Abstract
Background:
In October 2018, the US heart allocation system expanded the number of priority “Status” tiers from 3 to 6 and added cardiogenic shock requirements for some candidates listed with specific types of treatments.
Objective:
Determine the impact of the new policy on the treatment practices of transplant centers.
Methods:
Initial listing data on all adult heart candidates listed from December 1st, 2017 to April 30th, 2019 were collected from the Scientific Registry of Transplant Recipients. The Status-qualifying treatments (or exception requests) and hemodynamics at listing of a post-policy cohort (December 2018 to April 2019) were compared to a seasonally-matched pre-policy cohort (December 2017 to April 2018). Candidates in the pre-policy cohort were reclassified into the new priority system statuses using treatment, diagnosis, and hemodynamics.
Results:
Comparing the post-policy cohort (N =1,567) to the pre-policy cohort (N = 1,606), there were significant increases in listings with extra-corporeal membrane oxygenation (ECMO) (+1.2%), intra-aortic balloon pumps (IABP) (+ 4 %) and exceptions (+ 12%). Listings with low-dose inotropes (−18%) and high-dose inotropes (−3%) significantly decreased. The new priority status distribution had more Status 2 (+14%) candidates than expected and fewer Status 3 (− 5%), Status 4 (− 4%) and Status 6 (− 8%) candidates than expected (p-values < 0.01 for all comparisons).
Conclusion:
After implementation of the new heart allocation policy, transplant centers listed more candidates with ECMO, IABP and exception requests and fewer candidates with inotrope therapy than expected, leading to significantly more high-priority Status listings than anticipated. If these early trends persist, the new allocation system may not function as intended.
Keywords: Heart Transplantation, Allocation, Ethics
Condensed Abstract:
US heart allocation system expanded the number of priority “Status” tiers and added cardiogenic shock requirements for some candidates listed with specific types of treatments. Trends in the listing practices of heart transplant centers before and after policy implementation were determined using Scientific Registry of Transplant Recipients data. Compared to a seasonally matched pre-policy cohort, post-policy candidates were more likely to be listed with extra-corporeal membrane oxygenation (ECMO) (+1.2%), intra-aortic balloon pumps (IABP) (+ 4 %) and exceptions (+ 12%) and less likely to be listed with low-dose (−18%) or high-dose inotropes (−3%) inotropes. There were more Status 2 (+14%) candidates than expected and fewer lower priority candidates.
Introduction
End-stage heart failure kills over 250,000 Americans each year (1). Although heart transplantation remains the definitive treatment, the supply of deceased donor hearts is limited with just over 3,000 transplants performed each year (2). In 2016, the Organ Procurement and Transplant Network (OPTN) thoracic committee formally recognized “major problems” (3) with the ability of the US heart allocation system to meet federal requirements to make the “best use” of donor hearts by ranking candidates from “most to least medically urgent”(4). The system at the time consisted of 3 “status” tiers, with the top priority status 1A intended for a small minority of the most critically ill candidates (5). However, driven by expanding use of high-dose inotrope and continuous-flow left ventricular assist device (LVAD) therapies (6, 7), the majority of waiting adult heart transplant candidates in 2017 were status 1A (2), resulting in long wait times and wide variation in candidate severity of illness within this top priority tier. It became clear that the sickest patients were not being afforded the best chance for transplant.
To address these concerns, the OPTN updated the heart allocation system in October 2018, increasing the number of Status levels from 3 to 6 and implementing a cardiogenic shock requirement to restrict access to the top priority Status levels (8). This new priority system was intended to provide a more granular distinction between the most critically ill patients, enhancing the likelihood that the sickest patients would be transplanted faster. The policy was also designed to better incorporate new devices and technologies such as durable LVADs which can permit some patients to safely wait longer for an organ. Simulation modeling performed prior to implementation suggested that the new heart allocation system had the potential to reduce waitlist mortality without significant increases in post-transplant mortality (9). However, these simulations assumed that the treatment practices of heart transplant centers would remain unchanged after the policy was implemented. After the last major heart allocation policy change in 2006, there were significant changes in transplant center practices (6, 10, 11), suggesting this assumption may not be valid. The simulation also ignored the cardiogenic shock requirement, which had the potential shift over 600 adult heart transplant candidates a year from high priority statuses down to status 4 (12).
This registry cohort study aimed to identify the important early trends in the treatment practices of US adult heart transplant centers after the implementation of the new heart allocation policy. Specifically, we aimed to test the null hypothesis that center treatment practices would be unchanged in response to the policy, leading to a distribution of the new priority system Statuses that matched expectations.
Methods
Data Source and Study Population
This study used data from the SRTR (Scientific Registry of Transplant Recipients). The SRTR data system includes data on all donors, wait-listed candidates, and transplant recipients in the United States, submitted by the members of the OPTN. The Health Resources and Services Administration, U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors.
We identified all adult (≥18 years of age at listing) heart-alone candidates initially listed in the United States from December 1st, 2017 to April 30th, 2019 in the SRTR dataset. We seasonally matched a pre-policy cohort (December 1st, 2017 to April 30th 2018) to the post-policy cohort (December 1st, 2018 to April 30th 2019) for the main pre-post policy comparison. In the post-policy cohort, only new listings (candidates who were not listed in the pre-policy period) were considered. Candidates initially listed “inactive” in either cohort were excluded.
Primary Outcome
The primary outcome of the study was the treatment used on the first status justification form submitted for each candidate. Treatments were grouped into eight major categories, reflecting the typical clinical statuses of patients awaiting heart transplantation and based upon the treatment data available in SRTR: extra-corporeal membrane oxygenation (ECMO), intra-aortic balloon pump (IABP), durable left-ventricular assist devices (LVAD), other mechanical circulatory support (MCS), high-dose inotropes with invasive hemodynamic monitoring, low-dose inotropes (no hemodynamic monitoring), exception request for high priority status without treatment specified, and no treatment (without exception request for a higher status). Exception requests are for when the “transplant physician believes, using acceptable medical criteria, that a heart candidate has an urgency and potential for benefit comparable to that of other candidates at the requested status” and are adjudicated by regional review boards (13). Durable LVADs were distinguished from other MCS via an official list of device codes provided by the SRTR.
Reclassification of pre-policy cohort into the new priority system tiers
Following the approach taken during OPTN simulation modeling (9), we assigned each candidate in the pre-policy cohort a status by applying the new heart allocation policy (14) retrospectively based on the candidate’s old priority system status justification, listing diagnosis, and hemodynamics. This re-classification is possible for the same reason simulation modeling was possible- all the necessary treatment and clinical variables were recorded in the SRTR data pre-policy implementation. Our approach to this process has been published previously (12, 15, 16) and extends upon the OPTN simulation approach by applying the cardiogenic shock requirement to the candidates who are subject to it. Patients can meet the cardiogenic shock requirement via non-hemodynamic or the invasive hemodynamic criteria. The non-hemodynamic criteria are at least one of the following before support initiation: cardiopulmonary resuscitation (CPR), systolic blood pressure less than 70 mm Hg, arterial lactate greater than 4 mmol/l, or aspartate transaminase (AST) or alanine transaminase (ALT) greater than 1,000 U/L. For most candidates, the hemodynamic criteria require all of the following to be true within 24 hours: cardiac index below 2.0 L/min/m2, pulmonary capillary wedge pressure above 15 mmHg, and systolic blood pressure below 90 mm Hg. The exact hemodynamic cutoffs vary based on the type of support at the time measurement, and there are minimum dose requirements for inotropes. Full details are available in Supplemental Table 1. We only applied the hemodynamic portion of the criteria and conservatively assumed a patient met criteria for cardiogenic shock if any hemodynamic data were missing (Appendix, Supplemental Figure 1).
Treatments subject to the cardiogenic shock requirement
For candidates listed with IABP, high-dose inotropes, and low-dose inotropes (all subject to hemodynamic cardiogenic shock requirements in the new system), we determined trends in the number of candidates pre (stratified by whether or not the shock requirement was met) and post policy. Because of low numbers of listing and difficult interpretation of hemodynamics on support, candidates listed with ECMO and percutaneous temporary MCS were considered to have met the cardiogenic shock requirement and were excluded from the trend analysis.
In the SRTR dataset, candidates listed for heart transplantation have data drawn from two different forms: The Status justification form and the transplant candidate registration form. Status justification forms are submitted in real time when the patient is listed and are required to receive the corresponding priority status. The registration form contains many more fields and is typically completed by the transplant program coordinator and is used for research and risk-adjustment of waitlist outcomes. The data on the transplant candidate registration form is supposed to reflect the patient’s clinical state at the time of listing, however programs typically have 30 days after listing to complete it. Trends in the mean cardiac index recorded on the registration form and the mean cardiac index recorded on the Status justification form were determined. The mean difference between registration form and justification form cardiac index in the post-policy period was calculated by treatment group (paired t-test of the mean).
Statistical analysis
Unadjusted differences in treatment utilization rates for each treatment category between the pre and post policy cohorts were calculated and the difference evaluated with a Pearson’s chi-squared test. The predicted vs. observed new priority system status distributions were also compared in similar fashion. To adjust for potential confounding by changes in patient characteristics over time, we calculated a propensity score based on candidate age, sex, body mass index, race, cardiac diagnosis, history of diabetes, renal function, functional status, payor, education status, working status, history of smoking, and blood type. We used this score to perform inverse propensity score weighting (IPW) to balance candidate covariates (with a goal of standardized mean differences of <10%) (17) and then performed multinomial logistic regressions on the weighted sample to estimate adjusted differences in treatment rates.
This study was a secondary analysis of de-identified, pre-collected data and was granted exemption status by the University of Chicago Biological Sciences Division/University of Chicago Medical Center IRB to be performed without patient consent. Analyses were performed using R 3.4.3 (The R Foundation for Statistical Computing 2017) and Stata version 15 (StataCorp LLC, Austin TX). All statistical testing was two-sided with a p-value threshold of <0.05.
Results
Candidate characteristics
A total of 5,467 adult candidates were listed in an active Status during the study time period. The number of candidates in the seasonally matched pre-policy and post-policy cohorts were similar (1,606 vs. 1,567, p = 0.5 by Poisson means test). There were 60 candidates in the pre-policy cohort and 51 candidates in the post-policy cohort who were listed in inactive status and excluded from the analysis. Candidate characteristics in the pre and post policy cohort are displayed in Table 1 and were largely unchanged, with a few exceptions. The percentage of candidates working for income increased from 19% of candidates in the pre-policy cohort to 22% of candidates in the post-policy cohort (absolute difference 3%; 95% CI 0.15% to 5.9%), the rates of diabetes decreased from 29% of candidates in the pre-policy cohort to 27% of candidates in the post-policy cohort (absolute difference −1.7%; 95% CI −4.9% to 1.5%), and the number of candidates with “unknown” or missing functional status increased from 1.4% of candidates in the pre-policy cohort to 8.2% candidates in the post-policy cohort (absolute difference 6.7%; 95% CI 5.2% to 8.3%).
Table 1:
Candidate Characteristics by Policy Period
| variable | Dec 2017 – May 2018 (Pre-Policy) (N =1606) |
Dec 2018 – May 2019 (Post-policy) (N =1567) |
p-value |
|---|---|---|---|
| Median [IQR] | Median [IQR | ||
| Candidate Age at Listing | 56 [46–63] | 56 [45–63] | 0.58 |
| Body Mass Index | 28 [24–31] | 28 [24–32] | 0.89 |
| GFR | 62 [48–80] | 63 [48–79] | 0.42 |
| N (%) | N (%) | ||
| Female | 443 (28) | 412 (26) | 0.41 |
| Race | |||
| White | 1041 (65) | 971 (62) | 0.39 |
| Black | 349 (22) | 376 (24) | |
| Hispanic | 146 (9) | 147 (9) | |
| Other | 70 (4) | 73 (5) | |
| Blood type | |||
| A | 567 (35) | 594 (38) | 0.48 |
| AB | 77 (5) | 73 (5) | |
| B | 248 (15) | 225 (14) | |
| O | 714 (44) | 675 (43) | |
| Smoking history | 702 (44) | 674 (45) | 0.63 |
| Working for income | 310 (20) | 350 (23) | 0.016 |
| Educational Attainment | |||
| College | 905 (56) | 846 (54) | <0.01 |
| High School | 588 (37) | 553 (35) | |
| Less than high school or unknown | 113 (7) | 168 (11) | |
| Diagnosis | |||
| Dilated cardiomyopathy, non-ischemic | 670 (42) | 686 (44) | 0.25 |
| Ischemic cardiomyopathy | 496 (31) | 439 (28) | |
| Restrictive cardiomyopathy | 220 (14) | 235 (15) | |
| Other | 220 (14) | 207 (13) | |
| History of DM | 468 (29) | 430 (27) | <0.001 |
| Functional Status | |||
| Limited Impairment, 100–70% | 422 (26) | 345 (22) | <0.01 |
| Moderate Impairment, 50–60% | 400 (25) | 320 (20) | |
| Severe Impairment ≥ 40%% | 761 (47) | 774 (49) | |
| Unknown | 23 (1) | 128 (8) | |
| Payor: | |||
| Medicaid | 216 (13) | 206 (13) | <0.01 |
| Medicare | 559 (35) | 488 (31) | |
| Other | 69 (4) | 124 (8) | |
| Private | 762 (47) | 749 (48) | |
Summary statistics for candidate characteristics of both the pre and post-policy cohort. p-values for mean comparisons performed with paired t-test. p-values for categorical variables generated with chi-squared test.
Changes in exception and treatment rates at initial candidate listing
The by month trends for each treatment category during the study period are displayed in Central Illustration. The therapy with the largest decrease in utilization was low-dose inotropes, decreasing from 375 (23%) candidates in the pre-policy cohort to 88 (5.6%) candidates in the post-policy cohort (absolute difference −18%; 95% CI −20% to −15%). High-dose inotropes also significantly decreased, from 142 (8.8%) candidates in the pre-policy cohort to 91 (5.8%) candidates in the post-policy cohort (absolute difference −3%; 95% CI −4.9% to – 1.2%). The therapy with the largest increase in utilization was IABP, increasing from 73 (4.5%) candidates in the pre-policy cohort to 128 (8.2%) candidates in the post-policy cohort (absolute difference 3.7%; 95% CI 1.9% to 5.4%). ECMO utilization increased from 22 (1.4%) candidates in the pre-policy cohort to 41 (2.6%) candidates in the post-policy cohort (absolute difference 1.2%; 95% CI 0.21% to 2.3%).
Central illustration: Trends in treatments used to list adult heart transplant candidates during the transition to the new heart allocation policy.
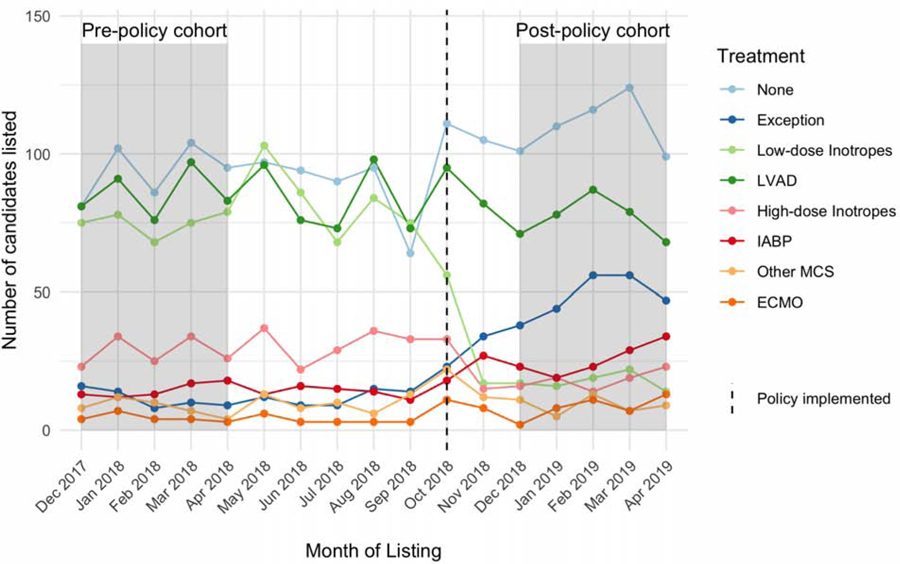
Trends in the number of adult heart transplant candidates listed in each month, stratified by treatment at initial listing. Colors correspond to the treatment listed on the Status justification form at initial listing. The dashed line represents October 2018, when the policy was implemented.
The number of patients listed at the lowest priority Status without a specific therapy on their justification form increased from 468 (29% candidates in the pre-policy cohort to 550 (35%) candidates in the post-policy cohort (absolute difference 6%; 95% CI 2.7% to 9.3%). Listings with exceptions (without a specific justifying treatment), increased from 57 (3.5%) candidates in the pre-policy cohort to 241 (15%) candidates in the post-policy cohort (absolute difference 12%; 95% CI 9.8% to 14%). All treatments had statistically significant changes post-policy (Table 2) except for LVADs and other/miscellaneous MCS. The inverse propensity score weighting on candidate characteristics was successful, with standardized differences between all variables < 1% (considered very well balanced, Supplemental Figure 2). The IPW model treatment rate estimates were not significantly different than the unadjusted results (Supplemental Table 2, Supplemental Table 3).
Table 2:
Treatment rates by policy period
| Treatment | Dec 2017 - Apr 2018 (Pre-Policy) N = 1,606 |
Dec 2018 - Apr 2019 (Post-policy) N = 1,567 |
absolute difference (%) |
|---|---|---|---|
| None | 468 (29) | 550 (35) | 6% [95% CI, 2.7% to 9.3%] |
| Exception | 57 (3.5) | 241 (15) | 12% [95% CI, 9.8% to 14%] |
| Low-dose Inotropes | 375 (23) | 88 (5.6) | −18% [95% CI, −20% to −15%] |
| LVAD | 428 (27) | 383 (24) | −2.2% [95% CI, −5.3% to 0.89%] |
| High-dose Inotropes | 142 (8.8) | 91 (5.8) | −3% [95% CI, −4.9% to −1.2%] |
| IABP | 73 (4.5) | 128 (8.2) | 3.7% [95% CI, 1.9% to 5.4%] |
| Other MCS | 41 (2.6) | 45 (2.9) | 0.3% [95% CI, −0.88% to 1.5%] |
| ECMO | 22 (1.4) | 41 (2.6) | 1.2% [95% CI, 0.21% to 2.3%] |
Observed vs. Expected new priority system tiers distribution
The expected status distribution generated from the pre-policy cohort was 2.9 % Status 1 candidates, 3.8 % Status 2 candidates, 17% Status 3 candidates, 45% Status 4 candidates, 0.6% Status 5 candidates, and 31% Status 6 candidates (Figure 1). The actual new priority status system distribution was significantly different from the expected Status distribution (chi-squared test statistic 196, p< 0.001). The proportion of Status 2 listings were significantly higher than expected compared to pre-policy cohort (+14%; 95% CI 12% to 16%). Status 3 was used less often than expected with (–4.5%; 95% CI −7% to −2%) listings compared to the pre-policy cohort. Status 4 listings were also significantly less than expected (−4.4%; 95% CI −7.9% to −0.9%). Finally, Status 6 was used significantly less often than expected, (–8%; 95% CI −11% to −4.9%) compared to pre-policy.
Figure 1: Predicted and Observed Status Distribution in the New US Heart Allocation System.
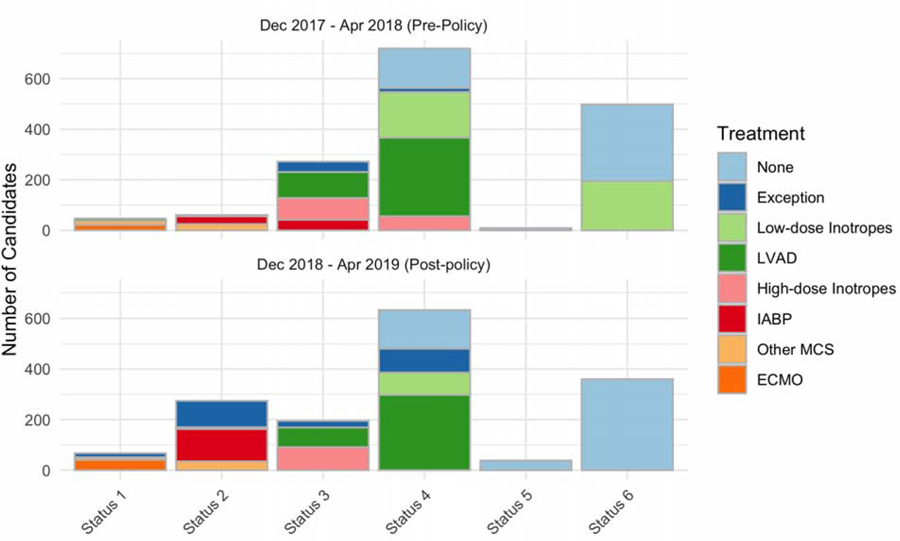
Predicted (top) Status distribution was created by applying the Status 1–6 listing criteria to the seasonally matched pre-policy cohort. Observed (bottom) Status distribution is histogram of actual listing Statuses utilized during the post-policy cohort. See supplement for details regarding classification of the pre-policy cohort into Statuses 1–6. Colors correspond to the treatment listed on the justification form for each status level.
Trends in the number and hemodynamics of candidates supported with treatments subject to the cardiogenic shock requirement
Figure 2 displays the monthly trends in listing with therapies subject to new hemodynamic requirements in the post-policy period. There was a significant increasing monotonic trend over time in IABP use (p<0.001), a significant decreasing monotonic trend in low-dose inotrope use (p=0.005), and no significant trend in high-dose inotropes (p=0.076) or ECMO listings (p=0.22). Figure 3 displays the mean cardiac index by month for each of these groups as recorded on the transplant candidate registration form and Status justification forms. In the old priority system, justification form hemodynamic data was only available for the high-dose inotrope candidates, as the other therapies did not require hemodynamics as part of the Status justification. There was no significant difference between Status justification form and registration form hemodynamics in the old priority system for candidates supported with high-dose inotropes. In the new priority system, registration form cardiac index was significantly higher than Status justification form cardiac index. Restricted to measurements with the same support (i.e., both on inotropes or off), the mean cardiac index on the justification form was less than mean cardiac index on the registration form across all treatments (p<0.01 for all comparisons). Specifically, the cardiac index used to justify status on the status justification form was −0.41 L/min/m2 lower for IABP, −0.28 L/min/m2 lower for high-dose inotropes, and −0.31 L/min/m2 lower for low-dose inotropes. There was no difference between the justification and registration form dates for any candidate in the analysis.
Figure 2: Trends in Adult Heart Transplant Candidates Subject to Hemodynamic Requirements in the New Heart Allocation System.
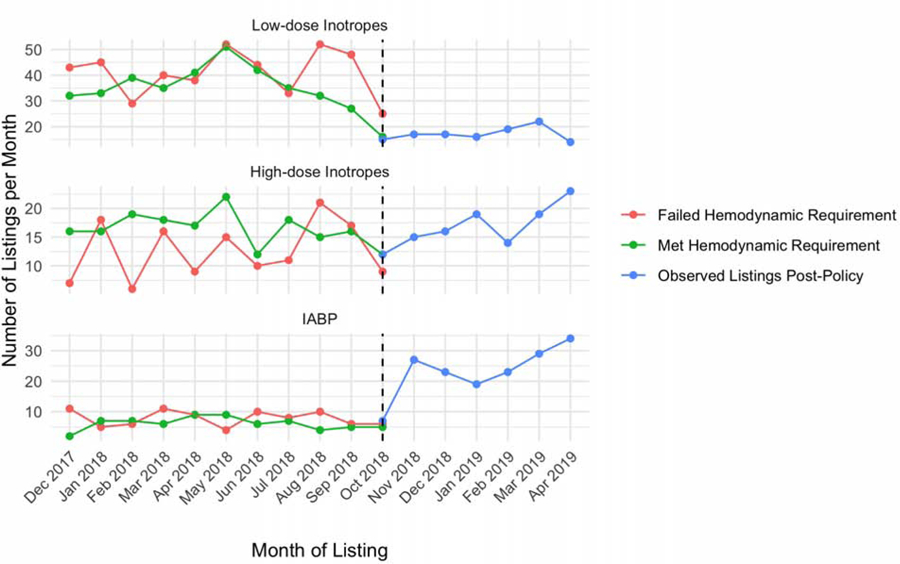
Trends in the number of candidates listed with low-dose inotropes, high-dose inotropes (with invasive hemodynamic monitoring), and IABP from December 2017 to April 2019. Listings prior to policy implementation are stratified by whether or not the candidates met the hemodynamic requirement for their respective therapy. ECMO and percutaneous temporary mechanical circulatory support devices were excluded due to low numbers of candidates listed each month (see Table 2).
Figure 3: Cardiac Index of Adult Heart Transplant Candidates subject to hemodynamic criteria in the new allocation system.
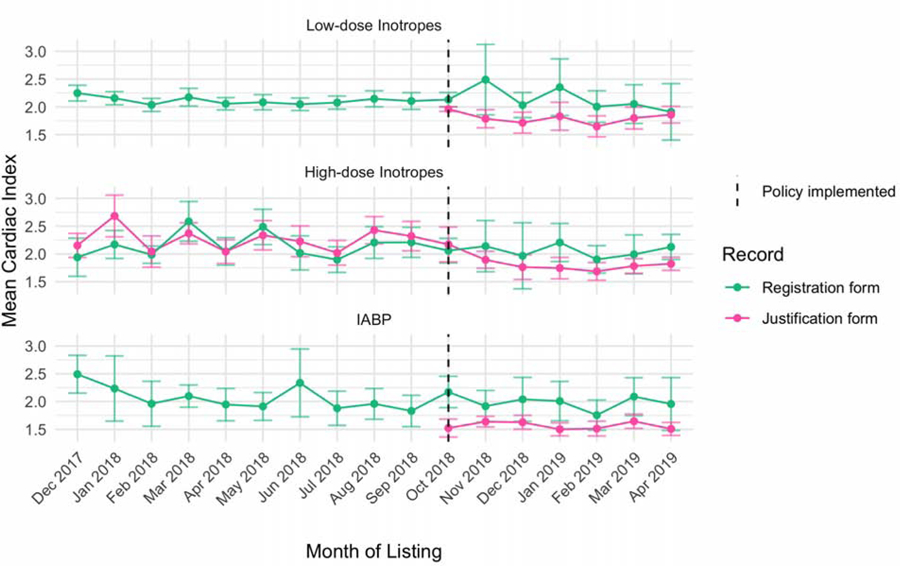
Trends in the mean cardiac index of candidates listed with low-dose inotropes, high-dose inotropes (with invasive hemodynamic monitoring), and IABP from December 2017 to April 2019. Mean cardiac index values recorded on the transplant candidate registration form (pink) are typically entered by a transplant coordinator after listing looking back in the medical record. Mean cardiac index values from the Status justification form are those used to qualify for status in real-time and are subject to the hemodynamic requirements post-policy. Only pairs of measurements where inotropic support at the time of measurement were concordant (i.e. both on or both off) were included. Error bars correspond to 95% confidence intervals of the means. ECMO and percutaneous temporary mechanical circulatory support devices were excluded due to low numbers of candidates listed each month (see Table 2).
Discussion
In this observational study of adult heart transplant listing before and after the new allocation policy change, we found significant changes in the transplant center treatment practices that were not explained by changes in candidates in the two-year time interval studied. The number of newly listed patients in both cohorts were similar and the patients had similar baseline characteristics and hemodynamics, but we found large increases in intra-aortic balloon pump utilization and lower utilization of inotropes. These changes in practice lead to a substantially different distribution of Statuses compared to expected, specifically higher rates of Status 2 and lower rates of Status 3, 4, and 6. After the cardiogenic shock requirement was implemented, we found a consistent difference between the cardiac index used for Status justification and the “baseline” registration form value that was highest for IABP candidates.
When the new priority system was designed, it was with the intention of reducing the clustering of candidates in the top-tier Status 1A and improving the geographic sharing of donor hearts. By splitting this tier into three separate tiers, the committee intended to meet the final rule requirement of a “sufficient number of categories…to avoid grouping together patients with substantially different medical urgency”(4). However several implicit assumptions underpinned the projections of success for this new policy. First, the cardiogenic shock requirement would work as an effective barrier to manipulation of waitlist priority via escalation of therapies, forcing centers to list stable candidates at lower Status. Second, aside from the impact of the shock requirement, treatment practices would remain stable. Third, the increased number of Status tiers would lead to better default prioritization of candidates and therefore fewer exceptions. The OPTN built the new priority system on the assumption that treatment is an adequate proxy for medical urgency, which means that the intensity of treatment reflects the patient’s underlying clinical state and need for transplant. If treatment practices are independent of considerations of priority for transplant, changes in the priority ranking tiers and the geographic sharing of donor hearts should not have affected treatment practices for the care of critically ill heart transplant candidates.
The results of this study challenge the validity of these assumptions. During the public comment period for preliminary version of the new priority system, fears about increasing utilization of ECMO in particular motivated the inclusion of the cardiogenic shock requirement (8). Therefore, while the absolute increase in listings with EMCO was small, it was statistically significant and potentially consistent with the fears that this therapy may be overused in the new system.
The high number of Status 2 listings from candidates treated with IABPs suggest the hemodynamic requirements are not limiting utilization of this treatment as much as expected. The shock requirement was expected to disqualify over 40% of candidates treated with IABPs (12), instead IABP listings increased after implementation. The large difference between registration and justification form cardiac index measurements for IABP candidates suggest that transplant centers may be influencing the recorded values on the justification form (what matters to receive status). Specifically, transplants centers can measure cardiac index by thermodilution and Fick methods. Without a standard protocol enforced by UNOS, this may allow centers to select the lower of the two cardiac indices measured by thermodilution and Fick methods for patients close to the hemodynamic thresholds. Why IABP utilization specifically increased can potentially be explained by its high Status and the low requirements for re-certification. While the initial listing requirements for IABP are more strict than high-dose inotropes, re-certification every 14 days is far easier. Only a pulmonary capillary wedge pressure of greater than 15 is required to re-certify for IABP. In contrast, high-dose inotrope candidates must demonstrate a cardiac index smaller than 2.2 (or other stricter physiologic criteria) in order to maintain Status 3 (a whole priority tier lower than IABP).
In contrast, the decrease in low-dose inotrope candidates exceeds what was expected from the cardiac index requirement. This is potentially because inotrope data for low-dose inotrope candidates was not available in the old priority system, so the minimum dose component of criteria could not be included in our reclassification methodology. Instead of being listed Status 6 with no support as intended, it appears many of these candidates were treated with alternative support therapies that garner higher priority status or listed via exception requests.
The new system has also dramatically increased exception requests, instead of decreasing exceptions as intended. Many of the granted exceptions are for Status 2, which potentially reduces the effective priority of the sickest candidates in this Status tier. While the higher number of exceptions could optimistically be attributed to transition effects, the number of exceptions per month had not begun to decline meaningfully as of April 2019, a full 6 months after policy implementation.
On the other hand, there are some potential positive interpretations of the trends in treatment practices post-policy implementation. The number of candidates listed at Status 6 without therapy increased (although fewer than expected), implying the hemodynamic criteria are partly effective. Specifically, the drop in high-dose inotrope candidates was consistent with expectations, suggesting the shock requirement is working for this group. And finally, while not perfect, the stratification Status 1A into Status 1, 2, and 3 is a clear improvement over the old priority system. The percentage of candidates listed at Status 1A was 45% in the old priority system. In the new priority system, the percentage of candidates listed at Status 1 was 4.3%. Hopefully this leads to increased allocation of hearts to the most urgent candidates and generates more lives saved from transplantation.
Limitations
Our study was mainly descriptive but has several limitations. First, our retrospective coding of old priority system, which had 3 statuses, to the new priority system, which has 6 statuses, is hypothetical in nature. Specifically, we had to assume candidates supported with ECMO or percutaneous temporary mechanical circulatory support met the hemodynamic criteria for listing at Status 1 and 2. However, our methodology has been published multiple times (12, 15, 16) and is similar to approach applied in the OPTN simulation studies. Second, in contrast to the tightly regulated Status justification cardiac index, the registration form cardiac index measurements are supposed to be “from the time of listing;” however, no specific date of measurement is recorded. So, while the date of the justification forms and the registration forms were the same for every patient in our analysis, it is possible that for some patients the registration form hemodynamics represent some pre-decompensation baseline clinical state prior to the worsening clinical state recorded on the justification form at listing. However, the strong correlation of the registration form and justification form hemodynamics for the high-dose inotrope candidates pre-policy suggest that both measurements capture more or less the same physiology. Also, the groups more likely to be disqualified by the requirement (low and high-dose inotrope candidates) had smaller gaps between the measurements than IABP candidates (which increased after the new policy was implemented). Third, even though the treatment rates were stable during our selected pre and post policy cohorts, treatment practices are likely to continue to evolve over time and follow-up studies are needed. Fourth, this study only examined the change in listing practices, but not its effect on wait-list mortality or post-transplant survival. More follow-up time will need to accrue to properly evaluate the effect of the new policy on these important outcomes.
Finally, the term “gaming” has at times been used to describe transplant center treatment practices and listing behavior. However, in the context of a therapy-based allocation system, the line between “gaming” and patient advocacy is blurred.(18) It is not possible from registry data to judge individual decisions regarding the optimal therapy for patients deemed by their physician team to be at high risk for death without imminent transplantation. Our study was descriptive as we aimed to test the null hypothesis that transplant center treatment practices are unresponsive to allocation system rules. The data in this study soundly reject this hypothesis, which has significant implications for the future of the new priority system.
Conclusion
After implementation of the new heart allocation policy, transplant centers listed more candidates with ECMO, IABP and exception requests and fewer candidates with inotrope therapy than expected, leading to significantly more high-priority Status listings than anticipated. If these early trends persist, the new allocation system may not function as intended.
Supplementary Material
Perspectives.
Competency in Systems-based Practice:
Changes in organ allocation policy lead to listing more candidates on mechanical circulatory support and exceptions and fewer candidates on inotropic medications, leading to more high-priority listings than anticipated.
Translation Outlook:
These trends call for further research of strategies that optimize outcomes of heart transplantation for the greatest number of patients.
Acknowledgments:
The authors would like to acknowledge Nikhil Narang for his assistance with the project by providing relevant clinical information. The data reported here have been supplied by the Hennepin Healthcare Research Institute (HHRI) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
Funding: William Parker was supported by a NIH grant K08 HL150291. Elbert Huang was supported by NIH grant numbers K24 DK105340 and P30 DK092949. Mathew Churpek was supported by NIH grant number R01 GM123193.
Abbreviations:
- OPTN
Organ Procurement and Transplant Network
- SRTR
Scientific Registry of Transplant Recipients
- ECMO
Extra-corporeal membrane oxygenation
- IABP
Intra-aortic balloon pump
- LVAD
Left-ventricular assist device
- MCS
Mechanical circulatory support
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Churpek reported receiving research support from EarlySense in Tel Aviv, Israel; and having a US patent pending for a risk stratification algorithm for hospitalized patients. The remaining authors have nothing to disclose.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics—2017 Update: A Report From the American Heart Association. Circulation 2017:CIR.0000000000000485. [DOI] [PMC free article] [PubMed]
- 2.Colvin M, Smith JM, Hadley N, et al. OPTN/SRTR 2017 Annual Data Report: Heart. Am. J. Transplant 2019;19:323–403. [DOI] [PubMed] [Google Scholar]
- 3.Proposal to Modify the Adult Heart Allocation System. Available at: https://optn.transplant.hrsa.gov/media/2006/thoracic_brief_201612.pdf. Accessed March 12, 2016.
- 4.e-CFR: Title 42: Public Health. Available at: http://www.ecfr.gov/cgi-bin/text-idx?SID=bb60e0a7222f4086a88c31211cac77d1&mc=true&node=pt42.1.121&rgn=div5#se42.1.121_18. Accessed December 23, 2015.
- 5.Colvin M, Smith JM, Hadley N, et al. OPTN/SRTR 2016 Annual Data Report: Heart. Am. J. Transplant 18:291–362. [DOI] [PubMed] [Google Scholar]
- 6.Parker WF, Garrity ER, Fedson S, Churpek MM. Trends in the Use of Inotropes to List Adult Heart Transplant Candidates at Status 1A. Circ. Heart Fail 2017;10:e004483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dardas T, Mokadam NA, Pagani F, Aaronson K, Levy WC. Transplant registrants with implanted left ventricular assist devices have insufficient risk to justify elective organ procurement and transplantation network status 1A time. J. Am. Coll. Cardiol 2012;60:36–43. [DOI] [PubMed] [Google Scholar]
- 8.Modify adult heart allocation 2016. 2nd round - OPTN. Available at: https://optn.transplant.hrsa.gov/governance/public-comment/modify-adult-heart-allocation-2016-2nd-round/. Accessed December 14, 2016.
- 9.Colvin M, Bolch C, Pyke J, Skeans M, Wang X, Zeglin J. Analysis Report: Data Request from the Heart Subcommittee of the OPTN Thoracic Organ Transplantation Committee. Data Request ID: HR2015_01. 2015.
- 10.Nativi JN, Kfoury AG, Myrick C, et al. Effects of the 2006 U.S. thoracic organ allocation change: analysis of local impact on organ procurement and heart transplantation. J. Heart Lung Transplant 2010;29:235–239. [DOI] [PubMed] [Google Scholar]
- 11.Schulze PC, Kitada S, Clerkin K, Jin Z, Mancini DM. Regional differences in recipient waitlist time and pre- and post-transplant mortality after the 2006 United Network for Organ Sharing policy changes in the donor heart allocation algorithm. JACC Heart Fail 2014;2:166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parker WF, Garrity ER, Fedson S, Churpek MM. Potential impact of a shock requirement on adult heart allocation. J. Heart Lung Transplant 2017;36:1013–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.OPTN Policy 6: Allocation of Hearts and Heart-Lungs. Available at: https://optn.transplant.hrsa.gov/governance/policies/. Accessed February 7, 2019.
- 14.Policies - OPTN. Available at: https://optn.transplant.hrsa.gov/governance/policies/. Accessed October 25, 2018.
- 15.Parker WF, Anderson AS, Hedeker D, et al. Geographic Variation in the Treatment of U.S. Adult Heart Transplant Candidates. J. Am. Coll. Cardiol 2018;71:1715–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parker WF, Anderson AS, Gibbons RD, et al. Association of Transplant Center With Survival Benefit Among Adults Undergoing Heart Transplant in the United States. JAMA 2019;322:1789–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Statist. Med 2015;34:3661–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prateeti Khazanie, Drazner Mark H. The Blurred Line Between Gaming and Patient Advocacy. Circulation 2019;140:2048–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


