Abstract
Head and neck cancer is disfiguring and deadly, and contemporary treatment has fallen short in terms of morbidity and mortality. The rich immune infiltrate within these tumors designates them as prime candidates for immunotherapy and success with these drugs has been documented for recurrent and metastatic head and neck cancer. Still, single-agent immunotherapy has generated either only transient responses or durable response in only a minority subset of patients. Mapping the immune escape mechanisms enacted by head and neck cancer within the tumor microenvironment allows for rational design of strategies to overcome this tolerance. We outline the immune pathway derangements within the head and neck cancer microenvironment and discuss combination treatment strategies to overcome the limitations of immunologic monotherapy.
Changing Paradigms in Head and Neck Cancer
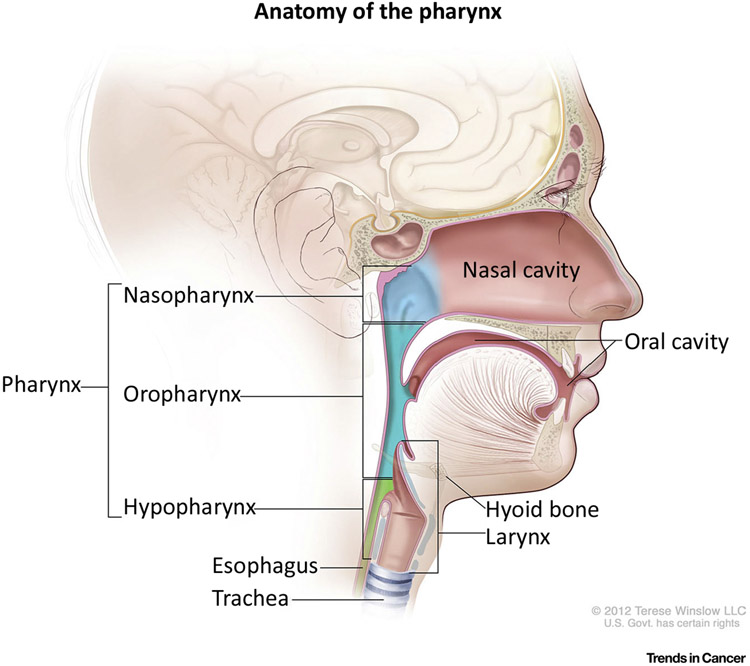
Head and neck squamous cell carcinoma (HNSCC), which includes cancers of the oral cavity, oropharynx, and larynx/hypopharynx, is the sixth most incident cancer worldwide, with an estimated 700 000 new cases in 2018, and portends a grave prognosis with 350 000 of these predicted to be fatal [1]. Treatment of head and neck cancer has evolved significantly over the past half century with improvements in surgical technique as well as advancements in the fields of medical and radiation oncology. Recently, a more detailed understanding of the molecular pathogenesis of HNSCC was made possible with whole genome sequencing of these tumors [2], invigorating the field of targeted chemotherapeutics. Despite these substantial technological advances, significant impact on the survival of patients afflicted by these cancers has not been observed. For example, the 5-year survival rate of patients with larynx cancer was 66% from 1975 to 1977 and 63% from 2007 to 2013i.
Much of the difficulty in studying and treating HNSCC lies in the fact that they are a heterogeneous group of cancers arising from distinct anatomic subsites, associated with varied risk factors and possessing diverse molecular pathology. Classically, tobacco and alcohol consumption were the primary risk factors associated with HNSCC and these factors demonstrate independent, synergistic, and dose-response increases in relative risk [3]. Chronic exposure to tobacco and alcohol is thought to promote diffuse and progressive molecular alterations in grossly normal epithelium. Additionally, as in other solid tumors, HNSCC has been associated with dysregulation of various oncogenes and tumor suppressor genes; the molecular disruption in HNSCC was eloquently reviewed recently [4].
The current paradigm for diagnosis and treatment of HNSCC is complicated by the varied roles of surgery, radiation, and chemotherapy that are dependent on anatomic subsite, stage, and tumor pathology (Box 1). Further complicating the picture was the rise in rates of oropharyngeal cancer over the past two decades, despite decreases in smoking and a decline in smoking-related HNSCC. Human papilloma virus (HPV; see Glossary) infection was eventually identified as the key risk factor for this aberration and HPV-related oropharyngeal cancer has since demarcated itself as a distinct clinical entity among HNSCC that has reached epidemic levels [5].
Box 1. Current Management of Head and Neck Cancer.
Diagnosis
Radiographic imaging (to include primary tumor, nodal drainage pathways, and distant pulmonary sites) and tissue sampling for pathologic diagnosis are the initial steps taken when encountering a new head and neck tumor. As treatment varies based on location, identifying the specific subsite of origin is of utmost importance (Figure I). Clinical assessment of the primary tumor (T stage), nodal disease (N stage), and distance metastasis (M stage) based on American Joint Committee on Cancer guidelines follows and guides treatment decision-making and prognosis [130].
Primary Treatment
For tumors of the oral cavity, extirpative surgical resection with neck dissection when indicated is the treatment of choice, with the goal being complete surgical cure with negative margins. This is an anatomically complex region with obvious speech, swallowing, and airway morbidity; extensive reconstruction is often necessary. For cancer of the oropharynx, primary radiotherapy is the therapeutic modality of choice, with treatment of the neck as indicated. Alternatively, trans-oral surgical resection of the tumor also provides similar outcomes and this option is typically offered to patients. For larger tumors (T3 or T4), concurrent primary chemoradiation is employed if surgical resection is deferred. In primary laryngeal/hypopharyngeal cancer, the modus operandum is preservation of function (voice and swallowing). Though the treatment algorithms for these anatomic regions and their subsites are complex, in general, small early lesions can be treated with primary radiotherapy or minimally invasive surgical extirpation. For larger lesions, primary chemoradiation or more aggressive surgical resection are necessary, both of which sacrifice functional outcomes.
Adjuvant Treatment
Postoperative radiation therapy is frequently employed for high-risk cohorts, including those with large tumors (T3 or T4), positive surgical margin, presence of lympho-vascular or perineural invasion, N2 or greater nodal disease, and gross extracapsular extension. Additionally, positive surgical margins or extracapsular extension are an indication for the addition of adjuvant chemotherapy in addition to radiation.
Recurrent/Metastatic Disease
Treatment options for recurrent HNSCC are often limited, as effects from previous treatments place patents at high risk for complications if salvage surgery or re-irradiation are attempted (e.g., life-threatening airway compromise or carotid-cutaneous fistula with exsanguination). Thus, systemic chemotherapeutic avenues are typically employed. In the case of distant metastasis, the disease is considered incurable and only chemotherapy is offered. The first line systemic chemotherapeutic protocol includes cisplatin or carboplatin plus 5-fluorouracil plus cetuximab [131].
Despite state-of-the-art therapeutic approaches to HNSCC, recurrence rates remain unacceptably high. Indeed, nearly half of oral cavity cancers in patients will recur [6-8] and 5-year survival in this scenario is a dismal 35%–45% [6,9]. In an attempt to quell these poor outcomes, and recognizing that HNSCC demonstrates one of the most inflamed tumor microenvironments (TME) among all solid tumors, the treatment of head and neck cancer has begun to shift towards a prominent role for immunotherapy. A detailed understanding of the host antitumor immune response and the abundant immune evasion strategies employed by these tumors is imperative as we move into the era of immunotherapy for head and neck cancer. As this understanding has evolved, foundations for rationally designed combination treatment strategies incorporating immunotherapy have emerged.
Immunity to Head and Neck Cancer
The past decade has seen great advances in the burgeoning fields of cancer immunogenomics and immunotherapy. In many cancers, preclinical and clinical data continue to mount in support of a more prominent (largely) T cell-mediated antitumor response than was previously thought. The T cell compartment delineates cancer antigen from host antigen in one of two ways: recognition of overexpressed but nonmutated native proteins that have escaped central tolerance (due to decreased tissue expression) or recognition of mutated, tumor-specific epitopes [10]. The latter is posited to play a more prominent role, as these ‘neoantigens’ should elicit a more robust T cell pool in the absence of regulation by tolerance mechanisms. The ability of the native host immune system to mount a response to tumor neoantigens is key to the development of effective immunotherapies for head and neck cancer.
Due to their inherent genetic instability and high mutagenic rate, HNSCCs routinely transcribe high levels of cancer-specific neoantigens [11]. In a mouse model of oral cavity cancer with high fidelity to human oral cavity cancer, a higher rate of unique tumor neoantigens was associated with responsiveness to immunotherapy [12]. Moreover, the tumors that were responsive to immunotherapy demonstrated more profound antigen-specific lymphocyte responses than the tumors with lower neoantigen load. Higher rates of nonsynonymous mutations and the corresponding neoantigenic load in other tumors correlate with a more pronounced immune response that portends better clinical outcomes in patients [13]. Additionally, HPV-related HNSCC express viral antigens, including the oncogenic drivers E6 and E7 and the transcriptional regulatory protein E2 [14]. High serum levels of antibodies to E2, E6, and E7 have been demonstrated in HPV+ HNSCC [15,16].
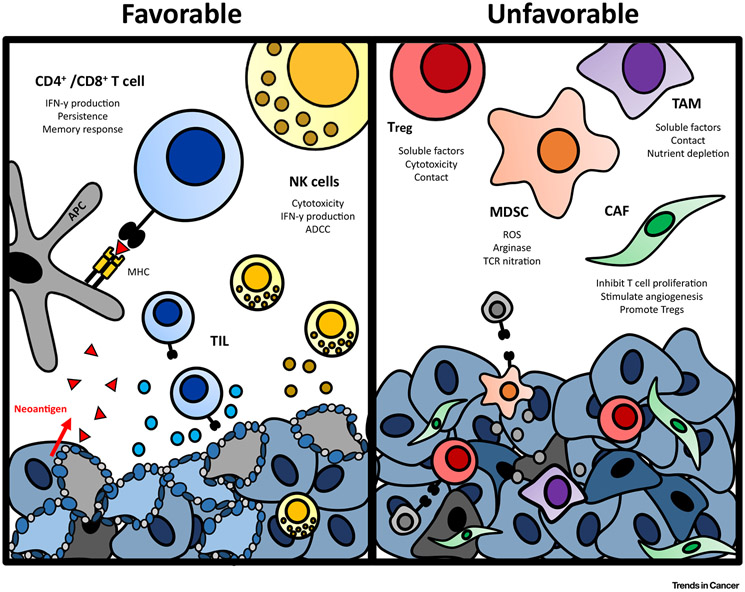
This neoantigenic catalog in both HPV− and HPV+ HNSCC elicits an antitumor immune response, the effector function of which is enacted by tumor infiltrating lymphocytes (TILs). Analysis of RNA sequencing data from a cohort of HNSCC from The Cancer Genome Atlas tumor bank revealed that both HPV– and HPV+ HNSCC demonstrate immune infiltrate that is among the most robust [CD8+ TILs as well as natural killer (NK) cells] compared with ten of the most highly immunogenic cancer types [17]. Higher levels of CD8+ TIL and CD56dim (activated) NK cell infiltration conferred a survival advantage compared with those with decreased immune infiltrate (Figure 1).
Figure 1. Immune Tumor Microenvironment of Head and Neck Cancer.
The final balance of favorable and unfavorable immune elements within the tumor microenvironment of head and neck cancer determines the inflammatory state of the tumor. ADCC, antibody-dependent cell-mediated cytotoxicity; APC, antigen presenting cell; CAF, cancer-associated fibroblast; MDSC, myeloid-derived suppressor cell; MHC, major histocompatibility complex; NK, natural killer; ROS, reactive oxygen species; TAM, tumor-associated macrophage; TCR, T cell receptor; TIL, tumor infiltrating lymphocyte; Treg, regulatory T cell.
The prognostic value of distinct populations of TILs was evaluated in a recent meta-analysis [18]. CD3+ TILs conferred a statistically significant overall survival advantage in the pooled analysis for both HPV– and HPV+ HNSCC [18]. Higher levels of CD4+ TILs demonstrated improved overall survival in HPV– tumors but the only two studies in HPV+ tumors yielded contradictory results [18]. As expected, increased infiltration with CD8+ TILs was most strongly associated with increased survival, disease free survival, and locoregional tumor control in both HPV– and HPV+ HNSCC [18]. Surprisingly, increasing infiltration with FoxP3+ T cells was also associated with improved survival [18]. While FoxP3 is classically associated with regulatory T cells (Tregs), it is not known whether these cells were truly Tregs as CD25 and CD127 levels were not characterized. It is plausible that this signal is an artifact of an overall increased immunoreactive TME, as FoxP3+ is also a marker of immune activation [19],
Immune Evasion by Head and Neck Cancer
Despite the rich immunogenicity of the TME, HNSCC have developed a diverse panel of strategies to thwart antitumor immunity. Many signaling molecules and cell types play a role in tumor-driven immune tolerance, from cytokines to both the innate and adaptive arms of the cellular immune system (Figure 2). It is the final balance of these immunopermissive and immunosuppressive mechanisms that determine the net immune response within these tumors and, consequently, their aggressiveness. Next, we will discuss four key mechanisms enacted by head and neck cancer to evade immunity, spanning direct T cell inhibition with soluble or surface inhibitory factors to the recruitment of suppressive immune cell populations, thereby permitting tumor outgrowth.
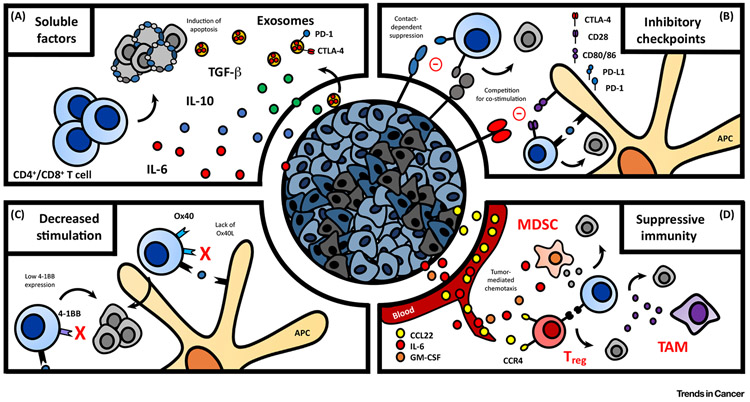
Figure 2. Immune Evasion by Head and Neck Cancer.
Head and neck cancer employs multiple mechanisms of immune escape, broadly divided into four categories. (A) Tumor-derived soluble factors emitted by cancer cells have direct immunosuppressive effects, including induction of T cell apoptosis. (B) Upregulation of inhibitory checkpoint molecules such as PD-1 and CTLA-4 impair memory T cell priming and promote exhaustion in the context of chronic stimulation. (C) Impaired co-stimulatory signaling prevents potent activation of antitumor immunity. (D) Recruitment of suppressive cell populations further establishes the immune inhibitory status of the microenvironment. APC, Antigen presenting cell; CTLA-4, cytotoxic T lymphocyte antigen 4; GM-CSF, granulocyte macrophage-colony stimulating factor; IL, interleukin; MDSC, myeloid-derived suppressor cell; PD-1, programmed death 1; TAM, tumor-associated macrophage; Treg, regulatory T cell.
Molecular Escape Mechanisms
Tumor-Derived Factors
HNSCC tumor cells directly evade immune responses through secretion of soluble factors to suppress T cell-mediated rejection as well as corruption of antigen presentation, thereby avoiding both T and NK cell detection. Production of immunosuppressive cytokines, including transforming growth factor (TGF)-β, interleukin (IL)-6, and IL-10 inhibit T cell proliferation and effector functions [20-22]. Tumor cells also deplete local micronutrients and overexpress indoleamine 2,3-dioxygenase (IDO), an enzyme responsible for depletion of tryptophan, which hinders T cell proliferation and activation [22]. It has also been shown that exosomes secreted by HNSCC are enriched for suppressive compounds [including cyclooxygenase-2 (COX2), TGF-β, programmed death 1 (PD-1), and cytotoxic T lymphocyte antigen 4 (CTLA-4)] that promote CD8+ T cell apoptosis, inhibit CD4+ T cell proliferation, upregulate Tregs, and impair NK cell function [23,24].
Beyond secreted cytokines and metabolites, HNSCC have developed mechanisms of human leukocyte antigen (HLA) modulation for immune escape. Complete loss of HLA signaling would be a potent stimulating signal for NK cell tumor killing [25]; therefore, tumor-mediated HLA dysfunction must be more nuanced to maintain immune evasion. Instead, HNSCC provoke genetic alterations in key genes associated with processing and presentation of neoantigens, including signal transducer and activator of signal (STAT) 1 deficiency and downregulated transporter for antigen processing, without significantly affecting HLA expression itself [26-30].
Immune Checkpoints
In the healthy state, effector functions of the immune system must be held in check to prevent damage to self (autoimmunity) or prolonged activation. The ‘rev limiters’ of the native immune system are the checkpoint molecules. Primarily surface molecules on immune cells, this class of receptors respond to binding of their ligands by quelling the activity of local effector immune cell populations. Characteristics of the local inflammatory environment, including cytokine profile and crosstalk between various immune cell subsets, can promote upregulation of these receptors or ligands on hematopoietic cells, lymphoidal cells, and nonimmune cells such as epithelia (including tumor cells). Coaptation of these checkpoint pathways is an important route of immune escape in HNSCC. Mapping of the complicated checkpoint highways within the TME yields many therapeutic targets and logic follows that intervening at multiple intersections on this highway simultaneously with combination therapy has the potential for improved treatment efficacy compared with monotherapy.
Multiple immune checkpoint receptors have been described and expression patterns and cell distribution are varied, giving these molecules diverse and tunable contributions to immunomodulation, as depicted in Figure 3 [31,32]. Checkpoint molecules are generally thought of as primarily immunosuppressive and the key inhibitory checkpoint receptors are PD-1 and CTLA-4. Lymphocyte activating gene 3 (LAG-3), T cell immunoglobulin and mucin domain-containing 3 (TIM-3), T cell Ig and ITIM domain (TIGIT), V-domain Ig suppressor of T cell activation (VISTA), glucocorticoid-induced TNFR-related protein (GITR), and killer immunoglobulin-like receptor (KIR) are more novel co-inhibitory checkpoints and will be discussed in turn below.
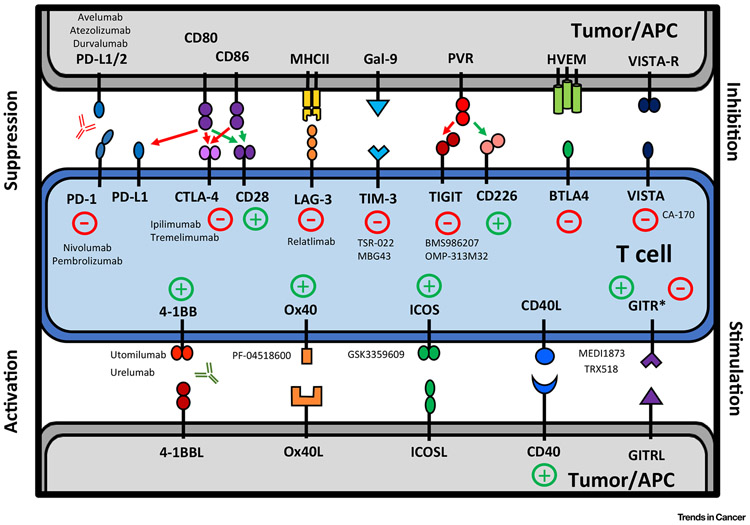
Figure 3. Targetable Immunomodulatory Pathways in Head and Neck Cancer.
Inhibitory and co-stimulatory immune axes are prominent within the head and neck cancer microenvironment and interactions between receptor and ligand represent therapeutic targets. Antagonistic drugs to inhibitory pathways and agonistic drugs to co-stimulatory pathways are the subject of numerous clinical trials and are listed near their target receptor where applicable. APC, Antigen presenting cell; GITR, glucocorticoid-induced TNFR-related protein; HVEM, herpes virus entry mediator; ICOS, inducible T cell co-stimulator; LAG-3, lymphocyte activating gene 3; MHC, major histocompatibility complex; PD-1, programmed death 1; PVR, poliovirus receptor; TIGIT, T cell Ig and ITIM domain; TIM-3, T cell immunoglobulin and mucin domain-containing 3; VISTA, V-domain Ig suppressor of T cell activation; BTLA-4, B- and T-lymphocyte attenuator 4.
PD-1 is primarily expressed on the surface of T cells [33] and interacts with two ligands: PD-L1 and PD-L2. PD-1 signaling supports immune tolerance and eventual exhaustion through reduced T cell receptor (TCR) signaling, reduced cytokine production, reduced target cell lysis, altered lymphocyte motility, and metabolic reprogramming in addition to inducing differentiation to a Treg phenotype. PD-L1 is the more constitutively expressed of the two and is present on lymphocytes, macrophages, and dendritic cells (DCs) [34] in addition to epithelia, including tumor cells. PD-1/PD-L1 interaction promotes tolerance by inhibiting effector T cell mechanisms, including cytokine production, proliferation, and cytotoxicity, and eventually leads to T cell exhaustion [35]. PD-L2 exhibits more restricted expression but elicits similar functional effects upon interaction with PD-1 [36]. In addition to decreased effector T cell functions, PD-1 signaling has also been shown to induce differentiation to a Treg phenotype [37]. Up to 60% of HNSCC demonstrate increased expression of PD-1 within the TME [38-41], highlighting this key mechanism for immune escape in HSNCC. PD-L1 is also upregulated in HNSCC, both in TILs and on the tumor surface [42-44], and expression of both PD-1 and PD-L1 is preferential for TILs relative to peripheral blood lymphocytes [45].
CTLA-4 expression on the surface of T cells is stimulated by TCR activation. CTLA-4 is the inhibitory counterpart to the stimulatory CD28 signal [46,47]. CTLA-4 has only transient expression patterns on CD8+ T cells, where it regulates memory/effector function [48]. The more predominant role of this checkpoint receptor is regulation of naïve CD4+ T cell differentiation upon antigen-specific priming by DCs. CTLA-4, when expressed, interacts with CD80/CD86 on DCs and out-competes CD28 co-stimulatory signaling and inhibits T cell activation [49]. Therefore, blockade of this receptor has obvious mechanistic rationale for treatment of HNSCC, with the goal being to restore DC priming of naïve T cells with neoantigenic specificity. CTLA-4 expression is upregulated in HNSCC tumor cells [50] and preferentially on TILs compared with peripheral lymphocytes [45,51]. CTLA-4 expression is also enriched on Treg TILs [52].
Among the more novel checkpoint molecules, LAG-3 and TIM-3 are the most well studied. LAG-3 is expressed on activated CD4+ and CD8+ T cells, NK cells, B cells, and DCs and functions as a co-inhibitory checkpoint molecule in HNSCC [53]. LAG-3 binds with major histocompatibility complex class II (MHCII) and is highly expressed on Tregs, and its blockade decreases both the inhibitory function of these cells as well as their overall quantity [53,54] . TIM-3 binds with galectin-9 and is expressed on both T cells and NK cells and, when specifically coexpressed with PD-1, signifies an exhausted T cell phenotype in HNSCC [51,55]. Blockade of TIM-3 in a mouse model of HNSCC produced a specific antitumor T cell response [56].
A number of other interesting co-inhibitory checkpoint molecules have come under investigation recently. TIGIT competes with the stimulatory molecule CD226 for binding with the poliovirus receptor (PVR) and exerts immunosuppressive effects by inhibiting T cell activation and decreasing DC cytokine production [57]. TIGIT is expressed on Tregs as well as memory and activated T cells [57]. B- and T-lymphocyte attenuator 4 (BTLA-4) binds herpes virus entry mediator (HVEM) and has T cell inhibitory functions similar to PD-1 and CTLA-4 [58]. VISTA is expressed on antigen-presenting cells and T cells and inhibits T cell proliferation and cytokine production upon interaction with its receptor (VISTA-R) [59]. GITR is expressed on T cells and modulates TCR-mediated apoptosis upon binding with its ligand (GITRL) [60].
Co-inhibitory checkpoint control is not limited to the adaptive arm of the immune system. KIR is expressed on NK cells, recognizes HLA, and is an important regulator of NK cell-mediated cytotoxicity [61]. There are activating and inhibitory KIRs, though inhibitory receptors bind with more affinity and dominate signaling when both are present. Upon binding an autologous HLA, inhibitory KIRs suppress NK cell-activating signals [62] and thus impair NK cell killing. Simultaneous checkpoint inhibition targeting both the adaptive and innate arms of the antitumor immune response has potential to be a powerful combination strategy and is discussed below.
Co-Stimulatory Pathways
In addition to upregulation of immune inhibitory pathways, perturbation of multiple co-stimulatory pathways is also seen in HNSCC and is summarized in Figure 3. Checkpoint inhibition combined with co-stimulatory pathway agonists is predicted to have synergistic effects in terms of promoting potent tumor clearance. One promising co-stimulatory molecule is Ox40, which is expressed on the surface of T cells and promotes proliferation, IFNγ production, and memory formation [63]. Expression of Ox40 has been demonstrated on the surface of HNSCC tumor cells and in draining lymph nodes [64], but expression of its ligand (Ox40L) is reduced in the tumor and thus this pathway is rendered ineffective at generating an antitumor immune response [65,66]. Therefore, potential exists to augment the Ox40 pathway in HNSCC with receptor agonists, which are currently being tested in this setting in clinical trials (discussed below).
Another co-stimulatory molecule expressed on the surface of activated T cells, NK cells, and DCs [67] is 4-1BB (CD137), which is downregulated in HNSCC [66]. In HNSCC treated with neoadjuvant cetuximab, 4-1BB expression was enhanced on TILs as well as intratumoral NK cells and DCs [68]. CD40 is also expressed on multiple cell types within the TME and has many downstream effects which sum to an increased adaptive immune response. In HNSCC, CD40 signaling promotes tumoricidal macrophages and is associated with lower stage of disease [69,70]. Finally, inducible T cell co-stimulator (ICOS) is expressed on activated T cells [71], promotes a Th2 response, and is enriched on the surface of CD4+ T cells in HSNCC [66].
Suppressive Cellular Tumor Infiltrate
The complex interplay of the various immune checkpoint and stimulatory pathways within the HNSCC TME occurs in the context of a diverse cellular milieu, a significant component of which are immunosuppressive cell populations [22]. Outside of direct effector cell suppression, HNSCC regulate and recruit other immune populations capable of modulating T and NK cell responses, including Tregs, myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs), and cancer-associated fibroblasts (CAFs), which are discussed below (Figures 1 and 2). Immunomodulation enacted by these various cell populations contributes to a tumor-promoted microenvironment. As such, inhibiting the recruitment or function of these cellular populations concurrently with other immune or nonimmune therapies may have additive effects.
Tregs
Immunosuppressive Tregs (CD4+CD25+FoxP3+) have been discussed in several contexts above, demonstrating their salient and ubiquitous role in immune evasion in HNSCC. A distinction must be made between natural Tregs, which develop in the thymus and are drawn to the TME subsequently, and inducible Tregs, which are naive tumor-resident T cells that are driven to regulatory status by the cytokine profile within the TME [72]. Recruitment of Tregs to the TME occurs via binding of CCR4 on the T cell surface by CCL22 produced by the tumor [73] . Interestingly, in melanoma, CCR4 expression is preferential for TIL Tregs as compared with peripheral circulating Tregs, supporting the importance of this receptor in Treg recruitment [74] . Data from other immunogenic solid cancers suggests that Tregs then expand within the TME in an apparently antigen-dependent [75] manner with support from IL-2 [76]. Tregs inhibit effector immune cell functions primarily through IL-10 and TGF-β [77]. Moreover, Tregs have demonstrated direct NK cell and CD8+T cell cytotoxicity through granzyme-B and perforin [78].
MDSCs
MDSCs are a CD11b+CD14+CD33+HLADR− immature myeloid cell population with potent immunosuppressive effects in HNSCC [79]. Recruitment of MDSCs to the TME occurs through chemotactic stimulus from multiple factors, including granulocyte macrophage-colony stimulating factor (GM-CSF), IL-6, IL-10, COX2, IL-1β, IDO, and vascular endothelial growth factor (VEGF) [22]. In vitro in HNSCC cell lines, constitutive expression of GM-CSF and IL-6 due to upstream genetic mutations have been shown to directly promote differentiation of peripheral blood mononuclear cells (PBMCs) into MDSCs [80]. Classic mechanisms of MDSC immunosuppression are at play within the HNSCC TME, including L-arginine, tryptophan, and cysteine depletion and production of T cell inhibitory reactive oxygen species such as peroxynitrate [22]. MDSC production of the enzymes arginase 1 (Arg-1) and nitric oxide synthase (iNOS), as well as IDO-mediated transformation of tryptophan to kynurenine metabolites, represent key MDSC immunosuppressive techniques [22]. The TME is a hostile, hypoxic environment with resultant upregulation of hypoxia-inducible factor 1alpha (HIF-1α), which in turn increases expression of PD-L1 on other TME-resident immune cells [81]. Another fascinating mechanism of MDSC-mediated immunosuppression involves presentation of neoantigen to experienced T cells, which results in nitration of the TCR and subsequent inability to be stimulated by antigen presenting cells (APCs) [82]. Finally, MDSCs also promote activation and expansion of Tregs [73].
TAMs
TAMs are a prominent member of the TME and their phenotype falls on a spectrum from antitumor (M1) to immunosuppressive and thus tumor supporting (M2). M1 TAMs are tumoricidal and M2 TAMs secrete tumor growth supporting cytokines [83,84]. In response to regulation by HNSCC, M2 TAMs dominate the TME and are associated with poor prognosis [85]. Mechanisms of TAM-mediated immunosuppression are similar to that of MDSCs [22], including depletion of local nutrients required for T cell function, secretion of immunosuppressive cytokines, expression of PD-L1 [44], and recruitment of Tregs [86].
CAFs
Beyond tumor and immune cells, the TME is composed of other supportive stromal cells, including fibroblasts. These TME stromal cells, namely CAFs, have distinct tumor-supporting properties compared with their normal tissue counterparts, by promoting angiogenesis, invasion, and metastasis through cytokine and growth factor production [87,88]. In HNSCC, CAFs were recently shown to inhibit T cell proliferation via the PD-1:PD-L1/2 axis, induce effector T cell apoptosis, and promote differentiation to a Treg phenotype [89].
Immunologics as Monotherapy: A Losing Battle?
We have described above a model in which the HNSCC TME is inherently immunogenic and provokes an antitumor infiltrate that is ‘primed’ to effectively clear the cancer, but that has been effectively subverted by the myriad tumor-mediated immune evasion mechanisms as summarized in Figures 1-3. By targeting key immunomodulatory pathways within this paradigm, the field of immunotherapy aims to unleash this primed immune response to invoke complete rejection of tumor and generate durable responses in patients. It has now been nearly 8 years since the first checkpoint inhibitor, ipilimumab (anti-CTLA-4 monoclonal antibody), demonstrated improved survival in late-stage melanoma patients [90]. Since then, checkpoint blockade has demonstrated repeated efficacy as a therapeutic option in subsets of patients with a number of solid cancers and has received FDAii approval in certain settings for metastatic melanoma, non-small cell lung cancer, renal cell carcinoma, Hodgkin lymphoma, urothelial cancer, colorectal cancer, gastroesophageal cancer, hepatocellular carcinoma, cervical cancer, and HNSCC [91]. Blockade of the PD-1/PD-L1 axis has thus far proved most promising for HNSCC, with two FDA approved drugs for recurrent/metastatic (r/m) disease and more in the pipeline.
Limited Efficacy of Single-Agent Immunotherapy
Pembrolizumab is a humanized IgG4 monoclonal antibody to the PD-1 receptor. Keynote-012 was an open-label, multicenter, Phase Ib trial of pembrolizumab for r/m HNSCC [43] that demonstrated an overall response rate of 18% (25% in HPV+ and 14% in HPV–). As a result of this study, FDA approval was expedited and pembrolizumab was approved as second line for r/m platinum-refractory HNSCC in August 2016. However, results of follow-up Phase III trials of pembrolizumab have been mixed, though a clinically meaningful benefit was observed [92,93]. Later in 2016, nivolumab was approved by the FDA for r/m HNSCC. Nivolumab is another humanized IgG4 anti-PD-1 monoclonal antibody, which had previously demonstrated efficacy in multiple tumor types. In the landmark open-label, Phase III Checkmate 141 trial, patients with platinum-refractory r/m HNSCC were randomized to either receive nivolumab or single-agent, investigator’s choice chemotherapy [94] and overall survival was significantly longer with nivolumab at 7.5 months compared with 5.1 months in the investigator’s choice arm. More recent investigation has turned to immune monotherapy as first-line treatment for head and neck cancer, and early results are promisingiii.
Unlike PD-1, blockade of CTLA-4 has not proven as efficacious for HNSCC and there are no current FDA approvals for this therapy. PD-1 and CTLA-4 blockade in HNSCC were reviewed very nicely in a recent publication in this journal and we direct the reader to this review for a more thorough discussion [95].
While blockade of the receptor side of the PD-1/PD-L1 axis has been the most studied and most successful to this point, targeting the PD-1 binding partner PD-L1 has gained traction in recent years. Three anti-PD-L1 antibodies recently gained FDA approval for treatment of urothelial cancer [96,97] (atezolizumab, avelumab, durvalumab), non-small cell lung cancer (atezolizumab, durvalumab), and Merkel cell carcinoma [98] (avelumab) and are currently under investigation in r/m HNSCC. Preliminary analysis of a single arm, Phase II trial for durvalumab as monotherapy for r/m HNSCC with high levels of PD-L1 expression demonstrated objective response rate of 16.1%, overall survival 7.1 months, and 12-month survival rate of 33.6%iv. Similarly, atezolizumab as monotherapy in PD-L1 positive r/m HNSCC demonstrated objective response rate of 24%v.
Despite promising results with immunotherapy in HNSCC, there continues to be a majority of patients who do not respond to single-agent immunologic drugs and we must not be satisfied with improvements in overall survival on the order of months. The multitude of immune molecules, receptors, pathways, and cell types at play within the TME and the multiple mechanisms of immune escape employed by HNSCC suggest that targeting only one may be a losing battle. Rather, combination strategies targeting multiple elements within the immune TME simultaneously, with or without traditional treatment modalities, promises to be a more successful strategy and has become a recent focus of HNSCC clinical trial activity.
Combination Treatment Strategies
In the past, the search for novel cancer therapies sought a ‘silver bullet’, a drug with significant efficacy as monotherapy. As the complexity of the immunologic derangements at play within the HNSCC TME is elucidated, it has become clear that a multipronged approach carries a greater potential for a robust and durable response. By targeting multiple immunologic pathways or combining an immunotherapeutic drug with a traditional treatment strategy, synergistic effects are being observed with better outcomes compared with monotherapy. Of the nearly 200 clinical trials underway for HNSCC, the vast majority are investigating combination therapies, including combinations of immunologic drugs with one another or with other treatment strategies (Figure 4 and Table 1).
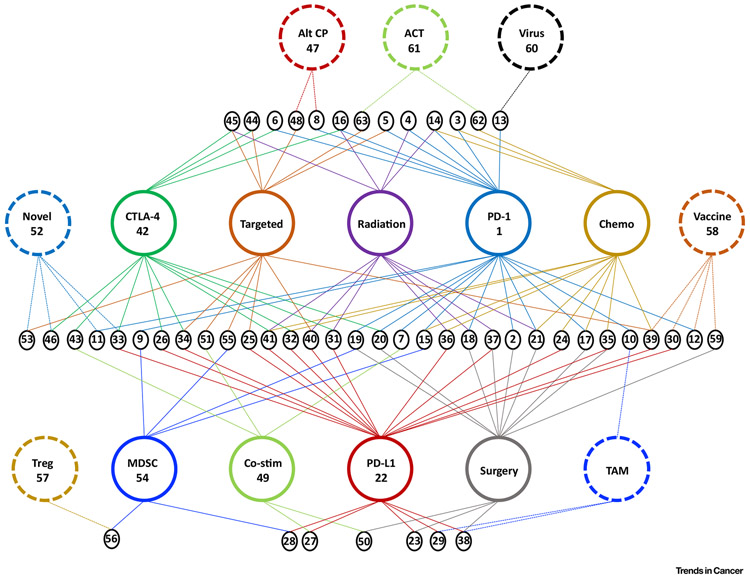
Figure 4. Combination Immunotherapy Clinical Trials in Head and Neck Cancer.
Of the nearly 200 clinical trials underway evaluating immunotherapy in head and neck cancer, the vast majority include combinations of therapy. Larger colored circles represent major treatment modalities in head and neck cancer. Smaller black circles at the nexus of multiple treatment modalities represent a unique combination treatment strategy under clinical trial evaluation. Numbers within the small black circles correspond to the numbers listed in the leftmost column of Table 1, where NCT numbers of active clinical trials (as of November 1, 2018) are listed. For example, number 45 in the upper left-most small black circle indicates the combination of CTLA-4 blockade with radiation and targeted chemotherapy and row 45 of Table 1 lists the corresponding clinical trials. ACT, Adoptive cell transfer; Alt CP, alternative checkpoint molecule; Chemo, chemotherapy; Co-stim, co-stimulatory pathway agonist; CTLA-4, cytotoxic T lymphocyte antigen 4; MDSC, myeloid-derived suppressor cell; Novel, novel immunologic treatment strategy; PD-1, programmed death 1; Targeted, target molecular therapy such as an epidermal growth factor receptor inhibitor; TAM, tumor-associated macrophage; Treg, regulatory T cell.
Table 1.
Clinical Trials of Immunologics for Head and Neck Cancer
Far left column number links to numbers in small black circles in Figure 4.
Combinations of Classic Checkpoint Inhibitors
The most well studied of these combination therapies is PD-1/PD-L1 axis blockade combined with CTLA-4 blockade, as this strategy is solidly based in the immune pathophysiology of the TME by targeting both cell priming and exhaustion post-chronic stimulation. Indeed, this therapy has demonstrated efficacy in melanoma and is currently being studied for r/m HNSCC (NCT02741570vi). Notably, immune-related side effects of this regimen are more significant than either therapy alone, which must be considered relative to its efficacy.
Combining checkpoint blockade, with its established efficacy and safety, with more novel immune-modulating drugs comprises a significant portion of the current clinical trials for HNSCC. Classic checkpoint inhibitors, typically PD-1 blockers, are being combined with nearly all other strategies, including alternative checkpoint inhibitors, co-stimulatory agonists, drugs targeting inhibitory immune cell populations (Tregs, MDSCs, TAMs), radiation, chemotherapy, and surgery.
Combinations with Alternative Checkpoint Inhibitors
Numerous monoclonal antibodies directed at immune inhibitory pathways outside of the PD-1 and CTLA-4 axes are under development. The LAG-3 inhibitor relatlimab (BMS-986016) recently entered clinical trials (NCT01968109vii) with or without nivolumab for solid tumors, including r/m HNSCC. In a mouse model of HNSCC, blockade of TIM-3 demonstrated promising preclinical efficacy [56], prompting two clinical trials evaluating novel TIM-3 inhibitors (TSR-022 and MBG43) with or without PD-1 blockade for r/m HNSCC (NCT02817633viii, NCT02608268ix). Inhibitors of the other novel inhibitory checkpoint molecules TIGIT, BTLA, VISTA, and KIR are also in development and at various points along the refinement and clinical trial pipeline in combination with classical checkpoint blockers.
Combinations with Co-stimulatory Agonists
Following treatment of HNSCC with cetuximab, 4-1BB is upregulated on NK cells, and stimulation of this pathway supported NK cell-mediated tumor cytotoxicity in preclinical models [68]. This has provoked clinical trial evaluation of urelumab, an agonistic 4-1BB monoclonal antibody, in combination with cetuximab (NCT02110082x) or tremelimumab (NCT02179918xi) for r/m HNSCC. Ox40 agonists are also under clinical trial evaluation as monotherapy versus combination with a 4-1BB agonist (NCT02315066xii) or as neoadjuvant presurgical therapy (NCT02274155xiii) for solid tumors, including r/m HNSCC.
CD40 agonistic antibodies and ligands have also been developed and showed some promise in melanoma [99], though they have yet to be evaluated in HNSCC clinical trials. Moreover, concerns of toxicity, including thromboembolism, cytokine storm, and tumor growth, have limited the excitement over this strategy [100]. ICOS agonists are also amidst clinical trial evaluation in combination with anti-PD-1, anti-CTLA-4, and classic chemotherapeutics (NCT03693612xiv, NCT02904226xv). GITR is expressed on the surface of APCs and CD4+ T cells and GITR signaling directly inhibits naïve Treg-mediated immunosuppression and demonstrated promising results in preclinical melanoma studies [101,102]. Two anti-GITR agonistic antibodies (TRX518 and MEDI1873) have reached clinical trials for solid tumors, including r/m HNSCC (NCT02628574xvi, NCT01239134xvii, NCT02583165xviii).
Targeting Tregs
As described above, Tregs exert a marked immunosuppressive effect within the TME of HNSCC and have emerged as an enticing target of immunotherapy. CD25, the alpha chain of the IL-2 receptor, is constitutively expressed at high levels on the surface of Tregs and is necessary for their inhibitory function. However, mixed results were observed in clinical studies of CD25 monoclonal antibodies in other cancers, potentially due to concomitant depletion of effector T cells in addition to Tregs [103,104].
CCR4 is preferentially expressed on Tregs, making it a promising immunotherapy target as well, and support for this hypothesis was demonstrated in T cell leukemia/lymphoma [74] and lung/esophageal cancer patients [105] with the anti-CCR4 monoclonal antibody mogamulizumab. Clinical trials are underway evaluating mogamulizumab in combination with other immunotherapy approaches in solid tumors, including r/m HNSCC (NCT02281409xix, NCT02867007xx). CTLA-4, Ox40, and GITR play prominent roles in Treg signaling, and their blockade (CTLA-4) or agonism (Ox40, GITR) is also the subject of multiple clinical trials (see above).
Targeting MDSCs
Given their important immunosuppressive effects, immunotherapeutic strategies targeting MDSCs are the subject of investigation and approaches include stimulating differentiation, inhibiting chemotaxis, and metabolic inhibition with small molecules. Forcing MDSC differentiation into the mature, APC phenotype with 1,25-dihydroxyvitamin D3 (vitamin D) in peripheral blood mitigates their suppressive capacity [106]. In combination with celecoxib, vitamin D was shown to induce a favorable cytokine profile when given in the neoadjuvant presurgical setting for HNSCC (NCT00953849xxi). A majority of MDSCs express the chemotactic receptor CXCR2 and most HNSCC tumors express its ligand CXCL1; consequently, drugging this interaction reduces MDSC migration into the TME and suppresses tumor growth [107,108]. Small molecule inhibitors are being developed and a clinical trial of an anti-CXCR2 monoclonal antibody in r/m HNSCC is underway (NCT02499328xxii).
IDO inhibitors, aimed at restoring tryptophan levels and removing suppressive kynurenine metabolites in the TME, are also under investigation (NCT02048709xxiii, NCT02327078xxiv, NCT02178722xxv, NCT02559492xxvi), as well as inhibition of STAT3, the upstream regulator of IDO (NCT02499328xxii). Disappointingly, IDO inhibitors have failed to meet early endpoint criteria and their role in the treatment of head and neck cancer may be in jeopardy. Finally, phosphodiesterase 5 inhibitors (e.g., tadalafil) impair MDSC functionality by interfering with production of Arg-1 and iNOS. Two placebo-controlled Phase II trials of neoadjuvant tadalafil in HNSCC demonstrated enhanced markers of antitumor immunity in the treatment groups [109,110], prompting further clinical trials of tadalafil in combination with other treatment approaches for HNSCC (NCT02544880xxvii, NCT01697800xxviii).
Targeting TAMs
As in MDSCs, targeting chemotaxis of TAMs to the TME is proving a promising strategy. TAMs rely on colony-stimulatory factor 1 receptor (CSF1R) binding with CSF1 for recruitment to the tumor. Antibody or small molecule blockade of this interaction depletes TAM from the TME [111,112] and promotes reprogramming to the antitumor M1 phenotype. Two trials combining CSF1R inhibition with PD-1 blockade are underway (NCT02452424xxix, NCT02526017xxx). Stimulator of interferon genes (STING) type I interferon signaling also provokes M2 TAM conversion to an M1 phenotype [113] and may represent an adjunct to other treatment modalities in the future.
Combinations with Radiation
Radiation therapy has been a mainstay as primary or adjuvant treatment of HNSCC for decades and is common as a partner with immunotherapy in clinical trials. Several local tissue effects of radiation make it an enticing combination with immunotherapy, including increasing production of tumor neoantigens due to mutagenic stimulation, increased rates of antigen presentation within the TME, increased CD8+ T cell killing, and improved cytokine profile due to induction of the inflammatory cascade [114]. The result is improved local control due to enhanced immune-mediated clearance and abscopal effect to improve immune-mediated control of distant disease. PD-L1 is also upregulated upon treatment with radiation and it remains to be seen if this will dampen the effector immune response or enhance the efficacy of checkpoint blockade. For this reason, combination of anti-PD-L1 therapies with radiation is hypothesized to have great potential in the treatment of HNSCC. The multitude of ongoing trials combining radiation with various combinations of immunotherapy and chemotherapy have been nicely summarized recently [38,115].
Combinations with Chemotherapy
Platinum-based chemotherapy plus 5-fluorouracil and the targeted agent cetuximab is the standard of care for r/m head and neck cancer (Box 1). As such, combining immunologic therapies with these strategies is enticing and often necessary for safe and meaningful clinical trial design. The most notable of these currently underway is Keynote-048 (NCT02358031iii), which recently reported promising results with pembrolizumab included with chemotherapy as first line therapy for r/m HNSCC. Figure 4 and Table 1 outline the numerous other combination approaches of this type currently under investigation.
Combination with Surgery
Surgical resection remains the standard of care for many primary HNSCCs, especially of the oral cavity, and little is known about the most efficacious and safe method to incorporate immunotherapy with surgical resection. One approach is to add immunotherapy in the adjuvant setting following definitive surgical resection, analogous to how traditional cytotoxic chemotherapeutics are used. Clinical trials are underway comparing immune drugs with standard adjuvant chemotherapy regimens (Figure 4 and Table 1). Importantly, immunotherapy is often much more well tolerated than traditional cytotoxic chemotherapy and can be used after surgery for patients that cannot tolerate the toxic side effects.
Perhaps more promising is the incorporation of immune agents in the neoadjuvant setting before surgical resection, a strategy which has several potential benefits. First, tumor shrinkage prior to surgery will allow for a less extensive and less morbid procedure, thereby improving functional, cosmetic, and psychological patient outcomes. Second, provocation of memory antitumor immunity in the host by immunotherapy may improve clearance of remnant cancer cells following resection and thus decrease rates of recurrence and metastasis. Finally, exposing treatment-naive tumors to single-agent or combination immunotherapy drugs creates a unique opportunity to study biomarkers of response to these therapies [116]. Untreated biopsy specimens and pathologic surgical resection specimens post-immunotherapy would be available to interrogate the microenvironmental changes incurred and these studies would provide unparalleled insight into the molecular and cellular effects of this therapy. It will then be possible to identify markers or cell populations predictive of response. Trials are underway evaluating neoadjuvant presurgical immunotherapy (NCT03021993xxxi) and final results are pending, though preliminary findings are promisingxxxii,xxxiii.
The immunologic landscape of head and neck cancer is multifaceted and complex. It follows that drugging an individual molecular or cellular immune target is destined to fail, as the cancer is poised to subvert single-agent approaches by employing one of a plethora of alternative immunosuppressive strategies. Therefore, the tumor must be outmaneuvered by predicting this failure and rationally designing synergistic therapeutic combinations as outlined above.
Novel Immunologic Therapies for Head and Neck Cancer
We have discussed the great strides that have been made in head and neck cancer treatment by drugging the various immunologic pathways at play within the TME and have proposed the superiority of combination therapy over monotherapy. As these techniques mature, investigators have turned towards development and implementation of even more innovative approaches to treating these cancers. Oncolytic viral therapy, vaccine therapies, and adoptive cell transfer (ACT) are promising fields that have the potential to play a role in the future of HNSCC treatment. Still, each of these novel treatments is unlikely to be efficacious in isolation, but rather would benefit from strategic combination with other therapies.
Oncolytic Viral Therapy
With their innate ability to invade, replicate, and induce target cell death, viruses possess the machinery necessary to kill tumor cells. Oncolytic therapies have the potential to debulk large tumors through direct infection and lysis of tumor cells, while additionally stimulating an adaptive immune response to eliminate any remnant cancer cells. The onus of current research efforts is to engineer either naturally occurring or genetically altered viruses that selectively target cancer cells without harming the human host cells. Talimogene laherparepvec (TVEC) is derived from herpes simplex virus type 1 and has been engineered to selectively replicate in tumor cells and to produce GM-CSF to boost the antitumor immune response. In HNSCC, TVEC is the oncolytic viral therapy furthest along the clinical development spectrum and demonstrated promising results in the early stage trials (including 100% pathologic response in post-treatment neck dissection specimens) [117].
While oncolytic viral strategies often stimulate robust responses in the short term, their long-term efficacy is limited by upregulation of the PD-1/PD-L1 pathway within the tumor. Preclinical melanoma models in which the oncolytic virus produces a soluble form of PD-1 within the TME have enhanced efficacy over both mono-viral therapy as well as the combination of viral therapy with antibody-based PD-1 blockade [118]. For HNSCC, TVEC is now under investigation in combination with pembrolizumab in the Phase Ib/III MASTERKEY232/KEYNOTE-137 clinical trial (NCT02626000xxxiv). Other oncolytic viruses are currently being developed for HNSCC, including those based on re-engineered reovirus and adenovirus [119,120]. In the future, local viral production of soluble receptors to inhibitory ligands, including PD-1, TIM-3, or LAG-3 (among others) may augment efficacy to generate long-term responses for HNSCC patients.
Vaccine Therapy
The benefits of prophylactic vaccination against HPV has been well documented [121,122] and multiple vaccines have received FDA approval, including Gardasil® and Cervarix®. Current recommendations from the Center for Disease Control and Prevention are for routine vaccination of children at age 11 or 12 years (as early as 9) and for vaccination of females age 13–26 years and males 13–21 years who were not previously vaccinatedxxxv. Additionally, a vaccination strategy targeting the HPV E6 and E7 viral proteins recently demonstrated safety and immunologic response [123].
For both HPV– and HPV+ head and neck cancers, therapeutic vaccines represent another promising avenue of research. This strategy is based on priming a specific antitumor immune response by administering a known tumor neoantigen with immunostimulatory support [124]. Multiple delivery methods have been devised, including transfection of the neoantigen within an expression plasmid (DNA vaccine), administration of the neoantigen itself (peptide vaccine), and cultured human or microbial cells engineered to express the neoantigen [125]. Multiple vaccine therapies are under clinical trial assessment for head and neck cancer, typically in combination with other immunotherapies [126].
Adoptive Cell Therapy
ACT is a treatment strategy in which tumor-specific T cells are expanded ex vivo and then returned to the patient to kill tumor cells and theoretically generate long-lasting memory against recurrence. Given the high recurrence rates in HNSCC, ACT is a highly promising modality for improving patient responses beyond the often transient effects of immunologic monotherapy. To generate tumor-specific T cell products, T cells with endogenous tumor-specificity can be expanded from the tumor site (TILs), or peripheral blood T cells can be engineered with tumor-specificity ex vivo via a TCR or chimeric antigen receptor (CAR) [127]. This therapeutic modality has demonstrated marked success in several hematologic malignancies, yielding FDA approval for CD19-based CAR T cells in acute lymphocytic leukemia and diffuse large B cell lymphoma [128]. However, several significant road blocks remain as this technology is transitioned into solid tumors such as HNSCC, including overcoming the suppressive nature of the TME and augmenting the ability of the T cells to migrate to and invade the tumor [128]. Clinical trials are underway currently of autologous TIL therapy (NCT03083873xxxvi), TCR-engineered T-cells (NCT03247309xxxvii), and CAR T cell therapy (NCT01818323xxxviii) for HNSCC.
Early results with these revolutionary avenues of head and neck cancer treatment are exciting. However, these therapies are in their infancy and our enthusiasm must be tempered until safe, durable, and reproducible efficacy is documented.
Concluding Remarks
We are amidst a paradigm shift in the treatment of head and neck cancer. Despite state-of-the-art comprehensive treatment approaches, including surgery, radiation, and chemotherapy, rates of recurrence and overall survival have not improved over the past several decades. Therefore, it is clear that novel treatment strategies for this cruel disease are desperately needed and immunotherapy has emerged as a promising alternative. Despite the ongoing enthusiasm, a significant proportion of patients are not responsive to single-agent immunologics and we must be proactive to continue to identify mechanisms of treatment failure and devise strategies to overcome them.
Subverting immunologic tolerance in head and neck cancer by combination therapy has solid theoretical rationale, as outlined above, and has proven effective in early studies. However, a deeper understanding of immune evasion mechanisms is warranted to identify the most effective combinations. One can imagine that the future of immunotherapy for head and neck cancer will be personalized [129], wherein particular immunologic derangements are identified in biopsy or peripheral blood specimens for a given patient and combinations of therapy are chosen based on their tumor’s unique repertoire of immune escape tactics. It remains to be seen which approach will prove most fruitful (see Outstanding Questions), but what is certain is that the immune system will play a central role in the future of head and neck cancer treatment.
Outstanding Questions.
Which subsets of patients are most suited for immunotherapy as part of combination treatment approaches (i. e., primary vs recurrent/metastatic, oral cavity vs oropharynx vs larynx/ hypopharynx, HPV positive vs HPV negative)?
Can biomarkers of response (tumor mutational burden, PD-L1 expression) be validated to better stratify patients into categories of potential response?
Which combinations of immunotherapy will prove the most efficacious?
What is the safety and adverse event profile of the various combinations of therapy?
What is the best strategy to combine immunotherapy with traditional treatments? Should immunotherapy be given in the neoadjuvant or adjuvant setting relative to surgery and/or radiation? Should immunotherapy be given concurrently with chemotherapy or targeted agents such as cetuximab?
How will more novel immune-based treatments such as oncolytic viruses, vaccines, and adoptive cell transfer be incorporated into the evolving treatment paradigm of head and neck cancer?
Figure I. Head and Neck Cancer Subsitesxxxix (for the National Cancer Institute © 2019, Terese Winslow LLC, US Govt. has certain rights).
Highlights.
A central role for immunotherapy in the management of head and neck cancer is emerging as a result of the elevated inflammatory state of its microenvironment.
Head and neck cancer hijacks numerous molecular and cellular immunomodulatory pathways to evade recognition and eradication by host immunity.
To date, only transient responses to single-agent immune checkpoint blockade have been observed in head and neck cancer or durable response only in a minority of patient subsets.
Combination treatment strategies targeting multiple immunologic pathways in conjunction with traditional approaches such as radiation or surgery may induce more robust and durable responses to immunotherapy in head and neck cancer.
Glossary
- Adoptive cell transfer (ACT):
a treatment strategy in which tumor-specific T cells are expanded ex vivo and then returned to the patient to kill tumor cells and generate long-lasting memory.
- Antigen presenting cell (APC):
a cell that processes a protein antigen and presents it to T cells in the context of MHC molecules.
- Cancer-associated fibroblast (CAF):
nonmalignant stromal cells within the tumor microenvironment with a largely tumor-supporting phenotype.
- Chimeric antigen receptor (CAR):
recombinant T cell receptor designed to recognize tumor antigen and rapidly generate tumor-targeted T cells.
- CytotoxicT lymphocyte antigen 4 (CTLA-4):
classic immune checkpoint molecule which competes with CD28 for CD80/86 binding and causes immunosuppression; target of the monoclonal antibodies ipilimumab and tremelimumab.
- Dendritic cells (DCs):
a subset of APCs that are uniquely suited for antigen presentation.
- Human leukocyte antigen (HLA):
the gene complex encoding the MHC. HLAs DP, DM, DO, DQ, and DR encode MHC class II.
- Human papilloma virus (HPV):
sexually transmitted virus with many subtypes, type 16 and 18 were recently associated with increasing rates of oropharyngeal cancer
- Major histocompatibility complex class II (MHCII):
molecules expressed on the surface of APCs that function to present extracellular antigen to target cells. They play a prominent role in presentation of tumor neoantigen to immune cells.
- Myeloid-derived suppressor cells (MDSC):
an immature subset of heterogeneous myeloid cells with potent immunosuppressive properties.
- Natural killer (NK) cells:
cytotoxic immune cell of the innate immune system, the effector arm of antibody-dependent cell-mediated cytotoxicity
- Neoantigen:
protein product expressed by tumor cells that is distinct from any host protein and serves as a unique target for the adaptive immune response to cancer.
- Programmed death 1 (PD-1):
classic immune checkpoint molecule which binds with PD-L1/L2, causing immunosuppression via reduced TCR signaling, reduced cytokine production, reduced target cell lysis, altered lymphocyte motility, and metabolic reprogramming, in addition to inducing differentiation to Tregs; target of the monoclonal antibodies nivolumab and pembrolizumab.
- Recurrent/metastatic (r/m):
reappearance of a previously treated cancer at the primary site, locoregional draining lymph nodes, or distant end-organs that requires new treatment.
- RegulatoryT cells (Tregs):
subset of T cells with potent immunosuppressive properties, characterized by FoxP3 and CD25 expression.
- Tumor-associated macrophages (TAM):
class of immune cells present in the microenvironment with variable antitumor (M1) or tumor-promoting (M2) phenotype.
- T cell receptor (TCR):
surface molecule on T cells responsible for recognizing antigen in the context of MHC.
- Tumor infiltrating lymphocytes (TIL):
lymphocytes that have migrated into the TME.
- Tumor microenvironment (TME):
the physical milieu in which a tumor exists, including tumor cells, blood vessels, immune cells, stromal cells, extracellular matrix, and signaling molecules.
Footnotes
Resources
https://oncologypro.esmo.org/Meeting-Resources/ESMO-2017-Congress/ Long-Term-Safety-and-Clinical-Outcomes-of-Atezolizumab-in-Head-and-Neck-Cancer-Phase-Ia-Trial-Results
xxxix: www.teresewinslow.com
Supplemental Information
Supplemental information associated with this article can be found, in the online version, at https://doi.org/10.1016/j.trecan.2019.02.007.
References
- 1.Bray F et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin 68, 394–424 [DOI] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Network (2015) Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature, 517, 576–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen N et al. (2018) Epidemiology and demographics of the head and neck cancer population. Oral Maxillofac. Surg. Clin. North Am 30, 381–395 [DOI] [PubMed] [Google Scholar]
- 4.Leemans CR et al. (2018) The molecular landscape of head and neck cancer. Nat. Rev. Cancer, 18, 269–282 [DOI] [PubMed] [Google Scholar]
- 5.Young D et al. (2015) Increase in head and neck cancer in younger patients due to human papillomavirus (HPV). Oral Oncol 51, 727–730 [DOI] [PubMed] [Google Scholar]
- 6.Kademani D et al. (2005) Prognostic factors in intraoral squamous cell carcinoma: the influence of histologic grade. J. Oral Maxillofac. Surg 63, 1599–1605 [DOI] [PubMed] [Google Scholar]
- 7.Haddad RI and Shin DM (2008) Recent advances in head and neck cancer. N. Engl. J. Med 359, 1143–1154 [DOI] [PubMed] [Google Scholar]
- 8.Koo BS et al. (2006) Recurrence and salvage treatment of squamous cell carcinoma of the oral cavity. Oral Oncol. 42,789–794 [DOI] [PubMed] [Google Scholar]
- 9.Bell RB et al. (2007) Tongue cancer: is there a difference in survival compared with other subsites in the oral cavity? J. Oral Maxillofac. Surg 65, 229–236 [DOI] [PubMed] [Google Scholar]
- 10.Schumacher TN and Schreiber RD (2015) Neoantigens in cancer immunotherapy. Science, 348, 69–74 [DOI] [PubMed] [Google Scholar]
- 11.Zolkind P et al. (2018) Cancer immunogenomic approach to neoantigen discovery in a checkpoint blockade responsive murine model of oral cavity squamous cell carcinoma. Oncotarget, 9, 4109–4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Onken MD et al. (2014) A surprising cross-species conservation in the genomic landscape of mouse and human oral cancer identifies a transcriptional signature predicting metastatic disease. Clin. Cancer Res 20, 2873–2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown SD et al. (2014) Neo-antigens predicted by tumor genome meta-analysis correlate with increased patient survival. Genome Res. 24, 743–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parfenov M et al. (2014) Characterization of HPV and host genome interactions in primary head and neck cancers. Proc. Natl. Acad. Sci. U. S. A 111, 15544–15549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson KS et al. (2015) HPV16 antibodies as risk factors for oropharyngeal cancer and their association with tumor HPV and smoking status. Oral Oncol. 51, 662–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Souza G et al. (2007) Case-control study of human papillomavirus and oropharyngeal cancer. N. Engl. J. Med 356,1944–1956 [DOI] [PubMed] [Google Scholar]
- 17.Mandal R et al. (2016) The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight, 1, e89829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Ruiter EJ et al. (2017) The prognostic role of tumor infiltrating T-lymphocytes in squamous cell carcinoma of the head and neck: a systematic review and meta-analysis. Oncoimmunology, 6, e1356148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ziegler SF (2007) FOXP3: not just for regulatory T cells anymore. Eur. J. Immunol. 37, 21–23 [DOI] [PubMed] [Google Scholar]
- 20.Lu SL et al. (2004) Overexpression of transforming growth factor beta1 in head and neck epithelia results in inflammation, angiogenesis, and epithelial hyperproliferation. Cancer Res. 64, 4405–4410 [DOI] [PubMed] [Google Scholar]
- 21.Pries R et al. (2006) Role of cytokines in head and neck squamous cell carcinoma. Expert Rev. Anticancer Ther 6, 1195–1203 [DOI] [PubMed] [Google Scholar]
- 22.Davis RJ et al. (2016) Overcoming barriers to effective immunotherapy: MDSCs, TAMs, and Tregs as mediators of the immunosuppressive microenvironment in head and neck cancer. Oral Oncol. 58, 59–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ludwig S et al. (2017) Suppression of lymphocyte functions by plasma exosomes correlates with disease activity in patients with head and neck cancer. Clin. Cancer Res 23, 4843–4854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Theodoraki MN et al. (2018) Exosomes in HNSCC plasma as surrogate markers of tumour progression and immune competence. Clin. Exp. Immunol 194, 67–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ljunggren HG and Karre K (1990) In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol. Today, 11, 237–244 [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Albaitero A et al. (2006) Role of antigen-processing machinery in the in vitro resistance of squamous cell carcinoma of the head and neck cells to recognition by CTL. J. Immunol 176, 3402–3409 [DOI] [PubMed] [Google Scholar]
- 27.Ferris RL et al. (2006) Immune escape associated with functional defects in antigen-processing machinery in head and neck cancer. Clin. Cancer Res 12, 3890–3895 [DOI] [PubMed] [Google Scholar]
- 28.Leibowitz MS et al. (2011) Deficiency of activated STAT1 in head and neck cancer cells mediates TAP1-dependent escape from cytotoxic T lymphocytes. Cancer Immunol. Immunother 60, 525–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferris RL et al. (2005) Human leukocyte antigen (HLA) class I defects in head and neck cancer: molecular mechanisms and clinical significance. Immunol. Res 33, 113–133 [DOI] [PubMed] [Google Scholar]
- 30.Matsui M et al. (2001) High expression of HLA-A2 on an oral squamous cell carcinoma with down-regulated transporter for antigen presentation. Biochem. Biophys. Res. Commun 280, 1008–1014 [DOI] [PubMed] [Google Scholar]
- 31.Deng WW et al. (2018) Co-inhibitory immune checkpoints in head and neck squamous cell carcinoma. Oral Dis. 24,120–123 [DOI] [PubMed] [Google Scholar]
- 32.Moy JD et al. (2017) Biological mechanisms of immune escape and implications for immunotherapy in head and neck squamous cell carcinoma. Eur. J. Cancer, 76, 152–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishida Y et al. (1992) Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 11, 3887–3895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong H et al. (1999) B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med 5, 1365–1369 [DOI] [PubMed] [Google Scholar]
- 35.Sharpe AH et al. (2007) The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat. Immunol 8, 239–245 [DOI] [PubMed] [Google Scholar]
- 36.Fife BT and Bluestone JA (2008) Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol. Rev 224, 166–182 [DOI] [PubMed] [Google Scholar]
- 37.Chen X et al. (2014) PD-1 regulates extrathymic regulatory T-cell differentiation. Eur. J. Immunol 44, 2603–2616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferris RL (2015) Immunology and immunotherapy of head and neck cancer. J. Clin. Oncol. 33, 3293–3304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allen CT et al. (2015) Anti-tumor immunity in head and neck cancer: understanding the evidence, how tumors escape and immunotherapeutic approaches. Cancers (Basel), 7, 2397–2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zandberg DP and Strome SE (2014) The role of the PD-L1: PD-1 pathway in squamous cell carcinoma of the head and neck. Oral Oncol. 50, 627–632 [DOI] [PubMed] [Google Scholar]
- 41.Badoual C et al. (2013) PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 73, 128–138 [DOI] [PubMed] [Google Scholar]
- 42.Concha-Benavente F et al. (2016) Identification of the cell-intrinsic and -extrinsic pathways downstream of EGFR and IFNgamma that induce PD-L1 expression in head and neck cancer. Cancer Res. 76, 1031–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seiwert TY et al. (2016) Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 17, 956–965 [DOI] [PubMed] [Google Scholar]
- 44.Lyford-Pike S et al. (2013) Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 73, 1733–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Montler R et al. (2016) OX40, PD-1 and CTLA-4 are selectively expressed on tumor-infiltrating T cells in head and neck cancer. Clin. Transl. Immunol 5, e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rudd CE et al. (2009) CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol. Rev 229, 12–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwartz RH (1992) Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell, 71, 1065–1068 [DOI] [PubMed] [Google Scholar]
- 48.Egen JG and Allison JP (2002) Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity, 16, 23–35 [DOI] [PubMed] [Google Scholar]
- 49.Collins AV et al. (2002) The interaction properties of costimulatory molecules revisited. Immunity, 17, 201–210 [DOI] [PubMed] [Google Scholar]
- 50.Yu GT et al. (2016) CTLA4 blockade reduces immature myeloid cells in head and neck squamous cell carcinoma. Oncoimmunology, 5, e1151594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jie HB et al. (2013) Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. Br. J. Cancer, 109, 2629–2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strauss L et al. (2007) The frequency and suppressor function of CD4 + CD25highFoxp3+ T cells in the circulation of patients with squamous cell carcinoma of the head and neck. Clin. Cancer Res 13, 6301–6311 [DOI] [PubMed] [Google Scholar]
- 53.Deng WW et al. (2016) LAG-3 confers poor prognosis and its blockade reshapes antitumor response in head and neck squamous cell carcinoma. Oncoimmunology, 5, e1239005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jie HB et al. (2015) CTLA-4(+) regulatory T cells increased in cetuximab-treated head and neck cancer patients suppress NK cell cytotoxicity and correlate with poor prognosis. Cancer Res. 75, 2200–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou Q et al. (2011) Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood, 117, 4501–4510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu JF et al. (2017) T-cell immunoglobulin mucin 3 blockade drives an antitumor immune response in head and neck cancer. Mol. Oncol 11, 235–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu X et al. (2009) The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol 10, 48–57 [DOI] [PubMed] [Google Scholar]
- 58.Watanabe N et al. (2003) BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat. Immunol 4, 670–679 [DOI] [PubMed] [Google Scholar]
- 59.Wang L et al. (2011) VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J. Exp. Med 208, 577–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nocentini G et al. (1997) A new member of the tumor necrosis factor/nerve growth factor receptor family inhibits T cell receptor-induced apoptosis. Proc. Natl. Acad. Sci. U. S. A 94, 6216–6221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thielens A et al. (2012) NK cell MHC class I specific receptors (KIR): from biology to clinical intervention. Curr. Opin. Immunol. 24, 239–245 [DOI] [PubMed] [Google Scholar]
- 62.Yusa S and Campbell KS (2003) Src homology region 2-containing protein tyrosine phosphatase-2 (SHP-2) can play a direct role in the inhibitory function of killer cell Ig-like receptors in human NK cells. J. Immunol 170, 4539–4547 [DOI] [PubMed] [Google Scholar]
- 63.Bauman JE and Ferris RL (2014) Integrating novel therapeutic monoclonal antibodies into the management of head and neck cancer. Cancer, 120, 624–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vetto JT et al. (1997) Presence of the T-cell activation marker OX-40 on tumor infiltrating lymphocytes and draining lymph node cells from patients with melanoma and head and neck cancers. Am. J. Surg 174, 258–265 [DOI] [PubMed] [Google Scholar]
- 65.Bell RB et al. (2016) OX40 signaling in head and neck squamous cell carcinoma: overcoming immunosuppression in the tumor microenvironment. Oral Oncol. 52, 1–10 [DOI] [PubMed] [Google Scholar]
- 66.Baruah P et al. (2012) Decreased levels of alternative co-stimulatory receptors OX40 and 4-1BB characterise T cells from head and neck cancer patients. Immunobiology, 217, 669–675 [DOI] [PubMed] [Google Scholar]
- 67.Pollok KE et al. (1993) Inducible T cell antigen 4-1BB. Analysis of expression and function. J. Immunol 150, 771–181 [PubMed] [Google Scholar]
- 68.Srivastava RM et al. (2017) CD137 stimulation enhances cetuximab-induced natural killer: dendritic cell priming of antitumor T-cell immunity in patients with head and neck cancer. Clin. Cancer Res 23, 707–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cao W et al. (2005) CD40 function in squamous cell cancer of the head and neck. Oral Oncol. 41, 462–469 [DOI] [PubMed] [Google Scholar]
- 70.Posner MR et al. (1999) Surface membrane-expressed CD40 is present on tumor cells from squamous cell cancer of the head and neck in vitro and in vivo and regulates cell growth in tumor cell lines. Clin. Cancer Res 5, 2261–2270 [PubMed] [Google Scholar]
- 71.Hutloff A et al. (1999) ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature, 397, 263–266 [DOI] [PubMed] [Google Scholar]
- 72.Zhou G and Levitsky HI (2007) Natural regulatory T cells and de novo-induced regulatory T cells contribute independently to tumor-specific tolerance. J. Immunol 178, 2155–2162 [DOI] [PubMed] [Google Scholar]
- 73.Nishikawa H and Sakaguchi S (2014) Regulatory T cells in cancer immunotherapy. Curr. Opin. Immunol 27, 1–7 [DOI] [PubMed] [Google Scholar]
- 74.Sugiyama D et al. (2013) Anti-CCR4 mAb selectively depletes effector-type FoxP3 + CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc. Natl. Acad. Sci. U. S. A 110, 17945–17950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bonertz A et al. (2009) Antigen-specific Tregs control T cell responses against a limited repertoire of tumor antigens in patients with colorectal carcinoma. J. Clin. Invest 119, 3311–3321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ahmadzadeh M and Rosenberg SA (2006) IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood, 107, 2409–2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bergmann C et al. (2008)T regulatory type 1 cells in squamous cell carcinoma of the head and neck: mechanisms of suppression and expansion in advanced disease. Clin. Cancer Res 14, 3706–3715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cao X et al. (2007) Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity, 27, 635–646 [DOI] [PubMed] [Google Scholar]
- 79.Pak AS et al. (1995) Mechanisms of immune suppression in patients with head and neck cancer: presence of CD34(+) cells which suppress immune functions within cancers that secrete granulocyte-macrophage colony-stimulating factor. Clin. Cancer Res 1, 95–103 [PubMed] [Google Scholar]
- 80.Lechner MG et al. (2010)Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J. Immunol 185, 2273–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Noman MZ et al. (2014) PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J. Exp. Med 211, 781–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nagaraj S et al. (2010) Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. J. Immunol 184, 3106–3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.O’Sullivan T et al. (2012) Cancer immunoediting by the innate immune system in the absence of adaptive immunity. J. Exp. Med 209, 1869–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Movahedi K et al. (2010) Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 70, 5728–5739 [DOI] [PubMed] [Google Scholar]
- 85.Costa NL et al. (2013) Tumor-associated macrophages and the profile of inflammatory cytokines in oral squamous cell carcinoma. Oral Oncol. 49, 216–223 [DOI] [PubMed] [Google Scholar]
- 86.Curiel TJ et al. (2004)Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat. Med 10, 942–949 [DOI] [PubMed] [Google Scholar]
- 87.Zhang J and Liu J (2013)Tumor stroma as targets for cancer therapy. Pharmacol. Ther 137, 200–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rasanen K and Vaheri A (2010) Activation of fibroblasts in cancer stroma. Exp. Cell Res 316, 2713–2722 [DOI] [PubMed] [Google Scholar]
- 89.Takahashi H et al. (2015) Immunosuppressive activity of cancer-associated fibroblasts in head and neck squamous cell carcinoma. Cancer Immunol. Immunother 64, 1407–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hodi FS et al. (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med 363, 711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ward FJ et al. (2018) On the road to immunotherapy-prospects for treating head and neck cancers with checkpoint inhibitor antibodies. Front. Immunol 9, 2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cohen E et al. (2017) Pembrolizumab (Pembro) vs Standard of Care (SOC) for Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma (R/M HNSCC): Phase 3 KEYNOTE-040 trial, KEYNOTE-040 Evaluates Pembrolizumab in Head and Neck Cancer, ESMO [Google Scholar]
- 93.Cohen EEW et al. (2019) Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head- and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet, 393, 156–167 [DOI] [PubMed] [Google Scholar]
- 94.Ferris RL et al. (2016) Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med 375, 1856–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Santuray RT et al. (2018) New therapies in head and neck cancer. Trends Cancer, 4, 385–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rosenberg JE et al. (2016) Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet, 387, 1909–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Massard C et al. (2016) Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J. Clin. Oncol 34, 3119–3125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kaufman HL et al. (2016) Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 17, 1374–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vonderheide RH et al. (2007) Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J. Clin. Oncol 25, 876–883 [DOI] [PubMed] [Google Scholar]
- 100.Henn V et al. (1998)CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature, 391,591–594 [DOI] [PubMed] [Google Scholar]
- 101.Ko K et al. (2005)Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3 + CD25 + CD4+ regulatory T cells. J. Exp. Med 202, 885–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shimizu J et al. (2002)Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat. Immunol 3, 135–142 [DOI] [PubMed] [Google Scholar]
- 103.Jacobs JF et al. (2010) Dendritic cell vaccination in combination with anti-CD25 monoclonal antibody treatment: a phase I/II study in metastatic melanoma patients. Clin. Cancer Res 16, 5067–5078 [DOI] [PubMed] [Google Scholar]
- 104.Rech AJ et al. (2012)CD25 blockade depletes and selectively reprograms regulatory T cells in concert with immunotherapy in cancer patients. Sci. Transl. Med 4, 134ra62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kurose K et al. (2015) Phase Ia study of FoxP3+ CD4 Treg depletion by infusion of a humanized anti-CCR4 antibody, KW-0761, in cancer patients. Clin. Cancer Res 21, 4327–4336 [DOI] [PubMed] [Google Scholar]
- 106.Garrity T et al. (1997) Increased presence of CD34+ cells in the peripheral blood of head and neck cancer patients and their differentiation into dendritic cells. Int. J. Cancer, 73, 663–669 [DOI] [PubMed] [Google Scholar]
- 107.Highfill SL et al. (2014) Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci. Transl. Med 6, 237ra67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Keane MP et al. (2004) Depletion of CXCR2 inhibits tumor growth and angiogenesis in a murine model of lung cancer. J. Immunol 172, 2853–2860 [DOI] [PubMed] [Google Scholar]
- 109.Weed DT et al. (2015) Tadalafil reduces myeloid-derived suppressor cells and regulatory T cells and promotes tumor immunity in patients with head and neck squamous cell carcinoma. Clin. Cancer Res 21, 39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Califano JA et al. (2015) Tadalafil augments tumor specific immunity in patients with head and neck squamous cell carcinoma. Clin. Cancer Res 21, 30–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mok S et al. (2014) Inhibition of CSF-1 receptor improves the antitumor efficacy of adoptive cell transfer immunotherapy. Cancer Res. 74, 153–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhu Y et al. (2014) CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 74, 5057–5069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Downey CM et al. (2014) DMXAA causes tumor site-specific vascular disruption in murine non-small cell lung cancer, and like the endogenous non-canonical cyclic dinucleotide STING agonist, 2′3′-cGAMP, induces M2 macrophage repolarization. PLoS One, 9, e99988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cavalieri S et al. (2018) Immuno-oncology in head and neck squamous cell cancers: news from clinical trials, emerging predictive factors and unmet needs. Cancer Treat. Rev 65, 78–86 [DOI] [PubMed] [Google Scholar]
- 115.Economopoulou P et al. (2016) The promise of immunotherapy in head and neck squamous cell carcinoma: combinatorial immunotherapy approaches. ESMO Open, 1, e000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Galot R et al. (2018) Personalized biomarker-based treatment strategy for patients with squamous cell carcinoma of the head and neck: EORTC position and approach. Ann. Oncol 29, 2313–2327 [DOI] [PubMed] [Google Scholar]
- 117.Harrington KJ et al. (2010) Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin. Cancer Res 16, 4005–4015 [DOI] [PubMed] [Google Scholar]
- 118.Bartee MY et al. (2017)Tumor-localized secretion of soluble PD1 enhances oncolytic virotherapy. Cancer Res. 77, 2952–2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fukuhara H et al. (2016) Oncolytic virus therapy: a new era of cancer treatment at dawn. Cancer Sci. 107, 1373–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Old MO et al. (2016) The current status of oncolytic viral therapy for head and neck cancer. World J. Otorhinolaryngol. Head Neck Surg 2, 84–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Frazer IH (2004) Prevention of cervical cancer through papillomavirus vaccination. Nat. Rev. Immunol 4, 46–54 [DOI] [PubMed] [Google Scholar]
- 122.Lowy DR and Schiller JT (2006) Prophylactic human papillomavirus vaccines. J. Clin. Invest 116, 1167–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Aggarwal C et al. (2019) Immunotherapy targeting HPV16/18 generates potent immune responses in HPV-associated head and neck cancer. Clin. Cancer Res 25, 110–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zolkind P et al. (2017) Neoantigens in immunotherapy and personalized vaccines: implications for head and neck squamous cell carcinoma. Oral Oncol. 71, 169–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bann DV et al. (2016) Novel immunotherapeutic approaches for head and neck squamous cell carcinoma. Cancers (Basel), 8, E87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang C et al. (2018) Targeting head and neck cancer by vaccination. Front. Immunol 9, 830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Paulos CM et al. (2008)Adoptive immunotherapy: good habits instilled at youth have long-term benefits. Immunol. Res 42, 182–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Knochelmann HM et al. (2018) CAR T cells in solid tumors: blueprints for building effective therapies. Front. Immunol 9, 1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Samstein RM et al. (2019) Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet 51, 202–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Amin MB et al. (2017)Head and neck cancer staging. In AJCC Cancer Staging Manual. (8th edn), Springer Science and Business Media LLC [Google Scholar]
- 131.Vermorken JB et al. (2008) Platinum-based chemotherapy plus cetuximab in head and neck cancer. N. Engl. J. Med 359,1116–1127 [DOI] [PubMed] [Google Scholar]