Abstract
Background
Proton therapy (PT) improves outcomes in nasal cavity (NC) and paranasal sinus (PNS) cancers. We present the largest series utilizing intensity-modulated proton therapy (IMPT) in their treatment.
Methods
Between 2013 and 2018, 86 consecutive patients (68 radiation-naive and 18 re-irradiation) received PT to median doses 70Gy and 67Gy RBE, respectively. 53% received IMPT.
Results
Median follow-up was 23.4 months [1.7–69.3] for all and 28.1 months [2.3–69.3] for surviving patients. Two-year local control (LC), distant control (DC), disease-free survival (DFS), and overall survival (OS) were 83%, 84%, 72%, and 81% for radiation-naïve and 77%, 80%, 54%, and 66% for reirradiation patients. For radiation-naïve patients, compared to 3D conformal proton technique (3DCPT), IMPT significantly improved LC (91% vs. 72%, p<0.01), and independently predicted LC (HR 0.14, p=0.01). Sixteen radiation-naïve patients (24%) experienced acute grade 3 toxicities; three (4%) experienced late grade 3 toxicities (osteoradionecrosis, vision loss, soft tissue necrosis). Similar LC was seen for re-irradiation patients with higher complications, 11% experienced late grade 3 toxicities (facial pain, brain necrosis). Reirradiation patients had more Grade 1–2 radionecrosis than radiation-naïve patients (brain: 33% vs. 7%, osteoradionecrosis: 17% vs. 4%).
Conclusions
PT achieved excellent LC for NC and PNS cancers with lower grade 3 toxicities relative to historical reports. IMPT has promise to improve the therapeutic ratio in these malignancies and is worthy of further investigation.
Keywords: Pencil Beam Proton therapy, Nasal cavity, Paranasal sinus, Reirradiation, Radiotherapy, Toxicity
Precis for use in the Table of Contents:
Radiation-naive and reirradiation patients achieve excellent local control with proton therapy techniques in the management of nasal cavity (NC) and paranasal sinus (PNS) cancers. This is the largest series reporting outcomes and toxicity with intensity-modulated proton therapy (IMPT), identifying IMPT as an independent predictor of local control in the treatment of these malignancies.
INTRODUCTION
Nasal cavity (NC) or paranasal sinus (PNS) cancers are rare in the United States [1]. Histology is widely varied with the most common being squamous cell carcinoma (SCC) (51.6%) [1]. Due to both their rarity and histological and anatomical heterogeneity, there are no high-level evidence-based treatment recommendations. In 2005, two reports from an international collaborative study across 17 institutions who performed craniofacial resections for malignant paranasal sinus and skull base tumors confirmed the importance of surgery as primary therapy [2–3]. These studies formed the benchmark against which all skull base outcomes are compared and guided management directives. The NCCN Guidelines recommend surgical resection as the preferred primary management for T3–T4 tumors, followed by aggressive multimodality management consisting of radiation therapy (RT) and/or chemotherapy depending on pathologic findings [4]. For unresectable disease or patients medically unfit for surgery, definitive RT is offered and typically with chemotherapy [5–6].
Prior to the development of intensity-modulated radiation therapy (IMRT), locoregional control (LRC) varied widely from 62% to 84% with the combination of surgery and RT, while the LRC for patients with unresectable disease who received conformal RT had been reported to be lower in the 20% to 47% range [7–10]. Utilization of IMRT has been associated with higher local control (LC) and reduced toxicity [11–14], demonstrating the potential of technological advancements in radiation towards improving the therapeutic ratio. However, there is still debate whether outcomes have improved significantly with IMRT [9,11], as photon dosimetry is still limited by close proximity to multiple critical normal structures.
Proton therapy (PT) has gained popularity in the past decade. The dosimetric benefits of PT are widely accepted, allowing for increased dose to target volumes and reduced doses to surrounding normal tissues, resulting in further decrease in toxicities. Studies have reported superior LC with PT over photon-based RT, and as such guidelines recommend the consideration of PT for cases in which constraints to critical structures are not achievable with standard IMRT techniques [15–16]. Given the rarity of this disease, comparing outcomes of photon- versus proton-based RT is impossible within a single institution. However, a meta-analysis of NC and PNS malignancies demonstrated significantly higher disease-free survival (DFS, RR 1.44, p=0.045) and LRC (1.26, p=0.011) with PT over IMRT [17].
We have previously reported our outcomes with NC and PNS malignancies using photon therapy in both the adjuvant and definitive settings [5,13,18–19]. Outcomes for unresectable disease were suboptimal with 2-year local progression-free rates of 45% [19]. Due to the superior dosimetry of protons over photon-based therapy as stated above, PT became our institutional preference in recent years, initially using a patch/match 3D conformal proton technique (3DCPT) and more recently intensity-modulated proton therapy (IMPT). We report our proton experience in a consecutive cohort of upfront treatment-naïve and recurrent, previously radiated NC and PNS malignancies.
MATERIALS/METHODS
Patients
Patients with NC or PNS malignancies treated at our proton facility between 2013 and 2018 were consecutively analyzed. Those treated with non-curative or palliative intent were excluded from analysis.
Evaluation/staging
Standard of care pre-treatment workup was done. Patients were staged according to the AJCC 7th Edition TNM staging system [20].
Surgery
Surgery included open and endoscopic approaches+/− neck dissection at the surgeon’s discretion. The head/neck multidisciplinary team assessed whether patients with resections at outside centers required further surgery prior to adjuvant radiation. Margins were defined as close if the tumor was within 1 mm of the resected margins.
Radiotherapy
Patients underwent CT simulation with a thermoplastic 3-point head/neck mask. Staging PET/CT and/or MRI studies were fused to the CT simulation scan to assist with target volume delineation. Target delineation and prescriptions were based on our previously published book [21]. For definitive cases, all gross disease was included in the gross tumor volume (GTV). For postoperative cases, the target volume was an expansion of preoperative and postoperative imaging findings at physician discretion. The subclinical clinical target volumes (CTV) included high-risk and/or lower-risk regions, including the adjacent sinuses and skull base. Bilateral elective neck irradiation was considered for esthesioneuroblastoma (except Kadish A) and high-grade advanced SCC. Elective nodal volumes included bilateral retropharyngeal nodes and levels IB–IV; level V was included only for tumors invading the nasopharynx. For well-lateralized tumors, ipsilateral neck RT was considered. For adenoid cystic carcinoma, the elective neck was not treated but the skull base was with cranial nerves deemed at risk and even up to Mecke’s Cave when indicated. For reirradiation tumors that had received prior full-course radiation, only the GTV or postoperative bed CTV was included. An MD volume was created based on the GTV or CTV with a 3–5 mm margin and was given the prescribed dose. Prescription doses for the MD volume GTV was 70–76Gy (relative biological effectiveness, RBE) and MD volume CTV received 60Gy (RBE) to high-risk regions and 66Gy (RBE) to microscopically affected margins. The skull base and elective nodal regions received 50–54Gy (RBE). PT was delivered once daily, five days a week, using 1.8–2Gy (RBE)/fraction to an intended dose of 70Gy (RBE). All patients received the prescription dose except for two who stopped early due to severe mucositis. A twice-daily schedule (total dose of 45Gy (RBE) was used in one patient with neuroendocrine carcinoma.
Proton therapy (PT)
Proton therapy was delivered using a Proteus 235 system (Ion Beam Applications, Belgium) treating with either uniform scanning (US) or pencil beam scanning (PBS). Patients earlier in the cohort were treated with a patch/match combination with US. PBS using 3–5 fields was the preferred modality once available. The majority of PBS patients were planned with single field uniform dose (SFUD), and multi-field optimization (MFO) was reserved for more complicated cases (typically larger or more locally advanced tumors) to maximize conformality and reduce dose to nearby organs at risk. In this paper, we use the nomenclature IMPT to refer to any PBS plan (both SFUD and MFO). IMPT treatments began in December 2015. For both US and PBS plans, setup variations were accounted for by compensator smear or simulated isocenter shift variations of between 3–5 mm respectively. Robustness tests, including range uncertainty of ±3.5%, were performed to ensure that CTV V95>95%. A generic relative biological effectiveness of 1.1 was used for dose evaluation. All patients were aligned using orthogonal radiographs on a 6 degree of freedom couch.
Systemic therapy
Systemic therapy was administered at the discretion of the treating medical oncologist with various agents including cisplatin, carboplatin, paclitaxel, etoposide, doxorubicin, and cetuximab. The preferred agent was high-dose cisplatin at 100mg/m2 given split over 2 days (50 mg/m2 per day) every three weeks as per our institution standard.
Follow up and Toxicity Evaluation
Patients were evaluated weekly during radiotherapy. After treatment, they were seen q3–4 months for the first two years, q6 months up to the fifth year, and yearly thereafter. Given the new adaptation of PT technology and to learn from our experience, patients were imaged with MRI more frequently than with typical historical surveillance schedules. Patients unable to follow-up in person were followed locally and results were communicated. The Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 was used to grade acute (from RT start to up to 3 months after the end of RT) and late (any time after 3 months until last follow-up) toxicities.
Statistical Methods
Local control (LC) was defined as absence of in-field recurrence within the primary site or high-dose volume. Regional control (RC) was defined as absence of progression in the neck or subclinical dose volumes. Distant control (DC) was defined as absence of progression in any region outside the head and neck. Disease-free survival (DFS) was defined as absence of local, regional, or distant recurrence of disease. The overall survival (OS), DFS, DC, and LC rates were calculated from the start of radiotherapy using the Kaplan-Meier method. The log-rank test provided estimates of statistical significance and variables with p-values ≤0.1 on univariate analysis (UVA) were entered into the multivariate analysis (MVA) using the Cox proportional hazards model. Frequency comparisons of various risk factors were evaluated by the Γ2 test. R project version 3.6.1 was used for statistical analysis. Survival package was used for survival outcomes, UVA and MVA analysis, and survminer package was used for survival curves. Statistical Product and Service Solutions (SPSS) version 21.0 was used for Descriptive Characteristics listed in Table 1 and Table 4.
Table 1.
Descriptive characteristics for the whole cohort
| Descriptive Characteristics | Whole Cohort n=86 (%) | RT-naïve Cohort n=68 | Re-RT Cohort n= 18 |
|---|---|---|---|
| Age | |||
| <70 years | 65 (76) | 52 (76) | 13 (72) |
| >=70 years | 21 (24) | 16 (24) | 5 (28) |
| Gender | |||
| Male | 56 (65) | 44 (65) | 12 (67) |
| Female | 30 (35) | 24 (35) | 6 (33) |
| Primary site | |||
| Nasal cavity or ethmoid sinus | 52 (60) | 45 (66) | 7 (39) |
| Other sinuses | 34 (40) | 23 (34) | 11 (61) |
| Histology | |||
| Squamous cell carcinoma | 35 (41) | 27 (40) | 8 (44) |
| Adenocarcinoma | 4 (4) | 4 (6) | 0 (0) |
| Adenoid cystic carcinoma | 11 (13) | 8 (12) | 3 (17) |
| Sinonasal undifferentiated carcinoma | 6 (7) | 6 (9) | 0 (0) |
| Olfactory neuroblastoma | 7 (8) | 5 (7) | 2 (11) |
| Neuroendocrine | 5 (6) | 4 (6) | 1 (6) |
| Other | 18 (21) | 14 (20) | 4 (22) |
| T category | |||
| T1–2 | 24 (35) | 18 (26) | 6 (33) |
| T3–4 | 62 (64) | 50 (74) | 12 (67) |
| N category | |||
| N0 | 70 (81) | 56 (82) | 14 (78) |
| N1 | 5 (6) | 2 (3) | 3 (17) |
| N2 | 10 (12) | 9 (13) | 1 (5) |
| N3 | 1 (1) | 1 (2) | 0 |
| Surgical Resection | |||
| No | 43 (50) | 33 (49) | 10 (56) |
| Yes | 43 (50) | 35 (51) | 8 (44) |
| Endoscopic | 16 (37) | 16 (46) | 0 (0) |
| Open | 27 (63) | 19 (54) | 8 (100) |
| Margin | |||
| Positive or Close | 26 (60) | 19 (54) | 7 (88) |
| Negative | 15 (35) | 14 (40) | 1 (12) |
| Unclear | 2 (5) | 2 (6) | 0 (0) |
| Proton Technique | |||
| 3DCPT | 40 (47) | 30 (44) | 10 (55) |
| IMPT | 46 (53) | 38 (56) | 8 (45) |
| Radiation dose (RBE) | |||
| Median (Range) | 70 (45–86) | 70 (45–86) | 68 (54–76) |
| <70 CGE | 34 (40) | 24 (35) | 10 (56) |
| ≥70 CGE | 52 (60) | 44 (65) | 8 (44) |
| Neck radiation | |||
| Yes | 41 (48) | 38 (59) | 1 (6) |
| No | 45 (52) | 28 (41) | 17 (94) |
| Chemotherapy | |||
| No | 31 (36) | 22 (32) | 9 (50) |
| Yes | 55 (64) | 46 (68) | 9 (50) |
| Cisplatin based | 41 (75) | 35 (76) | 6 (67) |
| Carboplatin based | 9 (16) | 9 (20) | 0 (0) |
| Cetuximab | 3 (5) | 1 (2) | 2 (22) |
| Other regimen | 2 (4) | 1 (2) | 1 (11) |
| 2 Year LC | 82% [73–92] | 83% [73–94] | 77% [57–100] |
| 2 Year DC | 84% [76–93] | 84% [75–95] | 80% [62–100] |
| 2 Year DFS | 70% [60–82] | 74% [63–87] | 54% [34–87] |
| 2 Year OS | 77% [68–88] | 81% [71–92] | 66% [46–92] |
Table 4.
Descriptive characteristics of RT-naïve 3DCPT compared to IMPT patients.
| Variable | 3DCPT n= 30 (%) | IMPT n=38 (%) | p-value |
|---|---|---|---|
| Age | |||
| <70 years | 21 (70) | 31 (82) | 0.17 |
| >=70 years | 9 (30) | 7 (18) | |
| Primary site | |||
| Nasal cavity or ethmoid sinus | 20 (67) | 25 (66) | 0.89 |
| Other sinuses | 10 (33) | 13 (34) | |
| Histology | |||
| Squamous cell carcinoma | 11 (37) | 16 (58) | 0.71 |
| Other | 19 (63) | 22 (42) | |
| T category | |||
| T1–2 | 7 (23) | 11 (29) | 0.68 |
| T3–4 | 23 (77) | 27 (71) | |
| N category | |||
| N0 | 25 (83) | 31 (82) | 0.82 |
| N+ | 5 (17) | 7 (18) | |
| Surgery | |||
| No | 15 (50) | 18 (47) | |
| Yes | 15 (50) | 20 (53) | 0.83 |
| Margin | |||
| Positive or Close | 10 (67) | 9 (45) | 0.28 |
| Negative | 5 (33) | 9 (45) | |
| Unclear | 0 (0) | 2 (10) | |
| Radiation Dose (RBE) | |||
| Median (Range) | 70(60–86) | 70 (45–77) | |
| ≥70CGE | 19 (63) | 25 (66) | 0.89 |
| <70CGE | 11 (37) | 13 (34) | |
| Neck radiation | |||
| Yes | 14 (47) | 26 (68) | 0.13 |
| No | 16 (53) | 12 (32) | |
| Chemotherapy | |||
| No | 12 (40) | 10 (26) | 0.38 |
| Yes | 18 (60) | 28 (74) | |
| Follow-up, median in months [range] | 29 [2–69] | 19 [2–47] | |
| 2yr LC | 72% [55–92] | 91% [80–100] | <0.01** |
| 2yr DC | 73% [58–93] | 94% [86–100] | 0.04** |
| 2yr DFS | 56% [40–78] | 88% [76–100] | <0.01** |
| 2yr OS | 75% [61–93] | 83% [70–100] | 0.21** |
RESULTS
Patient/Treatment Characteristics
Between 2013 and 2018, 122 patients with NC or PNS malignancies underwent PT, and 86 patients treated with curative intent were included for analysis (Figure 1). Cohort descriptive characteristics are shown in Table 1. Eighteen patients (21%) had prior RT (overlapping head and neck RT), with median dose 60 Gy [range 25–70]. Forty-three patients (50%) had surgical resection prior to adjuvant PT. Among patients who had surgery prior to PT, 19 (44%) were at outside centers. Sixteen (37%) patients received endoscopic surgery (9 with negative margins, 6 with positive/close margins, and 1 with unclear margins). Twenty-seven patients had open surgeries (most commonly: 12 maxillectomies, 7 rhinectomies, 6 craniofacial resections); 6 had negative, 20 had positive/close, and 1 had unclear margins. Forty-six patients (53%) were treated with IMPT. 41 patients (35 in RT-naïve cohort and 6 in re-RT cohort) received cisplatin-based chemotherapy, including 33 patients with cisplatin alone, 7 patients with etoposide plus cisplatin, 1 patient with doxorubicin and cisplatin. 9 patients received carboplatin-based regimen and were all from RT-naïve cohort, including 8 patients with carboplatin and paclitaxel, 1 patient with carboplatin alone. 3 patients received cetuximab alone as systemic treatment, of which 2 were re-RT patients. 1 RT-naïve patient received doxorubicin alone and 1 re-RT patient received docetaxel plus cetuximab.
Figure 1.
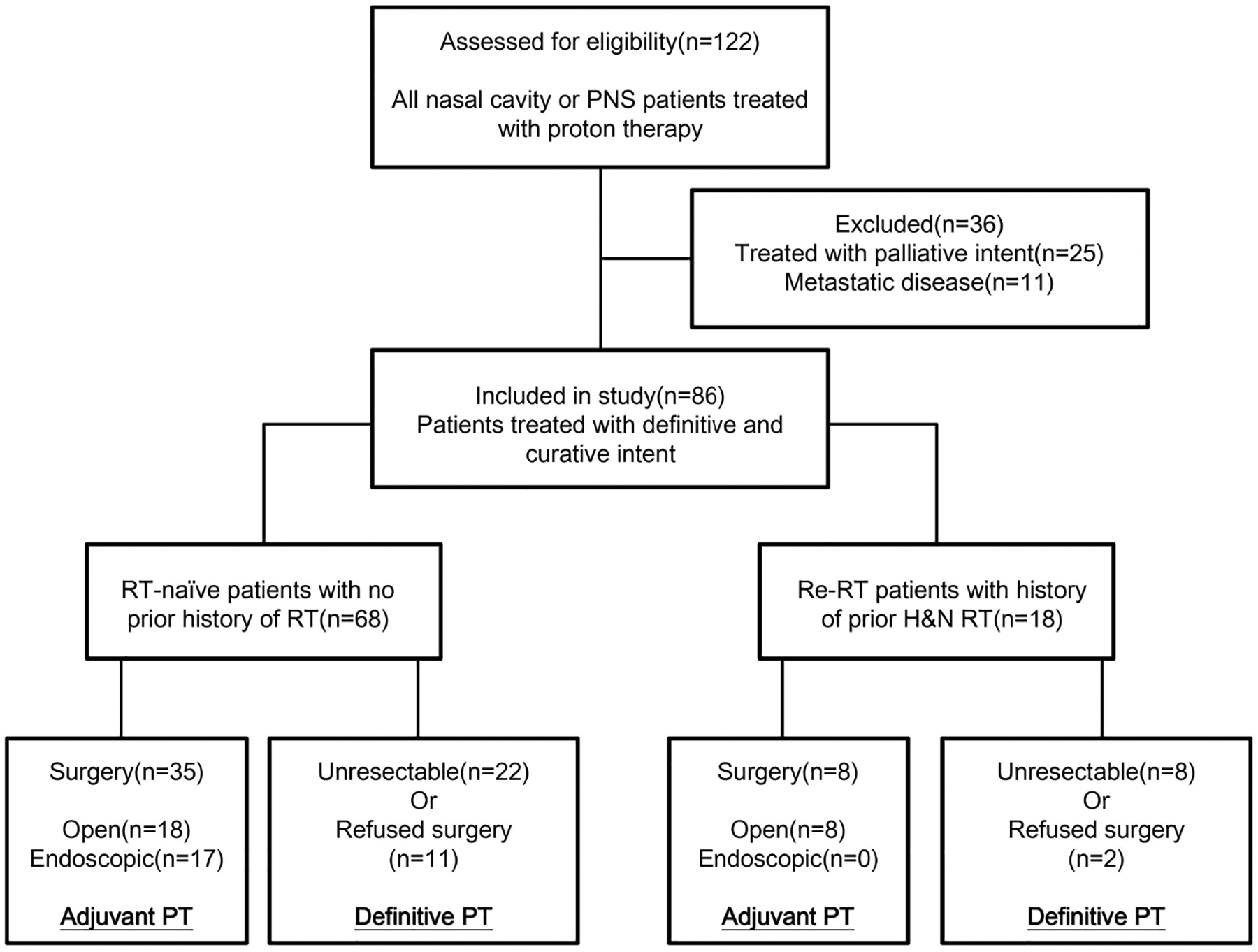
Consort diagram
Outcomes
Median follow-up was 23.4 months [1.7–69.3] for all and 28.1 months [2.3–69.3] among living patients. At last follow-up, 62 patients (72%) were alive and 28 patients had active disease (16 local recurrences, 6 regional recurrences, and 16 DM). Outcomes between RT-naïve and reirradiation patients were 2-year LC 83% and 77%; 2-year DC 84% and 80%, 2-year DFS 72% and 54%, 2-year OS 81% and 66%, Table 1).
RT-naïve patients
Table 2 reports univariate (UVA) and multivariate analysis (MVA) of predictors of local control in RT-naïve patients. IMPT was associated with robust 2-year LC (91% vs. 72%, p<0.01) advantages over 3DCPT. There was no LC detriment based on site, surgical resection (although the patient cohorts were very different), T stage, node-positivity, or chemotherapy. On MVA, PT technique was the only significant predictor of LC (3DCPT, HR 0.14, p=0.01).
Table 2.
Univariate and Multivariate Analysis of Predictors of local control in RT-naïve Patients
| UVA | MVA |
|---|---|
| P=0.56 | |
| P=0.11 | |
| P=0.70 | |
| P=0.63 | |
| P=0.93 | |
| 72% [55–92] vs. 91% [80–100] | HR 0.14 [0.03–0.66] |
Local recurrence occurred in twelve patients at a median time of 16 months [5 –50]. Salvage treatments included: surgical resection followed by adjuvant RT or systemic therapy (n=7), chemotherapy (n=2), reirradiation (n=1), and best supportive care (n=2). Regional failures developed in four patients. One patient received re-irradiation, one underwent salvage surgery, one received chemotherapy, and one received best supportive care. DM developed in thirteen patients in the lungs (n=6), bones (n=6), brain (n=2), liver (n=2), and axillary lymph node (n=1). Among these patients, six received radiation, two received systematic therapy, one underwent surgery, and the remaining received palliative treatment. Among deaths, five were cancer-related and nine were for unknown reasons.
Reirradiation patients
Two-year LC, DC, DFS, and OS were 75%, 80%, 50%, and 63% in those who had surgery, and 80%, 80%, 60%, and 68% in those who did not. Given small cohort size (n=18) and selection bias in patients treated with definitive reirradiation or offered surgical salvage, further analyses were not conducted.
Toxicities
Acute/late toxicities are listed in Table 3. One patient experienced grade 4 hematological toxicity attributed to chemotherapy, but there were no grade 4 or 5 toxicities due to radiation.
Table 3.
Acute and Late Toxicity, CTCAE v 5.0
| Whole Cohort, n=86 n (%) | No Prior RT, n=68 n (%) | Prior RT, n=18 n (%) | |
|---|---|---|---|
| Acute Grade 3 | |||
| Mucositis | 13 (15%) | 12 (18%) | 1 (6%) |
| Peg | 5 (6%) | 3 (4%) | 1 (6%) |
| Dermatitis | 6 (7%) | 3 (4%) | 2 (11%) |
| Fatigue | 1 (1%) | 1 (1%) | 0 |
| Soft tissue necrosis | 1 (1%) | 1 (1%) flap failure | 0 |
| Total: 18 (21%) patients | Total: 16 (24%) patients | Total: 2 (11%) patients | |
| Late Grade 3 | |||
| Vision loss | 2 (2%) | 2 (3%) | 0 |
| Osteoradionecrosis | 1 (1%) | 1 (1%) | 0 |
| Facial pain | 1 (1%) | 0 (0%) | 1 (6%) |
| Brain necrosis | 1 (1%) | 0 | 1 (6%) |
| Soft tissue necrosis | 1 (1%) | 1 (1%) flap failure | 0 |
| Soft tissue fibrosis | 1 (1%) | 1 (1%) | 0 |
| Total: 5 (6%) patients | Total: 3 (4%) patients | Total: 2 (11%) patients | |
| Late Grade 1–2 | |||
| Brain necrosis | 11 (13%) | 5 (7%) | 6 (33%) |
| Osteoradionecrosis | 5 (6%) | 2 (3%) | 3 (17%) |
| Soft tissue necrosis | 2 (2%) | 1 (1%) | 1 (6%) |
| Watering eyes | 15 (17%) | 15 (22%) | 0 |
| Trismus | 5 (6%) | 3 (4%) | 2 (11%) |
| Blurred vision | 2 (2%) | 1 (1%) | 1 (6%) |
| Glaucoma | 1 (1%) | 1 (1%) | 0 |
| Cataracts | 2 (2%) | 2 (3%) | 0 |
| Nasal congestion | 5 (6%) | 4 (6%) | 1 (6%) |
| Lymphedema | 1 (1%) | 0 | 1 (6%) |
| Oral cavity fistula | 1 (1%) | 1 (1%) | 0 |
| Ear disorder | 3 (3%) | 2 (3%) | 1 (6%) |
| Sinus disorder | 4 (5%) | 3 (4%) | 1 (6%) |
| Epistaxis | 1 (1%) | 1 (1%) | 0 |
| Total: 42 (49%) patients | Total: 32 (47%) patients | Total: 10 (50%) patients |
Sixteen RT-naïve patients (24%) experienced acute grade 3 toxicities, most commonly mucositis (n=12, of which 3 patients required a peg). All skin and mucosal toxicities recovered within three months. Only three patients (4%) experienced late grade 3 toxicities: osteoradionecrosis/osteomyelitis/flap failure requiring surgical debridement, severe optic nerve dysfunction (visual acuity 20/200), and blurred vision (visual acuity 20/150). Two reirradiation patients (11%) experienced acute grade 3 toxicities, most commonly dermatitis. All skin and mucosal toxicities recovered within three months. Two reirradiation patient had late Grade 3 toxicities including 1 facial pain requiring hospitalization and 1 brain necrosis.
Forty-seven percent of RT-naïve patients developed late grade 1–2 toxicities including most commonly watering eyes often requiring dacryocystorhinostomy, brain necrosis, and nasal/ear/sinus disorders. About 50% of reirradiation patients experienced late grade 1–2 toxicities, including most commonly brain necrosis and osteoradionecrosis. We observed one patient with severe hypopituitarism requiring intubation for respiratory failure about 8 months after endoscopic resection and 4 months after PT; MRI showed no findings to support radiation-induced brain injury and he made a full recovery with no known etiology for event.
In total, 15 patients experienced late toxicities required surgical intervention, including: 1 drainage of brain necrosis,1 debridement of osteonecrosis, 2 flap reconstructions,10 dacryocystorhinostomy, 5 nasal synechiae/stenosis/crusting, and 3 myringotomies.
Late radionecrosis was more common and appeared earlier in reirradiation patients than RT-naïve patients, with grade 1–2 brain necrosis (33% vs. 7%) and grade 1–2 osteoradionecrosis (17% vs. 4%). The median time to brain necrosis in all patients, RT-naïve patients, and re-RT patients were 20 months (3–48), 21 months (5–45), and 13 months (3–48), respectively. The median times [range] to osteonecrosis in all patients, RT-naïve patients, and re-RT patients were 24 months [3–41],35 months [21–41], and 7 months [3–28].
Comparison of RT-naïve 3DCPT and IMPT Patients
Figure 2 shows LC survival curves in RT-naïve patients based on PT technique. Patients treated with IMPT had better 2-year local control than those treated with 3DCPT (91% vs. 72%, p<0.01). Table 4 compares clinical and pathologic risk factors between 3DCPT and IMPT patients, and there were no significant differences between the two groups. Figure 3A shows a representative IMPT plan. IMPT was often utilized for larger and more infiltrative tumors, which would be expected to be more difficult to attain LC. Figure 3B shows MR findings before and after treatment.
Figure 2.
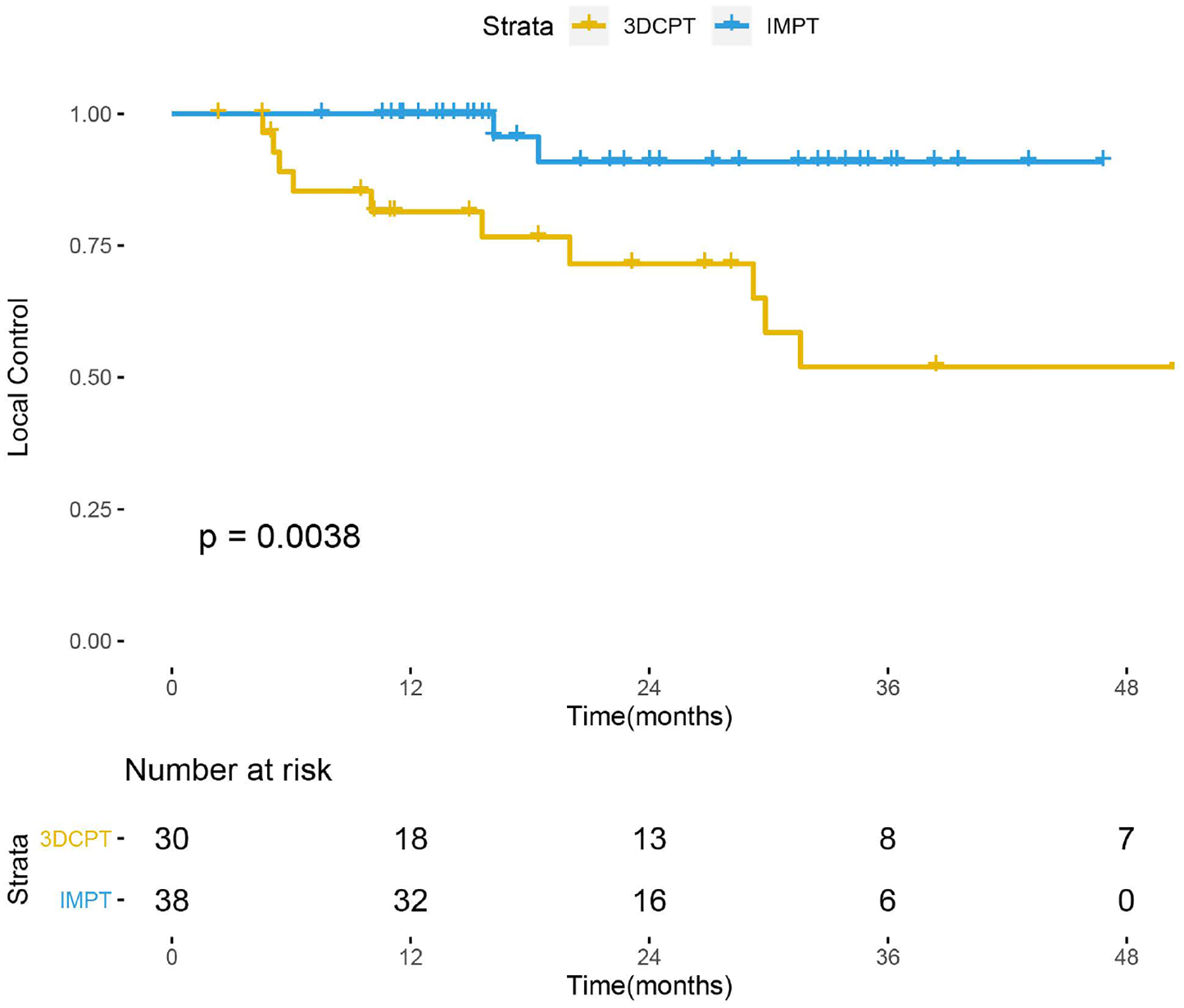
LC with IMPT vs. 3DCPT in RT-naïve patients.
Figure 3.
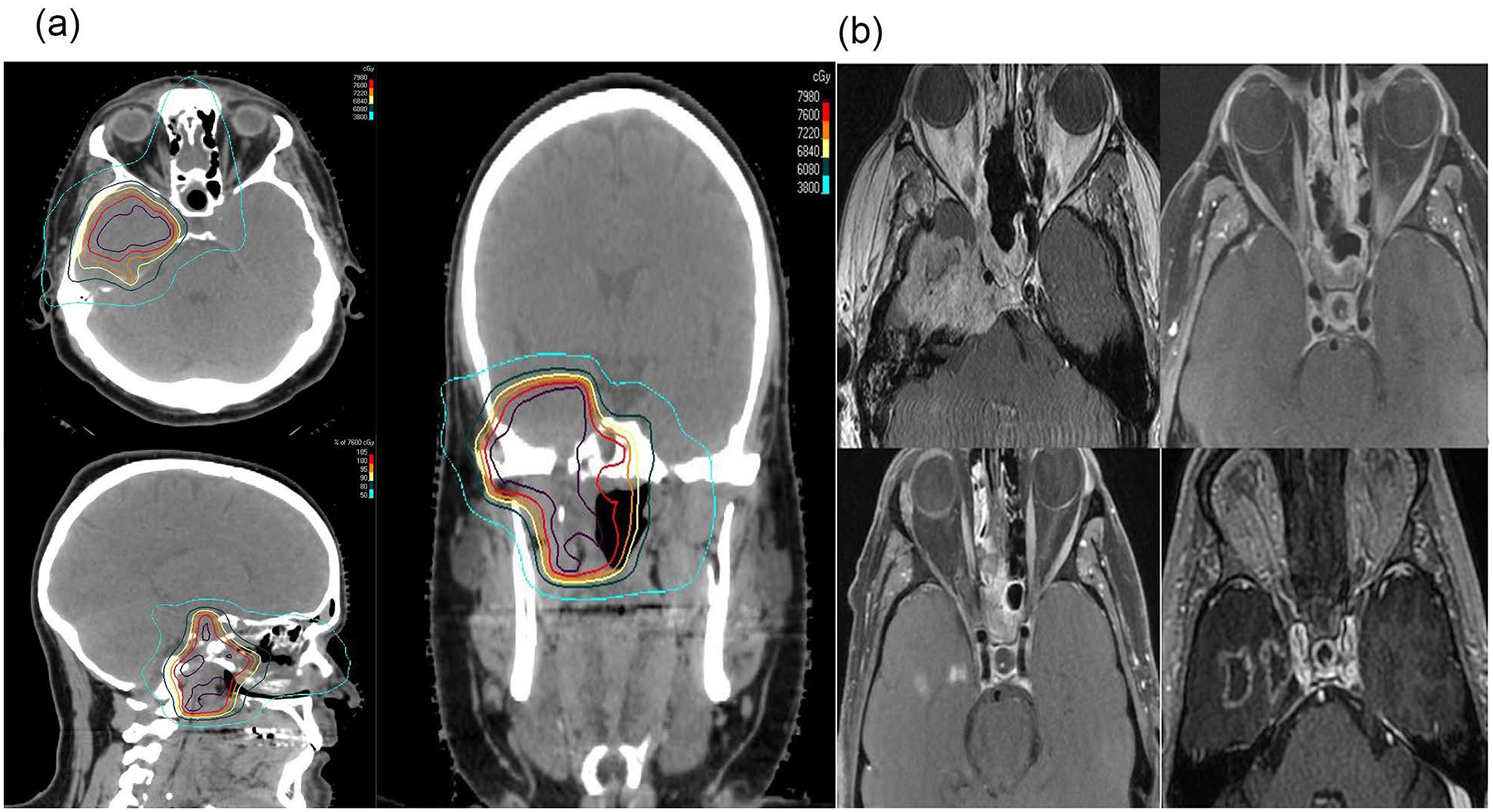
A 45-year old male diagnosed with an unresectable T4bN0M0 sphenoid sinus adenoid cystic carcinoma. There was significant temporal lobe invasion of this tumor. An IMPT plan (sagittal, axial, and coronal views) delivered using pencil beam scanning technique with a total dose of 76CGE (Figure 3A). Red isodose line encompassed the GTV (pre-treatment MRI in figure B shows clearly this tumor). The patient had complete response and is currently NED for 34 months but has developed Grade 2 temporal lobe necrosis gradually getting worse over time (Figure B): Representative T1 post MRI scans, pre-proton (upper left row ), 6 months post-proton (upper right row), 20 months post-proton (lower left row), and 32 months post-proton (lower right row). The serial MRI scans clearly show resolution of tumor and enlarging temporal lobe necrosis over time directly situated in the prior gross tumor volume (Figure 3B).
DISCUSSION
No randomized evidence has validated the optimal management of NC or PNS malignancies, and anatomic constraints from adjacent critical structures pose challenges to both surgical and radiation-based approaches. With photon RT, LC rates have been commonly estimated between 60–80% in the adjuvant setting and around 40–70% in the definitive setting, highlighting the difficulty of achieving local disease control with primary RT [6–14, 22–26]. It is known that high doses are required to control these tumors, as local recurrence is the predominant mode of failure [14,22,27]. IMRT technology allows for improved dose distributions and appears to have led to decreased complications, but there is debate over whether it has actually translated into improved outcomes over 3D-conformal RT [9,11,28].
Malignancies of the NC and PNS seem to be an ideal application for the use of PT considering additional physical and biological advantages, namely no exit dose (the Bragg’s Peak), over photon techniques including IMRT. Clinical data from several institutions show that high-dose PT is a definitive local therapy that can achieve excellent LC in locally advanced sinonasal cancer, irrespective of the extent of surgery [15,29]. Typically utilized for unresectable tumors, PT can achieve high 2-year LC of 83% compared to 45% with IMRT. In our cohort, we did not find that surgery prior to PT improved outcomes, but this should not be taken to suggest that surgery can be omitted. In our cohort, the majority (70%) of patients who did not receive surgery had unresectable disease; with definitive PT, their outcomes were comparable to patients with resectable disease who had surgery and adjuvant PT. In resectable cases, maximal safe complete surgical resection is important and permits a lower radiation dose to be administered to achieve excellent LC [5,6]. With lower radiation doses, decreased RT-related complications should follow.
Table 5 summarizes studies reporting outcomes with PT [29,31–33,35–37,40,42–43]. None reported outcomes with advanced proton technologies like IMPT, and research on their optimal clinical application has lingered.
Table 5:
Published studies of sinonasal malignancy and radiation therapy
| Investigator | Patients | Surgery | Pathology | Proton Therapy Technique | Follow-up (mos) | LC % (years) | OS % (years) |
|---|---|---|---|---|---|---|---|
| National Cancer Center Japan, 2011 [35] | Treatment naïve, unresectable N=39 |
none | 28% SCC | No IMPT | 45.4 | 77% (1y) | 59% (3y) |
| National Cancer Center Japan, 2012 [37] | Treatment naïve, T4b only N=13 |
none | 23% SCC | No IMPT | 56.5 | 77% (5y) | 76% (5y) |
| National Cancer Center Japan, 2015 [42] | Treatment-naïve, 70% T3–T4 N=90 |
14% | 24%SCC | No IMPT | 57.5 | 77% (5y) | 75% (3y) |
| National Cancer Center Japan, 2017 [31] | Treatment naïve N=42 |
none | Olfactory neuro-blastoma | No IMPT | 69 | 39–80% (5y) | 76–100% (5y) |
| Massaachusetts Eye and Ear, 2008 [29] | Treatment naïve, Locally advanced N=102 |
69% | 32% SCC | No IMPT | 43 | 49% (5y) in biopsy only patients | 87% (5y) in biopsy only patients |
| Massachusetts General, 2009 [43] | Treatment naïve, locally advanced N=20 |
35% | 50% SCC | No IMPT | 27 | 86% (2y) | 53% (2y) |
| Massachusetts General, 2016 [32] | Treatment naïve, T3–T4 only N=54 |
69% | 100% SCC | No IMPT | 82 | 80% (2y) | 67% (2y) |
| University of Florida, 2016 [33] | Treatment naïve, 8% recurrent N=84 |
87% | 26% SCC | No IMPT | 28.8 | 83% (3y) | 68% (3y) |
| Massachusetts General, 2006 [36] | 92% advanced, 8% recurrent N=36 |
78% | 28% SCC | No IMPT | 52.4 | 89% (5y) | 81% (5y) |
| University of Tsukubu, 2012 [40] | Recurrent or T4 N=17 |
none | 64% SCC | No IMPT | 23 | 35% (2y) | 47% (2y) |
| CURRENT STUDY, 2019 | 79% treatment naïve, 21% reirradiation N=86 |
41%SCC | RT-naïve | 24.0 | 83% (2y) | 81% (2y) | |
| Re-RT | 23.4 | 77% (2y) | 66% (2y) |
To our knowledge, this is the largest reported series of NC and PNS cancers treated with IMPT. Although these outcomes require additional follow-up for validation, we made several important observations:
PT on the whole is an effective definitive local therapy for primary treatment or reirradiation of malignancies of the NC and PNS.
LC is influenced robustly by treatment technique (IMPT being favorable).
Distant metastasis was a common mode of failure. The high LC rates seen with PT may shift the pattern of progression from predominantly local to distant, similar to what is observed for nasopharyngeal cancer [38] and human papillomavirus-related oropharyngeal cancer [39].
The possibility of IMPT to improve the therapeutic ratio is worth further investigation. There are clear dosimetric advantages with PT and IMPT over IMRT in head and neck malignances [15,30]. However, we are the first to report that IMPT is associated with the clinical advantages of improved LC over 3DCPT for NC and PNS cancers specifically. Our study was the only one to implement IMPT, which was identified as an independent favorable prognostic factor for LC.
The rates of serious late toxicities are much lower than historical series with photon IMRT. However, they still exist and can be severe (i.e, necrosis) and the onset of late complications appear sooner than IMRT. Low-grade late toxicities are in general under-reported in the photon literature, but they are common with treatments in this anatomic location (especially in reirradiation patients). Our paper has a detailed description of low-grade late toxicities confirmed by both radiation oncology and surgery, and can serve as a baseline for future reporting of all grades of toxicities.
Prior studies have shown inferior outcomes with SCC histology and recurrent tumors [7,14,40]. In our study, we found that SCC histology was an independent prognostic factor for DFS as well (data not show), but interestingly there was no LC detriment with the administration of PT. In our cohort, reirradiation patients still achieved high LC. This could be due to the fact that the RBE of protons may be higher resulting in improved tumor control.[41].
Serious treatment-related toxicity in our study was low compared to historical controls. In the photon IMRT literature, acute Grade ≥3 mucositis and dermatitis rates have been estimated at 37% and 15% of patients, respectively [14]. In our study, early grade 3 mucositis and dermatitis occurred in 15% and 7% of patients. Since the introduction of IMPT, we were able to significantly lower the dose delivered to the skin given IMPT’s ability to modulate the beam both distal and proximal to the tumor. This explains why the overall grade 3 dermatitis was half of historical rates. Without IMPT to treat skin as an avoidance structure, it will receive full prescription dose, resulting in higher cutaneous toxicity in proton versus photon-based treatments. Prior studies have also reported decreased acute toxicity with PT compared to IMRT [34]. Grade 3 late toxicity occurred in only five patients (6%): vision loss, osteonecrosis requiring surgical debridement, soft tissue necrosis requiring flap reconstruction, brain necrosis, and severe facial pain requiring hospitalization. Historical series have reported 12–28% rates of severe late toxicity, typically optic nerve disorder (3–6%), epiphora (3%), brain necrosis (1–6%), osteoradionecrosis (2–3%), soft tissue necrosis (1–9%), and endocrine disorders (5%) [32,33,35,40,42,43].
The incidence of low-grade toxicities is underreported in the literature but this should not be marginalized. For example, we observed one flap failure that began to develop even prior to the onset of adjuvant PT, but therapy was administered nonetheless due to the risks of tumor recurrence without treatment; consequently, we noted complete flap failure after PT. In our cohort, nearly half the cohort had grade 1–2 late toxicities and some were clinically very significant (brain/bone/soft tissue necrosis not requiring surgery, watering eyes, blurry vision). The high low-grade toxicity rates could in part be attributed to more frequent serial imaging employed in our proton patients versus historical controls, as we had been following them very closely given the adaptation of new technology. Additionally, since patients were living longer with disease control, they could be followed long enough to note complications, and complications continue to develop as patient’s live longer. With photon-based therapy, LC is known to be inferior and perhaps complications are overlooked in the setting of recurrence. It is also sometimes difficult to tease out what toxicities are related to tumor versus surgery versus RT complications. Given higher LC with PT, close clinical and radiographic surveillance of patients is important to ensure that complications are identified and managed expectantly. Therefore, patients should be followed with serial imaging to detect any complications as a result of treatment as these can occur much later after treatment. Furthermore, hormonal imbalances (like radiation-induced hypopituitarism) and visual toxicities can manifest years after treatment, so long-term follow-up is important [23,27].
Despite the promising results we reported, our study also has several limitations. First, this is a retrospective study of our experience in using proton therapy to treat a heterogeneous group of PNS tumors. Second, our toxicity data was done by chart review and there may potentially be more toxicities that were missed if these were not documented in the chart. Another limitation of our study is the relatively limited follow-up time. We will continue to follow these patients and will report long-term results in the future.
CONCLUSION
Our study shows that PT is a promising definitive treatment for patients with advanced or recurrent nasal cavity and paranasal sinus malignancies, with high likelihood of local control and lower toxicity than historical reports. IMPT was the most important predictor of LC. This is the largest series of IMPT, which shows its potential to improve the therapeutic ratio and suggests that its utilization as a definitive treatment in unresectable disease is worth further investigation and validation with longer follow-up. Given that late toxicities can occur after proton therapy, patients should be followed closely with serial imaging and appropriate care should be given when the complications are identified.
Funding information:
NIH/NCI Cancer Center, Grant/Award Number: P30 CA008748
Footnotes
Conflict of interests: None
REFERENCES
- 1.Turner JH and Reh DD Incidence and survival in patients with sinonasal cancer: A historical analysis of population‐based data. Head Neck, 2012;34: 877–885. [DOI] [PubMed] [Google Scholar]
- 2.Ganly I, Patel SG, Singh B, et al. Craniofacial resection for malignant paranasal sinus tumors: Report of an International Collaborative Study. Head Neck. 2005;27:575–84. [DOI] [PubMed] [Google Scholar]
- 3.Patel SG, Singh B, Polluri A, Bridger PG, Cantu G, Cheesman AD, deSa GM, Donald P, Fliss D, Gullane P, Janecka I, Kamata SE, Kowalski LP, Kraus DH, Levine PA, dos Santos LR, Pradhan S, Schramm V, Snyderman C, Wei WI, Shah JP. Craniofacial surgery for malignant skull base tumors: report of an international collaborative study. Cancer. 2003;98:1179–87. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. Head and Neck Cancer (Version 3.2019). https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. Accessed September 1, 2019.
- 5.Robbins KT, Ferlito A, Silver CE, et al. Contemporary management of sinonasal cancer. Head Neck. 2011;33:1352–65. [DOI] [PubMed] [Google Scholar]
- 6.Hoppe BS, Stegman LD, Zelefsky MJ, et al. Treatment of nasal cavity and paranasal sinus cancer with modern radiotherapy techniques in the postoperative setting The MSKCC experience. Int J Radiat Oncol Biol Phys 2007; 67:691–702. [DOI] [PubMed] [Google Scholar]
- 7.Dulguerov P, Jacobsen MS, Allal AS, et al. , Nasal and paranasal sinus carcinoma: Are we making progress? Cancer. 2001;92: 3012–3029. [DOI] [PubMed] [Google Scholar]
- 8.Mendenhall WM, Amdur RJ, Morris CG, et al. Carcinoma of the nasal cavity and paranasal sinuses. Laryngoscope. 2009; 119:899–906. [DOI] [PubMed] [Google Scholar]
- 9.Chen AM, Daly ME, Bucci MK, et al. Carcinomas of the paranasal sinuses and nasal cavity treated with radiotherapy at a single institution over five decades: Are we making improvement? Int J Radiat Oncol Biol Phys.2007; 69:141–147. [DOI] [PubMed] [Google Scholar]
- 10.Dirix P, Nuyts S, Geussens Y, et al. Malignancies of the nasal cavity and paranasal sinuses: long-term outcome with conventional or three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2007; 69:1042–50. [DOI] [PubMed] [Google Scholar]
- 11.Dirix P, Vanstraelen B, Jorissen M, et al. Intensity-modulated radiotherapy for sinonasal cancer: Improved outcome compared to conventional radiotherapy. Int J Radiat Oncol Biol Phys. 2010; 78:998–1004. [DOI] [PubMed] [Google Scholar]
- 12.Madani I, Bonte K, Vakaet L, Boterberg T, De Neve W. Intensity-modulated radiotherapy for sinonasal tumors: Ghent University Hospital update. Int J Radiat Oncol Biol Phys. 2009; 73:424–432. [DOI] [PubMed] [Google Scholar]
- 13.Hoppe BS, Wolden SL, Zelefsky MJ, et al. Postoperative intensity modulated radiation therapy for cancers of the paranasal sinuses, nasal cavity, and lacrimal glands: Technique, early outcomes, and toxicity. Head Neck. 2008; 30:925–932. [DOI] [PubMed] [Google Scholar]
- 14.Wiegner EA, Daly ME, Murphy JD, et al. Intensity-modulated radiotherapy for tumors of the nasal cavity and paranasal sinuses: clinical outcomes and patterns of failure. Int J Radiat Oncol Biol Phys. 2012; 83:243–51. [DOI] [PubMed] [Google Scholar]
- 15.Romesser PB, Cahlon O, Scher E, et al. Proton beam radiation therapy results in significantly reduced toxicity compared with intensity-modulated radiation therapy for head and neck tumors that require ipsilateral radiation. Radiotherapy and Oncology.2016;118:286–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siddiqui F, Smith RV, Yom SS, et al. Expert Panel on Radiation Oncology - Head and Neck Cancer. ACR appropriateness criteria® nasal cavity and paranasal sinus cancers. Head Neck. 2017; 39:407–418. [DOI] [PubMed] [Google Scholar]
- 17.Patel SH, Wang Z, Wong WW, et al. Charged particle therapy versus photon therapy for paranasal sinus and nasal cavity malignant diseases: a systematic review and meta-analysis. Lancet Oncol. 2014; 15:1027–38. [DOI] [PubMed] [Google Scholar]
- 18.Mimica X, Yu Y, McGill M, et al. Organ preservation for patients with anterior mucosal squamous cell carcinoma of the nasal cavity: Rhinectomy-free survival in those refusing surgery. Head Neck. 2019; 41:2741–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoppe BS, Nelson CJ, Gomez DR,et al. Unresectable carcinoma of the paranasal sinuses: outcomes and toxicities. Int J Radiat Oncol Biol Phys. 2008; 72:763–9. [DOI] [PubMed] [Google Scholar]
- 20.O’Sullivan B, Brierley J, Gospodarowicz M, et al. UICC TNM classification of Malignant Tumors. Eighth ed Chichester: Wiley; 2017. p. 17–54. [Google Scholar]
- 21.<j/>Lee NY, Riaz N, Lu JJ. Target volume delineation for conformal and intensity modulated radiation therapy. Berlin, Germany: Springer; 2015. [Google Scholar]
- 22.Gabriele AM, Airoldi M, Garzaro M, et al. Stage III-IV sinonasal and nasal cavity carcinoma treated with three-dimensional conformal radiotherapy. Tumori. 2008; 94:320–6. [DOI] [PubMed] [Google Scholar]
- 23.Becker C, Kayser G, Pfeiffer J. Squamous cell cancer of the nasal cavity: New insights and implications for diagnosis and treatment. Head Neck. 2016;38 Suppl 1: e2112–7. [DOI] [PubMed] [Google Scholar]
- 24.Gamez ME, Lal D, Halyard MY, et al. Outcomes and patterns of failure for sinonasal undifferentiated carcinoma (SNUC): The Mayo Clinic Experience. Head Neck. 2017; 39:1819–1824. [DOI] [PubMed] [Google Scholar]
- 25.Hollen TR, Morris CG, Kirwan JM, et al. Esthesioneuroblastoma of the nasal cavity. Am J Clin Oncol. 2015; 38:311–4. [DOI] [PubMed] [Google Scholar]
- 26.Allen MW, Schwartz DL, Rana V, et al. Long-term radiotherapy outcomes for nasal cavity and septal cancers. Int J Radiat Oncol Biol Phys. 2008; 71:401–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snyers A, Janssens GO, Twickler MB, et al. Malignant tumors of the nasal cavity and paranasal sinuses: long-term outcome and morbidity with emphasis on hypothalamic-pituitary deficiency. Int J Radiat Oncol Biol Phys. 2009; 73:1343–51. [DOI] [PubMed] [Google Scholar]
- 28.Daly ME, Chen AM, Bucci MK, et al. Intensity-modulated radiation therapy for malignancies of the nasal cavity and paranasal sinuses. Int J Radiat Oncol Biol Phys. 2007; 67:151–157. [DOI] [PubMed] [Google Scholar]
- 29.Resto VA, Chan AW, Deschler DG, Lin DT. Extent of surgery in the management of locally advanced sinonasal malignancies. Head Neck. 2008; 30:222–9. [DOI] [PubMed] [Google Scholar]
- 30.van Dijk LV, Steenbakkers RJ, ten Haken B, et al. Robust Intensity Modulated Proton Therapy (IMPT) Increases Estimated Clinical Benefit in Head and Neck Cancer Patients. PLoS One.2016; 11: e0152477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakamura N, Zenda S, Tahara M, et al. Proton beam therapy for olfactory neuroblastoma. Radiother Oncol. 2017; 122:368–372. [DOI] [PubMed] [Google Scholar]
- 32.Russo AL, Adams JA, Weyman EA, et al. Long-Term Outcomes After Proton Beam Therapy for Sinonasal Squamous Cell Carcinoma. Int J Radiat Oncol Biol Phys. 2016; 95:368–76. [DOI] [PubMed] [Google Scholar]
- 33.Dagan R, Bryant C, Li Z, et al. Outcomes of Sinonasal Cancer Treated with Proton Therapy. Int J Radiat Oncol Biol Phys. 2016; 95:377–85. [DOI] [PubMed] [Google Scholar]
- 34.McDonald MW, Liu Y, Moore MG, Johnstone PA. Acute toxicity in comprehensive head and neck radiation for nasopharynx and paranasal sinus cancers: cohort comparison of 3D conformal proton therapy and intensity modulated radiation therapy. Radiat Oncol. 2016; 11:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zenda S, Kohno R, Kawashima M, et al. Proton beam therapy for unresectable malignancies of the nasal cavity and paranasal sinuses. Int J Radiat Oncol Biol Phys. 2011; 81:1473–8. [DOI] [PubMed] [Google Scholar]
- 36.Weber DC, Chan AW, Lessell S, et al. Visual outcome of accelerated fractionated radiation for advanced sinonasal malignancies employing photons/protons. Radiother Oncol. 2006; 81:243–9. [DOI] [PubMed] [Google Scholar]
- 37.Okano S, Tahara M, Zenda S, et al. Induction chemotherapy with docetaxel, cisplatin and S-1 followed by proton beam therapy concurrent with cisplatin in patients with T4b nasal and sinonasal malignancies. Jpn J Clin Oncol.2012; 42:691–6. [DOI] [PubMed] [Google Scholar]
- 38.Lee N, Harris J, Garden AS, et al. Intensity-modulated radiation therapy with or without chemotherapy for nasopharyngeal carcinoma: radiation therapy oncology group phase II trial 0225. J Clin Oncol. 2009; 27:3684–3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Setton J, Caria N, Romanyshyn J, et al. Intensity-modulated radiotherapy in the treatment of oropharyngeal cancer: an update of the Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys. 2012; 82:291–8. [DOI] [PubMed] [Google Scholar]
- 40.Fukumitsu N, Okumura T, Mizumoto M, et al. Outcome of T4 (International Union Against Cancer Staging System, 7th edition) or recurrent nasal cavity and paranasal sinus carcinoma treated with proton beam. Int J Radiat Oncol Biol Phys. 2012; 83:704–11. [DOI] [PubMed] [Google Scholar]
- 41.Cuaron JJ, Chang C, Lovelock M, et al. Exponential Increase in Relative Biological Effectiveness Along Distal Edge of a Proton Bragg Peak as Measured by Deoxyribonucleic Acid Double-Strand Breaks. Int J Radiat Oncol Biol Phys. 2016; 95:62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zenda S, Kawashima M, Arahira S, et al. Late toxicity of proton beam therapy for patients with the nasal cavity, para-nasal sinuses, or involving the skull base malignancy: importance of long-term follow-up. Int J Clin Oncol. 2015; 20:447–54. [DOI] [PubMed] [Google Scholar]
- 43.Truong MT, Kamat UR, Liebsch NJ, et al. Proton radiation therapy for primary sphenoid sinus malignancies: treatment outcome and prognostic factors. Head Neck. 2009; 31:1297–308. [DOI] [PubMed] [Google Scholar]


