Abstract
While iron is a nutrient metal, iron overload can result in multiple organ failures. Iron chelators, such as deferoxamine, are commonly used to ameliorate iron overload conditions. However, their uses are limited due to poor pharmacokinetics and adverse effects. Many novel chelator formulations have been developed to overcome these drawbacks. In this review, we have discussed various nanochelators, including linear and branched polymers, dendrimers, polyrotaxane, micelles, nanogels, polymeric nanoparticles and liposomes. Although these research efforts have mainly been focused on nanochelators with longer half-lives, prolonged residence of polymers in the body could raise potential safety issues. We also discussed recent advances in nanochelation technologies, including mechanism-based, long-acting nanochelators.
Keywords: : chelation therapies, deferoxamine, hemoglobinopathies, polymeric chelators, secondary iron overload
Iron turnover
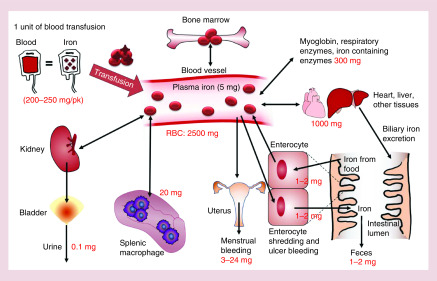
Iron is an essential metal for proper physiological function [1,2]. It is the component of heme in hemoglobin of red blood cells (RBCs) that carry oxygen to cells [3]. Iron is also a required element of the iron–sulfur complex involved in oxidative phosphorylation, which generates energy via ATP [4,5]. In addition, iron is an integral part of many enzymes, including peroxidases, cytochrome P450s, nitric oxide synthase, heme oxygenase and aconitase [5,6]. Iron turnover is highly regulated to support various metabolic processes (Figure 1). Dietary iron from vegetarian sources (nonheme iron) is taken up by DMT1 into the intestinal epithelial cells. In contrast, heme iron is transported by the proton-coupled folate transporter/heme carrier protein on enterocytes and metabolized to release iron. Iron is then exported from enterocytes into blood by an iron exporter FPN1 and binds to plasma Tf for blood circulation. The iron–Tf complex binds to the Tf receptor (TfR1) in various tissues and undergoes endocytosis. Iron is released from the endosome by DMT1 into the cytosol where it is utilized for erythropoiesis and cellular metabolism or stored in ferritin when in excess. When senescent RBCs are broken down in the macrophages, intracellular iron is exported into circulation by FPN1. Hepcidin, a hormone produced in the hepatocytes and released into circulation, binds to FPN1 and facilitates its degradation, thereby inhibiting iron transport in various tissues, including those involved in absorption (intestine), release (liver) and recycling (spleen) of iron.
Figure 1. . Iron turnover.
Approximately 1–2 mg of iron is absorbed from the intestine via food sources. Iron is then absorbed into the plasma and later incorporated into hemoglobin that is utilized to produce RBCs in the bone marrow. Approximately 2500 mg of iron is present in the RBC of the blood of an adult. Iron is also stored in different tissues, including liver and muscle. Iron is also utilized to make myoglobin and enzymes. About 0.8% of the RBCs are destroyed every day by macrophages to release iron to plasma. Iron is also lost from the body due to shredding of intestinal cell, bleeding and menstruation. An optimal level of iron is maintained in the body. In the case of secondary iron overload due to blood transfusion, iron is stored in the liver and other tissues in large quantities and damage these tissues due to oxidative stress. The excretion of iron in the urine and feces may be increased when iron chelators are used.
RBC: Red blood cell.
Iron disorders
Excess hepcidin results in anemia, whereas decreased hepcidin causes tissue iron overload. Hepcidin levels are modified by various factors. For example, matriptase-2 and erythroferrone negatively regulate hepcidin. In contrast, the HFE (high Fe or hyperferremia) protein controls hepcidin expression, and mutations in the HFE gene result in decreased hepcidin, which consequently increases iron absorption and promotes iron overload (also known as HFE-related hemochromatosis). Abnormally high iron is toxic because free iron can produce reactive oxygen species and cause tissue damage. While iron overload increases the risk of liver cirrhosis, cardiac dysfunction and diabetes, phlebotomy is the treatment of choice for hemochromatosis patients. Iron overload is also frequently found in anemic patients who suffer from hemoglobinopathies (e.g., thalassemia, sickle cell disease) and thereby need repeated blood transfusions. In such conditions, iron released from breakdown of RBCs is gradually accumulated in tissues, which leads to another major type of iron overload, known as secondary iron overload [7–9]. Cardiovascular complications, including hypertrophy, arrhythmia and heart failure, are the major health issues associated with secondary iron overload [8]. Liver dysfunction and development of diabetes and insulin resistance are also frequently reported [10,11]. Iron chelators are exclusively used to ameliorate disease conditions occurring in secondary iron overload, since phlebotomy is not indicated for anemic patients. Of interest, ferroptosis is a form of iron-dependent, nonapoptotic cell death associated with increased levels of cellular lipid peroxidation [12]. While ferroptosis has been implicated in several disease conditions, including cancer, ischemia/reperfusion injury, and cardiomyopathy [12–14], iron chelators have been shown to effectively inhibit ferroptosis [14], suggesting their clinical utilities beyond iron overload disorders.
Iron chelators
Clinically-available iron chelators
Only three iron chelators are clinically available to manage iron overload: deferoxamine (DFO), deferiprone (DFP) and deferasirox (DFX). The detailed comparisons of these chelators are discussed elsewhere [15–18]. Briefly, DFO is the oldest iron chelator that has been used in the USA since 1966. It is used in acute iron poisoning, secondary iron overload and aluminum toxicity. DFO is hexadentate and, therefore, can chelate an equimolar amount of iron. Although it is highly effective in chelating iron, it has a poor oral bioavailability with a very short half-life (20–30 min in humans), which requires multiple subcutaneous or intravenous administrations at a dose of 20–50 mg/kg/day for 8–24 h, 5 days a week. It is hydrophilic and is excreted in urine and feces. Adverse effects include local skin reaction, ophthalmological and auditory abnormality, allergic reaction, growth retardation and bone abnormality. It is recommended to have an annual ophthalmological and auditory check-up when a patient is treated with DFO. Other iron chelators are used when DFO is contraindicated or when DFO alone is not enough to chelate iron. More commonly, a combination of DFO and DFP or DFX are used to minimize adverse effects of a single chelator and to increase patient compliance. DFP, developed by Cipla, was available as an oral iron chelator in India since 1994 and subsequently marketed in Europe. Apotex Inc. conducted a clinical trial in the North America. However, DFP was not approved in the US until 2011 due to safety concerns. It is a hydrophilic drug with longer half-life; for example, 3–4 h compared with DFO. DFP is available as an oral formulation with a dose of 75–100 mg/kg/day in three divided doses. It is predominantly excreted in urine. The adverse effects of DFP are agranulocytosis, neutropenia, arthralgia and elevated liver enzymes. Thus, a weekly blood count is recommended for the patients taking DFP. The latest iron chelator is DFX which demonstrates the longest half-life; for example, 8–16 h. DFX is prescribed at a dose of 10–40 mg/kg/day, to be administered once or twice a day. It was developed by Novartis and was approved for use in the USA in 2005. DFX is predominantly excreted in the feces. DFX has gastrointestinal, kidney, and liver toxicity, which requires monthly evaluations of blood count and kidney and liver function. Due to the toxic potential of these iron chelators, many other iron chelators have been evaluated, as discussed below.
Experimental small molecule iron chelators
While readers are encouraged to consult the cited articles [15,19,20] for detailed information about the experimental iron chelators, here we briefly discuss several natural and synthetic iron chelators in the context of their efficacy in vitro and in animal models. Ohara et al. [21] reported on the development of super polyphenols (SP6 and SP10) which chelate iron and inhibit proliferation of cancer cell lines. Though it was safer than DFO at 300 mg/kg after intravenous (iv.) injection, iron chelation efficacy has not been evaluated in vivo [21]. Curcumin is an interesting example of a natural molecule that possesses iron chelation properties [20] although its iron binding affinity is lower than that of DFP or DFX [22,23]. Administration of curcumin via food or drinking water decreased iron in the liver of 1-year-old mice [24], as well as in FeSO4-induced iron-loaded rats [25]. However, its low water solubility and low intestinal absorption are major drawbacks to clinical translation [26]. Many iron chelators with additional pharmacological properties, including antioxidant activity, inhibition of monoaminoxidase or acetylcholine esterase, activation of dopamine receptor – among others – have been discovered [27–35]. Most of these novel iron chelators were either tested in vitro or in animal models related to neurological disease or cancer, but these agents have not been evaluated in secondary iron overload disease models. Kalinowski and Richardson [15] and Hatcher et al. [20] extensively discussed other new synthetic iron chelators, including desferrithiocin, tachpyridine, thiosemicarbazones, and analogs of pyridoxal isonicotinoyl hydrazone. However, none of these chelators have been clinically approved to date.
The need for advanced formulations
Although the available iron chelators effectively remove body iron and reduce iron-associated tissue damage, these small molecules have their unique drawbacks. For example, DFO requires repeated parenteral administrations, which can decrease patient compliance. Other issues include local irritation at the site of injection and auditory and visual disturbances, requiring yearly examination of the auditory and visual function. A weekly blood count is necessary for the patients taking DFP due to neutropenia. Similarly, monthly monitoring of blood count, kidney and liver function is needed for patients taking DFX. To overcome these issues, many research groups have tried to develop new formulations based on DFO due to its hexadentate nature and relatively low toxicity compared with DFX and DFP. In this review, several nanochelators made by incorporating DFO into various nano-supports will be discussed, with a focus on long-acting chelators to avoid continuous infusions and increase patient compliance.
Recent advances in the development of nanochelators
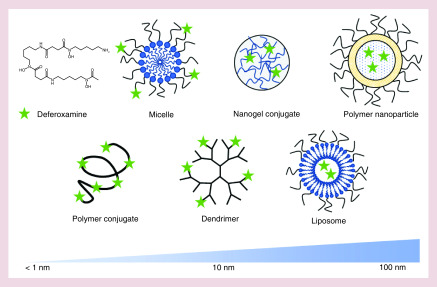
Research into extended half-life iron chelators began shortly following the approval of DFO. Initial efforts to improve the efficacy of DFO began with liposomal compositions in the late 1970s [36–38]. A liposomal formulation significantly extended the half-life of DFO [36] and improved its urinary iron excretion [37,38]. Additionally, liposomes accumulated in organs such as the liver and spleen, which increased DFO exposure in these key iron storage organs [37]. However, given that the first liposomal drug products were not US FDA approved until the 1990s [39], it is unsurprising that these early efforts with a nascent technology were not successfully translated to the clinic. Since these early efforts, novel drug-delivery technologies have been applied to remedy the short-comings of existing iron chelation therapies. These efforts have focused predominantly in two key areas: improving chelator's half-life to reduce administration frequency, and enhancing the safety profile to minimize side effects. As depicted in Figure 2, nanochelators have been formulated using both conjugation and controlled release approaches. Chelators have been conjugated to a number of moieties including linear and branched polymers, dendrimers, polyrotaxane, micelles and nanogels. Efforts to control the release of chelators have been more limited due to chelator hydrophilicity, but both polymeric and liposomal nanoparticles have been used to this end.
Figure 2. . Schematic description of nanochelators with approximate hydrodynamic diameter.
Deferoxamine (DFO) is a small molecule chelator that is often incorporated into nano-constructs to create nanochelators. Polymer conjugation can increase the hydrodynamic diameter of DFO dependent on the molecular weight of the polymer, often resulting in a range of approximately 5–10 nm [49–51,55,56]. Nanochelator micelles [45] can be formed by conjugating DFO to amphiphilic molecules, often yielding a size of approximately 10–100 nm, depending on amphiphile structure and molecular weight. Nanochelator dendrimers [52–54] can be formed by conjugating DFO to the dendrimer termini, resulting in a nanochelator size of approximately 10–100 nm depending on generation number and monomer chemistry. DFO can be incorporated into nanogels [57,58] via conjugation, generally resulting in a size range of approximately 50–200 nm. Nanochelators can also be formed by encapsulating DFO in the aqueous core of polymer nanoparticles [59] or liposomes [36–38,60], resulting in a nanochelator size range of approximately 50–200 nm, depending on formulation and process parameters.
A number of groups have reported on the development of nanochelators that have demonstrated efficacy in vitro, but have not yet been evaluated as iron overload therapies in vivo. Rossi et al. [40] reported on conjugates of DFO and PEG-acrylate copolymers that showed promising data on both iron binding and hemocompatibility in vitro. Tian et al. [41] reported on the development of alginate-DFO conjugates that reduced in vivo metabolism and in vitro cytotoxicity of the DFO. Zhou and Hider et al. [42–44] have reported on the development of novel iron chelating dendrimers. The dendrimers were formulated by conjugating a variety of chelators, including bidentate [44] and hexadentate [43,44] ligands, to poly-acid dendrimers. A dendrimer with hexadentate hydroxyl-pyridinone ligands has shown great promise of binding iron in vitro [42], but has not been evaluated in an animal model of iron overload. Huang et al. [45] recently reported on the development of DFP micelles formulated by conjugating DFP to PEG-poly-glutamic acid. While the micelles enhanced the chelation efficiency of DFP and showed efficacy in vitro, they were not evaluated in vivo [45]. Marzban et al. [46] reported on the development of a DFO nano-niosome, which is a nonionic surfactant-based vesicle that can encapsulate DFO. The nano-niosomal DFO formulation reduced cytotoxicity of DFO and enhanced in vitro iron chelation in hepatocytes [46]. Wang et al. [47] reported on the development of an iron chelating nanoparticle to treat Parkinson's’ disease (PD). The nanochelator was formulated by conjugating the chelator non-Fe hemin (NFH) to a nanoparticle formed from the radical polymerization of BSA-acrylate monomers. When targeted to the CNS with TAT peptide, the nanochelator showed efficacy in an MPTP-induced mouse model of PD [47]. Sulistyo et al. [48] reported on the development of a nanochelator based on encapsulating green tea extract in chitosan nanoparticles using the ionic gelation method. Though this nanoparticle reduced serum ferritin in a dietary model of iron overload in rats [48], there can be limited capability to clinically translate an extract-based formulation.
In the following sections, we discuss recent developments in the nanochelator field. Physicochemical properties of the nanochelators, including composition and hydrodynamic diameter (HD), are described in Table 1, and pharmacokinetic parameters, including excretion profiles, are described in Table 2. This review turns a focus to formulations that have been evaluated as iron overload therapies in vivo, and thus have the potential for clinical translation.
Table 1. . Summary of physicochemical characteristics of nanochelators.
| Nanochelator | Delivery system | Material | Primary excretion route | HD (nm) | DFO density | Ref. |
|---|---|---|---|---|---|---|
| Dextran-DFO | Branched polymer | Dextran | NR† | NR | 20–30% w/w | [49] |
| HES-DFO | Linear polymer | Hydroxy-ethyl starch | NR† | NR | 10–20% w/w | [49,50] |
| S-DFO | Linear polymer | Starch | NR† | NR | 26% w/w‡ | [51] |
| ULC-75 ULC-637 |
Dendrimer | Hyper-branched polyglycerol | Feces/urine§ Feces/urine§ |
9.8‡ 15.4‡ |
51% w/w‡ 20% w/w‡ |
[52,53] |
| BGD-60 BDD-200 |
Dendrimer | Hyper-branched polyglycerol | Feces Feces/urine |
21.2‡ 27.4‡ |
39% w/w 51% w/w |
[54] |
| hPR-DFO | Inclusion complex | Polyrotaxane | NR¶ | 3.5 | 35.7% w/w | [55] |
| DFO2-EPL DFO4-EPL DFO8-EPL |
Linear polymer | ε-Polylysine | Urine | 5.5 5.7 6.5 |
12.3% w/w‡ 22.1% w/w‡ 36.3% w/w‡ |
[56] |
| NG1-DFO NG2-DFO |
Nanogel | Acrylamide methacrylate PEG-diacrylate | NR¶ | 36 101 |
16.9% w/w 16.4% w/w |
[57] |
| rNG-DFO | Nanogel | Acrylamide methacrylate PEG-diacrylate | NR¶ | 240 | 7.23% w/w | [58] |
| DFO-NP | Nanoparticle | mPEG-PLGA | NR | 105.3 | 0.5–5% w/w‡ | [59] |
| LDFO | Liposome | Phosphatidyl choline Cholesterol |
NR | 88 119 |
354 g/mole lipid 266 g/mole lipid |
[60] |
Only urine excretion tested, so primary route cannot be determined.
Approximated values calculated based on available data.
ULC-75 has comparable excretion in urine/feces, ULC-637 excreted slightly more in feces than urine.
Amount of iron measured in urine/feces but not amount of nanochelator.
DFO: Deferoxamine; EPL: ε-Polylysine; HD: Hydrodynamic diameter; HES: Hydroxy-ethyl starch; LDFO: Liposomal deferoxamine; NP: Nanoparticle; NR: Not reported; PEG: Poly(ethylene glycol); PLGA: Poly(lactic-co-glycolic acid); ULC: Ultra-long circulating.
Table 2. . Pharmacokinetic properties of various nanochelators.
| Nanochelator | Species tested | DFO equivalent dose (mg/kg) | % Urine/feces excretion (time point) | t1/2, α | t1/2, β | LD50† (mg/kg) | Ref. |
|---|---|---|---|---|---|---|---|
| Free DFO | Mouse | NR | NR | NR | 5.5 min | 250 | [49] |
| Dex-DFO | Mouse | NR | NR / NR | NR | 67 min | 4000 | [49] |
| HES-DFO | Mouse | NR | NR / NR | NR | 84 min | NR | [49] |
| HES-DFO | Human | 5.1 15.3 51.2 153 |
35–55 / NR (48 h) | NR | 22.2 / 89.8 h 22.1 / 85.6 h 23.3 / 84.4 h 32.5 / 108.8 h |
NA | [50] |
| S-DFO | Human | 39.5‡ 79‡ 158‡ 237 |
68.6 / NR (7 days) 64.8 / NR (7 days) 64.3 / NR (7 days) 62.3 / NR (7 days) |
NR | 85 h 135 h 139 h 141 h |
NA | [51] |
| ULC-75 ULC-637 |
Mouse | 19‡ 10‡ |
8.77 / 8.18 (6 days) 4.76 / 6.45 (6 days) |
0.0562 h 2.271 h |
16.148 h 44.392 h |
>560 | [52,53] |
| BGD-60 BDD-200 |
Mouse | 3.9‡ 5.1‡ |
10.5 / 39.8 (6 days) 22.6 / 25.1 (6 days) |
6.69 h 6.488 h |
64.3 h 7.93 h |
NR | [54] |
| hPR-DFO | Mouse | NR | NR / NR | NR | NR | NR | [55] |
| DFO2-EPL DFO4-EPL DFO8-EPL |
Mouse | 0.32‡ 0.61‡ 1.38‡ |
52.34 / <0.2 (4 h) 38.73 / <0.2 (4 h) 39.06 / <0.2 (4 h) |
2.65 min 2.44 min 5.66 min |
43.00 min 36.93 min 76.83 min |
>330‡ | [56] |
| NG1-DFO NG2-DFO |
Rat | NR | NR / NR | NA | 47.91 h 49.06 h |
NR | [57] |
| rNG-DFO | Mouse | NR | NR / NR | NR | NR | NR | [58] |
| DFO-NP | Mouse | 100 | NR / NR | 1.13 h | 48.63 h | NR | [59] |
| LDFO | Mouse | NR | NR / NR | NR | NR | NR | [60] |
As DFO equivalents.
Approximated values calculated from reported parameters.
DFO: Deferoxamine; HES: Hydroxy-ethyl starch; LDFO: Liposomal deferoxamine; NP: Nanoparticle; NR: Not reported; ULC: Ultra-long circulating.
Polymer conjugates
Starch deferoxamine
Early efforts to extend the half-life of DFO involved conjugation to carbohydrates. As a part of Biomedical Frontiers Inc., Hallaway et al. [49] first demonstrated this approach by conjugating DFO to both dextran and hydroxy-ethyl starch (HES). When tested in Swiss-Webster mice, the conjugates did not alter the amount of urinary iron excretion. They did, however, markedly enhance half-life of DFO. Dextran-DFO and HES-DFO both had half-lives of 60–90 min (Table 2), which is a significant increase over the very short half-life of native DFO in mice (5.5 min) [49]. Furthermore, conjugation to dextran increased the LD50 by nearly eightfold compared with native DFO (Table 2). Additional safety studies were conducted in mongrel dogs to specifically evaluate DFO-induced hypotension, which is a common side effect. Whereas free DFO caused significant hypotension, HES-DFO caused no change, and dextran-DFO elevated blood pressure slightly [49].
Continuing with the clinical development of a starch–DFO conjugate, Biomedical Frontiers Inc. reported on the evaluation of HES-DFO in a Phase Ib clinical trial [50]. In this study, HES-DFO was administered via 4-h iv. infusion over a range of doses from 5.1 to 153 mg/kg DFO equivalents. In addition to finding no serious adverse events in the study, the authors noted a striking absence of hypotension, which is an acute side effect of DFO infusion. This lack of infusion-associated hypotension allowed HES-DFO to be administered at an initial rate of 200 mg/kg/h, which is significantly faster than the 15 mg/kg/h limit for free DFO [50]. This enhanced administration rate would be very valuable in instances of acute iron poisoning [61] where there is a need to rapidly chelate large amounts of iron. Evaluation of the pharmacokinetic data from the study showed that HES-DFO had a biphasic elimination profile, with a first half-life of approximately 20–30 h and the second half-life of approximately 85–105 h (Table 2) [50]. This biphasic disposition profile is due to the polydispersity of the HES stock, whereby low molecular weight HES fractions contribute to a rapid initial elimination, and the higher molecular weight fractions persist. Additional evaluation of urinary iron excretion showed that HES-DFO treatment induced nearly twice as much excretion of iron as an equivalent dose of DFO. Furthermore, additional chelation capacity was present beyond 48 h, due to the continued presence of high molecular weight HES-DFO at 48 h. Even with these promising initial results, clinical development of HES-DFO did not continue.
Subsequently, Biomedical Frontiers Inc. reported on a Phase Ib clinical trial of a second drug candidate, starch-DFO (S-DFO) [51]. This study evaluated the safety, pharmacokinetics, and iron excretion of S-DFO in patients with transfusional iron overload associated with β-thalassemia. S-DFO was administered via iv. infusion for 1 h at a range of doses from 39.5 to 237 mg/kg DFO equivalents. In addition to causing no serious adverse events, conjugation of DFO to starch increased the half-life of DFO to approximately 135 h at higher doses (Table 2). Due to this extended half-life, the authors indicated that a single dose of S-DFO provided excess iron chelation capacity (i.e., more chelator than transfused iron is present) for 57–159 h in a dose-dependent fashion. This excess chelation capacity would counter the excess iron uptake from an estimated 3–5 days of transfusions, thus greatly reducing dose frequency [51]. However, the clinical development of a starch–DFO conjugate halted following this study, and the company pursuing this development no longer exists.
Polyrotaxane deferoxamine
The Xiong lab has reported on the development of biodegradable polyrotaxane-DFO conjugates (hPR-DFO), which are formulated by threading α-cyclodextrin (α-CD) onto PEG chains, and conjugating DFO to the α-CD [55]. Enzymatically cleavable end groups are attached to the ends of the PEG chains to keep the cyclodextrin rings in place and impart biodegradability. Following cleavage, the α-CD–DFO conjugates can leave the PEG group and be cleared more readily. The efficacy of the hPR-DFO was evaluated in an iron-dextran model of murine iron overload, following three doses of 150 mg/kg DFO equivalent administered every other day [55]. While free DFO treatment did not reduce serum ferritin, treatment with hPR-DFO substantially reduced serum ferritin to approximately 20–25% lower than treatment with free DFO or saline. With respect to iron excretion, free DFO and hPR-DFO increased urinary iron excretion nearly four-times and two-times that of saline, respectively, indicating decreased efficacy of hPR-DFO on iron excretion into urine. Since both the hPR–DFO conjugate (3.5 nm in HD) and the free α-CD-DFO degradation product are well under the 6–8 nm kidney filtration threshold [62], this is a surprising observation. Both free DFO and hPR-DFO showed similar amounts of fecal elimination (nearly twofold higher than saline), so the fate of the hPR–DFO conjugate is unclear [55]. Since the vast majority of alpha-CD is eliminated through urine in <24 h [63], further characterization of hPR-DFO disposition is needed to understand the efficiency of iron binding and excretion for the hPR-DFO construct.
ε-Polylysine-deferoxamine
Kang et al. [56] recently reported on renal selective nanochelators that are composed of DFO conjugated on ε-polylysine (EPL). DFO-EPL polymeric nanoparticles were formulated by controlling the number of DFOs and their surface charges, which resulted in overall HD less than the renal filtration threshold (6–8 nm), and proper surface properties for renal clearance (Table 1). Pharmacokinetic analysis showed that DFO–EPL conjugates extended the terminal half-life of DFO (Table 2) and essentially restricted distribution of the conjugate to the plasma and extracellular spaces. Furthermore, the DFO–EPL conjugates were rapidly cleared in the urine in a dose-dependent fashion (~40–80% dose within 4 h post-injection) with negligible hepatobiliary elimination (<0.2% injection dose). DFO–EPL conjugates were distributed rapidly in the kidney and bladder with negligible amounts in other organs [56].
Evaluation of therapeutic efficacy revealed a number of benefits compared with equivalent amounts of free DFO. In a dietary iron overload mouse model, subcutaneous administration of 5 or 10 mg/kg DFO equivalents (as DFO4-EPL and DFO8-EPL, respectively) twice daily for 5 days reduced serum iron to near baseline levels, and significantly reduced levels of iron and ferritin in liver [56]. Moreover, DFO conjugation approximately doubled the amount of iron excreted in the urine within 4 h. Given the rapid urinary clearance of the DFO conjugates, sc. administration was a key aspect of enhancing the efficacy by slowing the release of DFO-EPL into the blood stream and increasing the contact time to bind iron released from tissue stores [56]. The DFO–EPL conjugates were also evaluated in the Belgrade rat, which is an established genetic model of thalassemia-like iron overload anemia with liver iron loading [64,65]. Again, sc. administration of DFO conjugates enhanced excretion of iron in urine and reduced levels of iron and ferritin in liver [56].
DFO–EPL conjugates were also evaluated in both single and repeated dose acute toxicity studies [56]. A single dose acute toxicity study on DFO4-EPL showed that at the highest iv. dose of approximately 330 mg/kg DFO equivalents, DFO-EPL caused no visible adverse events in mice, whereas DFO alone at the LD50 immediately killed all mice. Furthermore, histological evaluation of organs in mice 14 days after exposure showed no abnormalities with the DFO-EPL treatment, whereas mice treated with DFO showed numerous markers of toxicity. Serum analysis of biomarkers showed that DFO-EPL at the highest dose caused less damage than free DFO at 16 and 79 mg/kg. In a repeated dose study, five daily subcutaneous doses of approximately 20 mg/kg DFO equivalents (as DFO8-EPL) were administered, and kidneys were evaluated by histopathology. Both DFO and DFO-EPL treatment reduced signs of iron overload-associated kidney injury. However, free DFO induced acute kidney injury as evidenced by glomerular tubularization, whereas there was no evidence of renal tubule injury in the DFO-EPL groups [56]. Further studies are needed to understand the dose amounts and frequencies required to reduce iron load to normal levels. Sustained release technologies could be applied to further enhance the half-life of DFO-EPL while maintaining rapid renal clearance.
Dendrimer conjugates
Hyper-branched polyglycerol-deferoxamine
The Kizhakkedathu lab initially reported on the development of iron-chelating dendrimers that were formulated by conjugating DFO to hyper-branched polyglycerol (HPG) [52,53]. Using this system, the HD and the number of DFO per polymer could be appropriately tuned by controlling the molecular weight of the HPG and the conjugation conditions (Table 1). Compared with free DFO, conjugation significantly increased the half-life in a size-dependent manner to 15–45 h (Table 2). These ultra-long circulating (ULC) DFO conjugates showed very limited urinary excretion over 1 week with <10% injected dose (ID) in a size-dependent manner, with larger conjugates showing the lowest extent of renal clearance (Table 2) [53]. This is an expected observation since all tested conjugates were larger than the renal filtration threshold [62]. Subsequent studies of the ULC–DFO conjugates confirmed the initial observations of low urinary clearance (Table 2) and showed that both smaller (ULC-75) and larger (ULC-637) conjugates underwent limited (<10%) hepatobiliary clearance and fecal elimination over 6 days [52]. The biodistribution data on ULC–DFO conjugates align with expected data for nanoparticles above the renal filtration threshold [66], namely steady accumulation in RES organs such as liver and spleen over the time course of the experiment.
Preliminary evaluation of efficacy in an iron-dextran model of murine iron overload showed a number of benefits compared with free DFO [53]. After administering 150 mg/kg DFO equivalents of both small and large ULC–DFO conjugates every other day for 10 days (i.e., five total doses), ULC-DFO substantially enhanced urinary iron excretion compared with free DFO. While ULC-DFO significantly decreased plasma ferritin and organ iron compared with DFO, the reduction in iron burden did not reach the levels in healthy controls [53]. In a subsequent study evaluating once weekly dosing of the larger conjugate ULC-637, 4 weeks of treatment caused a statistically significant reduction of iron in liver, heart and spleen compared with free DFO. However, the total iron values were still three to tenfold higher than normal values [52]. In both studies of ULC–DFO conjugates, acute toxicity evaluations demonstrated that conjugation of DFO to HPG increased the LD50 to >500 mg/kg DFO equivalent, which is a twofold increase over free DFO [52,53].
While conjugation extended DFO half-life and reduced DFO toxicity, the ULC–DFO conjugates did not return iron levels to normal. Since only <20% ID was cleared through urine or feces in 6 days, it is possible that iron-bound ULC–DFO conjugates were trapped in the body, suggesting a prolonged accumulation. In order to enhance excretion, the Kizhakkedathu lab introduced biodegradable ketal groups to the HPG technology to enable fragmentation of the ULC–DFO conjugates [54]. Using this approach, two conjugates were prepared, each having different degradation rates. Pharmacokinetic and biodistribution studies showed that both conjugates had extended half-lives compared with native DFO; however, the more rapidly degradable conjugate BDD-200 had a half-life nearly 1/10th that of the more stable conjugate BGD-60 (Table 2). Consistent with the differences in degradation rates, BGD-60 accumulated in organs to a greater extent than BDD-200. Interestingly, while twice as much BDD-200 as BGD-60 was renally excreted (Table 2), the conjugates had comparable total excretion levels (near 50% ID), due to much higher fecal elimination of BGD-60 than BDD-200 (Table 2). For both conjugates, the addition of biodegradable linkers enhanced the total amount of dose cleared from the body while still enhancing half-life compared with free DFO. Interestingly, when the efficacy of these formulations was compared with DFO, the DFO conjugates showed mixed results; while BGD-60 and free DFO caused a similar reduction in serum ferritin and an increase in urinary iron excretion, BDD-200 reduced serum ferritin and liver iron further than other treatments, however without increasing total iron excretion [54]. It would be important to characterize both the excretion efficiency (i.e., bound vs unbound fraction) of the conjugates, as well as excretion over longer time frames, in order to understand how to better refine the system.
Nanogel conjugates
Nanogel-deferoxamine
The Xiong lab has reported on the development of nanochelators that were formulated by preparing acrylate gel-based nanoparticles (nanogels; NG) and subsequently conjugating DFO (NG-DFO) [57]. Using this approach, two conjugates of different diameters and DFO loads were prepared (Table 1). Conjugating DFO to the NG constructs significantly enhanced the half-life of DFO in healthy rats to nearly 50 h (Table 2). Interestingly, both NG–DFO conjugates had similar half-lives (48 and 49 h) even though they significantly differed in diameter (36 and 101 nm). This is unexpected since nanoparticle half-life typically decreases as the size of the nanoparticle increases, however it could be accounted for if the increased size also increased PEG coverage [66]. NG–DFO conjugates gradually accumulated in liver, lung, and spleen over the first 48 h and showed a persistent signal in liver through 168 h [57]. This observation would be expected given that the size of the NG–DFO conjugates precludes them from being cleared through urinary excretion.
Preliminary evaluation of efficacy in an iron dextran-induced iron overload model showed therapeutic benefits of the NG1-DFO conjugate compared with free DFO in mice [57]. After three doses of 150 mg/kg DFO equivalent, free DFO showed no decrease in serum ferritin, whereas NG1-DFO decreased serum ferritin by nearly 50% compared with saline control. Both free DFO and NG1-DFO increased iron excretion in urine and feces by comparable amounts, which is interesting considering the difference in serum ferritin levels. Since NG1-DFO is too large to be renally excreted, it is surprising to see comparable levels of urinary iron excretion between NG-DFO and free DFO. However, it is possible some DFO was released from the conjugate, thus enabling passage through the kidney. It is also interesting to see negligible difference in fecal elimination between NG-DFO and free DFO, given that NG-DFO extensively accumulates in the liver. Based on the persistent presence of NG-DFO over the 168-h biodistribution study [57], it is likely that biliary clearance of NG-DFO proceeds over a longer time-frame than was sampled in the efficacy study.
Since NG-DFO treatment significantly decreased serum ferritin without an increase in iron excretion, it is possible that the NG–DFO conjugates bound a significantly larger amount of iron due to the long circulation time, but were then trapped within the body due to slow clearance. To address this issue, the Xiong lab refined the NG-DFO technology to enable biodegradation of the polymer [58]. Thioketal-diamine linkers were introduced, which are cleaved in the presence of the reactive oxygen species (ROS) often associated with iron overload. When assessed in vitro, the cleavable linkers caused the 240 nm rNG–DFO conjugate to degrade to <10 nm fragments. In vivo efficacy of the degradable rNG–DFO conjugate was evaluated by administering 150 mg/kg DFO equivalents for a total of five doses every other day in an iron-dextran model of murine iron overload. Similar to the prior work with NG-DFO, the rNG-DFO conjugate decreased serum ferritin by nearly 50% compared with free DFO, which only had a marginal decrease compared with saline treatment. Both free DFO and rNG-DFO increased urine iron excretion by nearly threefold compared with saline treatment, though there was no significant difference between free DFO and rNG-DFO. Including the ROS-cleavable bonds in the rNG–DFO conjugate did significantly enhance fecal iron elimination, with a nearly twofold increase over saline and an approximately 30% increase over free DFO [58]. Based on the enhanced fecal elimination of rNG-DFO compared with NG-DFO, it is very likely that intact rNG-DFO accumulated in the liver, where it was slowly hydrolyzed by iron-induced ROS and removed from the body by hepatobiliary clearance. Though it is possible that the rNG-DFO fragments could be cleared through urinary excretion, most of the degradation likely occurs in organ tissues where the majority of excess iron is located [10]. While a standalone safety study was not executed, the safety of rNG-DFO was evaluated based on gross anatomy and histopathology during the course of the efficacy study [58]. For all treatment conditions, there were no changes in bodyweight or organ weight. Subsequent histopathological evaluation of the treated organs showed no drug specific toxicities for free DFO or rNG-DFO [58].
Controlled release nanoparticles
Polymeric deferoxamine nanoparticles
Guo et al. [59] reported on the development of polymeric nanoparticles (Table 1) for the controlled release of DFO. The nanoparticle DFO formulation significantly increased the DFO half-life to nearly 50 h (Table 2), which indicates prolonged vascular retention of DFO due to controlled release from the nanoparticle. Encapsulation also altered the distribution of DFO, including significantly increased liver accumulation [59], which would be expected of nanoparticles of the 100 nm size range [66]. Interestingly, encapsulation also increased total kidney accumulation and changed the kinetics of accumulation [59]. This is likely a result of the steady release of DFO from DFO-NP into the plasma, which enabled a larger fraction of the dose to enter the kidney.
Efficacy was evaluated in three murine models of iron overload following iv. injection of 40 mg/kg DFO equivalents once a week for 4 weeks. In an iron-dextran model, DFO-NPs reduced liver iron nearly 50% over DFO, and increased urinary iron excretion by approximately twofold [59]. In both β-thalassemia and hemochromatosis models, DFO-NPs reduced iron in both liver and spleen by nearly 50%, whereas free DFO reduced it marginally. Samples from the iron-dextran mice showed no tissue damage based on histology and no inflammatory response based on serum cytokines. Future studies are required to evaluate the safety of the nanoparticle DFO formulation, since free DFO is associated with known toxicities [18].
Liposomal deferoxamine
Recently, there has been renewed interest in the development of liposomal DFO. As a part of ZoneOne Pharma, Tran et al. [60] reported on liposomal DFO (LDFO) at the 58th annual meeting of American Society of Hematology. In the abstract from their presentation, the authors claimed that LDFO formulations increased iron excretion into urine more than two- to three-times compared with free DFO in an iron dextran model of iron overload. Additionally, 20–30% of the dose was found in plasma after 24 h, which indicates that liposome encapsulation increased the half-life of DFO [60]. A detailed report with efficacy of iron removal from liver and spleen is expected from the research group.
Perspectives on the development of nanochelators
A review of the recent developments in the field of nanochelators raises a few points of interest. Of notable interest are the varied methodologies of handling free DFO as a control. Given that DFO has been proven efficacious in humans, evaluation of novel nanochelators should involve DFO treatments that recapitulate the known efficacy. Since DFO is severely limited by the short half-life, it is traditionally infused for 8–12 h or administered subcutaneously [15]. The majority of recent publications on DFO nanochelators used iv. injection of DFO as a control, both as an appropriate control for DFO-associated nanochelators and to avoid difficult infusions in rodents. This does, however, make clinically-relevant evaluation of novel treatments difficult since it is iv. infusion, and not injection, that provides free DFO with adequate chelation efficacy. To avoid technical difficulties in slow iv. infusions in rodents, repeat subcutaneous injections should instead be employed to ensure that free DFO is an appropriate comparator for novel treatments.
When comparing safety evaluations of novel chelators, methodological differences become clear. Since adverse effects limit the utility of free DFO by narrowing the therapeutic window, novel nanochelators should demonstrate a superior safety profile and expanded therapeutic window. The majority of studies covered in this review did not conduct standalone safety assessments, which would be an important next step for the development of these technologies. However, many of these studies did evaluate safety markers while studying nanochelator efficacy [54,55,58,59]. Interestingly, free DFO treatment did not consistently produce signs of toxicity, however it is possible that organ damage associated with the iron overload disease state [7] reduced sensitivity. One exception was the publication by Kang et al. [56], which clearly showed multiple mechanisms of toxicity for free DFO. This is likely a result of the repeat subcutaneous administrations, which better recapitulate the administration patterns in humans. Since the side effects of DFO can cause patient harm and reduce patient compliance, it is important to demonstrate that novel nanochelators ameliorate DFO-associated toxicity.
A review of nanochelators also shows that plasma half-life can clearly be modulated by tuning nanochelator size, which has enabled the development of chelators with half-lives approaching or exceeding 4 days. While the extended half-life can reduce dose frequency, there is a clear need to balance extended half-life with adequate nanochelator excretion. Since larger nano-therapeutics cannot be excreted in the urine and tend to accumulate in highly perfused macrophage-laden tissues [66], long circulating nanochelators may not increase the excretion of iron, even though they can bind more total iron. Substantial and persistent accumulation of nanochelators could cause hepatic toxicity, as has been observed for other nanomaterials [67]. Therefore, there would be a benefit of having nanochelators that undergo rapid excretion when bound to iron. The evolution of nanochelators toward this goal can be seen in the development of both the ULC-DFO [52–54] and NG-DFO [57,58] technologies. For both technologies, introduction of biodegradable bonds enhanced total clearance without sacrificing the extended half-life. Kang et al. [56] demonstrated an alternative approach which was to formulate small nanochelators that are rapidly and exclusively excreted through the urine. Since these renally clearable nanochelators had a relatively short half-life for nanochelators, subcutaneous administration was employed to extend the residence time in plasma, which significantly enhanced the efficiency of iron excretion (i.e., fraction of chelator bound to iron) compared with iv. injection. In this particular instance, the combination of slow, prolonged release from the subcutaneous space with rapid renal clearance showed a benefit over comparable treatment with free DFO [56].
Future perspective for the development of nanochelators
Future approaches to nanochelator development should consider the combination of sustained release technologies with nanochelators that have increased half-life and efficient clearance. Since iron needs to be mobilized from tissue stores, sustained release technologies can be used to release nanochelator into the plasma at a rate that adequately matches iron mobilization. Subcutaneous depot technologies can be used to achieve this goal. Toliyat et al. [68] reported on a similar approach with free DFO, where DepoFoam was used as a depot for the slow release of DFO. This approach increased both the duration and extent of urinary iron excretion by threefold [68]. Polymeric depot formulations would be suitable for nanochelators as they can readily control the rate of nanochelator diffusion within the subcutaneous space. Typically, the polymer molecular weight and concentration can be tuned to modulate nanochelator release rates. Injectable hydrogels are one such sustained release technology that can be applied to nanochelators. Injectable hydrogels can be formulated using thermo-sensitive polymers including poloxamers and cellulose derivatives [69], shear-thinning polymers such as hyaluronic acids [70], and other stimulus sensitive or inducible polymers [71]. Microparticles are an additional technology that can be used to form sustained release nanochelator depots. They can be formulated using biocompatible polymers to control the release of nanochelators [72]. Microparticles in the appropriate size range can be injected through needles [73] and can avoid clearance by the immune system [74]. The utility of microparticles may be limited by the amount of drug that can be solubilized within the core. Large volume subcutaneous (sc.) injection is an alternative approach that can be used to increase dose size and reduce dose frequency for nanochelators. This technology, developed by Halozyme Therapeutics, uses recombinant human hyaluronidase (rHuPH20) to enable subcutaneous administration of large volumes [75]. Indeed, 150 units of rHuPH20 enables administration of up to 1000 ml drug solution. rHuPH20 can be administered prior to the therapeutic or co-administered with the therapeutic. This approach is currently being used in three approved therapies: a once monthly dose of IgG (HyQvia), a subcutaneous dose of trastuzumab (Herceptin sc./Herceptin Hylecta), and a subcutaneous dose of rituximab (MabThera sc./Rituxan Hycela) [75].
Sustained release technologies could also be applied to facilitate oral dosing of nanochelators. It has been established that a variety of nanomaterials including nanoparticles, liposomes and dendrimers have reasonable oral bioavailability [76], which indicates the potential for development of oral nanochelator therapies. Nanomaterials have been shown to accumulate in the liver following lymphatic and nonlymphatic GI uptake [77], which could provide additional therapeutic benefits of nanochelators since the liver is a major iron storage organ. Furthermore, the degree of oral absorption depends heavily on the surface characteristics of the nanoparticle [78], which indicates nanochelator chemistry could be modulated to enhance uptake. Sustained release technologies can be used to enhance GI residence time of nanochelators and thus promote oral absorption. Mucoadhesives have been used to enhance the oral bioavailability of large molecules such as insulin [79] and nanoparticles [78] by adhering to GI walls and increasing the residence time of the therapeutics. Another approach to enhance GI residence time is currently under development at Lyndra Therapeutics. They have created an ultra-long acting dosage form that expands within the stomach and reduces gastric transit rate due to a star shaped geometry [80]. This approach has been used for once weekly antiretroviral therapy for HIV [81] and biweekly antimalarial therapy [82]. It is unclear how this specific technology would be applied to nanochelators and how stable nanochelators would be in gastric acids; however, this needs to be empirically explored.
Ultimately, there is great promise for the application of sustained release technologies to the treatment of iron overload with nanochelators. Release rate of nanochelator from depot systems should be optimized to balance the availability of nanochelator with the availability of chelatable iron in both circulation and tissues. Achieving this balance can significantly enhance efficacy while reducing dose frequency, both of which would have a significant impact on the quality of life of patients suffering from iron overload disorders.
Conclusion
Secondary iron overload is a serious condition that impacts patients with certain anemic hemoglobinopathies, including thalassemia and sickle cell disease. Repeated transfusions associated with managing these diseases lead to accumulation of excess iron and increases the risk of serious toxicities, including death from heart failure. While current chelation therapies are effective at managing iron overload associated disease, therapeutic efficacy is highly dependent upon compliance. This is problematic since both potentially severe side effects and often complex dosing schemes can contribute to low compliance with iron chelation therapy. Nanochelators are a promising technology for overcoming these limitations of existing iron chelation therapies. A number of novel delivery technologies have been applied to develop nanochelators that show efficacy in treating preclinical animal models of iron overload. Conjugation and controlled release are the main approaches that have been employed to turn existing chelators into nanochelators.
Recent studies on nanochelators demonstrate a clear relationship between nanochelator size and resulting changes in pharmacokinetic properties and biodistribution. Reducing nanochelator size near the renal filtration threshold can still increase half-life compared with free chelator, followed by rapid renal excretion. On the other hand, increasing nanochelator size generally increases half-life; however, this comes with a decrease in the rate and extent of excretion of the iron-chelator complex and potential accumulation in organs. In addition to nanochelator size, biodegradability has a large impact on pharmacokinetic properties. Biodegradable bonds can decrease nanochelator size in vivo, which decreases half-life and increases excretion compared with non-degradable constructs. In this context, biodegradability is favored to enhance iron excretion and ensure that nanochelators do not accumulate in the body. A lack of persistent accumulation is required to ensure nanochelators can be clinically translated. The chronic nature of secondary iron overload necessitates repeat dosing, and persistent nanoparticle accumulation can be associated with toxicities.
While there is great promise for nanochelators, there are some intrinsic limitations to current technologies that will need to be addressed in order to enable clinical translation. Of clear importance is striking a balance between the extension of chelator half-life and rapid clearance of iron-bound chelators. Extended half-life can be useful to reduce dose frequency and to increase the exposure of the nanochelator to chelatable iron. However, the iron-chelator complex should be rapidly removed from the body to minimize toxicities and adverse events associated with polymer-based chelators. At the same time, nanochelators should not be excreted too rapidly since this can decrease chelation efficiency by causing the excretion of iron-unbound chelators. Since only a small fraction of iron is chelatable, the nanochelator must stay present in the plasma to compensate for the time it takes for iron to mobilize from iron-loaded tissue to circulation.
Progress toward this goal is evident when reviewing recent developments in the field. Large nanochelators with half-lives over 24 h have had their excretion increased by introducing biodegradable linkers that induce nanochelator fragmentation. These technologies can be optimized to fine tune the balance between extended plasma half-life and improved excretion. This could be done by modulating the identity and distribution of degradable linkers in order to control rate of degradation and the size of the final degradants. In contrast to these large constructs, small nanochelators with half-lives of hours have been developed that have exclusive urinary excretion due to their size being under the renal filtration threshold. The rapid clearance can be counter-balanced by slowing the rate of nanochelator administration. This has been demonstrated using subcutaneous administration, and can be further developed by applying controlled release technologies to better modulate nanochelator delivery. Future studies should achieve a balance between longer circulation half-life and rapid excretion in order to develop nanochelator formulations that are viable candidates for clinical translation.
Executive summary.
Iron disorders
Iron is an essential metal for life; however, excess concentrations of iron are toxic.
A number of disorders can result in dysregulation of iron homeostasis, including hemochromatosis and hemoglobinopathies, such as β-thalassemia and sickle cell disease.
Secondary iron overload results from repeated transfusions associated with hemoglobinopathies, since there is no mechanism to excrete the extra iron from transfused blood.
Iron overload disorders are associated with a number of pathological outcomes, including liver dysfunction, diabetes and cardiovascular complications that include heart failure.
Clinically available iron chelators
Iron chelation therapy is the primary treatment for secondary iron overload.
Clinically approved therapies include deferoxamine (DFO), deferiprone (DFP) and deferasirox (DFX), all of which require daily administration.
DFO treatment can be associated with hypotension, local skin reactions, and ophthalmic and auditory toxicities.
DFP and DFX treatments increase the risk of infection and renal and hepatic injury, respectively.
Inconvenient dosing regimens and adverse events are both key drivers of patient compliance with approved chelators, which reduces therapeutic efficacy and enables disease progression.
Recent advances in nanochelator development
Nanochelators are an attractive approach to iron chelation therapy. Not only can nanochelators enhance the efficiency of iron chelation, but they can also overcome the two key drivers of low patient compliance.
The use of nanochelators can reduce dose frequency and alleviate adverse events by increasing chelator half-life and altering tissue distribution.
Following early efforts that lead to some clinical trials, the past decade has seen a renewed interest in the application of nanochelators to iron overload disorders. DFO-based nanochelators have been of particular interest, possibly due to the lower intrinsic toxicity of DFO than that of DFP or DFX.
A variety of DFO-based nanochelator technologies are reviewed, including linear and branched polymers, dendrimers, polyrotaxane, micelles, nanogels, polymeric nanoparticles and liposomes.
These nanochelators possess a range of pharmacokinetic, efficacy, and safety profiles, in part due to each modality's unique physicochemical properties. Indeed, hydrodynamic diameter and surface chemistry have a large impact on the plasma-half life and clearance route of nanochelators.
Perspectives on nanochelator development
There is a clear need for more standardized approaches to readily enable comparison of the numerous available technologies.
Efficacy and safety studies should demonstrate clinically relevant readouts in order to reflect their actual therapeutic usage.
Future perspective
Continued development of nanochelators should consider the combination of sustained release technologies with chelators in order to increase efficacy and safety.
The slow mobilization of iron from tissue stores would be most compatible with slow release of drug. Injectable hydrogels, microparticles, large volume subcutaneous dosing technologies, and sustained release oral technologies could all be applied to this end.
Footnotes
Financial & competing interests disclosure
This work was supported by the US NIH/NHLBI R01 HL143020 and American Heart Association (AHA) 17GRNT33460134. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Disclaimer
The content expressed is solely the responsibility of the authors and do not necessarily represent the official views of the NIH and AHA.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Ganz T. Systemic iron homeostasis. Physiol. Rev. 93(4), 1721–1741 (2013). [DOI] [PubMed] [Google Scholar]; • An excellent review on iron homeostasis.
- 2.Andrews NC, Schmidt PJ. Iron homeostasis. Ann. Rev. Physiol. 69(1), 69–85 (2007). [DOI] [PubMed] [Google Scholar]
- 3.De Rosa MC, Carelli Alinovi C, Galtieri A, Scatena R, Giardina B. The plasma membrane of erythrocytes plays a fundamental role in the transport of oxygen, carbon dioxide and nitric oxide and in the maintenance of the reduced state of the heme iron. Gene 398(1–2), 162–171 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Brown GC, Borutaite V. Inhibition of mitochondrial respiratory complex I by nitric oxide, peroxynitrite and S-nitrosothiols. Biochim. Biophys. Acta 1658(1–2), 44–49 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Gomez M, Perez-Gallardo RV, Sanchez LA. et al. Malfunctioning of the iron-sulfur cluster assembly machinery in Saccharomyces cerevisiae produces oxidative stress via an iron-dependent mechanism, causing dysfunction in respiratory complexes. PLoS ONE 9(10), e111585 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poulos TL. Heme enzyme structure and function. Chem. Rev. 114(7), 3919–3962 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohgo Y, Ikuta K, Ohtake T, Torimoto Y, Kato J. Body iron metabolism and pathophysiology of iron overload. Int. J. Hematol. 88(1), 7–15 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]; • An excellent review on pathology of iron overload disorder.
- 8.Murphy CJ, Oudit GY. Iron-overload cardiomyopathy: pathophysiology, diagnosis, and treatment. J. Card. Fail. 16(11), 888–900 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Origa R. β-Thalassemia. Genet. Med. 19(6), 609–619 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Pietrangelo A. Iron and the liver. Liver Int. 36(Suppl. 1), 116–123 (2016). [DOI] [PubMed] [Google Scholar]
- 11.De Sanctis V, Soliman AT, Elsedfy H. et al. Diabetes and glucose metabolism in thalassemia major: an update. Exp. Rev. Hematol. 9(4), 401–408 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Dixon SJ, Lemberg KM, Lamprecht MR. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149(5), 1060–1072 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fang X, Wang H, Han D. et al. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl Acad. Sci. USA 116(7), 2672–2680 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Cao F, Yin H-L. et al. Ferroptosis: past, present and future. Cell Death Dis. 11(2), 88–88 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalinowski DS, Richardson DR. The evolution of iron chelators for the treatment of iron overload disease and cancer. Pharmacol. Rev. 57(4), 547–583 (2005). [DOI] [PubMed] [Google Scholar]; • An excellent review on small molecule iron chelators, both novel and approved.
- 16.Kontoghiorghe CN, Kontoghiorghes GJ. Efficacy and safety of iron-chelation therapy with deferoxamine, deferiprone, and deferasirox for the treatment of iron-loaded patients with non-transfusion-dependent thalassemia syndromes. Drug Des. Devel. Ther. 10, 465–481 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mobarra N, Shanaki M, Ehteram H. et al. A review on iron chelators in treatment of iron overload syndromes. Int. J. Hematol. Oncol. Stem Cell Res. 10(4), 239–247 (2016). [PMC free article] [PubMed] [Google Scholar]
- 18.Poggiali E, Cassinerio E, Zanaboni L, Cappellini MD. An update on iron chelation therapy. Blood Transfus. 10(4), 411–422 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andjelković M, Van Camp J, De Meulenaer B. et al. Iron-chelation properties of phenolic acids bearing catechol and galloyl groups. Food Chem. 98(1), 23–31 (2006). [Google Scholar]
- 20.Hatcher HC, Singh RN, Torti FM, Torti SV. Synthetic and natural iron chelators: therapeutic potential and clinical use. Future Med. Chem. 1(9), 1643–1670 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohara T, Tomono Y, Boyi X, Yingfu S, Omori K, Matsukawa A. A novel, nontoxic iron chelator, super-polyphenol, effectively induces apoptosis in human cancer cell lines. Oncotarget 9(67), 32751–32760 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buss JL, Greene BT, Turner J, Torti FM, Torti SV. Iron chelators in cancer chemotherapy. Curr. Top. Med. Chem. 4(15), 1623–1635 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Jiao Y, Wilkinson JT, Di X. et al. Curcumin, a cancer chemopreventive and chemotherapeutic agent, is a biologically active iron chelator. Blood 113(2), 462–469 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chin D, Huebbe P, Frank J, Rimbach G, Pallauf K. Curcumin may impair iron status when fed to mice for six months. Redox Biol. 2, 563–569 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Badria FA, Ibrahim AS, Badria AF, Elmarakby AA. Curcumin attenuates iron accumulation and oxidative stress in the liver and spleen of chronic iron-overloaded rats. PLoS ONE 10(7), e0134156–e0134156 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prasad S, Tyagi AK, Aggarwal BB. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res. Treat. 46(1), 2–18 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bar-Am O, Amit T, Kupershmidt L. et al. Neuroprotective and neurorestorative activities of a novel iron chelator-brain selective monoamine oxidase-A/monoamine oxidase-B inhibitor in animal models of Parkinson's disease and aging. Neurobiol. Aging 36(3), 1529–1542 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Das B, Kandegedara A, Xu L. et al. A novel iron(II) preferring dopamine agonist chelator as potential symptomatic and neuroprotective therapeutic agent for Parkinson's disease. ACS Chem. Neurosci. 8(4), 723–730 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Ghosh B, Antonio T, Reith ME, Dutta AK. Discovery of 4-(4-(2-((5-Hydroxy-1,2,3,4-tetrahydronaphthalen-2-yl)(propyl)amino)ethyl)piperaz in-1-yl)quinolin-8-ol and its analogues as highly potent dopamine D2/D3 agonists and as iron chelator: in vivo activity indicates potential application in symptomatic and neuroprotective therapy for Parkinson's disease. J. Med. Chem. 53(5), 2114–2125 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang T, Sun Y, Li Y. et al. Growth inhibition of a novel iron chelator, DpdtC, against hepatoma carcinoma cell lines partly attributed to ferritinophagy-mediated lysosomal ROS generation. Oxid. Med. Cell. Longev. 2018, 4928703 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalinowski DS, Yu Y, Sharpe PC. et al. Design, synthesis, and characterization of novel iron chelators: structure-activity relationships of the 2-benzoylpyridine thiosemicarbazone series and their 3-nitrobenzoyl analogues as potent antitumor agents. J. Med. Chem. 50(15), 3716–3729 (2007). [DOI] [PubMed] [Google Scholar]
- 32.Mao F, Huang L, Luo Z. et al. O-Hydroxyl- or O-amino benzylamine-tacrine hybrids: multifunctional biometals chelators, antioxidants, and inhibitors of cholinesterase activity and amyloid-beta aggregation. Bioorg. Med. Chem. 20(19), 5884–5892 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Nunez MT, Chana-Cuevas P. New perspectives in iron chelation therapy for the treatment of neurodegenerative diseases. Pharmaceuticals (Basel) 11(4), 108 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Potuckova E, Hruskova K, Bures J. et al. Structure-activity relationships of novel salicylaldehyde isonicotinoyl hydrazone (SIH) analogs: iron chelation, anti-oxidant and cytotoxic properties. PLoS ONE 9(11), e112059 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weinreb O, Amit T, Bar-Am O, Youdim MBH. Neuroprotective effects of multifaceted hybrid agents targeting MAO, cholinesterase, iron and β-amyloid in ageing and Alzheimer's disease. Br. J. Pharmacol. 173(13), 2080–2094 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guilmette RA, Cerny EA, Rahman YE. Pharmacokinetics of the iron chelator desperrioxamine as affected by liposome encapsulation: potential in treatment of chronic hemosiderosis. Life Sci. 22(4), 313–320 (1978). [DOI] [PubMed] [Google Scholar]
- 37.Lau EH, Cerny EA, Rahman YE. Liposome-encapsulated desferrioxamine in experimental iron overload. Br. J. Haematol. 47(4), 505–518 (1981). [DOI] [PubMed] [Google Scholar]
- 38.Young SP, Baker E, Huehns ER. Liposome entrapped desferrioxamine and iron transporting ionophores: a new approach to iron chelation therapy. Br. J. Haematol. 41(3), 357–363 (1979). [DOI] [PubMed] [Google Scholar]
- 39.Ventola CL. Progress in nanomedicine: approved and investigational nanodrugs. PT 42(12), 742–755 (2017). [PMC free article] [PubMed] [Google Scholar]
- 40.Rossi NA, Mustafa I, Jackson JK. et al. In vitro chelating, cytotoxicity, and blood compatibility of degradable poly(ethylene glycol)-based macromolecular iron chelators. Biomaterials 30(4), 638–648 (2009). [DOI] [PubMed] [Google Scholar]
- 41.Tian M, Chen X, Gu Z. et al. Synthesis and evaluation of oxidation-responsive alginate-deferoxamine conjugates with increased stability and low toxicity. Carbohydr. Polym. 144, 522–530 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Zhou T, Chen K, Kong LM. et al. Synthesis, iron binding and antimicrobial properties of hexadentate 3-hydroxypyridinones-terminated dendrimers. Bioorg. Med. Chem. Lett. 28(14), 2504–2512 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Zhou T, Liu ZD, Neubert H, Kong XL, Ma YM, Hider RC. High affinity iron(III) scavenging by a novel hexadentate 3-hydroxypyridin-4-one-based dendrimer: synthesis and characterization. Bioorg. Med. Chem. Lett. 15(22), 5007–5011 (2005). [DOI] [PubMed] [Google Scholar]
- 44.Zhou T, Neubert H, Liu DY. et al. Iron binding dendrimers: a novel approach for the treatment of haemochromatosis. J. Med. Chem. 49(14), 4171–4182 (2006). [DOI] [PubMed] [Google Scholar]
- 45.Huang X, Lu D, Ma Y. et al. From small deferiprone to macromolecular micelles: self-assembly enhances iron chelation. J. Colloid Interface Sci. 533, 375–384 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Marzban A, Akbarzadeh A, Ardestani MS, Ardestani F, Akbari M. Synthesis of nano-niosomal deferoxamine and evaluation of its functional characteristics to apply as an iron-chelating agent. Canadian J. Chem. Eng. 96(1), 107–112 (2018). [Google Scholar]
- 47.Wang N, Jin X, Guo D, Tong G, Zhu X. Iron chelation nanoparticles with delayed saturation as an effective therapy for Parkinson disease. Biomacromolecules 18(2), 461–474 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Sulistyo H, Kurniawan DW, Rujito L. Biochemical and histopathological effects of green tea nanoparticles in ironized mouse model. Res. Pharm. Sci. 12(2), 99–106 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hallaway PE, Eaton JW, Panter SS, Hedlund BE. Modulation of deferoxamine toxicity and clearance by covalent attachment to biocompatible polymers. Proc. Natl Acad. Sci. USA 86(24), 10108–10112 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Seminal paper demonstrated safety and chelation benefits of nanochelators.
- 50.Dragsten PR, Hallaway PE, Hanson GJ, Berger AE, Bernard B, Hedlund BE. First human studies with a high-molecular-weight iron chelator. J. Lab. Clin. Med. 135(1), 57–65 (2000). [DOI] [PubMed] [Google Scholar]
- 51.Harmatz P, Grady RW, Dragsten P. et al. Phase Ib clinical trial of starch-conjugated deferoxamine (40SD02): a novel long-acting iron chelator. Br. J. Haematol. 138(3), 374–381 (2007). [DOI] [PubMed] [Google Scholar]
- 52.Hamilton JL, Imran Ul-Haq M, Abbina S. et al. In vivo efficacy, toxicity and biodistribution of ultra-long circulating desferrioxamine based polymeric iron chelator. Biomaterials 102, 58–71 (2016). [DOI] [PubMed] [Google Scholar]
- 53.Imran Ul-Haq M, Hamilton JL, Lai BF. et al. Design of long circulating nontoxic dendritic polymers for the removal of iron in vivo. ACS Nano 7(12), 10704–10716 (2013). [DOI] [PubMed] [Google Scholar]; • First report of dendrimer nanochelator demonstrating thorough characterization of pharmacokinetics and efficacy in iron overload.
- 54.Abbina S, Abbasi U, Gill A, Wong K, Kalathottukaren MT, Kizhakkedathu JN. Design of safe nanotherapeutics for the excretion of excess systemic toxic iron. ACS Cent. Sci. 5(5), 917–926 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Demonstrates beneficial changes to nanochelator efficacy and pharmacokinetic profile through introduction of biodegradability.
- 55.Liu Z, Lin TM, Purro M, Xiong MP. Enzymatically biodegradable polyrotaxane-deferoxamine conjugates for iron chelation. ACS Appl. Mater. Interfaces 8(39), 25788–25797 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kang H, Han M, Xue J. et al. Renal clearable nanochelators for iron overload therapy. Nat. Commun. 10(1), 5134 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • First report on a short-residing nanochelator that demonstrates renal-specific clearance and minimal biodistribution along with efficacy and safety in iron overload.
- 57.Wang Y, Liu Z, Lin TM, Chanana S, Xiong MP. Nanogel-DFO conjugates as a model to investigate pharmacokinetics, biodistribution, and iron chelation in vivo. Int. J. Pharm. 538(1–2), 79–86 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Z, Qiao J, Nagy T, Xiong MP. ROS-triggered degradable iron-chelating nanogels: safely improving iron elimination in vivo. J. Control. Rel. 283, 84–93 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; • First report of nanochelator with biodegradable linkers to reduce size and enhance clearance in iron overload mode.
- 59.Guo S, Liu G, Frazer DM. et al. Polymeric nanoparticles enhance the ability of deferoxamine to deplete hepatic and systemic iron. Nano Lett. 18(9), 5782–5790 (2018). [DOI] [PubMed] [Google Scholar]
- 60.Tran DT, Hayes ME, Noble CO, Dai Z, Working PK, Szoka FC. Twice monthly liposome encapsulated deferoxamine (LDFO) has a high molar efficiency in removing total body iron in an iron dextran-overloaded mouse model. Blood 128(22), 2322–2322 (2016). [Google Scholar]
- 61.Wright SW, Valento M, Mazor SS, Chen BC. Severe iron poisoning treated with prolonged deferoxamine infusion: a case report. Toxicol. Comm. 2(1), 6–9 (2018). [Google Scholar]
- 62.Choi HS, Liu W, Misra P. et al. Renal clearance of quantum dots. Nat. Biotechnol. 25(10), 1165–1170 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Ommen B, De Bie ATHJ, Bär A. Disposition of 14C-alpha-cyclodextrin in germ-free and conventional rats. Regul. Toxicol. Pharmacol. 39(Suppl. 1), 57–66 (2004). [DOI] [PubMed] [Google Scholar]
- 64.Sladic-Simic D, Martinovitch PN, Zivkovic N. et al. A thalassemia-like disorder in Belgrade laboratory rats. Ann. New York Acad. Sci. 165(1), 93–99 (1969). [DOI] [PubMed] [Google Scholar]
- 65.Thompson K, Molina RM, Brain JD, Wessling-Resnick M. Belgrade rats display liver iron loading. J. Nutrition 136(12), 3010–3014 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoshyar N, Gray S, Han H, Bao G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine (Lond.) 11(6), 673–692 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Y-N, Poon W, Tavares AJ, McGilvray ID, Chan WCW. Nanoparticle-liver interactions: cellular uptake and hepatobiliary elimination. J. Control. Rel. 240, 332–348 (2016). [DOI] [PubMed] [Google Scholar]
- 68.Toliyat T, Jorjani M, Khorasanirad Z. An extended-release formulation of desferrioxamine for subcutaneous administration. Drug Deliv. 16(7), 416–421 (2009). [DOI] [PubMed] [Google Scholar]
- 69.Bodratti AM, Alexandridis P. Amphiphilic block copolymers in drug delivery: advances in formulation structure and performance. Exp. Opin. Drug Deliv. 15(11), 1085–1104 (2018). [DOI] [PubMed] [Google Scholar]
- 70.Huang G, Huang H. Application of hyaluronic acid as carriers in drug delivery. Drug Deliv. 25(1), 766–772 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nguyen QV, Huynh DP, Park JH, Lee DS. Injectable polymeric hydrogels for the delivery of therapeutic agents: a review. Eur. Polymer J. 72, 602–619 (2015). [Google Scholar]
- 72.Han FY, Thurecht KJ, Whittaker AK, Smith MT. Bioerodable PLGA-based microparticles for producing sustained-release drug formulations and strategies for improving drug loading. Front. Pharmacol. 7, 185–185 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Whitaker MA, Langston P, Naylor A, Azzopardi BJ, Howdle SM. Particle size and shape effects in medical syringe needles: experiments and simulations for polymer microparticle injection. J. Mater. Sci. Mater. Med. 22(8), 1975–1983 (2011). [DOI] [PubMed] [Google Scholar]
- 74.Pacheco P, White D, Sulchek T. Effects of microparticle size and Fc density on macrophage phagocytosis. PLoS ONE 8(4), e60989 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Locke KW, Maneval DC, Labarre MJ. ENHANZE(®) drug delivery technology: a novel approach to subcutaneous administration using recombinant human hyaluronidase PH20. Drug Deliv. 26(1), 98–106 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Florence AT. Issues in oral nanoparticle drug carrier uptake and targeting. J. Drug Target. 12(2), 65–70 (2004). [DOI] [PubMed] [Google Scholar]
- 77.Hillery AM, Jani PU, Florence AT. Comparative, quantitative study of lymphoid and non-lymphoid uptake of 60 nm polystyrene particles. J. Drug Target. 2(2), 151–156 (1994). [DOI] [PubMed] [Google Scholar]
- 78.Pridgen EM, Alexis F, Farokhzad OC. Polymeric nanoparticle drug delivery technologies for oral delivery applications. Exp. Opin. Drug Deliv. 12(9), 1459–1473 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Banerjee A, Wong J, Gogoi R, Brown T, Mitragotri S. Intestinal micropatches for oral insulin delivery. J. Drug Target. 25(7), 608–615 (2017). [DOI] [PubMed] [Google Scholar]
- 80.Altreuter DH, Kirtane AR, Grant T, Kruger C, Traverso G, Bellinger AM. Changing the pill: developments toward the promise of an ultra-long-acting gastroretentive dosage form. Exp. Opin. Drug Deliv. 15(12), 1189–1198 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kirtane AR, Abouzid O, Minahan D. et al. Development of an oral once-weekly drug delivery system for HIV antiretroviral therapy. Nat. Comm. 9(1), 2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bellinger AM, Jafari M, Grant TM. et al. Oral, ultra-long-lasting drug delivery: application toward malaria elimination goals. Sci. Trans. Med. 8(365), 365ra157 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]