Abstract
Background and Aims
The stomatal conductance (gs) of most plant species decreases in response to elevated atmospheric CO2 concentration. This response could have a significant impact on plant water use in a future climate. However, the regulation of the CO2-induced stomatal closure response is not fully understood. Moreover, the potential genetic links between short-term (within minutes to hours) and long-term (within weeks to months) responses of gs to increased atmospheric CO2 have not been explored.
Methods
We used Arabidopsis thaliana recombinant inbred lines originating from accessions Col-0 (strong CO2 response) and C24 (weak CO2 response) to study short- and long-term controls of gs. Quantitative trait locus (QTL) mapping was used to identify loci controlling short- and long-term gs responses to elevated CO2, as well as other stomata-related traits.
Key Results
Short- and long-term stomatal responses to elevated CO2 were significantly correlated. Both short- and long-term responses were associated with a QTL at the end of chromosome 2. The location of this QTL was confirmed using near-isogenic lines and it was fine-mapped to a 410-kb region. The QTL did not correspond to any known gene involved in stomatal closure and had no effect on the responsiveness to abscisic acid. Additionally, we identified numerous other loci associated with stomatal regulation.
Conclusions
We identified and confirmed the effect of a strong QTL corresponding to a yet unknown regulator of stomatal closure in response to elevated CO2 concentration. The correlation between short- and long-term stomatal CO2 responses and the genetic link between these traits highlight the importance of understanding guard cell CO2 signalling to predict and manipulate plant water use in a world with increasing atmospheric CO2 concentration. This study demonstrates the power of using natural variation to unravel the genetic regulation of complex traits.
Keywords: Arabidopsis thaliana, C24, CO2 response, stomata, stomatal conductance, gs, stomatal regulation, QTL mapping, RIL, NIL, water-use efficiency, water economy
INTRODUCTION
Stomata are microscopic pores in the epidermis, surrounded by two guard cells that regulate their aperture by changes in turgor pressure. Almost all gas exchange between plants and the atmosphere occurs through the stomata, hence the stomatal aperture is regulated to balance the trade-off between CO2 uptake for photosynthesis and transpirational water loss. Elevated CO2 concentration induces partial closure of stomata in most plant species (Morison, 1998; Ruszala et al., 2011; Franks and Britton-Harper, 2016). This reduces transpirational water loss and improves leaf-level water economy. With a projected doubling of the atmospheric CO2 concentration within the next 100 years (IPCC, 2013), the stomatal CO2 response could have a significant impact on global plant water use under future climatic conditions. However, the magnitude of the stomatal CO2 response and hence the potential for water conservation under elevated CO2 exhibit a large variation among and within species (Morison, 1998; Takahashi et al., 2015; Hõrak et al., 2017). Significant variation in the stomatal CO2 response among different accessions of the model plant Arabidopsis thaliana (Takahashi et al., 2015) provides an excellent opportunity to explore its genetic basis, as indicated by the recent discovery of a novel CO2 signalling component using natural A. thaliana accessions (Jakobson et al., 2016). Knowledge about the genetic regulation of stomatal conductance (gs) in response to elevated CO2 could facilitate the improvement of crop water-use efficiency in a future climate.
The pathway for stomatal closure in response to elevated CO2 consists of one CO2-specific branch that converges downstream with the pathway for abscisic acid (ABA)-induced stomatal closure (Webb and Hetherington, 1997; Engineer et al., 2016). The CO2 response is initiated by the conversion of CO2 to bicarbonate by the carbonic anhydrases CA1 and CA4 in guard cells (Hu et al., 2010), resulting in the activation of the mitogen-activated protein kinases MPK4 and MPK12 by a yet undescribed mechanism (Marten et al., 2008; Hõrak et al., 2016). These two MPKs inhibit the protein kinase HT1 (Hashimoto et al., 2006; Hõrak et al., 2016; Jakobson et al., 2016). Downstream of the CO2-specific branch are the kinases OST1 and GHR1. The inhibition of HT1 by MPK4/MPK12 releases the inhibition of OST1 and GHR1 (Hõrak et al., 2016), which results in the activation of the anion channel SLAC1 (Negi et al., 2008; Vahisalu et al., 2008; Geiger et al., 2009; Hua et al., 2012) and other ion channels in the plasma and vacuolar membranes, leading to loss of turgor and stomatal closure (Kollist et al., 2014; Hedrich and Geiger, 2017; Jezek and Blatt, 2017). Recent research has identified the BIG protein as an additional component of the CO2-specific branch of the signalling pathway. Although the exact molecular function of BIG is unknown, it was shown to induce anion currents in response to elevated HCO3− concentration (He et al., 2018). The mechanism by which changes in CO2 and/or HCO3− concentration are sensed is currently unknown and it is likely that more components and interactions of the guard cell CO2 response pathway remain to be discovered.
The current understanding of genetic and molecular controls of the stomatal CO2 response is largely based on studies of the response to short-term fluctuations in CO2 concentration, i.e. the change in gs that occurs within minutes to hours after a change in the atmospheric CO2 concentration (Vahisalu et al., 2008; Engineer et al., 2016). It is, however, unclear whether the short-term responsiveness is a good predictor of long-term changes in gs of plants grown under elevated CO2 concentration, i.e. changes in gs that occur over weeks to months (Morison, 1998; Haworth et al., 2013). Long-term responsiveness might represent both changes in aperture and density as it entails development of new leaves. Moreover, the potential links between short- and long-term gs responses on a molecular level have not been explored. In a synthesis of data from free air CO2 enrichment (FACE) experiments on trees, Hasper et al. (2017) observed a correlation between short-term stomatal responsiveness to changes in the CO2 concentration and long-term reductions in gs of plants grown under elevated CO2. However, other studies have indicated that gs may acclimate to growth under elevated CO2 (Šantrůček and Sage, 1996; Morison, 1998; Lodge et al., 2001; Medlyn et al., 2001), possibly as a result of altered stomatal sensitivity to CO2 (Onandia et al., 2011; Haworth et al., 2013, 2016). In addition, short- and long-term stomatal responses may be decoupled in cases where plants respond to prolonged CO2 exposure by adjusting stomatal size or density rather than aperture (Haworth et al., 2013, 2015).
In this study, we investigated the genetic controls of both short-term (within an hour) and long-term (within a month) responses of gs to elevated atmospheric CO2 concentration in A. thaliana. We identified genetic loci associated with short- and long-term gs responses to elevated CO2, and with several other traits related to stomatal regulation. We found that a major quantitative trait locus (QTL) associated with the short-term response to CO2 was also involved in the long-term regulation of gs in response to growth in elevated CO2 concentration. This QTL was related neither to the ABA-induced stomatal closure pathway nor to any known genetic components of stomatal regulation.
MATERIALS AND METHODS
Plant material and growth conditions
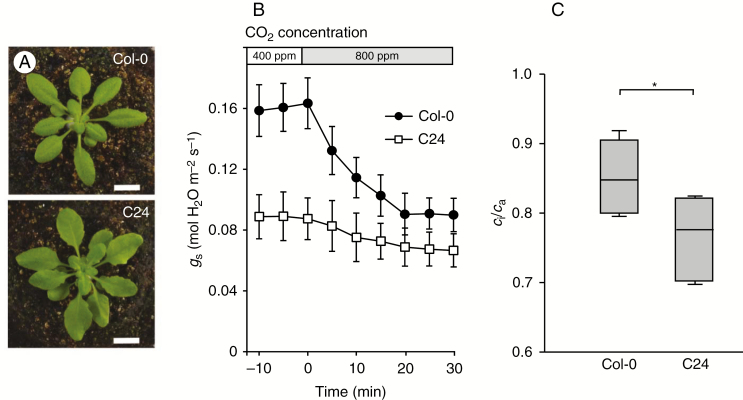
Recombinant inbred lines (RILs) originating from a reciprocal cross between the Arabidopsis thaliana accessions C24 and Col-0 (Törjék et al., 2006) were used in this study. These two accessions were selected based on a pilot study of the short-term stomatal response to elevated CO2 concentration among various A. thaliana accessions, where C24 was identified as a weak responder and Col-0 as a strong responder (Fig. 1). To confirm the location of a major QTL, we additionally used reciprocal near-isogenic lines (NILs) between C24 and Col-0 (Törjék et al., 2008).
Fig. 1.
Stomata-related traits of C24 and Col-0 grown in ambient CO2. (A) Plants grown side by side for 26 d in ambient CO2 concentration, 12:12 h photoperiod and a light intensity of 150 µmol photons m−2 s−1. Scale bar = 1 cm. (B) Short-term response of gs to elevation of CO2 concentration from 400 to 800 ppm during gas exchange measurements (n = 6, error bars show standard deviation). (C) Intrinsic water-use efficiency (iWUE) as measured by ci/ca, where low values represent high iWUE (n = 5). Boxes represent 25–75 % quartiles with the median as a horizontal line inside, and whiskers indicate the smallest and largest values. *P < 0.05, Welch’s t-test.
The following growth conditions were used for all plants except the RILs used in ABA response measurements and the NILs used for confirmation of a major QTL: seeds were sown on soil–perlite mix, stratified at 4 °C for 2 d and cultivated under short-day conditions (8 h light/16 h darkness; 22/18 °C) at ~60 % relative humidity and a photosynthetic photon flux density of 150–170 µmol photons m−2 s−1 in growth chambers (model AR-82L2/DE, Percival Scientific, Perry, IA, USA). Seedlings were transplanted to individual pots 2 weeks after germination. In the CO2 experiment, plants were grown in two separate, identical growth chambers (same as above) with contrasting CO2 concentrations. The ambient treatment had an average daytime CO2 concentration of 420 ppm and CO2 in the elevated treatment was maintained at an average daytime concentration of 820 ppm using a TKG-CO2-3011C CO2 control device (Tongdy Control Technology, Beijing, China). To avoid confounding effects of between- and within-chamber variation in environmental conditions, plants and CO2 treatment levels were shifted between the two growth chambers twice a week and trays with pots were rotated 180 °C.
Seeds used to generate plants for ABA response measurements and for confirmation of a major QTL were stratified in water for 2 d at 4 °C, sown on peat–vermiculite mix and grown through a hole in a glass plate covering the pot as described previously (Kollist et al., 2007) under short-day conditions (12 h light/12 h darkness, 23/20 °C) at 70 % relative humidity and a light intensity of 100–150 μmol m−2 s−1 in growth chambers (Microclima Arabidopsis MCA1600-3LP6-E, Snijders Scientific, Tilburg, the Netherlands).
Study design
The study comprised three experiments to investigate various aspects of stomatal regulation (as illustrated in Fig. 2).
Fig. 2.
Schematic overview of the experimental setup. Arabidopsis wild-type parental accessions Col-0 and C24, and recombinant inbred lines (RILs) and near-isogenic lines (NILs) originating from crosses between these accessions, were cultivated in the indicated conditions and subjected to measurement of gas exchange and carbon isotope ratios (not shown). Phenotypic data of RILs combined with genotype data of RILs were used for QTL mapping. Short-term stomatal response refers to a change in stomatal conductance within hours after the application of a stimulus, whereas the long-term response corresponds to the treatment effect when plants were grown in two different CO2 concentrations and measured at their respective growth concentration. The stomatal response to ABA was measured in a subset of RILs displaying the most extreme CO2 response phenotypes, to investigate whether a major CO2 response QTL was involved in the CO2-specific pathway for stomatal closure or in the downstream signalling pathway where CO2 and ABA responses converge. The short-term CO2 response of NILs was used to confirm the presence, location and effect of this major QTL.
QTL mapping of plants grown in ambient CO2
The initial QTL mapping experiment was designed to identify genetic loci associated with the short-term (within minutes to hours) response of gs to elevated CO2, with absolute gs at ambient and elevated CO2, and with the ratio of mole fractions of CO2 in the substomatal cavity and ambient air, ci/ca. The latter is a proxy for intrinsic water-use efficiency (iWUE), where low values represent high iWUE. We selected a subset of 100 RILs that displayed the largest number of chromosomal crossovers in the population, in order to maximize genetic variation (Supplementary Data Table S1). Of these RILs, 51 originated from a cross using C24 as pollen donor and Col-0 as pollen acceptor, and 49 where Col-0 was used as pollen donor to C24, to account for potential cytoplasmic effects. The RILs and their parental accessions were grown at ambient CO2 concentration. The short-term CO2 response, absolute gs, and ci/ca were quantified and these data were used for QTL mapping. Fine mapping of a major QTL controlling the short-term gs response to elevated CO2 was performed using additional RILs with crossovers in the region of interest and the location and the effect of the QTL was confirmed using NILs.
Long-term CO2 experiment
The aim of this experiment was to study the effects of growth in elevated CO2 on stomatal regulation in A. thaliana. Specifically, we wanted to (1) investigate whether growth in elevated CO2 concentration affected the short-term (within minutes to hours) CO2 responsiveness and absolute gs, as well as the detection of QTLs associated with CO2 responsiveness, and (2) map loci associated with the long-term (within weeks to months) response to elevated CO2. We used 50 RILs from the cross where C24 was the pollen donor and Col-0 the acceptor, which had been used in the previous experiment. The RILs were grown together with their parental accessions in ambient or elevated CO2 in two separate treatments for 4 weeks, which constitutes a large proportion of the A. thaliana life cycle (Boyes et al., 2001). Data on short-term CO2 response and absolute gs of plants from both CO2 treatments were used for QTL mapping, as well as data on the long-term gs response to elevated CO2.
ABA experiment.
The stomatal response to exogenously applied ABA was measured in ten RILs that showed the five strongest and the five weakest CO2 responses in the first experiment. The aim of the ABA experiment was to investigate the relationship between CO2- and ABA-induced stomatal closure in these lines.
Gas exchange measurements
In the first two experiments, gas exchange measurements on entire leaf rosettes of 4-week-old plants were conducted using two LI6400 systems (LI-COR Biosciences, Lincoln, NE, USA) fitted with 6400-17 Whole Plant Arabidopsis Chambers. Self-shading within rosettes was minimal at this growth stage. Leaf temperature was estimated using energy balance calculations (LI-COR Biosciences, 2011). The boundary layer conductance was estimated using a model of a leaf rosette made from filter paper, which was soaked in water that was allowed to evaporate inside the whole plant chamber. The boundary layer conductance was estimated to be 4 mol H2O m−2 s−1. The stomatal ratio (the ratio of gs on the leaf sides with lowest versus highest values) was assumed to be 0.5, which is recommended when the exact ratio is not known (LI-COR Biosciences, 2011). Fluxes of water vapour from the soil were prevented by covering the soil using household cling film. To test for the influence of water vapour exiting through the tiny gap in the plastic film surrounding the stem, we conducted measurements after cutting the plant rosette above the plastic film. The false conductance measured was <0.002 mol H2O m−2 s−1 (assuming a leaf area of 10 cm2) and was considered negligible.
Gas exchange measurements in the two first experiments were conducted at constant light (same as growth conditions) and temperature (22 °C). The vapour pressure deficit (VPD) of the air was set to a target value within the range 1 ± 0.2 kPa and was kept within ±0.03 kPa of the initial VPD throughout the measurement of a plant. The CO2 concentration was kept at 400 ppm until gs reached steady state (<2.5 % change in conductance over 5 min). When steady state had been reached, three measurements with 10 s between them were logged, after which the CO2 concentration was elevated to 800 ppm and the same procedure was repeated. Plant leaves were imaged using a flatbed scanner, leaf areas were calculated using the ROI manager function in ImageJ (version 1.48v, Schneider et al., 2012) and the conductance values were re-calculated to be expressed per unit leaf area. The percentage reduction in gs following elevation of the CO2 concentration was used as a measure of the short-term stomatal response to elevated CO2. In the CO2 experiment, we additionally calculated the long-term response to growth in elevated CO2 concentration. For the same genotype, we used gs values of plants from the two treatments measured at their respective growth CO2 concentration to calculate the percentage decrease in stomatal conductance resulting from growth in elevated CO2.
For the ABA response experiment and for the confirmation of a major QTL using NILs, gas exchange measurements were conducted on 25- to 30-d-old plants using a custom-made gas exchange system (Kollist et al., 2007). We quantified the percentage gs decrease in response to elevated CO2 (~800 ppm) in both experiments and in the ABA experiment also to spray application of 5 µm ABA solution (containing 0.012 % Silwet and 0.05 % ethanol). Measurements were conducted at a light intensity of 100–150 µmol photons m−2 s−1 and a temperature of 23–25 °C. Stomatal conductance was allowed to stabilize at ambient CO2 concentration and 65–70 % relative humidity for ~40 min before the stimulus was applied. The stomatal response was calculated as the percentage gs decrease 28 min after application of the stimulus. Leaf areas were measured using the polygon tool in ImageJ (version 1.48v, Schneider et al., 2012) on photographs of intact leaf rosettes.
Stable isotope analyses
The ratio of mole fractions of CO2 in the substomatal cavity (ci) and ambient air (ca), ci/ca, was used as a proxy for iWUE, where low ci/ca corresponds to a high iWUE (Condon et al., 2004; Pérez-Harguindeguy et al., 2013) as shown by the relationship:
A time-integrated measure of ci/ca was determined by analysing leaf stable carbon isotope composition. Leaves were dried for at least 24 h at 70 °C and homogenized with a pestle. The material (~1 mg per sample) was weighed into tin capsules and analysed for stable carbon isotope ratios using a PDZ Europa ANCA-GSL elemental analyser interfaced to a PDZ Europa 20-20 isotope ratio mass spectrometer (IRMS; Sercon, Crewe, UK) at the UC Davis Stable Isotope Facility, Davis, CA, USA. The photosynthetic 13C discrimination (Δ) was calculated from δ 13C values according to the following equation:
For this calculation we assumed a value of −8.44 ‰ for δ 13Cair, based on the average δ 13C ratio of CO2 in air measured at the Mauna Loa Observatory, HI, USA during 2014 (data downloaded from https://www.esrl.noaa.gov, Keeling et al., 2001). This value likely differed slightly from that in our experiment, but as the isotope data were only used to compare plants within the same experiment this error was considered negligible. The 13C discrimination was then used for the calculation of ci/ca as follows:
where a is the isotopic fractionation caused by diffusion (4.4 ‰) and b is the fractionation caused by carboxylation by Rubisco (27 ‰) (Farquhar et al., 1989). It should be noted that the above equations follow the simplified format presented by Farquhar et al. (1989), where ‰ is considered equivalent to 10–3; hence, all ‰ values were multiplied by 0.001 in our calculations.
Genotyping
The RIL population had previously been genotyped using SNP markers, as described by Törjék et al. (2003). The lines used in this study were partially re-genotyped in generations F9/F10 to confirm or correct double crossovers and to remove heterozygous regions. For this purpose, the SNaPshot® Multiplex System (Applied Biosystems, Waltham, MA, USA) was used according to the manufacturer’s protocol on an ABI 3730 Sequencer (Applied Biosystems). Peaks were identified using GeneMapper® (version 4.0, Applied Biosystems). In addition, simple sequence length polymorphism (SSLP) markers from the MSAT database (http://www7.inra.fr/vast/msat.php) were added to allow comparison with other A. thaliana RIL populations and single-feature polymorphism (SFP) markers were extracted from ATH1 GeneChip® (Affymetrix, Waltham, MA, USA) data as described by Schmidt et al. (2017). The SSLP fragments were PCR-amplified from genomic DNA and visualized on agarose gels. For large fragments and/or size differences above 10 bp, 1–2 % agarose gels (Carl Roth, Karlsruhe, Germany) were used. For smaller size differences, a 1:3 mixture of 4 % agarose/MetaPhor™ agarose (Lonza Group, Basel, Switzerland) was used. Fragment size was identified by comparison with the Generuler™ 1 kb Plus DNA Ladder (Thermo Fisher Scientific, Waltham, MA, USA) and genotypes were scored manually. Genetic maps for the two subsets of lines analysed in this study were constructed using the package R/qtl (version 1.41-6) in R (version 3.4.3) with the Kosambi mapping function (Broman et al., 2003). The NILs had been genotyped using the same set of SNP markers as the RILs, as described by Törjék et al. (2008).
For fine mapping of the major QTL on chromosome 2, 42 RILs with crossovers in the region of interest were used. Genotyping was performed using nine SSLP markers (Supplementary Data Table S2) in the QTL region following the methodology of Nilsson et al. (2016). Annealing temperatures were optimized for each primer pair using gradient PCR. PCR products were visualized on 3 % agarose gels (Seakem® LE, Lonza Group, Basel, Switzerland) and genotypes scored manually.
In order to confirm the lack of sequence variation in the MPK12 gene between C24 and Col-0 in publicly available sequence data (Berardini et al., 2015; Alonso-Blanco et al., 2016), a genomic fragment consisting of the coding region and 0.5-kb flanking region on both sides was PCR amplified from C24 using three sets of primers (Supplementary Data Table S2) and AccuPrime™ Pfx polymerase (Thermo Fisher Scientific, Waltham, MA, USA) to produce three overlapping fragments. The products were sequenced (Eurofins Genomics, Ebersberg, Germany) using the same set of primers, with an additional sequencing primer for one of the products (Supplementary Data Table S2).
Data analysis
Differences in the short-term CO2 response and absolute gs of accessions C24 and Col-0 were tested using Welch’s t-test. Welch’s t-test was also used to test for cytoplasmic effects by comparing trait averages from the reciprocal crosses. Differences in short-term response and absolute gs between RILs grown in ambient and elevated CO2 treatments were tested using the paired t-test. The relationship between short- and long-term stomatal CO2 responses was tested using linear regression and the paired t-test was used to test for a difference in magnitude of these responses. One-way ANOVA with Tukey’s post hoc test was used to test for differences between NILs and parental accessions. All statistical tests were performed with α = 0.05 using JMP (version 12.0.1, SAS Institute, Cary, NC, USA).
For QTL mapping, data from both crosses were analysed together as no significant cytoplasmic effect on any of the traits had been detected. Data from the first experiment, where 100 RILs were grown in ambient CO2, and the second experiment, where 50 RILs were grown in two CO2 treatments (ambient and elevated), were analysed separately. To increase mapping power and enable the identification of QTLs with pleiotropic effects, we used multi-trait analysis combining all phenotype data from each experiment. A step size of 10 cM, minimum cofactor proximity of 50 cM, a minimum separation of selected QTLs of 30 cM and a threshold of –log10P = 3.2 (based on Li and Ji, 2005) were used for QTL analysis. First, the whole genome was scanned for significant polymorphisms using simple interval mapping. Then, based on the selected cofactors, two rounds of composite interval mapping were run. Thereafter, a final QTL model was selected using backward selection on the selected cofactors, where the allelic effect and explained phenotypic variance of each QTL were estimated for each trait. All QTL analyses were performed in GenStat for Windows (16th edition, VSN International, Hemel Hempstead, UK).
RESULTS
Stomatal regulation of parental accessions
We observed significantly weaker short-term stomatal CO2 response, i.e. the percentage decrease in stomatal conductance (gs) following a doubling of the CO2 concentration (Welch’s t-test, P = 0.01, n = 6), as well as lower absolute gs at both 400 and 800 ppm CO2 of C24 compared with Col-0 (Welch’s t-test, P < 0.001 and P = 0.002, respectively, n = 6; Fig. 1B), confirming the results of the pilot study. Furthermore, C24 demonstrated a significantly lower ci/ca than Col-0, showing that C24 had a higher intrinsic water-use efficiency (iWUE) than Col-0 (Welch’s t-test, P = 0.043, n = 5; Fig. 1C). In summary, C24 generally has lower stomatal conductance and thus a more conservative regulation of transpirational water loss but at the same time its stomata are less responsive to increased CO2 concentration.
QTL mapping of stomatal regulation in 100 RILs grown in ambient CO2
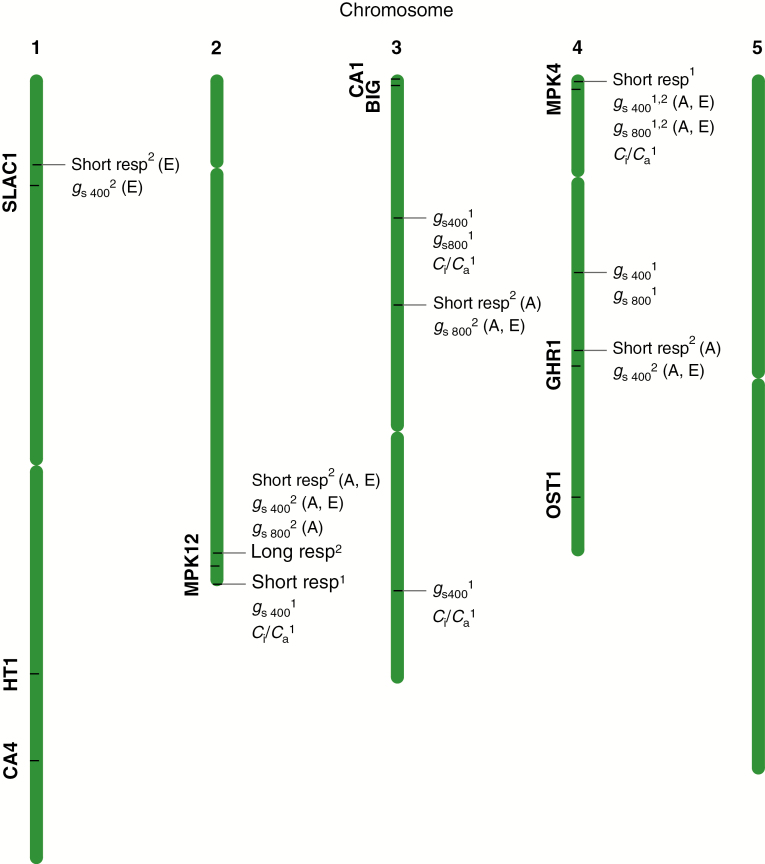
To investigate the genetic basis for the variation in stomatal regulation between Col-0 and C24, we quantified several stomata-related traits among 100 RILs originating from a reciprocal cross between these accessions and used these data for QTL mapping. The short-term gs response to elevated CO2 ranged from 13 to 64 % among the RILs (Supplementary Data Fig. S1, Table S3). The RILs also displayed a wide range in absolute gs (gs_400 0.062–0.174 mol m−2 s−1, gs_800 0.038–0.122 mol m−2 s−1) and ci/ca (0.668–0.905) (Supplementary Data Fig. S1, Tables S3 and S4). We detected a major QTL for the short-term CO2 response on chromosome 2, explaining 51 % of the variation in this trait. The Col-0 allele at this QTL conferred a stronger CO2 response (Table 1). An additional, minor QTL (explaining 3 % of the variation) for the short-term CO2 response was mapped to chromosome 4 (Fig. 3, Table 1). For this QTL, C24 was instead the high-value allele (Table 1).
Table 1.
QTLs detected using data from measurements of gas exchange and stable isotope composition of recombinant inbred lines grown in ambient CO2
| Trait | QTL location and confidence interval (cM) | Chromo-some | Closest marker | Variance explained (%) | High-value allele |
|---|---|---|---|---|---|
| Short-term CO2 response | 110 (105–110) | 2 | MASC02812/MSAT2.22 | 51 | Col-0 |
| g s_400 | 110 (105–110) | 2 | MASC02812/MSAT2.22 | 19 | Col-0 |
| c i/ca | 110 (105–110) | 2 | MASC02812/MSAT2.22 | 13 | Col-0 |
| g s_400 | 23 (0–108) | 3 | MASC04608 | 4 | Col-0 |
| g s_800 | 23 (0–108) | 3 | MASC04608 | 9 | Col-0 |
| c i/ca | 23 (0–108) | 3 | MASC04608 | 9 | Col-0 |
| g s_400 | 94 (0–108) | 3 | MASC03218 | 6 | Col-0 |
| c i/ca | 94 (0–108) | 3 | MASC03218 | 4 | Col-0 |
| Short-term CO2 response | 5 (0–35) | 4 | FRI | 3 | C24 |
| g s_400 | 5 (0–35) | 4 | FRI | 3 | Col-0 |
| g s_800 | 5 (0–35) | 4 | FRI | 14 | Col-0 |
| c i/ca | 5 (0–35) | 4 | FRI | 4 | Col-0 |
| g s_400 | 52 (6–92) | 4 | MASC09213 | 7 | C24 |
| g s_800 | 52 (6–92) | 4 | MASC09213 | 12 | C24 |
Fig. 3.
Chromosomal positions of all QTLs detected in this study and of known components of the stomatal CO2 response pathway. 1Detected in the first experiment with 100 recombinant inbred lines (RILs) grown in ambient CO2 concentration. 2Detected in the second experiment with 50 RILs grown in two CO2 treatments: ambient (A) and elevated (E) CO2. Short resp, short-term response; Long resp, long-term response.
Five QTLs related to absolute gs were detected, of which three were associated with gs at both 400 and 800 ppm CO2, and two were associated with gs only at 400 ppm (Fig. 3, Table 1). The amount of variation explained by these QTLs ranged from 3 to 19 %. Notably, the strongest QTL for gs measured at 400 ppm mapped to the same marker as the short-term CO2 response. The high-value allele was Col-0 for most of the QTLs regulating gs (Table 1), consistent with the higher gs of the Col-0 parental accession. Furthermore, four QTLs for ci/ca were detected (Fig. 3, Table 1). These QTLs explained 4–13 % of the variation in this trait and the high-value allele was Col-0 in all cases, consistent with the observation of lower iWUE in Col-0. The strongest QTL for ci/ca also mapped to the same locus as the strongest QTL for short-term CO2 response (Table 1).
Effects of long-term growth in elevated CO2
We next sought to investigate the relationship between the control of short- and long-term gs responses. To this end, 50 RILs from the cross where C24 was the pollen donor and Col-0 the pollen acceptor, which had been used in the previous experiment, were cultivated in ambient (~400 ppm) and elevated (~800 ppm) CO2 concentrations. After 4 weeks of the respective treatment, gas exchange measurements were conducted. The short-term response was calculated as the percentage decrease in gs between 400 and 800 ppm measured in sequence for each individual. The long-term response was calculated as the percentage decrease in gs resulting from growth in elevated CO2, i.e. for the same genotype we used gs values of plants from the two treatments measured at their respective growth CO2 concentration.
Among the tested RILs, growth in elevated CO2 concentration resulted in an average gs reduction of 26 % (paired t-test, P < 0.0001, n = 50; Fig. 4A, Supplementary Data Table S4). When the gs of plants grown in ambient and elevated CO2 was measured at the same CO2 concentration, plants from the elevated treatment generally displayed higher gs than plants from the ambient treatment. On average, plants from the elevated treatment had 11 % higher gs than plants from the ambient treatment when measured at 400 ppm and 20 % higher gs when measured at 800 ppm (paired t-test of gs_400, P = 0.004, n = 50; paired t-test of gs_800, P < 0.0001, n = 50; Fig. 4A, Supplementary Data Table S4), indicating gs acclimation of plants grown in elevated CO2.
Fig. 4.
Stomatal conductance (A) and short-term CO2 response (B) of 50 recombinant inbred lines grown in ambient and elevated CO2. Gas exchange was measured at 400 and 800 ppm CO2 in sequence. The short-term CO2 response was calculated as the percentage decrease in gs after elevation of the CO2 concentration from 400 to 800 ppm during gas exchange measurements. Boxes represent 25–75 % quartiles with the median as a horizontal line inside, and whiskers indicate the smallest and largest values. *P < 0.05, paired t-test (n = 50); note that the significant long-term response, i.e. paired t-test comparing gs of plants from the two CO2 treatments when measured at their respective growth CO2 concentration (P < 0.0001, n = 50), is not indicated in panel (A).
Growth under elevated CO2 concentration had a small but statistically significant effect on the short-term CO2 response (paired t-test, P = 0.0004, n = 50). RILs grown in ambient CO2 concentration showed an average gs decrease of 39 % in response to short-term elevation of the CO2 concentration, whereas the average short-term gs response of RILs grown in elevated CO2 was 34 % (Fig. 4B, Supplementary Data Table S4), indicative of a slight decrease in CO2 sensitivity of plants grown in elevated CO2.
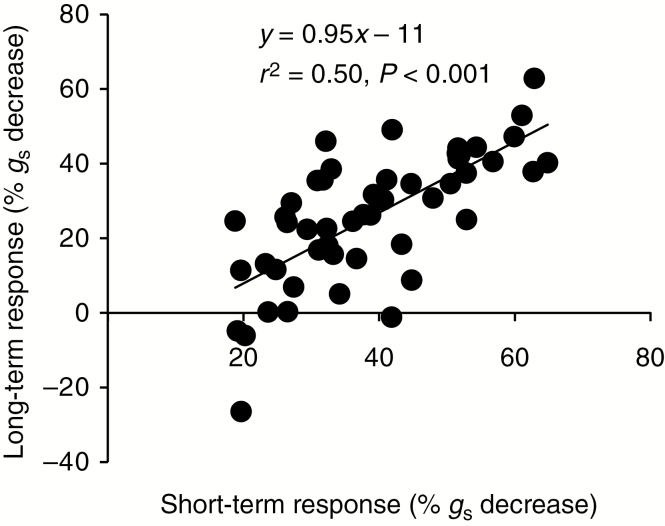
To test whether the short-term response could be used as a predictor of the long-term response, the short-term response of each genotype grown in ambient CO2 concentration was compared with the long-term response of the same genotype (Fig. 5). This showed a significant linear relationship between the responses (linear regression P < 0.0001, r2 = 0.53, F1,48 = 48.4). The long-term response was, however, significantly weaker than the short-term response (26 % versus 39 % gs decrease; paired t-test, P < 0.001, n = 50).
Fig. 5.
Long-term versus short-term stomatal response to elevated CO2 of 50 recombinant inbred lines cultivated in ambient or elevated CO2. Plants were grown in the respective CO2 treatments for 4 weeks and gas exchange was measured for each line and treatment at 400 and 800 ppm CO2 in sequence. The short-term CO2 response of each line was calculated as the percentage decrease in gs upon short-term elevation of the CO2 concentration of plants grown in ambient CO2. The long-term response of each line represents the percentage gs decrease in the elevated compared with the ambient treatment when plants were measured at their respective growth CO2 concentration. There was a significant linear relationship between the long- and short-term responses, but the long-term response was significantly weaker than the short-term response (paired t-test, P < 0.001, n = 50).
QTL mapping of stomatal regulation following long-term cultivation in elevated CO2
Data on short- and long-term stomatal CO2 responses and absolute gs from plants grown in two CO2 treatments were used for QTL mapping. The major QTL on chromosome 2 associated with the short-term CO2 response, identified in the previous experiment on plants grown in ambient CO2, was detected in plants grown in both ambient and elevated CO2. Using the subset of 50 RILs, this QTL mapped to the adjacent marker compared with the results from the previous experiment (Fig. 3, Table 2). Additionally, three other, minor QTLs for the short-term response were identified, explaining 7–10 % of the variation in this trait. These QTLs were detected only in one of the CO2 treatments (Table 2). For the long-term gs response to elevated CO2, one QTL explaining 14 % of the variation was identified (Fig. 3, Table 2). This QTL mapped to the same marker as the major QTL for the short-term response, suggesting that these traits are regulated by the same genetic component. Furthermore, five QTLs for absolute gs were detected, of which two were associated with gs measured at both 400 and 800 ppm, two with gs at 400 ppm and one with gs at 800 ppm. Most QTLs for absolute gs were detected in plants from both CO2 treatments (Fig. 3, Table 2).
Table 2.
QTLs detected using data from gas exchange measurements on recombinant inbred lines grown in ambient (A) or elevated (E) CO2 concentration
| Trait | QTL location and confidence interval (cM) | Chromo-some | Closest marker | Treatment | Variance explained (%) | High-value allele |
|---|---|---|---|---|---|---|
| Short-term CO2 response | 18 (0–138) | 1 | MASC09203 | E | 8 | C24 |
| g s_400 | 18 (0–138) | 1 | MASC09203 | E | 13 | C24 |
| Short-term CO2 response | 123 (104–125) | 2 | MASC00371 | A and E | A 36 E 27 | Col-0 |
| g s_400 | 123 (104–125) | 2 | MASC00371 | A and E | A 8 E 12 | Col-0 |
| g s_800 | 123 (104–125) | 2 | MASC00371 | A | 8 | C24 |
| Long-term CO2 response | 123 (104–125) | 2 | MASC00371 | – | 14 | Col-0 |
| Short-term CO2 response | 38 (0–101) | 3 | MSAT3.19/MASC04516 | A | 7 | C24 |
| g s_800 | 38 (0–101) | 3 | MSAT3.19/MASC04516 | A and E | A 18 E 9 | Col-0 |
| g s_400 | 6 (0–42) | 4 | FRI/MASC04123 | A and E | A 11 E 16 | Col-0 |
| g s_800 | 6 (0–42) | 4 | FRI/MASC04123 | A and E | A 9 E 25 | Col-0 |
| Short-term CO2 response | 79 (0–103) | 4 | MASC02548/F24J7ID/ G3883-1.4 | A | 10 | C24 |
| g s_400 | 79 (0–103) | 4 | MASC02548/F24J7ID/ G3883-1.4 | A and E | A 15 E 8 | C24 |
ABA response
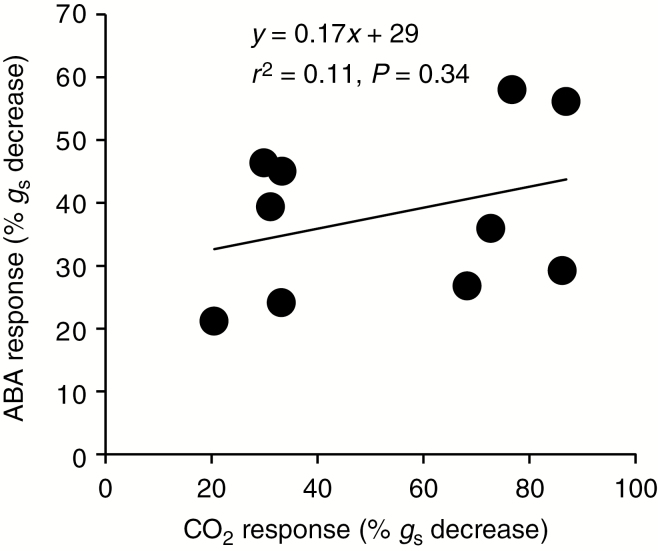
As the signalling pathway for the CO2-induced closure response is known to converge downstream with the pathway for ABA-induced stomatal closure, we tested whether the main loci involved in CO2-induced stomatal closure also affected the ABA-induced stomatal response. To this end, ten RILs representing contrasting genetic backgrounds and CO2-induced closure phenotypes were cultivated at ambient CO2 concentration and stomatal conductance was monitored after spray application of ABA. Measurements were performed on two or three replicates per line. This showed that the ability to close stomata in response to exogenous ABA was not correlated with the ability to respond to CO2 (Fig. 6), suggesting that the QTL for stomatal CO2 response identified in this study is not involved in ABA-induced stomatal closure.
Fig. 6.
There was no significant relationship between stomatal responses to exogenous abscisic acid (ABA) and elevated CO2. Five recombinant inbred lines displaying the weakest and five displaying the strongest CO2 responses were sprayed with 5 µm ABA. The percentage decrease in gs following application was quantified using gas exchange measurements. Measurements were performed on two or three replicates per line.
Fine mapping of a locus on chromosome 2 controlling CO2-induced closure
The major QTL associated with CO2-induced stomatal closure among the RILs mapped to the end of chromosome 2. To narrow down the region of interest, nine new SSLP markers (Supplementary Data Table S2) spanning the area between markers MASC06025 and MASC02812 were developed and used to genotype a subset of RILs with crossovers at the end of chromosome 2. This approach narrowed the region to a physical distance of 410 kb between markers MASC02812 and MASC00371 (Supplementary Data Table S5), consistent with previous mapping results that located this QTL to either of these two markers depending on the subset of RILs. This region contains the MPK12 gene, which encodes a kinase recently shown to be involved in CO2-induced stomatal closure (Hõrak et al., 2016; Jakobson et al., 2016). However, neither publicly available sequence data (Berardini et al., 2015; Alonso-Blanco et al., 2016) nor results from our own sequencing show any sequence differences between C24 and Col-0 for this gene or 0.5-kb flanking regions, except for a single SNP 0.47 kb downstream of the coding sequence.
Confirmation of the major QTL on chromosome 2 using NILs
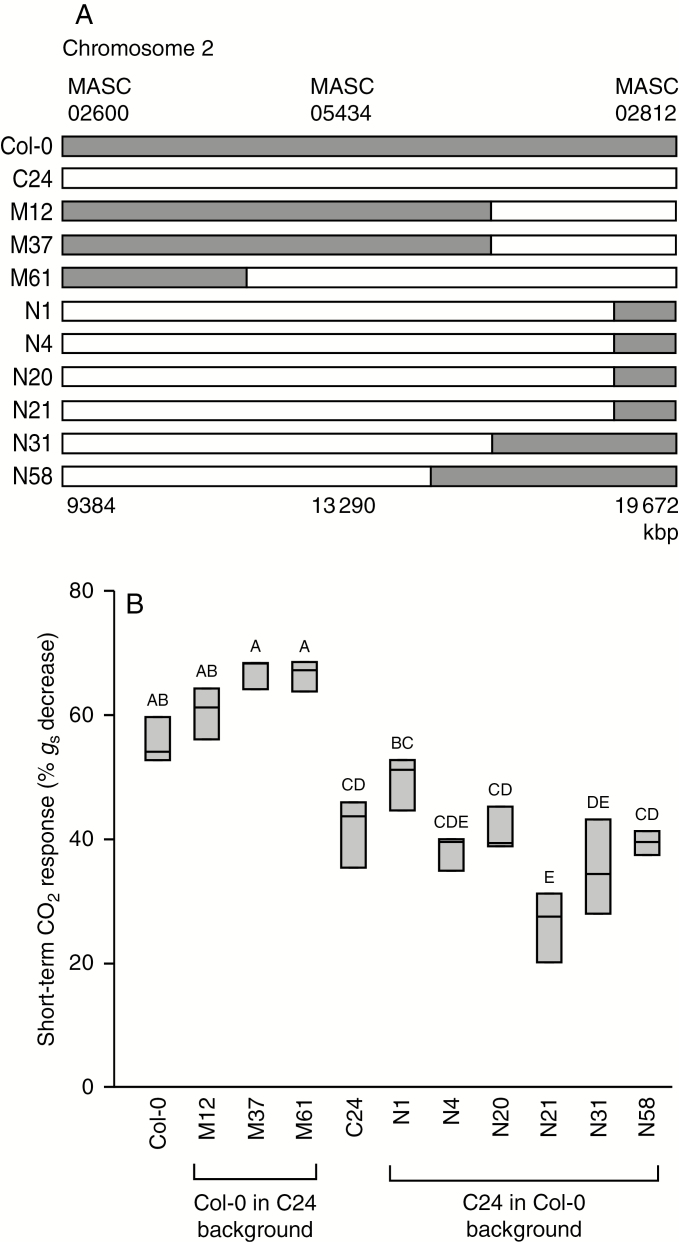
The short-term CO2 response of nine NILs (Supplementary Data Table S1; Törjék et al., 2008) measured in triplicate was used to confirm the location and effect of the major QTL identified and characterized in previous experiments with RILs. In these lines, the genome was predominantly from one of the parents, with a small introgression of the opposite genotype at the end of chromosome 2. Three lines were on the C24 background and six on the Col-0 background (Fig. 7A). Lines on the Col-0 background with introgression from C24 changed their CO2 response to the C24 phenotype and vice versa; lines on the C24 background with Col-0 introgression gained the Col-0 phenotype (Fig. 7B). One line (NIL number N1) could not be statistically distinguished from either of the parental accessions (Fig. 7B). The results of measurements of these independent lines confirm the location of the QTL between the two last markers on chromosome 2. Furthermore, these results show that the effect of the QTL was large enough to shift the phenotype from that of the background accession to one similar to the phenotype of the introgressed accession.
Fig. 7.
Genotype information (A) and short-term stomatal CO2 response (B) of the parental accessions (Col-0 and C24) and nine reciprocal near-isogenic lines with introgressions at the end of chromosome 2, i.e. in the region of a major QTL associated with the short-term CO2 response, which was identified by QTL mapping using recombinant inbred lines. Boxes represent 25–75 % quartiles with the median as a horizontal line inside, and whiskers indicate the smallest and largest values. Different letters indicate a statistically significant difference in Tukey’s post hoc test (P < 0.05, n = 3).
DISCUSSION
In this study, we used natural variation in stomatal regulation between the two A. thaliana accessions C24 and Col-0 to identify genetic loci associated with short- and long-term responses of gs to elevated CO2 concentration, as well as several other stomata-related traits. The short-term response represents the adjustment of gs that occurs within minutes to hours after change in atmospheric CO2 concentration, whereas the long-term response represents a change in gs seen after weeks to months of cultivation under elevated CO2 concentration. The use of RILs originating from a cross between C24 and Col-0 enabled the identification of a number of QTLs associated with stomatal regulation. Most notable was a QTL at the end of chromosome 2 explaining ~50 % of the variation in the short-term gs response to elevated CO2 concentration among the tested RILs. Interestingly, this QTL was also associated with the long-term gs response to growth under elevated CO2 concentration, suggesting that these traits are regulated by the same underlying gene. The same QTL was additionally associated with absolute gs at ambient CO2 concentration and water-use efficiency. The Col-0 genotype at this locus conferred stronger CO2 responsiveness in both the short and the long term, as well as higher gs at 400 ppm CO2. The C24 genotype was associated with higher water-use efficiency.
Exogenous application of ABA to a subset of RILs with the most extreme CO2 response phenotypes showed that there was no correlation between the stomatal closure responses to ABA and CO2. This implies that the identified major QTL is involved in the CO2-specific branch of the signalling pathway for stomatal closure, upstream of the convergence point for CO2- and ABA-induced responses. Analysis of short-term responsiveness to elevated CO2 in reciprocal NILs between C24 and Col-0 confirmed the location of the QTL at the end of chromosome 2. Introgressions in this region caused a significant change in responsiveness and shifted the phenotype to one similar to that of the introgressed parent. Fine mapping using RILs with crossovers in the region of interest allowed us to locate the QTL to a 410-kb region. This region contains the MPK12 gene, which was recently shown to have a pivotal role in CO2-induced stomatal closure (Jakobson et al., 2016). However, no sequence polymorphisms were found between C24 and Col-0 in MPK12 that could explain the phenotypic difference. The phenotype of C24, i.e. weak CO2 response in combination with low gs, also differs from the phenotype resulting from known loss-of-function mutations in MPK12, i.e. weak CO2 response in combination with very high gs (Jakobson et al., 2016; Tõldsepp et al., 2018). Finally, C24 and Col-0 show only a moderate difference in expression of MPK12 (60 % higher in C24; Xu et al., 2015). Taking these results together, it is thus unlikely that any polymorphism affecting the expression of MPK12 could explain the difference in CO2-dependent stomatal closure between C24 and Col-0. Besides MPK12, the mapped region does not contain any genes previously linked to stomatal behaviour. Further studies are required to accurately pinpoint the exact molecular difference underlying this QTL. Nevertheless, our data point to the presence of at least one gene in this region that encodes an important as yet unidentified component regulating gs and its response to elevated CO2 in both the short and the long term.
The fact that short- and long-term CO2 responses were significantly correlated and associated with the same locus suggests that knowledge about the signalling pathway for short-term gs regulation in response to elevated CO2 concentration could be used for manipulation of long-term gs responses under rising atmospheric CO2. Results from experimental field research corroborate the link between short- and long-term gs responses (Hasper et al., 2017), indicating that the short-term CO2 response may be a useful predictor of the long-term CO2 effect on gs also under ecologically realistic conditions. Data on short-term responsiveness among plant species and/or varieties could thus be valuable for projections of plant water use under rising atmospheric CO2 concentration. Cases where short-and long-term responses are decoupled due to a pronounced stomatal density response in plants with weak short-term responsiveness (Haworth et al., 2013) should, however, be a focus of further studies.
Growth under elevated CO2 concentration resulted in an average gs decrease of 26 % among the tested RILs, which is similar to the average long-term response observed in field experiments (21 %, Medlyn et al., 2001; 22 %, Ainsworth and Rogers, 2007). Plants grown under elevated CO2 exhibited slightly attenuated short-term responsiveness to CO2 and higher gs compared with plants grown under ambient CO2 when measured at the same CO2 concentration. These results show that both guard cell CO2 responsiveness and absolute gs acclimated to the CO2 concentration during growth. Previous research has shown that guard cells of plants grown under elevated CO2 may lose some of their sensitivity to short-term changes in CO2 concentration (Morison, 1998; Lodge et al., 2001; Medlyn et al., 2001; Onandia et al., 2011). The direction of gs acclimation in our study differed from previous observations. A meta-analysis on trees subjected to long-term CO2 exposure showed that photosynthetic capacity and gs were downregulated in parallel, resulting in lower gs of plants grown in elevated CO2 when plants from both treatments were measured at the same CO2 concentration (Medlyn et al., 1999, 2001). Similar results were observed in an experiment with A. thaliana in the reproductive stage (Teng et al., 2006). Experiments on earlier growth stages of A. thaliana, on the other hand, showed no downregulation of photosynthetic capacity or Rubisco content as long as plants were grown with an ample nitrogen supply (Tocquin et al., 2006; Jauregui et al., 2015). Tocquin et al. (2006) suggested that A. thaliana under controlled growth conditions simply responds to elevated CO2 by growth rate adjustment. One potential explanation for the upregulation of gs in our experiment is that increased photosynthetic efficiency under elevated CO2 stimulates leaf production and expansion in young A. thaliana plants, which not only maintains sink capacity but also increases the need for photosynthate. Consequently, photosynthesis may be stimulated further and result in the observed upregulation of gs, since changes in leaf CO2 demand and supply are typically well coupled (Wong et al., 1979). Indeed, plants grown under elevated CO2 in our experiment showed a 60 % increase in total leaf area (data not shown).
Stomatal regulation is a complex, tightly regulated trait of crucial importance for plant fitness and survival. As such, it can be expected to be controlled by the coordinated action of many genes with a certain degree of redundancy. Indeed, we identified numerous loci associated with stomatal regulation in addition to the major QTL on chromosome 2. Most of these QTLs explained minor proportions of the trait variation, but could potentially provide useful information about candidate genes if mapped more precisely. As the main focus of the present study was on the stomatal CO2 response, for which phenotyping is very time-consuming, it was necessary to work with a reduced set of lines. However, traits such as water-use efficiency and absolute gs could be quantified in a larger population, which may increase mapping power and resolution (Keurentjes et al., 2007). For several traits we identified QTLs with allelic effects opposite to those predicted by the parental phenotypes, corroborating the observation of transgressive segregation in the RIL population.
This study clearly demonstrates the large potential of using natural variation in A. thaliana to uncover the genetic basis of stomatal regulation and water economy in plants. The detection of two different QTLs in the same region using mapping populations with separate genetic backgrounds (the present study; Juenger et al., 2005; Brosché et al., 2010; Des Marais et al., 2014; Jakobson et al., 2016) highlights the importance of exploiting the variation among numerous accessions to fully resolve the genetic regulation of complex traits. The large differences in gs or water-use efficiency observed among cultivars of wheat (Lu et al., 1998; Condon et al., 2004), rice (Horie et al., 2006), maize (Ryan et al., 2016), legumes (Ehleringer et al., 1991; Ashok et al., 1999) cotton (Lu et al., 1998) and sugarcane (Basnayake et al., 2015) show that there is a large untapped potential in the genetic variation for stomatal traits in crop species as well. Breeding for low gs and high water-use efficiency may result in crop varieties suitable for cultivation in already dry areas (Araus et al., 2002), as shown by the successful development of transpiration-efficient wheat cultivars (Rebetzke et al., 2002; Condon et al., 2004). In the more moist and fertile areas currently suitable for highly productive crops with relatively high gs, it would be advantageous to grow cultivars exhibiting a gradual but substantial shift towards lower gs as the atmospheric CO2 concentration increases and the air, and perhaps also the soil, becomes progressively drier. While there is typically a trade-off between high gas exchange and high water-use efficiency, our results show that plants with high gs at the current atmospheric CO2 concentration may also exhibit large improvements in water economy under rising atmospheric CO2. In fact, high gs at present-day atmospheric CO2 concentration and strong stomatal responsiveness to CO2 were associated with the same QTL in the present study, and may even be regulated by the same gene.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Figure S1: histogram plots showing the distribution of trait data among RILs. Table S1: genotype data and genetic maps of RILs and NILs. Table S2: sequences and annealing temperatures of primers. Table S3: trait data from RILs grown in ambient CO2. Table S4: trait data from RILs grown in ambient and elevated CO2 concentration. Table S5: results of fine mapping of a major QTL at the end of chromosome 2.
ACKNOWLEDGEMENTS
The authors express their gratitude to Hoda Sadraei for conducting the pilot study that identified C24 and Col-0 as parental accessions, Dr Göran Wallin for expert advice on gas exchange measurements, Mats Räntfors for installation of the CO2 control system, and Atlanta Koraszewicz for preparation of isotope samples. We also thank the reviewers and Handling Editor Dr Jiri Šantrůček for valuable comments that significantly improved the manuscript.
FUNDING
This work was supported by Olle Engkvist Byggmästare Foundation; Carl Tryggers’ Foundation; Adlerbertska Research Foundation; the European Regional Development Fund (MOBJD78); the Estonian Ministry of Science and Education (IUT2-21); and the Academy of Finland (grant 307335, Center of Excellence in Molecular Biology of Primary Producers 2014–2019). The research presented in this paper is a contribution to the strategic research area Biodiversity and Ecosystem Services in a Changing Climate, BECC (www.becc.lu.se/).
LITERATURE CITED
- Ainsworth EA, Rogers A. 2007. The response of photosynthesis and stomatal conductance to rising CO2: mechanisms and environmental interactions. Plant, Cell & Environment 30: 258–270. [DOI] [PubMed] [Google Scholar]
- Alonso-Blanco C, Andrade J, Becker C, et al. 2016. 1,135 genomes reveal the global pattern of polymorphism in Arabidopsis thaliana. Cell 166: 481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araus JL, Slafer GA, Reynolds MP, Royo C. 2002. Plant breeding and drought in C3 cereals: what should we breed for? Annals of Botany 89: 925–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashok ISAH, Prasad TG, Wright GC, Kumar MU, Rao RCN. 1999. Variation in transpiration efficiency and carbon isotope discrimination in cowpea. Functional Plant Biology 26: 503–510. [Google Scholar]
- Basnayake J, Jackson PA, Inman-Bamber NG, Lakshmanan P. 2015. Sugarcane for water-limited environments. Variation in stomatal conductance and its genetic correlation with crop productivity. Journal of Experimental Botany 66: 3945–3958. [DOI] [PubMed] [Google Scholar]
- Berardini TZ, Reiser L, Li D, et al. 2015. The Arabidopsis Information Resource: making and mining the “gold standard” annotated reference plant genome. Genesis 53: 474–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyes DC, Zayed AM, Ascenzi R, et al. 2001. Growth stage-based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. Plant Cell 13: 1499–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broman KW, Wu H, Sen S, Churchill GA. 2003. R/qtl: QTL mapping in experimental crosses. Bioinformatics 19: 889–890. [DOI] [PubMed] [Google Scholar]
- Brosché M, Merilo E, Mayer F, et al. 2010. Natural variation in ozone sensitivity among Arabidopsis thaliana accessions and its relation to stomatal conductance. Plant, Cell & Environment 33: 914–925. [DOI] [PubMed] [Google Scholar]
- Condon AG, Richards RA, Rebetzke GJ, Farquhar GD. 2004. Breeding for high water-use efficiency. Journal of Experimental Botany 55: 2447–2460. [DOI] [PubMed] [Google Scholar]
- Ehleringer JR, Klasen S, Clayton C, et al. 1991. Carbon isotope discrimination and transpiration efficiency in common bean. Crop Science 31: 1611–1615. [Google Scholar]
- Engineer CB, Hashimoto-Sugimoto M, Negi J, et al. 2016. CO2 Sensing and CO2 regulation of stomatal conductance: advances and open questions. Trends in Plant Science 21: 16–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick KT. 1989. Carbon isotope discrimination and photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology 40: 503–537. [Google Scholar]
- Franks PJ, Britton-Harper ZJ. 2016. No evidence of general CO2 insensitivity in ferns: one stomatal control mechanism for all land plants? New Phytologist 211: 819–827. [DOI] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, et al. 2009. Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proceedings of the National Academy of Sciences of the USA 106: 21425–21430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Negi J, Young J, Israelsson M, Schroeder JI, Iba K. 2006. Arabidopsis HT1 kinase controls stomatal movements in response to CO2. Nature Cell Biology 8: 391–397. [DOI] [PubMed] [Google Scholar]
- Hasper TB, Dusenge ME, Breuer F, Uwizeye FK, Wallin G, Uddling J. 2017. Stomatal CO2 responsiveness and photosynthetic capacity of tropical woody species in relation to taxonomy and functional traits. Oecologia 184: 43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth M, Elliott-Kingston C, McElwain JC. 2013. Co-ordination of physiological and morphological responses of stomata to elevated [CO2] in vascular plants. Oecologia 171: 71–82. [DOI] [PubMed] [Google Scholar]
- Haworth M, Killi D, Materassi A, Raschi A. 2015. Coordination of stomatal physiological behavior and morphology with carbon dioxide determines stomatal control. American Journal of Botany 102: 677–688. [DOI] [PubMed] [Google Scholar]
- Haworth M, Killi D, Materassi A, Raschi A, Centritto M. 2016. Impaired stomatal control is associated with reduced photosynthetic physiology in crop species grown at elevated [CO2]. Frontiers in Plant Science 7: 1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Zhang RX, Peng K, et al. 2018. The BIG protein distinguishes the process of CO2-induced stomatal closure from the inhibition of stomatal opening by CO2. New Phytologist 218: 232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R, Geiger D. 2017. Biology of SLAC1-type anion channels – from nutrient uptake to stomatal closure. New Phytologist 216: 46–61. [DOI] [PubMed] [Google Scholar]
- Hõrak H, Kollist H, Merilo E. 2017. Fern stomatal responses to ABA and CO2 depend on species and growth conditions. Plant Physiology 174: 672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hõrak H, Sierla M, Tõldsepp K, et al. 2016. A dominant mutation in the HT1 kinase uncovers roles of MAP kinases and GHR1 in CO2-induced stomatal closure. Plant Cell 28: 2493–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie T, Matsuura S, Takai T, Kuwasaki K, Ohsumi A, Shiraiwa T. 2006. Genotypic difference in canopy diffusive conductance measured by a new remote-sensing method and its association with the difference in rice yield potential. Plant, Cell & Environment 29: 653–660. [DOI] [PubMed] [Google Scholar]
- Hu H, Boisson-Dernier A, Israelsson-Nordström M, et al. 2010. Carbonic anhydrases are upstream regulators of CO2-controlled stomatal movements in guard cells. Nature Cell Biology 12: 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua D, Wang C, He J, et al. 2012. A plasma membrane receptor kinase, GHR1, mediates abscisic acid- and hydrogen peroxide-regulated stomatal movement in Arabidopsis. Plant Cell 24: 2546–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC 2013. Climate change 2013 – the physical science basis. Working Group I Contribution to the IPCC Fifth Assessment Report. Cambridge: Cambridge University Press. [Google Scholar]
- Jakobson L, Vaahtera L, Tõldsepp K, et al. 2016. Natural variation in Arabidopsis Cvi-0 accession reveals an important role of MPK12 in guard cell CO2 signaling. PLoS Biology 14: e2000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauregui I, Aparicio-Tejo PM, Avila C, Rueda-López M, Aranjuelo I. 2015. Root and shoot performance of Arabidopsis thaliana exposed to elevated CO2: a physiologic, metabolic and transcriptomic response. Journal of Plant Physiology 189: 65–76. [DOI] [PubMed] [Google Scholar]
- Jezek M, Blatt MR. 2017. The membrane transport system of the guard cell and its integration for stomatal dynamics. Plant Physiology 174: 487–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juenger TE, McKay JK, Hausmann N, et al. 2005. Identification and characterization of QTL underlying whole-plant physiology in Arabidopsis thaliana: δ13C, stomatal conductance and transpiration efficiency. Plant, Cell & Environment 28: 697–708. [Google Scholar]
- Keeling CD, Piper SC, Bacastow RB, et al. 2001. Exchanges of atmospheric CO2 and 13CO2 with the terrestrial biosphere and oceans from 1978 to 2000. I. Global aspects. San Diego: Scripps Institution of Oceanography. [Google Scholar]
- Keurentjes JJ, Bentsink L, Alonso-Blanco C, et al. 2007. Development of a near-isogenic line population of Arabidopsis thaliana and comparison of mapping power with a recombinant inbred line population. Genetics 175: 891–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollist H, Nuhkat M, Roelfsema MR. 2014. Closing gaps: linking elements that control stomatal movement. New Phytologist 203: 44–62. [DOI] [PubMed] [Google Scholar]
- Kollist T, Moldau H, Rasulov B, et al. 2007. A novel device detects a rapid ozone-induced transient stomatal closure in intact Arabidopsis and its absence in abi2 mutant. Physiologia Plantarum 129: 796–803. [Google Scholar]
- Li J, Ji L. 2005. Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity 95: 221–227. [DOI] [PubMed] [Google Scholar]
- LI-COR Biosciences Inc. 2011. Using the LI6400/LI6400XT portable photosynthesis system. Lincoln, NE: LI-COR Biosciences. [Google Scholar]
- Lodge RJ, Dijkstra P, Drake BG, Morison JIL. 2001. Stomatal acclimation to increased CO2 concentration in a Florida scrub oak species Quercus myrtifolia Willd. Plant, Cell & Environment 24: 77–88. [Google Scholar]
- Lu ZM, Percy RG, Qualset CO, Zeiger E. 1998. Stomatal conductance predicts yields in irrigated Pima cotton and bread wheat grown at high temperatures. Journal of Experimental Botany 49: 453–460. [Google Scholar]
- Des Marais DL, Auchincloss LC, Sukamtoh E, et al. 2014. Variation in MPK12 affects water use efficiency in Arabidopsis and reveals a pleiotropic link between guard cell size and ABA response. Proceedings of the National Academy of Sciences of the USA 111: 2836–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marten H, Hyun T, Gomi K, Seo S, Hedrich R, Roelfsema MR. 2008. Silencing of NtMPK4 impairs CO2-induced stomatal closure, activation of anion channels and cytosolic Ca2+ signals in Nicotiana tabacum guard cells. Plant Journal 55: 698–708. [DOI] [PubMed] [Google Scholar]
- Medlyn BE, Badeck FW, De Pury DGG, et al. 1999. Effects of elevated [CO2] on photosynthesis in European forest species: a meta-analysis of model parameters. Plant, Cell & Environment 22: 1475–1495. [Google Scholar]
- Medlyn BE, Barton CVM, Broadmeadow MSJ, et al. 2001. Stomatal conductance of forest species after long-term exposure to elevated CO2 concentration: a synthesis. New Phytologist 149: 247–264. [DOI] [PubMed] [Google Scholar]
- Morison JIL. 1998. Stomatal response to increased CO2 concentration. Journal of Experimental Botany 49: 443–452. [Google Scholar]
- Negi J, Matsuda O, Nagasawa T, et al. 2008. CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 452: 483–486. [DOI] [PubMed] [Google Scholar]
- Nilsson AK, Fahlberg P, Johansson ON, Hamberg M, Andersson MX, Ellerström M. 2016. The activity of HYDROPEROXIDE LYASE 1 regulates accumulation of galactolipids containing 12-oxo-phytodienoic acid in Arabidopsis. Journal of Experimental Botany 67: 5133–5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onandia G, Olsson AK, Barth S, King JS, Uddling J. 2011. Exposure to moderate concentrations of tropospheric ozone impairs tree stomatal response to carbon dioxide. Environmental Pollution 159: 2350–2354. [DOI] [PubMed] [Google Scholar]
- Pérez-Harguindeguy N, Díaz S, Garnier E, et al. 2013. New handbook for standardised measurement of plant functional traits worldwide. Australian Journal of Botany 61: 167–234. [Google Scholar]
- Rebetzke GJ, Condon AG, Richards RA, Farquhar GD. 2002. Selection for reduced carbon isotope discrimination increases aerial biomass and grain yield of rainfed bread wheat. Crop Science 42: 739–745. [Google Scholar]
- Ruszala EM, Beerling DJ, Franks PJ, et al. 2011. Land plants acquired active stomatal control early in their evolutionary history. Current Biology 21: 1030–1035. [DOI] [PubMed] [Google Scholar]
- Ryan AC, Dodd IC, Rothwell SA, et al. 2016. Gravimetric phenotyping of whole plant transpiration responses to atmospheric vapour pressure deficit identifies genotypic variation in water use efficiency. Plant Science 251: 101–109. [DOI] [PubMed] [Google Scholar]
- Šantrůček J, Sage R. 1996. Acclimation of stomatal conductance to a CO2-enriched atmosphere and elevated temperature in Chenopodium album. Functional Plant Biology 23: 467–478. [Google Scholar]
- Schmidt R, Boudichevskaia A, Cao HX, He S, Meyer RC, Reif JC. 2017. Extracting genotype information of Arabidopsis thaliana recombinant inbred lines from transcript profiles established with high-density oligonucleotide arrays. Plant Cell Reports 36: 1871–1881. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Monda K, Negi J, et al. 2015. Natural variation in stomatal responses to environmental changes among Arabidopsis thaliana ecotypes. PLoS ONE 10: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng N, Wang J, Chen T, Wu X, Wang Y, Lin J. 2006. Elevated CO2 induces physiological, biochemical and structural changes in leaves of Arabidopsis thaliana. New Phytologist 172: 92–103. [DOI] [PubMed] [Google Scholar]
- Tocquin P, Ormenese S, Pieltain A, Detry N, Bernier G, Périlleux C. 2006. Acclimation of Arabidopsis thaliana to long-term CO2 enrichment and nitrogen supply is basically a matter of growth rate adjustment. Physiologia Plantarum 128: 677–688. [Google Scholar]
- Tõldsepp K, Zhang J, Takahashi Y, et al. 2018. Mitogen-activated protein kinases MPK4 and MPK12 are key components mediating CO2-induced stomatal movements. Plant Journal 96: 1018–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Törjék O, Berger D, Meyer RC, et al. 2003. Establishment of a high-efficiency SNP-based framework marker set for Arabidopsis. Plant Journal 36: 122–140. [DOI] [PubMed] [Google Scholar]
- Törjék O, Witucka-Wall H, Meyer RC, et al. 2006. Segregation distortion in Arabidopsis C24/Col-0 and Col-0/C24 recombinant inbred line populations is due to reduced fertility caused by epistatic interaction of two loci. Theoretical and Applied Genetics 113: 1551–1561. [DOI] [PubMed] [Google Scholar]
- Törjék O, Meyer RC, Zehnsdorf M, et al. 2008. Construction and analysis of 2 reciprocal Arabidopsis introgression line populations. Journal of Heredity 99: 396–406. [DOI] [PubMed] [Google Scholar]
- Vahisalu T, Kollist H, Wang YF, et al. 2008. SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 452: 487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AA, Hetherington AM. 1997. Convergence of the abscisic acid, CO2, and extracellular calcium signal transduction pathways in stomatal guard cells. Plant Physiology 114: 1557–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SC, Cowan IR, Farquhar GD. 1979. Stomatal conductance correlates with photosynthetic capacity. Nature 282: 424–426. [Google Scholar]
- Xu E, Vaahtera L, Hõrak H, Hincha DK, Heyer AG, Brosché M. 2015. Quantitative trait loci mapping and transcriptome analysis reveal candidate genes regulating the response to ozone in Arabidopsis thaliana. Plant, Cell & Environment 38: 1418–1433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.