Abstract
Prior work shows that compared to European Americans, East Asians show an enhanced propensity to take the perspective of another person. In the current work, we tested whether this cultural difference might be reflected in the gray matter (GM) volume of the temporoparietal junction (TPJ), a brain region selectively implicated in perspective taking and mentalizing. We also explored whether the cultural difference in the TPJ GM volume might be moderated by dopamine D4 receptor gene (DRD4) exon 3 variable-number tandem repeat polymorphism. Structural magnetic resonance imaging of 66 European Americans and 66 East Asian-born Asians were subjected to voxel-based morphometry. It was observed that the GM volume of the right TPJ was greater among East Asians than among European Americans. Moreover, this cultural difference was significantly more pronounced among carriers of the 7- or 2-repeat allele of DRD4 than among the non-carriers of these alleles. Our findings contribute to the growing evidence that culture can shape the brain.
Keywords: culture, mentalizing, gray matter volume, self-construal, DRD4
Inrtroduction
From the very beginning of psychology and neuroscience, the brain was considered plastic in its function and structure in response to environmental input (see Rosenzweig, 1996, for a historical review). The thesis of neuroplasticity has recently been brought back to the center stage of the field, with demonstrations of plastic increase of gray matter (GM) volume through extensive training in various tasks (e.g. Maguire et al., 2000; Draganski et al., 2004). One critical extension of the neuroplasticity hypothesis is to examine cross-cultural variation in regionally specific GM volume. Different cultures sanction varying sets of tasks (Kitayama et al., 2009). Hence, depending on the specific tasks that are positively sanctioned, cultures may engage different regions of the brain to varying extents. We may thus expect that the GM volume of relevant regions of the brain could vary across cultures. The field of cultural neuroscience has grown rapidly over the last decade (Kitayama et al., 2019). So far, however, it has not systematically tested the predicted cultural variation in cortical GM volume. The present work will build on recent evidence (Yu et al., 2019) and seek to fill this gap.
Our focus is on the GM volume of the temporoparietal junction (TPJ), a critical brain region involved in perspective taking. Since East Asians are more likely than European Americans to take perspectives of others (Cohen and Gunz, 2002), they are likely to use the TPJ more frequently over many occasions, as well as more extensively for many different purposes, which in turn could result in a relative increase of the TPJ GM volume. Moreover, building on prior evidence that the 7- or 2-repeat allele (7/2-R) of the dopamine D4 receptor gene (DRD4) augments effects of cultural experience (Kitayama et al., 2014, 2016; Tompson et al., 2018; Yu et al., 2019), we additionally explored whether the cultural difference at TPJ might be more pronounced for people who carry these alleles of DRD4.
Culture and perspective taking: the role of the temporoparietal junction (TPJ)
Compared to European Americans, East Asians are relatively more interdependent or less independent in their self-construal (Markus and Kitayama, 1991). Independence entails prioritizing personal preferences and goals over group goals or relational concerns. In contrast, interdependence involves attention to the needs and desires of others while inhibiting personal goal pursuit. Hence, more interdependent (less independent) individuals, including East Asians, may be expected to take others’ perspectives more (Cohen and Gunz, 2002). For example, Wu and Keysar (2007) used a location identification task and found that Chinese were more accurate and efficient in identifying locations from their partners’ perspective than European Americans. In another study, participants observed a video recording of actors who described life events that entailed social pain. They then inferred the emotions of the actors. The correspondence between the emotions reported by the actors and the emotions identified by the participants was higher for Asian participants than for British participants (Atkins et al., 2016).
Will the relative propensity toward perspective taking entail any structural consequences on the brain? Prior work on neuroplasticity demonstrates that extensive training in psycho-social, cognitive and motor tasks (e.g. navigation, chess playing, juggling) increases the GM volume of brain regions that are centrally relevant to the performing of the tasks (e.g. Maguire et al., 2000; Draganski et al., 2004). This literature would suggest that the GM volume of brain regions involved in perspective taking should be greater among East Asians than among European Americans. Although perspective taking can recruit a variety of regions, one region that is consistently engaged is the TPJ (Saxe and Kanwisher, 2003; Samson et al., 2004; Saxe and Wexler, 2005; Krall et al., 2015).1 Of importance, the specificity of TPJ in perspective taking is further corroborated by lesion studies as well as studies using transcranial magnetic stimulation. When TPJ is rendered unfunctional, there is a selective impairment in the reasoning about others’ beliefs, but not in other cognitive tasks (Apperly et al., 2004; Samson et al., 2004). A recent meta-analysis of neuroimaging studies shows that across many social cognitive tasks, East Asians recruit TPJ more than European Americans (Han and Ma, 2014). Altogether, we may expect a systematic cultural variation in the TPJ volume, which would be larger for East Asians than for European Americans.
Is the effect of perspective taking on TPJ bilateral or lateralized? Existing evidence is mixed. Whereas some studies find this effect bilaterally (Saxe and Kanwisher, 2003), some others show it to be right-lateralized. For example, one transcranial magnetic stimulation study finds that disruption of the right (rather than left) TPJ results in selective impairment of the ability in perspective taking (Young et al., 2010). This finding, in turn, is consistent with earlier evidence that the right TPJ is selectively linked to mentalizing (Saxe and Wexler, 2005). A recent meta-analysis arrived at the same conclusion (Krall et al., 2015). If these findings are applicable to cultural variations in TPJ volume, the predicted cultural variation could be more pronounced in the right (rather than left) TPJ.
Might the dopamine D4 receptor gene (DRD4) moderate the cultural difference?
The cultural difference predicted above by no means implies that people within each culture are homogeneous. To the contrary, some people are more likely than others to show a typical psychological or neural response that is associated with their cultural group (Kitayama et al., 2014; Tompson et al., 2018). One source of this individual difference could be genetic. A growing body of evidence shows that some common variants, or alleles, of the DRD4 augment environmental influences, including influences of cultural norms and values (Sheese et al., 2007; Kim and Sasaki, 2014; Kitayama et al., 2016). In recent work, Kitayama et al. (2014) report that the cultural difference observed in previous literature (European Americans being more independent and less interdependent than East Asians) is more pronounced among the 7/2-R allele carriers than among the non-carriers. Further evidence pertains to emotional experience. European Americans are typically quite positive in their daily emotional experience, whereas East Asians are typically more balanced between positive and negative emotions (Miyamoto and Ma, 2011; Sims et al., 2015). This cultural difference was observed among the carriers of 7/2-R, but not among the non-carriers (Tompson et al., 2018). These findings dovetail with evidence that the 7/2-R of DRD4 serves as a plasticity allele (Belsky and Pluess, 2009), which amplifies the effects of environment (Sheese et al., 2007; Bakermans-Kranenburg and Ijzendoorn, 2011; Weeland et al., 2015; King et al., 2016; Silveira et al., 2016).
Of importance, a recent study has shown a similar pattern with regional GM volume as the outcome variable (Yu et al., 2019). Specifically, prior evidence shows that as people become more interdependent, the GM volume of the orbitofrontal cortex (OFC) decreases (Kitayama et al., 2017). This association is supposedly observed because interdependence entails active suppression of psychological functions linked to OFC, such as personal goal pursuit and formation of strong preferences (O’Doherty et al., 2002; Rolls and Grabenhorst, 2008). As may be expected from this evidence, the OFC is smaller in GM volume for East Asians than for European Americans (Chee et al., 2010). Building on this evidence, Yu et al. (2019) observed that this cultural difference is more pronounced for carriers of the 7/2-R allele than for the non-carriers. Altogether, it would seem reasonable to explore whether the cumulative evidence on DRD4 might generalize to the cultural difference in TPJ GM volume. That is, the cultural difference predicted above (TPJ GM volume being greater for East Asians than for European Americans) might be more pronounced among the carriers than among the non-carriers.
Present study
In the present study, we acquired structural brain images of both European Americans and Asian-born East Asians. Our primary prediction was that TPJ GM volume would be greater for East Asians than for European Americans. In addition, we explored two subsidiary questions. First, DRD4 genotype of the participants was also assessed to test whether the predicted cultural difference in TPJ might be more pronounced among carriers of the 7/2-R allele than among non-carriers. Second, we tested a premise of our work that perspective taking (as indexed by the TPJ GM volume) is linked to a self-construal dimension of culture. We assessed the self-construal (Singelis, 1994) of the participants and examined whether the TPJ GM volume might be positively associated with interdependent self-construal and/or negatively associated with independent self-construal.
Method
Participants
We recruited 132 healthy right-handed young adults at the University of Michigan. Sixty-six of them (45 females and 21 males) were European Americans born and raised in the USA. The average age was 20.2 years, with a range of 18 and 23 years. The remaining 66 (40 females and 26 males) were Asian-born East Asians, including 43 Chinese, 17 South Koreans, 4 Taiwanese, 1 Japanese and 1 Singaporean. At the time of testing, they had been in the USA for <10 years. The average age was 21.2 years, with a range of 18 and 27 years. All the participants were from a larger participant pool (n = 635) dedicated to cultural psychology and genetic research we had built. Participants were selected based on their DRD4 genotype, such that approximately half of the participants were carriers of the 7/2-R allele of DRD4 and the other half did not carry these alleles. Six participants (3 from each cultural group) were excluded from further structural brain image analysis due to poor quality of their brain scan (although the results were no different when they were included). All data including structural brain images used in the present study are available at https://osf.io/wpfuv/. An analysis of the same dataset focusing on the OFC has been published (Yu et al., 2019). The current study was approved by the Internal Review Board of the University of Michigan, and all the participants provided informed consent and were paid for their participation.
Genotyping
The details of the genotyping procedure are included in Kitayama et al. (2014) and Yu et al. (2019). Genomic DNA was extracted from saliva samples collected from the participants, and the DRD4 exon 3 variable-number tandem repeat polymorphism was amplified with DRD4 forward and DRD4 reverse primers. Polymerase chain reaction products were then separated and visualized. The breakdown of different allelic frequency of DRD4 in the current sample is as follows: among European American participants, 10.3% 2-R, 4.0% 3-R, 65.9% 4-R, 0.8% 5-R, 16.7% 7-R and 2.4% 8-R and among East Asian participants, 22.2% 2-R, 0.8% 3-R, 76.2% 4-R and 0.8% 5-R. Following the procedure of prior work (Sasaki et al., 2013; Kitayama et al., 2014; Yu et al., 2019), individuals who carried 7/2-R variant were categorized as carriers, and individuals who carried other allelic variants (mostly 4-R/4-R, but also other less frequent genotypes) were categorized as non-carriers. There were 32 carriers and 31 non-carriers among European Americans and 26 carriers and 37 non-carriers among East Asians.
Questionnaire: self-construal scale
The Singelis self-construal (SC) Scale (Singelis, 1994) was used to assess SC. Fifteen items in the scale measured independent SC. Sample items included ‘I do my own thing, regardless of what others think’ and ‘My personal identity, independent of others, is very important to me’. The remaining 15 items measured interdependent SC. Sample items included ‘I often have the feeling that my relationships with others are more important than my own accomplishments’ and ‘I should take into consideration my parents’ advice when making education/career plans’. Participants rated each item on a 7-point Likert scale [1 (strongly disagree), 7 (strongly agree)]. Internal consistency of independent and interdependent SC was adequate for both European American participants (α = 0.65 and α = 0.62, respectively) and East Asian participants (α = 0.68 and α = 0.72, respectively). Participants completed the SC scale approximately 2 weeks before they underwent the scanning session.
Image acquisition
Scanning was performed using a Philips 3 Tesla MRI scanner (Philips Medical Systems, Andover, MA). A high-resolution T1-weighted structural image was acquired from all participants (echo time = 4.6 ms, repetition time = 9.8 ms, 256 × 200 matrix, flip angle = eight degrees, field of view = 256 × 256 × 180 (mm), 180 contiguous 1 mm sagittal slices per volume).
Image processing and analysis
Pre-processing and measurement
Structural brain images of the present study were processed and analyzed using voxel-based morphometry (VBM) (Ashburner and Friston, 2000), implemented in Statistical Parametric Mapping (SPM) software (SPM8; Wellcome Department of Cognitive Neurology, London, UK). VBM has been commonly used in studies examining the relationship between culture or culturally related psychological variables and structural aspects of the brain (Kitayama et al., 2017; Wang et al., 2017; Yu et al., 2019). Each participant’s structural image was first examined for its orientation and origin point and was adjusted if necessary to better match the template. The images were then segmented into different tissue classes including GM, white matter (WM) and cerebrospinal fluid (CSF) by using prior probability templates. Next, a study-specific template of GM was created using the ‘Diffeomorphic Anatomical Registration through Exponentiated Lie’ (DARTEL) algorithm (Ashburner, 2007), which was then affine-registered to Montreal Neurological Institute (MNI) space. All segmented GM images of the participants were then non-linearly warped to match the space of the DARTEL template, and a modulation step was performed as well by multiplying the warped tissue probability maps by the Jacobian determinant of the warp to preserve GM volume for later analysis. Finally, the modulated images were smoothed with a 10-mm full-width half-maximum Gaussian kernel. The global volume of GM, WM and CSF for all participants was calculated by multiplying the total number of voxels of each tissue type by the voxel size. Total intracranial volume (TIV) was then calculated by summing the global volume of GM, WM and CSF.
Note that VBM involves both (i) spatial normalization to warp all the brains into a study-specific template in MNI space and (ii) modulation to conserve volume so that the warping does not bias the result. The DARTEL tool creates a custom study-specific template of GM for the current sample composed of both European American and East Asian participants. This custom template thus takes into account the individual differences in brain shape. By normalizing the individual brains to this custom template in MNI space, it addresses individual difference in brain anatomy and thus produces valid results (see Ashburner, 2007, for more details).
Region of interest definition
Given our interest in the TPJ in the present study, we created an a priori anatomical region of interest (ROI) to bound our analysis (Figure 1). In particular, we took the two peak voxel coordinates of bilateral TPJ identified in Saxe and Kanwisher (2003), a foundational study on TPJ and perspective taking, and we created a 10-mm sphere around the peak coordinates in MNI space using the WFU PickAtlas toolbox (Maldjian et al., 2003).
Fig. 1.
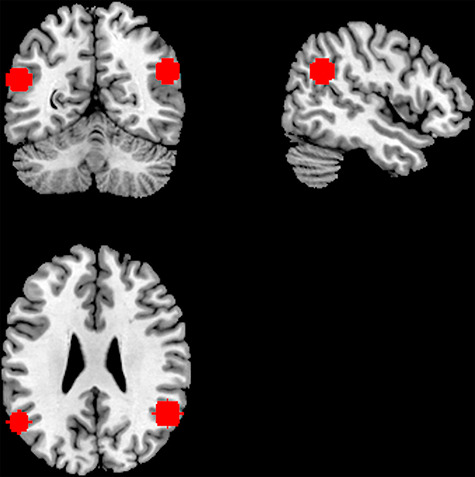
The anatomical region of interest (ROI) for the present analysis from a coronal (top left), sagittal (top right) and axial (bottom left) view. The ROI was defined by constructing a 10-mm sphere around the peak coordinates of temporoparietal junction (TPJ) in Saxe and Kanwisher (2003).
Statistical analysis
Following Yu et al. (2019), we carried out voxel-level analysis in the framework of general linear model with the pre-processed GM images to test our predictions. All voxels with a GM value of <0.2 were excluded to retain only the relatively homogenous voxels, given the potential edge effect at the border of GM and WM. Non-stationary cluster extent correction was also applied to correct for non-isotropic smoothness of VBM data (Hayasaka and Nichols, 2004). To test for the predicted correlation between TPJ GM volume and SC, we used a multiple regression design and included independent SC or interdependent SC as regressor, with age, sex and TIV included as covariates. To test for the predicted cultural difference as well as the culture x DRD4 status interaction on TPJ GM volume, we used a full factorial design and calculate the following two contrasts: (a) (East Asian carrier group + East Asian non-carrier group)—(European American carrier group + European American non-carrier group) for cultural difference and (b) (East Asian carrier group > European American carrier group)—(East Asian non-carrier group > European American non-carrier group) for culture x DRD4 status interaction. The same set of covariates was included in this model. The threshold of significance was set at P < 0.05 (FWE corrected at the voxel level), after small-volume correction using the TPJ ROI created as noted above. We also carried out additional exploratory whole brain analysis to test our predictions, and the threshold of significance was set at P < 0.05 (FWE corrected at the voxel-level), unless notified otherwise.
Results
Demographic and questionnaire data
In the current sample, East Asians were more interdependent than independent in their SC (t(62) = 2.490, P = 0.015). European Americans, in comparison, showed comparable level of interdependent and independent SC (t(62) = −0.160, P = 0.873). East Asians were more interdependent in their SC than European Americans as expected (t(124) = −2.565, P = 0.012). However, East Asians and European Americans were not different in their independent SC (t(124) = .380, P = 0.705). See Table 1 for detailed information on age, gender, DRD4 genotype breakdown and SC mean score as a function of culture.
Table 1.
Descriptive statistics of demographics, self-construal scores and DRD4 status information
| European Americans | East Asians | Significance | |
|---|---|---|---|
| n | 63 | 63 | |
| Mean age (SD) | 20.2 (1.61) | 21.2 (1.65) | t = −3.498, P = 0.001 |
| DRD4 (7/2-R vs other alleles) | 32/31 | 26/37 | χ 2 = 1.150, P = 0.284 |
| Sex (male vs female) | 20/43 | 24/39 | χ 2 = 0.559, P = 0.455 |
| Self-construal | |||
| Independent: mean (SD) | 4.87 (0.56) | 4.83 (0.59) | t = 0.380, P = 0.705 |
| Interdependent: mean (SD) | 4.85 (0.53) | 5.10 (0.56) | t = −2.565, P = 0.012 |
ROI analysis
First, we tested whether TPJ GM volume would be greater among East Asians than among European Americans, and if so, whether this cultural difference would be moderated by DRD4 status. After controlling for age, sex and TIV, we found that there were two clusters at the right TPJ that showed greater GM volume for East Asians than for European Americans (cluster 1: peak voxel MNI coordinates 57, −45, 28; Z value = 4.27; cluster size = 45; peak-level P = 0.001, FWE corrected; cluster 2: peak voxel MNI coordinates 52, −43, 28; Z value = 3.61; cluster size = 1; peak-level P = 0.012, FWE corrected) after the small-volume correction.
In addition, we found a significant Culture x DRD4 status interaction on GM volume at the right TPJ after controlling for the same set of covariates and after the small-volume correction (peak voxel MNI coordinates 52, −45, 22; Z value = 3.80; cluster size = 59; peak level P = 0.006, FWE corrected). In particular, among carriers of the 7/2-R allele of DRD4, East Asians showed significant greater GM volume at the right TPJ than European Americans. This cultural difference was absent among non-carriers (Figure 2). No significant effect was found at the left TPJ.
Fig. 2.
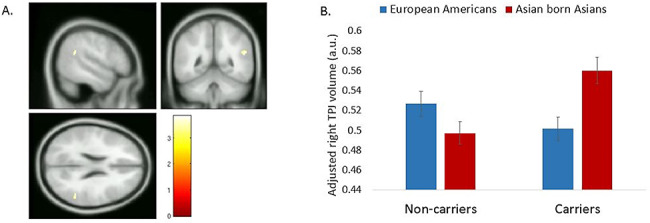
ROI analysis on Culture x DRD4 interaction using small-volume correction. (A) Cluster that shows significant interaction between culture and DRD4 status on gray matter (GM) volume within the TPJ ROI (thresholded at P value of <0.05 family-wise error (FWE) corrected). Peak voxel MNI coordinates 52, −45, 22; Z value = 3.80; cluster size = 59; peak-level P = 0.006, FWE corrected. (B) Mean cluster value as a function of culture and DRD4 status. Among carriers, Asian-born East Asians showed significantly greater right TPJ GM volume than European Americans. The pattern reversed among non-carriers as European Americans showed marginally greater right TPJ volume than East Asians.
Whole brain analysis
A whole brain voxel-level analysis was performed to explore if additional brain regions might show greater GM volume among East Asians than among European Americans. We also explored whether any regions might show a culture and DRD4 status interaction such that the cultural difference (East Asians > European Americans) is more pronounced in carriers than in non-carriers. A few findings are of note. First, a large area centering at the middle temporal gyrus bilaterally showed significant greater GM volume among East Asians than among European Americans (see Table 2 and Figure 3). The bilateral supramarginal gyrus and the somatosensory cortex showed a similar pattern. No region showed a significant interaction between culture and DRD4 status with the a priori threshold of P < 0.05 (FWE corrected). However, at a lower threshold of P < 0.001 (uncorrected), a few voxels near the bilateral TPJ, the right superior parietal lobe and the right temporal pole showed a significant interaction between culture and DRD4 (see Table 3 and Figure 4). The interaction evident in the right TPJ is consistent with the ROI analysis.
Table 2.
Regions where East Asians had greater GM volume compared to European Americans (cluster size > 10)
| MNI coordinates | ||||||
|---|---|---|---|---|---|---|
| Region (Brodmann’s area) | x | y | z | Z value | Cluster size | Cluster-level P value (FWE-corrected) |
| Right middle temporal gyrus (21) | 51 | -10 | -29 | 7.10 | 3179 | < 0.001 |
| Left middle temporal gyrus (21) | -56 | -9 | -29 | 6.82 | 1724 | < 0.001 |
| Right supramarginal gyrus (40) | 68 | -25 | 22 | 6.45 | 2277 | < 0.001 |
| Left supramarginal gyrus (40) | -63 | -30 | 43 | 5.40 | 286 | 0.002 |
| Right temporal pole (38) | 52 | 20 | -26 | 4.89 | 49 | 0.018 |
| Left somatosensory cortex (1) | -66 | -19 | 22 | 4.56 | 15 | 0.031 |
| Right retrosplenial area (30) | 20 | -40 | 0 | 4.60 | 15 | 0.031 |
| Right somatosensory cortex (1) | 52 | -25 | 49 | 4.48 | 14 | 0.032 |
| Right premotor cortex (6) | 16 | -7 | 70 | 4.48 | 13 | 0.033 |
Fig. 3.

Regions in which East Asians had greater GM volume than European Americans, shown in SPM render-style brain. The color bar shows the corresponding Z-scores. Threshold was set at T = 4.56 (corresponding to a P value of <0.05, FWE corrected).
Table 3.
Regions in which the GM volume showed Culture x DRD4 status interaction
| MNI coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Region (Brodmann’s area) | x | y | z | Z value | Cluster size | Peak-level P value (uncorrected) | Cluster-level P value (FWE-corrected) |
| Right angular gyrus (39) | 54 | -45 | 19 | 3.94 | 413 | < 0.001 | 0.341 |
| Right supramarginal gyrus (40) | 48 | -46 | 54 | 3.71 | 562 | < 0.001 | 0.229 |
| Right temporal pole (38) | 51 | 12 | -41 | 3.26 | 44 | < 0.001 | 0.873 |
| Left supramarginal gyrus (40) | -57 | -30 | 39 | 3.17 | 9 | < 0.001 | 0.939 |
Fig. 4.

Regions in which the GM volume showed Culture x DRD4 interaction in a pattern that cultural difference (East Asian > European American) is more pronounced in carriers than in non-carriers, shown in SPM render-style brain. The color bar shows the corresponding Z-scores. Threshold was set at T = 3.16 (corresponding to a P value of <0.001 uncorrected).
Correlation with self-construal
We also tested if TPJ GM volume would be correlated with independent or interdependent SC across cultural groups. After controlling for age, sex and TIV, we found a significant negative correlation between independent SC and right TPJ GM volume (Peak voxel MNI coordinates 54, −48, 33; Z value = 3.91; cluster size = 120; peak-level P = 0.004, FWE corrected) after small-volume correction using the bilateral TPJ ROI (Figure 5A). Figure 5B shows the scatterplot. The negative relationship can be found among both European Americans and East Asians. We did not find any significant correlation between interdependent SC and TPJ GM volume.
Fig. 5.
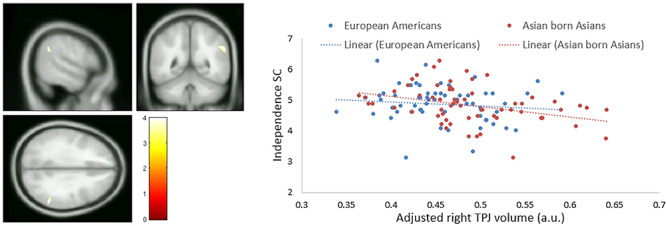
ROI analysis on the correlation between TPJ volume and independence self-construal (SC) using small-volume correction. (A) Cluster that shows significant negative correlations between independent SC and GM volume within the TPJ ROI (thresholded at P value of <0.05 family-wise error (FWE) corrected). Peak voxel MNI coordinates 54, −48, 33; Z value = 3.91; cluster size = 120; peak-level P = 0.004, FWE corrected. (B) Scatterplot on right TPJ GM volume (significant cluster) and interdependent SC as a function of culture.
Correlation with years in the US and TPJ GM volume among East Asians
In our previous work (Yu et al., 2019), we showed that the amount of time East Asians have stayed in the USA predicts GM volume of the OFC, especially among carriers of 7/2-R. Therefore, we also explored if the number of years in the USA predicts GM volume of TPJ. We carried out voxel-level analysis using a multiple regression design, which included the years in the USA as regressor. Age, sex and TIV were used as covariates. We found no correlation between GM volume of the TPJ and the number of years in the USA, among either carriers of 7/2-R or non-carriers.
Discussion
Cultural variation in brain volume
The critical contribution of the present work was to show that the GM volume of the right TPJ is greater for East Asians than for European Americans. This finding is consistent with prior evidence that East Asians engage in perspective taking more than European Americans do. Further, it is also consistent with some evidence showing that the right (rather than left) TPJ is more heavily involved in mentalizing (Saxe and Wexler, 2005; Young et al., 2010; Krall et al., 2015).
Our interpretation of the current evidence is that active engagement in cultural practices (called the cultural tasks) recruits certain brain regions more (Kitayama and Salvador, 2017). When behaviors performing the cultural tasks are positively reinforced, all neural mechanisms that are recruited to carry out the behaviors will be more frequently activated. More neural connections may develop, and perhaps, more neurons are devoted to the relevant circuitries. The last two decades of research links extensive training in cognitive and behavioral tasks to expansion of relevant cortical regions (Maguire et al., 2000; Draganski et al., 2004). Through this mechanism, culture is thought to be embrained. Thus, people’s brains are gradually shaped by culture. Moreover, those with culturally shaped brains will spontaneously think, feel and act in ways that are consistent with the values and norms of their culture. For them, to act naturally is already to act by their culture’s values and norms. Our work thus suggests that the previous work on neuroplasticity can be extended to novel cultural and social domains.
Moderation by DRD4
The cultural variation in the brain volume is correlational, and thus it is pre-mature to conclude from this evidence alone that it is caused by cultural experience. It is therefore important that we explored and found additional evidence for the moderation by DRD4. The cultural difference in the TPJ GM volume was more pronounced for people who carried the 7/2-R allele of DRD4, as compared to non-carriers. Prior evidence shows that the 7/2-R allele of DRD4 augments the effects of environmental influences (Belsky and Pluess, 2009; van IJzendoorn et al., 2011). In light of this evidence, we may suggest that the cultural variation in the right TPJ volume is likely caused by cultural experience.
As noted, we earlier examined a cultural variation in another brain region, the OFC. Yu et al. (2019) used the same dataset and observed that the OFC tended to be larger for European Americans than for East Asians. Moreover, the cultural difference in the OFC volume was more pronounced for carriers of the 7/2-R allele of DRD4 than for non-carriers. These findings are consistent with prior evidence that European Americans pursue their personal goals while forming clear preferences (two of the functions linked to OFC) to a greater extent than East Asians (see Markus and Kitayama, 1991; Kitayama et al., 2019, for reviews).
The 7/2-R allele of DRD4 is thought to increase the fidelity of the computation of cultural reward contingencies. If people get the cultural reward contingencies right, they ought to perform perspective taking more, and/or the OFC functions less, if they are brought up in East Asian, interdependent cultures. However, they may do perspective taking less and/or performing the OFC functions more, if they are brought up in European American, independent cultures. These culturally mediated behaviors may be responsible for the findings in both studies under discussion.
The mechanisms underlying the moderation effect of DRD4 have yet to be fully understood. However, the 7/2-R of DRD4 is associated with blunted D4 receptor activity (Wang et al., 2004). D4 receptors are inhibitory. Hence, the blunted activity of these receptors may activate the brain regions linked to them. These regions include the prefrontal cortex, which is densely connected to the striatal reward processing area. The 7/2-R allele of DRD4 may therefore be associated with more efficient reward processing (Glazer et al., 2020; Forbes et al., 2009), an important component in social and cultural learning. We might then hypothesize that DRD4 modulates the learning of cultural reward contingencies. This hypothesis would imply that the 7/2-R allele of this gene is associated with the acquisition of normatively sanctioned behavioral tendencies (Kitayama et al., 2016). Engagement in these behaviors may in turn cause structural effects on the brain over some substantial period.
It is worth noting that the 7/2-R allele of DRD4 emerged and was incorporated into the human genome over the last 50 000 years (Wang et al., 2004), around the same period that human culture and society became increasingly complex. Further, by that time, various components of reward processing, such as the computation of reward prediction errors and the computation of reward contingencies over some extended period, had already been firmly established, being tightly governed by elaborate gene networks. It would therefore seem possible that DRD4 started operating as a hub of the pre-existing gene signaling networks involved in reward processing. It might be precisely for this reason that the 7/2-R allele of DRD4 was positively selected. Further, this consideration might explain why DRD4 appears so special, not just one of the 20 000 genes. Unlike most genes, DRD4 does have unusually robust effects on the sensitivity to socialization agents (Sheese et al., 2007; Weeland et al., 2015; King et al., 2016), as well as on the degree to which culture’s modal tendencies are acquired (Sasaki et al., 2013; Kitayama et al., 2014; Tompson et al., 2018; Yu et al., 2019).
Linkage to self-construal
Yu et al. (2019) found that the OFC volume is associated with SC, and likewise, we observed that the TPJ volume is also associated with SC. Interestingly, however, the brain region thought to be related to independence SC (OFC) was inversely predicted by interdependence SC (Yu et al., 2019), consistent with earlier evidence by Kitayama et al. (2017). It could be argued that personal goal pursuit or formation of clear preferences is promoted by inhibition of competing tendencies of interdependence. However, it is not clear why the OFC volume is not positively associated with independent SC. A similar puzzle continues in the present study. We found that the brain region thought to be related to interdependence SC (TPJ) was inversely predicted by independence SC. It might be the case that psychological tendencies of, say, perspective taking are promoted by inhibition of competing psychological tendencies of independence. However, we find it hard to find a compelling argument about why the TPJ volume should not be positively associated with interdependent SC. Altogether, the existing evidence is consistent with the general expectation that the OFC volume should increase and the TPJ volume should decrease as a function of independent vs interdependent SC. But the exact measure of SC that shows these effects is hard to determine on an a priori basis. This combination of ‘global’ consistency with ‘local’ inconsistencies (of the specific measures that show the global effect) seems to extend to other areas of research in cultural neuroscience (Kitayama and Uskul, 2011, for a review). More work is needed to shed light on this admittedly curious absence of local consistencies in the literature.
Number of years in the USA and TPJ GM volume among East Asians
We found no correlation between the number of years in the USA and the TPJ GM volume among East Asians regardless of their DRD4 status. This null finding is of note since our prior work found a positive correlation between the number of years in the USA and an increase of OFC GM volume among East Asian carriers of the 7/2-R allele of DRD4 (Yu et al., 2019). One interpretation of the Yu et al. finding is that cultural experience in the USA causes the OFC to ‘grow’. At first glance, it might seem sensible to predict an inverse correlation between the number of years in the USA and the TPJ GM volume. However, while the use of a given brain region (e.g. OFC) can cause the region to expand (Maguire et al., 2000; Draganski et al., 2004; see also Pascual-Leone and Torres, 1993), it is not obvious if the reverse is also the case. That is, it is not clear whether decreased engagement of the brain region might also lead to the ‘shrinkage’ of it among healthy adults. This consideration might pose some limitations on the influence of a secondary culture on malleable brain changes. First, even though the OFC of East Asian plasticity allele carriers apparently increases when they are exposed to European American culture, the OFC of European American plasticity allele carriers might not necessarily decrease when they are exposed to East Asian culture. Second, even though the TPJ of East Asians shows no change in volume when they are exposed to European American culture, the TPJ of European Americans might show a reliable increase in volume when they are exposed to East Asian culture. These possibilities must be addressed in future work.
Limitations and conclusion
Our work showed clear evidence for the variation in cortical structure across cultural groups. However, some limitations must be acknowledged. First, it falls short of linking this evidence to functional differences in, say, propensities to perspective taking or competence in mind reading. Future work will benefit from simultaneous assessment of both the structure and functions of the brain, which would enable us to test the link between them. Second, the two cultural groups vary on a number of different dimensions, some arguably socio-cultural, but some others more ecological and biological, including genetic ancestry. While our work begins highlighting the significance of independence or interdependence as an active element of the cultural difference in TPJ GM volume we identified, more work is needed to identify specific mechanisms that support the cultural difference. Third, we only tested GM volume, a rather crude index of brain structure. Future work must extend the current work and test the degree to which culture might also influence properties of the structural and functional network of brain regions (TPJ and several other regions) that are involved in perspective taking. Fourth, the finding that DRD4 moderates the cultural difference observed in the TPJ GM volume is consistent with the growing body of evidence that cultural differences, both behavioral and neural, are often moderated by this particular gene. Moreover, as noted above, there is some ground to hypothesize that DRD4 is special in the domain of cultural evolution. Nevertheless, the current finding must be tested in larger independent samples in future work.
The limitations notwithstanding, we provide new evidence that culture plays an important role in affecting brain structure. Note that the cultural difference does not exist for non-carriers of the genetic alleles linked to environmental influence. This finding may be taken to suggest that culture might in fact serve as a causal agent in inducing the brain difference. More work is required before making a firm conclusion on this point. Nevertheless, we are optimistic that the present finding could open up a new avenue of research examining how sociocultural context might shape the brain. This effort has the potential of redefining the brain as a system that is open to the surrounding context. It may further provide empirical substance to an emerging view of humans as inherently embedded in, and interdependent with, ecological, social and cultural contexts (Henrich, 2017; Kitayama and Uskul, 2011).
Funding
This work was supported by a National Science Foundation grant (SES 1325881) and a Russell Sage Foundation Residential Fellowship to SK.
Footnotes
Two other regions that are often implicated in perspective taking are the medial prefrontal cortex (mPFC) and anterior temporal lobe (aTL) (Schurz et al., 2014). However, the functions of these two areas are far more general, extending over the function of perspective taking. First, mPFC is activated when reading about other’s appearance, character or bodily sensation (Aichhorn et al., 2006; Saxe and Powell, 2006). Thus, the function of mPFC is quite general, not limited to perspective taking. A similar consideration applies to aTL, which is traditionally known to be critical for storing semantic or conceptual knowledge (Patterson et al., 2007; Lambon Ralph et al., 2009). Thus, while it has been linked to the perspective taking, this linkage might occur because of its function in storing social conceptual knowledge (e.g. social affective descriptors, social rules, social relationships)—the knowledge that is required to infer another person’s mental states (Ross and Olson, 2010).
References
- Aichhorn, M., Perner, J., Kronbichler, M., Staffen, W., Ladurner, G. (2006). Do visual perspective tasks need theory of mind? NeuroImage, 30, 1059–68. doi: 10.1016/j.neuroimage.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Apperly, I.A., Samson, D., Chiavarino, C., Humphreys, G.W. (2004). Frontal and Temporo-parietal lobe contributions to theory of mind: neuropsychological evidence from a false-belief task with reduced language and executive demands. Journal of Cognitive Neuroscience, 16, 1773–84. doi: 10.1162/0898929042947928. [DOI] [PubMed] [Google Scholar]
- Ashburner, J. (2007). A fast diffeomorphic image registration algorithm. NeuroImage, 38(1), 95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner, J., Friston, K.J. (2000). Voxel-based Morphometry—the methods. NeuroImage, 11, 805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Atkins, D., Uskul, A.K., Cooper, N.R. (2016). Culture shapes empathic responses to physical and social pain. Emotion, 16, 587–601. doi: 10.1037/emo0000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg, M.J., vanIjzendoorn, M.H. (2011). Differential susceptibility to rearing environment depending on dopamine-related genes: new evidence and a meta-analysis. Development and Psychopathology, 23, 39–52. doi: 10.1017/S0954579410000635. [DOI] [PubMed] [Google Scholar]
- Belsky, J., Pluess, M. (2009). Beyond diathesis stress: differential susceptibility to environmental influences. Psychological Bulletin, 135, 885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Chee, M.W.L., Zheng, H., Goh, J.O.S., Park, D., Sutton, B.P. (2010). Brain structure in Young and old east Asians and westerners: comparisons of structural volume and cortical thickness. Journal of Cognitive Neuroscience, 23, 1065–79. doi: 10.1162/jocn.2010.21513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, D., Gunz, A. (2002). As seen by the other … perspectives on the self in the memories and emotional perceptions of easterners and westerners. Psychological Science, 13, 55–9. doi: 10.1111/1467-9280.00409. [DOI] [PubMed] [Google Scholar]
- Draganski, B., Gaser, C., Busch, V., Schuierer, G., Bogdahn, U., May, A. (2004). Changes in grey matter induced by training. Nature, 427, 311–2. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Forbes, E., Brown, S., Kimak, M., Ferrell, R., Manuck, S., Hariri, A. (2009). Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Molecular Psychiatry, 14, 60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer, J., King, A.P., Yoon, C., Liberzon, I., Kitayama, S. (2020). DRD4 polymorphisms modulate reward positivity and P3a in a gambling task: exploring a genetic basis for cultural learning. University of Michigan, unpublished manuscript. [DOI] [PubMed] [Google Scholar]
- Han, S., Ma, Y. (2014). Cultural differences in human brain activity: a quantitative meta-analysis. NeuroImage, 99, 293–300. doi: 10.1016/j.neuroimage.2014.05.062. [DOI] [PubMed] [Google Scholar]
- Hayasaka, S., Nichols, T.E. (2004). Combining voxel intensity and cluster extent with permutation test framework. NeuroImage, 23, 54–63. doi: 10.1016/j.neuroimage.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Henrich, J. (2017). The secret of our success: How culture is driving human evolution, domesticating our species, and making us smarter, Princeton University Press. [Google Scholar]
- van IJzendoorn, M.H., Bakermans-Kranenburg, M.J., Belsky, J., et al. (2011). Gene-by-environment experiments: a new approach to finding the missing heritability. Nature Reviews Genetics, 12, 881–1. doi: 10.1038/nrg2764-c1. [DOI] [PubMed] [Google Scholar]
- Kim, H.S., Sasaki, J.Y. (2014). Cultural neuroscience: biology of the mind in cultural contexts. Annual Review of Psychology, 65, 487–514. doi: 10.1146/annurev-psych-010213-115040. [DOI] [PubMed] [Google Scholar]
- King, A.P., Muzik, M., Hamilton, L., Taylor, A.B., Rosenblum, K.L., Liberzon, I. (2016). Dopamine receptor gene DRD4 7-repeat allele X maternal sensitivity interaction on child externalizing behavior problems: independent replication of effects at 18 months. PLoS ONE, 11, e0160473. doi: 10.1371/journal.pone.0160473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama, S., Salvador, C.E. (2017). Culture Embrained: going beyond the nature-nurture dichotomy. Perspectives on Psychological Science, 12, 841–54. doi: 10.1177/1745691617707317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama, S., Uskul, A.K. (2011). Culture, mind, and the brain: current evidence and future directions. Annual Review of Psychology, 62, 419–49. doi: 10.1146/annurev-psych-120709-145357. [DOI] [PubMed] [Google Scholar]
- Kitayama, S., Park, H., Sevincer, A.T., Karasawa, M., Uskul, A.K. (2009). A cultural task analysis of implicit independence: comparing North America, Western Europe, and East Asia. Journal of Personality and Social Psychology, 97, 236–55. doi: 10.1037/a0015999. [DOI] [PubMed] [Google Scholar]
- Kitayama, S., King, A., Yoon, C., Tompson, S., Huff, S., Liberzon, I. (2014). The dopamine D4 receptor gene (DRD4) moderates cultural difference in independent versus interdependent social orientation. Psychological Science, 25, 1169–77. doi: 10.1177/0956797614528338. [DOI] [PubMed] [Google Scholar]
- Kitayama, S., King, A., Hsu, M., Liberzon, I., Yoon, C. (2016). Dopamine-system genes and cultural acquisition: the norm sensitivity hypothesis. Current Opinion in Psychology, 8, 167–74. doi: 10.1016/j.copsyc.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama, S., Yanagisawa, K., Ito, A., Ueda, R., Uchida, Y., Abe, N. (2017). Reduced orbitofrontal cortical volume is associated with interdependent self-construal. Proceedings of the National Academy of Sciences, 114, 7969–74. doi: 10.1073/pnas.1704831114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitayama, S., Varnum, M., Salvador, C. (2019). Cultural Neuroscience. In: Cohen, D., Kitayama, S., editors. Handbook of Cultural Psychology, Second Edition, Guilford Publications, pp. 79–118. [Google Scholar]
- Krall, S.C., Rottschy, C., Oberwelland, E., et al. (2015). The role of the right temporoparietal junction in attention and social interaction as revealed by ALE meta-analysis. Brain Structure & Function, 220, 587–604. doi: 10.1007/s00429-014-0803-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph, M.A., Pobric, G., Jefferies, E. (2009). Conceptual knowledge is underpinned by the temporal pole bilaterally: convergent evidence from rTMS. Cerebral Cortex, 19, 832–8. doi: 10.1093/cercor/bhn131. [DOI] [PubMed] [Google Scholar]
- Maguire, E.A., Gadian, D.G., Johnsrude, I.S., et al. (2000). Navigation-related structural change in the hippocampi of taxi drivers. Proceedings of the National Academy of Sciences of the United States of America, 97, 4398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian, J.A., Laurienti, P.J., Kraft, R.A., Burdette, J.H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage, 19, 1233–9. doi: 10.1016/S1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Markus, H.R., Kitayama, S. (1991). Culture and the self: implications for cognition, emotion, and motivation. Psychological Review, 98, 224–53. doi: 10.1037/0033-295X.98.2.224. [DOI] [Google Scholar]
- Miyamoto, Y., Ma, X. (2011). Dampening or savoring positive emotions: a dialectical cultural script guides emotion regulation. Emotion, 11, 1346–57. doi: 10.1037/a0025135. [DOI] [PubMed] [Google Scholar]
- O’Doherty, J.P., Deichmann, R., Critchley, H.D., Dolan, R.J. (2002). Neural responses during anticipation of a primary taste reward. Neuron, 33, 815–26. doi: 10.1016/S0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone, A., Torres, F. (1993). Plasticity of the sensorimotor cortex representation of the reading finger in Braille readers. Brain, 116(1),39–52. doi: 10.1093/brain/116.1.39. [DOI] [PubMed] [Google Scholar]
- Patterson, C., Feightner, J., Garcia, A., Mac Knight, C. (2007). General risk factors for dementia: a systematic evidence review. Alzheimer’s & Dementia, 3, 341–7. doi: 10.1016/j.jalz.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Rolls, E.T., Grabenhorst, F. (2008). The orbitofrontal cortex and beyond: from affect to decision-making. Progress in Neurobiology, 86, 216–44. doi: 10.1016/j.pneurobio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Rosenzweig, M.R. (1996). Aspects of the search for neural mechanisms of memory. Annual Review of Psychology, 47, 1–32. doi: 10.1146/annurev.psych.47.1.1. [DOI] [PubMed] [Google Scholar]
- Ross, L.A., Olson, I.R. (2010). Social cognition and the anterior temporal lobes. NeuroImage, 49, 3452–62. doi: 10.1016/j.neuroimage.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson, D., Apperly, I.A., Chiavarino, C., Humphreys, G.W. (2004). Left temporoparietal junction is necessary for representing someone else’s belief. Nature Neuroscience, 7, 499–500. doi: 10.1038/nn1223. [DOI] [PubMed] [Google Scholar]
- Sasaki, J.Y., Kim, H.S., Mojaverian, T., Kelley, L.D.S., Park, I.Y., Janušonis, S. (2013). Religion priming differentially increases prosocial behavior among variants of the dopamine D4 receptor (DRD4) gene. Social Cognitive and Affective Neuroscience, 8, 209–15. doi: 10.1093/scan/nsr089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe, R., Kanwisher, N. (2003). People thinking about thinking people the role of the temporo-parietal junction in “theory of mind”. NeuroImage, 19, 1835–42. doi: 10.1016/S1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Saxe, R., Powell, L.J. (2006). It’s the thought that counts: specific brain regions for one component of theory of mind. Psychological Science, 17, 692–9. doi: 10.1111/j.1467-9280.2006.01768.x. [DOI] [PubMed] [Google Scholar]
- Saxe, R., Wexler, A. (2005). Making sense of another mind: the role of the right temporo-parietal junction. Neuropsychologia, 43, 1391–9. doi: 10.1016/j.neuropsychologia.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Schurz, M., Radua, J., Aichhorn, M., Richlan, F., Perner, J. (2014). Fractionating theory of mind: a meta-analysis of functional brain imaging studies. Neuroscience & Biobehavioral Reviews, 42, 9–34. doi: 10.1016/j.neubiorev.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Sheese, B.E., Voelker, P.M., Rothbart, M.K., Posner, M.I. (2007). Parenting quality interacts with genetic variation in dopamine receptor D4 to influence temperament in early childhood. Development and Psychopathology, 19, 1039–46. doi: 10.1017/S0954579407000521. [DOI] [PubMed] [Google Scholar]
- Silveira, P.P., Gaudreau, H., Atkinson, L., et al. (2016). Genetic differential susceptibility to socioeconomic status and childhood obesogenic behavior: why targeted prevention May be the best societal investment. JAMA Pediatrics, 170, 359–64. doi: 10.1001/jamapediatrics.2015.4253. [DOI] [PubMed] [Google Scholar]
- Sims, T., Tsai, J.L., Jiang, D., Wang, Y., Fung, H.H., Zhang, X. (2015). Wanting to maximize the positive and minimize the negative: implications for mixed affective experience in American and Chinese contexts. Journal of Personality and Social Psychology, 109, 292–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singelis, T.M. (1994). The measurement of independent and interdependent self-Construals. Personality and Social Psychology Bulletin, 20, 580–91. doi: 10.1177/0146167294205014. [DOI] [Google Scholar]
- Tompson, S.H., Huff, S.T., Yoon, C., King, A., Liberzon, I., Kitayama, S. (2018). The dopamine D4 receptor gene (DRD4) modulates cultural variation in emotional experience. Culture and Brain, 6, 118–29. doi: 10.1007/s40167-018-0063-5. [DOI] [Google Scholar]
- Wang, E., Ding, Y.-C., Flodman, P., et al. (2004). The genetic architecture of selection at the human dopamine receptor D4 (DRD4) gene locus. American Journal of Human Genetics, 74, 931–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, F., Peng, K., Chechlacz, M., Humphreys, G.W., Sui, J. (2017). The neural basis of independence versus interdependence orientations: a voxel-based morphometric analysis of brain volume. Psychological Science, 28, 519–29. doi: 10.1177/0956797616689079. [DOI] [PubMed] [Google Scholar]
- Weeland, J., Overbeek, G., deCastro, B.O., Matthys, W. (2015). Underlying mechanisms of gene–environment interactions in externalizing behavior: a systematic review and search for theoretical mechanisms. Clinical Child and Family Psychology Review, 18, 413–42. doi: 10.1007/s10567-015-0196-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, S., Keysar, B. (2007). The effect of culture on perspective taking. Psychological Science, 18, 600–6. doi: 10.1111/j.1467-9280.2007.01946.x. [DOI] [PubMed] [Google Scholar]
- Young, L., Camprodon, J.A., Hauser, M., Pascual-Leone, A., Saxe, R. (2010). Disruption of the right temporoparietal junction with transcranial magnetic stimulation reduces the role of beliefs in moral judgments. Proceedings of the National Academy of Sciences, 107, 6753–8. doi: 10.1073/pnas.0914826107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Q., Abe, N., King, A., Yoon, C., Liberzon, I., Kitayama, S. (2019). Cultural variation in the gray matter volume of the prefrontal cortex is moderated by the dopamine D4 receptor gene (DRD4). Cerebral Cortex, 29, 3922–31. doi: 10.1093/cercor/bhy271. [DOI] [PubMed] [Google Scholar]


