Abstract
Several recent studies have reported the phenomenon of late-onset neutropenia occurring usually several months following the administration of rituximab or rituximab-based therapies. While it appears that late-onset neutropenia is usually not clinically significant and is self-limited, it is important to recognize its existence given the expanding use of rituximab in both hematologic and nonhematologic disorders. Late-onset neutropenia is intriguing biologically and while its pathogenesis and mechanism are not completely understood, many interesting hypotheses have been proposed to explain its occurrence.
Rituximab is a monoclonal chimeric antibody that targets the CD20 B-cell antigen, expressed on normal and neoplastic B cells.1,2 In addition to playing a pivotal role in the treatment of newly diagnosed, relapsed and refractory indolent and aggressive CD20+ non-Hodgkin lymphoma (NHL) and chronic lymphocytic leukemia (CLL), rituximab’s investigation and use has recently extended to autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus (SLE).3–7 In the very early studies that assessed the use of rituximab, neutropenia was rarely reported (grade 3 or 4 neutropenia was only reported in 4.2% of patients in one early study), but monitoring for neutropenia was only performed at wide intervals after completion of therapy.8 We and others have more recently reported the occurrence of “late-onset neutropenia,” that is, neutropenia occurring at least 4 weeks after the administration of the antibody.9
The biologic functions of CD20 remain poorly understood. In vitro, the incubation of B cells with anti-CD20 antibody has variable effects on cell cycle progression and cell signaling and clinically causes the depletion of normal circulating B cells.10,11 The etiology of late-onset neutropenia is not well understood, although some investigators have suggested mechanisms such as the production of anti-neutrophil antibodies or the suppression of neutrophils by large granular lymphocytes (LGLs). We explored an alternative hypothesis that late-onset neutropenia is caused by perturbations of granulocyte homeostasis, mediated by a complex interaction between B-cell recovery, and stromal-derived factor-1 (SDF-1), a chemokine required for early B-cell development and retention of B-lineage and granulocytic precursors in the bone marrow.12,13
INCIDENCE AND ONSET OF LATE-ONSET NEUTROPENIA FOLLOWING RITUXIMAB
Late-onset neutropenia following rituximab has now been reported by a number of groups (Table 1).9,13–19 In our study, we evaluated 153 previously untreated patients treated on DA-EPOCH (dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin) protocols at the National Cancer Institute (NCI) and included the histologies diffuse large B-cell lymphoma (DLBCL), mantle cell lymphoma (MCL), and Burkitt lymphoma (BL).9 To control for confounding causes of neutropenia, we restricted the analysis to 130 patients who were in complete remission from their lymphoma, had hematopoietic recovery with an absolute neutrophil count (ANC) higher than 1.0 × 109/L after treatment, and had at least 12 months of observation time following the completion of therapy. Patients had complete blood cell counts (CBCs) performed every 3 months for the first year of follow-up and we defined late-onset neutropenia as neutropenia (ANC <0.5 × 109/L) occurring at least 60 days after the last treatment. Of the 130 patients in our study, 54 received DA-EPOCH alone and 76 received DA-EPOCH with rituximab (DA-EPOCH-R). We found that the incidence of late-onset neutropenia in patients who received rituximab-based therapy was 8% compared to 0% of patients who received DA-EPOCH alone (P = .04). The median time to onset of late-onset neutropenia was 175 days (range, 77-204 days) with a median neutrophil nadir of 0.2 × 109/L. The duration of late-onset neutropenia was between 11 and 16 days in patients who did not receive growth factors and the slopes of the neutrophil recovery curves were steep and complete. Only one patient with late-onset neutropenia presented with an infectious complication (buccal cellulitis) but this rapidly resolved after the initiation of filgrastim and intravenous antibiotics; in all other cases, late-onset neutropenia was detected incidentally and there were no clinical complications or sequelae.
Table 1.
Studies Showing the Incidence and Characteristics of Late-Onset Neutropenia Following Rituximab-Based Therapy
| Study | No. of Patients | Therapy | Disease Type | Incidence of Late-Onset Neutropenia | Median Time to Neutropenia | Median Duration of Neutropenia | Comments |
|---|---|---|---|---|---|---|---|
| Dunleavy et al9 | 76 54 |
DA-EPOCH-R DA-EPOCH |
DLBCL, ARL, MCL | 8% | 175 days | 14 days | Previously untreated patients |
| Nitta et al14 | 107 52 |
Chemotherapy + R Chemotherapy alone |
CD20+ B-cell lymphoma | 24.9% | 124 days | 28 days | Previously untreated patients |
| Lai et al15 | 121 | R-CHOP | DLBCL | 13.2% | 129 days | 69 days | Previously untreated patients |
| Lemieux et al16 | 39 | ACVB + R + ASCT | DLBCL | 15% | 114 days | 9 days | Previously untreated patients |
| Cattaneo et al18 | 9 50 18 |
R alone Chemotherapy + R R + chemotherapy + ASCT |
CD20+ B-cell lymphoma | 27.3% | 70 days | 77 days | Mix of previously untreated and treated patients |
| Voog et al13 | N/A | Rituximab-based therapy | DLBCL; FL; CLL | N/A | 112 days | 6 days | Previously treated patients |
| Chaiwatanatorn et al19 | 53 | Rituximab-based therapy | FL; MCL; DLBCL; red cell aplasia | 15% | 122 days | 9 days | Previously treated patients |
| Rios-Fernandez et al17 | 23 | Rituximab with various immunosuppressive drugs | Range of autoimmune diseases | 4% | 191 days (1 patient) | 5 days (1 patient) | Patients with autoimmune diseases |
Abbreviations: DA-EPOCH, dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin; R, rituximab; DLBCL, diffuse large B-cell lymphoma; ARL, AIDS-related lymphoma; MCL, mantle cell lymphoma; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; ACVB, doxorubicin, cyclophosphamide, vindesine, bleomycin; ASCT, autologous stem cell transplant; FL, follicular lymphoma; CLL, chronic lymphocytic leukemia.
As we only checked CBCs every 3 months and as late-onset neutropenia appears to be almost always asymptomatic, we estimated that the true incidence of late-onset neutropenia in our patient population was much higher than the 8% rate we detected on routine follow-up. We therefore developed a statistical model to estimate the true incidence of late-onset neutropenia. We assumed that the duration of neutropenia was normally distributed and recognized that it had an unknown mean and an unknown standard deviation of more than 1 day. In order to provide an estimate of the mean and variance of the duration of neutropenia, we combined data from two other published studies of late-onset neutropenia with our own data.13,19 Based on the estimates of neutropenia duration and assuming a 90-day interval between blood counts, we estimated that the proportion of late-onset neutropenia that went undetected in our study was 79%.13,19 Using this number along with the observed late-onset neutropenia rate of 8%, we estimated that the true rate of late-onset neutropenia following rituximab therapy is 35.5%.
Many other studies have investigated the incidence of late-onset neutropenia following rituximab (Table 1). However, conclusions are confounded due to the use of variable treatments, the lack of appropriate control groups and the inclusion of previously treated and high-dose therapy patients who have a higher incidence of chemotherapy-related bone marrow damage. This may complicate the detection of and distinction of late-onset neutropenia from other processes. However, some studies have evaluated the incidence of late-onset neutropenia in previously untreated patients. Nitta et al retrospectively reviewed the records of 120 patients treated with rituximab-containing chemotherapy as primary treatment for CD20+ B-cell lymphomas and found the incidence of late-onset neutropenia (defined as ANC ≤1.0 × 109/L without any apparent cause after the recovery of neutropenia following the completion of therapy) to be 24.9%, and in a control group of 52 patients who received similar therapy without rituximab, it was 0%.14 The median time to neutrophil nadir was 106 days after the last chemotherapy dose and the median neutrophil nadir was 0.61 × 109/L. Interestingly, in multivariate analyses, patients who received high-dose therapy followed by autologous stem cell transplantation, or who received high-dose methotrexate-containing regimens, were more likely than patients who received CHOP-like therapy (cyclophosphamide, doxorubicin, vincristine, prednisone) to develop late-onset neutropenia. Lai et al, using similar criteria (ANC ≤1.0 × 109/L) for late-onset neutropenia, retrospectively evaluated patients with DLBCL receiving rituximab plus CHOP (R-CHOP) and detected late-onset neutropenia in 13.2%; the median time to neutrophil nadir was 129 days and the median neutrophil nadir was 0.66 × 109/L.15 They also found that these cases of late-onset neutropenia were rarely associated with significant clinical sequelae. Lemieux et al reported six cases of late-onset neutropenia in 15% of patients with NHL following rituximab-based high-dose chemotherapy and autologous transplantation and the characteristics of the neutropenia were similar to that in other reports.16 Late-onset neutropenia occurred 76 to 192 days after the completion of rituximab and the duration of neutropenia ranged from 8 to 16 days. In contrast to other reports, 83% of patients required hospitalization for neutropenia-related complications, and in most cases, there was evidence of maturation arrest in the bone marrow. Recently, a small study looked at the incidence of late-onset neutropenia in patients receiving rituximab for autoimmune diseases and, of 23 patients, only one developed late-onset neutropenia.17 These patients were all receiving concurrent immunosuppressive therapy ranging from prednisone alone to combinations including agents such as azathioprine and methotrexate; interestingly, the amount of rituximab administered (two to four doses) was less than that administered in other studies. These studies suggest that the occurrence and severity of late-onset neutropenia may be related to the amount of rituximab and the myelotoxicity of chemotherapy administered.
Other studies have reported late-onset neutropenia in previously treated patients but as discussed earlier, interpretations and conclusions are confounded by factors such as pre-existing bone marrow damage and immune dyscrasias in patients with autoimmune diseases. In a study by Cattaneo et al, the reported incidence of late-onset neutropenia in 72 consecutive patients (some previously untreated) receiving rituximab-based therapy was 27%. Neutropenia developed after a median time of 10 weeks.18 Voog et al reported neutropenia in 8 previously treated patients occurring 8 to 23 weeks after the administration of rituximab for NHL or CLL.13 Patients’ neutrophil counts recovered after a median time of 6 days. Chaiwatanatorn et al evaluated 53 patients who received rituximab and identified late-onset neutropenia in 15%.19 The median time to neutropenia was 122 days and the median duration of neutropenia was 9 days.
POSTULATED MECHANISMS OF LATE-ONSET NEUTROPENIA: GRANULOCYTE HOMEOSTASIS AND THE ROLE OF SDF-1/CXC LIGAND 12
Several mechanisms for late-onset neutropenia following rituximab have been postulated but currently there is no direct evidence for one mechanism. While we have hypothesized that it may be related to perturbations in SDF-1 levels at the time of B-cell recovery interfering with neutrophil egress from the bone marrow, others have postulated alternative mechanisms including neutropenia due to autoantibody production following rituximab treatment and the expansion of T-LGL populations that may induce neutrophil apoptosis through Fas and Fas-ligand interactions.13,20
The median time to the development of late-onset neutropenia, which is similar to the time to B-cell recovery following rituximab, led us to hypothesize that this phenomenon is biologically related to B-cell recovery. To investigate this hypothesis, we evaluated the kinetics of B-cell recovery in a subgroup of patients following DA-EPOCH-R treatment. We measured B-cell kinetics at various time points before and after treatment and as expected, DA-EPOCH-R produced marked and protracted B-cell depletion at treatment completion and at 3 months following the end of treatment. By 9 months following therapy, 67% of patients had achieved significant B-cell recovery and in all cases this represented recovery of normal and not leukemic B cells. We then investigated if there was a correlation between declining granulocyte levels in the context of late-onset neutropenia and the period of rapid B-cell recovery and compared B-cell changes and granulocyte counts between the 3- and 9-month post-treatment time periods during which late-onset neutropenia occurred (Figure 1A). Indeed we found significant granulocyte declines in 56% of patients who had B-cell recovery during this time period.
Figure 1.
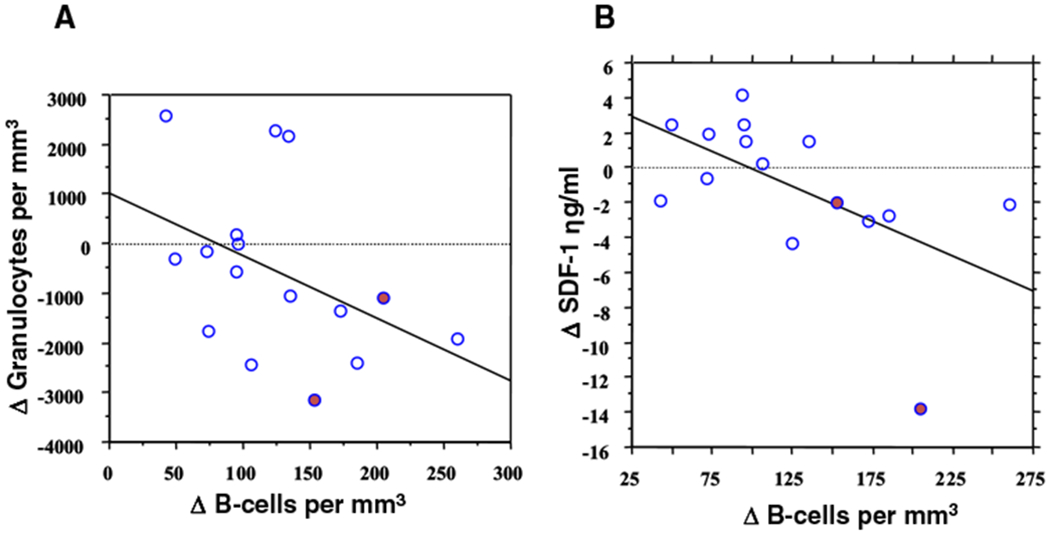
(A) B-cell recovery and granulocyte dynamics. Flow cytometric quantitation of circulating B cells and granulocytes was performed in 24 patients with mantle cell lymphoma. Between 3 and 9 months after treatment, the change (Δ) in B cells inversely correlated with the change in granulocytes, indicating that rapid B-cell recovery is associated with a fall in circulating granulocytes (R = −0.53; P = .04). (●) Designates the two patients with late-onset neutropenia. (B) SDF-1 kinetics and B-cell recovery. The kinetics of circulating SDF-1 following DA-EPOCH-R in 19 mantle cell patients are shown. Between 3 and 9 months after treatment, the change (Δ) in B cells inversely correlated with a reduction in circulating SDF-1 levels, indicating that SDF-1 levels decline as B cells recover (R = −0.67; P = .013). (●) Designates the 2 patients with late-onset neutropenia.
This suggested to us that late-onset neutropenia was due to perturbations in granulocyte homeostasis and led us to investigate the role of the SDF-1/CXCL12 chemokine, because of its dual central roles in regulating neutrophil egress from the bone marrow and in early B-cell lymphopoiesis.21–23 There is evidence that granulocyte egress from the bone marrow is regulated via interactions between the SDF-1 chemokine produced by stromal cells and its receptor, CXCR4, expressed on hematopoietic cells.22,24–26 SDF-1 is also needed for B-cell lymphopoiesis, where it triggers cell division and migration of early lineage B-cells.23,27 These observations suggest that SDF-1 concentrations may be disrupted during rapid B-cell expansion, causing neutrophil egress from the bone marrow. Though SDF-1 in the bone marrow was not directly accessible for analysis, we reasoned that SDF-1 changes in the bone marrow could be reflected in changes in circulating levels and assayed serum SDF-1 at various time points. Interestingly, we found that circulating SDF-1 levels obtained at 3 months following rituximab therapy correlated with later B-cell recovery at 9 months and felt that this reflected an increase in bone marrow SDF-1 early in the recovery period. We also found a correlation between a decrease in circulating SDF-1 levels after recovery and B-cell recovery at 9 months, in keeping with a subsequent decline in bone marrow SDF-1 following B-cell expansion (Figure 1B). (One would expect that in the absence of severe B-cell depletion, there would be absence of these B-cell-dependent SDF-1 level changes and, to demonstrate this, we analyzed SDF-1 levels in patients who received DA-EPOCH alone (ie, no rituximab) and found no significant change following treatment). Therefore, recognizing that SDF-1 regulation in the bone marrow microenvironment is poorly understood and complicated, we hypothesized that consumption by rapidly expanding B-cells could result in disruption of bone marrow SDF-1 gradients resulting in blockade of neutrophil egress from the bone marrow and therefore causing late-onset neutropenia (Figure 2).25,26,28 In support of our model, SDF-1 staining in bone marrow samples before treatment and at the time of late-onset neutropenia showed similar patterns, suggesting no significant changes in SDF-1 production by stromal cells during the neutropenia. In addition, bone marrow biopsies of patients with late-onset neutropenia showed evidence of granulocyte maturation and the administration of filgrastim produced rapid neutrophil mobilization, suggesting that neutrophil egress, rather than myeloid maturation, is affected in late-onset neutropenia. Our study suggested that late-onset neutropenia may be an epiphenomenon which is self-limited and not usually associated with infectious complications or clinical sequelae.
Figure 2.
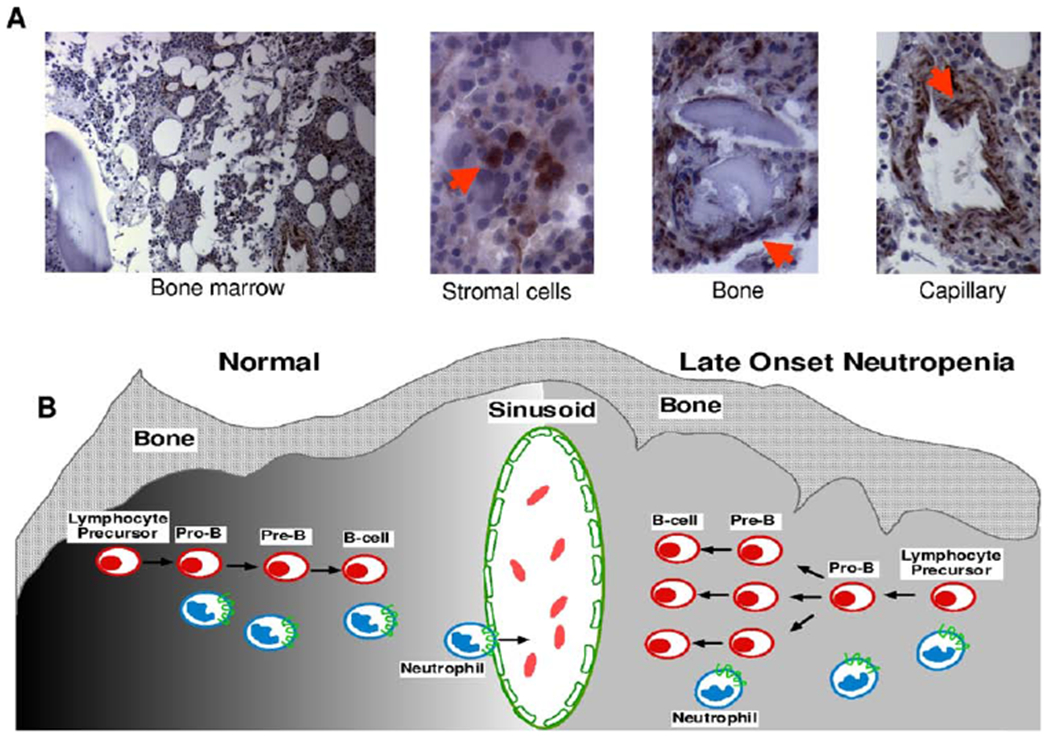
Bone marrow SDF-1 immunohistochemistry and model of late-onset neutropenia (LON). (A) SDF-1 detection in the bone marrow of a patient with LON. SDF-1 is expressed by bone marrow stromal cells, which are dispersed throughout the bone marrow; osteoblasts that are juxtaposed to the bone trabeculae;and endothelial cells that line the blood capillaries. Sections immunostained for SDF-1 with specific antibodies. Images were collected using a Nikon Eclipse 6600 microscope (10x/0.45 DICL and 40x/0.95 DICM lenses;Nikon, Tokyo, Japan) with a Retiga 1300 digital camera (QImaging, Burnaby, BC, Canada) and IP Lab acquisition software (Scanalytics, Fairfax, VA). Images were imported into Adobe Photoshop software (Adobe Systems, San Jose, CA). (B) A model of late-onset neutropenia is shown. Early B-cell lymphopoiesis and neutrophil egress into the bone marrow sinusoid is regulated by SDF-1 gradients (dark to light shading, left) within the bone marrow microenvironment, as shown on the left. In a patient with late-onset neutropenia, we hypothesize that the SDF-1 gradients are transiently disrupted (even gray shading, right) within the bone marrow microenvironment due to SDF-1 consumption by rapidly expanding B cells, resulting in the temporary inhibition of neutrophil egress across the sinusoid, as shown on the right. SDF-1–positive cells are indicated by arrows.
Interestingly, we observed a higher incidence of late-onset neutropenia in patients with AIDS-related lymphomas. While levels of circulating SDF-1 are generally normal in patients with AIDS, AIDS-related lymphomas have been associated with higher SDF-1 levels—this, as well as the potential dysregulation of CXCR4, which serves as both the SDF-1 receptor and a T-cell tropic human immunodeficiency virus-1 coreceptor, may predispose patients with AIDS-related lymphoma to late-onset neutropenia.29–31
GRANULOCYTE INHIBITION/DESTRUCTION
Several other mechanisms of late neutropenia following rituximab have been proposed by other groups. Papadaki et al reported the occurrence of neutropenia following rituximab treatment in patients who showed evidence of LGLs in the peripheral blood and bone marrow.12,20 These were characterized by a predominance of CD3+CD8+CD57+CD28− T cells. The authors hypothesized that LGLs caused neutropenia through CD95-induced apoptosis caused by Fas and Fas-ligand secretion by LGLs in addition to Fas and Fas-ligand independent mechanisms. Regarding the potential role of LGLs in late neutropenia following rituximab, one has to consider that benign reactive increases in LGLs have been observed in a wide variety of settings that have included clonal B-cell disorders, infections, transplantation, and old age.32,33 Chaiwatanatorn et al reported a high incidence of lymphopenia and hypogammaglobulinemia at the time of late-onset neutropenia and hypothesized an association between disordered immunological status following rituximab and aberrant B-cell reconstitution, leading to immune dyscrasias and the development of neutropenia in some patients.19 However, this hypothesis is vague considering the wide spectrum of immune dyscrasias that exist and does not mechanistically explain why some patients develop late neutropenias. Voog et al, using direct immunofluoresence testing, found IgG-type antibodies bound to the surface of neutrophils in two patients with late-onset neutropenia following rituximab and suggested that the transient production of autoantibodies may cause neutropenia following rituximab.13 However, their hypothesis is confounded by the fact that no antibody was found in the serum of either patient. Terrier et al evaluated the kinetics of various cytokines involved in B-cell and granulocyte homeostasis in a patient with Waldenstrom’s macroglobulinemia who developed late-onset neutropenia following rituximab and found an association, at the time of late-onset neutropenia, between a lack of granulopoiesis in the bone marrow and high levels of BAFF (B-cell activator belonging to the tumor necrosis factor [TNF] family), a strong stimulator of B-cell recovery.34 They hypothesized that late-onset neutropenia is caused by hematopoietic lineage competition with promotion of B-cell lymphopoiesis over granulopoiesis in the bone marrow.
While the current review focuses on late-onset neutropenia, rituximab is also associated with early neutropenia. It is reasonable to conjecture that early and late neutropenia are caused by different biological mechanisms, and that patients may experience both phenomena. A recent study of early rituximab-induced neutropenia following autologous transplant showed an association between the high-affinity FcRIIIa 158 V allele and the development of neutropenia.35 The authors hypothesized that high-affinity FcRs may mediate more vigorous ADCC on normal and malignant B lymphocytes with the release of granzyme and lysozyme, and neutrophil death via a bystander effect. Such a relatively acute process would not explain late-onset neutropenia, where the evidence points to perturbations of neutrophil homeostasis and not death.
CONCLUSIONS
Late-onset neutropenia following rituximab therapy is not uncommon and is likely to be significantly underdetected. While rituximab has been associated with the development of neutropenia early after administration and following high-dose therapy, the pathogenesis of late-onset neutropenia appears to be unique and distinct. Given the wide use of rituximab in the management of B-cell disorders and its ever-increasing indications in autoimmune and other diseases, it is important to recognize this phenomenon. In the vast majority of cases, late-onset neutropenia is self-limiting and rarely associated with infections or other serious problems. While the mechanism of late-onset neutropenia is uncertain, our results raise the intriguing notion that it may result from perturbations of SDF-1 during B-cell recovery that inhibit neutrophil egress from the bone marrow.
REFERENCES
- 1.Stashenko P, Nadler LM, Hardy R, Schlossman SF. Characterization of a human B lymphocyte-specific antigen. J Immunol. 1980;125:1678–85. [PubMed] [Google Scholar]
- 2.Bhan AK, Nadler LM, Stashenko P, McCluskey RT, Schlossman SF. Stages of B cell differentiation in human lymphoid tissue. J Exp Med. 1981;154:737–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maloney DG, Grillo-Lopez AJ, White CA, et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin’s lymphoma. Blood. 1997;90:2188–95. [PubMed] [Google Scholar]
- 4.Davis TA, Grillo-Lopez AJ, White CA, et al. Rituximab anti-CD20 monoclonal antibody therapy in non-Hodgkin’s lymphoma: safety and efficacy of re-treatment. J Clin Oncol. 2000;18:3135–43. [DOI] [PubMed] [Google Scholar]
- 5.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–42. [DOI] [PubMed] [Google Scholar]
- 6.O’Brien SM, Kantarjian H, Thomas DA, et al. Rituximab dose-escalation trial in chronic lymphocytic leukemia. J Clin Oncol. 2001;19:2165–70. [DOI] [PubMed] [Google Scholar]
- 7.McDonald V, Leandro M. Rituximab in non-haematological disorders of adults and its mode of action. Br J Haematol. 2009;146:233–46. [DOI] [PubMed] [Google Scholar]
- 8.McLaughlin P, Grillo-Lopez AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16: 2825–33. [DOI] [PubMed] [Google Scholar]
- 9.Dunleavy K, Hakim F, Kim HK, et al. B-cell recovery following rituximab-based therapy is associated with perturbations in stromal derived factor-1 and granulocyte homeostasis. Blood. 2005;106:795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tedder TFEP. CD20: a regulator of cell-cycle progression of B lymphocytes. Immunology Today;15:540–4. [DOI] [PubMed] [Google Scholar]
- 11.Reff ME, Carner K, Chambers KS, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83:435–45. [PubMed] [Google Scholar]
- 12.Papadaki T, Stamatopoulos K, Stavroyianni N, Paterakis G, Phisphis M, Stefanoudaki-Sofianatou K. Evidence for T-large granular lymphocyte-mediated neutropenia in rituximab-treated lymphoma patients: report of two cases. Leuk Res. 2002;26:597–600. [DOI] [PubMed] [Google Scholar]
- 13.Voog E, Morschhauser F, Solal-Celigny P. Neutropenia in patients treated with rituximab. N Engl J Med. 2003;348: 2691–4. [DOI] [PubMed] [Google Scholar]
- 14.Nitta E, Izutsu K, Sato T, et al. A high incidence of late-onset neutropenia following rituximab-containing chemotherapy as a primary treatment of CD20-positive B-cell lymphoma: a single-institution study. Ann Oncol. 2007;18:364–9. [DOI] [PubMed] [Google Scholar]
- 15.Lai GG, Lim ST, Tao M, Chan A, Li H, Quek R. Late-onset neutropenia following RCHOP chemotherapy in diffuse large B-cell lymphoma. Am J Hematol. 2009;84:414–7. [DOI] [PubMed] [Google Scholar]
- 16.Lemieux B, Tartas S, Traulle C, et al. Rituximab-related late-onset neutropenia after autologous stem cell transplantation for aggressive non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2004;33:921–3. [DOI] [PubMed] [Google Scholar]
- 17.Rios-Fernandez R, Gutierrez-Salmeron MT, Callejas-Rubio JL, Fernandez-Pugnaire M, Ortego-Centeno N. Late-onset neutropenia following rituximab treatment in patients with autoimmune diseases. Br J Dermatol. 2007;157: 1271–3. [DOI] [PubMed] [Google Scholar]
- 18.Cattaneo C, Spedini P, Casari S, et al. Delayed-onset peripheral blood cytopenia after rituximab: frequency and risk factor assessment in a consecutive series of 77 treatments. Leuk Lymphoma. 2006;47:1013–7. [DOI] [PubMed] [Google Scholar]
- 19.Chaiwatanatorn K, Lee N, Grigg A, Filshie R, Firkin F. Delayed-onset neutropenia associated with rituximab therapy. Br J Haematol. 2003;121:913–8. [DOI] [PubMed] [Google Scholar]
- 20.Papadaki T, Stamatopoulos K, Anagnostopoulos A, Fassas A. Rituximab-associated immune myelopathy. Blood. 2003;102:1557–8. [DOI] [PubMed] [Google Scholar]
- 21.Suratt BT, Petty JM, Young SK, et al. Role of the CXCR4/ SDF-1 chemokine axis in circulating neutrophil homeostasis. Blood. 2004;104:565–71. [DOI] [PubMed] [Google Scholar]
- 22.Martin C, Burdon PC, Bridger G, Gutierrez-Ramos JC, Williams TJ, Rankin SM. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19:583–93. [DOI] [PubMed] [Google Scholar]
- 23.Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20:707–18. [DOI] [PubMed] [Google Scholar]
- 24.Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 2002;30:973–81. [DOI] [PubMed] [Google Scholar]
- 25.Ma Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity. 1999;10:463–71. [DOI] [PubMed] [Google Scholar]
- 26.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–9. [DOI] [PubMed] [Google Scholar]
- 27.Egawa T, Kawabata K, Kawamoto H, et al. The earliest stages of B cell development require a chemokine stromal cell-derived factor/pre-B cell growth-stimulating factor. Immunity. 2001;15:323–34. [DOI] [PubMed] [Google Scholar]
- 28.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–64. [DOI] [PubMed] [Google Scholar]
- 29.Villalba S, Salvucci O, Aoki Y, et al. Serum inactivation contributes to the failure of stromal-derived factor-1 to block HIV-I infection in vivo. J Leukoc Biol. 2003;74: 880–8. [DOI] [PubMed] [Google Scholar]
- 30.Sei S, O’Neill DP, Stewart SK, et al. Increased level of stromal cell-derived factor-1 mRNA in peripheral blood mononuclear cells from children with AIDS-related lymphoma. Cancer Res. 2001;61:5028–37. [PubMed] [Google Scholar]
- 31.Forster R, Kremmer E, Schubel A, et al. Intracellular and surface expression of the HIV-1 coreceptor CXCR4/fusin on various leukocyte subsets: rapid internalization and recycling upon activation. J Immunol. 1998;160:1522–31. [PubMed] [Google Scholar]
- 32.Rose MG, Berliner N. T-cell large granular lymphocyte leukemia and related disorders. Oncologist. 2004;9: 247–58. [DOI] [PubMed] [Google Scholar]
- 33.Wong KF, Chan JC, Liu HS, Man C, Kwong YL. Chromosomal abnormalities in T-cell large granular lymphocyte leukaemia: report of two cases and review of the literature. Br J Haematol. 2002;116:598–600. [DOI] [PubMed] [Google Scholar]
- 34.Terrier B, Ittah M, Tourneur L, et al. Late-onset neutropenia following rituximab results from a hematopoietic lineage competition due to an excessive BAFF-induced B-cell recovery. Haematologica. 2007;92:e20–3. [DOI] [PubMed] [Google Scholar]
- 35.Weng W, Negrin RS, Lavori P, Horning SJ. Immunoglobulin G Fc receptor FcyRIIIa 158 V/F polymorphism correlates with rituximab-induced neutropenia after autologous transplantation in patients with non-Hodgkin’s lymphoma. J Clin Oncol 2010;28:279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]


