Abstract
Substituted hydroxamic acid is one of the most extensively studied pharmacophores because of their ability to chelate biologically important metal ions to modulate various enzymes, such as HDACs, urease, metallopeptidase, and carbonic anhydrase. Syntheses and biological studies of various classes of hydroxamic acid derivatives have been reported in numerous research articles in recent years but this is the first review article dedicated to their synthetic methods and their application for the synthesis of these novel molecules. In this review article, commercially available reagents and preparation of hydroxylamine donating reagents have also been described.
Keywords: Hydroxamic acids, hydroxy lamine, coupling reactions, catalytic reaction, mutagens, direct synthesis
1. INTRODUCTION
Hydroxamic acids are strong metal ion bidentate chelators that strongly chelate with Fe(III); siderophores [1], Zn(II); matrix metalloproteases (MMPs), carbonic anhydrase, and tumor necrosis factor-α converting enzyme (TACE), Ni(II); urease, and Cu(II) [2, 3]. Hydroxamic acids, pKa values in the order of 9, are approximately 1% ionized under physiological conditions and O-substitution changes the pKa only slightly [4]. Hydroxamic acids are readily hydrolyzed into carboxylic acids and hydroxylamine (a mutagen) under physiological conditions and this is the major limitation to therapeutic applications of these molecules [5]. Molecules with this functional group are well known epigenetic modulators [6, 7]. Hydroxamic acids act as modulators of anthrax lethal factor (LF) [8], botulinum neurotoxin [9, 10], carbonic anhydrase [11, 12], LpxC (UDP-3-O-[(R)-3-hydroxymyristoyl]-GlcNAc deacetylase) [13], Fe(II) and Mn(II) E. coli methionine aminopeptidase [14], peptide deformylase [15], tumor necrosis factor-α converting enzyme (TACE) [16], matrix metalloproteinase enzyme MMP-13 [17], tyrosinase [18] and aggrecanase [19]. These unique molecules are also known as trypanocidal agents [20], growth stimulating effector on queen bee larvae [21], high density lipoprotein (HDL) receptor CLA-1 up-regulating agents [22], in vitro antioxidant, antiradical, and SSAO inhibitors [23, 24], memory enhancer and mood stabilizers [25], and γ-aminobutyric acid(C) (GABAC) selective antagonists [26]. These molecules are potential drugs to treat Alzheimer’s disease [27], malaria [28] and many other anomalies [29,30]. Biological properties and therapeutic applications of hydroxamic acids have been reported in many recent review articles [31, 32], but there is no review article dedicated to the synthesis of hydroxamic acids sofar, except a short review article, which was published while we were finalizing this manuscript [33]. Monocyclic hydroxamic acids and siderophore based hydroxamic acids have been reported in many reviews recently [34–38]. These hydroxamic acid derivatives are beyond the scope of this review article.
Although hydroxamic acid derivatives are known to show a wide range of biological activities, these molecules are better known for their anticancer properties. Four hydroxamic acid derivatives (Fig. 1) have been approved to treat different types of cancers. Suberoylanilide hydroxamic acid (SAHA) or Zolinza (US brand name) with antineoplastic activity has been approved to treat cutaneous T cell lymphoma (CTCL) on October 6, 2006. SAHA (1) binds to the catalytic domain of HDACs that allows the hydroxamic moiety to chelate Zn(II) ion located in the active site of these enzymes, which inhibits the deacetylation and leads to the accumulation of both hyperacetylated histones and transcription factors. Hyperacetylation of histone proteins causes the upregulation of the cyclin-dependent kinase (CDK) p21 followed by G1 arrest. SAHA is known to affect hyperacetylation of non-histone proteins such as p53 (tumor suppressor), α-tubulin, and heat-shock protein 90 (HSP-90) to produce additional anti-proliferative effects. SAHA is also known to cross the Blood Brain Barrier (BBB). In combination with other antineoplastic drugs, SAHA is in different phases of clinical trials to treat a wide variety of cancers [39, 40]. Belinostat (BELEODAQ™, Spectrum Pharmaceuticals, Inc.) a sulfonamide derived cinnamic hydroxamic acid (2), was granted accelerated approval on July 3, 2014, by the Food and Drug Administration (FDA) to treat patients with relapsed or refractory peripheral T-cell lymphoma (PTCL). Belinostat (2) inhibits tumor cell proliferation, inducing apoptosis, stimulating cellular differentiation, and inhibiting angiogenesis by targeting HDAC enzymes [41]. Another cinnamic acid-derived hydroxamic acid, panobinostat (3), has been approved to treat multiple myeloma. Panobinostat (3) has been approved to be used with bortezomib and dexamethasone as a combination therapy [42]. Resminostat (4) is in clinical trials to treat advanced hepatocellular carcinoma in East Asian patients (Fig. 1) [43].
Fig. 1.
Approved hydroxamic acid based drugs.
A number of hydroxamic acid derivatives are different stages of clinical trials to treat a wide range of cancers. Pracinostat (5) is a cinnamic acid analogue of hydroxamic acid with potent HDAC inhibition activity. This small molecule is in phase II and III clinical trials to treat Myelodysplastic Syndromes (MDS) and Acute Myeloid Leukemia (AML), respectively [44]. Givinostat (6), a naphthalene-derived hydroxamic acid derivative, is a potent HDAC inhibitor, which is in clinical trials to treat different types of cancers including Hodgkin’s lymphoma, chronic lymphocytic leukemia (CLL), and multiple myeloma [45]. This novel drug (6) is also in phase III clinical trials to treat ambulant patients with Duchenne Muscular Dystrophy (DMD) [46]. Abexinostat (7), a benzofuran-derived hydroxamic acid, is in phase II clinical trials to treat Hodgkin’s lymphoma [47]. CHR-3996 (8) is a second generation HDAC inhibitor, which is in phase 1 clinical trials to treat patients with advanced or treatment refractory solid tumors [48]. CUDC-101 (9) is a multitarget inhibitor of HDACs, EGFR, and HER2, which is in clinical trials to treat head and neck squamous cell carcinoma (HNSCC) (Fig. 2) [49]. Tubastatin A is a potent HDAC6 inhibitor, which is at different stages of drug development [50].
Fig. 2.
Under clinical trials hydroxamic acid derivatives (5–9) [51] and a potent HDAC6 inhibitor (10) [50].
A large number of hydroxamic acid derivatives have been reported as potent antineoplastic agents to treat a wide range of cancers. Thus, the synthesis of molecules bearing a hydroxamic acid pharmacophore is of great interest for both organic and medicinal chemists.
Synthetic methods for hydroxamic acids are not so developed as compared to the biological application of these molecules. Sometimes synthetic chemists are content with as low as 10% yields of the hydroxamic acids from its precursors [52, 53]. According to literature reports sofar, there is not a particular reagent or condition that can be used for a wide variety of compounds. Nevertheless, different synthetic methods and reagents have been used to make a particular series of a few compounds [9]. Compilation of the following methods and reagents for the synthesis of hydroxamic acids will be helpful for synthetic and medicinal chemists to choose an appropriate or closely related reagent or method for a desired substrate.
2. COMMERCIALLY AVAILABLE HYDROXYLAMINE DONATING REAGENTS
Free hydroxylamine (NH2OH) is unstable, explosive, and mutagenic [54]. It is commercially available in the form of salts mostly as hydrochloride salt (11) and in the solution form (12). Free hydroxylamine is generated in situ from hydrochloride salt by treating with base. Solutions of NH2OH are used directly in a reaction without pretreatment or drying. Both N- and O-protected hydroxylamine s are available from commercial sources but mostly O-protected hydroxylamines (13–20) are used for hydroxamic acid synthesis.
3. SYNTHESIS OF HYDROXYLAMINE DONORS
Many reagents (Fig. 3) for hydroxamic acid synthesis are commercially available but some of them are quite a bit expensive. The following reagents can be prepared from readily available starting materials in multi-gram scale. p-Methoxybenzyl (PMB) protected hydroxylamine is synthesized by the reaction of N-hydroxyphthalimide (21) with p-methoxybenzylchloride (22) followed by hydrolysis with hydrazine and finally treating with IM HC1 in dichloromethane to make the hydrochloric salt (24) in very good overall yield [55, 56]. O-2-Methylprenyl hydroxylamine (27) is another hydroxylamine donating reagent that gives volatile by products after deprotection of the prenyl group (Scheme 1) [57].
Fig. 3.
Commonly available hydroxylamine donors.
Scheme 1.
Synthesis of hydroxylamine donors.
O-(Tetrahydro-2H-pyran-2-yl) hydroxylamine (20) is a commercially available masked hydroxylamine and it is one of the most frequently used reagents for hydroxamic acid synthesis. It can be prepared conveniently from readily available starting materials. Reaction of 21 with 3,4-dihydro-2H-pyran (28) in the presence of catalytic amount of p-toluenesulfonic acid (PTSA) followed by hydrolysis with methylhydrazine gives 20 in 75% overall yield [58]. tert-Butyldimethylsilyl protected hydroxylamine (13) is commercially available; albeit rather costly. It is prepared by reacting hydroxylamine hydrochloride with TBDMSC1 in the presence of ethylenediamine base to get the product 13 in very good yield (Scheme 1) [59].
The reagent (32) for the solid phase synthesis of hydroxamates is prepared by the reaction of hydroxylamine hydrochloride with N-(9-fluorenylmethoxycarbonyloxy) succinimide (Fmoc-OSu) to get N-Fmoc protected hydroxylamine (Fmoc-NHOH) as a white ciystalline solid in 80% yield and then Fmoc-NHOH is coupled with 2-chlorotrityl chloride (CTC) resin (30) in the presence of N,N’-diisopropylethylamine (DIPEA) in dichloromethane (CH2C12). The Fmoc group is removed by 20% piperidine/N-methyl-2-pyrrolidone (NMP) to get the free resin bound 2-chlorotrityl hydroxylamine derivative (32), which is reacted in situ for the hydroxamate precursors [18, 60]. The polystyrene supported reagent 34 for the solid phase synthesis of hydroxamic acids is prepared by shaking a pyridine solution of NH2OH.HCl with polystyrene sulfonyl chloride (33) in CH2C12 at room temperature for 12h, which has been used in Angeli-Rimini’s reaction on a solid support [61]. Hydroxylamine ChemMatrix® resin (36) is a novel reagent for the solid phase synthesis of peptide hydroxamic acids. Cal et al. have synthesized 36 from derivatized trityl ChemMatrix® resin (35) in three steps by treating with thionyl chloride followed by reaction with 11 and then hydrolysis with hydrazine to get the desired product (Scheme 1) [62].
4. SYNTHESIS OF SUBSTITUTED HYDROXAMIC ACIDS
4.1. A. Direct Reaction of Hydroxylamine with Ester Derivatives
Direct reaction of hydroxylamine has been frequently used to synthesize hydroxamic acids from its ester precursors. Free hydroxylamine in a solution is slightly volatile, so a large excess of the reagent is required (~10 equivalent) to complete the reaction. For the direct reaction of esters with in situ generated hydroxylamine, various polar solvents or solvent combinations are used (Scheme 2). Strong bases (CH3ONa, NaOH or KOH) in methanol are used to generate free hydroxylamine in situ from hydroxylamine hydrochloride; i.e. a highly selective HDAC6 inhibitor, tubastatin A (10) has been synthesized in 31% yield by treating the ester derivative (37) with hydroxylamine hydrochloride and 25% sodium methoxide in methanol (Scheme 2) [63, 64]. The simplest reagent for the synthesis of hydroxamic acids is the aqueous solution of NH2OH. This reagent system works well with benzylic esters and α,β-unsaturated esters. By using this reagent system, a thio analogue (40) of trichostatin has been synthesized in moderate yield. In this procedure, treatment of ester precursor (39) with 50% aqueous hydroxylamine (9 equivalent) followed by a solution of potassium hydroxide in methanol has afforded the trichostatin thio analogue 40 in 52% yield [65]. In a slightly modified procedure, 50% aqueous NH2OH has been used in the presence of IN NaOH in methanol at room temperature. By utilizing this procedure, a 18F analogue of SAHA has been synthesized in moderate yield (65%) from its ester precursor [66]. A series of triazolylphenyl-based potent and selective histone deacetylase inhibitors (e.g., 42) with activity against pancreatic cancer cells and Plasmodium falciparum have been synthesized by using hydroxylamine hydrochloride/KOH in MeOH from the ester precursors (e.g., 41) [67]. In another simple procedure, 1.76M NH2OH has been used to synthesize piperazenyl derived hydroxamic acids at room temperature. In this reagent system low to moderate to high yields of products have been obtained [68]. By using this reagent system chimeric quinazoline-derived hydroxamic acids (e.g., 44) have been synthesized in moderate to good yields. This molecule (44) has shown in vitro inhibitory activity against HDAC, EGFR, and HER2 with IC50 values of 4.4, 2.4, and 15.7 nM respectively (Scheme 2) [69].
Scheme 2.
Direct reaction of hydroxylamine with esters derivatives.
Salmi et al. have utilized 50% aqueous hydroxylamine for the synthesis of SAHA derivatives from its ester precursors at slightly elevated temperature (60 °C) in very good yields of the products [70]. In another slightly modified procedure, analogues of docinostat (46) have been converted into hydroxamic acids by 50% aqueous hydroxylamine and 25% NaOMe in MeOH at 0°C [71]. Pyridone based hydroxamic acids (e.g., 48) have been synthesized by using potassium hydroxy amide (H2NOK) in methanol to get the products in moderate yields [72]. Methanolic H2NOK in DMF solution has also been used for the synthesis of ferulic acid derived hydroxamic acids as potent histone deacetylase inhibitors [73]. Hydroxylamine in aqueous DMSO under weakly basic conditions has been used to synthesize 5-nitrosalicylic acid derived hydroxamic acids [74]. Hydroxamic acids of simple substrates (e.g., 50) have been synthesized by using NH2OH/NaOH in CH2Cl2:MeOH (1:2) solvent mixture [75]. Direct reaction of hydroxylamine with acyl chloride [76, 77], acylbenzotriazole, [78] and acyl imidazole [79] is also used to synthesize hydroxamic acids.
4.2. B. Catalytic KCN and Microwave Irradiation In Hydroxamic Acid Synthesis
Addition of a catalytic amount of potassium cyanide (KCN) in aqueous NH2OH increases the hydroxamic acid formation from ester derivatives both in solid and solution phase reactions. It is believed that this reaction proceeds via an acylcyanide intermediate followed by nucleophilic substitution by hydroxylamine [80]. Methanolic solution of hydroxylamine in the presence of a catalytic amount of KCN has been used to synthesize complex hydroxamic acids (e.g., 52) as LpxC inhibitors [81]. A highly potent and selective HDAC6 inhibitor, HPOB (55) has been synthesized by the direct reaction of ester derivatives with aqueous NH2OH in the presence of a catalytic amount of KCN [82].
Microwave irradiation (MW) has also been used to synthesize hydroxamic acids. By utilizing this procedure, syntheses of several quinazolin-4-one derivatives have been reported from ester derivatives (e.g., 56) as selective histone deacetylase-6 (HDAC6) inhibitors for the treatment of Alzheimer’s disease. Among these novel molecules, 57 has shown potent HDAC6 activity with an IC50 value of 8 nM and decreased zinc-mediated β-amyloid aggregation in vitro (Scheme 3) [27].
Scheme 3.
Reaction of hydroxylamine with esters under catalytic condition and microwave irradiation.
4.3. C. Reaction of Hydroxylamine with Activated Carboxylic Acids
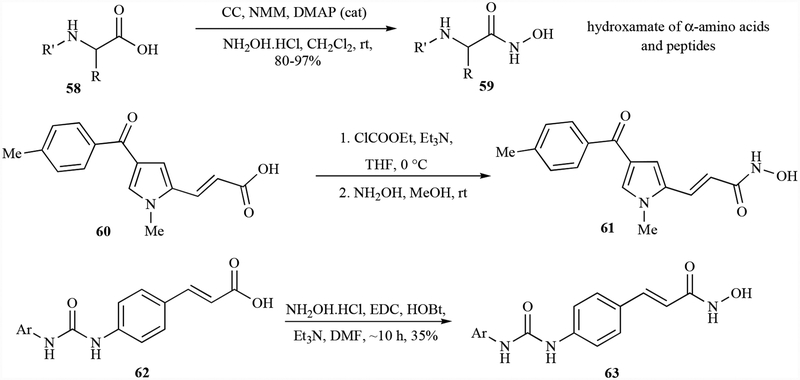
One of the simple and mild methods for hydroxamic acid synthesis is the coupling of hydroxylamine with the activated carboxylic acid derivatives in a one-pot reaction. Cyanuric chloride (CC) has been used to activate carboxylic acid derivatives (e.g., 58) followed by reaction with hydroxylamine to get the hydroxamic acids (59). This efficient method has been applied for the synthesis of enantiopure hydroxamates (e.g., 59) of α-amino acids and peptides in excellent yields [83]. The synthesis of chemically sensitive pyrrole-derived hydroxamic acids (e.g., 61) has been achieved by using ethyl chloroformate as an activating agent of the pyrrole derived carboxylic acids (e.g., 60) followed by treatment with hydroxylamine in a one-pot reaction. This reaction utilizes mild and neutral reaction conditions in which ethoxycarbonyl anhydride is the intermediate to react with the hydroxylamine to get the desired products [84, 85]. By utilizing similar procedures, a series of dual-action compounds were synthesized to target histone deacetylases and 3-hydroxy-3-methylglutaryl coenzyme a reductase (HMGR) as potential anticancer agents. This is one of the most frequently utilized methods for the synthesis of hydroxamic acids for sensitive substrates [86]. Hydroxylamine has also been coupled with carboxylic acids to make hydroxamic acids (e.g., 63) by using coupling agents such as EDC/HOBt to get the products in moderate yields (Scheme 4) [87, 88].
Scheme 4.
Reaction of hydroxylamine with activated carboxylic acids.
4.5. D. O-(Tetrahydro-2H-pyran-2-yl) Hydroxylamine (THPONH2) for hydroxamic Acid Synthesis
O-(Tetrahydro-2H-pyran-2-yl) hydroxylamine (20) has been used to synthesize hydroxamic acids of delicate substrates. For the synthesis of γ-aminobutyric acid (C) (GABAC) selective antagonist (e.g., 66), carboxylic acid derivative (64) is activated as a mixed anhydride (65) followed by in situ reaction with THPONH2 (23) to get the protected hydroxamates. Cleavage of O-(tetrahydro-2H-pyran-2-yl) group with 6M HC1 gives the target hydroxamic acids (e.g., 66) [26]. Preparation of azidosulfonyl-derived carboxylic acids into the corresponding hydroxamic esters (68) has been achieved by coupling with 23, with the help of EDC/HOBT and NMM in DMF followed by cleavage of the O-THP protecting group with hydrochloric acid containing dioxane/methanol mixtures [89]. One of the facile methods for the synthesis of hydroxamates (e.g., 70) is the coupling of 20 with EDC/HOBt followed by deprotection of tetrahydropyranyl group by anhydrous HC1 to get the target molecules in very good overall yield [90–92]. Trifluoroacetic acid has also been used to deprotect O-tetrahydro-2H-pyran-2-yl group of complex substrates [93]. PyBOP (benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate) reagent in DMF has been used to couple 20 with carboxylic acids followed by deprotection with aqueous HC1 to get hydroxamic acid products in good to moderate overall yields [94]. O-THP protecting group has been found to be very stable under basic conditions. After coupling with 20, several intermediate steps have been carried out for further functionalization of the molecules and then the tetrahydropyranyl protecting group has been deprotected in the final step to get the hydroxamic acids (e.g., 73) [95]. N,N’-Dicyclohexylcarbodiimide (DCC) has been used to couple THPONH2 (20) with carboxylic acid derivatives (e.g., 74) to get the protected hydroxamates (75), which on treatment with p-TsOH.H2O yields the hydroxamic acids (e.g. 76) in moderate yields [96]. O–THP group has been deprotected by solid phase macroporous sulfonic acid resin (MP-TsOH) in methanol to get the hydroxamic acids in good yields [97]. CDMT (2-chloro-4,6-dimethoxy-1,3,5-triazine)/NMM (N-methylmorpholine) mediated coupling followed by deprotection with HC1 has been used to make methylsulfone hydroxamic acid based (e.g., 78) LpxC inhibitors as gram-negative antibacterial agents (Scheme 5) [98].
Scheme 5.
THPONH2 (20) for hydroxamic acid synthesis.
4.6. E. O-Tritylhydroxylamine (TrONH2)
O-Trityl protected hydroxylamine (19) is used to introduce the hydroxy lamine group to synthesize hydroxamic acids. The advantage of using this reagent (19) is the facile deprotection of O-trityl group under mild acidic conditions. O-Trityl azido-hydroxamates have been utilized in Cu(I) catalyzed azide-alkyne cycloaddition reaction (“click” chemistry) to get ethynylestradiol (EED)-HDACI conjugates (80), which on deprotection with trifluoroacetic acid (TFA) gives the hybrid molecule containing the hydroxamic acid pharmacophore (81) [99].
Guerrant et al. [100] have utilized isobutylchloroformate (IBCF) mediated activation of carboxylic acid derivatives (e.g., 82) followed by reaction with 19 to get the protected hydroxamate (83). After a multi-step synthesis, the trityl group was removed by BF3–etherate to get the hybrid molecules (e.g., 84). For this reaction 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC or EDCI) and O-(benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium tetrafluoroborate (TBTU) mediated coupling gave very poor yield. The hydroxamate derivative (83) was coupled with daunorubicin by reductive amination followed by trityl deprotection to give the desired histone deacetylase and topoisomerase II dual inhibitor (84). EDC/HOBt has been used to couple 19 with the carboxylic acid derivatives followed by trityl deprotection with trifluoroacetic acid to get the hydroxamic acid derivatives (e.g., 86) as dual inhibitors of inosine monophosphate dehydrogenase (IMPDH) and histone deacetylases (HDAC) for cancer treatment (Scheme 6) [101]. Remiszewski et al. [102] have used many methods including EDC/HOBt mediated coupling of 19 with the carboxylic acids followed by deprotection with TFA to get hydroxamic acid derivatives. Direct reaction of 19 with activated acids has also been reported in the literature. Reaction of malonate-derived acylchlorides with 19 followed by trityl deprotection has been used to synthesize hydroxamic acids as antiplasmodial aminopeptidase-1 inhibitors [103].
Scheme 6.
Synthesis of hydroxamic acids by using O-tritylhydroxylamine.
4.7. F. Solid Phase Synthesis Of Hydroxamic Acids
Solid-phase synthesis of hydroxamic acid derivatives generally involves immobilization of the hydroxylamine group through O-linkage and in some cases through N-linkage and then coupling with the desired substrates. Solid-phase synthesis has also been achieved through the formation of protected resin-bound hydroxamates followed by nucleophilic displacement (Scheme 7) [104].
Scheme 7.
Solid supported synthesis of hydroxamic acids.
Nandurkar et al. have reported the solid-phase synthesis of hydroxamic acids on poly [acryloyl-bis(aminopropyl)polyethylene glycol] (PEGA) resins. This method is based on the nucleophilic displacement of esters immobilized on PEGA resins with hydroxylamine/sodium hydroxide in isopropanol [105]. A polymer-supported N-hydroxy benzene sulfonamide (HMBA-PEGA800), (87), has been successfully utilized to convert aldehydes to hydroxamic acids; adapting a century-old rarely used procedure (Angeli-Rimini’s reaction). The procedure is selective and tolerates the presence of other functional groups on the alkyl and aryl substrates [61]. Resin bound tritylated hydroxylamine followed by TFA deprotection has been utilized to synthesize cysteine derived complex hydroxamic acids (92). For this synthesis, a cysteine-derived carboxylic acid (89) is coupled with 32 to make the solid bound intermediate (90) in good yield (70%) using l-[bis(dimethylamino) methylene]-1H-1,2,3 -triazolo [4,5-b] pyridinium 3-oxidhexafluoro phosphate (HATU), and it was found to be more efficient than alternative coupling reagents such as N,N,N,N-tetramethyl-O-(1H-benzotriazol-1-yl)uronium hexafluorophosphate, (HBTU) or BOP reagent. Further functionalization of the resin bound material gives 91, which on deprotection with TFA afforded the desired hydroxamic acid (92) [106]. A solid phase method has been utilized for the rapid synthesis of benzothiophene hydroxamic acids (95). Fmoc protected amino benzothiophene-2-carboxylic acids (e.g., 93) were coupled with 32 using N,N′-diisopropylcarbodiimide (DIPC) to get the resin bound hydroxamic acid precursors (e.g., 94) and finally, cleavage from the resin with a trifluoroacetic acid (TFA)/triethylsilane (TES) solution in dichloromethane, afforded the corresponding hydroxamic acids (95), in many cases pure enough for testing [107]. Long peptide hydroxamic acids (e.g., 96) have been synthesized by using ChemMatrix® based hydroxamine resin (36) and found to be superior to commercially available hydroxylamine on Wang support in terms of both yield and purity [62]. Merrifield resin (97) has been utilized to synthesize hydroxamic acids in multi-step synthesis. SASRIN resin (99) has been prepared from Merrifield resin followed by reductive amination of O-2,4-dimethoxybenzyl hydroxylamine (DMB-ONH2) to make the acid labile O-benzyl protected hydroxylamine (e.g., 100). This resin supported dibenzyl derivative has been coupled with various acids by standard carbodiimide (CDI) coupling to get the dibenzyl derivatives (e.g., 101) followed by TFA deprotection to get various aryl hydroxamic acids (e.g., 102) up to 98% yield and 95% purity (Scheme 7) [108].
4.8. G. O-Benzylhydroxylamine (PMBONH2 and BnONH2) for Hydroxamic Acid Synthesis
O-(p-Methoxybenzyl)hydroxylamine (PMBONH2, 17) has been used as a hydroxylamine donor in several reports. Nucleophilic acyl substitution of acyl chloride (103) with PMBONH2 (17) gives the intermediate 104, which on further reaction followed by deprotection with TFA gives the hydroxamates as potent HDAC inhibitors (e.g., 105) [56,109]. BnONH2 (16) is a good source of hydroxylamine donor. Generally, BnONH2 is coupled with acid derivatives by standard coupling reagents followed by deprotection under neutral condition with H2, Pd/C to get the desired products in overall good yields [110]. BnONH2 has been coupled with tetrazole derived acids (e.g., 108) by EDC in THF followed by hydrogenolysis with H2, Pd/C to get the hydroxamic acids in low to moderate yields (e.g., 109) [52]. BnONH2 has also been coupled with acid derivatives (110) by BOP-C1 to get the O-benzyl hydroxamate (111) followed by debenzylation with H2, Pd/C to get hydroxamic acid derivatives (e.g., 112) [111, 112]. Reaction of O-benzylhydroxylamine with activated acid (113) by mixed anhydride gives O-benzyl hydroxamate without cyclization as an amide side product. Hydrogenolysis with H2, Pd/C gives hydroxamic acid (114) in excellent yields [113]. 1,1’-Carbonyldiimidazole (CDI) mediated coupling reactions of carboxylic acids (e.g., 115) with O-benzylhydroxylamine followed by hydrogenolysis of the benzyl group have been utilized to synthesize fosmidomycin based hydroxamic acids (e.g., 116) as IspC inhibitors (Scheme 8) [28].
Scheme 8.
Use of PMBONH2 and BnONH2 for hydroxamic acid synthesis.
4.9. H. O-Silylated Hydroxylamine for Hydroxamic Acid Synthesis
Ease of deprotection under neutral or mild acidic conditions makes the O-silylated hydroxylamine an important hydroxylamine donor for delicate substrates (Scheme 9).
Scheme 9.
O-Silyl hydroxylamines for hydroxamic acid synthesis.
Ethyl chloroformate-mediated reaction of acid (117) with O-(tert-butyldimethylsilyl)hydroxylamine (13) followed by silyl deprotection with trifluoroacetic acid in anisole has been used for the synthesis of very delicate hydroxamates such as 119 [114]. The hydroxamic acid group of (R)-trichostatin A (121) has been installed by reacting the acid precursor (120) with ethyl chloroformate to make a mixed anhydride followed by reaction with TBDMSONH2 (13) and finally the silyl group is removed by CsF to get the target molecule (121) in 92% overall yield [115]. This reagent (13) has been found particularly useful for the synthesis of trichostatin A as it can be easily prepared, readily handled, and storable solid reagent [116]. 13/EDC has been extensively used to install the hydroxamic acid group in complex substrates [117, 118]. Carboxylates (e.g., 122) have been condensed with 13 in the presence of EDC to give silyl intermediates, which afforded hydroxamates (e.g., 123) after acid cleavage with trifluoroacetic acid [19] Cycloaddition of silyl protected hydroxamate derivatives followed by silyl deprotection with TBAF has been utilized to introduce hydroxamic acid into complex substrates [119]. Silyl protected hydroxylamine has also been used to couple with acid derivatives by using EDC and then the silyl group is deprotected by TBAF to get the hydroxamic acid in moderate to good yields [120]. IN HC1 has been used to deprotect silylated hydroxamic acid [121]. Bis(2-oxo-3-oxazolidinyl)phosphinic chloride (BOP-C1) mediated coupling of 13 with acetylenic carboxylic acids (e.g., 124) followed by direct deprotection of silylated hydroxamic acid has been used to synthesize potential antileukemic agents (e.g., 125) in 20% yield [122]. Cyclophosphamide esters (126) were converted directly to the corresponding hydroxamic acids (e.g., 127) by treating with 14 and NaOMe in methanol. However, this method is not suitable for α-substituted compounds (e.g., 128) due to the steric hindrance. These compounds have been hydrolyzed to carboxylic acids (e.g., 128) followed by activation as mixed anhydrides and then reaction with 14 to get the hydroxamic acid derivatives (e.g., 129) (Scheme 9) [123].
5. MISCELLANEOUS METHODS OF HYDROXAMIC ACID PREPARATION
O-(2-Methoxy-2-propyl)hydroxylamine has been used as a source of hydroxylamine for the synthesis of hydroxamates of delicate substrates. For this synthesis, pyrrole-derived carboxylic acids (130) are activated by ethyl chloroformate followed by reaction with O-(2-methoxy-2-propyl)hydroxylamine and then deprotection of methoxyisopropyl by amberlyst-15 to afford hydroxamates in good yield (e.g., 131) [124]. O-Protected 3-hydroxy-oxazolidin-2,4-diones (e.g., 132) have been used to make α-hydroxy hydroxamates (e.g., 134) by treating with sodium methoxide in methanol followed by debenzylation with H2, Pd/C in excellent overall yield [125]. Acyltransferase activity of a bacterial strain Bacillus smithii (IITR6b2) has been utilized for the synthesis of nicotinic acid hydroxamate (136), from nicotinamide (135) [126]. This type of clean and green enzymatic reactions may have future use for the synthesis of biologically important hydroxamic acids. Zheng et. al. have reported the synthesis of hydroxamic acids from amide precursors by using amidase enzyme. This is an enantioselective methodology in which only the R enantiomer of the racemic mixture of cyclopropyl-derived amide (137) is converted into the corresponding hydroxamic acid (138) (Scheme 10). A continuous flow-tubing reactor has been used for the synthesis of hydroxamic acids by reacting methyl or ethyl carboxylic esters with hydroxylamine in the presence of sodium methoxide. This technique has many synthetic advantages including faster reaction rate and higher purity and this method was also successfully applied to the multistep preparation of suberoylanilide hydroxamic acid (SAHA) [127].
Scheme 10.
Miscellaneous methods.
Recently, an efficient photoorganocatalytic one-pot synthesis of hydroxamic acids (140) has been reported directly from the aldehyde precursor (139). This is a visible-light-mediated hydroacylation of dialkylazodicarboxylate followed by the addition of hydroxylamine hydrochloride (11) [128]. Porcheddu et. al. have reported the synthesis of indole-derived (142) and several other hydroxamic acids by using Angeli-Remini’s reaction of the aldehyde derivatives (e.g., 141) and solid-supported N-hydroxybenzene-sulfonamide (34) in one step [61]. Other oxidative transformations of aldehydes into hydroxamic acids have also been reported recently [129]. These enzymatic and photocatalytic methods are not so common. Nevertheless, they could be the used as green methodologies to synthesize hydroxamic acid derivatives.
CONCLUSIONS
Different methods of hydroxamic acid syntheses have been compiled. Although many synthetic methods and reagents are available, there is not a universal reagent or synthetic method that can be used for a wide range of substrates. From literate reports sofar, there are two reagents/methods worth trying for the new substrates. If the hydroxamic acid precursor is an ester then direct reaction of hydroxylamine in the presence of catalytic potassium cyanide may give the product in good yield. In case of carboxylic acid precursors, activation by a mixed anhydride followed by reaction with hydroxylamine could be the most promising. Based on the biological importance of hydroxamates, there is a need to develop synthetic methods/reagents for hydroxamic acids to get the products in good yield under mild and environmentally benign conditions.
ACKNOWLEDGEMENTS
This publication was made possible by the Arkansas INBRE start-up program, supported by grant funding from the National Institutes of Health (NIH) National Institute of General Medical Sciences (NIGMS) (P20 GM103429). MAA also thanks ABI-Astate for start-up (ABI-200127) funding.
FUNDING
None.
LIST OF ABBREVIATIONS
- BOP
benzotriazol-1-yloxytris(dimethylamino)phosphonium hexafluorophosphate
- BOP-C1
bis(2-oxo-3-oxazolidinyl)phosphinic chloride
- BnONH2
O-benzylhydroxylamine
- CC
cyanuric chloride
- CDI
1,1’-carbonyldiimidazole
- CDMT
2-chloro-4,6-dimethoxy-l,3,5-triazine
- DCC
N,N′-dicyclohexylcarbodiimide
- DIPC
N,N′-diisopropylcarbodiimide
- DMF
N,N-dimethylformamide
- EDC
1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride
- EGFR
epidermal growth factor receptor
- GABA
γ-aminobutyric acid
- HBTU
N,N,N,N-tetramethyl-O-(1H-benzotriazol-1-yl)uronium hexafluorophosphate
- HDAC
histone deacetylase
- HOBt
hydroxybenzotriazole
- IBCF
isobutylchloroformate
- IMPDH
inosine monophosphate dehydrogenase
- IspC
1-deoxy-d-xylulose 5-phosphate reductoi-somerase
- LpxC
UDP-3-O-[(R)-3-hydroxymyristoyl]-GlcNAc deacetylase
- MMP
matrix metalloproteinase
- NMM
N-methylmorpholine
- PEGA
poly[acryloyl-bis(aminopropyl)polyethylene glycol]
- SSAO
semicarbazide-sensitive amine oxidase
- TACE
tumor necrosis factor-α converting enzyme
- TBTU
O-(benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium tetrafluoroborate
- TES
triethylsilane
- TFA
trifluoro acetic acid
- THPONH2
O-(tetrahydro-2H-pyran-2-yl)hydroxylamine
- TrONH
O-tritylhydroxylamine
Footnotes
Publisher's Disclaimer: DISCLAIMER: The above article has been published in Epub (ahead of print) on the basis of the materials provided by the author. The Editorial Department reserves the right to make minor modifications for further improvement of the manuscript.
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
REFERENCES
- [1].Neff C; Bellot F; Waern JB; Lambert F; Brandei J; Serratrice G; Gaboriau F; Policar C, Glycosiderophores: Synthesis of tris-hydroxamate siderophores based on a galactose or glycero central scaffold, Fe(III) complexation studies. J. Inorg. Biochem, 2012,112, 59–67. [DOI] [PubMed] [Google Scholar]
- [2].Codd R, Traversing the coordination chemistry and chemical biology of hydroxamic acids. Coord. Chem. Rev, 2008, 252, 1387–1408. [Google Scholar]
- [3].Griffith DM; Szocs B; Keogh T; Suponitsky KY; Farkas E; Buglyo P; Marmion CJ, Suberoylanilide hydroxamic acid, a potent histone deacetylase inhibitor; its X-ray crystal structure and solid state and solution studies of its Zn(II), Ni(II), Cu(II) and Fe(III) complexes. J. Inorg. Biochem, 2011,705,763–9. [DOI] [PubMed] [Google Scholar]
- [4].Locock KES; Yamamoto I; Tran P; Hanrahan JR; Chebib M; Johnston GAR; Allan RD, γ-Aminobutyric Acid(C) (GABAC) Selective antagonists derived from the bioisosteric modification of 4-aminocyclopent-1-enecarboxylic acid: Amides and Hydroxamates. J. Med. Chem, 2013, 56, 5626–5630. [DOI] [PubMed] [Google Scholar]
- [5].Flipo M; Charton J; Hocine A; Dassonneville S; Deprez B; Deprez-Poulain R Hydroxamates: relationships between structure and plasma stability. J. Med. Chem, 2009, 52, 6790–802. [DOI] [PubMed] [Google Scholar]
- [6].Alam MA; Reddy YS; Ali MA New and under explored epigenetic modulators in search of new paradigms. Med. Chem, 2015,11, 271–285. [DOI] [PubMed] [Google Scholar]
- [7].Susanto JM; Colvin EK; Pinese M; Chang DK; Pajic M; Mawson A; Caldon CE; Musgrove EA; Henshall SM; Sutherland RL; Biankin AV; Scarlett CJ The epigenetic agents suberoylanilide hydroxamic acid and 5AZA2’ deoxycytidine decrease cell proliferation, induce cell death and delay the growth of MiaPaCa2 pancreatic cancer cells in vivo. Int. J. Oncol, 2015, 46, 2223–30. [DOI] [PubMed] [Google Scholar]
- [8].Calugi C; Trabocchi A; Lalli C; Guarna A D-proline-based peptidomimetic inhibitors of anthrax lethal factor. Eur. J. Med. Chem, 2012, 56,96–107. [DOI] [PubMed] [Google Scholar]
- [9].Silhar P; Silvaggi NR; Pellett S; Capkova K; Johnson EA; Allen KN; Janda KD Evaluation of adamantane hydroxamates as botulinum neurotoxin inhibitors: synthesis, crystallography, modeling, kinetic and cellular based studies. Bioorg. Med. Chem, 2013, 21, 1344–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Stowe GN; Silhar P; Hixon MS; Silvaggi NR; Allen KN; Moe ST.; Jacobson AR; Barbieri JT; Janda KD Chirality holds the key for potent inhibition of the botulinum neurotoxin serotype a protease. Org. Lett, 2010, 72,756–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Di Fiore A; Maresca A; Supuran CT; De Simone G Hydroxamate represents a versatile zinc binding group for the development of new carbonic anhydrase inhibitors. Chem. Commun, 2012, 48, 8838–40. [DOI] [PubMed] [Google Scholar]
- [12].Rodrigues GC; Feijo DF; Bozza MT; Pan P; Vullo D; Parkkila S; Supuran CT; Capasso C; Aguiar AP; Vermelho AB Design, Synthesis and evaluation of hydroxamic acid derivatives as promising agents for the management of chagas’ disease. J. Med. Chem, 2014, 57, 298–308. [DOI] [PubMed] [Google Scholar]
- [13].Fei Z; Kong W; Wang H; Peng J; Sun F; Yin Y; Bajwa J; Jiang X A scalable synthesis of a hydroxamic acid LpxC Inhibitor. Org. Process Res. Dev, 2012,16,1436–1441. [Google Scholar]
- [14].Huguet F; Melet A; Alves de Sousa R; Lieutaud A; Chevalier J; Maigre L; Deschamps P; Tomas A; Leulliot N; Pages JM; Artaud I Hydroxamic acids as potent inhibitors of Fe(II) and Mn(II) E. coli methionine aminopeptidase: Biological activities and X-ray structures of oxazole hydroxamate-EcMetAP-Mn complexes. Chem. Med. Chem, 2012, 7, 1020–30. [DOI] [PubMed] [Google Scholar]
- [15].Gao J; Cheng Y; Cui W; Chen Q; Zhang F; Du Y; Ji M, 3D-QSAR and molecular docking studies of hydroxamic acids as peptide deformylase inhibitors.Med. Chem. Res, 2011,27, 1597–1610. [Google Scholar]
- [16].Sengupta P; Puri CS; Chokshi HA; Sheth CK; Midha AS; Chitturi TR; Thennati R; Murumkar PR; Yadav MR, Synthesis, preliminary biological evaluation and molecular modeling of some new heterocyclic inhibitors of TACE. Eur. J. Med. Chem, 2011, 46, 5549–55. [DOI] [PubMed] [Google Scholar]
- [17].Tommasi RA; Weiler S; McQuire LW; Rogel O; Chambers M; Clark K; Doughty J; Fang J; Ganu V; Grob J; Goldberg R; Goldstein R; Lavoie S; Kulathila R; Macchia W; Melton R; Springer C; Walker M; Zhang J; Zhu L; Shultz M, Potent and selective 2-naphthylsulfonamide substituted hydroxamic acid inhibitors of matrix metalloproteinase-13. Bioorg. Med. Chem. Lett, 2011, 27, 6440–5. [DOI] [PubMed] [Google Scholar]
- [18].Kwak SY; Lee S; Choi HR; Park KC; Lee YS Dual effects of caffeoyl-amino acidyl-hydroxamic acid as an antioxidant and depigmenting agent. Bioorg. Med. Chem. Lett, 2011, 27, 5155–8. [DOI] [PubMed] [Google Scholar]
- [19].Nuti E; Santamaria S; Casalini F; Yamamoto K; Marinelli L; La Pietra V; Novellino E; Orlandini E; Nencetti S; Marini AM; Salerno S; Taliani S; Da Settimo F; Nagase H; Rossello A Arylsulfonamide inhibitors of aggrecanases as potential therapeutic agents for osteoarthritis: synthesis and biological evaluation. Eur. J. Med. Chem, 2013, 62, 379–94. [DOI] [PubMed] [Google Scholar]
- [20].Kelly JM; Taylor MC; Horn D; Loza E; Kalvinsh I; Bjorkling F Inhibitors of human histone deacetylase with potent activity against the african trypanosome trypanosoma brucei. Bioorg. Med. Chem. Lett, 2012, 22,1886–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Huang CY; Chi LL; Huang WJ; Chen YW; Chen WJ; Kuo YC; Yuan CM; Chen CN Growth stimulating effect on queen bee larvae of histone deacetylase inhibitors. J. Agric. Food. Chem, 2012, 60, 6139–49. [DOI] [PubMed] [Google Scholar]
- [22].Chen X; Wang L; Du Y; Wu Y; Jia X; Yang Y; Hong B, Design, synthesis and biological evaluation of hydroxamic acid derivatives as potential high density lipoprotein (HDL) receptor CLA-1 up-regulating agents.Molecules, 2011,16, 9178–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liu Y-H; Liang W-L; Lee C-C; Tsai Y-F; Hou W-C Antioxidant and sem icarbazide-sensitive amine oxidase inhibitory activities of glucuronic acid hydroxamate. Food Chem, 2011,129, 423–428. [DOI] [PubMed] [Google Scholar]
- [24].Koncic MZ; Barbaric M; Perkovic I; Zore B, Antiradical, chelating and antioxidant activities of hydroxamic acids and hydroxyureas. Molecules, 2011,16, 6232–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fass DM; Shah R; Ghosh B; Hennig K; Norton S; Zhao WN; Reis SA; Klein PS; Mazitschek R; Maglathlin RL; Lewis TA; Haggarty SJ Effect of inhibiting histone deacetylase with short-chain carboxylic acids and their hydroxamic acid analogs on vertebrate development and neuronal chromatin. ACS Med. Chem. Lett, 2010, 2, 39–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Locock KE; Yamamoto I; Tran P; Hanrahan JR; Chebib M; Johnston GA; Allan RD Gamma-Aminobutyric Acid(C) (GABA) selective antagonists derived from the bioisosteric modification of 4-Aminocyclopent-1-enecarboxylic Acid: Amides and hydroxamates. J. Med. Chem, 2013, 56, 5626–5630. [DOI] [PubMed] [Google Scholar]
- [27].Yu CW; Chang PT; Hsin LW; Chern JW Quinazolin-4-one Derivatives as selective histone deacetylase-6 inhibitors for the treatment of alzheimer’s disease. J. Med. Chem, 2013, 56, 6775–6791. [DOI] [PubMed] [Google Scholar]
- [28].Behrendt CT; Kunfermann A; Illarionova V; Matheeussen A; Pein MK; Grawert T; Kaiser J; Bacher A; Eisenreich W; Illarionov B; Fischer M; Maes L; Groll M; Kurz T Reverse fosmidomycin derivatives against the antimalarial drug target IspC (Dxr). J. Med. Chem, 2011, 54, 6796–802. [DOI] [PubMed] [Google Scholar]
- [29].Paris M; Porcelloni M; Binaschi M; Fattori D Histone deacetylase inhibitors: from bench to clinic. J. Med. Chem, 2008, 57, 1505–29. [DOI] [PubMed] [Google Scholar]
- [30].Supuran CT; Carta F; Scozzafava A Metalloenzyme inhibitors for the treatment of Gram-negative bacterial infections: A patent review (2009–2012). Expert Opin. Ther. Pat, 2013, 23, 777–88. [DOI] [PubMed] [Google Scholar]
- [31].Mai A Small-molecule chromatin-modifying agents: Therapeutic applications. Epigenomics, 2010, 2, 307–24. [DOI] [PubMed] [Google Scholar]
- [32].Mai A Hydroxamic Acids: Biological Properties and Potential Uses as Therapeutic Agents. Wiley Online Library, 2010. [Google Scholar]
- [33].Ganeshpurkar A; Kumar D; Singh SK, Strategies for the synthesis of Hydroxamic Acids. Curr. Org. Synth, 2018, 75, 154–165. [Google Scholar]
- [34].Trapencieris P; Strazdina J; Bertrand P Synthesis of small and medium size monocyclic hydroxamic acids (Review). Chem. Heterocycl. Com, 2012, 48, 833–855. [Google Scholar]
- [35].Codd R; Richardson-Sanchez T; Telfer TJ; Gotsbacher MP, Advances in the chemical biology of desferrioxamine B. ACS Chem. Biol, 2018, 13, II–25. [DOI] [PubMed] [Google Scholar]
- [36].Dhusia K; Bajpai A; Ramteke PW Overcoming antibiotic resistance: Is siderophore Trojan horse conjugation an answer to evolving resistance in microbial pathogens? J. Controlled Release, 2018, 269, 63–87. [DOI] [PubMed] [Google Scholar]
- [37].Sritharan M, Iron homeostasis in Mycobacterium tuberculosis: Mechanistic insights into siderophore-mediated iron uptake. J. Bacteriol, 2016, 198, 2399–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Shi YT; Jiang W; Auckloo BN; Wu B Several classes of natural products with metal ion chelating ability. Curr. Org. Chem, 2015, 19, 1935–1953. [Google Scholar]
- [39].NCI NCI Drug Dictionary. https://www.cancer.gov/publications/dictionaries/cancer-drug/def/vorinostat (February/24/2018),
- [40].Schobert R; Biersack B Multimodal HD AC inhibitors with improved anticancer activity. Curr. Cancer Drug Targets, 2018,18, 39–56. [DOI] [PubMed] [Google Scholar]
- [41].NCI NCI Drug Dictionary (Belinostat). https://www.cancer.gov/publications/dictionaries/cancer-drug/def/belinostat (February/27/2018),
- [42].NCI Panobinostat. Panobinostat is approved to be used with bortezomib and dexamethasone (February/27/2018),
- [43].Tak WY; Ryoo BY; Lim HY; Kim DY; Okusaka T; Ikeda M; Hidaka H; Yeon JE; Mizukoshi E; Morimoto M; Lee MA; Yasui K; Kawaguchi Y; Heo J; Morita S; Kim TY; Furuse J; Katayama K; Aramaki T; Hara R; Kimura T; Nakamura O; Kudo M Phase I/II study of first-line combination therapy with sorafenib plus resminostat, an oral HD AC inhibitor, versus sorafenib monotherapy for advanced hepatocellular carcinoma in east Asian patients. Invest New Drugs, 2018, 36, 1072–1084. [DOI] [PubMed] [Google Scholar]
- [44].NCI Clinical Trials Using Pracinostat. https://www.cancer.gov/about-cancer/treatment/clinical-trials/intervention/pracinostat (February/27/2018),
- [45].Mottamal M; Zheng S; Huang TL; Wang G, Histone deacetylase inhibitors in clinical studies as templates for new anticancer agents. Molecules, 2015, 20, 3898–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Trials, C. Clinical Study to Evaluate the Efficacy and Safety of Givinostat in Ambulant Patients With Duchenne Muscular Dystrophy. https://clinicaltrials.gov/ct2/show/NCT02851797 (February/28/2018),
- [47].Clinical Trials, Phase I/II Dose-Escalation Study of the Pan-Histone Deacetylase (HDAC) Inhibitor PCI-24781 in Lymphoma. 2014.
- [48].Clinical Trials. A Phase I Study to Evaluate the Safety and Tolerability of the Histone Deacetylase Inhibitor, CHR-3996, in Patients With Advanced Solid Tumours. https://clinicaltrials.gov/ct2/show/NCT00697879 (March/3/2018),
- [49].Galloway TJ; Wirth LJ; Colevas AD; Gilbert J; Bauman JE; Saba NF; Raben D; Mehra R; Ma AW; Atoyan R; Wang J; Burtness B; Jimeno A A Phase I Study of CUDC-101, a Multitarget Inhibitor of HDACs, EGFR, and HER2, in combination with chemoradiation in patients with head and neck squamous Cell Carcinoma. Clin. Cancer Res, 2015, 27, 1566–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sun S; Zhang Y; Zheng J; Duan B; Cui J; Chen Y; Deng W; Ye B; Liu L; Chen Y; Du J; Gu L HDAC6 inhibitor TST strengthens the antiproliferative effects of PI3K/mTOR inhibitor BEZ235 in breast cancer cells via suppressing RTK activation. Cell Death Dis, 2018, 9, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [51].Grassadonia A; Cioffi P; Simiele F; Iezzi L; Zilli M; Natoli C Role of hydroxamate-based histone deacetylase inhibitors (hb-hdacis) in the treatment of solid malignancies. Cancers, 2013, 5, 919–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Nguyen-Trung AT; Tritsch D; Grosdemange-Billiard C; Rohmer M Synthesis of tetrazole analogues of phosphonohydroxamic acids: An attempt to improve the inhibitory activity against the DXR. Bioorg. Med. Chem. Lett, 2013, 23,1643–7. [DOI] [PubMed] [Google Scholar]
- [53].Loppenberg M; Muller H; Pulina C; Oddo A; Teese M; Jose J; Holl R Synthesis and biological evaluation of flexible and conformationally constrained LpxC inhibitors. Org. Biomol. Chem, 2013, 77, 6056–70. [DOI] [PubMed] [Google Scholar]
- [54].Parker HL; Sherwood J; Hunt AJ; Clark JH Cyclic carbonates as green alternative solvents for the heck reaction. ACS Sustain. Chem. Eng, 2014,2,1739–1742. [Google Scholar]
- [55].Ramsay SL; Freeman C; Grace PB; Redmond JW; MacLeod JK Mild tagging procedures for the structural analysis of glycans. Carbohydr. Res, 2001, 333, 59–71. [DOI] [PubMed] [Google Scholar]
- [56].Shen J; Woodward R; Kedenburg JP; Liu X; Chen M; Fang L; Sun D; Wang PG Histone deacetylase inhibitors through click chemistry. J. Med. Chem, 2008, 51, 7417–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Nikitjuka A; Jirgensons A Synthesis of hydroxamic acids by using the acid labile O-2-Methylprenyl Protecting Group. Synlett. 2012, 2972–2974. [Google Scholar]
- [58].Martin NI; Woodward JJ; Marietta MA NG-hydroxyguanidines from primary amines. Org. Lett, 2006, 8, 4035–8. [DOI] [PubMed] [Google Scholar]
- [59].Miller MJ; Zhao G; Vakulenko S; Franzblau S; M?llmann U New C-3’ hydroxamate-substituted and more lipophilic cyclic hydroxamate cephalosporin derivatives as a potential new generation of selective antimicrobial agents. Org. Biomol. Chem, 2006, 4, 4178–4185. [DOI] [PubMed] [Google Scholar]
- [60].Kwak SY; Yang JK; Choi HR; Park KC; Kim YB; Lee YS Synthesis and dual biological effects of hydroxycinnamoyl phenylalanyl/prolyl hydroxamic acid derivatives as tyrosinase inhibitor and antioxidant. Bioorg. Med. Chem. Lett, 2013, 23, 1136–42. [DOI] [PubMed] [Google Scholar]
- [61].Porcheddu A; Giacomelli G Angeli-Rimini’s reaction on solid support: A new approach to hydroxamic acids. J. Org. Chem, 2006, 77, 7057–7059. [DOI] [PubMed] [Google Scholar]
- [62].Cal M; Jaremko M; Jaremko L; Stefanowicz P Solid phase synthesis of peptide hydroxamic acids on poly(ethylene glycol)-based support. J. Pept. Sei, 2013,79,9–15. [DOI] [PubMed] [Google Scholar]
- [63].Butler KV; Kalin J; Brochier C; Vistoli G; Langley B; Kozikowski AP Rational design and simple chemistry yield a superior, neuroprotective HDAC6 inhibitor, tubastatin A. J. Am. Chem. Soc, 2010,132, 10842–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kozlov MV; Kleymenova AA; Konduktorov KA; Kochetkov SN A new synthesis of 6-N-hydroxy-4-(2-methyl-1,2,3,4-tetrahydro-pyrido[4,3-b]indol-5-yhnethyl)benzamide, tubastatin a, a highly selective inhibitor of histone deacetylase. uss. J. Bioorg. Chem, 2013, 39,102–105. [DOI] [PubMed] [Google Scholar]
- [65].Marson CM; Savy P; Rioja AS; Mahadevan T; Mikol C; Veerupillai A; Nsubuga E; Chah wan A; Joel SP Aromatic sulfide inhibitors of histone deacetylase based on arylsulfinyl-2,4-hexadienoic acid hydroxyamides. J. Med. Chem, 2006, 49, 800–5. [DOI] [PubMed] [Google Scholar]
- [66].Hendricks JA; Keliher EJ; Marinelli B; Reiner T; Weissleder R; Mazitschek R In vivo PET imaging of histone deacetylases by 18F-suberoylanilide hydroxamic acid (18F-SAHA). J. Med. Chem, 2011, 54, 5576–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Chen Y; Lopez-Sanchez M; Savoy DN; Billadeau DD; Dow GS; Kozikowski AP A series of potent and selective, triazolylphenyl-based histone deacetylases inhibitors with activity against pancreatic cancer cells and Plasmodium falciparum. J. Med. Chem, 2008, 51, 3437–48. [DOI] [PubMed] [Google Scholar]
- [68].Kim DK; Lee JY; Kim JS; Ryu JH; Choi JY; Lee JW; Im GJ; Kim TK; Seo JW; Park HJ; Yoo J; Park JH; Kim TY; Bang YJ Synthesis and biological evaluation of 3-(4-substituted-phenyl)-N-hydroxy-2-propenamides, a new class of histone deacetylase inhibitors. J. Med. Chem, 2003, 46, 5745–51. [DOI] [PubMed] [Google Scholar]
- [69].Cai X; Zhai HX; Wang J; Forrester J; Qu H; Yin L; Lai CJ; Bao R; Qian C Discovery of 7-(4-(3-ethynylphenylamino)-7-methoxyquinazolin-6-yloxy)-N-hydroxyheptanamide (CUDc-101) as a potent multi-acting HD AC, EGFR, and HER2 inhibitor for the treatment of cancer. J. Med. Chem, 2010, 53, 2000–9. [DOI] [PubMed] [Google Scholar]
- [70].Salmi-Smail C; Fabre A; Dequiedt F; Restouin A; Castellano R; Garbit S; Roche P; Morelli X; Brunei JM; Collette Y Modified cap group suberoylanilide hydroxamic acid histone deacetylase inhibitor derivatives reveal improved selective antileukemic activity. J. Med. Chem, 2010, 53,3038–47. [DOI] [PubMed] [Google Scholar]
- [71].Cho YS; Whitehead L; Li J; Chen CH; Jiang L; Vogtle M; Francotte E; Richert P; Wagner T; Traebert M; Lu Q; Cao X; Dumotier B; Fejzo J; Rajan S; Wang P; Yan-Neale Y; Shao W; Atadja P; Shultz M Conformational refinement of hydroxamate-based histone deacetylase inhibitors and exploration of 3-piperidin-3-ylindole analogues of dacinostat (LAQ824). J. Med. Chem, 2010, 53, 2952–63. [DOI] [PubMed] [Google Scholar]
- [72].Cho M; Choi E; Yang JS; Lee C; Seo JJ; Kim BS; Oh SJ; Kim HM; Lee K; Park SK; Kwon HJ; Han G Discovery of pyridone-based histone deacetylase inhibitors: Approaches for metabolic stability. Chem. Med. Chem, 2013, 8, 272–9. [DOI] [PubMed] [Google Scholar]
- [73].Wang F; Lu W; Zhang T; Dong J; Gao H; Li P; Wang S; Zhang J Development of novel ferulic acid derivatives as potent histone deacetylase inhibitors. Bioorg. Med. Chem, 2013, 27, 6973–80. [DOI] [PubMed] [Google Scholar]
- [74].Kozlov MV; Kleymenova AA; Romanova LI; Konduktorov KA; Smirnova OA; Prasolov VS; Kochetkov SN Benzohydroxamic acids as potent and selective anti-HCV agents. Bioorg. Med. Chem. Lett, 2013, 23, 5936–40. [DOI] [PubMed] [Google Scholar]
- [75].Wagner FF; Olson DE; Gale JP; Kaya T; Weiwer M; Aidoud N; Thomas M; Davoine EL; Lemercier BC; Zhang YL; Holson EB Potent and selective inhibition of histone deacetylase 6 (HDAC6) does not require a surface-binding motif. J. Med. Chem, 2013, 56, 1772–6. [DOI] [PubMed] [Google Scholar]
- [76].Kozlov MV; Kleymenova AA; Romanova LI; Konduktorov KA; Smirnova OA; Prasolov VS; Kochetkov SN Benzohydroxamic acids as potent and selective anti-HCV agents. Bioorg. Med. Chem. Lett, 2013, 23, 5936–5940. [DOI] [PubMed] [Google Scholar]
- [77].Yang K; Nong K; Gu Q; Dong J; Wang J Discovery of N-hydroxy-3-alkoxybenzamides as direct acid sphingomyelinase inhibitors using a ligand-based pharmacophore model. Eur. J. Med. Chem, 2018,151, 389–400. [DOI] [PubMed] [Google Scholar]
- [78].Katritzky AR; Kirichenko N; Rogovoy BV Efficient conversions of carboxylic acids into O-alkyl, N-alkyl and O,N-dialkylhydroxamic acids. Synthesis, 2003, 2777–2780. [Google Scholar]
- [79].Kozlov MV; Kleymenova AA; Konduktorov KA; Malikova AZ; Kamarova KA; Novikov RA; Kochetkov SN Synthesis of (Z)-N-hydroxy-3-methoxy-3-phenylacrylamide as new selective inhibitor of hepatitis C virus replication. Russ. J. Bioorg. Chem, 2016, 42, 191–197. [Google Scholar]
- [80].Ho CY; Strobel E; Ralbovsky J; Galemmo RA Jr. Improved solution- and solid-phase preparation of hydroxamic acids from esters. J. Org. Chem, 2005,70,4873–5. [DOI] [PubMed] [Google Scholar]
- [81].Liang X; Lee CJ; Zhao J; Toone EJ; Zhou P Synthesis, structure, and antibiotic activity of aryl-substituted LpxC inhibitors. J. Med. Chem, 2013, 56, 6954–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Lee JH; Mahendran A; Yao Y; Ngo L; Venta-Perez G; Choy ML; Kim N; Ham WS; Breslow R; Marks PA, Development of a histone deacetylase 6 inhibitor and its biological effects. PNAS 2013,110,15704–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Giacomelli G; Porcheddu A; Salaris M Simple one-flask method for the preparation of hydroxamic acids. Org. Lett, 2003, 5, 2715–7. [DOI] [PubMed] [Google Scholar]
- [84].Massa S; Mai A; Sbardella G; Esposito M; Ragno R; Loidl P; Brosch G 3-(4-aroyl-lH-pyrrol-2-yl)-N-hydroxy-2-propenamides, a new class of synthetic histone deacetylase inhibitors. J. Med. Chem, 2001, 44, 2069–72. [DOI] [PubMed] [Google Scholar]
- [85].Mai A; Massa S; Ragno R; Esposito M; Sbardella G; Nocca G; Scatena R; Jesacher F; Loidl P; Brosch G Binding mode analysis of 3-(4-benzoyl-l-methyl-lH-2-pyrrolyl)-N-hydroxy-2-propenamide: A new synthetic histone deacetylase inhibitor inducing histone hyperacetylation, growth inhibition, and terminal cell differentiation. J. Med. Chem, 2002, 45, 1778–84. [DOI] [PubMed] [Google Scholar]
- [86].Chen JB; Chern TR; Wei TT; Chen CC; Lin JH; Fang JM, Design and synthesis of dual-action inhibitors targeting histone deacetylases and 3-hydroxy-3-methylglutaryl coenzyme A reductase for cancer treatment. J. Med. Chem, 2013, 56, 3645–55. [DOI] [PubMed] [Google Scholar]
- [87].Bouchain G; Leit S; Frechette S; Khalil EA; Lavoie R; Moradei O; Woo SH; Fournel M; Yan PT; Kalita A; Trachy-Bourget MC; Beaulieu C; Li Z; Robert MF; MacLeod AR; Besterman JM; Delorme D Development of potential antitumor agents. Synthesis and biological evaluation of a new set of sulfonamide derivatives as histone deacetylase inhibitors. J. Med. Chem, 2003, 46, 820–30. [DOI] [PubMed] [Google Scholar]
- [88].Woo SH; Frechette S; Abou Khalil E; Bouchain G; Vaisburg A; Bernstein N; Moradei O; Leit S; Allan M; Fournel M; Trachy-Bourget MC; Li Z; Besterman JM; Delorme D Structurally simple trichostatin A-like straight chain hydroxamates as potent histone deacetylase inhibitors. J. Med. Chem, 2002, 45, 2877–85. [DOI] [PubMed] [Google Scholar]
- [89].Hugenberg V; Riemann B; Hermann S; Schober O; Schafers M; Szardenings K; Lebedev A; Gangadharmath U; Kolb H; Walsh J; Zhang W; Kopka K; Wagner S Inverse 1,2,3-triazole-l-y 1-ethyl substituted hydroxamates as highly potent matrix metalloproteinase inhibitors: (radio)synthesis, in vitro and first in vivo evaluation. J. Med. Chem, 2013, 56, 6858–70. [DOI] [PubMed] [Google Scholar]
- [90].Varasi M; Thaler F; Abate A; Bigogno C; Boggio R; Carenzi G; Cataudella T; Dal Zuffo R; Fulco MC; Rozio MG; Mai A; Dondio G; Minucci S; Mercurio C Discovery, synthesis, and pharmacological evaluation of spiropiperidine hydroxamic acid based derivatives as structurally novel histone deacetylase (HDAC) inhibitors. J. Med. Chem, 2011, 54, 3051–64. [DOI] [PubMed] [Google Scholar]
- [91].Thaler F; Varasi M; Abate A; Carenzi G; Colombo A; Bigogno C; Boggio R; Zuffo RD; Rapetti D; Resconi A; Regalia N; Vultaggio S; Dondio G; Gagliardi S; Minucci S; Mercurio C Synthesis and biological characterization of spiro[2H-(l,3)-benzoxazine-2,4’-piperidine] based histone deacetylase inhibitors. Eur. J. Med. Chem, 2013, 64, 273–84. [DOI] [PubMed] [Google Scholar]
- [92].Thaler F; Colombo A; Mai A; Amici R; Bigogno C; Boggio R; Cappa A; Carrara S; Cataudella T; Fusar F; Gianti E; di Ventimiglia SJ; Moroni M; Munari D; Pain G; Regalia N; Sartori L; Vultaggio S; Dondio G; Gagliardi S; Minucci S; Mercurio C; Varasi M Synthesis and biological evaluation of N-hydroxyphenylacrylamides and N-hydroxypyridin-2-ylacrylamides as novel histone deacetylase inhibitors. J. Med. Chem, 2010, 53, 822–39. [DOI] [PubMed] [Google Scholar]
- [93].Freskos JN; Asmelash B; Gaston KR; Karwa A; Marzan TA; Nickols MA; Rogers TE; Schoenstein T; Sympson CJ; Vu B Design and synthesis of MMP inhibitors with appended fluorescent tags for imaging and visualization of matrix metalloproteinase enzymes. Bioorg. Med. Chem. Lett, 2013, 23, 5566–70. [DOI] [PubMed] [Google Scholar]
- [94].Mahboobi S; Sellmer A; Winkler M; Eichhorn E; Pongratz H; Ciossek T; Baer T; Maier T; Beckers T Novel chimeric histone deacetylase inhibitors: a series of lapatinib hybrides as potent inhibitors of epidermal growth factor receptor (EGFR), human epidermal growth factor receptor 2 (HER2), and histone deacetylase activity. J. Med. Chem, 2010, 53, 8546–55. [DOI] [PubMed] [Google Scholar]
- [95].Lai MJ; Huang HL; Pan SL; Liu YM; Peng CY; Lee HY; Yeh TK; Huang PH; Teng CM; Chen CS; Chuang HY; Liou JP Synthesis and biological evaluation of l-arylsulfonyl-5-(N-hydroxyacrylamide)indoles as potent histone deacetylase inhibitors with antitumor activity in vivo. J. Med. Chem, 2012, 55, 3777–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Suzuki T; Ota Y; Ri M; Bando M; Gotoh A; Itoh Y; Tsumoto H; Tatum PR; Mizukami T; Nakagawa H; Iida S; Ueda R; Shirahige K; Miyata N Rapid discovery of highly potent and selective inhibitors of histone deacetylase 8 using click chemistry to generate candidate libraries. J. Med. Chem, 2012, 55, 9562–75. [DOI] [PubMed] [Google Scholar]
- [97].Pabba C; Gregg BT; Kitchen DB; Chen ZJ; Judkins A Design and synthesis of aryl ether and sulfone hydroxamic acids as potent histone deacetylase (HDAC) inhibitors. Bioorg. Med. Chem. Lett, 2011, 21, 324–8. [DOI] [PubMed] [Google Scholar]
- [98].McAllister LA; Montgomery JI; Abramite JA; Reilly U; Brown MF; Chen JM; Barham RA; Che Y; Chung SW; Menard CA; Mitton-Fry M; Mullins LM; Noe MC; O’Donnell JP; Oliver RM 3rd; Penzien JB; Plummer M; Price LM; Shanmugasundaram V; Tomaras AP; Uccello DP Heterocyclic methylsulfone hydroxamic acid LpxC inhibitors as Gram-negative antibacterial agents. Bioorg. Med. Chem. Lett, 2012, 22, 6832–8. [DOI] [PubMed] [Google Scholar]
- [99].Gryder BE; Rood MK; Johnson KA; Patil V; Raftery ED; Yao LP; Rice M; Azizi B; Doyle DF; Oyelere AK, Histone deacetylase inhibitors equipped with estrogen receptor modulation activity. J. Med. Chem, 2013, 56, 5782–5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Guerrant W; Patil V; Canzoneri JC; Oyelere AK, Dual targeting of histone deacetylase and topoisomerase II with novel bifunctional inhibitors. J. Med. Chem, 2012, 55, 1465–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Chen L; Wilson D; Jayaram HN; Pankiewicz KW Dual inhibitors of inosine monophosphate dehydrogenase and histone deacetylases for cancer treatment. J. Med. Chem, 2007, 50, 6685–91. [DOI] [PubMed] [Google Scholar]
- [102].Remiszewski SW; Sambucetti LC; Bair KW; Bontempo J; Cesarz D; Chandramouli N; Chen R; Cheung M; Cornell-Kennon S; Dean K; Diamantidis G; France D; Green MA; Howell KL; Kashi R; Kwon P; Lassota P; Martin MS; Mou Y; Perez LB; Sharma S; Smith T; Sorensen E; Taplin F; Trogani N; Versace R; Walker H; Weltchek-Engler S; Wood A; Wu A; Atadja P N-hydroxy-3-phenyl-2-propenamides as novel inhibitors of human histone deacetylase with in vivo antitumor activity: Discovery of (2E)-N-hydroxy-3-[4-[[(2-hydroxyethyl)[2-(1H-indol-3-yl)ethyl]amino]methyl]phenyl]-2-propenamide (NVP-LAQ824). J. Med. Chem, 2003, 46, 4609–24. [DOI] [PubMed] [Google Scholar]
- [103].Deprez-Poulain R; Flipo M; Piveteau C; Leroux F; Dassonneville S; Florent I; Maes L; Cos P; Deprez B Structure-activity relationships and blood distribution of antiplasmodial aminopeptidase-1 inhibitors. J. Med. Chem, 2012, 55,10909–17. [DOI] [PubMed] [Google Scholar]
- [104].Zhang W; Zhang L; Li X; Weigel JA; Hall SE; Mayer JP Solid-phase synthesis of C-terminal peptide hydroxamic acids. J. Comb. Chem, 2001,5,151–3. [DOI] [PubMed] [Google Scholar]
- [105].Nandurkar NS; Petersen R; Qvortrup K; Komnatnyy VV; Taveras KM; Le Quem ent ST; Frauenlob R; Givskov M; Nielsen TE A convenient procedure for the solid-phase synthesis of hydroxamic acids on PEGA resins. Tetrahedron Lett, 2011, 52, 7121–7124. [Google Scholar]
- [106].Glenn MP; Kahnberg P; Boyle GM; Hansford KA; Hans D; Martyn AC; Parsons PG; Fairlie DP Antiproliferative and phenotype-transforming antitumor agents derived from cysteine. J. Med. Chem, 2004, 47, 2984–94. [DOI] [PubMed] [Google Scholar]
- [107].Marastoni E; Bartoli S; Berettoni M; Cipollone A; Ettorre A; Fincham CI; Mauro S; Paris M; Porcelloni M; Bigioni M; Binaschi M; Nardelli F; Parlani M; Maggi CA; Fattori D Benzofused hydroxamic acids: Useful fragments for the preparation of histone deacetylase inhibitors. Part 1: hit identification. Bioorg. Med. Chem. Lett, 2013, 23, 4091–5. [DOI] [PubMed] [Google Scholar]
- [108].Yin Z; Low K; Lye P N-linked hydroxylamine resin: Solid-phase synthesis of hydroxamic acids. Synth. Commun, 2005, 35, 2945–2950. [Google Scholar]
- [109].Hou J; Li Z; Fang Q; Feng C; Zhang H; Guo W; Wang H; Gu G; Tian Y; Liu P; Liu R; Lin J; Shi YK; Yin Z; Shen J; Wang PG Discovery and extensive in vitro evaluations of NK-HDAC-1: A chiral histone deacetylase inhibitor as a promising lead. J. Med. Chem, 2012, 55, 3066–75. [DOI] [PubMed] [Google Scholar]
- [110].Marek L; Hamacher A; Hansen FK; Kuna K; Marek L; Hamacher A; Hansen, Gohlke H; Kassack MU; Kurz T Histone deacetylase (HDAC) inhibitors with a novel connecting unit linker region reveal a selectivity profile for HDAC4 and HDAC5 with improved activity against chemoresistant cancer cells. J. Med. Chem, 2013, 56, 427–36. [DOI] [PubMed] [Google Scholar]
- [111].Lu Q; Yang YT; Chen CS; Davis M; Byrd JC; Etherton MR; Umar A; Chen CS Zn2+-chelating motif-tethered short-chain fatty acids as a novel class of histone deacetylase inhibitors. J. Med. Chem, 2004, 47, 467–74. [DOI] [PubMed] [Google Scholar]
- [112].Lu Q; Wang DS; Chen CS; Hu YD; Chen CS Structure-based optimization of phenylbutyrate-derived histone deacetylase inhibitors. J. Med. Chem, 2005, 48, 5530–5. [DOI] [PubMed] [Google Scholar]
- [113].Burkhart JL; Diehl B; Schmitt MJ; Kazmaier U A straightforward approach to MMP-2 and MMP-9 inhibitors based on chelate claisen rearrangements. Eur. J. Org. Chem, 2012, 2012, 567–575. [Google Scholar]
- [114].Nottingham M; Bethel CR; Pagadala SR; Harry E; Pinto A; Lemons ZA; Drawz SM; Akker F; Carey PR; Bonomo RA; Buynak JD Modifications of the C6-substituent of penicillin sulfones with the goal of improving inhibitor recognition and efficacy. Bioorg. Med. Chem. Lett, 2011,27,387–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Cosner CC; Bhaskara Reddy Iska V; Chatterjee A; Markiewicz JT; Corden SJ; Löfstedt J; Ankner T; Richer J; Hulett T; Schauer DJ; Wiest O; Helquist P evolution of concise and flexible synthetic strategies for Trichostatic Acid and the Potent Histone Deacetylase Inhibitor Trichostatin A. Eur. J. Org. Chem, 2013, 2013, 162–172. [Google Scholar]
- [116].Cosner CC; Helquist P Concise, convergent syntheses of (+/−)-trichostatin A utilizing a Pd-catalyzed ketone enolate alpha-alkenylation reaction. Org. Lett, 2011, 75, 3564–7. [DOI] [PubMed] [Google Scholar]
- [117].Nuti E; Casalini F; Santamaria S; Gabelloni P; Bendinelli S; DaPozzo E; Costa B; Marinelli L; La Pietra V; Novellino E; Margarida Bernardo M; Fridman R; Da Settimo F; Martini C; Rossello A Synthesis and biological evaluation in U87MG glioma cells of (ethynylthiophene)sulfonamido-based hydroxamates as matrix metalloproteinase inhibitors. Eur. J. Med. Chem, 2011, 46, 2617–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Fogli S; Banti I; Stefanelli F; Picchianti L; Digiacomo M; Macchia M; Breschi MC; Lapucci A Therapeutic potential of sulindac hydroxamic acid against human pancreatic and colonic cancer cells. Eur. J. Med. Chem, 2011, 46, 2617–29. [DOI] [PubMed] [Google Scholar]
- [119].Oyelere AK; Chen PC; Guerrant W; Mwakwari SC; Hood R; Zhang Y; Fan Y Non-peptide macrocyclic histone deacetylase inhibitors. J. Med. Chem, 2009,52,456–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Grolla AA; Podesta V; Chini MG; Di Micco S; Vallario A; Genazzani AA; Canonico PL; Bifulco G; Tron GC; Sorba G; Pirali T Synthesis, biological evaluation, and molecular docking of Ugi products containing a zinc-chelating moiety as novel inhibitors of histone deacetylases. J. Med. Chem, 2009, 52, 2776–85. [DOI] [PubMed] [Google Scholar]
- [121].Tazzari V; Cappelletti G; Casagrande M; Perrino E; Renzi L; Del Soldato P; Sparatore A New aryldithiolethione derivatives as potent histone deacetylase inhibitors. J. Med. Chem, 2009, 52, 2776–85. [DOI] [PubMed] [Google Scholar]
- [122].Guandalini L; Cellai C; Laurenzana A; Scapecchi S; Paoletti F; Romanelli MN Design, synthesis and preliminary biological evaluation of new hydroxamate histone deacetylase inhibitors as potential antileukemic agents. Bioorg. Med. Chem. Lett, 2008, 78, 5071–4. [DOI] [PubMed] [Google Scholar]
- [123].Sorensen MD; Blæhr LKA; Christensen MK; Hoyer T; Latini S; Hjarnaa P-JV; Björkling F Cyclic phosphinamides and phosphonamides, novel series of potent matrix metalloproteinase inhibitors with antitumour activity. Bioorg. Med. Chem. Lett, 2003, 77, 5461–5484. [DOI] [PubMed] [Google Scholar]
- [124].Mai A; Massa S; Pezzi R; Simeoni S; Rotili D; Nebbioso A; Scognamiglio A; Altucci L; Loidl P; Brosch G Class II (Ila)-selective histone deacetylase inhibitors. 1. Synthesis and biological evaluation of novel (aryloxopropenyl)pyrrolyl hydroxyamides. J. Med. Chem, 2005, 48, 3344–53. [DOI] [PubMed] [Google Scholar]
- [125].Kurz T; Widyan K O-Protected 3-hydroxy-oxazolidin-2,4-diones: Novel precursors in the synthesis of alpha-hydroxyhydroxamic acids. Org. Biomol. Chem, 2004,2,2023–7. [DOI] [PubMed] [Google Scholar]
- [126].Agarwal S; Gupta M; Choudhury B Bioprocess development for nicotinic acid hydroxamate synthesis by acyltransferase activity of Bacillus smithii strain IITR6b2. J. Ind. Microbiol. Biotechnol, 2013, 40, 937–46. [DOI] [PubMed] [Google Scholar]
- [127].Riva E; Gagliardi S; Mazzoni C; Passarella D; Rencurosi A; Vigo D; Martinelli M Efficient continuous flow synthesis of hydroxamic acids and suberoylanilide hydroxamic acid preparation. J. Org. Chem, 2009, 74, 3540–3. [DOI] [PubMed] [Google Scholar]
- [128].Papadopoulos GN; Kokotos CG Photoorganocatalytic One-Pot synthesis of Hydroxamic Acids from Aldehydes. Chem. Eur. J, 2016, 22, 6964–6967. [DOI] [PubMed] [Google Scholar]
- [129].Dettori G; Gaspa S; Porcheddu A; De Luca L One-Pot synthesis of Hydroxamic Acids from Aldehydes and Hydroxylamine. Adv. Synth. Catal, 2014,55(5,2709–2713. [Google Scholar]