Abstract
Prosthetic joint infection (PJI) is a devastating complication that results in substantial costs to society and patient morbidity. Advancements in our knowledge of this condition have focused on prevention, diagnosis, and treatment, in order to reduce rates of PJI and improve patient outcomes. Preventive measures such as optimization of patient comorbidities, and perioperative antibiotic usage are intensive areas of current clinical research to reduce the rate of PJI. Improved diagnostic tests such as synovial fluid α-defensin ELISA, and nucleic acid-based tests for serum, synovial fluid, and tissue cultures, have improved diagnostic accuracy and organism identification. Increasing the diversity of available antibiotic therapy, immunotherapy, and alternative implant coatings remain promising treatments to improve infection eradication in the setting of PJI.
Keywords: Prosthetic joint infection, osteomyelitis, total joint replacement, revision total joint replacement
Introduction
Prosthetic joint infection (PJI) is a devastating complication that results in substantial costs to society and patient morbidity.1 Despite being the focus of research efforts for many years, treatment failure of PJI remains high with failures rates up to 50%.2-4 Current advancements in knowledge have focused on prevention, diagnosis, and treatment to reduce rates of PJI and improve patient outcomes. Preventive measures such as optimization of patient comorbidities, and perioperative antibiotic usage currently generate significant research interest and controversy5-9. Diagnosis of PJI remains challenging in certain cases due to false-negative cultures, non-diagnostic laboratory tests, and heterogeneous patient presentation10-13. Improved synovial fluid diagnostic tests such as α-defensins and nucleic acid-based tests for serum, synovial fluid, and tissue cultures attempt to improve diagnostic accuracy and organism identification in PJI14-19. Lastly, treatment outcomes remain poor in PJI. Increasing the diversity of available antibiotic therapy, immunotherapy, and alternative implant coatings remain promising treatments to improve infection eradication in the setting of PJI19-23. In this review, we will focus on recent advancements in the prevention, diagnosis, and treatment of PJI.
New Advancements in Prevention of PJI
Patient-specific risk optimization
In recent years, preoperative optimization of medical comorbidities has received substantial research attention. Several modifiable risk factors have been independently correlated with the development of PJI, and large database and registry data have attempted to define the risk correlated with each factor (Table 1).5,6,24-43 The optimization of modifiable risk factors remains important in prevention efforts for PJI; however, significant controversies still exist regarding the best diagnosis and treatment strategies (Table 1).
Table 1.
Summary of recent modifiable risk factors independently correlated with PJI development.
| Risk Factor | Risk of Infection (Odds Ratio)5,6,25-27 |
Proposed Interventions | Controversies |
|---|---|---|---|
| Obesity | 1.7 – 6.4 | BMI cutoffs (>35-40)5,6,25-27,43 Weight loss/nutrition counseling39 Bariatric surgery37,38 Anthropometric indices of adiposity at site of surgery28 |
Weight loss counseling has not been reliably shown to lead to appropriate weight reduction in most patients. Outcomes of bariatric surgery to reduce post-operative complications remains controversial and has not consistently shown decreased perioperative risk. Thickness of subcutaneous fat at surgical site as opposed to body mass index may be more indicative of complication risk. May increase health disparities in TJA. Obesity alone may not be as strong a risk factor as other issues and patients benefit significantly from surgery. |
| Diabetes Mellitus | 1.6 – 6.1 | Hemoglobin A1C cutoffs (7.0, 7.5, 8.0) 29-31 Serum fructosamine cut offs (> 292μmol/L)32 Perioperative serum glucose measurement (> 150-200mg/dl)33 |
Improved perioperative glucose control does seem to improve postoperative outcomes. Low cutoffs < 7.0 may be difficult for patients to achieve. Hemoglobin A1C may not correlate as strongly as other markers of glycemic control. |
| Active Smoking | 1.4 – 3.7 | Smoking cessation counseling34-36 Nicotine replacement products34-36 Serum cotinine testing34 |
Smoking cessation does improve outcomes after TJA. Hard to monitor compliance. Serum cotinine testing may be useful to improve compliance with smoking cessation recommendations. |
| Malnutrition | 5.0 – 7.0 | Laboratory cut off values (albumin cutoffs <3.5 g/dL; total lymphocyte count <1,500 cells/mm3 ; transferrin level <200 mg/dL)40-42 Nutritional modification |
Need more data on whether this is modifiable or indicative of underlying poor host. |
The most important considerations for patient optimization are: 1) whether modification actually reduces perioperative risk, and 2) whether these risk factors can be successfully modified without reducing access to care. For example, morbid obesity, or a body mass index (BMI) greater than 40 (or 35 with obesity related health conditions), has been identified as an independent risk factor for PJI with a positive association with infection risk as BMI increases.44 This has led to some hospitals rationing care by restricting candidacy for total joint arthroplasty (TJA) to patients with BMI less than 35-40. Unfortunately, many obese patients don’t develop a PJI, and would otherwise benefit from surgery, making it difficult to identify the highest risk patients in this cohort. Other methods to define risk are needed; for example, anthropometric indices of adiposity, such as the thickness of subcutaneous fat, may be more reliable predictors of risk in obesity than BMI.28 A similar rationing of care is taking place regarding risk factors such as perioperative glucose control and tobacco use (Table 1). Preoperatively predicting perioperative glucose control remains challenging, however, and the validity of using hemoglobin A1c, which measures a collective 90-days of serum glucose control, as a surrogate measure has recently been questioned.29-31 Alternative tests such as perioperative serum glucose levels and fructosamine have been described as more sensitive measures of perioperative glucose control, and have demonstrated promise in preoperative screening for high risk patients.32,33. Multidisciplinary preoperative patient optimization strategies, such as the Perioperative Orthopedic Surgical Home model, as recently described by Kim et al., attempts to coordinate risk factor optimization amongst nutritional, medical and surgical specialists and may improve upon these optimization strategies.45,46 Further studies are needed to define the best methods to balance risk factor modification with access to care.
Perioperative Antibiotic Usage
Perioperative antibiotic usage is a proven strategy to reduce rates of PJI, and is routinely implemented as a part of existing national guidelines from the Center for Disease Control (CDC). Optimal antibiotic selection and dosages specifically for TJA, however, remain controversial. For instance, a recent large database study noted a 32% higher risk of PJI when non-cephalosporin antibiotics such as vancomycin were used for preoperative prophylaxis.7,47,48 This may be due to issues such as weight-based underdosage or improper administration protocols.48 Another controversial topic regarding perioperative antibiotic prophylaxis in TJA is the use of single versus multiple perioperative doses. The revised CDC guidelines in 2017 recommended against 24 hours of perioperative antibiotics in favor of a single perioperative dose.49 A recent meta-analysis and systematic review showed that single versus multiple doses of perioperative antibiotics does not seem to affect rates of PJI after TJA, however, the level of evidence supporting a single dose was low, and active randomized controlled trials will provide better guidance on these recommendations in the TJA population.50
Other prevention strategies have focused on the administration of local antibiotics during TJA. Unfortunately, this strategy has not proven effective in practice. The routine use of antibiotic loaded bone cement (ALBC) for cemented primary hip and knee arthroplasty is controversial, and it has not shown consistent efficacy or cost effectiveness in large scale studies to justify routine use in the United States.8,9,51 Local administration of vancomycin powder may be effective in reducing PJI rates after complex spine surgery; however, low-quality data has demonstrated there may only be a slight decrease in PJI risk when used preventatively in TJA and may increase rates of wound seroma.52-54 Intraoperative antiseptic prophylactic irrigation solutions have become more popular in the past decade. Recent large-scale studies have shown some benefit in the use of povidone-iodine solutions in infection prophylaxis prior to aseptic revision and primary TJA, however, it has not been consistent across all studies.56-58 Other irrigants such as chlorhexidine-gluconate have not been shown to improve infection rates, when compared with current antisepsis protocols.59
Another alternative antibiotic administration strategy that has generated interest is post-operative extended antibiotic prophylaxis, particularly in high-risk patients. Inabathula et al. published a retrospective cohort study comparing an extended oral antibiotic protocol for 7 days to standard perioperative antibiotic administration following elective TJA in groups of patients with high risk comorbidities.60 They found significantly reduced rates of PJI using this protocol with a 1% infection rate in the extended antibiotic group versus 2.2% in the perioperative administered group alone.60 While this is promising data, others have been critical of the methodology, and the potential global health impact of widespread adoption of these protocols needs to be balanced with maintaining appropriate antibiotic stewardship.61
New Advancements in Diagnosis of PJI
Serum-based Markers for PJI Diagnosis
Reliable identification of PJI typically involves invasive procedures such as joint aspiration or intra-operative tissue sampling. The use of blood-based biomarkers for diagnosis is advantageous as it: 1) can provide an organism-specific diagnosis without the need for tissue culture, 2) minimally-invasive, 3) easy to administer, and 4) less time-consuming. Table 2 provides a summary of recent promising diagnostic markers for PJI. Currently, serum inflammatory cell counts and biomarkers such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are measured routinely for diagnosis of PJI.62 Previous studies have shown that elevated white blood cell (WBC) is unreliable for identifying PJI with a pooled sensitivity of only 45% with specificity at 87%, and it is not currently recommended for PJI diagnosis.10,63 Serum-based biomarkers such as CRP and ESR are currently the recommended first-line serum tests for identifying PJI with estimates of sensitivity and specificity at 86% and 72% for ESR and 87% sensitivity and 79% specificity for CRP.11,64 As diagnostic tools, CRP, and ESR have limitations in monitoring response to treatment, diagnosis in the setting of low virulence organisms, and for those that have systemic inflammatory diseases. Given the limitations of current serum laboratory tests, recent studies have focused on the development of alternative serum biomarkers.
Table 2.
Summary of New Developments in Diagnostic Strategies in PJI.
| Tissue Source |
Proposed Diagnostic Markers |
Limitations |
|---|---|---|
| Serum | Interleukin 669,70,73 Procalcitonin71-73 D-dimer12,13 Tumor necrosis factor – α69 Intracellular adhesion molecule-1 70 Lipopolysaccharide-binding protein70 Blood Antibodies76-78 |
May not improve diagnostic accuracy of existing combination of ESR/CRP and clinical criteria and adds expense. Markers except organism-specific antibody production are not pathogen specific so need further tissue sampling for microbial culture. Serum antibodies may be confounded by pre-exposure to previous infection. |
| Synovial Fluid | α-defensins14-16,80-85 Synovial CRP12,13,81,82 Synovial D-dimer12,13,82 Leukocyte esterase12,81 Lipocalin86 Calprotectin87 |
High sensitivity and specificity as single agents or in combination ranging from 75% - 100% depending on the marker and study. Some available as point-of-care test (Synovasure lateral flow immunoassay kit for α-defensins) False positives can occur such as in the setting of metallosis, and a combination of markers may be better than any single agent alone. False negatives may occur in the setting of low virulence organisms. Not as useful to diagnose persistent infection in the setting of reimplantation surgery. |
| Periprosthetic Tissue and Implant | RT-PCR of RNA18,91 NGS19,92,93 Implant sonication97-100 |
May improve upon tissue or synovial fluid culture in identification of organisms especially in culture-negative settings. May be confounded by contamination from skin or non-infecting microorganisms. |
A few studies have explored proinflammatory cytokines produced in response to bacterial infections as potential serum biomarkers for identifying PJI.63,65,66 Randau et al. observed that serum IL-6 levels were significantly elevated in patients presented with PJI compared to healthy controls and those with aseptic loosening of the implants.67 A meta-analysis, based on 17 studies involving >700 patients, revealed that serum IL-6 could achieve diagnostic accuracies of 83%, with 72% sensitivity and 89% specificity.68 Intriguingly, combining serum IL-6 with other serological tests such as CRP can markedly improve PJI diagnostic accuracy.69,70 Non-cytokine biomarkers such as procalcitonin are also secreted during PJI-induced systemic inflammation, and therefore being explored as a potential biomarker. However, the lack of sensitivity may limit the usefulness of PCT for PJI diagnosis.71-73 Other serum-based biomarkers such as D-Dimer, TNF-α, intracellular adhesion molecule-1, and lipopolysaccharide-binding protein have shown diagnostic promise68-71. Nonetheless, rigorous clinical studies are required to evaluate their clinical utility.
One limitation of the previously described serum biomarkers is that they are not pathogen-specific, which often forces clinicians to prescribe broad empiric antimicrobial agents until tissue samples are surgically obtained and cultured. There has been a recent push to utilize pathogen-specific B cell responses and antibodies as diagnostic and prognostic markers of PJI, particularly S. aureus PJI. This pathogen has evolved many strategies to efficiently evade host immune responses to cause chronic PJI.74,75 Nonetheless, anti-S. aureus antibody responses during an infection can be utilized to diagnose S. aureus infections. The authors of this review have developed a serum-based multiplex immunoassay for reliably diagnosing S. aureus PJI.76 Utilizing this immunoassay, it was shown that antibody responses against certain S. aureus antigens dominate during PJI. Employing S. Aureus specific antigens either singly or in combination, this assay was able to achieve a diagnostic accuracy of 89%.76 Additionally, assessing anti-S. aureus antibody titers expressed by a subset of pathogen-specific B cells called “circulating plasmablasts” or antibody secreting cells (ASCs) achieved a greater than 85% diagnostic and prognostic accuracy to follow treatment response in patients with S. aureus diabetic foot infections.77 A recent study by Wood et al. demonstrated the utility of serologic anti-cytotoxin LukAB antibodies for diagnosing orthpaedic S. aureus infections in children. Interestingly, the authors observed that serum anti-LukAB antibody titers reliably discriminated children with S. aureus infections from uninfected controls with greater than 80% accuracy.78 Nonetheless, antibody-based diagnostic studies such as ours and others, are still at the proof-of-concept stage.76-78
Synovial-fluid Based Markers for PJI Diagnosis
The synovial spaces in joints are the sites where most infections actually occur, and they are consequently particularly apt places for tissue sampling of suspected infections. During an ongoing infection, synovial fluid experiences a drop in viscosity and a massive increase in innate and adaptive immune cells, predominantly polymorphonuclear cells (PMNs). These changes are reflected by other features including: 1) transformation from the normally clear, pale-yellow fluid to one that is dark yellow, and cloudy or opaque; 2) increase in volume; and 3) the presence of inflammatory mediators and anti-inflammatory products secreted by the resident synovial cells and by the infiltrating immune cells. Furthermore, these synovial products are retained in the joint and not diluted by rapid mixing with the plasma because they are constrained by the synovial capsule. Given these dramatic changes in the synovial fluid, many investigators have sought distinctive synovial markers to indicate the presence of an ongoing infection.12
Recent studies have attempted to identify unique biomarkers independent of traditional synovial fluid culture or cell count for the diagnosis and prognosis of infection. CRP and D-dimer have been examined for their increase in synovial fluid during ongoing PJI. 13,79 Their performance has generally been good-to-excellent with sensitivities as high as 99%, however, many wonder if they provide the sensitivity needed for identifying low-virulence pathogens such as S. epidermidis. In fact, a recent critical examination of the clinical utility of the serum-borne analytes, even in combination with WBC in the synovial fluid, revealed that while the analytic power is quite good, its accuracy remains only about 85% with substantial opportunity for improvement.12 Other promising synovial fluid biomarkers include PMN derived products such as neutrophil gelatinase-associated lipocalin and α-defensins.
Because the joint becomes heavily populated by PMN during infection, high abundance secretory proteins produced by those cells accumulate in the small volumes of the synovial capsule. One family of polypeptides that have demonstrated diagnostic promise in recent years are α-defensins. Produced primarily by PMN, α-defensins are a set of related low molecular weight polypeptides (called antimicrobial peptides) that have anti-bacterial activity.14 When used for the diagnosis of PJI in synovial fluid, it achieved sensitivity and specificity of over 90% in the hands of multiple investigators.14,80-85 In addition, a recently introduced lateral flow immunoassay kit, Synovasure™, is now recommended for rapid analysis (~20 minutes) in the surgical suite, though it was found to be slightly less sensitive.15,16,85 Leukocyte esterase (LE) is a 168-kDa enzyme also secreted primarily by PMN. Recent reports are very encouraging about the diagnostic power of LE in a conventional immunoassay format that is both sensitive and specific, and in an immunoassay strip format, like Synovasure™, that can be used in the surgical suite and can yield results in 20 minutes, albeit with slightly reduced sensitivity. Another promising synovial fluid biomarker produced by neutrophils is lipocalin-2.86 A recent study by Vergara et al. showed an 86% sensitivity and 77% specificity to discriminate PJI versus aseptic revision failures.86 Recently, a third neutrophil-expressed marker protein that has been examined: Calprotectin is a 24-kDa heterodimer that can be as much as 60% of the soluble protein in the cytoplasm of PMN. In one report, calprotectin had greater than 95% specificity and sensitivity in distinguishing infected from aseptic patients.87 All three of these biomarkers reflect the high abundance of PMN that enter the synovial space and there is promise for each to be a useful tool alone or in combination with other markers. With this impressive success in the recent past, one cautionary note in regards to low virulence pathogens like S. epidermidis or C. acnes. There is at least one report which claims that low virulence organisms elicit modest and not readily measurable changes in these assays potentially yielding false negatives.88 A second concern regards the impact of metallosis from certain implants that can cause false positives in the α-defensin assay. This can be corrected by a complementary marker like synovial CRP, which is typically not elevated in the setting of metallosis even with a positive α-defensin assay.17
An alternative approach that demonstrates promise includes nucleic acid-based techniques. Nucleic acid-based approaches such as polymerase chain reaction (PCR) or more recently next generation sequencing (NGS) have offered great hope for rapid and accurate identification of infecting pathogens. At the same time, they raised substantial concerns about the creation of confounding, potentially incorrect diagnostic information.89 As with culture, pathogens collected from the skin can be problematic contaminants. Possibly more confusing is the potential for residual nucleic acids from dead cells to be collected in the synovial samples taken for analysis90 Another concern in these nuclei acid-based methods is their potential vulnerability to recent antibiotic use.18,91 NGS approaches can readily distinguish multiple species but may also be vulnerable to similar contamination issues and further studies are warranted.19
Aware of these concerns, several investigators have begun to explore the utility of the following potentially powerful, and reasonably rapid, approaches. One alternative method to DNA-based methods of PCR, which may be complicated by false positive results, is the use of reverse transcriptase PCR (RT-PCR) of bacterial ribosomal RNA18. In contrast to DNA, rRNA degrades at the time of cell death, theoretically reducing the rates of false positive results. In support of this theory, a recent examination of SF samples from culture-positive PJI patients showed thatRT-PCR of 16S/28S rRNA genes yielded higher specificity and sensitivity than conventional markers like serum CRP and PMN.18 Additionally, Two recent papers have reported significant correlation with culture methods in culture positive samples. In addition, these groups have identified potential pathogens in culture negative synovial fluid raising the prospect that nucleic acid testing may be a significant advance in treatment of these culture negative cases in particular. 19,92
Implant-based Markers for PJI Diagnosis
Microbiological culture from periprosthetic tissue is a necessary step for correct identification of an infecting organism in PJIs. Despite modern diagnostic methods, isolation of the infecting organism in the setting of PJI can be challenging, and approximately 15% are reported as culture negative.75,93-96 One method to improve upon our current culture techniques includes implant sonication.
Implant sonication describes the process of subjecting an implant material to ultrasonic waves through a buffer fluid to mechanically disrupt intercellular connections. This disruption releases bacterial cells from the implant surface into the fluid medium, while dissociating large cell aggregates.97 Following sonication, the fluid can be cultured to diagnose the infecting organism. Since the introduction of implant sonication there have been mixed opinions on the ability for sonicate fluid culture to diagnose infection with more accuracy than periprosthetic tissue culture. Several studies have concluded that sonication alone is superior to periprosthetic tissue culture and especially when culturing in optimal conditions such as blood culture.98-100 Other studies have shown that tissue culture may be more sensitive than sonicate culture for the diagnosis of PJI.97,101-103 Ultimately, studies agree that the addition of sonicate culture to classic tissue culture methods may improve the likelihood of identifying the causative organism.101 Recently, Erivan et al. demonstrated that sonicate fluid recovered the causative organism in 10 patients with PJI, where periprosthetic tissue culture was negative.97 From these results, the authors suggested that if implant sonication is feasible in a particular clinic, it should be included in the diagnosis workflow as it has superior ability to recover the infecting organism. Alternatively, sonicate fluid can be analyzed using multiplex PCR methods in order to determine the infecting organisms without the need for culture.104 The addition of molecular diagnosis by PCR may improve the accuracy of sonicate fluid because it has the ability to identify viable and non-viable bacteria as well as decreasing the overall time to diagnose from days to hours.105 Ultimately, future studies are warranted to achieve consensus on the use of sonication in the clinic.
New Advancements in the Treatment of Musculoskeletal Infection
The failure rate of PJI treatment remains high despite surgical debridement, prosthetic exchange, and targeted systemic antimicrobial agents. Emerging antibiotic resistance, formation of biofilm, and migration of bacteria to immune privileged locations such as the osteocyte lacuna-canalicular network all contribute to the challenge of treating these infections.106-108 A summary of promising treatment strategies in PJI are shown in Table 3.
Table 3.
Summary of New Developments in Treatment Strategies.
| Treatment Category |
Promising Strategies | Examples |
|---|---|---|
| Antibiotics | Novel agents with activity against resistant bacteria. Biofilm active agents. Local antibiotic delivery systems |
New cyclic lipopeptides (daptomycin), oxazolidinones (tedizolid), or synthetic glycopeptides (oritavancin)20,111-113. Rifampin, doxycycline21,114,115. Calcium sulfate beads119, intra-articular catheter120, PMMA116,117 |
| Immunotherapy | Active Immunization (prevention) Passive Immunization (prevention and treatment) |
Vaccines targeting cell wall components of S. aureus (LTA acid, capsular polysaccharides), cell wall anchored proteins (IsdB), toxins (α-toxin, Panton-Valentine Leukicidin, SpA).121-128 Monoclonal antibodies to SpA, DNA binding proteins, α-toxin, ClfA.22,129,130 Antibody-antibiotic conjugates23,131 |
| Bacteriophages | Phages targeting biofilm. | Synergism between systemic antibiotics and phages, use against low metabolic persister cells132-135 |
| Local Implant Treatments | Silver Antibiotic coatings Chemotherapeutic Agents |
Silver coating, nanoparticle-based delivery systems138-141 Covalent bonding of antibiotic to titanium, antibiotic carriers such as hydrogels or phosphatidylcholine 143-147 Cisplatin, mitomycin C have anti-biofilm activity even in low metabolic cell states142 |
| Dispersal | Enzymatic Treatments Fibrinolytics Targeting quorum sensing |
Dispersin B, lysostaphin, DNases148,149 Streptokinase149,150 Autoinducing peptide type I, RNAIII-inhibiting peptide149,150,152 |
Novel Antibiotic Strategies
Systemic antibiotic therapy is a critical aspect to treating PJI, however, increasing bacterial resistance to conventional antimicrobial agents creates significant treatment challenges.109,110 Over the past decade, novel antibiotics with broad spectrum activity against gram-positive organisms such as daptomycin, which is a cyclic lipopeptide, and linezolid, an oxazolidinone, have been developed to expand treatment options for resistant infections.111-113 For instance, daptomycin had greater than an 80% treatment success rate for a mix of chronic and acute PJIs in the setting of resistant Staphylococcal infection when alternative antibiotics such as vancomycin could not be used due to resistance or patient intolerance.111 Next generation oxadolidinones or semisynthetic glycopeptides have shown excellent in vitro activity against resistant gram-positive infections, while improving upon the oral bioavailability (tedizolid) or half-life (oritavancin, dalbavancin) relative to previous agents, allowing for less frequent dosing periods or use of oral regimens.20
The addition of biofilm active agents and antibiotics that target metabolically quiescent bacterial colonies, termed small colony variants are also an important component of treating PJI. The minimum inhibitory concentrations (MIC) that are used as susceptibility tests for cultured bacteria do not reflect the susceptibility of the bacteria within a biofilm, which can require many fold higher concentrations to achieve the minimum biofilm eradication concentration (MBEC). In vitro studies from clinical isolates of S. aureus isolated from PJI suggest that rifampin and doxycycline, are among the few common antibiotics with measurable biofilm bactericidal concentrations (90% of S. aureus biofilms tested could be killed by rifampin and 50% by doxycycline).114 These findings are supported by clinical studies that show rifamycin-based antibiotics such as rifampin improve treatment of PJI, however, as a monotherapy, rapid resistance develops, emphasizing the importance of combination antimicrobial therapy in S. aureus PJI.21,115
The development of novel antibiotic or small molecule delivery systems may be a successful strategy to target biofilm formation and improve infection eradication. Both resorbable and non-resorbable antibiotic carriers such as polymethylmethacrylate (PMMA) or calcium sulfate respectively have been shown to increase local antibiotic concentrations well above MIC values for many different antibiotic combinations in vitro. 116,117 These local delivery systems are frequently used in the setting of PJI; however, there is no strong evidence to support their ability to improve eradication of clinical infection. For example, the use of gentamicin impregnated beads and/or sponges resulted in increased failure after debridement and implant retention for acute PJI with failure rates 40% in the local gentamicin group versus 26% in the control group after propensity matching.118 Additionally, antibiotic-impregnated calcium sulfate beads did not improve outcomes after debridement and implant retention in acute PJI.119 Another method of local antibiotic delivery by an intra-articular catheter showed some success in a limited number of patients.120 Well-controlled studies are still needed to justify the added cost, risk of resistant organisms, and morbidity of additional local antibiotic therapy to treat PJI.
Immunotherapy
Immunotherapy is another major area of interest to improve both the prevention and treatment of PJI. Both active and passive immunization strategies have been attempted in the past to target common PJI associated bacteria. Unfortunately, active immunization strategies focusing on prevention strategies utilizing vaccines to common sources of PJI such as S. aureus have not been successful beyond Phase I clinical trials.121-123 Vaccine strategies targeting components of the cell wall not universally expressed across strains such as poly-N-acetyl glucosamine, LTA acid and capsular polysaccharides have failed to reduce infection in the clinical setting.121,124 Another vaccine targeting iron-regulated surface determinant system (Isd) B, which is a cell wall-anchored protein that allows iron scavenging from hemoglobin in S. aureus, from Merck (V710) showed preclinical promise. However, it failed to reduce infection rates or mortality in a phase 2b/3 trial focused on prevention of S. aureus infection after cardiothoracic surgery.122 Additionally, increased rates of mortality due to sepsis was found in patients who did get infected, suggesting that this vaccine may have been detrimental to host immunity to S. aureus.122 Given these previous failures, newer strategies are utilizing a multi-agent vaccine with three or four S. aureus surface/capsular antigens, and these vaccines have shown improved immunogenicity in preclinical and early stage clinical trials in healthy volunteers.125 Further clinical trials in the setting of infection are needed to assess the efficacy of these approaches. Other approaches for active immunization against S. aureus have included targeting other virulence factors such as alpha-toxin, Panton-Valentine Leukocidin, surface protein A (SpA), and secretory proteins, however, these have not progressed beyond preclinical or early stage healthy human trials.126,128
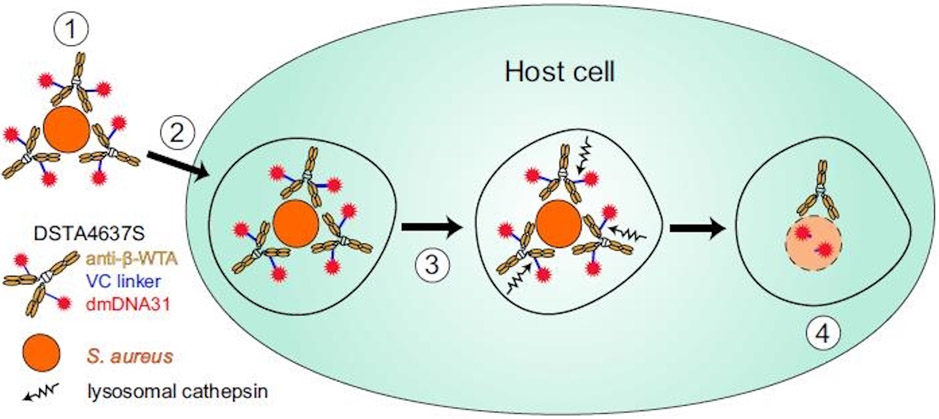
Passive immunization strategies may show more promise than vaccine-based approaches as a complement to systemic antimicrobial therapy in both the treatment and prevention setting, however, these are mostly in preclinical stages of development. For example, monoclonal antibodies (mAb) of the IgG3 class against SpA derived from human hosts are able to bind with high affinity to wild type S. aureus SpA even in the presence of other host antibodies, promote opsonization and associated phagocytic clearance, and reduced the rate of septic death in a mouse model of (methicillin resistant S. aureus) MRSA bacteremia in combination with vancomycin.129 Passive immunization can also be used to target components of biofilm, and a mAb against DNA binding proteins from the DNABII family, which have conserved homologs across many bacterial species, reduced both planktonic and adherent biofilm bacteria in a murine implant-associated infection model relative to daptomycin monotherapy alone.130 A combination of mAb to multiple S. aureus antigens has also shown promise in targeting biofilm formation, and mAb to α-toxin and clumping factor A (ClfA) resulted in decreased bacterial load in the periprosthetic tissue, reduced propensity for infection, and less biofilm aggregates in a murine model of hematogenous MRSA infection.22 Other novel strategies include targeting intracellular reservoirs of S. aureus. For example, an antibody-antibiotic conjugate (AAC) that consists of a monoclonal antibody recognizing specific sugars on wall teichoic acids (WTAs) bound to rifamycin class derivative antibiotic has been shown to bind to the surface of S. aureus, and then upon opsonization, the proteolytic environment of the phagolysosome of the host cell causes release of the active antibiotic form (Figure 1).23 This approach was effective in a wide range of host cells including murine and human macrophages, osteoblasts, endothelial and epithelial lining cells.23 In a murine model of hematogenous MRSA infection, use of the AAC reduced S. aureus bacteremia relative to systemic vancomycin alone in both wild type and severe combined immune deficiency spontaneous mutation (SCID) mice.23 A similar approach showed no toxicity in human phase I clinical trials.131
Figure 1. DSTA4637S mechanism for killing intracellular S. aureus.
Step 1, DSTA4637S binds S. aureus. Step 2, host cells internalize DSTA4637S-bound S. aureus. Step 3, fusion occurs with the phagolysosome where lysosomal cathepsins cleave the VC linker, releasing dmDNA31. Step 4, unconjugated dmDNA31 kills the intracellular bacteria. Reproduced with permission from: Peck M, Rothenberg ME, Deng R et al. A Phase 1, Randomized, Single-Ascending-Dose Study To Investigate the Safety, Tolerability, and Pharmacokinetics of DSTA4637S, an Anti-Staphylococcusaureus Thiomab Antibody-Antibiotic Conjugate, in Healthy Volunteers. Antimicrob Agents Chemother. 2019; 63(6). pii: e02588-18.
Bacteriophages
Bacteriophages (phage) are naturally occurring viruses that target bacterial cells with high specificity while causing minimal damage to host cells making them promising preclinical agents for treatment of PJI.132 Phages are able to adhere to the bacterial cell surface, insert its genomic material into the bacterial cell, replicate within the host cell, and lyse the bacterial cell wall resulting in cell death.132 Importantly, decreased cellular metabolic activity such as seen in small colony variants and biofilm can effectively be targeted and killed by phages in contrast to systemic antimicrobial agents.133 In vivo studies have shown synergism between the use of systemic antimicrobial agents and phage to treat biofilm associated infection in a rat model.134 Limited early clinical studies have shown that topically applied phages are safe in the setting otitis media and venous leg ulcers, however, no clinical data exists on its use in PJI.135,136
Local Implant Treatments
Improving the biofilm resistance of existing implant materials would serve both as primary prevention of PJI and reduce rates of treatment failure. One promising strategy that is used clinically for infection prevention in limited cases are silver-based implant coatings. Silver is one of the oldest antimicrobial agents known, exhibiting anti-bacterial effects to multiple intracellular and cell wall targets resulting in cell death, however, historical concerns about toxicity have limited its clinical use.137 Recently, silver coated implants have shown promising results in preclinical and limited clinical studies in orthopedic trauma and limb reconstruction.138-140 A silver coated megaprosthesis reduced rates of postoperative infection (11.8% versus 22.4%), and had improved success after debridement and implant retention relative to titanium implants in a small study.139 Alternative approaches involving the use of nanoparticle-based delivery systems have attempted to reduce off target effects to surrounding tissues while retaining the antimicrobial activity of the silver ions, and this may be a promising strategy in the future.141 Chemotherapeutic agents such as cisplatin have shown antibiofilm activity, however, research is limited to the preclinical setting.142
Other modifications to the implant surface have not progressed beyond preclinical studies, however, there are some promising strategies. Covalent bonding of an antibiotic to titanium may allow local antibiotic delivery without impairing host cell function or prosthetic osseointegration for both prevention and treatment strategies.143 Surface modifications that enhance osseointegration of titanium in combination with antibiotic coatings may be another strategy to increase biofilm resistance of existing implants while promoting host cell adhesion in favor of bacterial cell adhesion.144 Use of antibiotic carriers such as hydrogels or phosphatidylcholine-based materials may allow point of care application of antibiotic eluting implant coatings from the implant surface for both PJI prevention and treatment.145-147
Dispersal Agents
Another strategy that targets biofilm directly is the use of dispersal agents. Converting biofilm bacteria to their planktonic form may increase bacterial cell susceptibility to commonly used systemic antibiotics. Some examples that target the matrix components of biofilm include enzymatic treatments such as trypsin, Dispersin B, Lysostaphin, and DNases.148-150 Additionally, fibrinolytics like streptokinase or nattokinase break down the fibrin matrix within biofilm and decrease the MBEC of available systemic antibiotics.149,150 Another strategy for triggering biofilm dispersal includes targeting the quorum sensing system.For example, treatment in vitro with autoinducing peptide type I (AIP-1), a critical component of the quorum sensing system, was able to trigger dispersal of MRSA on titanium discs.150 RNAIII-Inhibiting peptide (RIP) is another peptide that targets the quorum sensing system in S. aureus by competing with RNAIII-activating peptide (RAP) which results in decreased cell adhesion and agr activation and has been investigated to reduce S. aureus adhesion to foreign materials.152 This also remains in the preclinical stages. Other possible local treatments to improve biofilm dispersal include the use of pulsed laser therapy and gold nanoparticles.153 One major concern about dispersal agents is that planktonic bacteria cells disassembled from their biofilms may be capable of exacerbating systemic infection. Therefore, dispersal agents must be used in combination with systemic therapies such as antibiotics.149-151 These treatments remain in the preclinical stages in the treatment of musculoskeletal infection.
Conclusion
In summary, promising prevention strategies include identifying high-risk patients for PJI and the use of the perioperative surgical home to optimize these risks before surgery. Alternative antibiotic strategies such as dual antibiotic therapy for preoperative administration, single versus multiple doses of preoperative antibiotics, the use of local antibiotic treatment such as vancomycin powder are promising, but need to be investigated further especially in high risk patient populations. Diagnostic strategies should focus on increasing the accuracy of synovial fluid- and serum-based tests and providing accurate, organism-specific diagnoses. Promising strategies include α-defensin and nucleic acid-based tests such as rRNA PCR or NGS. Further expansion of existing antibiotic classes such as the next generation oxadolidinones and improving the development of biofilm active agents such as rifampin to target biofilm more effectively is necessary for systemic antibiotic therapy improvements. Novel treatment strategies including immunotherapy, implant-based coatings, and dispersal agents may all improve biofilm treatment and eradication in PJI.
Supplementary Material
Acknowledgements
Conflict of interest: EMS have patents related to this work. EMS has received financial compensation and stock from Telephus Medical LLC. JLD is a co-founder of MicroB-plex, Inc. (Atlanta, GA.) and works there part-time. BFR, EAM, NK, GM have no conflicts of interest related to this work.
Grant sponsors: National Institutes of Health (EMS), Grant numbers: P30 AR069655 and P50 AR07200 (EMS), and AOTrauma Clinical Priority Program (EMS).
References
- 1.Schwarz EM, Parvizi J, Gehrke T et al. 2019. 2018 International Consensus Meeting on Musculoskeletal Infection: Research Priorities from the General Assembly Questions. J Orthop Res 37(5):997–1006. [DOI] [PubMed] [Google Scholar]
- 2.Bryan AJ, Abdel MP, Sanders TL et al. 2017. Irrigation and Debridement with Component Retention for Acute Infection After Hip Arthroplasty: Improved Results with Contemporary Management. J Bone Joint Surg Am 99(23):2011–2018. [DOI] [PubMed] [Google Scholar]
- 3.Lora-Tamayo J, Murillo O, Iribarren JA et al. 2013. A large multicenter study of methicillin-susceptible and methicillin-resistant Staphylococcus aureus prosthetic joint infections managed with implant retention. Clin Infect Dis 56(2):182–94. [DOI] [PubMed] [Google Scholar]
- 4.Nodzo SR, Boyle KK, Spiro S et al. 2017. Success rates, characteristics, and costs of articulating antibiotic spacers for total knee periprosthetic joint infection. Knee 24(5):1175–1181. [DOI] [PubMed] [Google Scholar]
- 5.Edwards PK, Mears SC, Stambough JB et al. 2018. Choices, compromises, and controversies in total knee and total hip arthroplasty modifiable risk factors: what you need to know. J Arthroplasty 33(10): 3101–3106. [DOI] [PubMed] [Google Scholar]
- 6.Kee JR, Mears SC, Edwards PK, Barnes CL. 2017. Modifiable risk factors are common in early revision hip and knee arthroplasty. J Arthroplasty 32(12): 3689–3692. [DOI] [PubMed] [Google Scholar]
- 7.Wyles CC, Hevesi M, Osmon DR et al. 2019. Increased risk of periprosthetic joint infection following primary total knee and hip arthroplasty with the use of alternative antibiotics to cefazolin: the value of allergy testing for antibiotic prophylaxis. Bone Joint J 101-B(6Supple_B): 9–15. [DOI] [PubMed] [Google Scholar]
- 8.Cummins JS, Tomek IM, Kantor SR et al. 2009. Cost-effectiveness of antibiotic-impregnated bone cement used in primary total hip arthroplasty. J Bone Joint Surg Am 91(3) 634–641. [DOI] [PubMed] [Google Scholar]
- 9.King JD, Hamilton DH, Jacobs CA, Duncan ST. 2018. The hidden cost of commercial antibiotic-loaded bone cement: A systematic review of clinical results and cost implications following total knee arthroplasty. J Arthroplasty 33(12): 3789–3792. [DOI] [PubMed] [Google Scholar]
- 10.Parvizi J, Tan TL, Goswami K, et al. 2018. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J Arthroplasty 33:1309–1314 e1302. [DOI] [PubMed] [Google Scholar]
- 11.Huerfano E, Bautista M, Huerfano M, et al. 2017. Screening for Infection Before Revision Hip Arthroplasty: A Meta-analysis of Likelihood Ratios of Erythrocyte Sedimentation Rate and Serum C-reactive Protein Levels. J Am Acad Orthop Surg 25:809–817. [DOI] [PubMed] [Google Scholar]
- 12.Lee YS, Koo KH, Kim HJ et al. 2017. Synovial Fluid Biomarkers for the Diagnosis of Periprosthetic Joint Infection: A Systematic Review and Meta-Analysis. J Bone Joint Surg Am 99(24):2077–84. [DOI] [PubMed] [Google Scholar]
- 13.Qin L, Li F, Gong X et al. 2019. Combined Measurement of D-Dimer and C-Reactive Protein Levels: Highly Accurate for Diagnosing Chronic Periprosthetic Joint Infection. J Arthroplasty Epub. [DOI] [PubMed] [Google Scholar]
- 14.Berger P, Van Cauter M, Driesen R et al. 2017. Diagnosis of prosthetic joint infection with alpha-defensin using a lateral flow device: a multicentre study. Bone Joint J 99-B(9):1176–82. [DOI] [PubMed] [Google Scholar]
- 15.Gehrke T, Lausmann C, Citak M et al. 2018. The Accuracy of the Alpha Defensin Lateral Flow Device for Diagnosis of Periprosthetic Joint Infection: Comparison with a Gold Standard. J Bone Joint Surg Am 100(1):42–8. [DOI] [PubMed] [Google Scholar]
- 16.Marson BA, Deshmukh SR, Grindlay DJC, and Scammell BE. 2018. Alpha-defensin and the Synovasure lateral flow device for the diagnosis of prosthetic joint infection: a systematic review and meta-analysis. Bone Joint J 100-B(6):703–11. [DOI] [PubMed] [Google Scholar]
- 17.Stone WZ, Gray CF, Parvataneni HK et al. 2018. Clinical Evaluation of Synovial Alpha Defensin and Synovial C-Reactive Protein in the Diagnosis of Periprosthetic Joint Infection. J Bone Joint Surg Am 100(14):1184–90. [DOI] [PubMed] [Google Scholar]
- 18.Kuo FC, Lu YD, Wu CT et al. 2018. Comparison of molecular diagnosis with serum markers and synovial fluid analysis in patients with prosthetic joint infection. Bone Joint J 100-B(10):1345–51. [DOI] [PubMed] [Google Scholar]
- 19.Ivy MI, Thoendel MJ, Jeraldo PR et al. 2018. Direct Detection and Identification of Prosthetic Joint Infection Pathogens in Synovial Fluid by Metagenomic Shotgun Sequencing. J Clin Microbiol 56(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crotty MP, Krekel T, Burnham CA, Ritchie DJ. 2016. New Gram-Positive Agents: the Next Generation of Oxazolidinones and Lipoglycopeptides. J Clin Microbiol 54(9):2225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jørgensen NP, Skovdal SM, Meyer RL et al. 2016. Rifampicin-containing combinations are superior to combinations of vancomycin, linezolid and daptomycin against Staphylococcus aureus biofilm infection in vivo and in vitro. Pathog Dis 74(4):ftw019. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Cheng LI, Helfer DR et al. 2017. Mouse model of hematogenous implant-related Staphylococcus aureus biofilm infection reveals therapeutic targets. Proc Natl Acad Sci U S A 114(26):E5094–E5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehar SM, Pillow T, Xu M et al. 2015. Novel antibody-antibiotic conjugate eliminates intracellular S. aureus. Nature 527(7578):323–8. [DOI] [PubMed] [Google Scholar]
- 24.Alamanda VK, Springer BD. 2018. Perioperative and modifiable risk factors for periprosthetic joint infections (PJI) and recommended guidelines. Curr Rev Musculoskelet Med 11(3): 325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kunutsor SK, Whitehouse MR, Blom AW et al. 2016. Patient-related risk factors for periprosthetic joint infection after total joint arthroplasty: A systematic review and meta-analysis. PLoS One 11(3): e0150866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mont MA, Tanzer M. Orthopaedic Knowledge Update: Hip and Knee Reconstruction 5. Rosemont: American Academy of Orthopaedic Surgeons; 2017. [Google Scholar]
- 27.Namba RS, Inacio MC, Paxton EW. 2013. Risk factor associated with deep surgical site infections after primary total knee arthroplasty: an analysis of 56,216 knees. J Bone Joint Surg Am. 95(9): 775–782. [DOI] [PubMed] [Google Scholar]
- 28.Armstrong JG, Morris TR, Sebro R et al. 2018. Prospective study of central versus peripheral obesity in total knee arthroplasty. Knee Surg Relat Res 30(4): 319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cancienne JM, Werner BC, Browne JA. 2017. Is there an association between hemoglobin A1c and deep postoperative infection after TKA? Clin Orthop Relat Res 475(6): 1642–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shohat N, Muhsen K, Gilat R et al. 2018. Inadequate glycemic control is associated with increased surgical site infection in total joint arthroplasty: A systematic review and meta-analysis. J Arthroplasty 33(7): 2312–2321. [DOI] [PubMed] [Google Scholar]
- 31.Tarabichi M, Shohat N, Kheir MM et al. 2017. Determining the threshold for HbA1c as a predictor for adverse outcomes after total joint arthroplasty: A multicenter, retrospective study. J Arthroplasty 32(9S): S263–S267. [DOI] [PubMed] [Google Scholar]
- 32.Chrastil J, Anderson MB, Stevens V et al. 2015. Is hemoglobin A1c or perioperative hyperglycemia predictive of periprosthetic joint infection or death following primary total joint arthroplasty? J Arthroplasty 30(7): 1197–1202. [DOI] [PubMed] [Google Scholar]
- 33.Shohat N, Tarabichi M, Tan TL et al. 2019. Fructosamine is a better glycaemic marker compared with glycated haemoglobin (HbA1c) in predicting adverse outcomes following total knee arthroplasty: a prospective multicentre study. Bone Joint J 101-B(7_Supple_C): 3–9. [DOI] [PubMed] [Google Scholar]
- 34.Hart A, Rainer WG, Taunton MJ et al. 2019. Cotinine testing improves smoking cessation before total joint arthroplasty. J Arthroplasty 34(7S): S148–S151. [DOI] [PubMed] [Google Scholar]
- 35.Boylan MR, Bosco JA 3rd, Slover JD. 2019. Cost-effectiveness of preoperative smoking cessation interventions in total joint arthroplasty. J Arthroplasty 34(2): 215–220. [DOI] [PubMed] [Google Scholar]
- 36.Matharu GS, Mouchti S, Twigg S et al. 2019. The effect of smoking on outcomes following primary total hip and knee arthroplasty: a population-based cohort study of 117,024 patients. Acta Orthop 2: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee GC, Ong K, Baykal D et al. 2018. Does prior bariatric surgery affect implant survivorship and complications following primary total hip arthroplasty/total knee arthroplasty? J Arthroplasty 33(7): 2070–2074. [DOI] [PubMed] [Google Scholar]
- 38.Li S, Luo X, Sun H, Wang K et al. 2019. Does prior bariatric surgery improve outcomes following total joint arthroplasty in the morbidly obese? A meta-analysis. J Arthoplasty 34(3): 577–585. [DOI] [PubMed] [Google Scholar]
- 39.Lui M, Jones CA, Westby MD. 2015. Effect of non-surgical, non-pharmacological weight loss interventions in patients who are obese prior to hip and knee arthroplasty surgery: a rapid review. Syst Rev 4: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bohl DD, Shen MR, Kayupov E, Della Valle CJ. 2016. Hypoalbuminemia independently predicts surgical site infection, pneumonia, length of stay, and readmission after total joint arthroplasty. J Arthroplasty 31(1): 15–21. [DOI] [PubMed] [Google Scholar]
- 41.Courtney PM, Rozell JC, Melnic CM et al. 2016. Effect of malnutrition and morbid obesity on complication rates following primary total joint arthroplasty. J Surg Orthop Adv 25(2): 99–104. [PubMed] [Google Scholar]
- 42.Golladay GJ, Satpathy J, Jiranek WA. 2016. Patient optimization-strategies that work: Malnutrition. J Arthroplasty 31(8): 1631–1634. [DOI] [PubMed] [Google Scholar]
- 43.Springer BD, Roberts KM, Bossi KL et al. 2019. What are the implications of withholding total joint arthroplasty in the morbidly obese? A prospective, observational study. Bone Joint J 101-B(7_Supple_C):28–32. [DOI] [PubMed] [Google Scholar]
- 44.Houdek MT, Wagner ER, Watts CD et al. 2015. Morbid obesity: a significant risk factor for failure of two-stage revision total hip arthroplasty for infection. J Bone Joint Surg Am 97(4):326–32. [DOI] [PubMed] [Google Scholar]
- 45.Levine B, Caccavallo P, Springer BD, Meneghini RM. 2018. Preoperative patient preparation for total knee arthroplasty Instr Course Lect. 67: 177–190. [PubMed] [Google Scholar]
- 46.Kim KY, Anoushiravani AA, Chen KK et al. 2019. Perioperative Orthopedic Surgical Home: Optimizing total joint arthroplasty candidates and preventing readmission. J Arthroplasty 34(7S): S91–S96. [DOI] [PubMed] [Google Scholar]
- 47.Smith EB, Wynne R, Joshi A, Liu H, Good RP. 2012. Is it time to include vancomycin for routine perioperative antibiotic prophylaxis in total joint arthroplasty patients? J Arthroplasty 27 (8 Suppl): 55–60. [DOI] [PubMed] [Google Scholar]
- 48.Kheir MM, Tan TL, Azboy I et al. 2017. Vancomycin prophylaxis for total joint arthroplasty: Incorrectly dosed and has a higher rate of periprosthetic infection than cefazolin. Clin Orthop Relat Res 475(7): 1767–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berŕıos-Torres SI, Umscheid CA, Bratzler DW et al. 2017. Healthcare Infection Control Practices Advisory Committee. Centers for Disease Control and Prevention guideline for the prevention of surgical site infection. JAMA Surg 152(8):784–91. [DOI] [PubMed] [Google Scholar]
- 50.Siddiqi A, Forte SA, Docter S et al. 2019. Perioperative Antibiotic Prophylaxis in Total Joint Arthroplasty: A Systematic Review and Meta-Analysis. J Bone Joint Surg Am 101(9):828–842. [DOI] [PubMed] [Google Scholar]
- 51.Gutowski CJ, Zimstowski BM, Clyde CT, Parvizi J. 2014. The economics of using prophylactic antibiotic-loaded bone cement in total knee replacement. Bone Joint J 96-B(1): 65–69. [DOI] [PubMed] [Google Scholar]
- 52.Cohen EM, Marcaccio S, Goodman AD et al. 2019. Efficacy and cost-effectiveness of topical vancomycin powder in primary cementless total hip arthroplasty. Orthopedics 42(5): e430–e436. [DOI] [PubMed] [Google Scholar]
- 53.Johnson JD, Nessler JM, Horazdovsky RD et al. 2017. Serum and wound vancomycin levels after intrawound administration in primary total joint arthroplasty. J Arthroplasty 32(3): 924–928. [DOI] [PubMed] [Google Scholar]
- 54.Qadir R, Ochsner JL, Chimento GF et al. 2014. Establishing a role for vancomycin powder application for prosthetic joint infection prevention-results of a wear simulation study. J Arthroplasty 29(7): 1449–1456. [DOI] [PubMed] [Google Scholar]
- 55.Dial BL, Lampley AJ, Green CL, Hallows R. 2018. Intrawound vancomycin powder in primary total hip arthroplasty increases rate of sterile wound complications. Hip Pelvis 30(1): 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Calkins TE, Culvern C, Nam D et al. 2019. Dilute Betadine Lavage Reduces the Risk of Acute Postoperative Periprosthetic Joint Infection in Aseptic Revision Total Knee and Hip Arthroplasty: A Randomized Controlled Trial. J Arthroplasty September 12 E-pub. [DOI] [PubMed] [Google Scholar]
- 57.Hart A, Hernandez NM, Abdel MP et al. 2019. Povidone-iodine wound lavage to prevent infection after revision total hip and knee arthroplasty: an analysis of 2,884 cases. J Bone Joint Surg Am 101(13): 1151–1159. [DOI] [PubMed] [Google Scholar]
- 58.Hernandez NM, Hart A, Taunton MJ et al. 2019. Use of Povidone-iodine irrigation prior to wound closure in primary total hip and knee arthroplasty: an analysis of 11,738 cases. J Bone Joint Surg Am 101(13): 1144–1150. [DOI] [PubMed] [Google Scholar]
- 59.Frisch NB, Kadri OM, Tenbrunsel T et al. 2017. Intraoperative chlorhexidine irrigation to prevent infection in total hip and knee arthroplasty. Arthroplast Today 3(4): 294–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Inabathula A, Dilley JE, Ziemba-Davis M et al. 2018. Extended oral antibiotic prophylaxis in high-risk patients substantially reduces primary total hip and knee arthroplasty 90-day infection rate. J Bone Joint Surg Am 100(24): 2103–2109. [DOI] [PubMed] [Google Scholar]
- 61.DeFrancesco CJ, Fu MC, Kahlenberg CA et al. 2019. Extended antibiotic prophylaxis may be linked to lower peri-prosthetic joint infection rates in high-risk patients: an evidence-based review. HSS J 15(3): 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gabay C, Kushner I. 1999. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 340:448–454. [DOI] [PubMed] [Google Scholar]
- 63.Berbari E, Mabry T, Tsaras G, et al. 2010. Inflammatory blood laboratory levels as markers of prosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg Am 92:2102–2109. [DOI] [PubMed] [Google Scholar]
- 64.Yuan K, Chen HL, Cui ZM. 2014. Diagnostic accuracy of C-reactive protein for periprosthetic joint infection: a meta-analysis. Surg Infect (Larchmt) 15:548–559. [DOI] [PubMed] [Google Scholar]
- 65.Gollwitzer H, Dombrowski Y, Prodinger PM, et al. 2013. Antimicrobial peptides and proinflammatory cytokines in periprosthetic joint infection. J Bone Joint Surg Am 95:644–651. [DOI] [PubMed] [Google Scholar]
- 66.Shah K, Mohammed A, Patil S, et al. 2009. Circulating cytokines after hip and knee arthroplasty: a preliminary study. Clin Orthop Relat Res 467:946–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Randau TM, Friedrich MJ, Wimmer MD, et al. 2014. Interleukin-6 in serum and in synovial fluid enhances the differentiation between periprosthetic joint infection and aseptic loosening. PLoS One 9:e89045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xie K, Dai K, Qu X, et al. 2017. Serum and Synovial Fluid Interleukin-6 for the Diagnosis of Periprosthetic Joint Infection. Sci Rep 7:1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bottner F, Wegner A, Winkelmann W, et al. 2007. Interleukin-6, procalcitonin and TNF-alpha: markers of peri-prosthetic infection following total joint replacement. J Bone Joint Surg Br 89:94–99. [DOI] [PubMed] [Google Scholar]
- 70.Ettinger M, Calliess T, Kielstein JT, et al. 2015. Circulating biomarkers for discrimination between aseptic joint failure, low-grade infection, and high-grade septic failure. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 61:332–341. [DOI] [PubMed] [Google Scholar]
- 71.Vijayan AL, Vanimaya, Ravindran S, et al. 2017. Procalcitonin: a promising diagnostic marker for sepsis and antibiotic therapy. J Intensive Care 5:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xie K, Qu X, Yan M. 2017. Procalcitonin and alpha-Defensin for Diagnosis of Periprosthetic Joint Infections. J Arthroplasty 32:1387–1394. [DOI] [PubMed] [Google Scholar]
- 73.Yoon JR, Yang SH, Shin YS. 2018. Diagnostic accuracy of interleukin-6 and procalcitonin in patients with periprosthetic joint infection: a systematic review and meta-analysis. Int Orthop 42:1213–1226. [DOI] [PubMed] [Google Scholar]
- 74.Ricciardi BF, Muthukrishnan G, Masters E, et al. 2018. Staphylococcus aureus Evasion of Host Immunity in the Setting of Prosthetic Joint Infection: Biofilm and Beyond. Curr Rev Musculoskelet Med 11(3):389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Masters EA, Trombetta RP, de Mesy Bentley KL, et al. 2019. Evolving concepts in bone infection: redefining “biofilm”, “acute vs. chronic osteomyelitis”, “the immune proteome” and “local antibiotic therapy”. Bone Res 7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nishitani K, Beck CA, Rosenberg AF, et al. 2015. A Diagnostic Serum Antibody Test for Patients With Staphylococcus aureus Osteomyelitis. Clin Orthop Relat Res 473:2735–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oh I, Muthukrishnan G, Ninomiya MJ, et al. 2018. Tracking Anti-Staphylococcus aureus Antibodies Produced In Vivo and Ex Vivo during Foot Salvage Therapy for Diabetic Foot Infections Reveals Prognostic Insights and Evidence of Diversified Humoral Immunity. Infection and immunity 86(12): pii: e00629–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wood JB, Jones LS, Soper NR, et al. 2019. Serologic Detection of Antibodies Targeting the Leukocidin LukAB Strongly Predicts Staphylococcus aureus in Children With Invasive Infection. J Pediatric Infect Dis Soc 8:128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lehrer RI, and Lu W. 2012. alpha-Defensins in human innate immunity. Immunol Rev 245(1):84–112. [DOI] [PubMed] [Google Scholar]
- 80.Bonanzinga T, Ferrari MC, Tanzi G et al. 2019. The role of alpha defensin in prosthetic joint infection (PJI) diagnosis: a literature review. EFORT Open Rev 4(1):10–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Vecchi E, Romano CL, De Grandi R et al. 2018. Alpha defensin, leukocyte esterase, C-reactive protein, and leukocyte count in synovial fluid for pre-operative diagnosis of periprosthetic infection. Int J Immunopathol Pharmacol 32(2058738418806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Deirmengian C, Kardos K, Kilmartin P et al. 2014. Combined measurement of synovial fluid alpha-Defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am 96(17):1439–45. [DOI] [PubMed] [Google Scholar]
- 83.Deirmengian C, Kardos K, Kilmartin P et al. 2014. Diagnosing periprosthetic joint infection: has the era of the biomarker arrived? Clin Orthop Relat Res 472(11):3254–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Deirmengian C, Kardos K, Kilmartin P et al. 2015. The Alpha-defensin Test for Periprosthetic Joint Infection Responds to a Wide Spectrum of Organisms. Clin Orthop Relat Res 473(7):2229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Han X, Xie K, Jiang X et al. 2019. Synovial fluid alpha-defensin in the diagnosis of periprosthetic joint infection: the lateral flow test is an effective intraoperative detection method. J Orthop Surg Res 14(1):274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vergara A, Fernández-Pittol MJ, Muñoz-Mahamud E et al. 2019. Evaluation of Lipocalin-2 as a Biomarker of Periprosthetic Joint Infection. J Arthroplasty;34(1):123–125. [DOI] [PubMed] [Google Scholar]
- 87.Salari P, Grassi M, Cinti B et al. 2019. Synovial Fluid Calprotectin for the Preoperative Diagnosis of Chronic Periprosthetic Joint Infection. J Arthroplasty Epub. [DOI] [PubMed] [Google Scholar]
- 88.Kheir MM, Tan TL, Shohat N et al. 2018. Routine Diagnostic Tests for Periprosthetic Joint Infection Demonstrate a High False-Negative Rate and Are Influenced by the Infecting Organism. J Bone Joint Surg Am 100(23):2057–65. [DOI] [PubMed] [Google Scholar]
- 89.Vaishya R, Sardana R, Butta H, and Mendiratta L. 2019. Laboratory diagnosis of Prosthetic Joint Infections: Current concepts and present status. J Clin Orthop Trauma 10(3):560–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Everhart JS, DiBartola AC, Dusane DH et al. 2018. Bacterial Deoxyribonucleic Acid Is Often Present in Failed Revision Anterior Cruciate Ligament Reconstructions. Arthroscopy 34(11):3046–52. [DOI] [PubMed] [Google Scholar]
- 91.Li M, Zeng Y, Wu Y, Si H et al. 2019. Performance of Sequencing Assays in Diagnosis of Prosthetic Joint Infection: A Systematic Review and Meta-Analysis. J Arthroplasty 34(7):1514–22 e4. [DOI] [PubMed] [Google Scholar]
- 92.Namdari S, Nicholson T, Abboud J. 2019. Comparative study of cultures and next-generation sequencing in the diagnosis of shoulder prosthetic joint infections. J Shoulder Elbow Surg 28(1):1–8. [DOI] [PubMed] [Google Scholar]
- 93.Vasoo S 2018. Improving the diagnosis of orthopedic implant-associated infections: optimizing the use of tools already in the box. J of clin microbiol 56: e01379–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tande AJ and Patel R. Prosthetic Joint Infection. 2014. Clin Microbiol Rev 27: 302–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Flemming H-C and Wingender J. 2010. The biofilm matrix. Nat Rev Microbiol 8: 623–33. [DOI] [PubMed] [Google Scholar]
- 96.Stoodley P, Nistico L, Johnson S, et al. 2008. Direct demonstration of viable Staphylococcus aureus biofilms in an infected total joint arthroplasty: a case report. J Bone and Joint Surg Am 90: 1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Erivan R, Villatte G, Eymond G et al. 2018. Usefulness of sonication for diagnosing infection in explanted orthopaedic implants. Orthopaedics & Traumatology: Surgery & Research 104: 433–8. [DOI] [PubMed] [Google Scholar]
- 98.Trampuz A, Piper KE, Jacobson MJ, et al. 2007. Sonication of removed hip and knee prostheses for diagnosis of infection. N Engl J of Med 357: 654–63. [DOI] [PubMed] [Google Scholar]
- 99.Dapunt U, Lehner B, Burckhardt I et al. 2014. Evaluation of implant sonication as a diagnostic tool in implant-associated infections. Journal of applied biomaterials & functional materials 12: 135–40. [DOI] [PubMed] [Google Scholar]
- 100.Portillo ME, Salvadó M, Trampuz A, et al. 2015. Improved diagnosis of orthopedic implant-associated infection by inoculation of sonication fluid into blood culture bottles. J Clin Microbiol 53: 1622–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dudareva M, Barrett L, Figtree M, et al. 2018. Sonication versus tissue sampling for diagnosis of prosthetic joint and other orthopedic device-related infections. J Clin Microbiol 56: e00688–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Van Diek FM, Albers CG, Van Hooff ML et al. 2017. Low sensitivity of implant sonication when screening for infection in revision surgery. Acta orthop 88: 294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maniar HH, Wingert N, McPhillips K, et al. 2016. Role of sonication for detection of infection in explanted orthopaedic trauma implants. J Orthop Trauma 30: e175–e80. [DOI] [PubMed] [Google Scholar]
- 104.Hischebeth GT, Gravius S, Buhr JK, et al. 2017. Novel diagnostics in revision arthroplasty: implant sonication and multiplex polymerase chain reaction. JoVE (Journal of Visualized Experiments). e55147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Achermann Y, Vogt M, Leunig M et al. 2010. Improved diagnosis of periprosthetic joint infection by multiplex PCR of sonication fluid from removed implants. J Clin Microbiol 48: 1208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bjerke-Kroll BT, Christ AB, McLawhorn AS et al. 2014. Periprosthetic joint infections treated with two-stage revision over 14 years: an evolving microbiology profile. J Arthroplasty 29(5):877–82. [DOI] [PubMed] [Google Scholar]
- 107.de Mesy Bentley KL, Trombetta R, Nishitani K et al. 2017. Evidence of Staphylococcus Aureus Deformation, Proliferation, and Migration in Canaliculi of Live Cortical Bone in Murine Models of Osteomyelitis. J Bone Miner Res 32(5):985–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.de Mesy Bentley KL, MacDonald A, Schwarz EM, Oh I. 2018. Chronic Osteomyelitis with Staphylococcus aureus Deformation in Submicron Canaliculi of Osteocytes: A Case Report. JBJS Case Connect 8(1):e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rosteius T, Jansen O, Fehmer T et al. 2018. Evaluating the microbial pattern of periprosthetic joint infections of the hip and knee. J Med Microbiol 67(11):1608–1613. [DOI] [PubMed] [Google Scholar]
- 110.Siljander MP, Sobh AH, Baker KC et al. 2018. Multidrug-Resistant Organisms in the Setting of Periprosthetic Joint Infection-Diagnosis, Prevention, and Treatment. J Arthroplasty 33(1):185–194. [DOI] [PubMed] [Google Scholar]
- 111.Chang YJ, Lee MS, Lee CH et al. 2017. Daptomycin treatment in patients with resistant staphylococcal periprosthetic joint infection. BMC Infect Dis 17(1):736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Byren I, Rege S, Campanaro E et al. 2012. Randomized controlled trial of the safety and efficacy of Daptomycin versus standard-of-care therapy for management of patients with osteomyelitis associated with prosthetic devices undergoing two-stage revision arthroplasty. Antimicrob Agents Chemother 56(11):5626–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Deroche L, Plouzeau C, Bémer P et al. 2019. Probabilistic chemotherapy in knee and hip replacement infection: the place of linezolid. Eur J Clin Microbiol Infect Dis 38(9):1659–1663. [DOI] [PubMed] [Google Scholar]
- 114.Mandell JB, Orr S, Koch J et al. 2019. Large variations in clinical antibiotic activity against Staphylococcus aureus biofilms of periprosthetic joint infection isolates. J Orthop Res 37(7):1604–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zimmerli W, Widmer AF, Blatter M et al. 1998. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA 279(19):1537–41. [DOI] [PubMed] [Google Scholar]
- 116.Moley JP, McGrath MS, Granger JF et al. 2018. Reduction in Pseudomonas aeruginosa and Staphylococcus aureus biofilms from implant materials in a diffusion dominated environment. J Orthop Res 36(11):3081–3085. [DOI] [PubMed] [Google Scholar]
- 117.van Vugt TAG, Arts JJ, Geurts JAP. 2019. Antibiotic-Loaded Polymethylmethacrylate Beads and Spacers in Treatment of Orthopedic Infections and the Role of Biofilm Formation. Front Microbiol 10:1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wouthuyzen-Bakker M, Löwik CAM, Knobben BAS et al. 2018. Use of gentamicin-impregnated beads or sponges in the treatment of early acute periprosthetic joint infection: a propensity score analysis. J Antimicrob Chemother 73(12):3454–3459. [DOI] [PubMed] [Google Scholar]
- 119.Flierl MA, Culp BM, Okroj KT et al. 2017. Poor outcomes of irrigation and debridement in acute periprosthetic joint infection with antibiotic-impregnated calcium sulfate beads. J Arthroplasty 32(8): 2505–2507. [DOI] [PubMed] [Google Scholar]
- 120.Whiteside LA, Roy ME. 2017. One-stage Revision With Catheter Infusion of Intraarticular Antibiotics Successfully Treats Infected THA. Clin Orthop Relat Res 475(2):419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bhattacharya M, Wozniak DJ, Stoodley P, Hall-Stoodley L.2015. Prevention and treatment of Staphylococcus aureus biofilms. Expert Rev Anti Infect Ther 13(12):1499–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fowler VG, Allen KB, Moreira ED et al. 2013. Effect of an investigational vaccine for preventing Staphylococcus aureus infections after cardiothoracic surgery: a randomized trial. JAMA 309(13):1368–78. [DOI] [PubMed] [Google Scholar]
- 123.McNeely TB, Shah NA, Fridman A et al. 2014. Mortality among recipients of the Merck V710 Staphylococcus aureusvaccine after postoperative S. aureus infections: an analysis of possible contributing host factors. Hum Vaccin Immunother 10(12):3513–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shinefield H, Black S, Fattom A et al. 2002. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med 346(7):491–6. [DOI] [PubMed] [Google Scholar]
- 125.Dupont CD, Scully IL, Zimnisky RM et al. 2018. Two Vaccines for Staphylococcus aureus Induce a B-Cell-Mediated Immune Response. mSphere 3(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xu C, Zhang BZ, Lin Q et al. 2018. Live attenuated Salmonella typhimurium vaccines delivering SaEsxA and SaEsxB via type III secretion system confer protection against Staphylococcus aureus infection. BMC Infect Dis 18(1):195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Landrum ML, Lalani T, Niknian M et al. 2017. Safety and immunogenicity of a recombinant Staphylococcus aureus α-toxoid and a recombinant Panton-Valentine leukocidin subunit, in healthy adults. Hum Vaccin Immunother 13(4):791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yang Y, Yu R, Yang X et al. 2017. Protection against Staphylococcus aureus and tetanus infections by a combined vaccine containing SasA and TeNT‑Hc in mice. Mol Med Rep 15(4):2369–2373. [DOI] [PubMed] [Google Scholar]
- 129.Varshney AK, Kuzmicheva GA, Lin J et al. 2018. A natural human monoclonal antibody targeting Staphylococcus Protein A protects against Staphylococcus aureus bacteremia. PLoS One 13(1):e0190537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Estellés A, Woischnig AK, Liu K et al. 2016. A High-Affinity Native Human Antibody Disrupts Biofilm from Staphylococcus aureus Bacteria and Potentiates Antibiotic Efficacy in a Mouse Implant Infection Model. Antimicrob Agents Chemother 60(4):2292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Peck M, Rothenberg ME, Deng R et al. 2019. A Phase 1, Randomized, Single-Ascending-Dose Study To Investigate the Safety, Tolerability, and Pharmacokinetics of DSTA4637S, an Anti-Staphylococcus aureus Thiomab Antibody-Antibiotic Conjugate, in Healthy Volunteers. Antimicrob Agents Chemother 63(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Akanda ZZ, Taha M, Abdelbary H. 2018. Current review-The rise of bacteriophage as a unique therapeutic platform in treating peri-prosthetic joint infections. J Orthop Res 36(4):1051–1060. [DOI] [PubMed] [Google Scholar]
- 133.Doolittle MM, Cooney JJ, Caldwell DE. 1996. Tracing the interaction of bacteriophage with bacterial biofilms using fluorescent and chromogenic probes. J Ind Microbiol 16:331–341. [DOI] [PubMed] [Google Scholar]
- 134.Yilmaz C, Colak M, Yilmaz BC, et al. 2013. Phage therapy in implant-related infections: an experimental study. J Bone Joint Surg Am 95:117–125. [DOI] [PubMed] [Google Scholar]
- 135.Rhoads DD, Wolcott RD, Kuskowski MA, et al. 2009. Bacteriophage therapy of venous leg ulcers in humans: results of a phase I safety trial. J Wound Care 238:240–243. [DOI] [PubMed] [Google Scholar]
- 136.Wright A, Hawkins CH, Anggard EE, et al. 2009. A controlled clinical trial of a therapeutic phage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clin Otolaryngol 34:349–357. [DOI] [PubMed] [Google Scholar]
- 137.Siddiqi KS, Husen A, Rao RAK. 2018. A review on biosynthesis of silver nanoparticles and their biocidal properties. J Nanobiotechnology 16(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hardes J, von Eiff C, Streitbuerger A et al. 2010. Reduction of periprosthetic infection with silver-coated megaprostheses in patients with bone sarcoma. J Surg Oncol 101(5):389–95. [DOI] [PubMed] [Google Scholar]
- 139.Wafa H, Grimer RJ, Reddy K et al. 2015. Retrospective evaluation of the incidence of early periprosthetic infection with silver-treated endoprostheses in high-risk patients: case-control study. Bone Joint J 97-B(2):252–7. [DOI] [PubMed] [Google Scholar]
- 140.Alt V 2017. Antimicrobial coated implants in trauma and orthopaedics-A clinical review and risk-benefit analysis. Injury 48(3):599–607. [DOI] [PubMed] [Google Scholar]
- 141.van Hengel IAJ, Riool M, Fratila-Apachitei LE et al. 2017. Selective laser melting porous metallic implants with immobilized silver nanoparticles kill and prevent biofilm formation by methicillin-resistant Staphylococcus aureus. Biomaterials 140:1–15. [DOI] [PubMed] [Google Scholar]
- 142.Chowdhury N, Wood TL, Martínez-Vázquez M et al. 2016. Biotechnol Bioeng. 113(9):1984–1992. [DOI] [PubMed] [Google Scholar]
- 143.Kucharíková S, Gerits E, De Brucker K et al. 2016. Covalent immobilization of antimicrobial agents on titanium prevents Staphylococcus aureus and Candida albicans colonization and biofilm formation. J Antimicrob Chemother 71(4):936–45. [DOI] [PubMed] [Google Scholar]
- 144.Diefenbeck M, Schrader C, Gras F et al. 2016. Gentamicin coating of plasma chemical oxidized titanium alloy prevents implant-related osteomyelitis in rats. Biomaterials 101:156–64. [DOI] [PubMed] [Google Scholar]
- 145.Drago L, Boot W, Dimas K et al. 2014. Does implant coating with antibacterial-loaded hydrogel reduce bacterial colonization and biofilm formation in vitro? Clin Orthop Relat Res 472(11):3311–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Giavaresi G, Meani E, Sartori M et al. 2014. Efficacy of antibacterial-loaded coating in an in vivo model of acutely highly contaminated implant. Int Orthop 38(7):1505–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jennings JA, Carpenter DP, Troxel KS et al. 2015. Novel Antibiotic-loaded Point-of-care Implant Coating Inhibits Biofilm. Clin Orthop Relat Res 473(7):2270–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hogan S, Zapotoczna M, Stevens NT et al. 2017. Potential use of targeted enzymatic agents in the treatment of Staphylococcus aureus biofilm-related infections. J Hosp Infect 96(2):177–182. [DOI] [PubMed] [Google Scholar]
- 149.Hogan S, O’Gara JP, O’Neill E. 2018. Novel Treatment of Staphylococcus aureus Device-Related Infections Using Fibrinolytic Agents. Antimicrob Agents Chemother 62(2). pii: e02008–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jørgensen NP, Zobek N, Dreier C et al. Streptokinase Treatment Reverses Biofilm-Associated Antibiotic Resistance in Staphylococcus aureus. Microorganisms 4(3). pii: E36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lauderdale KJ, Malone CL, Boles BR et al. 2010. Biofilm dispersal of community-associated methicillin-resistant Staphylococcus aureus on orthopedic implant material. J Orthop Res 28(1):55–61. [DOI] [PubMed] [Google Scholar]
- 152.Giacometti A, Cirioni O, Gov Y et al. 2003. RNA III inhibiting peptide inhibits in vivo biofilm formation by drug-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 47(6):1979–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kirui DK, Weber G, Talackine J, Millenbaugh NJ. 2019. Targeted laser therapy synergistically enhances efficacy of antibiotics against multi-drug resistant Staphylococcus aureus and Pseudomonas aeruginosa biofilms. Nanomedicine 20:102018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.