Abstract
Background
We have shown that genetic overexpression of cell cycle proteins can increase the proliferation of transplanted cardiomyocytes derived from human induced-pluripotent stem cells (hiPSC-CMs) in animal models of myocardial infarction (MI). Here, we introduce a new, non-genetic approach to promote hiPSC-CM cell cycle activity and proliferation in transplanted human cardiomyocyte patches (hCMPs).
Methods
Mice were randomly distributed into 5 experimental groups (n = 10 per group). One group underwent Sham surgery, and the other 4 groups underwent MI induction surgery followed by treatment with hCMPs composed of hiPSC-CMs and nanoparticles that contained CHIR99021 and FGF1 (the NPCF-hCMP group), with hCMPs composed of hiPSC-CMs and empty nanoparticles (the NPE-hCMP group); with patches containing the CHIR99021/FGF-loaded nanoparticles but lacking hiPSC-CMs (the NPCF-Patch group), or patches lacking both the nanoparticles and cells (the E-Patch group). Cell cycle activity was evaluated via Ki67 and Aurora B expression, bromodeoxyuridine incorporation, and phosphorylated histone 3 levels (immunofluorescence); engraftment via human cardiac troponin T or human nuclear antigen expression (immunofluorescence) and bioluminescence imaging; cardiac function via echocardiography; infarct size and wall thickness via histology; angiogenesis via isolectin B4 expression (immunofluorescence); and apoptosis via TUNEL and caspace 3 expression (immunofluorescence).
Results
Combined CHIR99021- and FGF1-treatment significantly increased hiPSC-CM cell cycle activity both in cultured cells (by 4- to 6-fold) and in transplanted hCMPs, and compared to treatment with NPE-hCMPs, NPCF-hCMP transplantation increased hiPSC-CM engraftment by ~4-fold and was associated with significantly better measurements of cardiac function, infarct size, wall thickness, angiogenesis, and hiPSC-CM apoptosis four weeks after MI induction.
Conclusions
Nanoparticle-mediated CHIR99021 and FGF1 delivery promotes hiPSC-CM cell cycle activity and proliferation, as well as the engraftment and regenerative potency of transplanted hCMPs, in a mouse MI model.
Keywords: CHIR99021, FGF1, Nanoparticle, Patch, Stem cell, Cell cycle, Myocardial infarction, Heart failure
1. Introduction
Eighty percent of deaths from cardiovascular disease are attributable to coronary obstructions that lead to myocardial infarction (MI) [1]. Conventional nonsurgical therapies fail to remuscularize the infarcted region, because the regenerative capacity of mammalian hearts is limited, and although treatment via heart transplantation can be successful, it is frequently precluded by the shortage of donor hearts [2]. The results from decades of preclinical studies [3–8] and more recent clinical trials [9,10], suggest that a construct composed of cardiac cells (cardiomyocytes and/or nonmyocytes) suspended in a fibrin patch may be a promising treatment for MI when delivered to the epicardium of the injured heart. However, the fabrication of functional, stably engrafted, engineered myocardium that efficiently integrates with both the electrical and circulatory systems of the host heart remains a daunting challenge in the field of cardiac cell therapy [11–13].
Fibroblast growth factor 1 (FGF1, also known as acidic FGF) is involved in a range of physiological processes, including development, morphogenesis, wound healing, and proliferation [14–16], and transgenic FGF1 overexpression, as well as intramyocardial infusion of molecules that mimic FGF1, is cardioprotective in mouse MI models [17,18]. Furthermore, knockout of the gene coding for glycogen synthase kinase 3 (GSK3) was associated with cardiomyocyte hyperproliferation and increases in the expression of cell cycle regulatory proteins (GATA4, cyclin D2, and c-myc) [19], which is consistent with evidence that GSK-3β inhibits cardiomyocyte proliferation [20–22]. Here, we show that when a human cardiomyocyte patch (hCMP) containing cardiomyocytes (CMs) derived from human induced-pluripotent stem cells (hiPSCs) was administered to the hearts of mice after MI, concomitant treatment with FGF1 and CHIR99021 (a potent, highly selective inhibitor of GSK-3) significantly improved the effectiveness of the hCMP for myocardial repair and recovery. FGF1 and CHIR99021 were released over an extended period from nanoparticles that had been suspended in the hCMP [23,24] and combined to promote hiPSC-CM proliferation while impeding apoptosis in the transplanted cells. Collectively, these observations suggest that this novel strategy can significantly remuscularize the infarcted regions of mouse hearts.
2. Materials and methods
A detailed description of the experimental procedures used in this investigation is provided in the Online Supplement. All experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Alabama at Birmingham and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication No 85–23). All assessments were conducted by an investigator who was blinded to the experimental condition and/or treatment group. Data were presented as mean ± SEM, and significance (P < .05) was determined via the Student’s t-test for comparisons between two groups or one-way analysis of variance (ANOVA) for comparisons among three or more groups.
3. Results
3.1. CHIR99021 and FGF1 activate the cell cycle, increase proliferation, and reduce apoptosis in cultured hiPSC-CMs
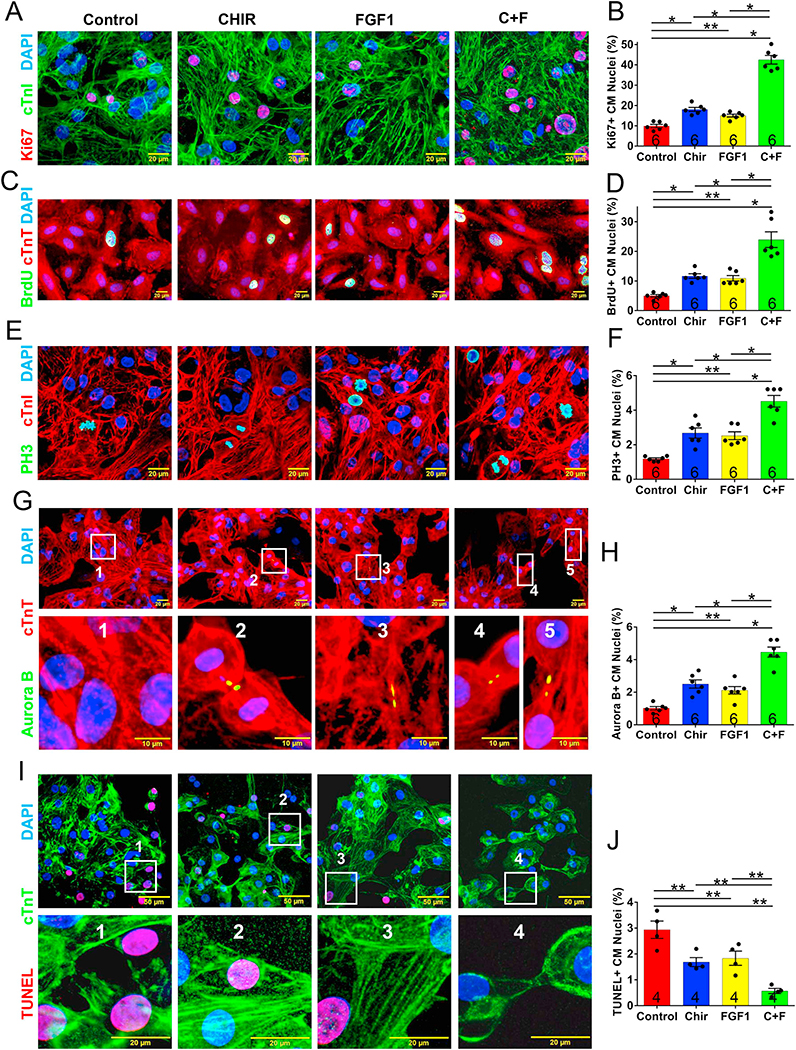
The effect of CHIR99021 and FGF1 treatment on proliferation and cell cycle activity in cultured hiPSC-CMs was evaluated via immunofluorescence analyses of markers for proliferation (Ki67), for S-phase (the incorporation of bromodeoxyuridine [BrdU],) and M-phase (histone 3 phosphorylation [PH3]) of the cell cycle, and for cytokinesis (Aurora B). The expression of all four markers became significantly more common as CHIR99021 concentrations increased from 0 to 5 μM (Supplemental Fig. 1), as FGF1 levels increased from 0 to 100 ng/mL (Supplemental Fig. 2), and when the cells were cultured with both CHIR99021 (5 μM) and FGF1 (100 ng/mL) than with either individual treatment (Fig. 1A–H), but declined at higher concentrations. The combined treatment also significantly reduced hiPSC-CM apoptosis (Fig. 1I–J).
Fig. 1.
CHIR99021 and FGF1 promoted cell cycle progression and impeded hypoxia-induced apoptosis in cultured hiPSC-CMs. The cell cycles of hiPSC-CMs were synchronized via serum starvation; then, the cells were treated with CHIR99021 (5 μM), FGF1 (100 ng/mL), or both CHIR99021 and FGF1 (CHIR99021 + FGF1) (5 μM and 100 ng/mL, respectively) for 24 h and immunofluorescently stained for the presence of cTnI and cTnT; nuclei were identified via DAPI staining. (A) Proliferation was evaluated via immunofluorescence analyses of Ki67 expression and (B) quantified as the percentage of positively stained cells. (C) hiPSC-CMs in the S-phase of the cell cycle were identified via immunofluorescence analyses of BrdU expression and (D) quantified as the percentage of positively stained cells. (E) hiPSC-CMs in the M-phase of the cell cycle were identified via analyses of PH3 expression and (F) quantified as the percentage of positively stained cells. (G) hiPSC-CMs undergoing cytokinesis were identified via Aurora B expression and (H) quantified as the percentage of positively stained cells. (I) Apoptosis was evaluated via immunofluorescence analyses of TUNEL expression and (J) quantified as the percentage of positively stained cells. For each treatment group, the number of independent experiments with new cell cultures is displayed at the base of the corresponding column; Five randomly selected viewing fields were evaluated per group per experiment. *p < .01, **p < .05, one-way ANOVA with Tukey correction for multiple comparisons.
3.2. CHIR99021- and FGF1-containing nanoparticles increase hiPSC-CM engraftment when hCMPs are transplanted into infarcted mouse hearts
A major roadblock for the effectiveness of myocardial cell therapy is the exceptionally low engraftment rate; thus, we investigated whether the increase in hiPSC-CM proliferation, cell cycle activity, and apoptosis resistance associated with CHIR99021 and FGF1 treatment in cultured cells could improve the engraftment and regenerative potential of hiPSC-CMs when the cells were administered as a human myocardial patch (hCMP). To extend the duration of CHIR99021 and FGF1 treatment, the molecules were encapsulated in nanoparticles, which were suspended with the hiPSC-CMs in a fibrin scaffold. The nanoparticles were composed of polylactic-co-glycolic acid (PLGA), ~125 nm in diameter (CHIR99021: 123.63 ± 44.48 nm, FGF1: 129.57 ± 45.94 nm), and contained 8.07 μg/mg CHIR99021 or 1.26 ± 0.03 μg/mg FGF1. The encapsulation efficiency was 50.41 ± 1.1% for CHIR99021 and 62.8 ± 1.6% for FGF1, and when 1000 μg of the nanoparticles were incubated in 1000 μL of phosphate-buffered saline (pH 7.4 at 37 °C), 54.69 ± 6.14% of the encapsulated CHIR99021 was released during the first day and 78.76 ± 1.74% was released by day 10, while 54.56 ± 3.14% and 62.62 ± 2.98% of the FGF1 was released by day 3 and day 10, respectively (Supplemental Table 1, Supplemental Fig. 3A). The release kinetics strictly followed the Hixson-Crowell model, and when the nanoparticles were loaded with coumairin-6 and added to cultured hiPSC-CMs, fluorescence images confirmed that the cells consistently internalized the fluorescent label (Supplemental Fig. 3).
53 mice were randomly distributed into five experimental groups; MI was surgically induced in four of the five groups via ligation of the left-anterior descending coronary artery (LAD), and the fifth (Sham) group underwent all surgical procedures for MI induction except LAD ligation. Animals in the NPCF-hCMP group were treated with hCMPs fabricated from hiPSC-CMs (1×106 cells/patch per animal), CHIR99021-loaded nanoparticles (300 μg), and FGF1-loaded nanoparticles (200 μg), animals in the NPE-hCMP group were treated with an hCMP composed of hiPSC-CMs and empty nanoparticles, animals in the NPCF-Patch group were treated with a cell-free patch that contained only the CHIR99021- and FGF1-loaded nanoparticles, and animals in the E-Patch group were treated with empty patches lacking both the cells and nanoparticles; the patches were positioned over the infarcted region of the left-ventricular (LV) anterior wall and sutured to the visceral layer of the pericardium. Two mice (one each in the E-Patch and NPCF-Patch groups) died during LAD ligation and one (in the NPE-hCMP group) died 3 days after MI induction; all remaining mice survived until sacrifice on Day 28.
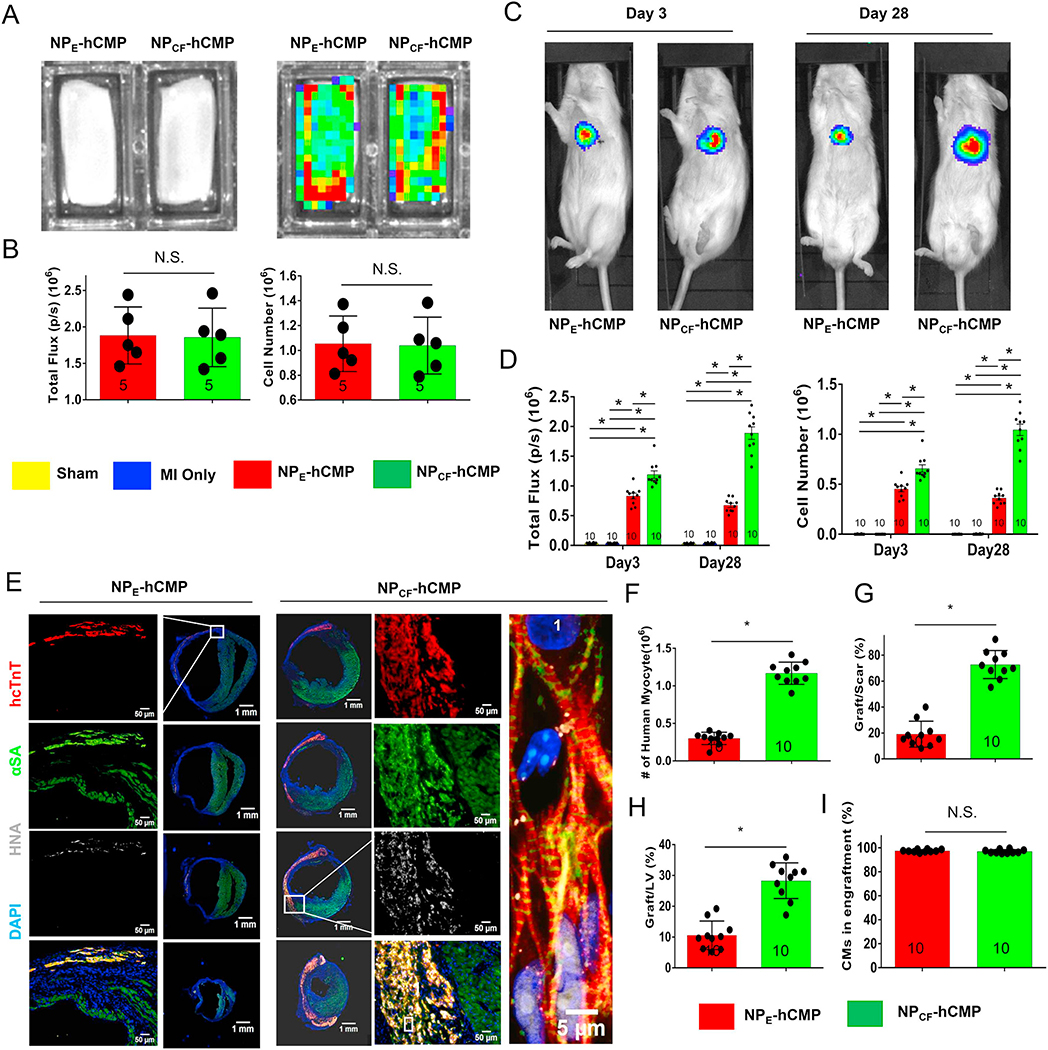
Because the hiPSC-CMs within the transplanted patch carried a luciferase reporter plasmid and were of human origin, engraftment was evaluated in living animals via bioluminescence imaging (BLI) (Fig. 2A–D, Supplemental Fig. 4) 3 and 28 days after transplantation and by identifying cells that co-expressed the human variant of cardiac troponin T (hcTnT) and human nuclear antigen (HNA) in myocardial tissues collected from animals sacrificed on Day 28 (Fig. 2E–F). Both assessments indicated that the transplanted hiPSC-CMs were significantly more common in the NPCF-hCMP group than in NPE-hCMP animals. Measurements of graft size (Fig. 2G–H) were also 3–4 times greater in NPCF-hCMP–treated than in NPE-hCMP–treated hearts and suggested that the hiPSC-CMs occupied ~70% of the myocardial scar and ~25% of the LV in NPCF-hCMP animals. Notably, > 97% of HNA-positive cells also expressed hcTnT on Day 28 (Fig. 2I), which confirms that the population of engrafted cells was composed almost exclusively of cardiomyocytes.
Fig. 2.
hiPSC-CM engraftment was greater in the hearts of mice treated with NPCF-hCMPs than with NPE-hCMPs after MI. Mice were randomly distributed into 5 experimental groups. One group underwent Sham surgery, and the other 4 groups underwent MI induction surgery followed by treatment with hCMPs composed of hiPSC-CMs and nanoparticles that contained CHIR99021 and FGF1 (the NPCF-hCMP group), with hCMPs composed of hiPSC-CMs and empty nanoparticles (the NPE-hCMP group); with patches containing the CHIR99021/FGF-loaded nanoparticles but lacking hiPSC-CMs (the NPCF-Patch group), or patches lacking both the nanoparticles and cells (the E-Patch group). The hiPSC-CMs carried a luciferase reporter plasmid and were of human origin; thus, engraftment was evaluated via (A–D) bioluminescence imaging (BLI) and (E–F) by identifying cells that expressed the human variant of cardiac troponin T (hcTnT) and human nuclear antigen (HNA). (A) Photographic (left) and BLI (right) images of NPE-hCMPs and NPCF-hCMPs were taken 1 week before transplantation, and (B) BLI signal intensity (left) was measured and compared to a standard curve to determine the total number of hiPSC-CMs (right). (C) Three and 28 days after MI induction and treatment, mice in the NPE-hCMP and NPCF-hCMP groups were injected with luciferin, and BLI images were collected 10 min later; then, (D) BLI signal intensity (left) was measured and compared to a standard curve to determine the number of engrafted cells. (E) Serial sections from the hearts of NPE-hCMP and NPCF-hCMP mice that had been sacrificed 4 weeks after MI induction and treatment were stained for the presence of hcTnT, HNA, and α sarcomeric actin (αSA) and nuclei were counterstained with DAPI; then, (F) engraftment was quantified by counting the number of cells that expressed both hcTnT and HNA. (G–H) The area occupied by the engrafted cells was measured and expressed as a percentage of the area of (G) the scar and (H) the left ventricle. (I) Cells that expressed HNA and both HNA and hcTnT were counted; then, the proportion of transplanted cells that displayed a cardiomyocyte identity was calculated as the ratio of double-positive to HNA-positive cells and expressed as a percentage. The number of animals evaluated for each treatment group is displayed at the base of the corresponding column. Five randomly selected viewing fields were evaluated per section and twenty sections were evaluated per animal. *p < .01, two-way ANOVA with Sidak correction for multiple comparisons in panel D; *p < .01, two-tailed Student’s t-test for panels B and F–I.
3.3. CHIR99021- and FGF1-containing nanoparticles increase the potency of transplanted hCMPs for myocardial repair and recovery after MI in mice
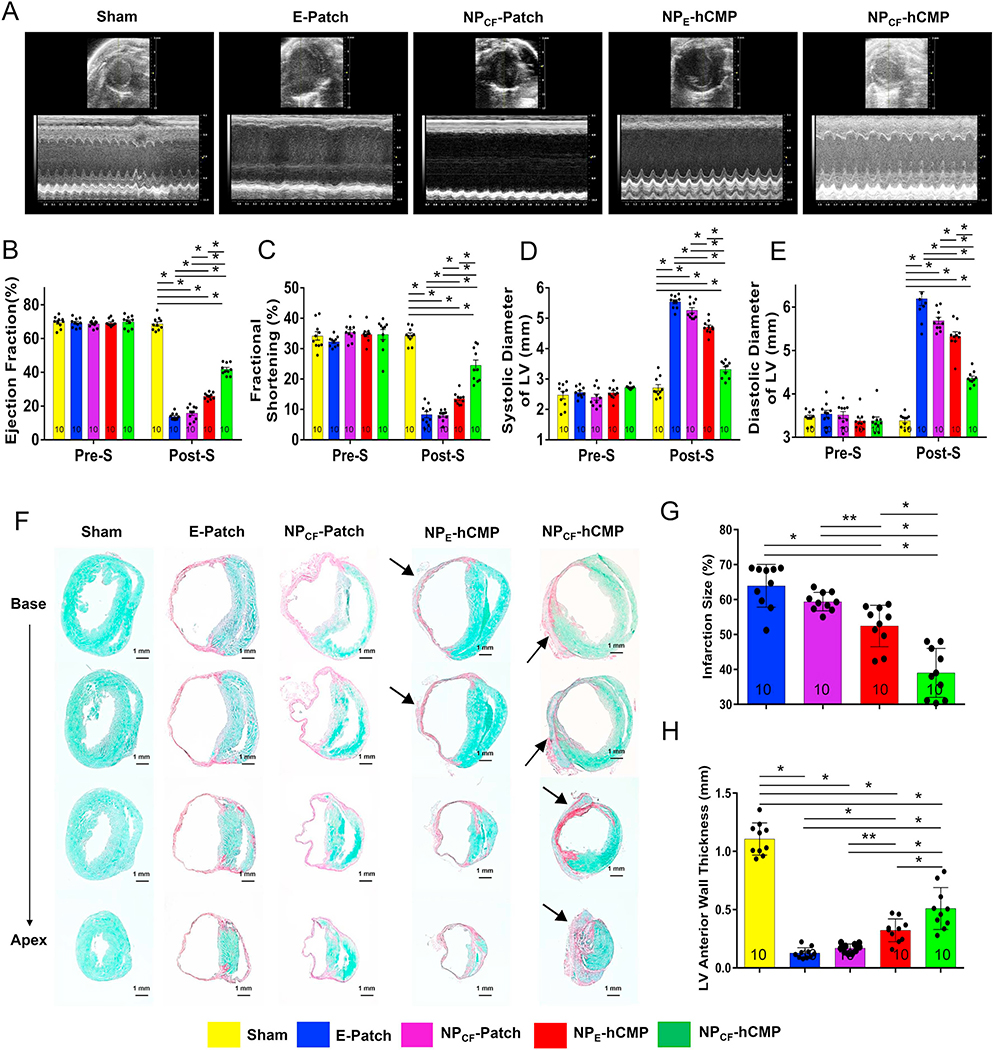
Echocardiographic assessments (Fig. 3A) of cardiac function confirmed that measurements of LV ejection fraction (Fig. 3B), fractional shortening (Fig. 3C), end-systolic diameter (Fig. 3D), and end-diastolic diameter (Fig. 3E) in the five treatment groups were equivalent before MI or Sham surgery and significantly better in Sham animals than in any of the four groups that underwent MI induction on Day 28. However, all four echocardiographic parameters, as well as histological assessments (Fig. 3F) of infarct size (Fig. 3G) and LV anterior wall thickness (Fig. 3H), were significantly better on Day 28 in NPCF-hCMP animals than in any other group that underwent MI induction. Measurements on Day 28 were also significantly better in NPE-hCMP animals than in NPCF-Patch or E-Patch animals, while assessments in the latter two treatment groups were similar.
Fig. 3.
NPCF-hCMPs were more potent than NPE-hCMPs for myocardial repair and functional recovery after MI in mice. (A) Echocardiographic assessments of left ventricular function were performed in animals from the Sham, E-Patch, NPCF-Patch, NPE-hCMP, and NPCF-hCMP groups before MI induction or Sham surgery (Pre-S) and 4 weeks afterward (Post-S) and then used to calculate the left ventricular (LV) (B) ejection fraction (EF), (C) fractional shortening (FS), and diameters during (D) systole and (E) diastole. (F–H) Serial sections from the hearts of mice sacrificed 4 weeks after MI were (F) stained with fast green to identify functional myocardium and with Sirius red to identify scar tissue; then, (G) infarct size was measured and expressed as a percentage of the total LV surface area, and (H) the thickness of the LV anterior wall was measured and expressed in millimeters. The number of animals evaluated for each treatment group is displayed at the base of the corresponding column. Also, for the histological experiments, five randomly selected viewing fields were evaluated per section and at least twenty sections were evaluated per animal. *p < .01, two-way ANOVA with Tukey correction for multiple comparisons in panels B–E; *p < .01, **p < .05, one-way ANOVA with Tukey correction for multiple comparisons in panels G and H. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. CHIR99021- and FGF1-containing nanoparticles promote hiPSC-CM cell cycle activity and proliferation, reduce hiPSC-CM apoptosis, and increase vascularity in hCMP-treated infarcted mouse hearts
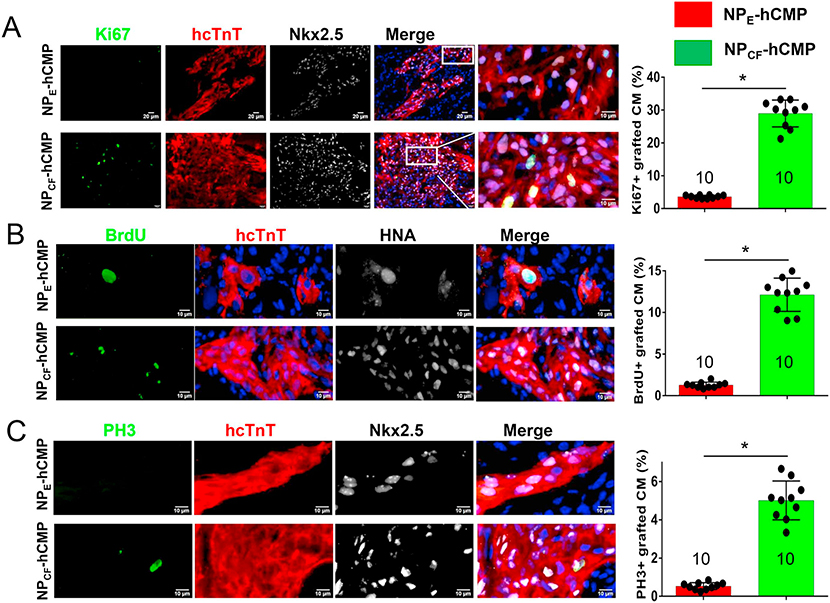
Our BLI assessments (Fig. 2A–D) appeared to be higher on Day 28 than on Day 3 in the NPCF-hCMP group but not in NPE-hCMP animals, which suggests that the CHIR99021- and FGF1-loaded nanoparticles promoted hiPSC-CM proliferation after engraftment. This supposition was confirmed by quantifying the proportion of engrafted hiPSC-CMs that expressed Ki67 and PH3 or incorporated BrdU on Day 28. Despite our in-vitro observation that most of the CHIR99021 and FGF1 will be released from cultured nanoparticles well before this time point, each of the three markers for proliferation and/or cell cycle activity colocalized with HNA/hcTNT expression more frequently in tissues harvested from NPCF-hCMP–treated than from NPE-hCMP–treated hearts (Fig. 4). Notably, even relatively low concentrations of CHIR99021 and FGF1 were sufficient to drive cell cycle progression in cultured hiPSC-CMs (Supplemental Figs. 1 and 2), and PH3-positive cells were significantly more common in NPCF-hCMPs than in NPE-hCMPs when cultured for 7, 14, and 21 after fabrication (Supplemental Fig. 5). Collectively, these observations could suggest that once the hiPSC-CM cell cycle was activated by the higher initial concentrations of CHIR99021 and FGF1, cell cycle activity could be maintained by the much lower, residual concentrations present weeks later.
Fig. 4.
hiPSC-CM cell cycle activity and proliferation were greater in NPCF-hCMPs than in NPE-hCMPs four weeks after transplantation into infarcted mouse hearts. (A-C) Engrafted hiPSC-CMs in the NPCF-hCMPs and in the NPE-hCMPs were identified in mouse hearts at week 4 after MI induction and treatment via immunofluorescent staining for the expression of hcTnT and HNA or hNkx2.5; then, the proportion of hcTnT-hNkx2.5 double-positive cells (A and C) or hcTnT-HNA double-positive cells (B) that also stained positively for (A) Ki67 (a proliferation marker) (B) BrdU incorporation (an S-phase marker), and (C) PH3 (an M-phase marker) was determined and expressed as a percentage. The number of animals evaluated for each treatment group is displayed at the base of the corresponding column. Five randomly selected viewing fields were evaluated per section and at least six sections were evaluated per animal. *p < .01, two-tailed Student’s t-test.
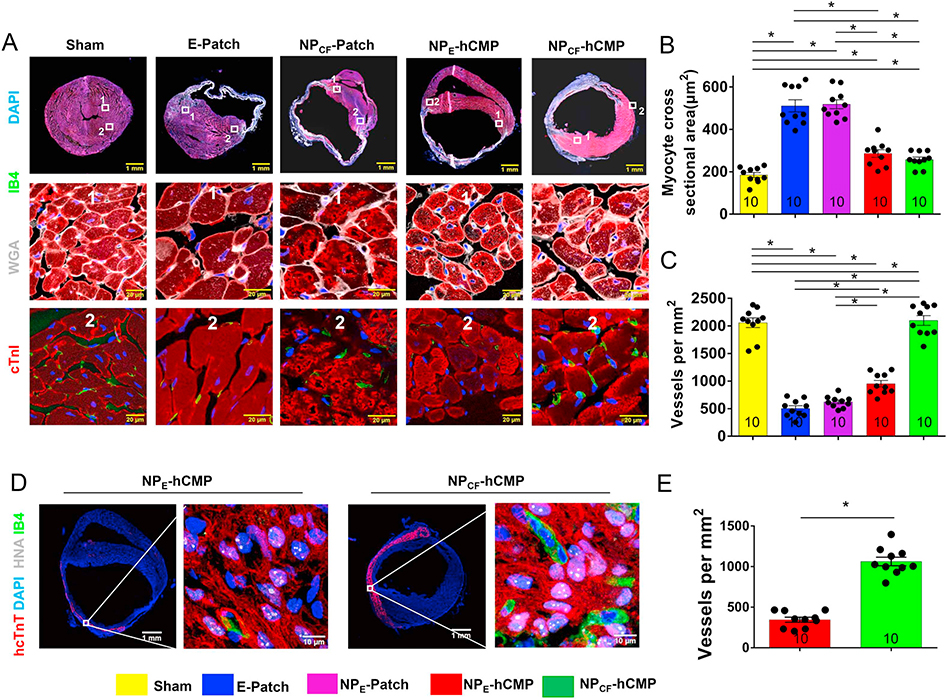
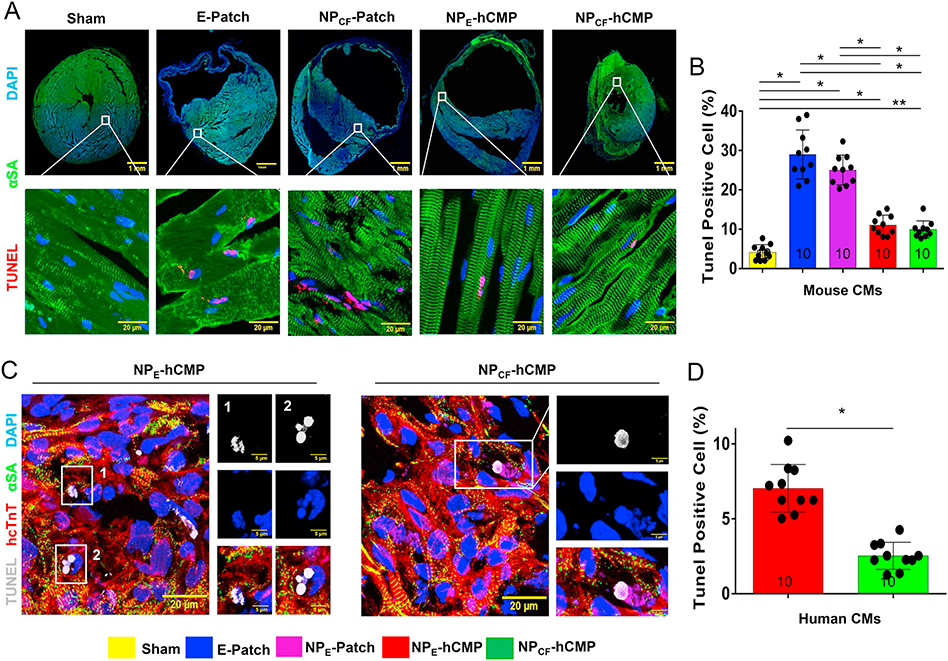
When compared to assessments conducted in samples from the border zones (BZ) of hearts from NPCF-Patch or E-Patch animals, cardiomyocyte cross-sectional surface areas were significantly smaller (Fig. 5A–B), the proportion of cells that expressed the endothelial marker isolectin B4 was significantly larger (Fig. 5A, C), and cardiomyocytes that were positive in apoptosis assays (TUNEL staining [25,26] and caspace 3 expression [27]) were significantly less common (Fig. 6A–B, Supplemental Fig. 6) in the BZs of NPCF-hCMP– and NPE-hCMP–treated hearts. Cells in the infarct patch-transplanted area which expressed IB4 (Fig. 5D) were significantly more abundant in NPCF-hCMP–treated animals than in the NPE-hCMP–treated group (Fig. 5E). Thus, both of the cell-containing hCMPs appeared to impede cardiomyocyte hypertrophy, promote vascularization, and reduce apoptosis; however, the pro-angiogenic effect was significantly more pronounced in both the BZ and the infarct zone (IZ) of hearts from the NPCF-hCMP group than in NPE-hCMP–treated hearts. Furthermore, despite some evidence suggesting that the loss of GSK-3α/β activity leads to cell cycle progression, checkpoint activation, and subsequent apoptosis in the cardiomyocytes of adult mice [28], TUNEL-positive cells were significantly less common in the IZ (Fig. 6C–D), but not the BZ, of NPCF-hCMP–treated than NPE-hCMP–treated hearts, which suggests that the CHIR99021- and FGF1-loaded nanoparticles increased apoptosis resistance in the transplanted hiPSC-CMs.
Fig. 5.
hCMP transplantation reduced cardiomyocyte hypertrophy and promoted angiogenesis four weeks after transplantation into infarcted mouse hearts. Animals were sacrificed 4 weeks after MI induction or Sham surgery; then, (A–C) sections from the border-zone of the infarct were collected and (A) stained with wheat germ agglutinin (WGA) to visualize cell borders, and for the expression of cardiac troponin I (cTnI) and isolectin B4 (IB4) to visualize cardiomyocytes and endothelial cells, respectively. Nuclei were counterstained with DAPI. (B) Cardiomyocyte cross-sectional surface areas were measured and expressed in square micrometers. (C) Vessel density was quantified as the number of IB4-positive vascular structures per square millimeter. (D–E) Sections from the infarcted zone in hearts from NPCF-hCMP and NPE-hCMP animals were (D) stained for the expression of human nuclear antigen (HNA), human cardiac troponin T (hcTnT), and IB4 expression; nuclei were counterstained with DAPI. (E) Vessel density was quantified as the number of IB4-positive vascular structures per square millimeter. The number of animals evaluated for each treatment group is displayed at the base of the corresponding column. Five randomly selected viewing fields were evaluated per section and at least ten sections were evaluated per animal. *p < .01, one-way ANOVA with Tukey correction for multiple comparisons in panels B and C; *p < .01, two-tailed Student’s t-test in panel E.
Fig. 6.
hiPSC-CM apoptosis was lower in NPCF-hCMPs than in NPE-hCMPs four weeks after transplantation into infarcted mouse hearts. Mice were sacrificed 4 weeks after MI or Sham surgery. (A–B) Sections were collected from the border zone (BZ) of the infarct and (A) stained for the expression of α sarcomeric actin (αSA) to visualize cardiomyocytes, and TUNEL-stained to identify apoptotic cells; nuclei were counterstained with DAPI. (B) Apoptosis among all cardiomyocytes (i.e., both native cardiomyocytes and any hiPSC-CMs that may have been present) was quantified as the proportion of αSA-expressing cells that were also positive for TUNEL staining and expressed as a percentage. (C–D) Sections from the infarcted zone in hearts from NPCF-hCMP and NPE-hCMP animals were (C) stained for the expression of αSA and human cardiac troponin T (hcTnT) to visualize hiPSC-CMs, and then TUNEL-stained; nuclei were counterstained with DAPI. (D) hiPSC-CM apoptosis was quantified as the proportion of hcTnT-expressing cells that were also positive for TUNEL staining and expressed as a percentage. The number of animals evaluated for each treatment group is displayed at the base of the corresponding column. Five randomly selected viewing fields were evaluated per section and at least ten sections were evaluated per animal. *p < .01, **p < .05, one-way ANOVA with Tukey correction for multiple comparisons in panel B; *p < .01, two-tailed Student’s t-test in panel D.
3.5. hiPSC-CMs remain immature 28 days after NPCF- or NPE-hCMP transplantation in infarcted mouse hearts
Phenotypically, hiPSC-CMs more closely resemble neonatal cardiomyocytes than the cardiomyocytes of adult mammals [29], and some evidence suggests that immature hiPSC-CMs may cause ventricular arrhythmia after transplantation into primate hearts [30]. Thus, we evaluated the maturity of transplanted hiPSC-CMs in both hCMP-treatment groups by visually comparing fluorescently stained sections from the IZ and the remote (i.e., noninfarcted) zone (RZ) of the same heart (Supplemental Fig. 7). In the RZ, native cardiomyocytes displayed prominent and well-localized expression of cardiac troponin I (cTnI) in sarcomeres, of N-Cadherin and Connexin 43 (adherens and gap junctions) in the intercalated disks, and of Caveolin-3 (t-tubules) at the cell border, whereas the same proteins in hiPSC-CMs of the IZ (and, to a lesser extent, in native cardiomyocytes of the BZ) were disorganized and expressed at lower levels.
4. Discussion
Transplanted stem-cell–derived cardiac cells are a promising treatment for repairing damaged myocardium and improving function in infarcted hearts, but their therapeutic potency is hampered by the poor rate of cell engraftment and survival, which has become one of the primary concerns associated with the development of these therapies [3]. Previously, we have shown that the engraftment of transplanted hiPSC-CMs can be increased via the overexpression of cyclin D2, which dramatically induced cell cycle activity and enabled the cells that survived transplantation to proliferate and repopulate the infarct [31]. In our current study, we developed a nongenetic method for enhancing the cell cycle activity of hiPSC-CMs by suspending them in a fibrin-based patch with nanoparticles that released FGF1 and CHIR99021 over an extended period; then, we compared these engineered constructs (NPCF-hCMPs) with patches containing hiPSC-CMs and chemical-free nanoparticles (NPE-hCMPs) in a mouse MI model. The number of engrafted cells was significantly greater in the NPCF-hCMP–treatment group than in NPE-hCMP animals, and NPCF-hCMP administration was associated with significantly greater improvements in LV performance, infarct size, wall thickness, angiogenesis, and hiPSC-CM apoptosis. To our knowledge, these results are the first to show that the benefits associated with hCMP transplantation in infarcted hearts can be significantly increased via chemical activation of the cell cycle and support the therapeutic benefits of NPCF-hCMP administration.
Because the proliferation of transplanted hiPSC-CMs was chemically induced, the nongenetic approach employed here may be more easily translated to the clinic than the method described in our earlier study, in which cyclin D2 cDNA was linked to the myosin heavy chain promoter and delivered to the hiPSCs before differentiation. Furthermore, although the use of nanoparticles for long-term release of cytokines or other chemical agents remains novel in the cardiovascular field, they have been extensively developed for the treatment of cancer [23]. Nanoparticles can also be created from a variety of polymers, including chitosan-alginate [24], which has been tested in a rat MI model; the PLGA polymer used here and in our previous investigation is approved for use in humans by the US Food and Drug Administration [32].
In animals from the NPCF-hCMP group, the number of engrafted hiPSC-CMs appeared to increase from Day 3 to Day 28 after transplantation, which strongly suggests that the improvement in graft size evolved at least partially via the proliferation of an initially smaller number of engrafted cells. However, the engraftment rate was also higher on Day 3, and vascular density was greater on Day 28, in NPCF-hCMP than in NPE-hCMP animals, so CHIR99021 and FGF1 may also have induced activity in native cardiac cells (e.g., the proliferation of endothelial and smooth-muscle cells) that increased survival of the transplanted hiPSC-CMs and contributed to functional improvement. Our observations are also limited by the study’s duration (4 weeks), which precluded us from examining the long-term fate of the transplanted cells. Notably, the engrafted cells did not appear fully mature after hCMP administration, which could have important implications for patient safety, because some evidence suggests that immature hiPSC-CMs may cause ventricular arrhythmia [30]. Thus, future investigations are needed to fully characterize the mechanisms by which nanoparticle-mediated CHIR99021 and FGF1 administration improved myocardial recovery from MI, and whether hCMP maturation can be increased via electrical/mechanical stimulation or the inclusion of pro-maturation hormones/chemicals [4,33,34], as well as the long-term safety and effectiveness of treatment.
In conclusion, we demonstrate for the first time that the effectiveness of a transplanted, hiPSC-CM–containing hCMP for the treatment of MI in mice can be significantly increased via the inclusion of nanoparticles carrying CHIR99021 and FGF1, which promoted hiPSC-CM cell cycle activity and proliferation 4 weeks after hCMP administration. The combined chemical treatment also increased angiogenesis at the border of the infarct and reduced hiPSC-CM apoptosis, which may have contributed to the observed improvements in LV remodeling and chamber function.
Supplementary Material
Acknowledgments
The authors wish to thank W. Kevin Meisner, PhD, ELS, for his editorial assistance with manuscript preparation.
Funding
This work was supported by National Institute of Health grants (NHLBI R01 grants: HL95077, HL114120, HL131017, U01 HL134764 to JZ, and NHLBI R01 HL142627 to WZ). In addition, in part by an award from the American Heart Association Scientist Development grant (16SDG30410018).
Abbreviations
- hiPSC-CMs
Human induced pluripotent stem cell-derived cardiomyocytes
- hCMP
Patch containing human induced pluripotent stem cell-derived cardiomyocytes
- NPCF-hCMP
hCMP containing hiPSC-CMs and nanoparticles formulated with CHIR99021 and FGF1
- NPE-hCMP
hCMP containing hiPSC-CMs and empty nanoparticles
- NPCF-Patch
Patch containing nanoparticles formulated with CHIR99021 and FGF1 but lacking cells
- E-Patch
Patch lacking both nanoparticles and cells
- BZ
Border zone
- IZ
Infarct zone
- MI
Myocardial infarction
- RZ
Remote zone
- EF
Ejection fraction
- FS
Fractional shortening
Footnotes
Declaration of Competing Interest
The authors have declared that no conflict of interest exists.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.yjmcc.2020.03.003.
References
- [1].Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, American E Heart Association Council on, C. Prevention Statistics, S. Stroke Statistics, Heart disease and stroke statistics-2018 update: a report from the American Heart Association, Circulation 137 (12) (2018) (e67–e492). [DOI] [PubMed] [Google Scholar]
- [2].Soonpaa MH, Field LJ, Survey of studies examining mammalian cardiomyocyte DNA synthesis, Circ. Res. 83 (1) (1998) 15–26. [DOI] [PubMed] [Google Scholar]
- [3].Yanamandala M, Zhu W, Garry DJ, Kamp TJ, Hare JM, Jun HW, Yoon YS, Bursac N, Prabhu SD, Dorn GW 2, Bolli R, Kitsis RN, Zhang J, Overcoming the roadblocks to cardiac cell therapy using tissue engineering, J. Am. Coll. Cardiol. 70 (6) (2017) 766–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gao L, Gregorich ZR, Zhu W, Mattapally S, Oduk Y, Lou X, Kannappan R, Borovjagin AV, Walcott GP, Pollard AE, Fast VG, Hu X, Lloyd SG, Ge Y, Zhang J, Large cardiac muscle patches engineered from human induced-pluripotent stem cell-derived cardiac cells improve recovery from myocardial infarction in swine, Circulation 137 (16) (2018) 1712–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wendel JS, Ye L, Tao R, Zhang J, Zhang J, Kamp TJ, Tranquillo RT, Functional effects of a tissue-engineered cardiac patch from human induced pluripotent stem cell-derived Cardiomyocytes in a rat infarct model, Stem Cells Transl. Med. 4 (11) (2015) 1324–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Xiong Q, Ye L, Zhang P, Lepley M, Swingen C, Zhang L, Kaufman DS, Zhang J, Bioenergetic and functional consequences of cellular therapy: activation of endogenous cardiovascular progenitor cells, Circ. Res. 111 (4) (2012) 455–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ye L, Basu J, Zhang J, Fabrication of a myocardial patch with cells differentiated from human-induced pluripotent stem cells, Methods Mol. Biol. 1299 (2015) 103–114. [DOI] [PubMed] [Google Scholar]
- [8].Ye L, Chang YH, Xiong Q, Zhang P, Zhang L, Somasundaram P, Lepley M, Swingen C, Su L, Wendel JS, Guo J, Jang A, Rosenbush D, Greder L, Dutton JR, Zhang J, Kamp TJ, Kaufman DS, Ge Y, Zhang J, Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells, Cell Stem Cell 15 (6) (2014) 750–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Menasche P, Vanneaux V, Hagege A, Bel A, Cholley B, Cacciapuoti I, Parouchev A, Benhamouda N, Tachdjian G, Tosca L, Trouvin JH, Fabreguettes JR, Bellamy V, Guillemain R, Suberbielle Boissel C, Tartour E, Desnos M, Larghero J, Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report, Eur. Heart J. 36 (30) (2015) 2011–2017. [DOI] [PubMed] [Google Scholar]
- [10].Menasche P, Vanneaux V, Hagege A, Bel A, Cholley B, Parouchev A, Cacciapuoti I, Al-Daccak R, Benhamouda N, Blons H, Agbulut O, Tosca L, Trouvin JH, Fabreguettes JR, Bellamy V, Charron D, Tartour E, Tachdjian G, Desnos M, Larghero J, Transplantation of human embryonic stem cell-derived cardiovascular progenitors for severe ischemic left ventricular dysfunction, J. Am. Coll. Cardiol. 71 (4) (2018) 429–438. [DOI] [PubMed] [Google Scholar]
- [11].Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song L, Sirabella D, Morikawa K, Teles D, Yazawa M, Vunjak-Novakovic G, Advanced maturation of human cardiac tissue grown from pluripotent stem cells, Nature 556 (7700) (2018) 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ye L, Zimmermann WH, Garry DJ, Zhang J, Patching the heart: cardiac repair from within and outside, Circ. Res. 113 (7) (2013) 922–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhang J, Zhu W, Radisic M, Vunjak-Novakovic G, Can we engineer a human cardiac patch for therapy? Circ. Res. 123 (2) (2018) 244–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Beenken A, Mohammadi M, The FGF family: biology, pathophysiology and therapy, Nat. Rev. Drug Discov. 8 (3) (2009) 235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Engel FB, Schebesta M, Duong MT, Lu G, Ren S, Madwed JB, Jiang H, Wang Y, Keating MT, p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes, Genes Dev. 19 (10) (2005) 1175–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Engel FB, Hsieh PC, Lee RT, Keating MT, FGF1/p38 MAP kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction, Proc. Natl. Acad. Sci. U. S. A. 103 (42) (2006) 15546–15551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Htun P, Ito WD, Hoefer IE, Schaper J, Schaper W, Intramyocardial infusion of FGF-1 mimics ischemic preconditioning in pig myocardium, J. Mol. Cell. Cardiol. 30 (4) (1998) 867–877. [DOI] [PubMed] [Google Scholar]
- [18].Buehler A, Martire A, Strohm C, Wolfram S, Fernandez B, Palmen M, Wehrens XH, Doevendans PA, Franz WM, Schaper W, Zimmermann R, Angiogenesis-independent cardioprotection in FGF-1 transgenic mice, Cardiovasc. Res. 55 (4) (2002) 768–777. [DOI] [PubMed] [Google Scholar]
- [19].Kerkela R, Kockeritz L, Macaulay K, Zhou J, Doble BW, Beahm C, Greytak S, Woulfe K, Trivedi CM, Woodgett JR, Epstein JA, Force T, Huggins GS, Deletion of GSK-3beta in mice leads to hypertrophic cardiomyopathy secondary to cardiomyoblast hyperproliferation, J. Clin. Invest. 118 (11) (2008) 3609–3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Titmarsh DM, Glass NR, Mills RJ, Hidalgo A, Wolvetang EJ, Porrello ER, Hudson JE, Cooper-White JJ, Induction of human iPSC-derived cardiomyocyte proliferation revealed by combinatorial screening in high density microbioreactor arrays, Sci. Rep. 6 (2016) 24637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Uosaki H, Magadum A, Seo K, Fukushima H, Takeuchi A, Nakagawa Y, Moyes KW, Narazaki G, Kuwahara K, Laflamme M, Matsuoka S, Nakatsuji N, Nakao K, Kwon C, Kass DA, Engel FB, Yamashita JK, Identification of chemicals inducing cardiomyocyte proliferation in developmental stage-specific manner with pluripotent stem cells, Circ. Cardiovasc. Genet. 6 (6) (2013) 624–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hara H, Takeda N, Kondo M, Kubota M, Saito T, Maruyama J, Fujiwara T, Maemura S, Ito M, Naito AT, Harada M, Toko H, Nomura S, Kumagai H, Ikeda Y, Ueno H, Takimoto E, Akazawa H, Morita H, Aburatani H, Hata Y, Uchiyama M, Komuro I, Discovery of a small molecule to increase Cardiomyocytes and protect the heart after ischemic injury, JACC Basic Transl. Sci. 3 (5) (2018) 639–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Oduk Y, Zhu W, Kannappan R, Zhao M, Borovjagin AV, Oparil S, Zhang JJ, VEGF nanoparticles repair the heart after myocardial infarction, Am. J. Physiol. Heart Circ. Physiol. 314 (2) (2018) H278–H284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Binsalamah ZM, Paul A, Khan AA, Prakash S, Shum-Tim D, Intramyocardial sustained delivery of placental growth factor using nanoparticles as a vehicle for delivery in the rat infarct model, Int. J. Nanomedicine 6 (2011) 2667–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zhu W, Soonpaa MH, Chen H, Shen W, Payne RM, Liechty EA, Caldwell RL, Shou W, Field LJ, Acute doxorubicin cardiotoxicity is associated with p53-induced inhibition of the mammalian target of rapamycin pathway, Circulation 119 (1) (2009) 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhu W, Zhang W, Shou W, Field LJ, P53 inhibition exacerbates late-stage anthracycline cardiotoxicity, Cardiovasc. Res. 103 (1) (2014) 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Toischer K, Zhu W, Hunlich M, Mohamed BA, Khadjeh S, Reuter SP, Schafer K, Ramanujam D, Engelhardt S, Field LJ, Hasenfuss G, Cardiomyocyte proliferation prevents failure in pressure overload but not volume overload, J. Clin. Invest. 127 (12) (2017) 4285–4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhou J, Ahmad F, Parikh S, Hoffman NE, Rajan S, Verma VK, Song J, Yuan A, Shanmughapriya S, Guo Y, Gao E, Koch W, Woodgett JR, Madesh M, Kishore R, Lal H, Force T, Loss of adult cardiac myocyte GSK-3 leads to mitotic catastrophe resulting in fatal dilated cardiomyopathy, Circ. Res. 118 (8) (2016) 1208–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Garg P, Garg V, Shrestha R, Sanguinetti MC, Kamp TJ, Wu JC, Human induced pluripotent stem cell-derived cardiomyocytes as models for cardiac channelopathies: a primer for non-electrophysiologists, Circ. Res. 123 (2) (2018) 224–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shiba Y, Gomibuchi T, Seto T, Wada Y, Ichimura H, Tanaka Y, Ogasawara T, Okada K, Shiba N, Sakamoto K, Ido D, Shiina T, Ohkura M, Nakai J, Uno N, Kazuki Y, Oshimura M, Minami I, Ikeda U, Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts, Nature 538 (7625) (2016) 388–391. [DOI] [PubMed] [Google Scholar]
- [31].Zhu W, Zhao M, Mattapally S, Chen S, Zhang J, CCND2 overexpression enhances the regenerative potency of human induced pluripotent stem cell-derived cardiomyocytes: remuscularization of injured ventricle, Circ. Res. 122 (1) (2018) 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Khan I, Gothwal A, Sharma AK, Kesharwani P, Gupta L, Iyer AK, Gupta U, PLGA nanoparticles and their versatile role in anticancer drug delivery, Crit. Rev. Ther. Drug Carrier Syst. 33 (2) (2016) 159–193. [DOI] [PubMed] [Google Scholar]
- [33].Parikh SS, Blackwell DJ, Gomez-Hurtado N, Frisk M, Wang L, Kim K, Dahl CP, Fiane A, Tonnessen T, Kryshtal DO, Louch WE, Knollmann BC, Thyroid and glucocorticoid hormones promote functional T-tubule development in human-induced pluripotent stem cell-derived Cardiomyocytes, Circ. Res. 121 (12) (2017) 1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jackman CP, Ganapathi AM, Asfour H, Qian Y, Allen BW, Li Y, Bursac N, Engineered cardiac tissue patch maintains structural and electrical properties after epicardial implantation, Biomaterials 159 (2018) 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.