Abstract
Objective:
To identify whether a high Pao2 (hyperoxemia) at the time of presentation to the PICU is associated with in-hospital mortality.
Design:
Single-center observational study.
Setting:
Quaternary-care PICU.
Patients:
Encounters admitted between January 1, 2009, and December 31, 2018.
Interventions:
None.
Measurements and Main Results:
Encounters with a measured Pao2 were included. To account for severity of illness upon presentation, we calculated a modified Pediatric Risk of Mortality IV score excluding Pao2 for each encounter, calibrated for institutional data. Logistic regression was used to determine whether hyperoxemia (Pao2 ≥ 300 torr [39.99 kPa]) in the 12 hours surrounding PICU admission was associated with in-hospital mortality. We reperformed our analysis using a cutoff for hyperoxemia obtained by comparisons of observed versus predicted mortality when encounters were classified by highest Pao2 in 50 torr (6.67 kPa) bins. Results are reported as adjusted odds ratios with 95% CIs. Of 23,719 encounters, 4,093 had a Pao2 recorded in the period −6 to +6 hours after admission. Two hundred seventy-four of 4,093 (6.7%) had in-hospital mortality. The prevalence of hyperoxemia increased with rising modified Pediatric Risk of Mortality IV and was not associated with mortality in multivariable models (adjusted odds ratio, 1.38; 95% CI, 0.98–1.93). When using a higher cutoff of hyperoxemia derived from comparison of observed versus predicted rates of mortality of greater than or equal to 550 torr (73.32 kPa), hyperoxemia was associated with mortality (adjusted odds ratio, 2.78; 95% CI, 2.54–3.05).
Conclusions:
A conventional threshold for hyperoxemia at presentation to the PICU was not associated with in-hospital mortality in a model using a calibrated acuity score. Extreme states of hyperoxemia (≥ 73.32 kPa) were significantly associated with in-hospital mortality. Prospective research is required to identify if hyperoxemia before and/or after PICU admission contributes to poor outcomes.
Keywords: hyperoxemia, hyperoxia, oxygen toxicity, Pediatric Risk of Mortality, pediatric
The deleterious effects of supratherapeutic oxygen (hyperoxemia) have been documented in both neonates and adults (1, 2). In children, some studies have reported an association between hyperoxemia and mortality (3–5), whereas others have not identified this finding (6–8). At least one study has suggested that hyperoxemia may be beneficial (9).
Previous studies evaluating the association between presenting hyperoxemia and mortality among critically ill children performed adjustment with uncalibrated measures of illness severity (4, 8). Such severity of illness requires recalibration before application to single-center data (10, 11). Our objective was to identify whether early hyperoxemia among patients admitted to a PICU is associated with mortality. We hypothesized that early hyperoxemia is associated with mortality after adjusting for illness severity.
METHODS
We performed an observational study of all PICU encounters (unique hospitalizations) admitted between January 1, 2009, and December 31, 2018. Patients admitted to the cardiac or neonatal ICUs were not included. Data were extracted from a clinical data warehouse using the business intelligence platform SAP BusinessObjects (SAP, Walldorf, Germany). This study was approved by a local Institutional Review Board.
We performed risk adjustment for early hyperoxemia using an electronic Pediatric Risk of Mortality IV score, modified by excluding Pao2 values (modified Pediatric Risk of Mortality IV [m-PRISM-IV]), calculated using data from −2 to +4 hours surrounding PICU admission, per previously published methods (12, 13). Calibration was performed by randomly selecting 75% of all encounters as a development cohort and assessing model performance among the remaining 25%. We assessed predictive validity of m-PRISM-IV by examining the C-statistic and examined calibration using the Hosmer-Lemeshow goodness-of-fit test, defining inadequate fit as p value less than 0.05.
Encounters with a measured Pao2 during the period −6 to +6 hours after PICU admission were included. We used a threshold for hyperoxemia of 300 torr (≥ 40.0 kPa) associated with poor outcomes in previous studies (14, 15). Our exposure of interest was highest Pao2 for each encounter during early admission, classified as normoxemia, hypoxemia (Pao2 < 60 mm Hg [8.0 kPa]), and hyperoxemia. For a secondary analysis, we determined a hyperoxemia cutoff by plotting rates of observed versus predicted mortality for encounters with highest Pao2 classified into 50 torr (6.7 kPa) bins and identifying where observed exceeded the predicted mortality. Chart review was performed for patients with a presenting Pao2 greater than or equal to 550 torr (73.3 kPa) to determine if extracorporeal life support (ECLS) was present at the time of admission.
We performed univariable logistic regression to identify associations between m-PRISM-IV and oxygen category with mortality. A multivariable model was constructed incorporating variables with univariable p value of less than 0.1. Results are reported as odds ratios and adjusted odds ratios with 95% CIs. Analyses were conducted using R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Of 23,719 encounters during the inclusion period, 491 (2.1%) ended with in-hospital mortality. There were 4,093 encounters (17.3%; 55.3% male; mean age, 8.7 ± sd, 6.7 yr) with at least one Pao2 measurement during the period from −6 to +6 hours after admission, of which 274 of 4,093 (6.7%) resulted in inhospital mortality. Using a cutoff of 300 torr (40.0 kPa) for hyperoxemia, in-hospital mortality occurred in 141 of 2,721 (4.9%) with normoxemia, 22 of 203 (9.8%) with hypoxemia, and 111 of 895 (11.0%) with hyperoxemia.
The m-PRISM-IV C-statistic was 0.86 (95% CI, 0.84–0.88) for the derivation cohort (n = 17,816) without acceptable calibration (Hosmer-Lemeshow goodness-of-fit test p < 0.001). After recalibration, the C-statistic was 0.85 (95% CI, 0.81–0.89) in the test cohort (n = 5,939) and demonstrated acceptable calibration (Hosmer-Lemeshow p = 0.362).
In univariable analysis, hyperoxemia and hypoxemia were associated with mortality. After adjustment with m-PRISM-IV, only hypoxemia was associated with in-hospital mortality (Table 1). Stratifying the cohort by maximum Pao2 demonstrated that observed mortality surpassed predicted mortality at multiple points above the 300 torr (40.0 kPa) range, but most substantially in the Pao2 greater than or equal to 550 torr (73.3 kPa) bin (Fig. 1). Using this cutoff, mortality was observed in 220 of 3,755 encounters (5.9%) with normoxemia, 22 of 225 (9.8%) with hypoxemia, and 32 of 113 (28.3%) with hyperoxemia. An association was observed between hyperoxemia greater than or equal to 550 torr (73.3 kPa) and in-hospital mortality in adjusted models. Two patients with extreme hyperoxemia were on ECLS at the time of admission.
TABLE 1.
Association of the Maximum Pao2 During Hospitalization and In-Hospital Mortality Before and After Adjustment With the Modified Pediatric Risk of Mortality IV Score in (A) Model Using a Pao2 Greater Than or Equal to 39.99 kPa Cutoff for Hyperoxemia and (B) Model Using a Pao2 Greater Than or Equal to 73.32 kPa Cutoff for Hyperoxemia
| Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| Model (A): Pao2 ≥ 39.99 kPa (300 torr) Cutoff for Hyperoxemia | ||||
| Variable | OR (95% CI) | p | aOR (95% CI) | p |
| Maximum Pao2 | ||||
| Normoxemia | Reference | Reference | ||
| Hypoxemia (< 7.99 kPa [60 torr]) | 2.09 (1.31–3.35) | 0.002 | 2.11 (1.09–4.10) | 0.027 |
| Hyperoxemia (≥ 39.99 kPa [300 torr]) | 2.39 (1.85–3.10) | < 0.001 | 1.38 (0.98–1.93) | 0.063 |
| m-PRISM-IV score | 2.81 (2.56–3.08) | < 0.001 | 2.79 (2.54–3.06) | < 0.001 |
| Model (B): Pao2 ≥ 73.32 kPa (550 torr) Cutoff for Hyperoxemia | ||||
| OR (95% CI) | p | aOR (95% CI) | p | |
| Maximum Pao2 | ||||
| Normoxemia | Reference | Reference | ||
| Hypoxemia (< 7.99 kPa [60 torr]) | 1.74 (1.10–2.76) | 0.018 | 2.00 (1.04–3.83) | 0.037 |
| Hyperoxemia (≥ 73.32 kPa [550 torr]) | 6.34 (4.12–9.77) | < 0.001 | 2.44 (1.32–4.50) | 0.004 |
| m-PRISM-IV score | 2.81 (2.56–3.08) | < 0.001 | 2.78 (2.54–3.05) | < 0.001 |
aOR = adjusted odds ratio, m-PRISM-IV = modified Pediatric Risk of Mortality IV, OR = odds ratio.
Figure 1.
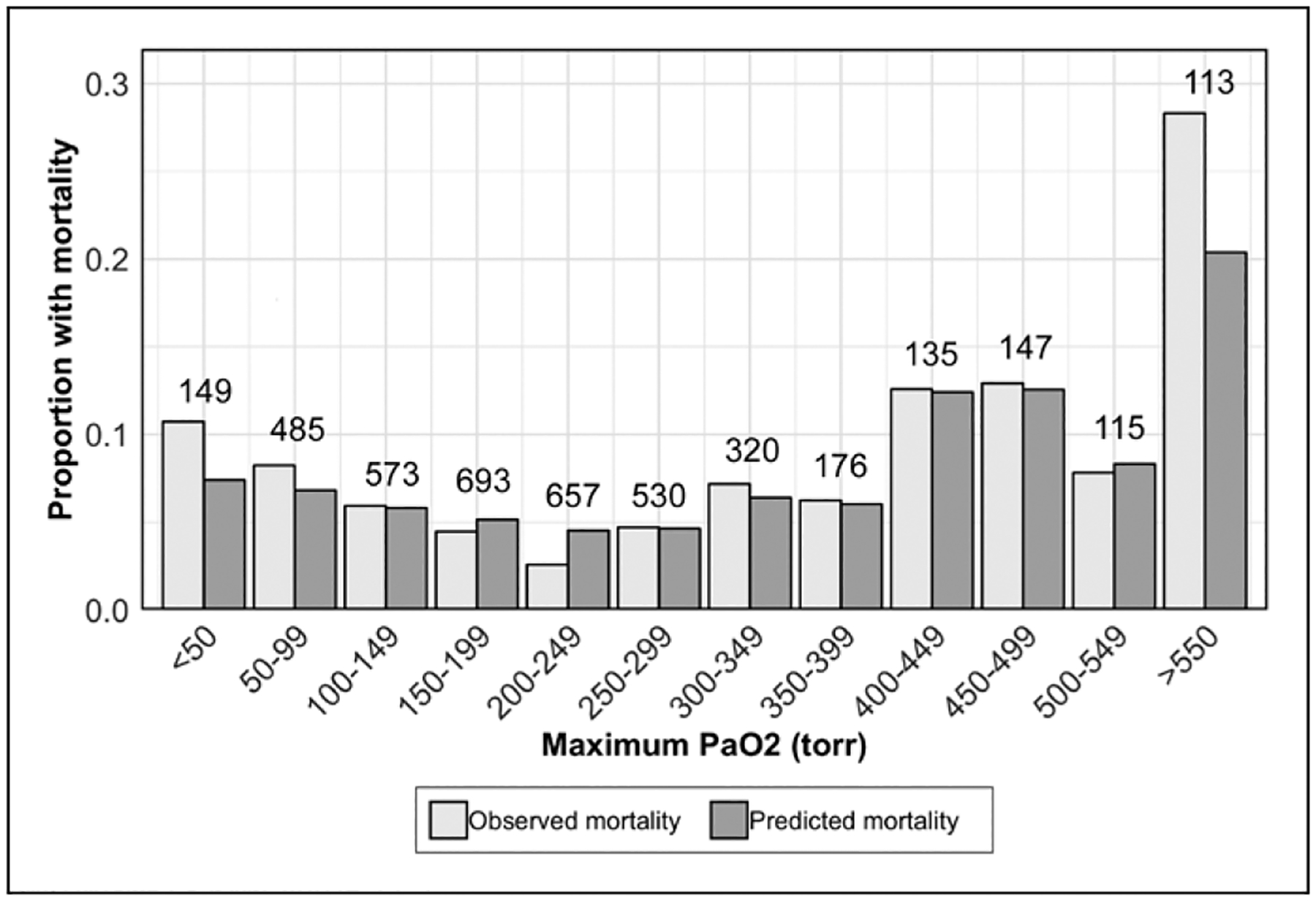
Clustered bar chart of proportions of observed and predicted (using modified Pediatric Risk of Mortality IV) mortality for included patients grouped by maximum Pao2 during early PICU hospitalization (−6 to +6 hr). The number of encounters in each Pao2 bin is displayed above the bars.
DISCUSSION
In this evaluation of patients admitted to a PICU, we found that early hyperoxemia, defined using a conventional cutoff of greater than or equal to 300 torr (40.0 kPa), was not associated with mortality in models adjusted using a calibrated measure of illness severity, although encounters meeting an extreme definition of hyperoxemia (Pao2 ≥ 550 torr [73.3 kPa]) had increased odds of mortality. In an evaluation of 7,410 patients, Raman et al (8) identified a U-shaped association with admission Pao2 and mortality in models risk adjusted using a modified Pediatric Index of Mortality (PIM) 2 score after removal of Fio2/Pao2 (F:P) ratio. Risk of mortality was observed to rise below a Pao2 less than 188 torr (25 kPa) or greater than 300 torr (40.0 kPa) (8). The rise in mortality in the patients with greatest Pao2 did not significantly exceed the nadir in mortality which was present in the Pao2 188–300 torr (25.1–40.0 kPa) group. In another investigation with 1,447 patients, Numa et al (4) identified an association with rising mortality in a model incorporating an unmodified PIM-3 score. Notably, the PIM-3 score includes the F:P ratio, with the net effect that patients with higher Pao2 would be expected to have lower PIM-3 (expected mortality) (16). Failure to remove this F:P ratio makes it more likely that predicted mortality by PIM-3 (and PIM-2) would be lowered in the hyperoxemic patients, perhaps exaggerating the standardized mortality ratio within these patients. PRISM-IV, which we used, incorporates Pao2 within the nonneurologic variable subscore, but this score only increases in the presence of hypoxemia.
Although this study did not identify an association between hyperoxemia and mortality using a commonly used threshold, this investigation is notable for identifying that hyperoxemia was associated with higher m-PRISM-IV scores and corresponding mortality risk. The implication is that the presence of hyperoxemia associates with a number of other non–oxygen-based predictors of outcome captured by the m-PRISM-IV score. This would be more likely to be noted as a result of a confounding association rather than a cause and effect relationship, since the other components of PRISM are not known to be impacted by blood oxygen. Our finding of an increased mortality associated with hyperoxemia at very high oxygen tensions (Pao2 > 550 torr) implies that perhaps in these extreme situations, oxygen may have a more profound, independent relationship with mortality. On the basis of these findings, continued attention is warranted by clinicians to avoid unnecessary extreme hyperoxemia, particularly as initial stabilization is achieved following resuscitation.
Importantly, the lack of a calibrated acuity measure may have confounded previous studies. Severity of illness measurements are frequently used to control for patient acuity for research purposes, but these are developed using multicenter data and require recalibration before application in a single institution (10, 11). Our use of a calibrated risk-adjusted model allowed for better model fitting to institutional data and superior calibration while avoiding overfitting.
This study is limited by its retrospective design. The association between extremely high Pao2 and mortality may be due to confounding by indication. The timing and frequency of Pao2 values obtained during admission were determined by the treating clinical team. As structured data related to the initiation or continuation of ECLS early during a PICU encounter were unreliable at the study center across the 10-year study period, we were unable to adjust for the presence of ECLS, although chart review identified only two patients with extreme hyperoxemia who were on ECLS at the time of admission. An association was observed between extreme states of hyperoxemia and mortality, although causal inference will require prospective evaluation.
CONCLUSIONS
We did not identify an association between early hyperoxemia and in-hospital mortality using a conventional Pao2 threshold and after adjusting with a calibrated measure of illness severity;extreme levels of hyperoxemia or hypoxemia in the 12 hours surrounding PICU admission appear to be independently associated with death. Future work should examine factors associated with extreme hyperoxemia and evaluate optimal approaches to assess for hyperoxemia in the absence of arterial blood gas data. As oxygen overexposure may represent an iatrogenic source of injury and therefore a modifiable risk factor, this is an issue that merits evaluation in critically ill children.
ACKNOWLEDGMENTS
We acknowledge Ms. Sajel Kantawala for her assistance with data queries.
Supported, in part, by the Children’s Hospital of Pittsburgh Trust Young Investigator Award (to Dr. Horvat).
Dr. Dezfulian’s institution received funding from Mallinckrodt Pharmaceuticals, Investigator Initiated Award “inhaled nitric oxide (iNO) after outof-hospital cardiac arrest (OHCA)” (trial principal investigator), and he received support for article research from the National Institutes of Health. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Chu DK, Kim LH, Young PJ, et al. : Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): A systematic review and meta-analysis. Lancet 2018; 391:1693–1705 [DOI] [PubMed] [Google Scholar]
- 2.Vento M, Moro M, Escrig R, et al. : Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics 2009; 124:e439–e449 [DOI] [PubMed] [Google Scholar]
- 3.Ferguson LP, Durward A, Tibby SM: Relationship between arterial partial oxygen pressure after resuscitation from cardiac arrest and mortality in children. Circulation 2012; 126:335–342 [DOI] [PubMed] [Google Scholar]
- 4.Numa A, Aneja H, Awad J, et al. : Admission hyperoxia is a risk factor for mortality in pediatric intensive care. Pediatr Crit Care Med 2018; 19:699–704 [DOI] [PubMed] [Google Scholar]
- 5.Ramgopal S, Dezfulian C, Hickey RW, et al. : Association of severe hyperoxemia events and mortality among patients admitted to a pediatric intensive care unit. JAMA Netw Open 2019; 2:e199812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Del Castillo J, López-Herce J, Matamoros M, et al. ; Iberoamerican Pediatric Cardiac Arrest Study Network RIBEPCI: Hyperoxia, hypocapnia and hypercapnia as outcome factors after cardiac arrest in children. Resuscitation 2012; 83:1456–1461 [DOI] [PubMed] [Google Scholar]
- 7.Guerra-Wallace MM, Casey FL 3rd, Bell MJ, et al. : Hyperoxia and hypoxia in children resuscitated from cardiac arrest. Pediatr Crit Care Med 2013; 14:e143–e148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raman S, Prince NJ, Hoskote A, et al. : Admission PaO2 and mortality in critically ill children: A Cohort Study and systematic review. Pediatr Crit Care Med 2016; 17:e444–e450 [DOI] [PubMed] [Google Scholar]
- 9.van Zellem L, de Jonge R, van Rosmalen J, et al. : High cumulative oxygen levels are associated with improved survival of children treated with mild therapeutic hypothermia after cardiac arrest. Resuscitation 2015; 90:150–157 [DOI] [PubMed] [Google Scholar]
- 10.Marcin JP, Pollack MM: Review of the acuity scoring systems for the pediatric intensive care unit and their use in quality improvement. J Intensive Care Med 2007; 22:131–140 [DOI] [PubMed] [Google Scholar]
- 11.Pollack MM, Holubkov R, Funai T, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network: Simultaneous prediction of new morbidity, mortality, and survival without new morbidity from pediatric intensive care: A new paradigm for outcomes assessment. Crit Care Med 2015; 43:1699–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pollack MM, Holubkov R, Funai T, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network: The pediatric risk of mortality score: Update 2015. Pediatr Crit Care Med 2016; 17:2–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horvat CM, Ogoe H, Kantawala S, et al. : Development and performance of electronic Pediatric Risk of Mortality and pediatric logistic organ dysfunction-2 automated acuity scores. Pediatr Crit Care Med 2019; 20:e372–e379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kilgannon JH, Jones AE, Shapiro NI, et al. ; Emergency Medicine Shock Research Network (EMShockNet) Investigators: Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA 2010; 303:2165–2171 [DOI] [PubMed] [Google Scholar]
- 15.Roberts BW, Kilgannon JH, Hunter BR, et al. : Association between early hyperoxia exposure after resuscitation from cardiac arrest and neurological disability: Prospective multicenter protocol-directed Cohort Study. Circulation 2018; 137:2114–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Straney L, Clements A, Parslow RC, et al. ; ANZICS Paediatric Study Group and the Paediatric Intensive Care Audit Network: Paediatric index of mortality 3: An updated model for predicting mortality in pediatric intensive care. Pediatr Crit Care Med 2013; 14: 673–681 [DOI] [PubMed] [Google Scholar]


