Table 1.
Optimization of the Suzuki–Miyaura Reaction between 2-Naphthyl tert-Butylcarbonate and 4-Methoxyphenylboronic Acid Using LNiII (o-tol)(Cl) (L = dppf, dippf, dcypf) as Precatalystsa
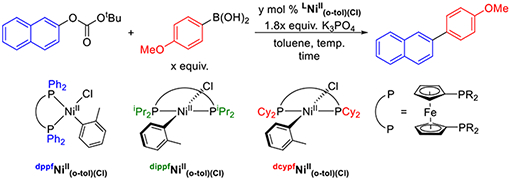 | |||||||
|---|---|---|---|---|---|---|---|
| entry | ligand | temp (°C) | time (h) | boronic acid (equiv) | catalyst loading(mol %) | water (equiv) | yield (%) |
| 1 | dppf | 80 | 24 | 2.5 | 2.5 | 0 | 5 |
| 2 | dippf | 80 | 24 | 2.5 | 2.5 | 0 | 61 |
| 3 | dcypf | 80 | 24 | 2.5 | 2.5 | 0 | >99 |
| 4 | dcypf | 80 | 4 | 2.5 | 2.5 | 0 | >99 |
| 5 | dcypf | 80 | 2 | 2.5 | 2.5 | 0 | 90 |
| 6 | dcypf | 60 | 4 | 2.5 | 2.5 | 0 | 92 |
| 7 | dcypf | 40 | 4 | 2.5 | 2.5 | 0 | 31 |
| 8 | dcypf | 60 | 4 | 1 | 2.5 | 0 | 64 |
| 9 | dcypf | 60 | 4 | 1.5 | 2.5 | 0 | 74 |
| 10 | dcypf | 60 | 4 | 2 | 2.5 | 0 | 87 |
| 11 | dcypf | 60 | 4 | 2.5 | 0.5 | 0 | 31b |
| 12 | dcypf | 60 | 4 | 2.5 | 1 | 0 | 57b |
| 13 | dcypf | 60 | 4 | 2.5 | 2 | 0 | 74b |
| 14 | dcypf | 60 | 4 | 2.5 | 2.5 | 50 | 91 |
| 15 | dcypf | 60 | 4 | 2.5 | 1.0 | 50 | 59 |
Reaction conditions: 2-naphthyl tert-butylcarbonate (0.133 mmol), 4-methoxyphenylboronic acid (0.133 mmol, 1 equiv; 0.200 mmol, 1.5 equiv; 0.266 mmol, 2 equiv; 0.333 mmol, 2.5 equiv), K3PO4 (1.8 equiv relative to boronic acid), naphthalene internal standard (0.0665 mmol, 0.5 equiv), toluene (1 mL). Yields were determined by GC and are reported as the average of at least two trials.
The reaction went to completion after 12 h of reaction time; however, the reactions at 0.5 and 1.0 mol % did not reach completion even after 24 h.
