Abstract
Background
We report the prevalence, risk factors and mortality associated with multimorbidity in urban South Asian adults.
Methods
Hypertension, diabetes, heart disease, stroke and chronic kidney disease were measured at baseline in a sample of 16 287 adults ages ≥20 years in Delhi, Chennai and Karachi in 2010–11 followed for an average of 38 months. Multimorbidity was defined as having ≥2 chronic conditions at baseline. We identified correlates of multimorbidity at baseline using multinomial logistic models, and we assessed the prospective association between multimorbidity and mortality using Cox proportional hazards models.
Results
The adjusted prevalence of multimorbidity was 9.4%; multimorbidity was highest in adults who were aged ≥60 years (37%), consumed alcohol (12.3%), body mass index ≥25 m/kg2 (14.1%), high waist circumference (17.1%) and had family history of a chronic condition (12.4%). Compared with adults with no chronic conditions, the fully adjusted relative hazard of death was twice as high in adults with two morbidities (hazard ratio [HR] = 2.3; 95% confidence interval [CI]: 1.6, 3.3) and thrice as high in adults with ≥3 morbidities (HR = 3.1; 95% CI: 1.9, 5.1).
Conclusion
Multimorbidity affects nearly 1 in 10 urban South Asians, and each additional morbidity carries a progressively higher risk of death. Identifying locally appropriate strategies for prevention and coordinated management of multimorbidity will benefit population health in the region.
Keywords: chronic disease, mortality, multimorbidity, South Asia
Introduction
Rapid aging of populations in low- and middle-income countries (LMIC) has resulted in a rise in chronic conditions, and there is heightened concern regarding the health of individuals living with multiple chronic conditions, or multimorbidity.1 Experiencing multimorbidity—compared with single morbidity in isolation—is associated with higher disability, lower self-rated health and lower quality of life.2–5 Furthermore, multimorbidity is costly and taxing to patients and the health system,6,7 especially in LMICs such as India and Pakistan7 where the complexities of managing multiple conditions poses a significant challenge for primary healthcare physicians.8
The few recent studies on multimorbidity in South Asia have been limited to self-reported morbidity data without objective measures to ascertain undiagnosed conditions. Furthermore, we are not aware of any population-based studies that document the risk of death associated with multimorbidity in South Asia. To fill this gap, we draw on a population-based cohort of urban adults in India and Pakistan with objective assessments on five highly prevalent chronic conditions: hypertension, diabetes, heart disease, stroke and chronic kidney disease. The aims of the present study were 2-fold: (i) to examine the sociodemographic and health correlates of prevalent single and multimorbidity at baseline and (ii) to prospectively examine the association of single and multimorbidity with mortality.
Methods
Study design and analytic sample
Data were from the Cardiometabolic Risk Reduction in South Asia Surveillance Study (CARRS Surveillance Study). The participants constitute a population-based, representative sample of urban adults in Chennai, New Delhi and Karachi in 2010–119 followed to measure cardiometabolic diseases and their risk factors. Details of the CARRS study design have previously been published.9 In brief, participants were selected in each of the three cities using a multistage cluster random sampling technique. At the first stage, primary sampling units were municipal sub-divisions (wards in Chennai and Delhi and clusters in Karachi). Next, households were sampled with equal opportunity for selection. In each household, one man and one woman aged ≥20 years were selected using the Kish method.10 Pregnant women (as the body and blood measurements would be different from general population) and bed-ridden individuals (inability to complete detailed questionnaire and measurements) were excluded. All the participants provided written informed consent prior to enrollment/participation in the study.
Trained field teams gathered data using standardized techniques.9 Data were collected from participants through interviews in local languages, clinical examinations and laboratory analysis of blood samples collected either at mobile clinics set up in participants’ neighborhoods (Chennai, Delhi) or in participants’ homes (Karachi). The baseline response rate was 94.7% for questionnaire completion and 84.3% for blood sample collection.
The present study includes baseline data from the three CARRS study sites collected between October 2010 and November 2011 with mortality follow-up through June 2014. The primary analysis includes data from all 16 287 men and women enrolled at baseline.
Chronic conditions and multimorbidity at baseline
We assessed the presence of five chronic conditions at baseline using the following criteria: hypertension (self-report of prior diagnosis, measured blood pressure [BP] of 140/90 mmHg or above, or taking medication); diabetes (self-report, fasting plasma glucose of ≥126 mg/dL or HbA1c of ≥6.5, or taking medication); heart disease (self-report); stroke (self-report); chronic kidney disease (self-report [Karachi site], probable kidney disease based on applying the 2012 Kidney Disease International Global Outcomes CKD guidelines of albumin-to-creatinine ratio ≥30 mg/g, or Estimated Glomerular Filtration Rate [eGFR] <60 ml/min/1.73 m [New Delhi and Chennai sites]).11 We relied on self-reported CKD at the Karachi site due to the unavailability of appropriate assay technology needed to measure creatinine per internationally recognized standards. Multimorbidity was defined as having two or more of these five chronic conditions at baseline.
Participant follow-up and ascertainment of death
Participants were contacted annually by phone (third follow-up assessment) or in-person (first and second follow-up assessment). Vital status for deceased participants was verified through verbal autopsy interviews with next of kin. Participants included in the mortality analysis were followed for an average of 38 months (range: minimum of 13 months to maximum of 51 months). All of the 320 deaths ascertained through June 2014 were included in this analysis.
Covariates
Demographic variables of interest included age, sex, occupation and education. We classified participant occupation into five mutually exclusive categories: students and housewives (not in the labor force); retired (not presently in the labor force but previously working); unemployed (in the labor force, but not currently working); manual profession or small business (employed in a physically demanding job); professional (employed in a non-physically demanding job). Education was measured using the highest level of education and categorized as up to primary schooling (up to Class 4), high school to secondary (Classes 5–12), and college graduation (Bachelor’s degree) and above. Due to a strong correlation between education and an index of household assets (r = 0.38) we did not include the index of household assets in the analysis.
The health risk factors consisted of current tobacco use (current daily versus non-daily users), current alcohol consumption (regular or occasional user in the last 6 months versus no alcohol use in the last >6 months), body mass index (BMI, kg/m2), waist circumference (cm) and family history (history of hypertension, diabetes, stroke and heart disease versus no family history).
Statistical analysis
We used multinomial logistic regression to estimate the adjusted prevalence (marginal predicted probability) of zero, single (one chronic condition in isolation at baseline) and multimorbidity (two or more chronic conditions at baseline) by sociodemographic and health-related factors of interest. We also report the associations (prevalence ratios) of sociodemographic and health-related factors with single and multimorbidity (reference = no chronic conditions) from multinomial logistic regression models including all sociodemographic and health-related factors simultaneously.
We next used Poisson regression models to compute age-adjusted mortality (deaths per 1000 person-years) by sociodemographic and health-related variables of interest. Finally, we evaluated the hazard ratio (HR) of death associated with multimorbidity and sociodemographic and health-related factors using Cox proportional hazards models that were fully adjusted for all predictors.
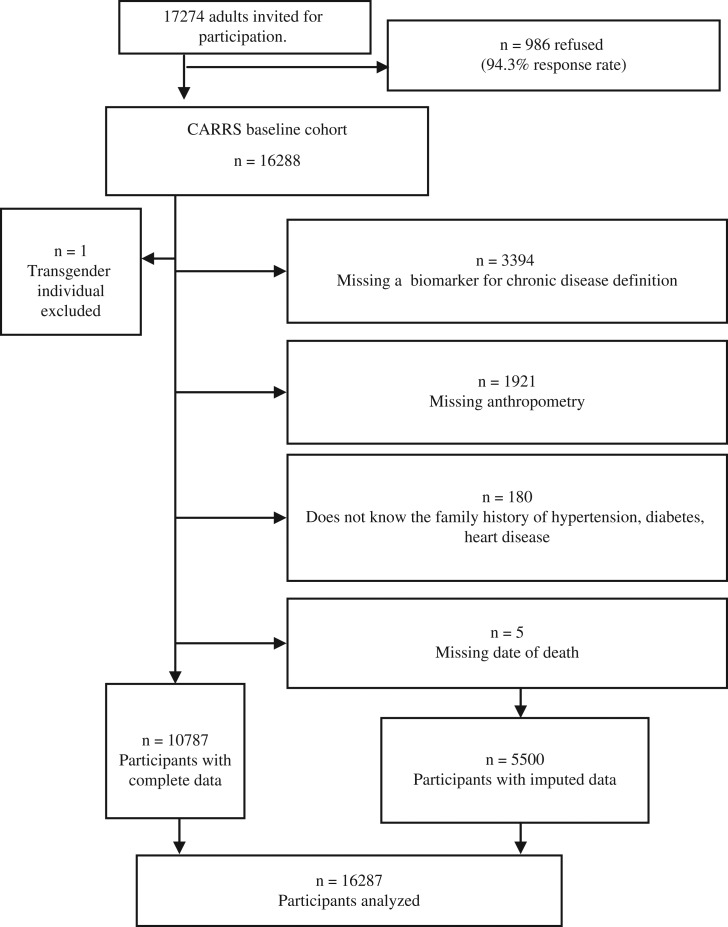
We found that 32% of participants were missing a covariate needed for the analysis (Fig. 1). To utilize data from all participants while minimizing bias due to exclusions, analyses were performed using multiple imputed data to account for missing covariates. Multiple imputation was conducted using the chained equation approach.12 We used a fully conditional specification method to impute missing covariates. The imputation model contained age; sex; education; occupation; family history of hypertension, diabetes, stroke and heart disease; height; weight; waist; FPG; HbA1c level; and systolic and diastolic BP. We also assessed convergence by plotting means and standard deviations by iteration and imputation. We conducted 10 imputations and analyzed the results according to convention.12 All primary analyses are based on the multiply imputed data.
Fig. 1.
Participant exclusion criteria and sample size after each stage of restriction.
A supplemental sensitivity analysis of prevalent multimorbidity at baseline was restricted to the 10 792 participants with complete laboratory assessments (FPG, systolic BP and albumin), anthropometric measurements (height, weight and waist), awareness of family history and sociodemographic data of interest. A second supplemental sensitivity analysis of mortality was conducted using data from the 10 787 participants for whom we knew the exact date of last follow-up or death, needed for survival analysis.
We accounted for the complex survey design in all analyses by adjusting standard errors for clustering and incorporating sampling weights. Estimates of prevalence and associations were age- and sex-standardized to the 2010 South Asia regional population.13 All statistical test values were two-sided, and a P < 0.05 was considered as statistically significant. Analyses were performed using Stata software (version 12.1; Stata Corporation, College Station, TX).
Results
Table 1 displays the baseline characteristics of the 16 287 participants comprising the CARRS cohort. The average age of the participants at baseline was 41.0 (95% confidence interval [CI]: 40.0, 42.0) years. Overall, 52.7% were women, 18.1% had completed college and 47.8% were employed. The prevalence of each individual condition was as follows: hypertension, 31.1% (29.4%, 32.9%); diabetes, 23.5% (22.0%, 25.0%); heart disease, 2.4% (2.1%, 2.8%); stroke, 0.4% (0.3%, 0.5%); and chronic kidney disease, 7.3% (5.8%, 8.9%). With respect to specific comorbidities, diabetes and hypertension was the most prevalent combination observed (11.0% in the total population; data not shown). Over half (56.6%) of the sample had no chronic conditions, 28.6% experienced single morbidity and 14.7% experienced multimorbidity at baseline.
Table 1.
Participant characteristics
| Sociodemographic characteristics | Prevalence or mean [95% CI] |
|---|---|
| Age, years, mean | 41.0 [40.0,42.0] |
| Gender, % | |
| Men | 47.3 [42.0,52.6] |
| Women | 52.7 [47.4,58.0] |
| Educational attainment, % | |
| Up to primary schooling | 20.9 [19.1,22.7] |
| High school to secondary | 61.0 [59.0,63.0] |
| College and above | 18.1 [16.1,20.0] |
| Employment, % | |
| Not in the labor force, student/housewives | 44.3 [40.0,48.7] |
| Not in the labor force, retired | 3.8 [2.9,4.6] |
| Unemployed | 4.2 [3.6,4.7] |
| Employed in a manual profession | 34.5 [31.1,37.9] |
| Employed in a non-manual professional | 13.3 [11.5,15.0] |
| Health-related risk factors | |
| Current alcohol consumption, % | 14.7 [12.8,16.5] |
| Current tobacco consumption, % | 23.0 [21.1,25.1] |
| Anthropometry | |
| BMI, m/kg2, mean | 25.4 [25.1,25.6] |
| Waist circumference, cm, mean | 85.8 [85.2,86.4] |
| Family history of chronic conditions, % | |
| Hypertension | 21.5 [20.2,22.8] |
| Diabetes | 26.8 [25.2,28.4] |
| Heart disease | 10.5 [9.4,11.5] |
| Chronic condition status | |
| Single chronic conditions | |
| Hypertension,% | 31.1 [29.4,32.9] |
| Diabetes,% | 23.5 [22.0,25.0] |
| Heart disease,% | 2.4 [2.1,2.8] |
| Stroke, % | 0.4 [0.3,0.5] |
| Chronic kidney disease, % | 7.3 [5.8,8.9] |
| Total number of chronic conditions | |
| 0 | 56.6 [54.6,58.6] |
| 1 (Single morbidity) | 28.6 [27.4,29.8] |
| ≥2 (Multimorbidity) | 14.7 [13.5,16.0] |
Multimorbidity and its correlates
Table 2 shows adjusted prevalence and the adjusted prevalence ratios of demographic and socioeconomic factors associated with single and multimorbidity relative to no chronic conditions from a multinomial logistic regression model. We found that 30.2% (28.9%, 31.5%) participants had at least one chronic condition and that 9.4% (8.7%, 10.1%) had two or more chronic conditions. The prevalence of multimorbidity was 10% in those younger than 60 years and 37% in adult’s aged ≥60 years, and each year of age was associated with 11% higher relative prevalence of multimorbidity (PR = 1.11; 1.10, 1.11). Compared with those with primary school education or below, college graduates and higher had lower single morbidity (PR = 0.85; 0.72, 0.99) and multimorbidity (PR = 0.76; 0.61, 0.96). Similarly, those employed in manual labor were less likely to have multimorbidity than the professionally employed (PR = 0.68; 0.53, 0.87). Alcohol consumption (PR = 1.68; 1.39, 2.01), BMI (PR = 1.04; 1.02, 1.06) and waist circumference (PR = 1.05; 1.04, 1.06) were positively associated with the prevalent multimorbidity. Having a positive family history for any one of the conditions was associated with nearly twice the prevalence of multiple comorbidities (PR = 1.79; 1.58, 2.03). There were no associations between gender or tobacco consumption and single or multimorbidity after adjusting for other covariates.
Table 2.
Sociodemographic and risk factor correlates of multimorbidity in urban South Asian adults
| Adjusted prevalence of single and multimorbiditya, % | Adjusted prevalence ratiosb | ||||
|---|---|---|---|---|---|
| No chronic conditions | Any one chronic condition | ≥2 Chronic conditions | Any one chronic condition versus no chronic conditions | ≥2 Chronic condition versus no chronic conditions | |
| MP [CI] | MP [CI] | MP [CI] | PR [CI]a | PR [CI] | |
| Total population | 60.4 [59.0,61.8] | 30.2 [28.9,31.5] | 9.4 [8.7,10.1] | ||
| Agec | |||||
| Age < 60 years | 62.6 [60.8,64.4] | 27.2 [25.9,28.5] | 10.2 [9.2,11.1] | n/a | n/a |
| Age ≥ 60 years | 21.9 [19.8,23.9] | 41.2 [39.1,43.4] | 36.9 [34.2 ,39.6] | n/a | n/a |
| Age, years | n/a | n/a | n/a | 1.06 [1.05,1.07] | 1.11 [1.10,1.11] |
| Gender | |||||
| Men | 56.2 [54.1,58.4] | 34.5 [32.4,36.5] | 9.3 [8.4,10.2] | Ref | Ref |
| Women | 62.1 [60.2,64.0] | 28.3 [26.7,30.0] | 9.6 [8.6,10.6] | 0.96 [0.78,1.19] | 1.23 [0.92,1.58] |
| Education | |||||
| Up to primary schooling | 60.2 [57.8,62.5] | 30.2 [28,32.3] | 9.7 [8.5,10.9] | Ref | Ref |
| High school to secondary | 59.2 [57.4,61] | 31 [29.2,32.8] | 9.8 [9,10.6] | 1.04 [0.92,1.18] | 1.02 [0.89,1.18] |
| Graduation and above | 64.5 [61.9,67.2] | 27.6 [25.3,29.8] | 7.9 [6.7,9.1] | 0.85 [0.72,0.99] | 0.76 [0.61,0.97] |
| Occupation | |||||
| Employed, professional | 57.9 [54.2,61.6] | 31.3 [28,34.5] | 10.8 [8.7,12.9] | Ref | Ref |
| Not in the labor force, student or housewife | 59.9 [57.3,62.6] | 29.8 [27.4,32.3] | 10.2 [9.1,11.4] | 0.92 [0.75,1.14] | 0.91 [0.68,1.23] |
| Not in the labor force, retired | 59.9 [52,67.9] | 30 [23.9,36.2] | 10 [6.9,13.1] | 0.93 [0.65,1.33] | 0.89 [0.56,1.42] |
| Unemployed | 57.2 [52.2,62.3] | 33.5 [29,38.1] | 9.3 [6.8,11.8] | 1.08 [0.84,1.39] | 0.87 [0.62,1.20] |
| Employed, manual professional | 62.3 [59.9,64.6] | 29.8 [27.7,31.9] | 7.9 [6.9,8.9] | 0.89 [0.75,1.04] | 0.68 [0.53,0.87] |
| Current alcohol consumption | |||||
| No | 61.9 [60.5,63.4] | 29.1 [27.8,30.5] | 8.9 [8.2,9.6] | Ref | Ref |
| Yes | 51.1 [48.4,53.8] | 36.6 [33.9,39.2] | 12.3 [10.6,14.1] | 1.52 [1.34,1.72] | 1.68 [1.39,2.01] |
| Current tobacco consumption | |||||
| No | 60.4 [58.7,62] | 30.2 [28.6,31.8] | 9.4 [8.7,10.2] | Ref | Ref |
| Yes | 60.5 [58.1,62.9] | 30.2 [28.2,32.3] | 9.3 [8.1,10.5] | 0.99 [0.88,1.14] | 0.98 [0.83,1.16] |
| Anthropometryc | |||||
| Body mass index | |||||
| BMI < 25 m/kg2 | 63.4 [61,65.7] | 26.8 [25,28.5] | 9.9 [8.7,11.1] | n/a | n/a |
| BMI ≥ 25 m/kg2 | 54 [51.9,56.1] | 31.9 [30.2,33.6] | 14.1 [12.7,15.6] | n/a | n/a |
| BMI, m/kg2 | n/a | n/a | n/a | 1.03 [1.01,1.05] | 1.04 [1.02,1.06] |
| Waist circumference | |||||
| WC ≤ 80 cm women, ≤90 cm men | 69.6 [67.8,71.5] | 22.8 [21.3,24.3] | 7.5 [6.6,8.4] | n/a | n/a |
| WC > 80 cm women, > 90 cm men | 47.8 [45.3,50.2] | 35.1 [33.3,36.8] | 17.1 [15.5,18.8] | n/a | n/a |
| Waist circumference, cm | n/a | n/a | n/a | 1.03 [1.02,1.04] | 1.05 [1.04,1.06] |
| Family history of a chronic conditiond | |||||
| No family history | 62.9 [61.2,64.5] | 29.4 [27.9,31] | 7.7 [7,8.4] | Ref | Ref |
| Any family history | 56.5 [54.7,58.3] | 31.1 [29.4,32.8] | 12.4 [11.4,13.4] | 1.18 [1.07,1.29] | 1.79 [1.58,2.03] |
BMI, body mass index; CI, confidence interval; WC, waist circumference.
aThe adjusted prevalence estimates are the predicted marginal prevalence of multimorbidity within groups defined by the covariate.
bThe adjusted prevalence ratios were estimated using a multinomial logistic regression with all covariates shown in the table included simultaneously.
cVariable was divided into categories to estimate adjusted prevalence but was treated as a continuous factor in the prevalence ratio model.
dIncludes family history of hypertension, diabetes or heart disease.
Multimorbidity and age-adjusted mortality
Table 3 displays age-adjusted mortality alongside adjusted HR by sociodemographic and health factors of interest. The total age-adjusted mortality was 8.2 (95% CI: 7.9, 8.5) deaths per 1000 person-years. For individuals with no chronic conditions, age-adjusted mortality was 3.2 per 1000; the corresponding mortality in adults with single and multimorbidity were 3.6 and 5.1 per 1000 person years, respectively. After adjusting for sociodemographic and health-related factors, mortality was 36% (HR = 1.36; 0.94, 1.97) higher in those with single morbidity and 145% (HR = 2.45; 1.72, 3.49) higher in those with multimorbidity. In Fig. S2 and Supplemental Table 1, we show that even among those with multimorbidity, having more than three conditions was associated with higher mortality than having only two conditions (HR = 2.3; 1.6, 3.3 for two morbidities versus none; HR = 3.1; 1.9, 5.1 for three conditions versus none).
Table 3.
Age-adjusted mortality and mortality hazard ratios associated with multimorbidity, sociodemographic and health-related factors
| Variable | Age-adjusted deaths per 1000 person-yearsa | Unadjusted hazard ratiob [95% CI] | Age- and sex- adjusted hazard ratioc [95% CI] | Fully adjusted hazard ratiod [95% CI] |
|---|---|---|---|---|
| Overall | 8.2 [7.3,9.1] | |||
| Number of chronic conditionse | ||||
| 0 | 3.2 [2.2,4.2] | Ref | Ref | Ref |
| 1 | 3.6 [2.6,4.5] | 2.02 [1.41,2.89] | 1.11 [0.77,1.59] | 1.36 [0.94,1.97] |
| ≥2 | 5.1 [3.8,6.4] | 4.42 [3.12,6.26] | 1.65 [1.18,2.32] | 2.45 [1.72,3.49] |
| Agef | ||||
| Age < 60 years | 4.8 [4.1,5.6] | n/a | n/a | n/a |
| Age ≥ 60 years | 31.2 [26.6,36.5] | n/a | n/a | n/a |
| Age, years | n/a | 1.08 [1.07,1.09] | 1.07 [1.06,1.08] | 1.06 [1.05,1.07] |
| Gender | ||||
| Men | 4.5 [3.3,5.7] | Ref | Ref | Ref |
| Women | 2.9 [2.2,3.6] | 0.51 [0.36,0.73] | 0.63 [0.47,0.85] | 0.91 [0.55,1.52] |
| Education | ||||
| Up to primary schooling | 5.4 [3.9,7] | Ref | Ref | |
| High school to secondary | 3.7 [2.8,4.6] | 0.49 [0.37,0.64] | 0.66 [0.48,0.89] | |
| Graduation and above | 1.6 [0.9,2.2] | 0.20 [0.12,0.32] | 0.32 [0.19,0.53] | |
| Occupation | ||||
| Employed, professional | 2.3 [1.3,3.4] | Ref | Ref | |
| Not in the labor force, student or housewife | 2.9 [2.1,3.6] | 1.25 [0.75,2.08] | 1.03 [0.54,1.98] | |
| Not in the labor force, retired | 4.8 [2.7,6.8] | 8.84 [5.26,14.85] | 1.55 [0.85,2.81] | |
| Unemployed | 11.5 [6.6,16.4] | 9.60 [5.61,16.41] | 2.91 [1.58,5.35] | |
| Employed, manual profession | 4.5 [3.0,5.9] | 1.73 [1.00,3.00] | 1.14 [0.63,2.07] | |
| Current alcohol consumption | ||||
| No | 3.2 [2.5,3.9] | Ref | Ref | |
| Yes | 5.9 [3.5,8.4] | 1.63 [1.06,2.49] | 1.41 [0.95,2.1] | |
| Current tobacco consumption | ||||
| No | 3.0 [2.4,3.6] | Ref | Ref | |
| Yes | 5.5 [3.8,7.2] | 1.91 [1.4,2.6] | 1.27 [0.93,1.73] | |
| Anthropometryf | ||||
| BMI, m/kg2 | n/a | 0.92 [0.88,0.95] | 0.93 [0.87,0.99] | |
| BMI < 25 m/kg2 | 5.1 [3.7,6.5] | n/a | n/a | |
| BMI ≥ 25 m/kg2 | 2.4 [1.9,2.9] | n/a | n/a | |
| Waist circumference (WC), cm | n/a | 0.99 [0.98,1.00] | 0.99 [0.97,1.01] | |
| WC ≤ 80 cm women, ≤90 cm men | 5.0 [3.8,6.2] | n/a | n/a | |
| WC > 80 cm women, > 90 cm men | 2.6 [2,3.2] | n/a | n/a | |
| Family historyg | ||||
| No family history | 3.7 [2.9,4.6] | Ref | Ref | |
| Any family history | 3.5 [2.4,4.5] | 0.66 [0.48,0.91] | 1.22 [0.89,1.66] | |
BMI, body mass index; CI, confidence interval; WC, waist circumference.
aAge-adjusted deaths per 1000 person-years were computed the predicted rates from Poisson regression models including the covariate of interest and age groups.
bUnadjusted hazard ratios for mortality were estimated using Cox proportional hazard models adjusted for the complex survey design.
cSex- and age-adjusted hazard ratios for mortality were estimated using Cox proportional hazard models and included only multimorbidity, sex and age.
dFully adjusted hazard ratios for mortality were estimated using Cox proportional hazard models and included multimorbidity, sex, age and all other variables shown in the table.
eThe presence of hypertension, diabetes, heart disease, chronic kidney disease and stroke were summed for the number of chronic conditions.
fVariable was divided into categories to estimate adjusted mortality rates but was treated as a continuous factor in the adjusted Cox regression model.
gIncludes family history of hypertension, diabetes or heart disease.
With respect to demographic factors, we observed a positive association between age and mortality in the fully adjusted model, but no differences in mortality were observed by gender. Education was strongly and inversely associated with mortality in the fully adjusted models (relative to the group with less than primary school: HR = 0.66; 0.48, 0.89 for high school to secondary; HR = 0.32; 0.19, 0.53 for college graduates and above). Relative to individuals with professional employment, the unemployed were three times more likely to experience death (HR = 2.91; 1.58, 5.35); we found no other statistically significant differences between employment groups and mortality. Although not statistically significant in the fully adjusted model, tobacco and alcohol were positively, whereas BMI and waist circumference were inversely, related to mortality.
We repeated the analyses using complete-case data and observed similar results (see Supplemental Tables 2 and 3).
Discussion
Main findings of this study
We evaluated the prevalence, correlates, and mortality risks associated with multimorbidity in adults in three large cities in South Asia. To our knowledge, this is among the first population-based reports of mortality risk associated with multiple morbidities in this region. We found that roughly 40% of participants had at least one of the five chronic conditions examined, while 9.4% of participants suffered from multimorbidity. Relative to those with no morbidities, having either single or multiple chronic morbidities increased the risk death from any cause. After adjustment for potentially confounding factors, the relative hazard of death for single morbidity and multimorbidity compared to no morbidity was higher by 36 and 145%, respectively.
Our findings suggest that in the urban South Asian context, chronic conditions often manifest simultaneously. Particularly the combination of diabetes and hypertension was highly prevalent. As public health systems are adapting to address the burden of non-communicable diseases, it will be important to consider such conditions in combination for effective response.
What is already known on this topic
The information on prevalence, risk factors, and impacts of multimorbidity in LMICs is limited. Recently, WHO’s Global Ageing and Adult Health (SAGE) study estimated prevalence of multimorbidity in India as 24%, which was comparable to the prevalence observed in Mexico (27%), Ghana (23%) and China (22%) but lower than South Africa (32%) or Russia (50%).14 However, other studies of South Asian adults report highly variable prevalence estimates of multimorbidity—from 5 to 83% according to one review—in part due to differences regarding which chronic conditions are included in the definition of multimorbidity.15
Because morbidities accumulate with age, the most consistent risk factor for multimorbidity in the South Asian studies is older age.14,15 Although there is some controversy in South Asia regarding whether chronic conditions are an affliction of the wealthy and have lesser impact on the poor16 or present among individuals across the socioeconomic spectrum,17 multimorbidity tends to be higher among individuals with higher socioeconomic position.14,15,18 Drawing on cross-national comparison of China and Ghana, researchers have suggested that the burden of multimorbidity is expected to shift from advantaged to disadvantaged populations as a country economically develops.19
Prior studies have shown that a higher number of chronic conditions within individuals is associated with higher disability levels.17 Adults with multimorbidity also have more complex care needs which may affect quality of life and strain carers.20 Additionally, empirical evidence from around the world suggest that multimorbidity places higher demand on the health system. It was associated with more outpatient visits (China); with higher hospital visit expenditure (Russia); with greater frequency of hospitalization (India).21 Patient advocates cite this evidence as the need for patient-centered care as opposed to disease-centered care.
What this study adds
Our study replicates the positive association between age and multimorbidity observed in previous studies.22–24 Of the behavioral risk factors examined, alcohol consumption, BMI and waist circumference were associated with multimorbidity,25 highlighting the important role of lifestyle factors in the emergence of multimorbidity. These subgroups may be targeted for screening and appropriate clinical management.
With respect to the social patterning of multimorbidity in three large South Asian metropolises, we found a nuanced picture. First, we note that the prevalence of multimorbidity was over 8% in all subgroups, regardless of educational or occupational background. However, we found an inverse association between and education and multimorbidity (i.e. multimorbidity was less frequent in the most educated adults) but a positive association between occupational class and multimorbidity (i.e. service professionals were more likely that those employed in a manual profession to experience multimorbidity). We note that the directions of associations for both of these social factors are consistent across single morbidity and multimorbidity. The lower prevalence of the examined comorbidities in adults in manual professions could potentially be due to occupation-related physical activity, dietary factors, selection of physically abled individuals in manual professions, or other factors altogether. While this should be further investigated, it is beyond the scope of the current study. The differences between our findings and past studies may be related to true differences driven by the urban context, differing conditions included in the multimorbidity definition, and variations in assessment methods (e.g. self-report versus laboratory) used to identify conditions.
Finally, this is the first study examining multimorbidity and mortality in South Asia. We show that the presence of multimorbidity substantially affects risk of death independent of age and other confounding factors.
Strengths and limitations
Strengths of this study include the large and population-based sample used for the analysis, objective assessment for several conditions, and prospective follow-up of participants for mortality outcomes. There were also some limitations. We were unable to examine the impact of specific combinations or control status of morbidities on health. We instead relied on a simple count of diseases as the measure of multimorbidity. Our multimorbidity definition was a relatively simple measure compared with previous approaches,8,24,26–29 that did not take severity of disease into account. For diabetes, hypertension, and chronic kidney disease, we supplemented self-reported prior diagnosis with laboratory measurements. For heart disease and stroke, however, we relied exclusively on self-report. These conditions are subject to measurement error due to under-diagnosis.26–29 Future investigation may also include other prevalent chronic conditions like cancers, chronic obstructive pulmonary disease, depression and infectious diseases such as tuberculosis and HIV as data becomes available. Finally, though the findings may generalize to other large metropolises on the subcontinent they may not apply to rural settings where the majority of the Indian population continues to reside.
Conclusion
This study is a step forward in quantifying the burden of multimorbidity so that we can develop more coherent set of responses around multiple co-occurring chronic conditions. Disease guidelines around the world tend to be developed for single conditions and fail to account for the multitude of conditions that may be present in an adult simultaneously. Conventions to seek specialized treatment for a condition lead to treatment silos, which can be costly to the health system and imprudent for patient care.30 Such guidelines focused on single morbidity are thus of limited value for the nearly one in ten adults we observed to have multimorbidity in three large, ethnically diverse megacities of South Asia. Given that multimorbidity carries a substantially higher risk for death than having a single condition, improving our understanding of how to manage multiple chronic conditions simultaneously in patients could be an important leverage point for further progress in life expectancy in India.
Supplementary Material
Supplementary data
Supplementary data are available at the Journal of Public Health online.
Funding
The Cardiometabolic Risk Reduction in South Asia Surveillance Study was funded in part by the National Heart, Lung, and Blood Institute of the National Institutes of Health, Department of Health and Human Services [Contract no. HHSN268200900026C] and the United Health Group (Minneapolis, MN, USA). The sponsors were not involved with study design, data collection, data analysis, or decision to submit the manuscript for publication. Roopa Shivashankar is supported by a Wellcome Trust Capacity Strengthening Strategic Award Extension phase to the Public Health Foundation of India and a consortium of UK universities [WT084754/Z/08/A].
References
- 1. Arokiasamy P, Uttamacharya U, Jain K et al. The impact of multimorbidity on adult physical and mental health in low- and middle-income countries: what does the study on global ageing and adult health (SAGE) reveal? BMC Med [Internet] 2015[cited 2016 Sep 12];13:178 http://www.ncbi.nlm.nih.gov/pubmed/26239481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McCarron M, Swinburne J, Burke E et al. Patterns of multimorbidity in an older population of persons with an intellectual disability: results from the intellectual disability supplement to the Irish longitudinal study on aging (IDS-TILDA). Res Dev Disabil [Internet] 2013[cited 2016 Sep 12];34(1):521–7. http://www.ncbi.nlm.nih.gov/pubmed/23085501. [DOI] [PubMed] [Google Scholar]
- 3. Tooth L, Hockey R, Byles J et al. Weighted multimorbidity indexes predicted mortality, health service use, and health-related quality of life in older women. J Clin Epidemiol [Internet] 2008[cited 2016 Sep 12]61(2):151–9. http://www.ncbi.nlm.nih.gov/pubmed/18177788. [DOI] [PubMed] [Google Scholar]
- 4. Brilleman SL, Salisbury C. Comparing measures of multimorbidity to predict outcomes in primary care: a cross sectional study. Fam Pract [Internet] 2013[cited 2016 Sep 12];30(2):172–8. http://www.ncbi.nlm.nih.gov/pubmed/23045354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kubinger KD. On artificial results due to using factor analysis for dichotomous variables. Psychol Sci 2003;45:106–10. [Google Scholar]
- 6. Orueta JF, García-Álvarez A, García-Goñi M et al. Prevalence and costs of multimorbidity by deprivation levels in the basque country: a population based study using health administrative databases. Schooling CM, editor.PLoS One [Internet] 2014[cited 2016 Nov 30];9(2):e89787 http://dx.plos.org/10.1371/journal.pone.0089787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vogeli C, Shields AE, Lee TA et al. Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J Gen Intern Med [Internet] 2007[cited 2016 Nov 30];22(Suppl 3):391–5. http://www.ncbi.nlm.nih.gov/pubmed/18026807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hudon C, Fortin M, Vanasse A. Cumulative Illness rating scale was a reliable and valid index in a family practice context. J Clin Epidemiol [Internet] 2005[cited 2016 Sep 12];58(6):603–8. http://www.ncbi.nlm.nih.gov/pubmed/15878474. [DOI] [PubMed] [Google Scholar]
- 9. Nair M, Ali MK, Ajay VS et al. CARRS surveillance study: design and methods to assess burdens from multiple perspectives. BMC Public Health [Internet] 2012[cited 2016 Sep 12];12:701 http://www.ncbi.nlm.nih.gov/pubmed/22928740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. WHO | STEPwise approach to surveillance (STEPS) WHO [Internet] 2015[cited 2016 Sep 12]. http://www.who.int/chp/steps/en/.
- 11. Kidney Disease: Improving Global Outcomes Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl [Internet] 2013;3:1–150. [Google Scholar]
- 12. Marchenko VY, Eddings W. A Note on How to Perform Multiple-imputation Diagnostics in Stata 2011 [cited 2016 Sep 12]. www.stata.com/users/ymarchenko/midiagnote.pdf
- 13. World Bank Population Projection Tables by Country and Group. 2011. (HNP Stats).
- 14. Agrawal S, Agrawal PK. Association between body mass index and prevalence of multimorbidity in low-and middle-income countries: a cross-sectional study. Int J Med Public Heal [Internet] 2016[cited 2017 Oct 24];6(2):73–83. http://www.ncbi.nlm.nih.gov/pubmed/28894693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pati S, Hussain MA, Swain S et al. Development and validation of a questionnaire to assess multimorbidity in primary care: an Indian experience. Biomed Res Int [Internet] 2016[cited 2017 Apr 3];2016:1–9. http://www.hindawi.com/journals/bmri/2016/6582487/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boutayeb A, Boutayeb S. The burden of non communicable diseases in developing countries. Int J Equity Health [Internet] 2005[cited 2016 Sep 12];4(1):2 http://www.ncbi.nlm.nih.gov/pubmed/15651987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Basu S, King AC. Disability and chronic disease among older adults in India: detecting vulnerable populations through the WHO SAGE Study. Am J Epidemiol [Internet] 2013[cited 2016 Sep 12];178(11):1620–8. http://www.ncbi.nlm.nih.gov/pubmed/24049156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arokiasamy P, Uttamacharya U, Jain K. Multi-morbidity, functional limitations, and self-rated health among older adults in India. SAGE Open 2015;5(1):5. [Google Scholar]
- 19. Kunna R, San Sebastian M, Stewart Williams J. Measurement and decomposition of socioeconomic inequality in single and multimorbidity in older adults in China and Ghana: results from the WHO study on global AGEing and adult health (SAGE). Int J Equity Health [Internet] 2017[cited 2017 Oct 24];16(1):79 http://equityhealthj.biomedcentral.com/articles/10.1186/s12939-017-0578-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boyd CM, Fortin M. Future of multimorbidity research: how should understanding of multimorbidity inform health system design? Public Heal Rev [Internet] 2010[cited 2017 Nov 8];32(2):451–74. https://publichealthreviews.biomedcentral.com/track/pdf/10.1007/BF03391611?site=publichealthreviews.biomedcentral.com. [Google Scholar]
- 21. Lee JT, Hamid F, Pati S et al. Impact of noncommunicable disease multimorbidity on healthcare utilisation and out-of-pocket expenditures in middle-income countries: cross sectional analysis. Correa-Velez I, editor.PLoS One [Internet] 2015[cited 2017 Oct 27];10(7):e0127199 http://dx.plos.org/10.1371/journal.pone.0127199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Agborsangaya CB, Lau D, Lahtinen M et al. Multimorbidity prevalence and patterns across socioeconomic determinants: a cross-sectional survey. BMC Public Health [Internet] 2012[cited 2016 Sep 12];12(1):201 http://bmcpublichealth.biomedcentral.com/articles/10.1186/1471-2458-12-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marengoni A, Angleman S, Melis R et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev [Internet] 2011[cited 2016 Sep 12];10(4):430–9. http://www.ncbi.nlm.nih.gov/pubmed/21402176. [DOI] [PubMed] [Google Scholar]
- 24. Kirchberger I, Meisinger C, Heier M et al. Patterns of multimorbidity in the aged population. Results from the KORA-Age study. PLoS One [Internet] 2012[cited 2016 Sep 12];7(1):e30556 http://www.ncbi.nlm.nih.gov/pubmed/22291986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pati S, Swain S, Hussain MA et al. Prevalence and outcomes of multimorbidity in South Asia: a systematic review. BMJ Open [Internet] 2015[cited 2017 Apr 3];5(10):e007235 http://www.ncbi.nlm.nih.gov/pubmed/26446164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Valderas JM, Starfield B, Sibbald B et al. Defining comorbidity: implications for understanding health and health services. Ann Fam Med [Internet] 2009[cited 2016 Sep 12];7(4):357–63. http://www.ncbi.nlm.nih.gov/pubmed/19597174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Diederichs C, Berger K, Bartels DB. The measurement of multiple chronic diseases—a systematic review on existing multimorbidity indices. J Gerontol A Biol Sci Med Sci [Internet] 2011[cited 2016 Sep 12];66(3):301–11. http://www.ncbi.nlm.nih.gov/pubmed/21112963. [DOI] [PubMed] [Google Scholar]
- 28. Huntley AL, Johnson R, Purdy S et al. Measures of multimorbidity and morbidity burden for use in primary care and community settings: a systematic review and guide. Ann Fam Med [Internet] 2012[cited 2016 Sep 12];10(2):134–41. http://www.ncbi.nlm.nih.gov/pubmed/22412005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pati S, Swain S, Hussain MA et al. Prevalence, correlates, and outcomes of multimorbidity among patients attending primary care in Odisha, India. Ann Fam Med [Internet] 2015[cited 2017 Apr 3];13(5):446–50. http://www.ncbi.nlm.nih.gov/pubmed/26371265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moffat K, Mercer SW. Challenges of managing people with multimorbidity in today’s healthcare systems. BMC Fam Pract [Internet] 2015[cited 2017 Oct 27];16:129 http://www.ncbi.nlm.nih.gov/pubmed/26462820. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.