Abstract
This article comments on:
Sebastià Capó-Bauçà, Marcel Font-Carrascosa, Miquel Ribas-Carbó, Andrej Pavlovč and Jeroni Galmés, Biochemical and mesophyll diffusional limits to photosynthesis are determined by prey and root nutrient uptake in the carnivorous pitcher plant Nepenthes × ventrata, Annals of Botany, Volume 126, Issue 1, 29 June 2020, Pages 25–37, https://doi.org/10.1093/aob/mcaa041.
Keywords: Carnivorous plant, CO2 assimilation, photosynthesis, Nepenthes, nutrient stress, Rubisco
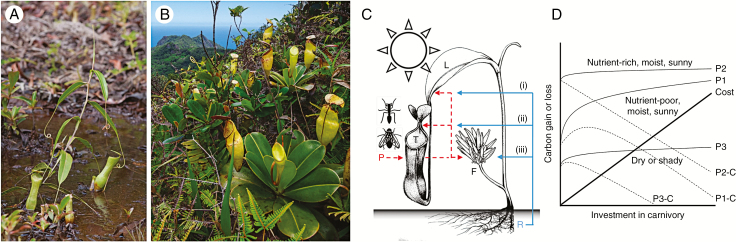
Carnivorous plants have inspired generations of scientists. About 800 species have evolved various forms of leaf-derived trap that attract, capture, retain, kill and digest animal prey, as a mode of survival in nutrient-poor environments. Since Charles Darwin provided the first evidence for carnivory in plants, the benefits of this life history mode have been well documented, including the enhancement of photosynthetic efficiency in response to nutrient uptake from prey. Givnish et al. (1984) presented a compelling cost–benefit model predicting that carnivory is an evolutionarily stable strategy in habitats where nutrients – in particular N – are limiting, but light and water are plentiful (Fig. 1A,D). According to the model (Fig. 1D), prey-derived N should boost the production of chlorophyll and Rubisco, thereby enhancing photosynthetic assimilation rates. While the photosynthesis-enhancing effects of prey intake are well documented and appear to be consistent across carnivorous lineages (Pavlovič and Saganová, 2015), the underlying physiological mechanisms remain poorly understood. In this issue of Annals of Botany, Capó-Baucà et al. (2020) present the most comprehensive investigation of how prey capture affects the photosynthetic apparatus in a carnivorous pitcher plant to date.
Fig. 1.
(A) Nepenthes gracilis pitcher plant growing in a typical sunny, wet, nutrient-poor habitat in northern Borneo. (B) Nepenthes pervillei showing green, chlorophyll-rich leaf laminae and yellow, chlorophyll-poor traps. (C) The complex interplay between the investment of soil-borne nutrients (blue line) from the roots (R), and prey-borne nutrients (red broken line) from the trap (T) into either (i) photosynthesis, especially by the photosynthetically efficient leaf lamina (L), (ii) trapping efficiency (of different forms of prey, P) by the photosynthetically inefficient pitcher trap and (iii) to the flowers/fruits (F), i.e. the plant’s reproductive potential. (C) A simplified cost–benefit model for carnivory showing the rate of increase in photosynthesis (P) under different conditions (1–3). Grey broken lines represent the net difference between photosynthetic benefit and cost (C) of obtaining nutrients by carnivory. The greatest rate (1) is in nutrient-poor, moist, sunny environments where the benefit of a small investment in carnivory exceeds its own cost. Adapted from Givnish et al. (2018).
The genus Nepenthes (Fig. 1A,B), which is the focus of the current study, is the most species-rich of the three major (unrelated) lineages of carnivorous pitcher plants, and is distributed predominantly in the Malay Archipelago. Despite growing interest in the genus as models for evolutionary research (Whitewoods et al., 2020) and natural blueprints for bioinspired technologies (Bushan, 2009), surprisingly few studies have addressed fundamental questions on the physiology of carnivorous plants. The few that have indicate that a complex interplay exists between nutrient quantity, source and mode of uptake on the one hand, and nutrient allocation and growth rate, photosynthetic efficiency, and reproductive success on the other (Fig. 1C) (Adamec and Pavlovič, 2018). Together, this body of work suggests a fundamental trade-off: leaves are modified into traps at the expense of photosynthetic efficiency because traits that make an effective insect trap are different from, and largely incompatible with, those that make an efficient light trap. As a result, traps contribute little to the, already comparatively low, photosynthetic performance of carnivorous plants (Fig. 1B). In pitcher plants this conundrum is solved either by spatial separation (insect and light-trapping functions in different parts of the leaf) or by temporal separation (the production of two distinct leaf types). A remarkable developmental plasticity enables pitcher plants to respond to nutrient availability by reallocating investment to photosynthetically active (non-carnivorous) parts of the plant when sufficient N is available (Ellison and Farnsworth, 2008). When N is limiting, investment is shifted towards the production of traps, thereby investing in enhanced nutrient acquisition through carnivory. Several studies have shown that the N-content of pitchers is significantly lower than that of photosynthetic organs (Ellison and Farnsworth, 2008; Osunkoya et al., 2008), and that this is at least partly due to the low Rubisco content of pitchers (Pavlovič and Saganová, 2015).
Together, this recent research corroborates the predictions of Givnish’s cost–benefit model, although the underlying physiological mechanisms remain elusive. In particular, it remains unclear what limits photosynthesis (and hence growth) in carnivorous plants: stomatal conductance, internal transport processes or the biochemical efficiency of the photosystems? In this issue, Capó-Bauçà et al. help to close this knowledge gap by quantifying photosynthesis in response to nutrient application, whether that is by adding insect prey to traps or mineral nutrients via the roots. In contrast to previous studies, they measure not only a suite of key photosynthesis parameters – gas exchange, chlorophyll fluorescence and photosynthesis-related leaf proteins together with the mineral composition and N and C isotopic discrimination of both leaves and prey insects – but also the effects of supplying four different types of prey, representative of the natural prey spectrum of the plants.
Capó-Bauçà et al. show for the first time that photosynthesis in nutrient-stressed N. × ventrata is limited by parenchymatic CO2 diffusion rather than by intrinsic biochemical efficiency. Remarkably, this limitation is lifted when sufficient nutrients are supplied, and biochemistry becomes the new limiting factor. The addition of nutrients from prey or mineral sources led to a general accumulation of leaf protein complexes involved in the photochemical assimilation process: chlorophyll, chlorophyll-binding proteins, components of the oxygen evolving complex and electron transport chain, as well as ATPase and Rubisco. The increase in photosynthetic rate was matched by enhanced growth rates after nutrient addition. In line with previous studies, Capó-Bauçà et al. observed a shift in biomass investment towards photosynthetic leaves (and away from traps) in root-fertilized treatments. Interestingly, they observed differences in N bioavailability among the four different types of insects fed to the pitchers, highlighting the importance of examining a range of prey species. Significantly, it remains unclear whether the observed difference in biomass allocation to vegetative organs was due to the nature of the nutrient source (insects or inorganic solution), or to the mode of administration (via the roots rather than the pitchers). An additional treatment with mineral fertilizer applied to the pitchers would address this question. As Capó-Bauçà et al. assert, the investment in carnivory is likely to be controlled by both the quantity and the form of nutrient input, highlighting the importance of robust experimental design when examining the effects of carnivory on plant physiology.
The last two decades have seen a step change in our understanding of the physiology of carnivorous plants in relation to nutrient acquisition. Capó-Bauçà et al. shed further light on the functional consequences of plant carnivory, which have received significantly less scientific attention than, for example, the mechanisms of prey capture and digestion. Laboratory experiments using commercial cultivars cannot capture the diversity of interactions in natural systems, for example among microclimate, prey diversity and pitcher-inhabiting microfauna. Nevertheless, the present study does provide new insights into the benefits of carnivory that form a key assumption of Givnish’s cost–benefit model. Future studies should attempt to study the interplay of nutrient intake and allocation on the one hand, and photosynthesis and growth on the other, in natural habitats. Furthermore, we need to explore how the physiological limitations identified by Capó-Bauçà et al. apply to other carnivorous plants such as sundews, Venus flytraps and bladderworts. Both construction and maintenance costs of traps vary considerably among species (Osunkoya et al., 2008; Pavlovič and Saganová, 2015), and photosynthesis in aquatic bladderworts is likely to be limited by CO2 availability rather than by nutrient supply (Adamec, 2008). When further species are added to the picture, along with data on their prey and nutrient assimilation in natural systems, the needle will move once again on our understanding of the effects of carnivory on plant physiology.
LITERATURE CITED
- Adamec L. 2008. Mineral nutrient relations in the aquatic carnivorous plant Utricularia australis and its investment in carnivory. Fundamental and Applied Limnology 171: 175–183. [Google Scholar]
- Adamec L, Pavlovič A. 2018. Mineral nutrition of terrestrial carnivorous plants. In: Ellison AM, Adamec L, eds. Carnivorous plants: physiology, ecology, and evolution. Oxford: Oxford University Press, 221–231. [Google Scholar]
- Bushan B. 2009. Biomimetics: lessons from nature – an overview. Philosophical Transactions of the Royal Society A 367: 1445–1486. [DOI] [PubMed] [Google Scholar]
- Capó-Bauçà S, Font-Carrascosa M, Ribas-Carbó M, Pavlovič A, Galmés J. 2020. Biochemical and mesophyll diffusional limits to photosynthesis are determined by prey and root nutrient uptake in the carnivorous pitcher plant Nepenthes × ventrata. Annals of Botany. 126. 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison AM, Farnsworth EJ. 2008. Prey availability directly affects physiology, growth, nutrient allocation and scaling relationships among leaf traits in 10 carnivorous plant species. Journal of Ecology 96: 213–221. [Google Scholar]
- Givnish TJ, Burkhardt EL, Happel RE, Weintraub JD. 1984. Carnivory in the bromeliad Brocchinia reducta, with a cost/benefit model for the general restriction of carnivorous plants to sunny, moist, nutrient-poor habitats. American Naturalist 124: 479–497. [Google Scholar]
- Givnish TJ, Sparks KW, Hunter SJ, Pavlovič A. 2018. Why are plants carnivorous? Cost/benefit analysis, whole-plant growth, and the context-specific advantages of botanical carnivory. In: Ellison AM, Adamec L, eds. Carnivorous plants: physiology, ecology, and evolution. Oxford: Oxford University Press, 233–255. [Google Scholar]
- Osunkoya OO, Daud SD, Wimmer FL. 2008. Longevity, lignin content and construction cost of the assimilatory organs of Nepenthes species. Annals of Botany 102: 845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovič A, Saganová M. 2015. A novel insight into the cost–benefit model for the evolution of botanical carnivory. Annals of Botany 115: 1075–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitewoods CD, Gonçalves B, Cheng J, et al. 2020. Evolution of carnivorous traps from planar leaves through simple shifts in gene expression. Science (New York, N.Y.) 367: 91–96. [DOI] [PubMed] [Google Scholar]