ABSTRACT
We described a 32-year-old man who developed severe drug-induced liver injury after using Ligandrol (LGD-4033). The diagnosis was confirmed by a liver biopsy that showed cholestatic hepatitis with a mild portal, periportal, and perisinusoidal fibrosis. Ligandrol is a selective androgen receptor modulator that is available over the counter and via the internet.
INTRODUCTION
Unlike anabolic androgenic steroids, selective androgen receptor modulators (SARMs) have tissue-selective activities. They express anabolic effects in the bone and muscle tissues, without causing androgenic side effects such as acne and prostate enlargement.1–4 Therefore, they became popular among athletes. They are under investigation for the treatment of muscle wasting in cancer patients and osteoporosis; however, their use is not approved by the US Food and Drug Administration (FDA) and by the World Anti-Doping Agency.1–6 FDA issued a warning against using SARM because of potential liver injury.7–10 We report a case of severe drug-induced liver injury (DILI) secondary to Ligandrol (LGD-4033).
CASE REPORT
A 32-year-old white man without any chronic medical problem was admitted to our hospital for elevated liver enzymes and jaundice. His other symptoms included fatigue, pruritus, and weight loss that started approximately 50 days before presentation. He reported that he took 1 mL (10 mg) of Ligandrol (LGD-4033) daily for approximately 2 weeks for muscle building (approximately a total of 15 mL of Ligandrol). Subsequently, he started feeling ill, had diffuse pruritus and body aches, and followed by jaundice. He was admitted to an outside hospital for 24 hours. He denies taking any other supplements, over the counter, or prescribed medications. His medical, social, surgical, and family histories were noncontributory. The review of systems included diffuse itching, jaundice, acholic stool, intermittent abdominal pain that was associated with nausea, and 40 lbs of weight loss. Physical examination revealed an ill-appearing malnourished man with icteric sclerae and excoriations on the extremities. He had no asterixis or other signs of hepatic encephalopathy.
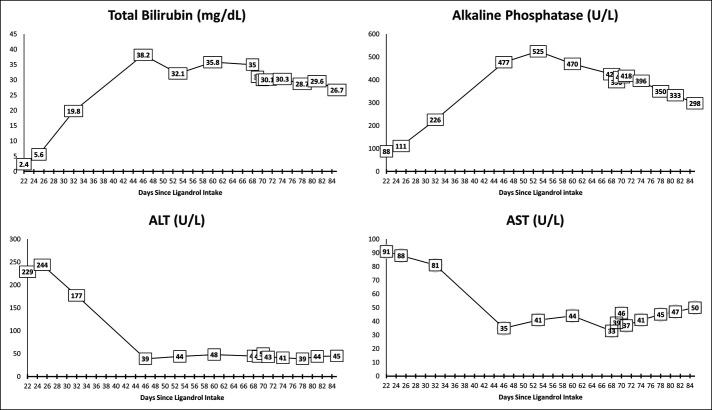
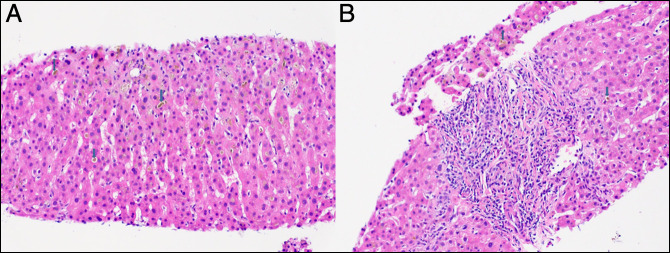
The initial laboratory test results were aspartate aminotransferase 91 IU/L, alanine aminotransferase 229 IU/L, alkaline phosphatase 88 IU/L, total bilirubin 2.4 mg/dL, and albumin 3.8 g/dL. Laboratory results at our hospital were aspartate aminotransferase 33 IU/L, alanine aminotransferase 45, alkaline phosphatase 425 mg/dL, total bilirubin 35.0 mg/dL, direct bilirubin 26.8, and albumin 3.5, international normalized ratio 1.1 (Figure 1). Serological markers for acute hepatitis A, B, and C were negative. Iron level was 56 mcg/dL, total iron-binding capacity 246 mcg/dL, iron % saturation 23%, ferritin 422 ng/mL, ceruloplasmin 57 mg/dL, and alpha-1-antitrypsin 196.4 mg/dL. Serum antinuclear antibody, antimitochondrial antibody, antismooth muscle antibody, and anti–liver-kidney microsomal antibody were negative. Immunoglobulin G level was 748 mg/dL. Abdominal ultrasound and computed tomography revealed hepatomegaly. The magnetic resonance cholangiopancreatogram showed small hepatic cyst and splenomegaly and no intrahepatic or extrahepatic biliary dilatation. The patient underwent a transjugular liver biopsy that showed cholestatic hepatitis with mild portal, periportal, and perisinusoidal fibrosis consistent with a DILI in the setting of Ligandrol use (Figure 2). No hyaline globules were seen on periodic acid–Schiff–diastase stain. Iron stain showed 2+ staining of the hepatocytes.
Figure 1.
Serum total bilirubin, alkaline phosphatase level, and aminotransferase levels since the patient started taking 1 mL (10 mg) of Ligandrol daily for 2 weeks and then stopped. Day 22 shows the laboratory values approximately 1 week after the patient stopped Ligandrol. ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Figure 2.
Liver biopsy showing (A) liver parenchyma with canalicular bile plugs (arrows) and (B) portal tracts with mild chronic lymphocyte-predominant inflammation and canalicular bile plugs (arrows). The bile duct is preserved and unremarkable (Hematoxylin and Eosin stain 200×).
DISCUSSION
The patient took Ligandrol, which was sold over the counter as a muscle-building supplement. Ligandrol contains LGD-4033 [4-((R)-2-((R)-2,2,2-Trifluoro-1-hydroxyethyl)pyrrolidin-1-yl)-2-(trifluoromethyl)benzonitrile] that is also known as VK5211.11 SARM acts as a ligand that enters the cell by diffusion and binds to the androgen receptor in the cytoplasm, forming receptor/ligand complex that translocates to the nucleus where it binds to the DNA and acts as a transcriptional regulator of androgen-responsive genes.4,12,13
A placebo-controlled randomized clinical trial of LGD-4033 conducted on 76 healthy men showed no serious adverse effects and no significant change in serum aminotransferases at daily doses of 0.1, 0.3, and 1 mg over 21 days.14 Our patient took 10 mg of LGD-4033 daily, which is 10 to 100 times higher than the daily doses (0.1 mg, 0.3 mg, and 1.0 mg) administered in this clinical trial.14 Hepatotoxicity because of drugs can be intrinsic (dose-dependent) or idiosyncratic (not dose-dependent), and most cases of DILI are due to idiosyncratic reactions, and drugs with idiosyncratic DILI have a dose-dependent component.15 This information suggests that the patient's DILI was either intrinsic or idiosyncratic with a dose-dependent component. We did not do genetic testing to test for hepatic transporter protein abnormalities or other genetic abnormalities that can predispose the patient to DILI. However, polymorphisms of hepatic transporter proteins increase the risk of drug-induced cholestatic injury.16 It is also possible that the Ligandrol that the patient took had other different unapproved drugs.5
After ruling out other possible causes of acute liver injury, a diagnosis was made based on the timing of the administration of Ligandrol, the improvement of total bilirubin, alkaline phosphatase, and aminotransferases after the patient stopped taking it, and the liver biopsy findings. In conclusion, we reported a severe Ligandrol (LGD-4033)-induced hepatotoxicity. Despite FDA's warning, Ligandrol and other SARMs continue to be sold over the counter and via the internet as body-building products.7–10 Therefore, a higher level of regulations for these products is required to prevent future life-threatening hepatotoxicity and potential cardiovascular adverse events.
DISCLOSURES
Author contributions: M. Barbara and AL Mindikoglu wrote and reviewed the manuscript for important intellectual content. S. Dhingra reviewed the manuscript for important intellectual content. AL Mindikoglu is the article guarantor.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
REFERENCES
- 1.Dalton JT, Taylor RP, Mohler ML, Steiner MS. Selective androgen receptor modulators for the prevention and treatment of muscle wasting associated with cancer. Curr Opin Support Palliat Care. 2013;7:345–51. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann DB, Komrakova M, Pflug S, et al. Evaluation of ostarine as a selective androgen receptor modulator in a rat model of postmenopausal osteoporosis. J Bone Miner Metab. 2019;37:243–55. [DOI] [PubMed] [Google Scholar]
- 3.Morimoto M, Aikawa K, Hara T, Yamaoka M. Prevention of body weight loss and sarcopenia by a novel selective androgen receptor modulator in cancer cachexia models. Oncol Lett. 2017;14:8066–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solomon ZJ, Mirabal JR, Mazur DJ, et al. Selective androgen receptor modulators: Current knowledge and clinical applications. Sex Med Rev. 2019;7:84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Wagoner RM, Eichner A, Bhasin S, et al. Chemical composition and labeling of substances marketed as selective androgen receptor modulators and sold via the internet. JAMA. 2017;318:2004–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thevis M, Schanzer W. Detection of SARMs in doping control analysis. Mol Cell Endocrinol. 2018;464:34–45. [DOI] [PubMed] [Google Scholar]
- 7.FDA In Brief: FDA Warns Against Using SARMs in Body-Building Products. (https://www.fda.gov/news-events/fda-brief/fda-brief-fda-warns-against-using-sarms-body-building-products). Accessed October 15, 2019. [Google Scholar]
- 8.Warning Letter. (https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/infantry-labs-llc-535333-10232017). Accessed October 15, 2019. [Google Scholar]
- 9.Warning Letter. (https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/panther-sports-nutrition-535341-10232017). Accessed October 15, 2019. [Google Scholar]
- 10.FDA Analysis Shows Body-Building Products Labeled to Contain Steroid and Steroid-Like Substances Continue to Inflict Serious Liver Injury. (https://www.fda.gov/drugs/drug-safety-and-availability/fda-analysis-shows-body-building-products-labeled-contain-steroid-and-steroid-substances-continue). Accessed October 14, 2019. [Google Scholar]
- 11.National Center for Biotechnology Information. PubChem Database. CID=44137686, (https://pubchem.ncbi.nlm.nih.gov/compound/lgd-4033) Accessed January 25, 2020. [Google Scholar]
- 12.Saeed A, Vaught GM, Gavardinas K, et al. 2-Chloro-4-[[(1R,2R)-2-hydroxy-2-methyl-cyclopentyl]amino]-3-methyl-benzonitrile: A transdermal selective androgen receptor modulator (SARM) for muscle atrophy. J Med Chem. 2016;59:750–5. [DOI] [PubMed] [Google Scholar]
- 13.Gao W, Bohl CE, Dalton JT. Chemistry and structural biology of androgen receptor. Chem Rev. 2005;105:3352–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basaria S, Collins L, Dillon EL, et al. The safety, pharmacokinetics, and effects of LGD-4033, a novel nonsteroidal oral, selective androgen receptor modulator, in healthy young men. J Gerontol A Biol Sci Med Sci. 2013;68:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lammert C, Einarsson S, Saha C, et al. Relationship between daily dose of oral medications and idiosyncratic drug-induced liver injury: Search for signals. Hepatology. 2008;47:2003–9. [DOI] [PubMed] [Google Scholar]
- 16.Pan G. Roles of hepatic drug transporters in drug disposition and liver toxicity. Adv Exp Med Biol. 2019;1141:293–340. [DOI] [PubMed] [Google Scholar]