graft-vs-host disease | ||||
| 1-year | 19% (17–21) | 23% (20–26) | 20% (16–25) | 0.10 |
| Chronic graft-vs-host disease | ||||
| 5-year | 52% (49–54) | 50% (46–54) | 53% (48–59) | 0.68 |
| Relapse | ||||
| 1-year | 27% (25–29) | 25% (22–28) | 20% (16–24) | 0.01 |
| 5-year | 36% (34–38) | 32% (29–36) | 26% (21–31) | <0.001a |
| Non-relapse mortality | ||||
| 5-year | 28% (25–30) | 32% (29–36) | 34% (28–39) | 0.02 |
| Disease-free survival | ||||
| 5-year | 37% (34–39) | 35% (32–39) | 40% (35–46) | 0.29 |
| Overall survival | ||||
| 5-year | 42% (39–44) | 40% (36–44) | 45% (39–50) | 0.39 |
Abbreviations: CI, confidence interval; Gy, Gray.
Significant at p < 0.01 level
Table 4.
Total body irradiation (TBI) dose in multivariate models of treatment with cyclophosphamide plus TBI as myeloablative conditioning regimen for allogeneic transplant using matched sibling and unrelated donor (2001–2013).
| Outcome | n | Events | HR | Upper | Lower | P-value |
|---|---|---|---|---|---|---|
| Overall Survival | ||||||
| TBI dose | 0.18 | |||||
| 12 Gy | 1732 | 1024 | 1.0 | |||
| 13–13.75 Gy | 647 | 394 | 1.06 | 0.94 | 1.19 | 0.36 |
| 14 Gy | 325 | 190 | 0.89 | 0.76 | 1.05 | 0.17 |
| 14 Gy vs. TBI 13–13.75 Gy (Ref.) | 0.85 | 0.71 | 1.01 | 0.06 | ||
| Disease-Free Survival | ||||||
| TBI dose | 0.04 | |||||
| 12 Gy | 1727 | 1098 | 1.0 | |||
| 13–13.75 Gy | 644 | 423 | 1.01 | 0.90 | 1.13 | 0.90 |
| 14 Gy | 323 | 199 | 0.83 | 0.71 | 0.97 | 0.02 |
| 14 Gy vs. TBI 13–13.75 Gy (Ref.) | 0.82 | 0.69 | 0.97 | 0.02 | ||
| Non-Relapse Mortality | ||||||
| TBI dose | 0.009a | |||||
| 12 Gy | 1734 | 486 | 1.0 | |||
| 13–13.75 Gy | 645 | 215 | 1.25 | 1.06 | 1.48 | 0.007 |
| 14 Gy | 326 | 117 | 1.25 | 1.02 | 1.53 | 0.03 |
| 14 Gy vs. TBI 13–13.75 Gy (Ref.) | 0.99 | 0.79 | 1.25 | 0.96 | ||
| Relapse | ||||||
| TBI dose | 0.008a | |||||
| 12 Gy | 1737 | 618 | 1.0 | |||
| 13–13.75 Gy | 646 | 209 | 0.92 | 0.78 | 1.08 | 0.29 |
| 14 Gy | 323 | 84 | 0.69 | 0.55 | 0.88 | 0.002a |
| 14 Gy vs. TBI 13–13.75 Gy (Ref.) | 0.76 | 0.59 | 0.98 | 0.03 | ||
| Acute GVHD Grade II-IV | ||||||
| TBI doseb | . | . | . | . | . | 0.01 |
| 12 Gy | 1724 | 739 | 1.0 | . | . | . |
| 13–13.75 Gy | 640 | 313 | 1.15 | 1.00 | 1.31 | 0.05 |
| 14 Gy | 326 | 137 | 0.85 | 0.71 | 1.03 | 0.09 |
| 14 Gy vs. TBI 13–13.75 Gy (Ref.) | 0.74 | 0.61 | 0.91 | 0.004 | ||
| Acute GVHD Grade III-IV | ||||||
| TBI dose | 0.21 | |||||
| 12 Gy | 1728 | 329 | 1.0 | |||
| 13–13.75 Gy | 641 | 148 | 1.18 | 0.97 | 1.44 | 0.10 |
| 14 Gy | 324 | 66 | 0.96 | 0.73 | 1.27 | 0.79 |
| 14 Gy vs. TBI 13–13.75 Gy (Ref.) | 0.82 | 0.61 | 1.10 | 0.18 | ||
| Chronic GVHD | ||||||
| TBI dose (<=8 months) | 0.0001c | |||||
| 12 Gy | 1127 | 622 | 1.0 | |||
| 13–13.75 Gy | 401 | 205 | 0.83 | 0.71 | 0.97 | 0.02 |
| 14 Gy | 193 | 96 | 0.64 | 0.52 | 0.80 | 0.0001a |
| 14 Gy vs. TBI 13–13.75 Gy (Ref.) | 0.78 | 0.61 | 1.00 | 0.05 | ||
| TBI dose (>8 months) | 0.02 | |||||
| 12 Gy | 588 | 217 | 1.0 | |||
| 13–13.75 Gy | 239 | 102 | 1.10 | 0.98 | 1.24 | 0.12 |
| 14 Gy | 133 | 75 | 1.00 | 0.62 | 1.62 | 0.99 |
| 14 Gy vs. TBI 13–13.75 Gy (Ref.) | 0.91 | 0.51 | 1.63 | 0.75 | ||
| VOD/SOS | ||||||
| TBI dose | 0.03 | |||||
| 12 Gy | 1737 | 88 | 1.0 | |||
| 13–13.75 Gy | 648 | 36 | 1.16 | 0.78 | 1.71 | 0.46 |
| 14 Gy | 322 | 30 | 1.77 | 1.17 | 2.69 | 0.007 |
| 14 Gy vs. TBI 13–13.75 Gy (Ref.) | 1.53 | 0.94 | 2.49 | 0.08 | ||
| IPS | ||||||
| TBI dose | 0.80 | |||||
| 12 Gy | 1715 | 131 | 1.0 | |||
| 13–13.75 Gy | 634 | 53 | 1.08 | 0.78 | 1.49 | 0.63 |
| 14 Gy | 318 | 30 | 1.12 | 0.75 | 1.67 | 0.57 |
| 14 Gy vs. TBI 13–13.75 Gy (Ref.) | 1.04 | 0.66 | 1.63 | 0.87 |
Abbreviations: GVHD, graft-versus-host disease; Gy, Gray; HR, hazard ratio; IPS, interstitial pneumonia syndrome; TBI, total body irradiation; VOD, veno-occlusive disease.
Significant at p<0.01 level
All patients received cyclophosphamide with TBI
Significant at p < 0.01 level
Figure 1.
Cumulative incidence function of non-relapse mortality by dose of TBI
Overall survival
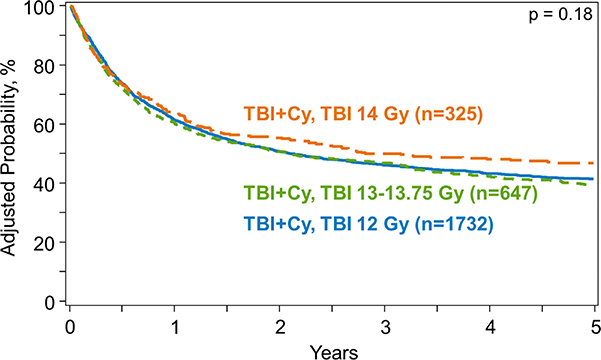
OS after allogeneic HCT was similar across the three TBI dose groups on univariate analysis: 5-year OS was 42% (95%CI, 39–44%), 40% (95%CI, 36–44%) and 45% (95%CI, 39–50%) in SD-TBI, IH-TBI and HD-TBI groups, respectively (p=0.39) (Table 3). The multivariate analysis also showed no significant association between the TBI dose and OS (p=0.18) (Table 4) (Figure 4). The analysis also demonstrated that younger patients (<20 years vs. >40 years), those with CML (vs. AML), with matched sibling donor (vs. unrelated donor), and those receiving CNI/MTX (vs. CNI/MMF), with KPS ≥90 (vs. <90) had significantly improved OS (Supplemental Table S1). OS also improved significantly with each time interval (e.g., 2011–2013 vs. 2001–2003, HR 0.6, p<0.0001).
Figure 4.
Kaplan-meier curve of overall survival by dose of TBI
Disease-free survival
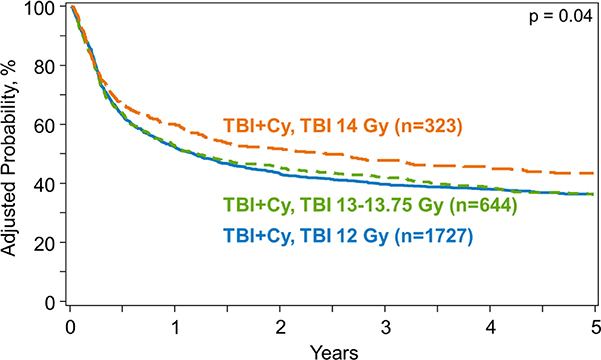
Univariate analysis demonstrated that the 5-year probability of DFS did not differ significantly among TBI dose groups and was 37% (95%CI, 34–39%), 35% (95%CI, 32–39%) and 40% (95%CI, 35–46%) in SD-TBI, IH-TBI and HD-TBI groups, respectively (p= 0.36) (Table 3). There was no significant difference in DFS among the three TBI dose groups on multivariate analysis (Table 4) (Figure 3).
Figure 3.
Disease-free survival by dose of TBI
Relapse
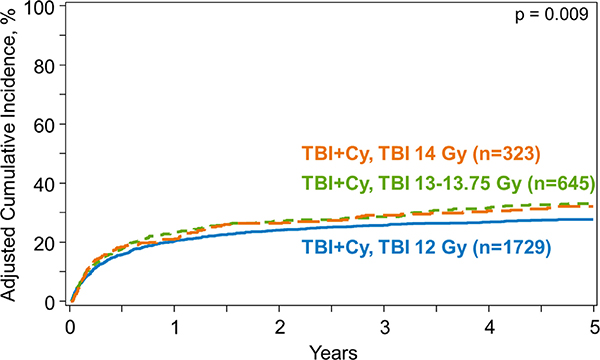
The risk of disease relapse post-HCT differed significantly among the TBI dose groups on univariate analysis: 5-year cumulative incidence of relapse was 36% (95%CI, 34–38%) in SD-TBI group, 32% (95%CI, 29–36%) in IH-TBI and 26% (95%CI, 21–31%) in HD-TBI group (p<0.001) (Table 3). Multivariate analysis showed that HD-TBI recipients had a significantly lower relapse risk compared to SD-TBI group (hazards ratio [HR] 0.69, p=0.002) (Table 4) (Figure 2). Patients with MDS (vs. AML) and early (vs. intermediate or advanced) disease and with matched sibling donor (vs. unrelated) had a lower risk of relapse (Supplemental Table S4).
Figure 2.
Cumulative incidence function of relapse by dose of TBI
Acute GVHD
Univariate analysis revealed 1-year cumulative incidence of grade II-IV acute GVHD in the IH-TBI group was 49% (95%CI, 45–53%) compared to 43% in the SD-TBI group (95%CI, 40–45%) and 42% in HD-TBI group (95%CI, 37–47%) (p=0.02) (Table 3). On multivariate analysis, TBI dose was not associated with grade II-IV acute GVHD (p=0.01), or grade III-IV acute GVHD (p=0.21) (Table 4).
Chronic GVHD
On univariate analysis, 5-year cumulative incidence of chronic GVHD was not significantly different among the three groups: 52% (95%CI, 49–54%) in SD-TBI group, 50% (95%CI, 46–54%) in IH-TBI group and 53% (95%CI, 48–59%) in HD-TBI group (p=0.68) (Table 3). However, multivariate analysis suggested that the risk of chronic GVHD among the three cohorts was time-dependent: TBI was significantly associated with chronic GVHD in the first 8 months post-HCT (p=0.0001), but not beyond 8 months after HCT (p=0.02) (Table 4). HD-TBI conferred a lower risk of chronic GVHD compared to SD group (HR 0.64, p=0.0001) early on after allogeneic HCT.
TBI-associated post-transplant organ dysfunction
On univariate analysis, the 100-day cumulative incidence of VOD/SOS following allogeneic HCT was 5% (95%CI, 4–6%), 6% (95%CI, 4–7%) and 9% (95%CI, 6–12%) in SD-TBI, IH-TBI and HD-TBI groups, respectively (Table 3). Multivariate analysis showed TBI dose was not significantly associated with risk of VOD/SOS (p=0.03) (Table 4). TBI dose also had no significant association with IPS after allogeneic HCT, which carried a 2-year cumulative incidence of 8–9% in the three cohorts (Tables 3–4).
Causes of death
Relapse of primary disease was the most common cause of death in all three groups (Table 5). However, there were more relapse-related deaths with SD-TBI (55%) compared to the other two groups (47% in IH-TBI and 40% in HD-TBI group). The proportion of deaths due to organ failure increased with higher doses of TBI (19% in HD-TBI and 9% in SD-TBI group). Respiratory and multi-organ failure were most common, followed by heart failure and hepatic dysfunction (Table 5a).
Table 5.
Causes of death after myeloablative conditioning allogeneic transplant using cyclophosphamide and total body irradiation (TBI) as conditioning, by dose of TBI (2001–2013).
| Cause of death | 12 Gy (n=1034) n (%) |
13–13.75 Gy (n=395) n (%) |
14 Gy (n=192) n (%) |
|---|---|---|---|
| Primary disease | 564 (55) | 184 (47) | 77 (40) |
| New malignancy | 9 (1) | 4 (1) | 3 (2) |
| Graft-versus-host disease | 112 (11) | 63 (16) | 24 (13) |
| Interstitial pneumonitis | 52 (5) | 15 (4) | 9 (5) |
| Infection | 131 (13) | 47 (12) | 27 (14) |
| Organ failure | 96 (9) | 46 (12) | 37 (19) |
| Other cause | 62 (6) | 29 (7) | 13 (7) |
| Not reported | 8 (1) | 7 (2) | 2 (1) |
Table 5a.
Organ failure as cause of death following myeloablative conditioning allogeneic transplant using cyclophosphamide and total body irradiation (TBI) as conditioning, by dose of TBI (2001–2013).
| Cause of death, n | 12 Gy | 13–13.75 Gy | 14 Gy |
|---|---|---|---|
| Liver (n=21) | 10 | 7 | 4 |
| Veno-occlusive disease/SOS (n=14) | 4 | 4 | 6 |
| Cardiac (n=28) | 17 | 8 | 3 |
| Pulmonary (n=61) | 36 | 10 | 15 |
| Central nervous system (n=5) | 3 | 2 | 0 |
| Renal (n=6) | 4 | 0 | 2 |
| Multiple organ (n=39) | 20 | 13 | 6 |
| Other (n=4) | 1 | 2 | 1 |
DISCUSSION
This contemporary observational study compared MAC regimens containing Cy combined with three TBI dose groups in allogeneic HCT recipients with AML, ALL, CML and MDS. HD-TBI group had a more frequent use of peripheral blood graft, unrelated donors, had fewer patients with KPS>90 and had a higher median age: all variables were included in multivariate modelling to account for the baseline differences. Compared to 12 Gy TBI, we observed increased NRM with intermediate TBI dose of 13–13.75 Gy and lower relapse with high TBI dose (14 Gy). While the analysis showed significant difference in NRM risk between SD-TBI and IH-TBI groups, there was no significant difference in the risk of NRM between HD-TBI and other two groups. However, the impact on NRM seemed to be equal once the TBI dose increased beyond SD-TBI: comparing the NRM risk between HD-TBI and SD-TBI groups in the multivariate model showed HR for death was 1.25 (the same as HR with IH compared to SD group). It is likely that the statistical significance was not reached given the small sample size population in the HD group hindering power to detect a difference; a larger population may have shown significant results. With regards to the relapse model, there is a linear relationship with increments on the TBI dose: HR (for death) of 1.0 for SD-TBI, 0.92 for IH-TBI and 0.69 for HD-TBI, though not statistically significant. With the potentially opposing effects of TBI dose on relapse and NRM, there was no significant difference observed in OS and DFS among the TBI dose groups in the study.
There was no statistically significant difference in the risk of grade II-IV or III-IV acute GVHD. Furthermore, no association of TBI dose with the risk of IPS was found. The risk of chronic GVHD (in the early post-HCT period) was lower in patients receiving HD-TBI, an unexpected finding, particularly given the absence of significant difference in acute GVHD risk. While there is no good explanation for having increased risk chronic GVHD after SD TBI compared to the higher doses; one possibility is that SD-TBI patients received early interventions to prevent or treat relapse, such as withdrawal of immunosuppression or donor leukocyte infusions, which would then be expected to result in increased risk of early onset chronic GVHD. However, we did not have access to the post-transplant data to support this hypothesis. There is also a possibility of residual confounding by other variables that were not included in the analysis such as post-transplant therapeutic interventions. The study demonstrated no significant association between the TBI dose and the risk of IPS after allogeneic HCT. While the incidence of VOD/SOS of liver was higher with higher doses of TBI, this observation did not meet statistical significance.
Radiation is a potent anti-tumor therapy that is not dependent on cell cycle, growth or metabolism, and is not affected by common methods of chemotherapy resistance such as P-glycoprotein pumps38–40 and so chemotherapy-resistant clones may still be radio-sensitive41. Furthermore, radiation is directly toxic to hematopoietic stem cells21,22, and can reach potential sanctuary sites such as testis and brain41, making TBI an important component of the conditioning regimens before allogeneic HCT for treatment of hematologic malignancies. TBI has traditionally been a part of MAC regimens with the objective of eradicating malignant cells and also providing the immunosuppression needed to prevent rejection of donor hematopoietic cells41. Dose escalation of TBI in MAC has been investigated and demonstrated to be feasible with acceptable non-relapse mortality in several single-center studies23,24,26. Myeloablative TBI dose cohorts have been compared in a few studies and have shown reduced relapse risk of AML27,42, CML43 and ALL44 with higher dose TBI in the conditioning. A randomized study by Clift et al. published in 1990s evaluated a conditioning regimen of Cy 120 mg/kg in combination with TBI 15.75 Gy with 7 consecutive daily fractions of 2.25 Gy (n=37), and demonstrated a lower relapse risk compared with TBI 12 Gy with 6 consecutive daily fractions of 2 Gy (n=34) in patients with AML in first complete remission42. The 3-year probabilities of relapse were 35% for the 12 Gy group and 12% for the 15.75 Gy group (p=0.06). However, the 3-year NRM was 12% and 32% for the two respective groups (p=0.04). In essence, the increased dose of TBI significantly reduced the probability of relapse but did not improve OS because of increased NRM.
Baseline demographics show the HD-TBI recipients were older, with poorer KPS: this suggests the possibility of selection bias by clinicians to target a higher risk patient population with increased TBI dose. However, multivariate analysis should account for these differences. Similarly, the analysis accounted for the higher proportion of AML patients in the HD-TBI group. The analysis demonstrated significantly better OS in CML patients (vs. AML; HR 0.8, p=0.006); MDS patients experienced higher NRM (vs. AML; HR 1.82, p=0.0001) and lower relapse risk (vs. AML; HR 0.45, p<0.0001) on multivariate analysis. With regards to donor-recipient HLA matching, since the proportion of 7/8 matched unrelated donors was similar across all three groups, and our multivariate analysis adjusted for degree of HLA matching, this small group of patients is unlikely to have altered our results. It is worth noting that we tested for interaction between the TBI dose and disease type, disease risk and all other variables, for each endpoint, and found none. The study covered a period of 14 years and as expected, the patients receiving allogeneic HCT in more recent years experienced significantly less NRM (36% better in 2008–2010 and 54% improvement in 2011–2013, as compared to 2001–03, respectively) and OS (23% and 40% improvement over 2001–03, respectively) (Supplemental Tables S1 and S3). The lack of significant interaction between the TBI dose and the categorical variable of year of HCT indicates that the improvement in NRM over time has been observed in all TBI-based MAC allogeneic HCTs regardless of the TBI dose. Stated differently, the results suggest that despite the improvement in supportive care over the years, which may allow for a higher dose of TBI, NRM continues to be higher with HD-TBI.
This study has many limitations, including those inherent with the retrospective nature of the study arising from non-random assignment to the TBI groups, institutional variability in TBI dosing and fractionation, as well as variation in Cy dosing (HD group that had a lower median Cy dose to allow a higher TBI dose) (Table 2). It is important to point out that the reason for selecting the doses of TBI is not known; the TBI doses were most likely decided by the institutions as a matter of preference and were likely not based on the disease risk category, as evident from Table 1. Nonetheless, we cannot exclude potential selection bias in the higher dose TBI groups and residual confounding that could not be addressed by the analysis. The much smaller number of HD-TBI conditioned transplant in the recent time periods (6% in 2011–13 vs. 12% in 2001–05 vs. 13% in 2006–10) may indicate that this bias is present (Table 1). From a radiobiologic perspective, a major shortcoming of this analysis is anchoring the analysis on total TBI dose; we were unable to incorporate dose rate and/or protractionation. These fundamental variables are known to be associated with the biological consequences of ionizing radiation exposure and interpreting the data in the absence of these variables can be difficult. This variability in clinical practice with regards to the use of TBI among centers is exemplified by the study by European Group for Blood and Marrow Transplantation that surveyed 56 centers from 23 countries and demonstrated significant differences in the treatment technique, dose per fraction, in the organs shielded and the maximum accepted total delivered dose to those organs45. Furthermore, we did not evaluate TBI dose in combination with chemotherapy agents other than Cy such as etoposide, melphalan or fludarabine and this limits the generalizability of the study findings. The question of optimal TBI dose for other types of allogeneic transplant, such as umbilical cord blood and haploidentical transplants in the myeloablative setting, remains unanswered.
In conclusion, TBI dose of over 12Gy was demonstrated to reduce relapse risk, but this advantage was hampered by the increase in NRM, and that likely translated into no significant impact on OS. The study results suggest that Cy/TBI 12 Gy, therefore, should be considered the optimal conditioning regimen for patients with AML, ALL, MDS, and CML undergoing MAC allogeneic HCT. Higher TBI dosing may be associated with greater morbidity, as evidenced by the higher incidence of organ failure as the cause of death (Table 5a). We can speculate that young adults (<40 years), with robust performance status (KPS ≥90), advanced disease (myeloid malignancy) and a matched sibling donor may derive greater survival benefit from HD-TBI (compared to <14 Gy TBI; Supplemental Table S1). Future research should focus on novel strategies to protect patients against the adverse effects of high dose TBI. Its potency in disease control is clear; reducing TBI’s toxicity and NRM may therefore help overcome relapse, the most significant barrier to long-term survival after allogeneic HCT. Developing safer methods to deliver radiation, sparing sensitive organs, continues to be an important area of research to maximize the effectiveness of high dose TBI in allogeneic HCT recipients.
Supplementary Material
HIGHLIGHTS.
Total body irradiation (TBI) is an important component of myeloablative conditioning regimens for allogeneic hematopoietic cell transplantation. Long-term disease control appears to be dose-dependent, but doses >12 Gray (Gy) have previously been associated with excess toxicity and higher non-relapse mortality (NRM).
In the current era, TBI doses higher than 12 Gy offer no survival advantage over standard dose TBI (12 Gy) when used in combination with cyclophosphamide as conditioning for allogeneic transplant, as a decrease in relapse risk is offset by increased risk of NRM. The study supports the recommendation for fractionated TBI 12 Gy in myeloablative conditioning for hematologic malignancies.
Acknowledgments
The authors thank Jennifer Motl for editorial support in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). Funding: The CIBMTR is supported primarily by Public Health Service Grant/Cooperative Agreement 5U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 1U24HL138660 from NHLBI and NCI; a contract HHSH250201700006C with Health Resources and Services Administration (HRSA/DHHS); Grants N00014 1712388, N000141712850 and N000141812045 from the Office of Naval Research HHSH250201700006C; and grants from Adaptive Biotechnologies; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; Atara Biotherapeutics, Inc.; Be the Match Foundation; *bluebird bio, Inc.; *Bristol Myers Squibb Oncology; *Celgene Corporation; *Chimerix, Inc.; *CytoSen Therapeutics, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Gilead Sciences, Inc.; HistoGenetics, Inc.; Immucor; *Incyte Corporation; Janssen Scientific Affairs, LLC; *Jazz Pharmaceuticals, Inc.; Karius, Inc.; Karyopharm Therapeutics, Inc.; *Kite Pharma, Inc.; Medac, GmbH; *Mediware; The Medical College of Wisconsin; *Merck & Co, Inc.; *Mesoblast; MesoScale Diagnostics, Inc.; Millennium, the Takeda Oncology Co.; *Miltenyi Biotec, Inc.; Mundipharma EDO; National Marrow Donor Program; Novartis Pharmaceuticals Corporation; PCORI; *Pfizer, Inc; *Pharmacyclics, LLC; PIRCHE AG; *Sanofi Genzyme; *Seattle Genetics; Shire; Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; Swedish Orphan Biovitrum, Inc.; *Takeda Oncology; and University of Minnesota. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
* Corporate Members
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Barrett AJ, Battiwalla M: Relapse after allogeneic stem cell transplantation. Expert Review of Hematology 3:429–441, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Majhail NS, Chitphakdithai P, Logan B, et al. : Significant Improvement in Survival after Unrelated Donor Hematopoietic Cell Transplantation in the Recent Era. Biology of Blood and Marrow Transplantation 21:142–150, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horan JT, Logan BR, Agovi-Johnson M-A, et al. : Reducing the Risk for Transplantation-Related Mortality After Allogeneic Hematopoietic Cell Transplantation: How Much Progress Has Been Made? Journal of Clinical Oncology 29:805–813, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.PLM Hahn T Jr, Hassebroek A, et al. : Significant Improvement in Survival After Allogeneic Hematopoietic Cell Transplantation During a Period of Significantly Increased Use, Older Recipient Age, and Use of Unrelated Donors. Journal of Clinical Oncology 31:2437–2449, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gooley TA, Chien JW, Pergam SA, et al. : Reduced Mortality after Allogeneic Hematopoietic-Cell Transplantation. New England Journal of Medicine 363:2091–2101, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chhabra S, Ahn KW, Hu Z-H, et al. : Myeloablative vs reduced-intensity conditioning allogeneic hematopoietic cell transplantation for chronic myeloid leukemia. Blood Advances 2:2922–2936, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ringdén O, Labopin M, Ehninger G, et al. : Reduced Intensity Conditioning Compared With Myeloablative Conditioning Using Unrelated Donor Transplants in Patients With Acute Myeloid Leukemia. Journal of Clinical Oncology 27:4570–4577, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Bornhäuser M, Kienast J, Trenschel R, et al. : Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: a prospective, open-label randomised phase 3 trial. The Lancet Oncology 13:1035–1044, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Aoudjhane M, Labopin M, Gorin NC, et al. : Comparative outcome of reduced intensity and myeloablative conditioning regimen in HLA identical sibling allogeneic haematopoietic stem cell transplantation for patients older than 50 years of age with acute myeloblastic leukaemia: a retrospective survey from the Acute Leukemia Working Party (ALWP) of the European group for Blood and Marrow Transplantation (EBMT). Leukemia 19:2304, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Scott BL, Pasquini MC, Logan BR, et al. : Myeloablative Versus Reduced-Intensity Hematopoietic Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndromes. Journal of Clinical Oncology 35:1154–1161, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ringden O, Ruutu T, Remberger M, et al. : A randomized trial comparing busulfan with total body irradiation as conditioning in allogeneic marrow transplant recipients with leukemia: a report from the Nordic Bone Marrow Transplantation Group. Blood 83:2723–2730, 1994 [PubMed] [Google Scholar]
- 12.Nagler A, Rocha V, Labopin M, et al. : Allogeneic Hematopoietic Stem-Cell Transplantation for Acute Myeloid Leukemia in Remission: Comparison of Intravenous Busulfan Plus Cyclophosphamide (Cy) Versus Total-Body Irradiation Plus Cy As Conditioning Regimen—A Report From the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Journal of Clinical Oncology 31:3549–3556, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Copelan EA, Hamilton BK, Avalos B, et al. : Better leukemia-free and overall survival in AML in first remission following cyclophosphamide in combination with busulfan compared with TBI. Blood 122:3863–3870, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bredeson C, LeRademacher J, Kato K, et al. : Prospective cohort study comparing intravenous busulfan to total body irradiation in hematopoietic cell transplantation. Blood 122:3871–3878, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granados E, de La Camara R, Madero L, et al. : Hematopoietic cell transplantation in acute lymphoblastic leukemia: better long term event-free survival with conditioning regimens containing total body irradiation. Haematologica 85:1060–1067, 2000 [PubMed] [Google Scholar]
- 16.Wu Q, Zhang R, Wang H, et al. : Comparison of outcomes of idarubicin intensified TBI-CY and traditional TBI-CY conditioning regimen for high-risk acute lymphoblastic leukemia undergoing allogeneic hematopoietic stem cell transplantation: A single center experience. Leuk Res, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Tachibana T, Tanaka M, Hagihara M, et al. : Clinical significance of the administration of cytarabine or thiotepa in addition to total body irradiation and cyclophosphamide for allogeneic hematopoietic cell transplantation in patients with acute leukemia. International Journal of Hematology 102:451–459, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Stein AS, O’Donnell MR, Synold TW, et al. : Phase-2 trial of an intensified conditioning regimen for allogeneic hematopoietic cell transplant for poor-risk leukemia. Bone Marrow Transplantation 46:1256, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mengarelli A, Iori A, Guglielmi C, et al. : Standard versus alternative myeloablative conditioning regimens in allogeneic hematopoietic stem cell transplantation for high-risk acute leukemia. Haematologica 87:52–58, 2002 [PubMed] [Google Scholar]
- 20.Li Q-b, Li L, You Y, et al. : A comparative study of outcomes of idarubicin- and etoposide-intensified conditioning regimens for allogeneic peripheral blood stem cell transplantation in patients with high-risk acute leukemia. Acta Pharmacologica Sinica 30:1471, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lijian S, Yi L, Daohong Z: Hematopoietic Stem Cell Injury Induced by Ionizing Radiation. Antioxidants & Redox Signaling 20:1447–1462, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo C-Y, Luo L, Urata Y, et al. : Sensitivity and dose dependency of radiation-induced injury in hematopoietic stem/progenitor cells in mice. Scientific reports 5:8055–8055, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sobecks RM, Daugherty CK, Hallahan DE, et al. : A dose escalation study of total body irradiation followed by high-dose etoposide and allogeneic blood stem cell transplantation for the treatment of advanced hematologic malignancies. Bone Marrow Transplantation 25:807, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Petersen FB, Deeg HJ, Buckner CD, et al. : Marrow transplantation following escalating doses of fractionated total body irradiation and cyclophosphamide—a phase I trial. International Journal of Radiation Oncology*Biology*Physics 23:1027–1032, 1992 [DOI] [PubMed] [Google Scholar]
- 25.Girinsky T, Benhamou E, Bourhis J-H, et al. : Prospective Randomized Comparison of Single-Dose Versus Hyperfractionated Total-Body Irradiation in Patients With Hematologic Malignancies. Journal of Clinical Oncology 18:981–981, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Alyea E, Neuberg D, Mauch P, et al. : Effect of total body irradiation dose escalation on outcome following T-cell-depleted allogeneic bone marrow transplantation. Biology of Blood and Marrow Transplantation 8:139–144, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Clift RA, Buckner CD, Appelbaum FR, et al. : Long-Term Follow-Up of a Randomized Trial of Two Irradiation Regimens for Patients Receiving Allogeneic Marrow Transplants During First Remission of Acute Myeloid Leukemia. Blood 92:1455–1456, 1998 [PubMed] [Google Scholar]
- 28.Wong JYC, Rosenthal J, Liu A, et al. : Image-Guided Total-Marrow Irradiation Using Helical Tomotherapy in Patients With Multiple Myeloma and Acute Leukemia Undergoing Hematopoietic Cell Transplantation. International Journal of Radiation Oncology*Biology*Physics 73:273–279, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong JYC, Forman S, Somlo G, et al. : Dose Escalation of Total Marrow Irradiation With Concurrent Chemotherapy in Patients With Advanced Acute Leukemia Undergoing Allogeneic Hematopoietic Cell Transplantation. International Journal of Radiation Oncology*Biology*Physics 85:148–156, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stein A, Palmer J, Tsai N-C, et al. : Phase I Trial of Total Marrow and Lymphoid Irradiation Transplantation Conditioning in Patients with Relapsed/Refractory Acute Leukemia. Biology of Blood and Marrow Transplantation 23:618–624, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horowitz MM: The role of registries in facilitating clinical research in BMT: examples from the Center for International Blood and Marrow Transplant Research. Bone Marrow Transplantation 42:S1, 2008 [DOI] [PubMed] [Google Scholar]
- 32.JONES RJ, LEE KSK, BESCHORNER WE, et al. : VENOOCCLUSIVE DISEASE OF THE LIVER FOLLOWING BONE MARROW TRANSPLANTATION. Transplantation 44:778–783, 1987 [DOI] [PubMed] [Google Scholar]
- 33.Mcdonald GB, Sharma P, Matthews DE, et al. : Venocclusive Disease of the Liver after Bone Marrow Transplantation: Diagnosis, Incidence, and Predisposing Factors. Hepatology 4:116–122, 1984 [DOI] [PubMed] [Google Scholar]
- 34.Clark JG, Hansen JA, Hertz MI, et al. : Idiopathic Pneumonia Syndrome after Bone Marrow Transplantation. American Review of Respiratory Disease 147:1601–1606, 1993 [DOI] [PubMed] [Google Scholar]
- 35.Przepiorka D, Weisdorf D, Martin P, et al. : 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 15:825–8, 1995 [PubMed] [Google Scholar]
- 36.Filipovich AH, Weisdorf D, Pavletic S, et al. : National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 11:945–56, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Fine JP, Gray RJ: A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association 94:496–509, 1999 [Google Scholar]
- 38.Juliano RL, Ling V: A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochimica et Biophysica Acta (BBA) - Biomembranes 455:152–162, 1976 [DOI] [PubMed] [Google Scholar]
- 39.Shareef MM, Brown B, Shajahan S, et al. : Lack of P-Glycoprotein Expression by Low-Dose Fractionated Radiation Results from Loss of Nuclear Factor-κB and NF-Y Activation in Oral Carcinoma Cells. Molecular Cancer Research 6:89–98, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Ruth AC, Roninson IB: Effects of the Multidrug Transporter P-Glycoprotein on Cellular Responses to Ionizing Radiation. Cancer Research 60:2576–2578, 2000 [PubMed] [Google Scholar]
- 41.Wong JYC, Filippi AR, Dabaja BS, et al. : Total Body Irradiation: Guidelines from the International Lymphoma Radiation Oncology Group (ILROG). International Journal of Radiation Oncology • Biology • Physics 101:521–529, 2018 [DOI] [PubMed] [Google Scholar]
- 42.Clift R, Buckner C, Appelbaum F, et al. : Allogeneic marrow transplantation in patients with acute myeloid leukemia in first remission: a randomized trial of two irradiation regimens [see comments]. Blood 76:1867–1871, 1990 [PubMed] [Google Scholar]
- 43.Scarpati D, Frassoni F, Vitale V, et al. : Total body irradiation in acute myeloid leukemia and chronic myelogenous leukemia: influence of dose and dose-rate on leukemia relapse. International Journal of Radiation Oncology*Biology*Physics 17:547–552, 1989 [DOI] [PubMed] [Google Scholar]
- 44.Marks DI, Forman SJ, Blume KG, et al. : A Comparison of Cyclophosphamide and Total Body Irradiation with Etoposide and Total Body Irradiation as Conditioning Regimens for Patients Undergoing Sibling Allografting for Acute Lymphoblastic Leukemia in First or Second Complete Remission. Biology of Blood and Marrow Transplantation 12:438–453, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Giebel S, Miszczyk L, Slosarek K, et al. : Extreme heterogeneity of myeloablative total body irradiation techniques in clinical practice: A survey of the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Cancer 120:2760–2765, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.