Abstract
Tremendous efforts have been directed to investigate the ontogeny of drug transporters in the fetus, neonates, infants, and children based on their importance for understanding drug pharmacokinetics. During development (i.e. in the fetus and newborn infant), there is a special interest in transporters expressed in the placenta which modulate placental drug transfer. Many of these transporters can decrease or increase drug concentrations in the fetus and at birth stressing the relevance of elucidating the expression in the placenta and potential gestational age-dependent changes therein. Hence, the main objective of this review was to summarize the current knowledge about expression and ontogeny of transporters in the human placenta in healthy pregnant women. Additionally, various in vitro, ex vivo and in silico models that can be used to investigate placental drug transfer, namely placental cancer cell lines, ex vivo cotyledon perfusion experiments and physiologically based pharmacokinetic (PBPK) models, are discussed together with their advantages and shortcomings. A particular focus was placed on PBPK models because these models can integrate different types of information, such as expression data, ontogeny information and observations obtained from the ex vivo cotyledon perfusion experiment. Such a mechanistic modeling framework may leverage the available information and ultimately help to improve the knowledge about the adequacy and safety of pharmacotherapy in pregnant women and their fetus.
Introduction
Drug transporters are of paramount importance for drug exposure and many research efforts have consequently been directed to elucidate the ontogeny of these transporters in the fetus, neonates, infants, and children.1–3 In addition to the expression of these transporters in tissues such as the intestinal tract, drug exposure in preterm and term neonates may also be modulated by drug transfer via the placenta.4 In fact, numerous transporters are expressed in the placenta transferring nutrients such as amino acids, vitamins and glucose, and xenobiotics such as drugs and environmental pollutants across the blood-placenta barrier. Many drug transporters may have a fetoprotective effect by acting as efflux transporters and lowering drug concentrations on the fetal side. For example, breast cancer resistance protein (BCRP) has been found to substantially limit glyburide transfer across the human placenta in both in vitro and ex vivo experiments.5,6 Consistent with these findings, a clinical study involving pregnant women with gestational diabetes mellitus reported that glyburide concentrations in the umbilical vein were on average significantly lower compared to those measured in maternal plasma. Specifically, the reported median umbilical-to-maternal plasma glyburide concentration ratio was 0.81 (interquartile range: 0.46 – 1.27).7
Hence, a detailed understanding of transporters in the placenta including their ontogeny constitutes a crucial aspect for elucidating drug exposure in utero and in neonates. Quantitative information on the expression can be obtained from various experiments (e.g. with cultured placental cancer cell lines, such as the BeWo cell line, or primary (cyto)trophoblasts isolated from fresh placentae). Importantly, the latter experiment can also be used to study the ontogeny of transporters. Ex vivo cotyledon perfusion experiments are suitable to assess the kinetics of drug transfer across the placental barrier. Data from these experiments can eventually be leveraged in a mechanistic modeling framework, such as physiologically-based pharmacokinetic (PBPK) models, to investigate and predict the rate and extent of placental drug transfer and ultimately fetal and neonatal drug exposure in silico.8 Since much of the success of integrating placental drug transfer in vivo depends on the availability and quality of data on transporter expression, the primary objective of this article is to review the expression and ontogeny of various transporters in the healthy human placenta. Additionally, in vitro, ex vivo and in silico methods that can be used to investigate placental drug transfer are discussed along with their advantages and shortcomings.
Although animal models have also been used for the investigation of placental drug transfer, histological and morphological differences between different species and humans make an extrapolation to pregnant women difficult.9 Therefore, the current review does not include animal models.
Placental Transporters
The maternal-fetal interface in the placenta consists of a layer of syncytiotrophoblasts and cytotrophoblasts that separate the maternal from the fetal circulation. This cellular layer constitutes the blood-placenta-barrier and various transporters may be expressed in the apical or basolateral membrane of these cells. Transporters may additionally be expressed in the endothelial membrane lining the interior surface of the fetal blood vessels and separating the fetal blood from the stroma and trophoblasts.
Regulation of placental transporter expression
Knowledge about the specific effect of pregnancy on the regulation of transporter expression in the placenta is incomplete and most information is limited to the expression of the adenosine triphosphate-binding cassette (ABC) B1 (encoding P-glycoprotein) and ABCG2 (encoding BCRP). There is some evidence that treatment with progesterone and estrogen may upregulate the expression of ABCB1 and ABCG2 in placental cancer cell lines10 and in primary trophoblasts isolated from term placentae.11 Interestingly, although the plasma concentrations of these hormones rise dramatically during pregnancy, no increase in placental expression of ABCB1 and ABCG2 is observed in vivo stressing the importance of parallel pathways regulating the expression of these genes. For example, treatment of primary term trophoblasts with the inflammatory mediators tumor necrosis factor a (TNF-α) and interleukin (IL)-1β in vitro has been shown to decrease the expression of ABCB1 and ABCG2V Since the human placenta constitutively produces these cytokines, their inhibitory effect on ABCB1 and ABCG2 expression may outweigh the activating effect exerted by progesterone and estrogen. Of note, a number of disorders are associated with elevated plasma concentrations of these cytokines compared to healthy controls, e.g. preeclampsia,12 gestational diabetes,13 intrauterine growth restriction (IUGR)14 and intrauterine infection.15 This raises the question to which extent the pregnancy-induced change in placental transporter expression is modified by diseases and drugs.
Furthermore, hypoxic conditions have been observed to significantly increase the production of TNF-α and IL-1β in cultured villous explants from human placentae.16 However, although the placenta is poorly vascularized during early pregnancy,17 the resulting hypoxia does not appear to translate into reduced placental transporter expression in the first trimester, stressing again the importance of parallel signalling pathways.
In addition to these factors regulating gene expression, the cellular tumor antigen p53 is another protein interacting with the ABCB1 promotor region in cancer cell lines. This interaction leads either to repression or activation of the gene expression depending on the relative orientation of p53 binding sites in the ABCB1 promoter region.18 In placentae collected at term pregnancy, very high levels of p53 have been observed in the syncytiotrophoblast19 which might be linked to the declining expression of ABCB1. In summary, a mechanistic understanding of the processes involved in the regulation of placental drug transporter expression is currently lacking and needs to be addressed by future studies.
Expression and ontogeny of placental transporters
Despite these knowledge gaps on the factors controlling gene expression, many studies have determined whether and to which extent drug transporters are expressed in the human placenta and how the expression pattern changes during the course of pregnancy. The subsequent sections address these aspects for each transporter. For reasons outlined below, the review of transporter expression has been limited to studies analysing fresh placenta tissue from healthy pregnant women since many diseases change the transporter expression in placenta, whereas transporter expression in placental cancer cell lines, such as BeWo cells, will not be reported here. Hence, the available information was generally derived from placentae collected at the end of the first trimester following abortion or in the second half of pregnancy following preterm or term delivery. Data from preterm delivery associated with intrauterine infections or other complications were not included here; if no such condition was reported, the pregnant women were assumed to be healthy and data from preterm delivery were included in this analysis. Figure 1 schematically illustrates the drug transporters known to be expressed in the human placenta. The localization and change in transporter expression during pregnancy is also listed in Table 1 together with alternative names and examples of substrates of each transporter. The following information on transporter expression is exclusively derived from human placentae.
Figure 1.
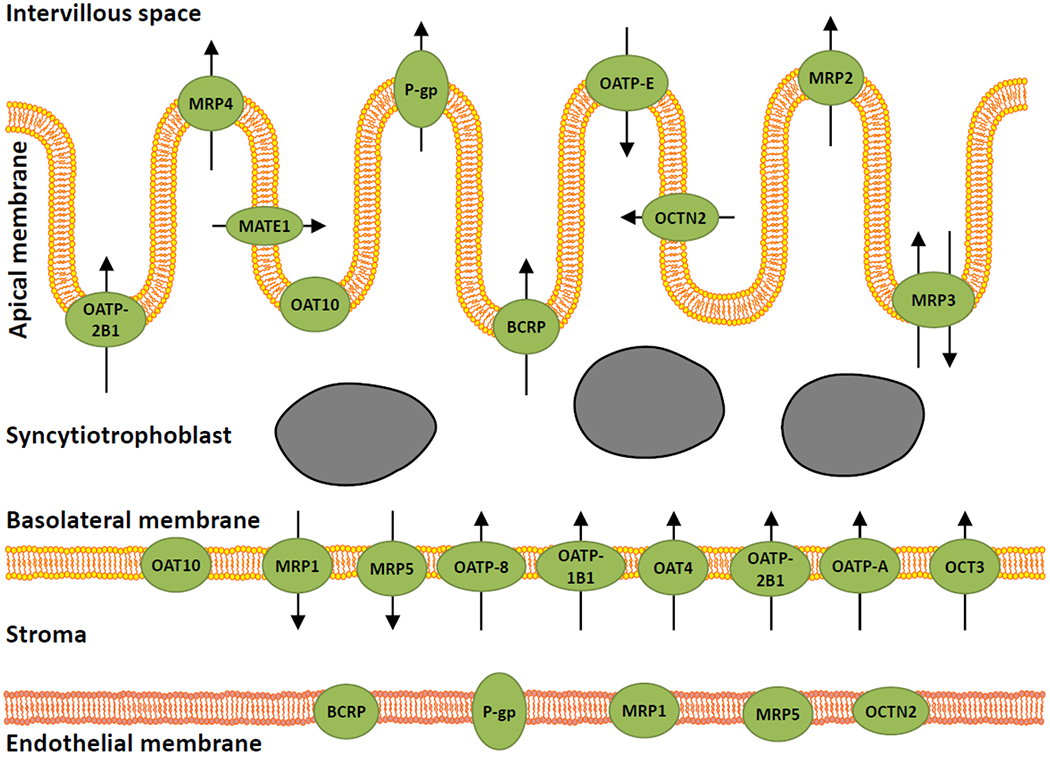
Expression and localization of drug transporters in the placenta. Transporters are shown as green symbols, and the arrows indicate the direction of the transport, if reported.BCRP, breast cancer resistance protein; MATE, multidrug and toxin extrusion; MRP, multidrug resistance-associated protein; OAT, organic anion transporter; OATP, organic anion transporting polypeptide; OCT, organic cation transporter; OCTN, organic cation/carnitine transporter; P-gp, P-glycoprotein.
Table 1:
Transporters Expressed in the Human Placenta and Examples of Substrates
| Transporter name | Alternative names | Localization in the placenta | Change in expression during pregnancy | Substrate(s) |
|---|---|---|---|---|
| P-gp | ABCB1; MDR1 | Syncytiotrophoblast, cytotrophoblast, fetal and maternal endothelium | ↓, ↔ | Dabigatran etexilate, dexamethasone, digoxin, fexofenadine, glyburide, methotrexate, carvedilol, methotrexate, talinolol |
| BCRP | ABCG2; MXR | Syncytiotrophoblast, cytotrophoblast, fetal endothelium | ↓ | glyburide, atorvastatin, rosuvastatin, zidovudine, cimetidine, methotrexate |
| OAT 1 | SLC22A6 | No expression in placenta | NA | beta-lactam antibiotics, non-steroidal antiinflammatory drugs, furodemide, tenofovir |
| OAT2 | SLC22A7 | No expression in placenta | NA | salicylate, furosemide, acyclovir, ganciclovir |
| OAT3 | SLC22A8 | No expression in placenta | NA | beta-lactam antibiotics, cephalosporin antibiotics non-steroidal antiinflammatory drugs, cimetidine, furosemide, methotrexate |
| OAT4 | SLC22A9; SLC22A11 | Syncytiotrophoblast, cytotrophoblast | NA | uric acid, methotrexate |
| OAT 10 | SLC22A13 | Syncytiotrophoblasta | NA | urate |
| OATP-A | OATP1A2; SLCO1A2; SLC21A3 | Trophocytes | ↓ | ciprofloxacin, fexofenadine, glyburide, atorvastatin, pravastatin, rosuvastatin, atenolol, methotrexate, sumatriptan |
| OATP-1B1 | SLCO1B1 ; OATP-C; HBLRR; LST1; SLC21A6 | Trophoblast | ↓ | bile acid, enalapril, cefazolin, atorvastatin, pravastatin, rosuvastatin, methotrexate, glyburide, fexofenadine |
| OATP-2B1 | OATP-B; SLC21A9 | Syncytiotrophoblast, cytotrophoblast | ↓ | atorvastatin, pravastatin, rosuvastatin, glyburide, fexofenadine, sumatriptan, methotrexate |
| OATP-D | SLCO3A1 ; OATP3A1; SLC21A11 | NA | ↓ | thyroxine, vasopressin |
| OATP-E | SLCO4A1; OATP4A1; OATP1; OATPE; OATPRP1; SLC21A12 | Syncytiotrophoblast | ↔ | benzylpenicillin, thyroxine |
| OATP-8 | SLC21A8 | NA | NA | bile acid |
| MRP1 | ABCC1 | Syncytiotrophoblast, fetal endothelium | ↔ | Ritonavir, methotrexate |
| MRP2 | ABCC2, CMRP, CMOAT | Syncytiotrophoblast | ↑ | atenolol, carvedilol, pravastatin, methotrexate |
| MRP3 | ABCC3 | Syncytiotrophoblast | ↔ | methotrexate |
| MRP4 | ABCC4 | Syncytiotrophoblast | NA | tenofovir, methotrexate |
| MRP5 | ABCC5 | Syncytiotrophoblast, fetal endothelium | ↓ | cGMP, methotrexate |
| OCTN1 | SLC22A4 | NA | NA | gabapentin |
| OCTN2 | SLC22A5 | Syncytiotrophoblast, fetal endothelium | ↓ | carnitine |
| OCT1 | SLC22A1 | NA | ↔ | Metformin, sumatriptan, atenolol |
| OCT2 | SLC22A2 | NA | ↓ | Atenolol, , metformin, , procainamide |
| OCT3 | SLC22A3; EMT | Syncytiotrophoblast | ↓ | metformin |
| MATE1 | SLC47A1 | Syncytiotrophoblast | ↔ | Atenolol, metformin, ganciclovir, cimetidine, acyclovir |
| MATE2 | SLC47A2 | Syncytiotrophoblast | ↓ | Atenolol, metformin, acyclovir, ganciclovir, cimetidine |
| PMAT | SLC29A4; ENT4 | NA | NA | Metformin, atenolol |
| BSEP | ABCB11 | NA | ↓ | pravastatin, fexofenadine |
| MDR3 | ABCB4 | NA | ↑ | digoxin, ursodiol |
| FIC1 | ATP8B1 | NA | ↓ | phosphatidylserine, phosphatidylethanolamine |
Expression in placenta not detected in all studies
Note: ↑ denotes increasing expression during pregnancy, ↓ denotes decreasing expression during pregnancy and ↔ denotes constant expression during pregnancy
Abbreviations’. ABC: Adenosine triphosphate-binding cassette; ATP8B1: ATPase phospholipid transporting 8B1; BCRP: breast cancer resistance protein; BSEP: Bile salt export pump; CMOAT: Canalicular multispecific organic anion transporter; CMRP: Canalicular multidrug resistance protein; EMT: Extraneuronal monoamine transporter; ENT: Equilibrative nucleoside transporter; FIC: Familial intrahepatic cholestasis; HBLRR: Hyperbilirubinemia, Rotor type; LST: Liver specific transporter; MATE: Multidrug and toxin extrusion; MDR: Multidrug resistance protein; MRP: Multidrug resistance-associated protein; MXR: Multixenobiotic resistance; NA: not available; OAT: Organic anion transporter; OATP: Organic anion transporting polypeptide; OATPRP: Organic anion transporting polypeptide-related protein; OCT: Organic cation transporter; OCTN: Organic cation/carnitine transporter; P-gp: P-glycoprotein; PMAT: Plasma membrane monoamine transporter; SLC: Solute carrier; SLCO: Solute carrier organic anion transporter.
P-glycoprotein (P-gp):
P-gp is an efflux transporter localized in the syncytiotrophoblasts and cytotrophoblasts as well as in the fetal and maternal endothelium of placental blood vessels.20–23 Due to its importance in drug transport, numerous studies have investigated P-gp expression in the placenta from the 7th gestational week until term. The expression in these studies was quantified in relation to housekeeping genes, i.e. constitutive genes required for the maintenance of basic cellular functions that are expressed in all cells under normal and pathophysiological conditions, such as GAPDH, HPRT, YWHAZ, beta-actin and mRNA 18s. Although the results of one study suggested that P-gp was unchanged from the first trimester to term,24 most studies show that P-gp expression decreases with advancing gestational age.23,25–32
Breast cancer resistance protein (BCRP):
There are numerous studies on BCRP transporter expression in the placenta. Staining results indicate that the transporter is expressed in the apical membrane of the syncytiotrophoblasts and cytotrophoblasts as well as in fetal endothelial cells from the first trimester to term.23,25,33–37 Various studies have quantified the expression of BCRP relative to the expression of housekeeping genes such as GAPDH, HPRT, YWHAZ, β-actin and 18S.23,25–28,33,36,38–44 Although no clear pattern emerges from these studies, BCRP expression seems to decrease or remain unchanged between the first trimester and term.23,25–28,39,43
Organic anion transporter (OAT) 1:
No expression of OAT1 was detected in the placenta using Northern Blot analysis.45
OAT 2:
There is only one study investigating OAT2 expression in pregnancy suggesting that OAT2 is not expressed in the placenta.46
OAT3:
Findings from two studies using Northern Blotting techniques suggest that OAT3 is not expressed in the placenta.45,47
OAT4:
OAT4 is an influx transporter which has been detected in the basolateral membrane of the syncytiotrophoblast and cytotrophoblast cells of the placenta in the third trimester.37,47–49 Its ontogeny in the placenta is yet unknown.
OAT 10:
Using RT-PCR and immunohistochemical staining, Uehara et al. demonstrated that OAT10 was expressed in syncytiotrophoblast cells of placentae collected between 26 and 41 weeks of gestation.37
Organic-anion-transporting polypeptide (OATP)-A:
OATP-A is an influx transporter expressed in trophocytes responsible for the uptake of substrates, such as unconjugated bilirubin, from the fetal blood.50,51 Semi-quantitative expression analysis using real-time reverse transcriptase (RT) polymerase chain reaction (PCR) suggested that OATP-A expression relative to 18S rRNA decreases between the first and third trimester of pregnancy.51
OATP-1B1:
Studies on OATP-1B1 (also known as OATP-C) expression in the placenta are not yet fully conclusive. In a previous study with isolated trophoblast cells from term placentae, mRNA of OATP-1B1 could not be detected by analytical RT-PCR, whereas weak levels were detected by real-time quantitative RT-PCR.50 Another study that also used real-time quantitative RT-PCR with different primer and probe sequences detected a very low level of OATP-1B1 expression in 4 of the 8 placentae obtained between 9 and 12 weeks gestation and no expression in 6 placentae obtained between 38 and 40 weeks gestation.51
OATP-2B1:
OATP-2B1, also called OATP-B, is an influx transporter expressed in the basolateral membrane of syncytiotrophoblast and cytotrophoblast cells.48,52–54 Additionally, OATP-2B1 has been found to be also expressed to a much lesser extent in the apical membrane of these cells. Petrovic et al. reported that the expression of OATP-2B1, normalized to β-actin mRNA expression, is lower in placental samples obtained at term delivery (gestational age: 38.8 ± 0.8 (mean ± SD)) as compared with those at preterm delivery (gestational age: 32.8 ± 2.5).28
OATP-D:
In a previous study, mRNA of OATP-D could be detected in microvillus fragments of the placenta through real-time quantitative RT-PCR expression.51 Importantly, this study also compared the expression between the first and third trimester (9–12 weeks gestation and 38 and 40 weeks gestation, respectively). The results showed that OATP-D expression is significantly down-regulated towards the end of pregnancy with an averaged normalized expression of only 5.9% of that measured in the first trimester.51 The cellular localization of OATP-D in the placenta is yet unknown.
OATP-E:
OATP-E is an influx transporter located on the apical side of syncytiotrophoblast cells.54 In the study by Patel et al., no significant difference in the expression levels between the first and third trimester was found.51
OATP-8:
Expression of OATP-8 has been detected in term placentae where it may act as an influx transporter.50
Multidrug resistance protein (MRP) 1:
There are several studies that show MRP1 expression in the placenta. The location of MRP1 was found to be on the basolateral side of syncytiotrophoblasts of the terminal and intermediate villi and, although not consistently observed in all studies, to a lesser extent also in fetal endothelial cells.21,33,35,52,20,55 Weak expression signals have, although rarely, also been detected in the apical membrane of syncytiotrophoblasts.33 Three studies investigated the quantification of MRP1 expression normalized to that of housekeeping genes (GAPDH, HPRT and mRNA-18s). The results are somewhat conflicting showing that MRP1 expression between the 10th gestational week and term either increases or remains unchanged.25,56
MRP2:
A few studies were conducted to investigate MRP2 expression in pregnancy.25,52,53,57 The results show that MRP2 is an efflux transporter located on the apical side of syncytiotrophoblast cells.52,53,57 Several studies have quantified MRP2 expression through real-time PCR demonstrating a relatively consistent increase in MRP2 expression during pregnancy.53,56,58 One study reported a 2-fold increase in expression between 9–10 weeks of gestation and term53, while another reported a 4.4-fold and 1.8-fold increase between gestational week 28 and term, and gestational week 34 and term, respectively.59 Interestingly, the difference between gestational week 34 and term was not found in the MRP2 protein amount quantified by immunoblot analysis.59
MRP3:
Among all MRPs investigated, MRP3 has been found to be the most abundantly expressed transporter in the placenta. It is an efflux transporter with transport direction from the fetus to the maternal side. Several studies showed that MRP3 is located on the apical side of the syncytiotrophoblast cell predominantly in small terminal villi.25,52,57 Quantitative real-time PCR showed no significant difference between the MRP3 expression in samples obtained at 9 – 10 weeks of gestation and those obtained at term. Although this study also reported that Western blot analysis did not detect MRP3 protein amount in term placentae, this negative result was noted to be likely due to a low reactivity of the antibodies used.56
MRP4:
Real-time PCR and immunoblotting analysis indicated that MRP4 is present in the apical membrane of syncytiotrophoblast cells.57
MRP5:
There are a few studies that show that MRP5 is located on the basolateral side of syncytiotrophoblast cells and fetal endothelial cells.59 Quantification of MRP5 mRNA through real-time PCR indicated that MRP5 expression decreases significantly throughout pregnancy.56,59 The same pattern could also be confirmed for the protein amount in term and pre-term placentae through quantification of band intensity of Western blot analysis.
Multidrug and toxin extrusion (MATE) 1:
MATE1 is an efflux transporter located on the apical side of syncytiotrophoblast cells. Northern Blot analysis and quantitative RT-PCR indicated no expression or very weak expression of MATE1 in placentae collected in the first trimester and at term.60–62
MATE2:
MATE2 is another efflux transporter expressed in the apical side of syncytiotrophoblast cells.60–62 Analysis of MATE2 expression through RT-PCR showed a decrease between the first trimester and term, although the decrease was not significant due to large variability.60
Organic cation transporter (OCT) 1:
Several studies demonstrate that OCT 1 is expressed in the placenta both at early and late stages of pregnancy; however, the exact localization has not yet been identified.61,63–66 In one study by Ahmadimoghaddam et al., the OCT 1 expression quantified by RT-PCR was unchanged between the first trimester and term.66
OCT2:
Several studies show that OCT2 is expressed in the placenta in the first and third trimester, but the localization is yet unknown.61,63–65 OCT2 gene expression measured via RT-PCR has been observed to decrease approximately 4-fold between placentae collected in the late first trimester and at term.66
OCT3:
OCT3 is an influx transporter located in the basolateral membrane of syncytiotrophoblast cells.61,67–69 Two studies have quantified the ontogeny of OCT3 by RT-PCR reporting varying results. Ahmadimoghaddam et al. observed a ~3.6-fold decrease between the late first trimester and term60, whereas Lee et al. reported constant expression levels between the first trim ester, second trimester and term.61 Interestingly, quantitative LC-MS/MS analysis by the latter authors revealed another pattern showing that protein levels of OCT3 were on average increased by 65% in the second trimester and by 57% at term compared to those in the first trimester.61
Organic cation/carnitine transporter (OCTN) 1:
Two studies detected OCTN1 expression in the term placenta using Northern Blot and quantitative PCR.70,71 The localization of OCTN1 in the placenta has not been determined yet.
OCTN2:
OCTN2 is an influx transporter expressed in the apical membrane of syncytiotrophoblast cells and in fetal endothelial cells.53,72 Compared to OCTN1, higher expression signals have been measured via quantitative PCR for OCTN2 in the term placenta. Additionally the mRNA expression, normalized to 18S rRNA, was observed to be around 15% higher in placentae obtained at around 32 weeks of gestation compared to those around 39 weeks of gestation, but this difference did not reach statistical significance.53,70,72
Plasma membrane monoamine transporter (PMAT):
Weak expression levels of PMAT has been reported in the term placenta.61 The localization of this transporter in the placenta is still unknown.
Bile salt export pump (BSEP):
RT-PCR analysis indicated a weak expression of BSEP in placental specimens collected between 9 and 12 weeks of gestation and no expression at term.51
Multidrug resistance protein (MDR3):
RT-PCR analysis showed that MDR3 is expressed in the placenta and that the expression increases between 9 – 12 weeks of gestation and term.51
Familial intrahepatic cholestasis (FIC) 1:
FIC expression in the placenta as assessed by RT-PCR has been reported to decrease between 9 – 12 weeks of gestation and term.51
Models for placental drug transfer
In vitro models
Multiple in vitro, ex vivo and in silico models have been used to study transcellular drug transfer through the placenta.73,74 In vitro model systems typically rely on different cell cultures that are experimentally applied in transport studies. For example, non-proliferative primary cytotrophoblasts can be isolated from human term placentae. Although easily cultured on semi-permeable membranes, these cells do not form a confluent cell monolayer but aggregate with disrupted tight junctions.75 Hemmings et al. reported a technique to grow multiple overlapping layers of syncytialised cells overcoming some of the problems observed with discontinuous membrane cultures of primary trophoblasts.76 Human cancer cell lines constitute another in vitro system used for placental transfer studies. These cell lines include the JEG-3, the Jar, and the BeWo cell line (b30 clone),77,79 which are all derived from choriocarcinoma cells. Among these, the BeWo cell line is the most frequently used for transfer studies. It grows relatively fast and forms confluent, polarized monolayers that are morphologically similar to normal trophoblasts.80 Although these characteristics make it attractive for placental transfer studies, the BeWo cell line has a few disadvantages, in particular a different gene expression and protein amount of various transporters. For example, the mRNA level and protein amount of MDR1 and MDR3 has been found to be significantly lower in BeWo cells compared to primary trophoblasts isolated from term human placentae, whereas those of BCRP and MRP1 are much higher in BeWo cells.81 Despite this discrepancy, a previous study has observed that the transport of antipyrine, benzoic acid, caffeine, and glyphosate across BeWo cell monolayers was overall in adequate agreement with results obtained from the ex vivo cotyledon perfusion experiment. However, the transfer rate measured in BeWo cells was much lower than in the ex vivo cotyledon perfusion experiment.82 Consistent with these findings, another study demonstrated a good correlation between the relative transport rates in BeWo cells and the transfer indices measured in the ex vivo cotyledon perfusion experiment for a set of nine model compounds (including the previously investigated compounds antipyrine, benzoic acid, caffeine, and glyphosate).83 Similar findings were also reported for non-dioxin-like polychlorinated biphenyls.84 Recently, BeWo cells have also been employed to bioprint a 3D, bioengineered in vitro placenta model with biophysical properties that closely mimic those of the maternal decidua.85 This model was developed to capture the chemotactic effect of epidermal growth factor on the migration of trophoblasts and human mesenchymal cells for studying preeclampsia. In future studies, this model might serve as a platform for printing more complex, physiological placental models capturing the in vivo physiology better than current 2D cell line models, thereby providing a more realistic framework for placental drug transfer.
Ex vivo cotyledon perfusion experiments
Ex vivo cotyledon perfusion experiments provide the only experimental model available to study human placental transfer within the physiological tissue architecture that is present in vivo. In this experiment, the physiological circulations in the placenta are reestablished by cannulating fetal and maternal blood vessels of an isolated cotyledon. Thereafter, a perfusate is circulated through the catheters at specific flow rates mimicking the physiological fetal and maternal blood flow rates through the placenta observed in vivo.74 The circulation of the placenta can be either non-recirculating (open/single-pass) or recirculating (closed). The usage of this model started in 1962 by Panigel86 and was subsequently refined by various groups.87,88 Since the tissue for this experiment is collected from both vaginal birth and C-sections, the gestational age of the placentae utilized is usually around term gestation, which is one of the main limitations of the ex vivo perfusion experiment as earlier stages of pregnancy cannot be investigated. Another shortage of the experiment is that the concentrations of drug-binding proteins (e.g. albumin and α1-acid glycoprotein) are often equal for the maternal and fetal circuit, which may not reflect the physiological conditions in vivo. Since only the unbound drug fraction can cross the placenta, the balance between maternal and fetal concentrations of drug-binding proteins is of particular importance. For example, the placental transfer of digoxin in the perfused cotyledons has been observed to be dependent on the albumin concentrations in both circuits.89,90 Also, as discussed later on, the difference in the pH value between the maternal and fetal blood may be another relevant factor influencing the transfer of weakly basic drugs, which should be accounted for in these experiments. Despite these challenges, a recent study of 26 drugs showed that the prediction of ex vivo placenta perfusion matches the observed in vivo data well.91
PBPK models
PBPK models constitute another method for investigating placental drug transfer. Various models have previously been presented that allow the prediction of concentrations in the fetal blood or tissues.92,93,94–97 The two most promising approaches for predicting the transfer of a compound across the placenta were those presented by Mendes et al.94,95 and by Zhang et al. 96 The approach suggested by Mendes et al. relies on experimental data obtained from the ex vivo cotyledon perfusion experiment. Briefly, a four compartment model is used to describe these data and the key parameters governing the placental transfer (the placental transfer constant and the partition coefficient) were initially fitted to the experimental data. Thereafter, these parameters were integrated in a whole-body PBPK model. It was shown that through this approach, the pharmacokinetics of emtricitabine, tenofovir and nevirapine observed at delivery could adequately be predicted by the PBPK model.94,95 One shortcoming of this approach is that it cannot be readily extrapolated to other compounds for which no data from the ex vivo cotyledon perfusion experiment are available. Additionally, in case of highly protein-bound drugs, the experiment may not adequately reflect the in vivo situation unless different protein concentrations are used in the maternal and fetal circuit. Still, this approach can provide important insights into the placental transfer of compounds.
Another approach was presented by Zhang et al. for compounds crossing the placenta exclusively via passive diffusion.96,98 In this case, the fetal-to-maternal partition ratio was assumed to equal 1.0. According to this approach, the placental permeability of a compound is estimated from its apparent permeability measured in vitro (in e.g. Caco-2 cell lines) using midazolam as a calibrator compound. Although promising results were obtained for zidovudine and theophylline, 96 more examples are needed to corroborate these findings. The advantage of this approach is that it provides a rather simple and quick estimate of placental permeability. Yet, the main drawback remains the limitation to compounds crossing the placenta exclusively via passive diffusion.
To date, many maternal-fetal PBPK models have in common that they do not account for an explicit integration of placental drug transporters. While the active influx or efflux of drugs in the placenta can implicitly be factored into the permeability rate, a deconvolution of passive and active transfer processes is still desirable as it provides a greater mechanistic understanding of the transfer processes which ultimately facilitates extrapolation to earlier stages of pregnancy or even other compounds. Hence, the development of more mechanistic PBPK models for the study of maternal-fetal drug transfer may benefit from the herein presented information on the expression and ontogeny of transporters in the placenta.
Apart from the explicit integration of placental transporters, these models may also benefit from a finer representation of the fetal physiology. Recently, several repositories have been published that review physiological changes in the fetus, such as organ growth, change in blood flow rates, tissue composition and drug-binding protein concentrations.98–100 In addition, drug transfer may also be influenced by the pH difference between the fetal and maternal blood. Under normal conditions, the pH of the umbilical cord blood is about 0.1 log units lower than that of the maternal blood which can, in principle, lead to an increased fetal/maternal concentration ratio of weakly basic drugs. While the pH difference under normal conditions is probably insignificant, it may become relevant in cases of fetal acidosis and maternal alkalosis. For example, a lower pH value in the umbilical cord blood, as might occur in case of fetal asphyxia, may result in trapping the ionized form of weak bases, whereas in cases of maternal alkalosis, the non-dissociated fraction of weak bases available for transfer to the fetus may be increased.101,102 Several reports in the literature demonstrate that lower pH values in umbilical cord blood at delivery are associated with increased fetal blood concentrations of the weakly basic anesthetics bupivacaine, mepivacaine and lidocaine which may lead to toxic effects in the fetus.103–105 Placental drug transfer is also influenced by metabolism within the syncytiotrophoblasts and hence the expression of drug-metabolizing enzymes should also be considered. Although many of the previously published maternal-fetal PBPK models did not account for these factors, incorporating all these inherent physiologic functions, and possibly others, may substantially improve the mechanistic level of the model and lead to more accurate predictions of drug concentrations in the fetus.
Outlook
In summary, little is known about the mechanisms regulating the expression of drug transporters in the human placenta. Despite this knowledge gap, many studies investigated whether and to which extent drug transporters are present in the placenta and numerous transporters were found to be expressed at different levels throughout gestation. While there is abundant information on the ontogeny of some transporters in the placenta, such as P-gp, for other transporters this information is either conflicting or lacking. For these transporters, this review identifies important knowledge gaps where further research is needed. The ex vivo cotyledon perfusion experiment is currently being considered the gold standard for studying maternal-fetal drug transfer. However, attention should be given to those cases where the experimental conditions do not ideally reflect all factors affecting drug transfer in vivo, such as an altered pH value or protein concentration in the fetal circulation. Still, the results from these experiments provide very suitable information that can be incorporated in a mechanistic modeling framework. First attempts to inform PBPK models on the basis of ex vivo perfusion cotyledon experiments have shown promising results. To increase the mechanistic understanding of placental drug transfer, a refinement of these models with respect to the explicit incorporation of placental drug transporters seems necessary. This review provides important information on the expression and ontogeny of placental transporters that could be leveraged within a PBPK modeling context. Ultimately, these models can help to improve the knowledge about the adequacy and safety of pharmacotherapy in pregnant women and their fetuses.
Acknowledgements
The authors wish to acknowledge the assistance of Dr. Xinning Yang from the US Food and Drug Administration.
Footnotes
Publisher's Disclaimer: Disclaimer: The opinions expressed in this article are those of the authors and should not be interpreted as the position of the U.S. Food and Drug Administration.
Disclosure: The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Brouwer KL, Aleksunes LM, Brandys B, et al. Human Ontogeny of Drug Transporters: Review and Recommendations of the Pediatric Transporter Working Group. Clin Pharmacol Ther. 2015;98(3):266–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Wildt SN. Profound changes in drug metabolism enzymes and possible effects on drug therapy in neonates and children. Expert Opin Drug Metabo Toxicol. 2011;7(8):935–948. [DOI] [PubMed] [Google Scholar]
- 3.Hines RN. Developmental expression of drug metabolizing enzymes: impact on disposition in neonates and young children. Int J Pharm. 2013;452(1–2):3–7. [DOI] [PubMed] [Google Scholar]
- 4.Iqbal M, Audette MC, Petropoulos S, Gibb W, Matthews SG. Placental drug transporters and their role in fetal protection. Placenta. 2012;33(3):137–142. [DOI] [PubMed] [Google Scholar]
- 5.Gedeon C, Anger G, Piquette-Miller M, Koren G. Breast cancer resistance protein: mediating the trans-placental transfer of glyburide across the human placenta. Placenta. 2008;29(1):39–43. [DOI] [PubMed] [Google Scholar]
- 6.Pollex E, Lubetsky A, Koren G. The role of placental breast cancer resistance protein in the efflux of glyburide across the human placenta. Placenta. 2008;29(8):743–747. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz RA, Rosenn B, Aleksa K, Koren G. Glyburide transport across the human placenta. Obstet Gynecol. 2015;125(3):583–588. [DOI] [PubMed] [Google Scholar]
- 8.Dallmann A, Pfister M, van den Anker J, Eissing T. Physiologically Based Pharmacokinetic Modeling in Pregnancy: A Systematic Review of Published Models. Clin Pharmacol Ther. 2018;104(6):1110–1124. [DOI] [PubMed] [Google Scholar]
- 9.Grigsby PL. Animal Models to Study Placental Development and Function throughout Normal and Dysfunctional Human Pregnancy. Semin Reprod Med. 2016;34(1):11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coles LD, Lee IJ, Voulalas PJ, Eddington ND. Estradiol and progesterone-mediated regulation of P-gp in P-gp overexpressing cells (NCI-ADR-RES) and placental cells (JAR). Mol Pharm. 2009;6(6):1816–1825. [DOI] [PubMed] [Google Scholar]
- 11.Evseenko DA, Paxton JW, Keelan JA. Independent regulation of apical and basolateral drug transporter expression and function in placental trophoblasts by cytokines, steroids, and growth factors. Drug Metab Dispos. 2007;35(4):595–601. [DOI] [PubMed] [Google Scholar]
- 12.Dong W, Yin L. Expression of lipoxin A4, TNFalpha and IL-1beta in maternal peripheral blood, umbilical cord blood and placenta, and their significance in pre-eclampsia. Hypertens Pregnancy. 2014;33(4):449–456. [DOI] [PubMed] [Google Scholar]
- 13.Gomes CP, Torloni MR, Gueuvoghlanian-Silva BY, Alexandre SM, Mattar R, Daher S. Cytokine levels in gestational diabetes mellitus: a systematic review of the literature. Am J Reprod Immunol. 2013;69(6):545–557. [DOI] [PubMed] [Google Scholar]
- 14.Holcberg G, Huleihel M, Sapir O, et al. Increased production of tumor necrosis factor-alpha TNF-alpha by IUGR human placentae. Eur J Obstet Gynecol Reprod Biol. 2001;94(1):69–72. [DOI] [PubMed] [Google Scholar]
- 15.Saji F, Samejima Y, Kamiura S, Sawai K, Shimoya K, Kimura T. Cytokine production in chorioamnionitis. J Reprod Immunol. 2000;47(2):185–196. [DOI] [PubMed] [Google Scholar]
- 16.Benyo DF, Miles TM, Conrad KP. Hypoxia stimulates cytokine production by villous explants from the human placenta. J Clin Endocrinol Metab. 1997;82(5):1582–1588. [DOI] [PubMed] [Google Scholar]
- 17.Dallmann A, Ince I, Meyer M, Willmann S, Eissing T, Hempel G. Gestation-specific changes in the anatomy and physiology of healthy pregnant women: an extended repository of model parameters for physiologically based pharmacokinetic modeling in pregnancy. Clin pharmacokinet. 2017;56(11):1303–1330. [DOI] [PubMed] [Google Scholar]
- 18.Johnson RA, Ince TA, Scotto KW. Transcriptional repression by p53 through direct binding to a novel DNA element. J Biol Chem. 2001;276(29):27716–27720. [DOI] [PubMed] [Google Scholar]
- 19.Jeschke U, Schiessl B, Mylonas I, et al. Expression of the proliferation marker Ki-67 and of p53 tumor protein in trophoblastic tissue of preeclamptic, HELLP, and intrauterine growth-restricted pregnancies. Int J Gynecol Pathol. 2006;25(4):354–360. [DOI] [PubMed] [Google Scholar]
- 20.Nagashige M, Ushigome F, Koyabu N, et al. Basal membrane localization of MRP1 in human placental trophoblast. Placenta. 2003;24(10):951–958. [DOI] [PubMed] [Google Scholar]
- 21.Atkinson DE, Greenwood SL, Sibley CP, Glazier JD, Fairbairn LJ. Role of MDR1 and MRP1 in trophoblast cells, elucidated using retroviral gene transfer. Am J Physiol Cell Physiol. 2003;285(3):C584–591. [DOI] [PubMed] [Google Scholar]
- 22.Cordon-Cardo C, O’Brien JP, Boccia J, Casals D, Bertino JR, Melamed MR. Expression of the multidrug resistance gene product (P-glycoprotein) in human normal and tumor tissues. J Histochem Cytochem. 1990;38(9):1277–1287. [DOI] [PubMed] [Google Scholar]
- 23.Lye P, Bloise E, Dunk C, et al. Effect of oxygen on multidrug resistance in the first trimester human placenta. Placenta. 2013;34(9):817–823. [DOI] [PubMed] [Google Scholar]
- 24.Hodyl NA, Stark MJ, Butler M, Clifton VL. Placental P-glycoprotein is unaffected by timing of antenatal glucocorticoid therapy but reduced in SGA preterm infants. Placenta. 2013;34(4):325–330. [DOI] [PubMed] [Google Scholar]
- 25.Mason CW, Buhimschi IA, Buhimschi CS, Dong Y, Weiner CP, Swaan PW. ATP-binding cassette transporter expression in human placenta as a function of pregnancy condition. Drug Metab Dispos. 2011;39(6):1000–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lye P, Bloise E, Javam M, Gibb W, Lye SJ, Matthews SG. Impact of bacterial and viral challenge on multidrug resistance in first- and third-trimester human placenta. Am J Pathol. 2015;185(6):1666–1675. [DOI] [PubMed] [Google Scholar]
- 27.Mathias AA, Hitti J, Unadkat JD. P-glycoprotein and breast cancer resistance protein expression in human placentae of various gestational ages. Am J Physiol Regul Integr Comp Physiol. 2005;289(4):R963–969. [DOI] [PubMed] [Google Scholar]
- 28.Petrovic V, Kojovic D, Cressman A, Piquette-Miller M. Maternal bacterial infections impact expression of drug transporters in human placenta. Int Immunopharmacol. 2015;26(2):349–356. [DOI] [PubMed] [Google Scholar]
- 29.Sun M, Kingdom J, Baczyk D, Lye SJ, Matthews SG, Gibb W. Expression of the multidrug resistance P-glycoprotein, (ABCB1 glycoprotein) in the human placenta decreases with advancing gestation. Placenta. 2006;27(6–7):602–609. [DOI] [PubMed] [Google Scholar]
- 30.Hemauer SJ, Nanovskaya TN, Abdel-Rahman SZ, Patrikeeva SL, Hankins GD, Ahmed MS. Modulation of human placental P-glycoprotein expression and activity by MDR1 gene polymorphisms. Biochem Pharmacol. 2010;79(6):921–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bremer S, Hoof T, Wilke M, et al. Quantitative expression patterns of multidrug-resistance P-glycoprotein (MDR1) and differentially spliced cystic-fibrosis transmembrane-conductance regulator mRNA transcripts in human epithelia. Eur J Biochem. 1992;206(1):137–149. [DOI] [PubMed] [Google Scholar]
- 32.Gil S, Saura R, Forestier F, Farinotti R. P-glycoprotein expression of the human placenta during pregnancy. Placenta. 2005;26(2–3):268–270. [DOI] [PubMed] [Google Scholar]
- 33.Afrouzian M, Al-Lahham R, Patrikeeva S, et al. Role of the efflux transporters BCRP and MRP1 in human placental bio-disposition of pravastatin. Biochem Pharmacol. 2018;156:467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ceckova M, Libra A, Pavek P, et al. Expression and functional activity of breast cancer resistance protein (BCRP, ABCG2) transporter in the human choriocarcinoma cell line BeWo. Clin Exp Pharmacol Physiol. 2006;33(1–2):58–65. [DOI] [PubMed] [Google Scholar]
- 35.Kozlowska-Rup D, Czekaj P, Plewka D, Sikora J. Immunolocalization of ABC drug transporters in human placenta from normal and gestational diabetic pregnancies. Ginekol Pol. 2014;85(6):410–419. [DOI] [PubMed] [Google Scholar]
- 36.Lye P, Bloise E, Nadeem L, Gibb W, Lye SJ, Matthews SG. Glucocorticoids modulate multidrug resistance transporters in the first trimester human placenta. J Cell Mol Med. 2018;22(7):3652–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uehara I, Kimura T, Tanigaki S, et al. Paracellular route is the major urate transport pathway across the blood-placental barrier. Physiol Rep. 2014;2(5): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bircsak KM, Moscovitz JE, Wen X, et al. Interindividual Regulation of the Breast Cancer Resistance Protein/ABCG2 Transporter in Term Human Placentas. Drug Metab Dispos. 2018;46(5):619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeboah D, Sun M, Kingdom J, et al. Expression of breast cancer resistance protein (BCRP/ABCG2) in human placenta throughout gestation and at term before and after labor. Can J Physiol Pharmacol. 2006;84(12):1251–1258. [DOI] [PubMed] [Google Scholar]
- 40.Javam M, Audette MC, Iqbal M, Bloise E, Gibb W, Matthews SG. Effect of oxygen on multidrug resistance in term human placenta. Placenta. 2014;35(5):324–330. [DOI] [PubMed] [Google Scholar]
- 41.Kobayashi D, Ieiri I, Hirota T, et al. Functional assessment of ABCG2 (BCRP) gene polymorphisms to protein expression in human placenta. Drug Metab Dispos. 2005;33(1):94–101. [DOI] [PubMed] [Google Scholar]
- 42.Memon N, Bircsak KM, Archer F, et al. Regional expression of the BCRP/ABCG2 transporter in term human placentas. Reprod Toxicol. 2014;43:72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sieppi E, Vahakangas K, Rautio A, Ietta F, Paulesu L, Myllynen P. The xenoestrogens, bisphenol A and para-nonylphenol, decrease the expression of the ABCG2 transporter protein in human term placental explant cultures. Mol Cell Endocrinol. 2016;429:41–49. [DOI] [PubMed] [Google Scholar]
- 44.Pfeifer E, Parrott J, Lee GT, et al. Regulation of human placental drug transporters in HCV infection and their influence on direct acting antiviral medications. Placenta. 2018;69:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Race JE, Grassl SM, Williams WJ, Holtzman EJ. Molecular cloning and characterization of two novel human renal organic anion transporters (hOAT1 and hOAT3). Biochem Biophys Res Commun. 1999;255(2):508–514. [DOI] [PubMed] [Google Scholar]
- 46.Cropp CD, Komori T, Shima JE, et al. Organic anion transporter 2 (SLC22A7) is a facilitative transporter of cGMP. Mol Pharmacol. 2008;73(4):1151–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cha SH, Sekine T, Kusuhara H, et al. Molecular cloning and characterization of multispecific organic anion transporter 4 expressed in the placenta. J Biol Chem. 2000;275(6):4507–4512. [DOI] [PubMed] [Google Scholar]
- 48.Ugele B, St-Pierre MV, Pihusch M, Bahn A, Hantschmann P. Characterization and identification of steroid sulfate transporters of human placenta. Am J Physiol Endocrinol Metab. 2003;284(2):E390–398. [DOI] [PubMed] [Google Scholar]
- 49.Noguchi S, Nishimura T, Fujibayashi A, Maruyama T, Tomi M, Nakashima E. Organic Anion Transporter 4-Mediated Transport of Olmesartan at Basal Plasma Membrane of Human Placental Barrier. J Pharm Sci. 2015;104(9):3128–3135. [DOI] [PubMed] [Google Scholar]
- 50.Briz O, Serrano MA, MacIas RI, Gonzalez-Gallego J, Marin JJ. Role of organic anion-transporting polypeptides, OATP-A, OATP-C and OATP-8, in the human placenta-maternal liver tandem excretory pathway for foetal bilirubin. Biochem J. 2003;371(Pt 3):897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patel P, Weerasekera N, Hitchins M, Boyd CA, Johnston DG, Williamson C. Semi quantitative expression analysis of MDR3, FIC1, BSEP, OATP-A, OATP-C,OATP-D, OATP-E and NTCP gene transcripts in 1st and 3rd trimester human placenta. Placenta. 2003;24(1):39–44. [DOI] [PubMed] [Google Scholar]
- 52.St-Pierre MV, Serrano MA, Macias RI, et al. Expression of members of the multidrug resistance protein family in human term placenta. Am J Physiol Regul Integr Comp Physiol. 2000;279(4):R1495–1503. [DOI] [PubMed] [Google Scholar]
- 53.Grube M, Meyer Zu Schwabedissen H, Draber K, et al. Expression, localization, and function of the carnitine transporter octn2 (slc22a5) in human placenta. Drug Metab Dispos. 2005;33(1):31–37. [DOI] [PubMed] [Google Scholar]
- 54.Wang H, Zhou L, Gupta A, et al. Regulation of BCRP/ABCG2 expression by progesterone and 17beta-estradiol in human placental BeWo cells. American Journal of Physiology - Endocrinology & Metabolism. 290(5):E798–807. [DOI] [PubMed] [Google Scholar]
- 55.Straka E, Ellinger I, Balthasar C, et al. Mercury toxicokinetics of the healthy human term placenta involve amino acid transporters and ABC transporters. Toxicology. 2016;340:34–42. [DOI] [PubMed] [Google Scholar]
- 56.Pascolo L, Fernetti C, Pirulli D, Crovella S, Amoroso A, Tiribelli C. Effects of maturation on RNA transcription and protein expression of four MRP genes in human placenta and in BeWo cells. Biochem Biophys Res Commun. 2003;303(1):259–265. [DOI] [PubMed] [Google Scholar]
- 57.Azzaroli F, Mennone A, Feletti V, et al. Clinical trial: modulation of human placental multidrug resistance proteins in cholestasis of pregnancy by ursodeoxycholic acid. Aliment Pharmacol Ther. 2007;26(8):1139–1146. [DOI] [PubMed] [Google Scholar]
- 58.Meyer zu Schwabedissen HE, Jedlitschky G, Gratz M, et al. Variable expression of MRP2 (ABCC2) in human placenta: influence of gestational age and cellular differentiation. Drug Metab Dispos. 2005;33(7):896–904. [DOI] [PubMed] [Google Scholar]
- 59.Meyer Zu Schwabedissen HE, Grube M, Heydrich B, et al. Expression, localization, and function of MRP5 (ABCC5), a transporter for cyclic nucleotides, in human placenta and cultured human trophoblasts: effects of gestational age and cellular differentiation. Am J Pathol. 2005;166(1):39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahmadimoghaddam D, Zemankova L, Nachtigal P, et al. Organic cation transporter 3 (OCT3/SLC22A3) and multidrug and toxin extrusion 1 (MATE1/SLC47A1) transporter in the placenta and fetal tissues: expression profile and fetus protective role at different stages of gestation. Biol Reprod. 2013;88(3):55. [DOI] [PubMed] [Google Scholar]
- 61.Lee N, Hebert MF, Prasad B, et al. Effect of gestational age on mRNA and protein expression of polyspecific organic cation transporters during pregnancy. Drug Metab Dispos. 2013;41(12):2225–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Otsuka M, Yasuda M, Morita Y, et al. Identification of essential amino acid residues of the NorM Na+/multidrug antiporter in Vibrio parahaemolyticus. J Bacteriol. 2005;187(5):1552–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bottalico B, Pilka R, Larsson I, Casslen B, Marsal K, Hansson SR. Plasma membrane and vesicular monoamine transporters in normal endometrium and early pregnancy decidua. Mol Hum Reprod. 2003;9(7):389–394. [DOI] [PubMed] [Google Scholar]
- 64.Bottalico B, Noskova V, Pilka R, et al. The organic cation transporters (OCT1, OCT2, EMT) and the plasma membrane monoamine transporter (PMAT) show differential distribution and cyclic expression pattern in human endometrium and early pregnancy decidua. Mol Reprod Dev. 2007;74(10):1303–1311. [DOI] [PubMed] [Google Scholar]
- 65.Gorboulev V, Ulzheimer JC, Akhoundova A, et al. Cloning and characterization of two human polyspecific organic cation transporters. DNA Cell Biol. 1997;16(7):871–881. [DOI] [PubMed] [Google Scholar]
- 66.Ahmadimoghaddam D, Staud F. Transfer of metformin across the rat placenta is mediated by organic cation transporter 3 (OCT3/SLC22A3) and multidrug and toxin extrusion 1 (MATE1/SLC47A1) protein. Reprod Toxicol. 2013;39:17–22. [DOI] [PubMed] [Google Scholar]
- 67.Lee N, Hebert MF, Wagner DJ, et al. Organic Cation Transporter 3 Facilitates Fetal Exposure to Metformin during Pregnancy. Mol Pharmacol. 2018;94(4):1125–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sata R, Ohtani H, Tsujimoto M, et al. Functional analysis of organic cation transporter 3 expressed in human placenta. J Pharmacol Exp Ther. 2005;315(2):888–895. [DOI] [PubMed] [Google Scholar]
- 69.Kekuda R, Prasad PD, Wu X, et al. Cloning and functional characterization of a potential-sensitive, polyspecific organic cation transporter (OCT3) most abundantly expressed in placenta. J Biol Chem. 1998;273(26):15971–15979. [DOI] [PubMed] [Google Scholar]
- 70.Karahoda R, Ceckova M, Staud F. The inhibitory effect of antiretroviral drugs on the L-carnitine uptake in human placenta. Toxicol Appl Pharmacol. 2019;368:18–25. [DOI] [PubMed] [Google Scholar]
- 71.Tamai I, Yabuuchi H, Nezu J, et al. Cloning and characterization of a novel human pH-dependent organic cation transporter, OCTN1. FEBS Lett. 1997;419(1):107–111. [DOI] [PubMed] [Google Scholar]
- 72.Chang TT, Shyu MK, Huang MC, et al. Hypoxia-mediated down-regulation of OCTN2 and PPARalpha expression in human placentas and in BeWo cells. Mol Pharm. 2011;8(1):117–125. [DOI] [PubMed] [Google Scholar]
- 73.Bode CJ, Jin H, Rytting E, Silverstein PS, Young AM, Audus KL. In vitro models for studying trophoblast transcellular transport. Methods Mol Med. 2006;122:225–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Myllynen P, Vahakangas K. Placental transfer and metabolism: an overview of the experimental models utilizing human placental tissue. Toxicol In Vitro. 2013;27(1):507–512. [DOI] [PubMed] [Google Scholar]
- 75.Feinman MA, Kliman HJ, Caltabiano S, Strauss JF 3rd. 8-Bromo-3’,5’-adenosine monophosphate stimulates the endocrine activity of human cytotrophoblasts in culture. J Clin Endocrinol Metab. 1986;63(5):1211–1217. [DOI] [PubMed] [Google Scholar]
- 76.Hemmings DG, Lowen B, Sherburne R, Sawicki G, Guilbert LJ. Villous trophoblasts cultured on semi-permeable membranes form an effective barrier to the passage of high and low molecular weight particles. Placenta. 2001;22(1):70–79. [DOI] [PubMed] [Google Scholar]
- 77.Pattillo RA, Gey GO. The establishment of a cell line of human hormone-synthesizing trophoblastic cells in vitro. Cancer Res. 1968;28(7):1231–1236. [PubMed] [Google Scholar]
- 78.Kohler PO, Bridson WE. Isolation of hormone-producing clonal lines of human choriocarcinoma. J Clin Endocrinol Metab. 1971;32(5):683–687. [DOI] [PubMed] [Google Scholar]
- 79.Azizkhan JC, Speeg KV Jr., Stromberg K, Goode D. Stimulation of human chorionic gonadotropin by JAr line choriocarcinoma after inhibition of DNA synthesis. Cancer Res. 1979;39(6 Pt 1):1952–1959. [PubMed] [Google Scholar]
- 80.Liu F, Soares MJ, Audus KL. Permeability properties of monolayers of the human trophoblast cell line BeWo. Am J Physiol. 1997;273(5 Pt 1):C1596–1604. [DOI] [PubMed] [Google Scholar]
- 81.Evseenko DA, Paxton JW, Keelan JA. ABC drug transporter expression and functional activity in trophoblast-like cell lines and differentiating primary trophoblast. Am J Physiol Regul Integr Comp Physiol. 2006;290(5):R1357–1365. [DOI] [PubMed] [Google Scholar]
- 82.Poulsen MS, Rytting E, Mose T, Knudsen LE. Modeling placental transport: correlation of in vitro BeWo cell permeability and ex vivo human placental perfusion. Toxicol In Vitro. 2009;23(7):1380–1386. [DOI] [PubMed] [Google Scholar]
- 83.Li H, van Ravenzwaay B, Rietjens IM, Louisse J. Assessment of an in vitro transport model using BeWo b30 cells to predict placental transfer of compounds. Arch Toxicol. 2013;87(9):1661–1669. [DOI] [PubMed] [Google Scholar]
- 84.Correia Carreira S, Cartwright L, Mathiesen L, Knudsen LE, Saunders M. Studying placental transfer of highly purified non-dioxin-like PCBs in two models of the placental barrier. Placenta. 2011;32(3):283–291. [DOI] [PubMed] [Google Scholar]
- 85.Kuo CY, Eranki A, Placone JK, et al. Development of a 3D Printed, Bioengineered Placenta Model to Evaluate the Role of Trophoblast Migration in Preeclampsia. ACS Biomater. Sci. Eng 2016;2:1817–1826. [DOI] [PubMed] [Google Scholar]
- 86.Panigel M Placental perfusion experiments. Am J Obstet Gynecol. 1962;84(11):1664–1683. [Google Scholar]
- 87.Schneider H, Panigel M, Dancis J. Transfer across the perfused human placenta of antipyrine, sodium and leucine. Am J Obstet Gynecol. 1972;114(6):822–828. [DOI] [PubMed] [Google Scholar]
- 88.Miller RK, Wier PJ, Shah Y, di Sant’Agnese PA, Perez-D’Gregorio R. Criteria for in vitro dual perfusions in the human placental lobule: perfusions excess of 12 h In: Genbacev AK O, Beaconsfield R, ed. Placenta as a Model and Source. New York: Plenum; 1989:27–38. [Google Scholar]
- 89.Schmolling J, Jung S, Reinsberg J, Schlebusch H. Digoxin transfer across the isolated placenta is influenced by maternal and fetal albumin concentrations. Reprod Fertil Dev. 1996;8(6):969–974. [DOI] [PubMed] [Google Scholar]
- 90.Tsadkin M, Holcberg G, Sapir O, et al. Albumin-dependent digoxin transfer in isolated perfused human placenta. Int J Clin Pharmacol Ther. 2001;39(4):158–161. [DOI] [PubMed] [Google Scholar]
- 91.Hutson JR, Garcia-Bournissen F, Davis A, Koren G. The human placental perfusion model: a systematic review and development of a model to predict in vivo transfer of therapeutic drugs. Clin Pharmacol Ther. 2011;90(1):67–76. [DOI] [PubMed] [Google Scholar]
- 92.Loccisano AE, Longnecker MP, Campbell JL Jr., Andersen ME, Clewell HJ, 3rd. Development of PBPK models for PFOA and PFOS for human pregnancy and lactation life stages. J Toxicol Environ Health A. 2013;76(1):25–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yoon M, Schroeter JD, Nong A, et al. Physiologically based pharmacokinetic modeling of fetal and neonatal manganese exposure in humans: describing manganese homeostasis during development. Toxicol Sci. 2011;122(2):297–316. [DOI] [PubMed] [Google Scholar]
- 94.De Sousa Mendes M, Hirt D, Vinot C, et al. Prediction of human fetal pharmacokinetics using ex vivo human placenta perfusion studies and physiologically based models. Br J Clin Pharmacol. 2016;81(4):646–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.De Sousa Mendes M, Lui G, Zheng Y, et al. A Physiologically-Based Pharmacokinetic Model to Predict Human Fetal Exposure for a Drug Metabolized by Several CYP450 Pathways. Clin Pharmacokinet. 2017;56(5):537–550. [DOI] [PubMed] [Google Scholar]
- 96.Zhang Z, Unadkat JD. Development of a Novel Maternal-Fetal Physiologically Based Pharmacokinetic Model II: Verification of the model for passive placental permeability drugs. Drug Metab Dispos.2017;45(8):939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schalkwijk S, Buaben AO, Freriksen JJM, et al. Prediction of Fetal Darunavir Exposure by Integrating Human Ex-Vivo Placental Transfer and Physiologically Based Pharmacokinetic Modeling. Clin Pharmacokinet. 2018;57(6):705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang Z, Imperial MZ, Patilea-Vrana GI, Wedagedera J, Gaohua L, Unadkat JD. Development of a Novel Maternal-Fetal Physiologically Based Pharmacokinetic Model I: Insights into Factors that Determine Fetal Drug Exposure through Simulations and Sensitivity Analyses. Drug Metab Dispos. 2017;45(8):920–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.El-Masri H, Kleinstreuer N, Hines RN, et al. Integration of Life-Stage Physiologically Based Pharmacokinetic Models with Adverse Outcome Pathways and Environmental Exposure Models to Screen for Environmental Hazards. ToxicolSci. 2016;152(1):230–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Abduljalil K, Jamei M, Johnson TN. Fetal Physiologically Based Pharmacokinetic Models: Systems Information on the Growth and Composition of Fetal Organs. Clin Pharmacokinet. 2019;58(2):235–262. [DOI] [PubMed] [Google Scholar]
- 101.Haberer JP, Monteillard C. Effects of peridural obstetrical anesthesia on the fetus and the newborn infant. Ann Fr Anesth Reanim. 1986;5(4):381–414. [DOI] [PubMed] [Google Scholar]
- 102.Garland M Pharmacology of drug transfer across the placenta. Obstet Gynecol Clin North Am. 1998;25(1):21–42. [DOI] [PubMed] [Google Scholar]
- 103.Brown WU Jr., Bell GC, Alper MH. Acidosis, local anesthetics, and the newborn. Obstet Gynecol. 1976;48(1):27–30. [PubMed] [Google Scholar]
- 104.Datta S, Alper MH, Ostheimer GW, Brown WU Jr., Weiss JB. Effects of maternal position on epidural anesthesia for cesarean section, acid-base status, and bupivacaine concentrations at delivery. Anesthesiology. 1979;50(3):205–209. [DOI] [PubMed] [Google Scholar]
- 105.Bozynski ME, Rubarth LB, Patel JA. Lidocaine toxicity after maternal pudendal anesthesia in a term infant with fetal distress. Am J Perinatol. 1987;4(2):164–166. [DOI] [PubMed] [Google Scholar]


