Abstract
Background
Due to supply chain disruption, the COVID-19 pandemic has caused severe shortages in personal protective equipment for health care professionals. Local fabrication based on 3D printing is one way to address this challenge, particularly in the case of products such as protective face shields. No clear path exists, however, for introducing a locally fabricated product into a clinical setting.
Methods
We describe a research protocol under Institutional Review Board supervision that allowed clinicians to participate in an iterative design process followed by real-world testing in an emergency department. All designs, materials used, testing protocols, and survey results are reported in full to facilitate similar efforts in other clinical settings.
Findings
Clinical testing allowed the incident command team at a major academic medical center to introduce the locally fabricated face shield into general use in a rapid but well-controlled manner. Unlike standard hospital face shields, the locally fabricated design was intended to be reusable. We discuss the design and testing process and provide an overview of regulatory considerations associated with fabrication and testing of personal protective equipment, such as face shields.
Conclusions
Our work serves as a case study for robust, local responses to pandemic-related disruption of medical supply chains with implications for health care professionals, hospital administrators, regulatory agencies, and concerned citizens in the COVID-19 and future health care emergencies.
Funding
: This work was supported by the Harvard MIT Center for Regulatory Sciences, NIH/NCI grants U54-CA225088 and T32-GM007753, and the Harvard Ludwig Center. M.-J.A. is a Friends of McGovern Graduate Fellow.
Keywords: COVID-19, FDA regulations, personal protective equipment, PPE, face shield, 3D printing, regulatory science, additive manufacturing, local fabrication, maker communities
Context and Significance
The COVID-19 pandemic has disrupted medical device supply chains and caused severe shortages in personal protective equipment needed for infection control. To mitigate these shortages, local companies and maker communities have come together to use 3D printing and public domain designs to fabricate protective equipment, such as face shields. However, using unapproved versions of regulated protective equipment in hospitals is problematic. The authors describe the use of a research protocol supervised by an ethical review panel to test a 3D-printed face shield in the emergency department of a major academic medical center and deploy the face shield widely. They make available designs, materials, testing protocols, and provider surveys so that others can reproduce their efforts and make pandemic response more resilient.
Graphical Abstract
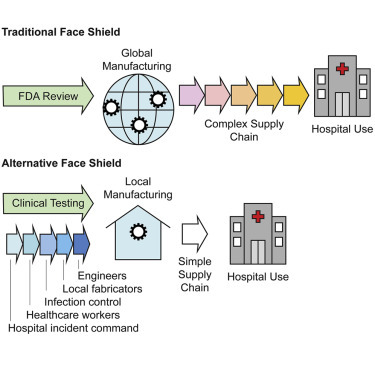
In response to pandemic-related shortages in medical supplies, the authors use an institutional review board-supervised research protocol to clinically test a 3D-printed face shield in a hospital emergency department. This allowed a major academic medical center to incorporate locally manufactured personal protective equipment into the care of COVID-19 patients.
Introduction
In the face of a rapidly expanding COVID-19 pandemic, severe shortages have emerged in personal protective equipment (PPE), putting both health care professionals and patients at increased risk of infection.1, 2, 3 The origins of these shortages are varied but reflect the fragility of medical supply chains in which distribution of critical medical product is dominated by a small number of suppliers reliant on widely distributed manufacturing operations.4 Because hospitals commonly use just-in-time inventory management, supply chain problems rapidly deplete hospital supplies and prevent restocking from traditional vendors. Faced with shortages of unknown duration, many caregivers and medical centers have turned to local fabricators to see whether they can provide replacements for products such as face shields, filtering respirators, and even ventilator components.5 The substitution of conventionally sourced products with non-traditional local products is made feasible by rapid expansion in inexpensive additive manufacturing capabilities (“3D printing”) by small businesses and hobbyists (“maker communities”).6 Computer-aided design (CAD) software has also become widely available, making it possible to share designs in public forums, including the NIH 3D Print Exchange.7 This has resulted in dozens of open-sourced designs, online videos, and blogs dedicated to fabricating different types of PPE.
This article describes the local fabrication and testing of a face shield, one of the simpler types of PPE in terms of design and regulation, from prototyping through clinical testing and adoption by the incident command8 of a major US hospital system. We discuss how hospitals and other health care providers can most effectively test and make use of innovative products in the face of life-threatening disease and supply shortages while ensuring staff and patient safety. Multiple projects led by small companies and citizens have developed creative alternatives to traditional PPE. However, they find that even hospitals in need have difficulty using such products because PPE is normally subject to significant regulation and it is unclear how non-traditional products should be evaluated. The “home stretch” of alternative supply chains is therefore problematic. Also unclear are policies pertaining to PPE life cycle, including whether a product adopted in crisis should remain in inventory after the crises has passed.
Face shields are used in hospitals for infection control9 and are also required PPE in many research and industrial settings. A face shield is a type of PPE in which a transparent, impact- and moisture-resistant visor (commonly made of plastic) is mounted on a frame that encircles the head and positions the visor in front of a user’s face. An example of a traditional health care face shield is the Critical Cover Coverall Face Shield sold by AlphaProtech.10 In health care settings, a face shield is commonly used to protect mucous membranes in the nose, mouth, and eyes from splashes of body fluid that could transmit disease. During the COVID pandemic, face shields are being widely used in conjunction with other protective equipment and are considered to be particularly important for high-risk procedures, such as endotracheal intubation for mechanical ventilation.
Although face shields are simple-appearing devices, they are subject to regulation. The US Food and Drug Administration (FDA) classifies medical devices either as class I (low-risk), class II (moderate risk), or class III (high risk), depending on the degree of risk to a user or patient. Face shields are categorized by the FDA as class I medical devices. The US American National Standards Institute (ANSI)/International Safety Equipment Association (ISEA) Z.87.1-2015 standard specifies nearly twenty required physical features of a face shield as well as testing requirements for visual resolving power, resistance to high-velocity impacts, and protection from droplets and splashes.11 Similar standards exist in Europe and other countries. The need for such standards is obvious given that defective face shields can expose users, particularly those in industry, to serious and life-threatening injuries (e.g., in welding).
Typically, a face shield manufacturer passes an ANSI/ISEA Z.87.1-2015 certification and then notifies the FDA of compliance. The FDA (and in some cases the Centers for Disease Control and Prevention [CDC]) maintains a list of approved products.12 Unlike more-complex medical products, a 510(k) filing is not required; 510(k) filings involve a demonstration that a new device is substantially equivalent to an existing, legally marketed device and are a major route for introducing new medical devices into the US market. Except in rare circumstances, local manufacturers, maker communities, and hospitals are unlikely to have the necessary expertise to fabricate and test face shields to ANSI/ISEA Z.87.1-2015. In the US, the immediate issue of face shield regulation was addressed by an April 2020 FDA letter stating that it “does not intend to object to individuals’ distribution and use of improvised PPE when no alternatives, such as FDA-cleared masks or respirators, are available.”13 This provides a legal framework for use of locally fabricated (“improvised”) PPE in the US, but it does not address the more-general issue of introducing non-traditional PPE into health care environments, what to do if emergency guidance is lacking (as it currently is in many countries), and how other non-traditional devices might be introduced into the hospital supply chain in a rational, safe, and controlled manner.
Options for face shields being pursued by individual citizens, nonprofit institutions, academic medical centers, and small- and large-scale manufacturers include flat plastic shields that can be rapidly assembled by users; three-part designs consisting of a shield, elastic headband, and brow foam, which are being manually assembled by volunteers across the country; and 3D-printed shields, including the Prusa design and its derivatives (Table 1 ). These designs have been introduced with different use cases and fabrication capabilities in mind. Unlike industrial face shields, which can be expensive and are often used for extended periods of time, the vast majority of medical face shields are low cost and intended to be discarded after a single use. Some non-regulated designs (e.g., developed by Prusa14 in the Czech Republic) are intended for multiple uses and are potentially superior in fit and function to regulated disposable face shields. As a practical matter, at a time when PPE is in extremely short supply, even lower quality face shields are unlikely to be discarded after a single use. This raises questions about procedures for face shield sterilization, which are not generally available, even for commercial products.
Table 1.
Examples of Ongoing, Non-traditional Face Shield Fabrication Designs and Specific Efforts
| Face Shield Design Description | Links to Specific Design Efforts |
|---|---|
| Flat plastic face shields that can be rapidly assembled by users | https://project-manus.mit.edu/fs |
| https://open-face-website.now.sh/ | |
| 3-part machine-less face shield requiring volunteer assembly | https://making.engr.wisc.edu/shield/ |
| 3D-printed face shields requiring manufacturer assembly | https://www.prusaprinters.org/prints/25857 |
| https://www.protohaven.org/proto-shield/ | |
| https://3dprint.nih.gov/discover/3dpx-013359 |
In this paper, we describe the production and implementation of a 3D-printed face shield (modified from the Prusa design)14 and its introduction into the Brigham and Women’s Hospital (BWH), a major US academic medical center. In conjunction with the members of BWH Incident Command, we obtained user feedback from surveys of emergency department staff under a protocol approved by our local Institutional Review Board (IRB) (similar to Ethics Committees in the EU).15 We also describe modifications to our design to prepare it for large-scale manufacturing through industry partnerships. The use of a research protocol made it possible to introduce an untested device, and ready it for deployment, in advance of FDA guidance and in a manner that greatly increased the confidence of hospital leadership in the final product. All of the designs and protocols generated through this effort are being freely shared for reuse and improvement, and the results for our testing at the BWH emergency department are reported in full to facilitate the execution of similar face shield efforts in other clinical settings. We anticipate that this work will provide a framework for the design and implementation of similar approaches to PPE manufacturing for current and future medical emergencies.
Results
We sought to develop a locally fabricated face shield that would meet the requirements of an academic hospital when traditional supply chains failed. The requirements included a simple design that limited aerosol and splatter exposure coming from the front and above, that was resistant to fogging, that did not adversely affect a user’s vision, and that was comfortable enough to be worn all day by health care professionals in a high-intensity clinical setting. Conventional disposable face shields on the US market are commonly available in 3/4 length (178 mm; 7 in) and full length (230 mm; 9 in) versions. We produced a full-length visor but increased the width from 230 mm to 305 mm so as to maximize facial protection without obstructing hearing or impeding a user’s range of motion.9 , 16 The face shield was designed for reuse by a single individual following cleaning and disinfection procedures recommended by the CDC for reprocessing protective eyewear; this involves use of US Environmental Protection Agency (EPA)-registered sanitizing wipes (Super Sani cloth, EPA registration number 9480-4; Figures 1A and 1B) or 70% isopropanol. We also tested a more-sophisticated form of sterilization that used ionized hydrogen peroxide (iHP).17
Figure 1.
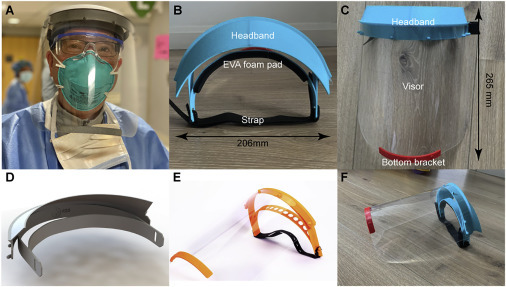
The BWH/PanFab Mk 1.0 Face Shield
(A) An anesthesiologist wearing the BWH/PanFab face shield in a hospital emergency department.
(B) Headband, foam pad, and strap image with dimensions as indicated.
(C) Headband, visor, and bottom bracket image with dimensions as indicated.
(D) 3D model of the face shield.
(E and F) Image of Prusa design14 (E) and final PanFab face shield prototype (F).
Starting with the Prusa RC2 design (Figure 1C),14 we made iterative modifications based on clinician feedback and user testing. The final design, shown in Figure 1D, is similar in many respects to the DtM-v3.1 face shield that was subsequently released via the NIH 3D Print Exchange.18 The similarity between the DtM-v3.1 and PanFab designs is likely to reflect a convergence on a core set of features and the shared desire of a product applicable to multiple health care settings. In the PanFab design process, user feedback from health care and fabrication experts was an essential component. For example, potential users were concerned that the original Prusa design did not provide adequate liquid protection at the top and sides of the visor (this feature is also missing from commercial products as well). We therefore added a fin above the headband to prevent fluid from entering the top of the face shield during high-risk procedures in which a clinician is required to lean forward; this includes endotracheal intubation, one of the riskier procedures that must be performed on COVID-19 patients. We also added a lip above the visor so that any liquid that did fall on the fin would be retained by the lip and would not spread over the visor and affect a user’s ability to see through the visor. Overall, four substantial design modifications were made based on clinical feedback, as outlined in Table 2 . We note that the resulting design includes many features that are absent from disposable commercial face shields commonly stocked in US hospitals.
Table 2.
Examples of Original Design Features, Clinical Feedback for Improvement, and Final Product
| Original Prusa Design | Clinical Feedback for Design Improvement | Final Design |
|---|---|---|
| Open gap between outer face shield envelope and user | limited fluid protection on top of visor when performing procedures (e.g., intubation) | added fin on top of the prototype headband and additional plastic lip to retain fluid and prevent it from obstructing face shield view |
| Single attachment point for face shield strap | difficulty attaching strap and suboptimal fit for different face types | used hook and loop Velcro to adapt each visor to individual users |
| 240 mm width and 240 mm length for face shield outer envelope dimensions | original length not sufficiently protective for all user facial lengths and height | outer envelope length modified to be 240 mm wide and 305 mm long without obstructing hearing or access to ears for stethoscope |
| Anchor point for straps placed lateral to the headband | shield uncomfortable to wear for an extended time | anchor points for hook and loop strap placed in line with the headbands, reducing tightness |
Testing in a Clinical Environment
A total of 97 adults (≥18 years of age) in a variety of clinical roles at the BWH main campus Emergency Department were enrolled in the study. Five participants were lost to follow-up and were excluded from the analysis. Enrollment occurred during two shifts (daytime [n = 52] and overnight [n = 40]) to account for potentially varied attitudes, patient volume, available resources, or other confounders. Demographic information and roles are summarized in Table 3 . As described in the STAR Methods, all study participants passed splash and fit tests before using the face shield in their typical duties.
Table 3.
Demographics (Total Respondents: 92)
| Feature | Number | Percent |
|---|---|---|
| Sex | ||
| Male | 25 | 27.2% |
| Female | 67 | 72.8% |
| Size | ||
| Mean height (in) | 66.2 | |
| Mean weight (lb) | 164.3 | |
| Role | ||
| Attending | 4 | 4.3% |
| Resident | 4 | 4.3% |
| Nurse | 45 | 48.9% |
| Tech | 16 | 17.4% |
| Physician assistant | 6 | 6.5% |
| Environmental | 6 | 6.5% |
| Registration | 2 | 2.2% |
| Radiology | 5 | 5.4% |
| Other | 4 | 4.3% |
Each subject completed a questionnaire on baseline experiences and attitudes. The great majority of study subjects (81.4%) identified themselves as having a patient-facing, clinical role (e.g., physician, physician assistant, nurse, or technician); similarly, most (n = 88; 96%) reported having recently been directly involved in the care of a person under investigation (PUI) for possible COVID-19 infection. Nearly all subjects had recently worn some form of eye protection (n = 91; 99%), and most had exclusively used PPE that was hospital standard issue (n = 57; 62%). Most respondents trusted hospital standard-issue PPE (n = 70; 76%), although some reported being unsure (n = 12; 13%; Table 4 ).
Table 4.
Baseline Experience and Attitudes
| Ever Been Involved in the Care of a Person with Suspected COVID-19 |
Worn Eye Protection in the Past Week |
Used Non-hospital-Supplied PPE |
Trust Hospital-Supplied PPE |
|||||
|---|---|---|---|---|---|---|---|---|
| Answer | Number | Percent | Number | Percent | Number | Percent | Number | Percent |
| Yes | 88 | 95.7% | 91 | 98.9% | 35 | 38.0% | 70 | 76.1% |
| No | 2 | 2.2% | 1 | 1.1% | 57 | 62.0% | 10 | 10.9% |
| Unsure | 2 | 2.2% | 0 | 0.0% | 0 | 0.0% | 12 | 13.0% |
| Total | 92 | 100.0% | 92 | 100.0% | 92 | 100.0% | 92 | 100.0% |
No respondents reported that the PanFab face shield was worse than the hospital standard-issue model in splash protection, durability, ease of use, or comfort; in fact, many preferred it over the hospital-issued model. Average scores in each of four categories (on a 5-point Likert scale) for splash protection, durability, ease of use, and comfort were 4.7, 4.6, 4.3, and 4.4, respectively (Table 5 ). This indicates a better experience with the PanFab face shield as compared to the hospital standard-issue model in all surveyed categories. Most participants rated the novel face shield as offering slightly better or much better splash protection (n = 87; 95%) and durability (n = 84; 91%; Table 5). Nearly all participants reported feeling comfortable or very comfortable using this face shield (n = 88; 96%), with only 1 person (1%) stating she/he felt neither comfortable nor uncomfortable using the face shield (Table 6 ). With respect to continued use, 92% (n = 85) of users planned to continue using the PanFab face shield; four respondents reported being unsure about continued use, but none were opposed (Table 5).
Table 5.
Response across Domains to the Question: “Compared to the Standard Issue Face Shield, How Would You Rate the Prototype Face Shield?”
| Responsea |
Criterion (Number of Users) |
|||
|---|---|---|---|---|
| Comfort Level with Splash Protection | Sturdiness and Reliability | Ease of Use | Comfort | |
| Much worse | 0 | 0 | 0 | 0 |
| Slightly worse | 0 | 2 | 3 | 5 |
| Not worse/not better | 4 | 5 | 17 | 10 |
| Slightly better | 16 | 17 | 21 | 17 |
| Much better | 71 | 67 | 48 | 55 |
| Average scorea | 4.7 | 4.6 | 4.3 | 4.4 |
Individual scores starting at 1 for “much worse” and extending to 5 for “much better”
Table 6.
How Comfortable Are You Using This Shield in a Clinical Scenario Where You Did Not Have Another Option?
| Response | Number | Percent |
|---|---|---|
| Very uncomfortable | 0 | 0.0% |
| Uncomfortable | 0 | 0.0% |
| Neither comfortable nor uncomfortable | 1 | 1.1% |
| Comfortable | 27 | 30.3% |
| Very comfortable | 61 | 68.5% |
Participant Comments
Anonymous respondent comments were also collated. Many individuals expressed gratitude and thanks for the opportunity to use the PanFab face shield and felt that our efforts demonstrated support for frontline clinicians. The impact on morale is a positive aspect of community-resourced PPE, particularly when health care providers are working under extremely difficult conditions. Participants’ anonymous verbatim comments included “I prefer these [new] shields to our old shields,” “This is very sturdy, comfortable and it doesn’t fog! [...] I plan to wear this every day,” and “[The] area of protection is amazing, feels sturdy and secure to head. [While there is] mild pressure on [the] forehead, [this is] preferable to [a different shield] that offers less protection.” Other feedback included concern that the Velcro strap might be a problem for some users with longer hair and that the shield length could be an issue for shorter users. These are issues than can be addressed with simple modifications to the current design.
Discussion
The BWH/PanFab Mk 1.0 face shield provides protection in a reusable design that can be cleaned using standard hospital disinfectants. Using a team that self-organized on-line in response to a request of hospital incident command, PanFab was able to proceed from project inception to implementation in 3 weeks. Critically, using a clinical testing approach, we were able to introduce a non-traditionally manufactured product into a hospital supply chain in a safe and controlled manner (and prior to relaxation of FDA requirements on PPE). To date, we have fabricated approximately 3,000 face shields, all of which remain in regular use. We have determined that PanFab Mk 1.0 face shields can be cleaned with wipes commonly available in hospitals (e.g., Super Sani cloths), 70% isopropanol, and by ionized hydrogen peroxide sterilization (TOMI SteraMist),17 a powerful sterilant also being used on N95 filtering facepiece respirators (masks). Keys to successful introduction of our face shield into a hospital setting included a dedicated liaison within incident command, the willingness of the IRB to work closely and quickly with designers, and the ability of the BWH legal and leadership teams to quickly rule on policy issues.
This project was undertaken at a time of substantial disruption in hospital supply chains and normal infection control procedures as a result of the COVID-19 pandemic. The project was greatly facilitated by a close collaboration between PanFab’s volunteer engineers and the BWH incident command and by the participation of a clinician-scientist (author S.H.Y.) in both organizations. The group involved in face shield specification included emergency room clinicians, infection control specialists, and individuals from the hospital’s environmental health and safety office. In addition to traditional face shield requirements for transparency, splash protection (from the front), and comfort, the design team added features not found in existing hospital PPE, including protection from splashes coming from the side (a broader visor), forehead protection (a fin above the headband), and cleanability and reusability (using wipes and iHP sterilization). The final version of the face shield was formally approved by hospital incident command for use during a period of PPE shortage caused by the COVID-19 pandemic. With the current design in hand, we expect that others can fabricate face shields in 2 weeks or less. In some cases, teams seeking to replicate our approach will need to modify the PanFab design due to shortages of raw materials or differences in fabrication capabilities. Under these circumstances, it is possible that additional user testing under an IRB protocol might be required. We therefore recommend that groups interested in manufacturing face shields coordinate with local incident commands and oversight structures to ensure that essential requirements are met. As discussed below, we also recommend that hospital incident commands include knowledgeable individuals tasked with reaching out to local manufacturing and maker communities.
Additional Considerations for Large-Scale Manufacturing and Dissemination
Moving from prototyping to large-scale manufacturing is a process that traditionally takes many months but in the context of a pandemic must be completed in a matter of weeks. 3D printing and laser cutting are efficient methods for prototyping a design and improving it iteratively, but they are not ideal for large-scale manufacturing. Alternative approaches include rotary die cutting to produce transparent visors and injection molding to fabricate the headband and support bracket. The transition from laser cutting to rotary die cutting is straightforward, but injection molding the headband requires several adjustments to the design. This includes subtly changing the shapes of specific elements and selecting appropriate materials. Subsequent to the introduction of the V1.0 design described here, our group made changes to PanFab face shield design that facilitates rapid-turnaround injection molding for large-scale production (PanFab Mk V1.1; Material 4, available from Mendeley Data at https://dx.doi.org/10.17632/558s8cwfty.1). Injection molding has the added advantage of a more consistent product than 3D printing and is more likely to pass ANSI/ISEA testing. Injection-molded parts can also be sterilized using a range of technologies, whereas concerns have been raised about sterilization of 3D parts made from polylactic acid (PLA).19
Regulatory Considerations and Proposed Improvements
An IRB-approved protocol was used in the current study to oversee the introduction of non-traditional PPE into a health care environment and to allow for testing with informed consent. However, use of a research protocol in this setting may have wider applicability if we consider a non-traditionally manufactured face shield as an “investigational device.” For non-significant risk devices, such as face shields, the FDA authorizes IRBs to conduct the necessary risk assessment, and an investigational device exemption (IDE) is unlikely to be required from the FDA. We note, however, that the relevant US regulations in 21 CFR 812.2 do not cover circumstances in which a normally approved device (i.e., a face shield meeting ANSI/ISEA Z.87.1-2015) that has become unavailable might be replaced by a non-approved variant (i.e., the PanFab face shield) that would be tested via research protocol. In the specific case of our deployment of the PanFab face shield, the latest emergency guidance from the FDA13 would appear to apply; some US state governments have issued their own guidance.20 However, existing emergency guidance is not necessarily adequate for all anticipated needs in the current COVID-19 epidemic, and it is neither guaranteed nor permanent. Moreover, countries such as Canada have more restrictive policies in place.21 Thus, we believe that it would be highly desirable to establish procedures whereby research protocols overseen by IRBs (or ethics committees in the EU) could be used to facilitate future responses to medical emergencies and also promote much-needed innovation in PPE. Regulatory clarification or modification is specifically needed to cover circumstances likely to arise in pandemic emergencies, when local fabrication is needed to augment failing supply chains.
When an emergency is over, devices that have not met prevailing regulatory requirements will likely need to be withdrawn from service to prevent continued use of products with unknown durability and performance characteristics in a health care setting. Precisely when and how this should occur remains unclear. Is it ethical for a hospital that no longer needs products made under emergency conditions to destroy them if other hospitals are in need? Conversely, is it ethical or legal to transfer unused unapproved products or used but sterilized products? From a practical perspective, it is unclear whether users will object to withdrawing a product, such as the BWH/PanFab Mk 1.0, that appears to be superior in fit and function to the low-cost face shields routinely issued by hospitals. These issues remain largely unexplored.
Lessons Learned
The global COVID-19 pandemic has put extreme pressure on health care systems and highlighted many weaknesses in the highly centralized supply chains that have developed for critical medical supplies. Designing, testing, and producing the first batch of PanFab face shields took approximately 3 weeks; with a design in place, others could introduce the same design in less than 2 weeks. In contrast, as of this writing, traditional supply chains remain substantially disrupted 2 months into the COVID-19 pandemic in the US, and much-advertised alternative sources of supply from large companies have not yet been widely distributed. Thus, it appears that local fabrication and testing can make essential supplies available 6–8 weeks more rapidly than waiting for large-scale but less-agile manufacturing. In the longer term, products made by large manufacturers are likely to predominate, but the period covered by this work coincides with the first wave of very high COVID-19 hospitalization rates in Boston. We conclude that local manufacturing is likely to represent an effective source of supply for PPE in an emergency with the potential to rapidly adapt to local demands. In the future, the availability of open-sourced and clinically validated designs and test procedures could result in the local production of PPE within days of a crisis. With this in mind, we are currently subjecting an alternative face shield design to clinical testing in a Boston hospital to create another resource for future use.
Community-level disaster resilience is well-recognized as essential in responses to both natural disasters and public health emergencies,22 but the role of local manufacturing and maker communities in medical supply chains has not previously been considered part of such resiliency. We strongly believe that this should change and that refinement of regulatory and institutional policies is necessary. Hospitals should integrate individuals with engineering and manufacturing expertise into their incident command structure and prioritize longitudinal relationships with the local fabrication and maker communities well before an emergency happens. Our experience highlights the fact that individuals with the necessary medical, engineering, and managerial experience already exist in many academic medical centers; such individuals need to be included in future pandemic planning.
The creation of research protocols for PPE testing could also bring much needed innovation in normal times. Studies over a period of at least 15 years by the US National Academies of Sciences and other US government bodies23 have repeatedly highlighted the need for innovation in PPE, but little progress has been made. Practitioners and ordinary citizens should demand a much more transparent and distributed system for providing essential medical products of all types. Designs for key products should be tested clinically, published in peer-reviewed journals, and demonstrated to meet existing fabrication standards well in advance. Unpatented designs for essential medical products should be made publicly available under non-restrictive Creative Commons or similar licenses. Patented designs should be placed in a patent pool for free use during public health emergencies or be subject to compulsory licensing at a reasonable cost. National suppliers and local fabricators must be compensated for their work, but in extreme cases, 28 US Code § 1498 (“section 1498”) gives the US federal government the “right to use patented inventions without permission, while paying the patent holder ‘reasonable and entire compensation’,” with immunity from patent claims.
The current crisis has shown that, when a pandemic is spreading and health care workers are placed at high risk, we require a distributed and robust community level approach to essential medical supplies, not a secretive and centralized one. The resulting devices, developed and produced largely by volunteers, are not only likely to decrease the risk of hospital infection in the current example but also send a powerful message to frontline medical staff that the local community stands behind them. Although a pandemic was required to galvanize these insights and promote rapid change, our hope is that the spirit of thoughtful collaboration and rapid innovation does not dissipate after the resolution of the current COVID-19 crisis.
Limitations of Study
There are several limitations to this study. First, we have not subjected our final design (printed or molded) for testing or certification under ANSI/ISEA Z.87.1-2015. Testing represents a substantial additional expense and does not cover design modifications made necessary by local circumstances during an emergency situation. Moreover, ANSI/ISEA compliance requires use of an ISO 9001 quality management system, which is not feasible for the rapid, non-traditional manufacturing described here. Nonetheless, having a set of tested and approved designs would increase resiliency during a public health emergency, such as COVID-19, by allowing hospitals to have comprehensive plans in place for expanding PPE when necessary; new regulations will be required for approving emergency-use-only products that satisfy a predefined subset of ANSI/ISEA standards. Second, procedures for sterilizing and reusing face shields have not been evaluated by the FDA. PanFab Mk 1.0 face shields do not appear to be damaged by standard anti-bacterial wipes or 70% isopropanol, and they can be exposed to iHP under conditions that result in a 9-log10 kill of bacterial spore biological indicators.24 In contrast, biological indicators placed in the headband foam of a disposable Fisher-brand face shield of a type typically issued to US health care workers were not successfully sterilized. However, these types of studies need to be repeated with larger sample sizes and more rigorous post-sterilization functional testing. A third limitation of our study is that the PanFab face shield has not undergone long-term testing for durability and usability. PLA, which was used to print the headband, is known to be biodegradable via hydrolysis. PLA was used due to its availability in the face of supply chain disruptions that made other 3D printing materials, such as PET-G, difficult to acquire (the label provided with all PanFab face shields includes the warning that the face shields should be considered a temporary solution for use in a medical crisis and are not equivalent to an FDA-approved product; Material 3; available from Mendeley Data at https://dx.doi.org/10.17632/558s8cwfty.1). A final limitation of the PanFab face shield is that it cannot be flat packed for efficient storage and shipping: it is instead optimized for local fabrication. We are therefore prototyping a flat-packed face shield that has many of the same features of the PanFab Mk 1.0 design without requiring 3D printing capabilities.
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited Data | ||
| Material 1: Associated study IRB questionnaire, related to Table 3, 4, 5, and 6 | N/A | https://dx.doi.org/10.17632/558s8cwfty.1 |
| Material 2: Design files of face shield parts: .STL + DXF, related to Figure 1 | N/A | https://dx.doi.org/10.17632/558s8cwfty.1 |
| Material 3: Instruction for use and overview of product, related to Figure 1 | N/A | https://dx.doi.org/10.17632/558s8cwfty.1 |
| Material 4: Design files of injection molding compatible face-shield, related to Figure 1 | N/A | https://dx.doi.org/10.17632/558s8cwfty.1 |
| Material 5: IRB questionnaire individual results and summary statistics, related to Table 3, 4, 5, and 6 | N/A | https://dx.doi.org/10.17632/558s8cwfty.1 |
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Prof. Peter Sorger (peter_sorger@hms.harvard.edu, cc: sorgeradmin@hms.harvard.edu).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
Additional Material are available from Mendeley Data at https://dx.doi.org/10.17632/558s8cwfty.1.
Experimental Model and Subject Details
Please see Table 3 of the text for study subjects’ demographic information. Verbal consent was obtained from all study subjects. The study was approved by Partners Healthcare IRB: 2020P00910I.
Method Details
Initial Design and Serial Prototyping
We recruited a team of five clinicians, including physicians specializing in internal medicine, infectious disease, emergency medicine and dermatology who worked in tandem with a safety officer to solicit feedback and serially prototype potential face shield designs. Starting with the open source Prusa-design, we iteratively modified, 3D-printed, and obtained clinician feedback on specific features (described in Table 2). Four design iterations led to consensus on a design with acceptable fit, comfort and degree of protection that made use of readily-available materials. This model was officially evaluated by infection control and safety officers and approved for clinical testing. The final model, the BWH/PanFab Mk1.0 face shield (henceforth the PanFab face shield), is composed of five components: (i) a transparent visor made of biaxially-oriented polyethylene terephthalate (BoPET, also known as Mylar; LEVOSHUA brand from Amazon.com), (ii-iii) a 3D printed headband and bottom reinforcement bracket made of polylactic acid (PLA, 1.75mm diameter, Hatchbox), (iv a hook and loop strap (VELCRO® Brand ONE-WRAP; Manchester NH) and (v) a foam pad made of ethylene-vinyl acetate (EVA 6mm - unknown manufacturer, donated) for added comfort.
Design and printing of the headband and bottom reinforcement bracket
Based on the Prusa RC2 open-source model, a 3D mesh model was imported into Fusion 360 (Autodesk, V2.0.7830) software and converted into a solid body for editing using the boundary representation (BRep). The design was then modified iteratively based on clinician feedback (summarized in Table 2). For each prototype, the model was exported into .STL format before being imported into the open-source 3D-printing software CURA (Ultimaker), where it was sliced using the following parameters: 0.2mm layer height, 15% gyroid infill. Following slicing, the printer-specific g-code was sent to fused filament fabrication (FFF) 3d-printers (Ender 3 Pro, Creality). PLA was 3D printed at 90mm/s, the overall material volume used, and part envelope were 53.2 × 103 mm3 and 215 × 152 × 50 mm for the headband, and 4260 mm3 and 120 × 30 × 13mm for the reinforcement bracket. These parameters were optimized to decrease print time and material, while retaining functionality. Material 2 (available from Mendeley Data at https://dx.doi.org/10.17632/558s8cwfty.1) includes design .STL files and Material 3 describes associated material considerations (available from Mendeley Data at https://dx.doi.org/10.17632/558s8cwfty.1).
Design and cutting of the transparent visor and the foam pad
The transparent visor was designed using InkScape software to match the pegs of the 3D-printed headband. The visor was 240 mm long and 305 mm wide, ensuring that the user’s face would be fully covered without obstructing hearing. The model was outputted into .DXF format and then laser cut from 0.007” BoPET using a GlowForge or GlowForge plus laser cutter (1 pass, speed setting: 500 mm/sec, 40% power, focus height: 0.178 mm). GlowForge and GlowForge plus maximum laser powers were 40 W and 45 W respectively. The foam pad was designed in InkScape software before being outputted into .DXF format and laser cut from 6mm EVA foam (1 pass, speed setting: 155 mm/sec, 30% power, focus height: 6.13 mm). The overall dimensions of the foam were 6 mm in thickness, 20 mm in width, and 190 mm in length.
Face shield assembly
Following printing of the headband and bottom bracket, and laser cutting of the foam pad and the transparent visor, the face shield was assembled using the instructions in Material 3 (available from Mendeley Data at https://dx.doi.org/10.17632/558s8cwfty.1). Briefly, the foam pad was attached to the inner band of the headband using either super glue or hot glue (unknown manufacturers), hook and loop straps (VELCRO Brand ONE-WRAP Double Sided Roll 0.75 in) were cut to 330 mm in length and secured to the headband by looping the hook and loop straps inside the hole at the posterior side of the headband, and then attaching the straps onto itself. The transparent visor was then mounted onto the headband by first securing one of the outer holes of the visor onto the headband peg. The visor was pulled across the headband so that each visor hole was aligned with the pegs of the headband. Prior to delivery for testing, face shields were cleaned using sanitizing wipes (Super Sani cloth, EPA registration number 9480-4) and placed under 254 nm ultraviolet light for 5 min in a germicidal cabinet (Monitor 2000, Sellstrom).
Testing and Validation in Clinical Setting Subject Selection
To assess face shield usability and safety, a cohort of physicians, physician assistants, emergency department technicians, environmental service staff, and other individuals with patient-facing roles were recruited to the study from the BWH Emergency Department. To account for different workflows and preferences, participants were recruited from both day and night shifts. Study subjects were provided with a fact sheet and verbal consent was obtained (Partners Healthcare IRB: 2020P00910, Material 1, available from Mendeley Data at https://dx.doi.org/10.17632/558s8cwfty.1 ).
Quality assessments
To assess quality, fabrication staff performed the following assessments in accordance with testing procedures reported for existing face shield designs18:
-
(1)
Visually inspected each component, checking for printing defects, cracks, and crevices.
-
(2)
Donned and doffed the face shield 10 times. Donning and doffing of the face shields were done in accordance with CDC guidelines. The face shield passed the test if it took less than 10 s to don or doff the face shield.
-
(3)
Qualitative visibility assessment: The qualitative vision test was passed if the fabrications staff reported no adverse effect on vision when wearing a face shield for > 30 min.
-
(4)
Cleanability/reusability of the face shield was assessed through a pilot study using ionized hydrogen peroxide sterilization (iHP; TOMI SteraMist)17. The face shield passed the test if this sterilization produced a 9-log10 kill as assessed by bacterial spore biological indicators 24.
-
(5)
Compatibility with commonly used hospital disinfecting wipes was assessed by cleaning the PETG visor ten times ten times with EPA-registered sanitizing wipes (Super Sani cloth, EPA registration number 9480-4), waiting for the visor to dry completely between each disinfection. A similar test was performed with an 70% isopropanol wipe. The face shield passed the test if the fabrication staff noted no fogging, distortion or any changes affecting vision.
Functionality assessments
To assess functionality, research subjects were fitted with an unused PanFab face shield and the following tests were performed:
-
(1)
Test of splash resistance: a spray of water was delivered using a spray at the center of the visor. The visor passed the test if a subject did not feel any droplets on her/his face or neck.
-
(2)
Wearability testing: With the face shield on, subjects were asked to look left, right, up, down, and shake their heads, say yes and no. The face shield passed the test if none of the motions were impeded and the face shield did not fall off.
Fogging testing
The face shield was worn with and without a facemask for an extended period (min. 30 mins) under physical stress (e.g., an exercise machine) by one participant and it was not observed to undergo excessive fogging.
User Feedback
An initial survey was administered to evaluate baseline demographics and attitudes toward PPE. After fit and splash testing, subjects returned to their work and used the face shield during their regular workflow for one hour, at which time a second survey was administered to obtain feedback on face shield performance.
Quantification and Statistical Analysis
Summary statistics were computed using Microsoft Excel.
Acknowledgments
Above all, we thank the members of the Greater Boston Pandemic Fabrication Team (PanFab) for technical, administrative, and logistic support necessary for the execution of this project. Membership is found at https://www.panfab.org/the-team-and-the-project/consortium-members. We also thank Michael Klompas, MD, Jon Boyer, ScD CIH, and Charles M. Morris, MD for their clinical and safety feedback; Kevin T. Giordano, MBA FACHE, Douglas Carney, AIA MBA, Julia Sinclair, MBA, Allison Moriarty, MPH, and Bernard R. Jones, EdM for their support with implementation, operations, and approval process; Demetrio Anaya for his CAD expertise; Peter Chai, MD for helping with protocol development; as well as all of the health care workers that participated in the study. We thank BoroBot, iRobot, and Velcro, in addition to the Harvard Graduate School of Design, Salesforce, SunPe Prototype, the Shin Laboratory at the Brigham and Women’s Hospital’s Stepping Strong Foundation for Trauma Innovation, and the Wentworth Institute of Technology for generously lending their design expertise and manufacturing capabilities for the production of the BWH/PanFab Mk 1 Face Shield. Local fabricators, makers, and citizens generously donated their time and resources and were essential for all stages of the project. This work was also supported by the Harvard MIT Center for Regulatory Sciences, NIH/NCI grants U54-CA225088 (to P.K.S., N.R.L., and D.P.) and T32-GM007753 (to D.P.), and the Harvard Ludwig Center. M.-J.A. is a recipient of the Friends of McGovern Graduate Fellowship. The ORCID IDs for the authors are as follows: (A.M.) 0000-0002-6084-5617, (M.-J.A.) 0000-0002-9774-1483, (D.P.) 0000-0002-4218-1693, (P.D.A.) 0000-0002-4573-3609, (E.W.B.) 0000-0002-3454-1310, (J.F.) 0000-0002-5198-835X, (M.S.S.) 0000-0002-9165-8611, (P.K.S.) 0000-0002-3364-1838, (N.R.L.) 0000-0002-8264-834X, and (S.H.Y.) 0000-0002-1432-9128.
Author Contributions
Face Shield Design Prototyping and Production, M.-J.A., B.B., P.D.A., E.W.B., A.F., J.F., R.O., L.S., C.V., and S.H.Y.; Face Shield Design Clinical Testing, A.M., P.D.A., N.R.L., and S.H.Y.; Writing, A.M., M.-J.A., D.P., M.S.S., P.K.S., N.R.L., and S.H.Y.; Greater Boston Pandemic Fabrication Team (PanFab) Consortium Coordination, D.P., H.Y., and P.K.S.
Declaration of Interests
A.M. is a consultant or has received honoraria from Pfizer, 3Derm, and hims and has equity in Lucid Dermatology and hims. He is an associate editor for JAMA Dermatology. A.M. declares that none of these relationships are directly or indirectly related to the content of this manuscript. P.K.S. is a member of the SAB or Board of Directors of Applied Biomath, Glencoe Software, and RareCyte, Inc. and has equity in these companies. In the last 5 years, the Sorger lab has received research funding from Novartis and Merck. P.K.S. declares that none of these relationships are directly or indirectly related to the content of this manuscript. N.R.L. is a consultant for or has received honoraria from the following companies: Seattle Genetics, Sanofi, and Bayer. E.W.B. is funded by NIH grants R01DA047236, HL R01HL 126911, and HD093655; DARPA award FA8750-18-C-0025; and Philips Healthcare. E.W.B. declares that none of these relationships are directly or indirectly related to the content of this manuscript. P.D.A. has equity in Hallandia V. P.D.A. declares that none of these relationships are directly or indirectly related to the content of this manuscript.
Published: June 19, 2020
References
- 1.WHO . 2020. Shortage of personal protective equipment endangering health workers worldwide.https://www.who.int/news-room/detail/03-03-2020-shortage-of-personal-protective-equipment-endangering-health-workers-worldwide [Google Scholar]
- 2.Jacobs A., Richtel M., Baker M. 2020. ‘At war with no ammo’: doctors say shortage of protective gear is dire. The NY Times, March 19, 2020.https://www.nytimes.com/2020/03/19/health/coronavirus-masks-shortage.html [Google Scholar]
- 3.Schlanger Z. 2020. Begging for thermometers, body bags, and gowns: U.S. health care workers are dangerously ill-equipped to fight COVID-19. Time, April 20, 2020.https://time.com/5823983/coronavirus-ppe-shortage/ [Google Scholar]
- 4.Volkin S. 2020. How has COVID-19 impacted supply chains around the world? The Hub, April 6, 2020.https://hub.jhu.edu/2020/04/06/goker-aydin-global-supply-chain/ [Google Scholar]
- 5.Livingston E., Desai A., Berkwits M. Sourcing personal protective equipment during the COVID-19 pandemic. JAMA. 2020;323:1912–1914. doi: 10.1001/jama.2020.5317. [DOI] [PubMed] [Google Scholar]
- 6.Statt N. 2020. 3D printers are on the front lines of the COVID-19 pandemic. The Verge, May 25, 2020.https://www.theverge.com/2020/5/25/21264243/face-shields-diy-ppe-3d-printing-coronavirus-covid-maker-response [Google Scholar]
- 7.NIH . 2020. NIH 3D Print Exchange.https://3dprint.nih.gov/ [Google Scholar]
- 8.Fletcher B., Knight A., Pockrus B., Wain M.J., Lehman-Huskamp K. Hospital incident command: First responders or receiving centers? Am. J. Disaster Med. 2016;11:125–130. doi: 10.5055/ajdm.2016.0231. [DOI] [PubMed] [Google Scholar]
- 9.Christensen R.P., Robison R.A., Robinson D.F., Ploeger B.J., Leavitt R.W. Efficiency of 42 brands of face masks and 2 face shields in preventing inhalation of airborne debris. Gen. Dent. 1991;39:414–421. [PubMed] [Google Scholar]
- 10.AlphaProTech . 2020. Critical Cover Coverall Face Shields.http://www.alphaprotech.com/product/166.aspx?catid=50 [Google Scholar]
- 11.CDC . 2015. ANSI/ISEA Z87.1-2015.https://wwwn.cdc.gov/PPEInfo/Standards/Info/ANSI/ISEAZ8712015 [Google Scholar]
- 12.FDA . 2020. 2019 device approvals.https://www.fda.gov/medical-devices/recently-approved-devices/2019-device-approvals [Google Scholar]
- 13.FDA . 2020. Enforcement policy for face masks and respirators during the coronavirus disease (COVID-19) public health emergency (revised)https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enforcement-policy-face-masks-and-respirators-during-coronavirus-disease-covid-19-public-health [Google Scholar]
- 14.PrusaPrinters . 2020. Prusa Face Shield.https://www.prusaprinters.org/prints/25857-prusa-face-shield [Google Scholar]
- 15.Moon M.R., Khin-Maung-Gyi F. The history and role of institutional review boards. Virtual Mentor. 2009;11:311–321. doi: 10.1001/virtualmentor.2009.11.4.pfor1-0904. [DOI] [PubMed] [Google Scholar]
- 16.Roberge R.J. Face shields for infection control: a review. J. Occup. Environ. Hyg. 2016;13:235–242. doi: 10.1080/15459624.2015.1095302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McEvoy B., Rowan N.J. Terminal sterilization of medical devices using vaporized hydrogen peroxide: a review of current methods and emerging opportunities. J. Appl. Microbiol. 2019;127:1403–1420. doi: 10.1111/jam.14412. [DOI] [PubMed] [Google Scholar]
- 18.NIH . 2020. DtM-v3.1 Face Shield PPE, 3D printable headband NO LOGO.https://3dprint.nih.gov/discover/3dpx-013359 [Google Scholar]
- 19.Guvendiren M., Molde J., Soares R.M.D., Kohn J. Designing biomaterials for 3D printing. ACS Biomater. Sci. Eng. 2016;2:1679–1693. doi: 10.1021/acsbiomaterials.6b00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelley E.D. 2020. Memorandum: comprehensive personal protective equipment (PPE) guidance.https://archives.lib.state.ma.us/handle/2452/825190 [Google Scholar]
- 21.Government of Canada . 2020. 3D printing and other manufacturing of personal protective equipment in response to COVID-19.https://www.canada.ca/en/health-canada/services/drugs-health-products/medical-devices/covid-19-unconventional-manufacturing-personal-protective-equipment.html [Google Scholar]
- 22.Plough A., Fielding J.E., Chandra A., Williams M., Eisenman D., Wells K.B., Law G.Y., Fogleman S., Magaña A. Building community disaster resilience: perspectives from a large urban county department of public health. Am. J. Public Health. 2013;103:1190–1197. doi: 10.2105/AJPH.2013.301268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Academies of Sciences, Engineering, and Medicine . 2020. Standing Committee on Personal Protective Equipment for Workplace Safety and Health.https://www.nationalacademies.org/our-work/standing-committee-on-personal-protective-equipment-for-workplace-safety-and-health [Google Scholar]
- 24.Cramer A., Plana D., Yang H.L., Carmack M., Tian E., Sinha M.S., et al. medRxiv; 2020. Analysis of SteraMist Ionized Hydrogen Peroxide Technology as a Method for Sterilizing N95 Respirators and Other Personal Protective Equipment.https://www.medrxiv.org/content/10.1101/2020.04.19.20069997v2 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Additional Material are available from Mendeley Data at https://dx.doi.org/10.17632/558s8cwfty.1.


