Abstract
Background:
Sedentary behaviors (SB) may exacerbate loss of muscle mass and function, independent of physical activity levels. This study examined the associations of SB with abdominal muscle area and density, a marker of muscle quality, in adults.
Methods:
A total of 1895 participants from the Multi-Ethnic Study of Atherosclerosis completed detailed health history, physical activity and SB questionnaires, computed tomography to quantify body composition, and measurements of inflammatory markers. Analyses included linear and nonlinear regression.
Results:
The mean age and body mass index were 64.6 years and 28 kg·m−2, respectively, and 50% were women. On average, participants engaged in 28 metabolic equivalent hours·week−1 of SB. With adjustment for age, sex, race/ethnicity, physical activity, cardiovascular disease risk factors, and inflammation, multivariable regression modeling revealed a nonlinear (quadratic) relationship between SB and locomotor, stability, and total abdominal muscle density (P < .01) but not muscle area. The SB inflection point at which locomotor, stability, and total abdominal muscle density began to decrease was 38.2, 39.6, and 39.2 metabolic equivalent hours·week−1 of SB, respectively.
Conclusions:
SB is associated with reduced muscle density when practiced as little as 5.5 metabolic equivalent hours·day−1. These findings may have important implications for SB guidelines for targeting skeletal muscle health in older adults.
Keywords: inflammation, muscle mass, sitting, obesity, physical activity, computed tomography
Adults in the United States spend an average of 8 hours· day−1 in sedentary behaviors,1 with studies reporting older adults may spend up to 85% of their waking hours engaged in sedentary behaviors.2 Increased sedentary time has been associated with an elevated risk for cardiovascular disease, type 2 diabetes, functional disability, and premature death.1
Skeletal muscle accounts for 40% of total body mass and is the main determinant of energy expenditure among sedentary individuals. Although regular physical activity is fundamental for preserving skeletal muscle mass, strength and function with aging, sedentary behaviors may exacerbate the loss of muscle mass and strength, independent of physical activity.2,3 In this regard, sedentary time has been shown to be inversely related to physical function and muscular strength in older adults.3–5 Notably, the few cross-sectional studies investigating the association of sedentary behavior and skeletal muscle mass have measured muscle area, but not density, and reported conflicting results.6,7 Muscle density is a marker of muscle quality, with higher density values associated with greater voluntary muscle strength, independent of muscle cross-sectional area, and lower fat infiltration within the muscle.8
Although the mechanisms for loss of muscle mass and function with age are unclear, higher levels of inflammatory markers have been hypothesized to play a role through catabolic effects on muscle tissue.9–11 Thus, it is of growing interest to examine the associations between time spent in sedentary behaviors and muscle area and density and to determine whether these associations are independent of relevant covariates. Given this, the purpose of this study was to examine the associations between sedentary behavior and abdominal muscle area and density, and to determine if the associations were independent of time spent in moderate to vigorous physical activity (MVPA) and other relevant risk factors. We hypothesized that sedentary behavior would be inversely associated with abdominal muscle area and density, independent of MVPA, cardiovascular disease risk factors, adiposity, and markers of inflammation.
Methods
Participants
The Multi-Ethnic Study of Atherosclerosis (MESA) is a longitudinal cohort study of adults from 6 regions across the United States. The overall design of the MESA study has been published.12 In brief, the cohort included a total of 6814 men and women aged 45–84 years who were free from clinically apparent cardiovascular disease at the time of enrollment (July 2000–August 2002). The racial/ethnic groups of participants included African American, Chinese American, Hispanic and non-Hispanic White. Participants who were enrolled in the study returned for follow-up clinic visits approximately 2, 4, 6, and 10 years after the baseline clinic visit.
At clinic visits 2 and 3 (from 2002 to 2005), a random subset of 1970 participants was enrolled in an ancillary study where abdominal computed tomography scans were obtained and subsequently used to quantify abdominal muscle area, muscle density, visceral fat, and subcutaneous fat. Participants with complete data on sedentary behavior and muscle density and area (n = 1895) comprise the sample for the current study. The MESA studies were approved by the institutional review board of each study site, and all participants provided written informed consent.
Standard questionnaires were used to obtain information on participant sociodemographics, ethnicity, and health history. Cigarette smoking was defined as current, former, or never smoker, with ever smoker defined as ≥100 cigarettes in a lifetime. Height and weight were measured to the nearest 0.1 cm and 0.5 kg, respectively. Body mass index, waist circumference, and blood pressure were measured using standard procedures. Hypertension was defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or taking antihypertensive medication.
Participants self-reported their activity levels using the Typical Week Physical Activity Survey. This survey was adapted from the Cross-Cultural Activity Participation Study13 and designed to identify the frequency of and time spent in leisure sedentary behavior (sedentary behavior) and in various physical activities during a typical week in the previous month. Questions specific to sedentary behavior included time spent reading, sitting, watching television, and recreational computer use. Respondents were first asked whether they participated in each category of activity. If they answered yes, they were asked questions regarding the average number of days per week and time per day engaged in these activities. Minutes of activity were summed for each discrete activity type and multiplied by the metabolic equivalent (MET) level.14 Sitting, reclining, and watching TV were multiplied by 1.0 METs, and reading, knitting, sewing, and recreational computer use were multiplied by 1.5 METs. Survey responses were quantified into MET hours per week of sedentary behavior and MVPA defined as moderate and vigorous activities from all categories.
Laboratory
Venous blood was collected after a 12-hour fast. Samples were shipped to the MESA central laboratory for measurement of total and high-density lipoprotein cholesterol, triglycerides, and glucose concentrations. Insulin, C-reactive protein, adiponectin, leptin, tumor necrosis factor-alpha, interleukin-6, and resistin concentrations were measured in stored samples from visits 2 or 3 using Bio Rad Luminex flow cytometry (Millipore, Billerica, MA) at the laboratory for Clinical Biochemistry Research (University of Vermont, Burlington, VT). Average analytic coefficients of variation across several control samples ranged from 6.0% to 13.0%. Dyslipidemia was defined as a total cholesterol/high-density lipoprotein cholesterol ratio >5.0 or if the participant was taking medication to reduce cholesterol. Diabetes was defined as fasting glucose ≥126 mg·dL−1 or use of diabetes medication.
Abdominal Muscle Measurements
Abdominal muscle and visceral and subcutaneous fat were measured from computed tomography scans. Abdominal slices from these scans were processed using MIPAV 4.1.2 software (National Institutes of Health, Bethesda, MD) that measured fat, lean, and total tissue using a semiautomated method. Fat tissue was identified as being between −190 and −30 Hounsfield units (HU), whereas lean tissue was identified as being between 0 and 100 HU. Densities between 0 and −30 HU were labeled as undefined tissue type.
Using the pixel intensities of a single slice obtained at L4/L5, and the HU criteria provided above, fat and muscle areas were calculated for subcutaneous and visceral fat, as well as abdominal muscle groups. Bilateral oblique, rectus abdominis, paraspinal, and psoas muscles were defined within their unique fascial planes. These muscles were grouped into muscles of stabilization (oblique, rectus abdominis and paraspinal muscles); muscles of locomotion (psoas muscle); and total abdominal muscle (oblique, rectus abdominis, paraspinal muscles, and psoas). For each muscle, area was determined by summing the number of pixels of 0 to 100 HU within that muscle’s corresponding fascial plane. Muscle density was the average HU measurement within the muscle’s distinct fascial plane. Subcutaneous adipose tissue was defined as the fat outside of the visceral cavity, not including the fat located within the muscular fascia. Visceral fat area was computed as the sum of the pixels of the appropriate HU range and within the visceral cavity.
Statistical Analysis
The data analyses for this study were conducted in 2017. Sedentary behavior was treated as a continuous and categorical variable (ie, quartiles). Analysis of covariance was used to determine the means of demographic characteristics and muscle density and area by quartile of sedentary behavior, after adjusting for age, sex, and race/ethnicity.
The independent associations between sedentary behavior and muscle density and area were assessed by multivariable linear regression. Because the results of the regression analyses utilizing quartiles of sedentary behavior showed a significant, nonlinear association with the different muscle outcomes, we included both linear and quadratic terms in the multivariable models. The initial model (model 1) adjusted for age, sex, race/ethnicity, and MVPA. Model 2 included model 1 plus dyslipidemia, hypertension and antihypertensive medication use, diabetes, and smoking. Model 3 included model 2 plus height and subcutaneous and visceral adiposity. Model 4 included model 3 plus inflammatory markers (adiponectin, leptin, resistin, C-reactive protein, tumor necrosis factor-alpha, and interleukin-6). The inflection point and confidence intervals (CIs) were calculated as the point at which the effect of sedentary behavior on muscle density switches from positive to negative.
Multiplicative interactions between sedentary behavior and race/ethnicity, sex, and age for the different muscle groups were assessed. When significant interactions were present, models were run separately for each level of the covariate to understand how the associations differed. All statistical analyses were conducted using SPSS (version 24; IBM, Armonk, NY), and a P value of .05 was used to determine statistical significance.
Results
The characteristics of the study cohort are presented in Table 1. The mean age was 64.6 years, and nearly 50% of participants were women. The mean level of sedentary behavior was 28 MET hours·week−1. Thirteen participants (<1%) reported no sedentary behavior, and 21 participants (1%) reported no MVPA.
Table 1.
Characteristics of the Study Cohort (n = 1895): The Multi-Ethnic Study of Atherosclerosis
| Characteristics | Mean (SD)/% (frequency) |
|---|---|
| Age, mean (SD), y | 64.6 (9.6) |
| Female, % (frequency) | 49.8 (944) |
| Race/ethnicity, % (frequency) | |
| White | 40.0 (758) |
| Chinese American | 13.2 (251) |
| African American | 20.9 (397) |
| Hispanic | 25.8 (498) |
| Ever smoker, % (frequency) | 54.0 (1023) |
| BMI, mean (SD), kg·m−2 | 28.0 (5.1) |
| Dyslipidemia, % (frequency) | 38.4 (728) |
| Diabetes, % (frequency) | 14.2 (270) |
| Hypertension, % (frequency) | 46.7 (885) |
| Waist circumference, mean (SD), cm | 97.9 (13.9) |
| Subcutaneous fat area, mean (SD), cm2 | 253.7 (117.7) |
| Visceral fat area, mean (SD), cm2 | 146.4 (68.3) |
| Locomotor muscle area, mean (SD), cm2 | 23.7 (7.4) |
| Locomotor muscle density, mean (SD), HU | 50.3 (5.2) |
| Stability muscle area, mean (SD), cm2 | 74.6 (21.8) |
| Stability muscle density, mean (SD), HU | 39.5 (6.1) |
| Total abdominal muscle area, mean (SD), cm2 | 98.3 (27.5) |
| Total abdominal muscle density, mean (SD), HU | 42.2 (5.5) |
| Sedentary behavior, median (IQR), MET h·wk−1 | 24.5 (24) |
| MVPA, median (IQR), MET h·wk−1 | 59.8 (75.5) |
| Insulin, mean (SD), pmol·L−1 | 287.7 (347.8) |
| Glucose, mean (SD), mg·dL−1 | 98.2 (27.8) |
| Triglycerides, mean (SD), mg·dL−1 | 133.3 (95.6) |
| High-density lipoprotein, mean (SD), mg·dL−1 | 51.6 (15.1) |
| C-Reactive protein, median (IQR), mg·L−1 | 1.5 (2.6) |
| Adiponectin, median (IQR), μg·mL−1 | 17.5 (14.5) |
| Leptin, median (IQR), ng·mL−1 | 13.2 (22.6) |
| Resistin, median (IQR), ng·mL−1 | 15.0 (7.2) |
| Interleukin-6, median (IQR), pg·mL−1 | 1.8 (1.7) |
| Tumor necrosis factor-alpha, median (IQR), pg·mL−1 | 4.6 (2.9) |
Abbreviations: BMI, body mass index; HU, Hounsfield units; IQR, interquartile range; MET, metabolic equivalents; MVPA, moderate to vigorous physical activity. Note: SI conversion factors: To convert glucose, cholesterol, and triglycerides to mmol·L−1, multiply values by 0.0555, 0.0259, and 0.0113, respectively. To convert C-reactive protein to nmol·L−1, multiply values by 9.524.
Older participants reported higher levels of sedentary behavior (Table 2). After adjustment for age, sex, and race/ethnicity, there were higher mean levels of body mass index, waist circumference, subcutaneous fat, visceral fat, insulin, C-reactive protein, leptin, and interleukin-6, and lower mean levels of total abdominal muscle density across higher quartiles of sedentary behavior (P < .05).
Table 2.
Adjusted Mean Characteristics by Quartile of Sedentary Behavior
| Characteristic, unit | Sedentary Behavior | P value | |||
|---|---|---|---|---|---|
| Q1 (n = 498) | Q2 (n = 523) | Q3 (n = 453) | Q4 (n = 490) | ||
| Age, y | 62.1 | 63.1 | 65.8 | 67.8 | <.001 |
| BMI,a kg·m−2 | 27.5 | 27.6 | 28.1 | 28.7 | .001 |
| Waist,a cm | 96.5 | 97.2 | 98.0 | 99.9 | .002 |
| Subcutaneous fat area,a cm2 | 242.4 | 250.4 | 258.6 | 265.6 | .02 |
| Visceral fat area,a cm2 | 139.7 | 143.6 | 146.7 | 155.9 | .002 |
| Locomotor muscle area,a cm2 | 23.4 | 23.8 | 23.8 | 23.8 | .52 |
| Stability muscle area,a cm2 | 73.6 | 75.0 | 74.8 | 75.2 | .39 |
| Abdominal muscle area,a cm2 | 97.0 | 98.8 | 98.6 | 99.0 | .32 |
| Locomotor muscle density,a HU | 50.6 | 50.4 | 50.4 | 49.8 | .06 |
| Stability muscle density,a HU | 39.7 | 39.8 | 39.6 | 39.1 | .08 |
| Abdominal muscle density,a HU | 42.4 | 42.5 | 42.3 | 41.7 | .05 |
| Insulin,a pmol·L−1 | 251.8 | 275.8 | 270.7 | 351.4 | <.001 |
| Glucose,a mg·dL−1 | 97.8 | 97.4 | 97.2 | 100.4 | .27 |
| Triglycerides,a mg·dL−1 | 129.7 | 131.2 | 131.0 | 141.4 | .22 |
| HDL cholesterol,a mg·dL−1 | 51.9 | 51.7 | 52.2 | 50.8 | .49 |
| High sensitivity CRP,a mg·L−1 | 2.7 | 2.6 | 3.9 | 3.7 | .01 |
| Adiponectin,a μg·mL−1 | 20.5 | 21.5 | 21.2 | 20.1 | .23 |
| Leptin,a ng·mL−1 | 17.1 | 19.4 | 22.3 | 23.7 | <.001 |
| Resistin,a ng·mL−1 | 16.2 | 15.9 | 16.9 | 16.5 | .31 |
| Interleukin-6,a pg·mL−1 | 2.1 | 2.2 | 2.3 | 2.8 | <.001 |
| TNF-a,a pg·mL−1 | 6.1 | 6.1 | 5.3 | 5.5 | .53 |
| MVPA,a MET h·wk−1 | 83.5 | 80.7 | 83.5 | 82.1 | .93 |
Abbreviations: BMI, body mass index; CRP, C-reactive protein; HDL, high-density lipoprotein; HU, Hounsfield units; MET, metabolic equivalents; MVPA, moderate to vigorous physical activity; Q, quartile; TNF-α, tumor necrosis factor-alpha. Note: Quartile cutpoints (MET h·wk−1): Q1 < 14, Q2 = 14–24.5, Q3 = 24.6–38, and Q4 > 38.
Boldface indicates statistical significance from analysis of covariance.
Adjusted for age, sex, and race/ethnicity.
There were few significant linear associations between sedentary behavior and muscle area or density. Therefore, to determine if the associations were nonlinear, regression analyses were performed using quartiles of ascending sedentary time. After adjusting for age, sex, race/ethnicity, and MVPA, compared with the first quartile (lowest), the fourth quartile (highest) of sedentary behavior was associated with significantly lower locomotor muscle density (standardized β = −0.07 HU, P < .01); stability muscle density (standardized β = −0.05 HU, P = .04); and total abdominal muscle density (standardized β = −0.06 HU, P = .02) (Figure 1). With the addition of dyslipidemia, hypertension, diabetes, and smoking into the model (model 2), only locomotor muscle density remained significant (P = .04). The addition of visceral and subcutaneous fat into the model attenuated the associations (P > .05). Quartiles 2 and 3 were not different than quartile 1 for any of the muscle density groups (P > .05). There were no associations between quartile of sedentary behavior and muscle area in any of the models.
Figure 1 —
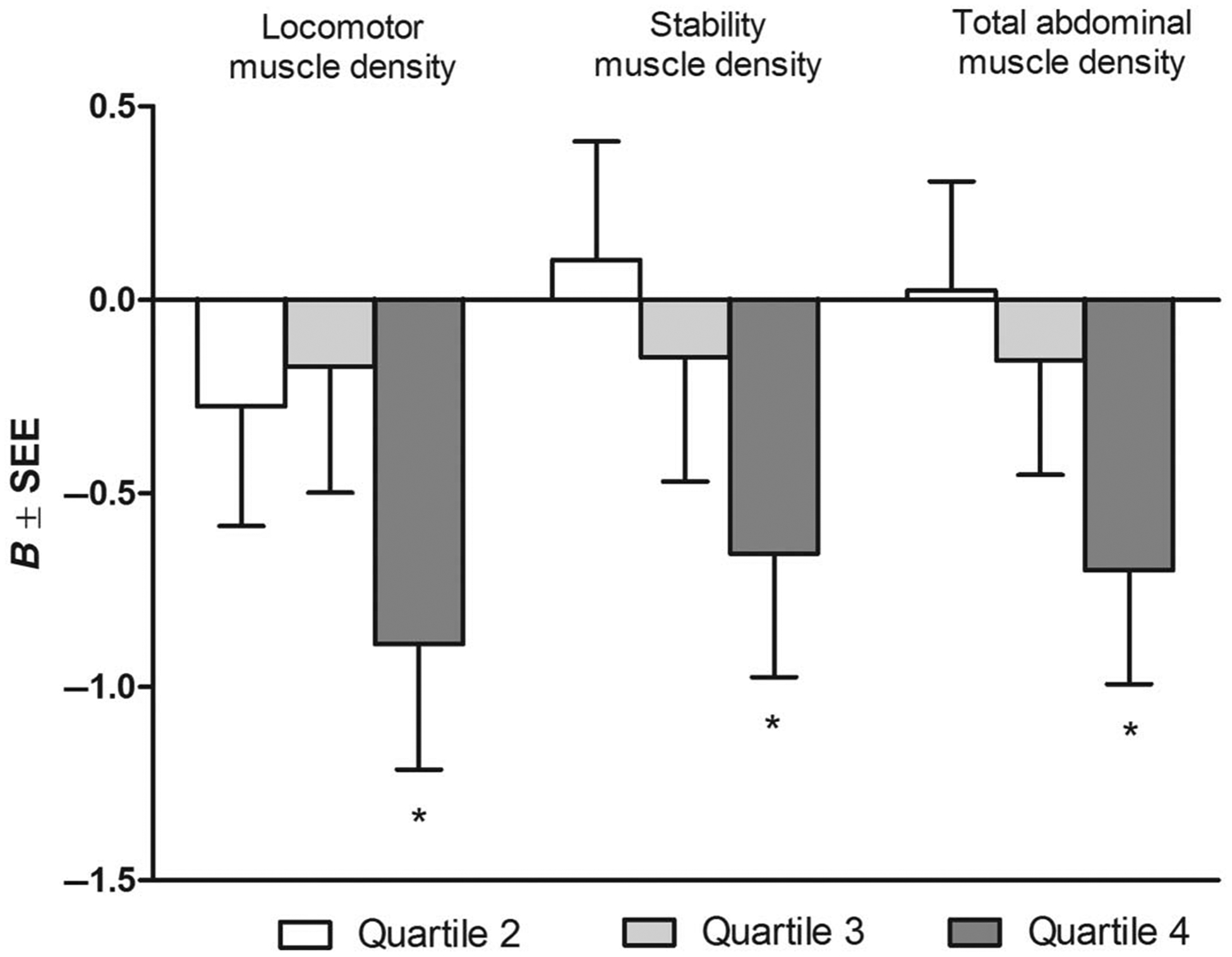
Multivariable-adjusted associations between quartiles of sedentary behavior and muscle density. Referent category: Quartile 1. Quartile cutpoints (MET h·wk−1): Q1 < 14, Q2 = 14–24.5, Q3 = 24.6–38, Q4 > 38. Adjusted for age, sex, race/ethnicity, and MVPA. B indicates slope; SEE, standard error of the estimate. *P < .05.
We further examined the nonlinear association between sedentary behavior and muscle area and density (Table 3). There was no association between sedentary behavior and muscle area in any of the models. However, the nonlinear (quadratic) term was significant for the associations between sedentary behavior and density of the locomotor, stability, and total abdominal muscles after adjustment for adiposity (model 3, P < .05) and inflammation (model 4, P < .01). The significant positive linear term and negative quadratic term in the models indicate that although there is a linear relationship between sedentary behavior and muscle density (positive linear term), the slope becomes less positive with greater sedentary behavior (negative quadratic term). The sedentary behavior inflection point at which the muscle density curve began to decrease was 38.2 MET hours·week−1 (CI, 30.1–46.3 MET h·wk−1), 39.6 MET hours·week−1 (CI, 29.3–49.1 MET h·wk−1), and 39.2 MET hours·week−1 (CI, 30.6–47.8 MET h·wk−1) for locomotor, stability, and total abdominal muscle density, respectively. Overall, and after adjustment for all covariates, with every 18 MET hour·week−1 higher sedentary behavior (1 SD) muscle density was lower by −0.22, −0.14, and −0.17 HU for locomotor, stability, and total abdominal muscle, respectively (P < .01 for all).
Table 3.
Multivariable Linear and Quadratic Associations Between Sedentary Behavior and Muscle Area and Density
| Sedentary Behavior per 1 SD Increment (18 MET h wk−1) | ||||
|---|---|---|---|---|
| Model | ||||
| 1 | 2 | 3 | 4 | |
| Locomotor muscle area | ||||
| sedentary behavior | 0.076 | 0.071 | 0.052 | 0.057 |
| sedentary behaviorquadratic | −0.069 | −0.065 | −0.047 | −0.048 |
| Stability muscle area | ||||
| sedentary behavior | 0.051 | 0.033 | 0.046 | 0.052 |
| sedentary behaviorquadratic | −0.028 | −0.021 | −0.041 | −0.043 |
| Total abdominal muscle area | ||||
| sedentary behavior | 0.061 | 0.045 | 0.050 | 0.056 |
| sedentary behaviorquadratic | −0.041 | −0.034 | −0.045 | −0.047 |
| Locomotor muscle density | ||||
| sedentary behavior | 0.055 | 0.090 | 0.196** | 0.218** |
| sedentary behaviorquadratic | −0.112 | −0.133* | −0.213** | −0.227** |
| Stability muscle density | ||||
| sedentary behavior | 0.021 | 0.065 | 0.126* | 0.142** |
| sedentary behaviorquadratic | −0.062 | −0.095 | −0.137* | −0.143** |
| Total abdominal muscle density | ||||
| sedentary behavior | 0.032 | 0.077 | 0.151 | 0.169** |
| sedentary behaviorquadratic | −0.080 | −0.112* | −0.164** | −0.172** |
Abbreviations: MET, metabolic equivalent; MVPA, moderate to vigorous physical activity. Note: Data are presented as standardized betas for linear and quadratic terms from multivariable regression analyses. Model 1: age, sex, race/ethnicity, and MVPA; model 2: model 1 + smoking, diabetes, hypertension, and dyslipidemia; model 3: model 2 + height, subcutaneous and visceral fat; model 4: model 3 + inflammatory markers. Boldface indicates statistical significance
P < .05,
P < .01.
Using multiplicative interaction terms, we tested for significant differences in the magnitudes of the associations between sedentary behavior and muscle area and density by race/ethnicity, sex, and age. There were significant interactions of sedentary behavior with sex for stability muscle density (P = .02), total abdominal muscle density (P = .03), and locomotor muscle area (P = .01). The sedentary behavior by sex interaction is presented for predicted total abdominal muscle density in Figure 2. The shape of the curve was similar but slightly flatter in men compared with women, with the inflection point occurring earlier for men (33.1 vs 44.6 MET h·wk−1). There were also significant interactions of sedentary behavior with race/ethnicity for total abdominal muscle area and density (P = .02 for both), as well as stability muscle density (P = .02) and locomotor muscle area (P < .01), with Hispanics significantly different than Whites (referent group). The shape of the curve was flatter for Whites compared with Hispanics, but the inflection point was similar (32.1 vs 34.6 MET h·wk−1, respectively). The interactions were not significant for Chinese or African Americans. There were no significant interactions between sedentary behavior and age for muscle area or density (P > .05).
Figure 2 —
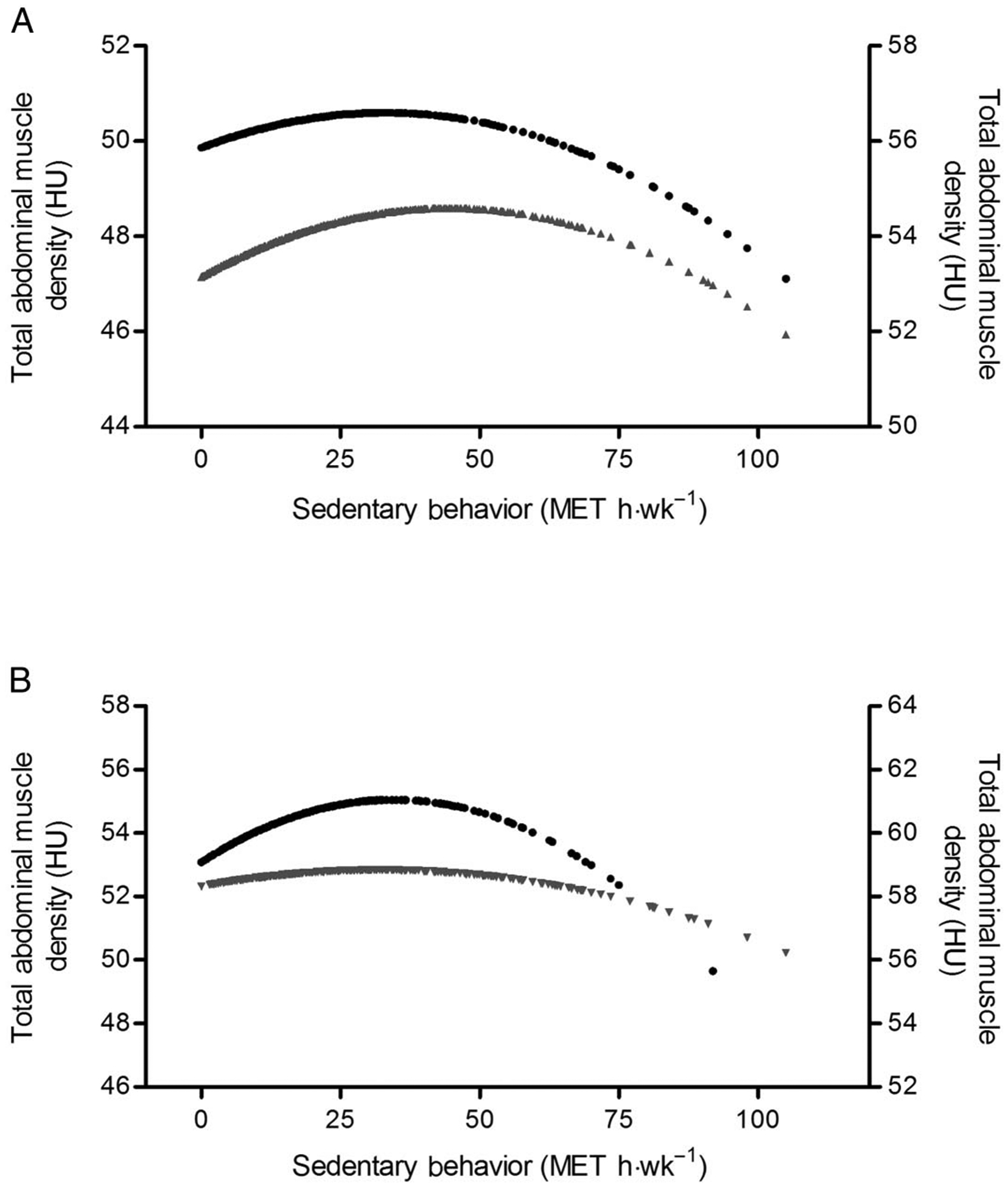
Predicted total abdominal muscle density for fully adjusted model (model 4). (A) • = men, right axis; ▴ = women, left axis; (B) • = Hispanic, right axis; ▾ = White, left y-axis. HU indicates Hounsfield units; MET, metabolic equivalent.
Discussion
In this cross-sectional analysis of a relatively large multiethnic cohort from 6 centers located across the United States, sedentary behavior was associated with lower abdominal muscle density but not muscle area. The association appears to be nonlinear with higher levels of sedentary behavior associated with lower muscle density. Notably, the associations between sedentary behavior and muscle density were independent of relevant covariates suggesting that sedentary behavior may contribute to the progression of age-related sarcopenia and that the effect is independent of comorbidities, cardiovascular disease risk factors, and/or measures of adiposity. Our findings have clinical relevance and suggest that a lower muscle quality is associated with as little as 5.5 MET hours·day−1 or 38 MET hours·week−1 of sedentary behavior and that there may be differences across race/ethnicity and sex.
The nonlinear association between sedentary behavior and muscle density indicates there may be a threshold for when sedentary behavior becomes detrimental for muscle density in older adults. Adults over 60 years of age spend the majority (≥8.5 h·d−1) of their waking hours being sedentary, with men more sedentary than women.1,15 Accumulating evidence showing a link between sedentary behavior and cardiometabolic health has prompted development of national guidelines in Australia and the United Kingdom to encourage individuals to minimize their sedentary time. However, these guidelines are nonspecific and have focused on nonquantitative recommendations such as decreasing time spent sitting for extended periods. Our finding may be helpful to inform evidence-based quantitative guidelines on reducing sedentary behavior.
While previous research has shown that physical inactivity and prolonged periods of bed rest are associated with a decrease in muscle mass,16,17 this study is the first to demonstrate that sedentary behavior is inversely associated with muscle density, independent of MVPA and other important determinants of muscle health and function. To our knowledge, only 2 studies to date investigated muscle mass and sedentary behavior with conflicting results. Larsen et al7 reported no significant associations between leisure-time sedentary behavior, measured by a single item, and abdominal muscle area measured by computed tomography in community-dwelling older adults. In contrast, Gianoudis et al6 reported significant, independent associations between television time and muscle mass measured by dual-energy X-ray absorptiometry in older adults. Our data are consistent with Gianoudis et al6 and extend the literature by investigating abdominal muscle area and density, which are strongly correlated with total body muscle mass,18,19 and including a measure of sedentary behavior that captures different types of leisure-time activities. Our findings suggest that reducing daily sitting time may play an important role in preventing the age-related loss of muscle.
Emerging evidence suggests that muscle density (ie, quality) may be more important than muscle area when examining muscle performance and health. In this respect, density of muscle derived from computed tomography has been inversely associated with body fat, fat infiltration of muscle, and muscle strength and function.8,20 Whether sedentary behavior independently contributes to loss of muscle density is unknown, but recent evidence suggests that sedentary behavior is independently associated with reduced muscle strength and functional performance.4–6 Our study adds to the understanding of the detrimental effects of sedentary behavior on skeletal muscle health and is the first to demonstrate leisure sedentary behavior is associated with skeletal muscle density independent of MVPA.
The underlying mechanisms of the deleterious effects of sedentary behavior on health outcomes are not completely understood. It has been suggested that prolonged sitting and too little physical activity may lead to distinct physiologic effects. The lack of muscle contractions and reduced energy expenditure while engaged in sedentary behaviors may suppress insulin sensitivity and alter lipoprotein metabolism, which are important for metabolic health.21 Furthermore, the consequences of energy surplus, insulin resistance, and increased adiposity from sedentary behaviors are associated with inflammation and increased risk of sarcopenia6 and cardiometabolic disease.1
Interactions were observed between sedentary behavior and race/ethnic groups, as well as sex, such that the associations between sedentary behavior and muscle density were different in Hispanic participants compared with Whites. These associations were also different between women and men. The differences may be due to variance in muscle mass and fat mass across ethnicities, with higher body fat relative to body mass index in Hispanic adults, when compared with other ethnicities.22,23 Furthermore, there may be ethnic differences in the relative loss of skeletal muscle with aging,22 and this may be exacerbated by sedentary behavior. The differences between men and women could be partially due to a greater muscle mass volume in men than women. In this regard, height and weight are the main determinants of appendicular muscle mass in adults.22 In addition, it has been shown that men and women have different patterns of sedentary behavior with older men typically more sedentary than older women.1,15
Our study has many strengths including the use of a well-characterized, gender-balanced, ethnically diverse population, with validated assessments of physical activity and sedentary behaviors, and careful assessment of many potentially confounding factors. Nonetheless, our findings should be considered in the context of several limitations. First, measures of physical activity and leisure sedentary behavior were self-reported and subject to recall bias. Second, this was a cross-sectional analyses, and direction of associations cannot be determined. It is possible that individuals became more sedentary as the result of muscle weakness (ie, reverse causation). Given this, prospective studies are needed to determine relationships of sedentary behaviors and change in muscle over time. Finally, muscle density and area were measured from an abdominal computed tomography in this study, but traditionally, sarcopenia definitions are based on appendicular lean skeletal muscle mass measured by dual-energy X-ray absorptiometry. Computed tomography-derived abdominal muscle mass are strongly correlated with whole-body muscle mass and appendicular skeletal muscle mass as measured by dual-energy X-ray absorptiometry.18
In summary, high levels of sedentary behavior are associated with low levels of abdominal muscle density, independent of relevant covariates including MVPA, cardiovascular disease risk factors, adiposity, and markers of inflammation. These results suggest that reduced muscle quality is associated with as little as 5.5 MET hours day−1 or 38 MET hour’s week−1 of sedentary behavior and that these associations may differ based on sex and race/ethnicity. The findings may have important implications for sedentary behavior guidelines for maintaining skeletal muscle health in older adults.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 and grant R01HL088451 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from NCATS. The article contents have not been previously presented elsewhere. No financial disclosures were reported by the authors of this paper.
Contributor Information
Chantal A. Vella, Department of Movement Sciences, University of Idaho, Moscow, ID.
Erin D. Michos, Johns Hopkins School of Medicine, Baltimore, MD.
Dorothy D. Sears, University of California San Diego, La Jolla, CA.
Mary Cushman, University of Vermont, Burlington, VT..
Rachel B. Van Hollebeke, University of California San Diego, La Jolla, CA.
Michelle M. Wiest, University of Idaho, Moscow, ID.
Matthew A. Allison, University of California San Diego, La Jolla, CA.
References
- 1.Young DR, Hivert MF, Alhassan S, et al. Sedentary behavior and cardiovascular morbidity and mortality. A science advisory from the American Heart Association. Circulation. 2016;134:e262–e279. doi: 10.1161/CIR.0000000000000440 [DOI] [PubMed] [Google Scholar]
- 2.Shad BJ, Wallis G, van Loon LJ, Thompson JI. Exercise prescription for the older population: the interactions between physical activity, sedentary time, and adequate nutrition in maintaining musculoskeletal health. Maturitas. 2016;93:78–82. doi: 10.1016/j.maturitas.2016.05.016 [DOI] [PubMed] [Google Scholar]
- 3.Wirth K, Klenk J, Brefka S, et al. Biomarkers associated with sedentary behaviour in older adults: a systematic review. Ageing Res Rev. 2017;35:87–111. doi: 10.1016/j.arr.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 4.Reid N, Healy GN, Daly RM, et al. Twelve-year television viewing time trajectories and physical function in older adults. Med Sci Sports Exerc. 2017;49(7):1359–1365. doi: 10.1249/MSS.0000000000001243 [DOI] [PubMed] [Google Scholar]
- 5.van der Velde JHPM, Savelberg HHCM, van der Berg JD, et al. Sedentary behavior is only marginally associated with physical function in adults aged 40–75 years—the Maastricht Study. Front Physiol. 2017;8:242. doi: 10.3389/fphys.2017.00242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gianoudis J, Bailey CA, Daly RM. Associations between sedentary behavior and body composition, muscle function and sarcopenia in community-dwelling older adults. Osteoporos Int. 2015;26:571–579. doi: 10.1007/s00198-014-2895-y [DOI] [PubMed] [Google Scholar]
- 7.Larsen BA, Allison MA, Kang E, et al. Associations of physical activity and sedentary behavior with regional fat deposition. Med Sci Sports Exerc. 2014;46(3):520–528. doi: 10.1249/MSS.0b013e3182a77220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in elderly: the Health ABC Study. J Appl Physiol. 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157 [DOI] [PubMed] [Google Scholar]
- 9.Schaap LA, Pluijm SMF, Deeg DJH, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119:526.e9–526.e17. doi: 10.1016/j.amjmed.2005.10.049 [DOI] [PubMed] [Google Scholar]
- 10.Toth MJ, Matthews DE, Tracy RP, Previs MJ. Age-related differences in skeletal muscle protein synthesis: relation to markers of immune activation. Am J Physiol Endocrinol Metab. 2005;288: E883–E991. doi: 10.1152/ajpendo.00353.2004 [DOI] [PubMed] [Google Scholar]
- 11.Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57(5):M326–M332. doi: 10.1093/gerona/57.5.M326 [DOI] [PubMed] [Google Scholar]
- 12.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9): 871–881. doi: 10.1093/aje/kwf113 [DOI] [PubMed] [Google Scholar]
- 13.Ainsworth BE, Irwin ML, Addy CL, Whitt MC, Stolarczyk LM. Moderate physical activity patterns of minority women: the Cross-Cultural Activity Participation Study. J Womens Health Gend Based Med. 1999;8(6):805–813. doi: 10.1089/152460999319129 [DOI] [PubMed] [Google Scholar]
- 14.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000;32(9 suppl):S498–S504. doi: 10.1097/00005768-200009001-00009 [DOI] [PubMed] [Google Scholar]
- 15.Diaz KM, Howard VJ, Hutto B, et al. Patterns of sedentary behavior in US middle-age and older adults: the REGARDS study. Med Sci Sports Exerc. 2016;48(3):430–438. doi: 10.1249/MSS.0000000000000792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martone AM, Marzetti E, Calvani R, et al. Exercise and protein intake: a synergistic approach against sarcopenia. BioMed Res Int. 2017. doi: 10.1155/2017/2672435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paddon-Jones DP, Sheffield-Moore M, Urban RJ, et al. Essential amino acid and carbohydrate supplementation ameliorates muscle protein loss in humans during 28 days bedrest. J Clin Endocrinol Metab. 2004;89(9):4351–4358. doi: 10.1210/jc.2003-032159 [DOI] [PubMed] [Google Scholar]
- 18.Mourtzakis M, Prado CMM, Lieffers JR, Reiman T, McGargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008; 33:997–1006. doi: 10.1139/H08-075 [DOI] [PubMed] [Google Scholar]
- 19.Shen W, Punyanitya M, Wang ZM, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. 2004;97:2333–2338. doi: 10.1152/japplphysiol.00744.2004 [DOI] [PubMed] [Google Scholar]
- 20.Mikkelsen UR, Agergaard J, Couppe C, et al. Skeletal muscle morphology and regulatory signalling in endurance-trained and sedentary individuals: the influence of ageing. Exp Gerontol. 2017;93:54–67. doi: 10.1016/j.exger.2017.04.001 [DOI] [PubMed] [Google Scholar]
- 21.Engeroff T, Fuzeki E, Vogt L, Banzer W. Breaking up sedentary time, physical activity, and lipoprotein metabolism. J Sci Med Sport. 2017; 20(7):678–683. doi: 10.1016/j.jsams.2016.11.018 [DOI] [PubMed] [Google Scholar]
- 22.Gallagher D, Visser M, DeMeersman RE, et al. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol. 1997;83(1):229–239. doi: 10.1152/jappl.1997.83.1.229 [DOI] [PubMed] [Google Scholar]
- 23.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732 [DOI] [PMC free article] [PubMed] [Google Scholar]


