Abstract
Background:
Over the course of the last 30 years, autism spectrum disorder (ASD) diagnoses have increased, thus identifying a large group of aging individuals with ASD. Currently, little is known regarding how aging will affect these individual’s neuroanatomy, compared to the neurotypical (NT) population. Because of the anatomical overlap of ASD-related cortical pathology and age-related cortical thinning, both following an anterior-to-posterior severity gradient, we hypothesize adults with ASD will show larger age-related cortical thinning than NT adults.
Methods:
We analyzed cortical measurements using available data from the multi-site Autism Brain Imaging Data Exchange I (ABIDE I; n=282) and our own cohort of middle-age to older adults with and without ASD (n=47) mostly available in ABIDE II (n=35). We compared correlations between cortical measures and age in right-handed adults with ASD (n=157) and similar NT adults (n = 172), controlling for IQ and site. Participants were 18 to 64 years of age (mean=29.8 years; median=26 years).
Results:
We found significant differences between diagnosis groups in the relationship between age and cortical thickness for areas of left frontal lobe (pars opercularis), temporal lobe (inferior gyrus, middle gyrus, banks of the superior temporal sulcus, and entorhinal cortex), parietal lobe (inferior gyrus), and lateral occipital lobe. For all areas, adults with ASD showed a greater negative correlation between age and cortical thickness than NT adults.
Conclusion:
As hypothesized, adults with ASD demonstrated exacerbated age-related cortical thinning, compared to NT adults. These differences were the largest and most extensive in the left temporal lobe. Future longitudinal work is warranted to investigate whether differences in brain age trajectories will translate to unique behavioral needs in older adults with ASD.
Keywords: ASD, Autism, Aging, Cortical Thickness, MRI, Temporal Lobe, Brain, Gray Matter
Introduction
The United States population is rapidly aging, expecting to approximately double the number of individuals over the age of 65 by 2030 (Knickman & Snell, 2002). Diagnosis rates of the developmental disorder, autism spectrum disorder (ASD), have also rapidly increased over the past 30 years (Weintraub, 2011). Combined, these two phenomenon are leading to a growing population of elderly with ASD, which is expected to reach over 700,000 individuals by 2030 (Piven & Rabins, 2011). Because the first children identified as autistic by Dr. Leo Kanner (Kanner, 1943) are only recently elderly, there is a dearth of research describing age-related biological changes in this population. Thus, there is a need to better understand the unique aging challenges adults with ASD may face, with the goal of maximizing quality of life and longevity.
Brain aging is of particular concern for adults with ASD. Research demonstrates abnormal cortical growth and other ASD-related pathology does not affect each lobe of the brain equally. Specifically, there is an anterior-to-posterior severity gradient where the frontal and temporal lobes are most severely affected, the parietal lobe is affected in some but not all cases, and the occipital lobe is relatively spared (Carper & Courchesne, 2005; Courchesne et al. 2011; van Rooij et al., 2017; Wallace et al., 2015). This has direct relevance to brain aging because normal cortical atrophy follows a similar anterior-to-posterior severity gradient (Galluzzi et al., 2008; Pfefferbaum et al., 2013). The anatomic overlap between ASD-related pathology and normal age-related cortical atrophy suggests the anterior cortices of adults with ASD may be vulnerable to exacerbated age-related changes.
There is support from limited cross-sectional reports that the brain may degenerate faster in adults with ASD. When investigating total brain size, faster decline is seen in early middle-age adults with ASD compared to NT adults (Courchesne et al., 2011). Across studies there is somewhat of an age-related pattern of cortical thickness/volume differences: those that center on adolescent and young-adults with ASD report larger gray matter volume (Bonilha et al., 2008; Hyde et al., 2010; Ke et al., 2008; Rojas et al., 2006; Waiter et al., 2005); and thicker cortices (Chen, Jiao, & Herskovits, 2011; Hyde et al., 2010; Khundrakpam et al., 2017) than NT, but a report of slightly older adults reveals thinner cortices in the ASD group (Hadjikhani et al., 2006). However, two studies found both thicker and thinner cortices in participants with ASD, depending on the region examined (Ecker et al., 2013; van Rooij et al., 2017), and another study that spanned adolescents to young adulthood found only thinner cortices in the ASD group (Wallace et al., 2010). Strikingly, the anterior cortices are more robustly affected in nearly every report.
Cross-sectional studies examining interactions between age and diagnosis from childhood to older adults are contradictory, with some showing increased thinning with age in ASD (Libero et al., 2014; Wallace et al., 2010), decreased thinning with age (Raznahan et al., 2010; Scheel et al., 2011), both increased and decreased thinning, depending on the region (Rooji et al., 2018), and no difference from NT participants (Koolschijn & Geurts, 2016). These inconsistencies likely arise from three reasons: 1) relatively small sample sizes, 2) demographic differences between groups (e.g. IQ, sex, handedness, etc.), and 3) different age ranges (e.g. inclusion of children vs. adults only). In this investigation, we used the Autism Brain Imaging Data Exchange (ABIDE I and ABIDE II [BNI site only]; Di Martino et al. 2014, 2017) to examine relationships between cortical thickness and age for ASD and NT groups. This study included adults only (18+ years of age) to focus on the effects of brain aging rather than development. This is among the largest sample sizes to investigate cortical thickness and age in adults with ASD. In alignment with most cortical research across age ranges in adults with ASD, we hypothesized adults with ASD would have larger age-related differences in cortical thickness, than NT adults. Further, we hypothesized divergent trajectories would be most evident in anterior cortices (frontal and temporal lobes), with relative sparing of the occipital lobe.
Methods
Participants
We analyzed structural MRI data available through the ABIDE I database, which includes 539 ASD and 573 NT participants with MRI scans and phenotypic information from 17 different sites. Participants under the age of 18 were excluded from this study (n=762), to focus on the effects of brain aging in adults with ASD. Of note, the adult age cutoff is not universally agreed upon. We define adult as over 18 years to allow the most complete comparison to previous literature. Participants whose data contained motion artifacts (n=31, see below for details) and who were not right handed (n=37; see limitations section for details) were also excluded, leaving 282 participants (ASD n=132, NT n=150). Because middle-age and older adults are sparsely represented in ABIDE I, we added our own cohort of 47 adults over the age of 40 (ASD n=25, NT n=22), most of which (n=35) are available in ABIDE II under the site Barrow Neurological Institute (BNI). These groups combined (n=329) allowed us to compare relationships between cortical thickness and age as a continuous variable in adults with and without ASD.
ASD Diagnosis and NT Controls
Inclusion in the ASD group was based on DSM-IV-TR criteria for a clinician diagnosis of ASD. Diagnostic methods varied by site; however, all diagnoses were supported by review of clinical data and administration of the Autism Diagnostic Observation Schedule (Lord et al. 2000) and/or the Autism Diagnostic Interview—Revised (Lord et al.,1994). Two-hundred forty-one participants (73.2%) reported current medication status. Of that, 26 participants (10.8%) were taking psychotropic medications (ASD n=25, NT n=1). Comorbidity reporting was even more sparse (n=18, 5.4%), of which nine participants with ASD (50%) reported a comorbid psychiatric condition (e.g. ADHD, anxiety, specific phobia, or dysthymia). Participants were classified as NT if their medical history was absent of any neurological conditions or Axis-I disorders by DSM-IV criteria. See http://fcon_1000.projects.nitrc.org/indi/abide/for full diagnostic procedures by site. All work was carried out in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Declaration of Helsinki as revised in 2000. All participants provided informed consent at their respective institution.
MRI Acquisition
For this multi-site sample, scanner type and structural MRI sequence parameters differed across sites (see http://fcon_1000.projects.nitrc.org/indi/abide/). Structural scans from the ABIDE I dataset have been extensively examined; according to Turner et al. (2016), we excluded any participants’ data determined via visual examination by blinded members of this research group to have motion artifacts. These criteria were used to minimize the probability that signal differences between groups could be due to motion artifacts, poor image quality, or segmentation errors (Reuter et al., 2015). Motion excluded participant’s demographics can be found in Supp. Table 1, and the number of excluded participants by ABIDE I site can be found in Supp. Table 2. The largest percentage of excluded participants were from the Social Brain Lab (SBL) at the Netherlands Institute for Neuroscience, and mostly NT participants. Other demographic features (age, IQ, ADOS, and sex) were very similar to the participants retained for analyses (Supp. Table 1).
Image analysis
T1-weighted images were processed to generate surface models and obtain cortical thickness using FreeSurfer version 5.3.0 image analysis suite. These processing steps have been described in detail elsewhere (Cosman Jr, Fischl, Wells III, & Dale, 1999; Fischl & Dale, 2000; Han et al., 2006). Cortical thickness was defined as the distance between the white and gray matter boundary to the pial surface. We focus on cortical thickness because others have previously identified this as the primary contributor to age-related gray matter changes in ASD (Wallace et al., 2015) and typical aging (Storsve et al., 2014). Segmentations were visually inspected to ensure accuracy of automated processes. All images examined in this study met acceptable segmentation registration with no large deformities requiring manual editing. This is likely due to the exclusion of participants containing artifacts prior to the FreeSurfer pipeline and is consistent with other studies (Koolschijn and Geurts, 2016; Libero et al., 2014). Individual surfaces were aligned to an average spherical representation (fsaverage) to improve correspondence of measurement points. Cortical thickness data was smoothed with a 15 mm full-width-at-half-maximum Gaussian kernel prior to statistical analysis, which is common throughout the literature to provide a normal distribution of results (e.g. Wallace et al., 2010, 2015; Koolschijn and Geurts 2016; Scheel et al., 2011).
Statistical analyses
Whole-brain vertex-wise analysis was performed with FreeSurfer’s general linear modeling tool “mri_glmfit”. Our primary analyses of interest were univariate linear models to assess the main effects and interaction between diagnosis group (ASD vs. NT) and age on cortical thickness. Covariates included IQ and site. Statistical maps were generated with an FDR threshold of p<0.05. Whole-brain cortical thickness measurements were mapped onto the “inflated” surface. Plots were generated to show the relationship between age and cortical thickness for ASD and NT groups in regions with statistical significance.
Results
Demographic variables
There were no significant differences in age or sex between the ASD and NT groups (Table 1). To further assure groups were equivalently represented across this broad age range (18–64 years), we identified the number of participants in each group appearing within five-year age windows (Supp. Table 3; Supp. Figure 1). Analysis revealed statistically similar distribution across the five-year age windows between groups X2(9, N=329)=7.27, p=0.61. There was a significant differences between ASD and NT groups for IQ (Table 1), thus this variable was included as a covariate in cortical thickness analyses in addition to site (Supp. Table 4 shows group demographics by ABIDE Site).
Table 1.
Demographic variables
| ASD Mean (SD) Range | NT Mean (SD) Range | Test statistic | p-value | Effect size | |
|---|---|---|---|---|---|
| Participants (M/F) | 144/13 | 154/18 | X2 =0.46 | 0.50 | d=0.07 |
| Age (years) | 30.9 (12.4) 18–64 | 28.8 (10.5) 18–64 | t-score = 1.60 | 0.11 | d=0.18 |
| FIQa | 109.0 (15.3) 53–137 | 113.9 (10.9) 89–148 | t-score = −3.37 | <0.001 | d=0.37 |
| ADOSb total | 11.9 (3.6) 2–21 | NA | - | - | - |
Full Scale Intelligence Quotient;
Autism Diagnostic Observation Schedule
Correlations between age and cortical thickness: Diagnosis group differences
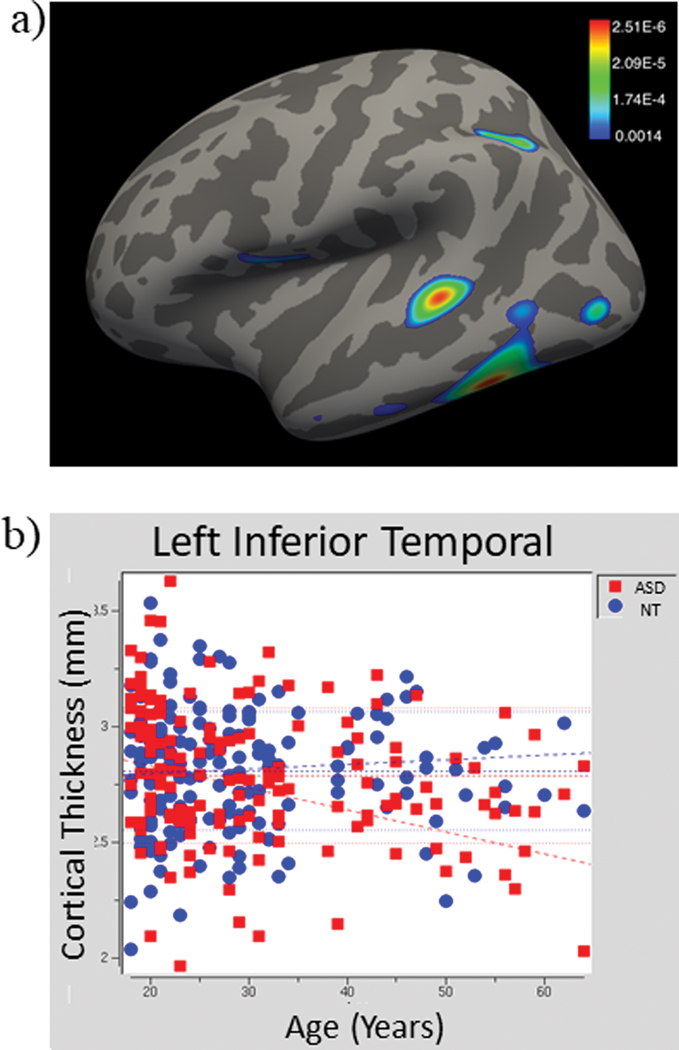
The correlation between cortical thickness and age was significantly different between ASD and NT groups in areas of the left frontal lobe (pars opercularis), temporal lobe (inferior gyrus, middle gyrus, banks of the superior temporal sulcus, and entorhinal cortex), parietal lobe (inferior gyrus), and lateral occipital lobe (Fig. 1a, Table 2). Specifically, participants in the ASD group showed a stronger negative correlation between age and cortical thickness in these regions than NT participants (Fig. 1b, example scatterplot; see Supp. Fig. 2 for scatterplots from each cluster). The results remained largely the same when excluding participants with ASD who had known psychiatric comorbidities or taking psychotropic medication (n=31); however, there were no differences between groups in the occipital lobe (Supp. Figure 3, Supp. Table 5).
Fig. 1:
(a) Areas where the relationship between cortical thickness and age are significantly (p < 0.05, false discovery rate corrected) different between the ASD and NT groups on the left lateral surface. (c) A representative significant cluster (left inferior temporal gyrus) showing a negative correlation with age in the ASD group and no correlation in the NT group.
Table 2.
Cluster summary information for correlations between age and cortical thickness: Diagnosis group differences
| Lobe | Cluster | Max1 | VtxMax2 | Size(mm2)3 | TalX, TalY, TalZ4 | NVtxs5 | WghtVtx6 |
|---|---|---|---|---|---|---|---|
| Frontal | Pars Opercularis | 3.417 | 54559 | 130.58 | −24.8, 33.3, −9.2 | 451 | 1416.95 |
| Temporal | Inferior temporal | 5.601 | 70062 | 873.65 | −28.6, −42.9, −40.5 | 1792 | 6811.41 |
| Banks of superior temporal sulcus | 5.414 | 117701 | 244.07 | −39.9, −24.4, −16.2 | 930 | 3648.63 | |
| Inferior temporal | 3.056 | 148752 | 42.5 | −36.5, −10.4, −48.1 | 88 | 259.26 | |
| Middle temporal | 2.878 | 53069 | 3.84 | −32.4, 12.2, −52.4 | 9 | 25.76 | |
| Parietal | Inferior parietal | 4.528 | 147160 | 272.85 | −20, −50.1, 32.2 | 1152 | 4092.6 |
| Occipital | Lateral occipital | 3.866 | 31882 | 108.14 | −19.9, −80.4, −18.5 | 243 | 806.3 |
Maximum −log10(pvalue) in cluster;
Vertex number at maximum;
Surface area of cluster;
Talairach (MNI305) coordinate of maximum;
Number of vertices in cluster;
Weight of cluster (size x intensity)
Age and diagnosis main effects
Across all participants, controlling for diagnosis, IQ, and site, there were widespread main effects of age on cortical thickness. Age-related cortical thickness reductions were bilateral, but strongest in the right frontal lobe (medial and lateral), lateral temporal lobe, and medial parietal lobes, with relative sparing of the occipital lobe (Supp. Fig. 4). There were also increases with age found in medial temporal regions (Supp. Fig. 4). There were no main effects of diagnosis on cortical thickness without respect to age.
Discussion
The present study investigated age-related cortical differences in adults with ASD, compared to NT, in a large, multi-site dataset. As hypothesized, adults with ASD showed larger age-related differences in cortical thickness than NT adults, such that as age increased there was a greater reduction in cortical thickness in adults with ASD than NT adults. Effects were seen all lobes of the left hemisphere, however the strongest and most extensive areas affected were in the temporal lobe. Despite widespread main effects of age, diagnosis groups main effects were not detected when age was not considered, highlighting the importance of including age as a measure of interest in all cortical investigations of adults with ASD.
This relatively large study provides compelling evidence of vulnerability to accelerated cortical thinning in adults with ASD. This work builds on previous investigations of cortical growth patterns in ASD, first identifying larger cortices in children with ASD (Courchesne et al., 2011), which can be seen in the youngest adults in our sample. This is consistent with other young-adult ABIDE I investigations (Khundrakpam et al., 2017; Haar et al., 2016). Gray matter volume has also been shown to be significantly larger in children to young adults with ASD, with relative sparing of the occipital lobe (Bonilha 2008; Waiter 2004). Longitudinal studies have identified abnormal gray matter growth trajectories in children with ASD (Chen et al., 2011). Our results suggest abnormal cortical growth trajectories continue and change in adulthood and are most pronounced in the left temporal lobe.
The first neuroimaging investigation of older adults in ASD identified larger age-related declines in total brain volumes (Courchesne et al., 2011) similar to age-related cortical thickness patterns in our results. Age-related cortical thinning in adults with ASD has also been identified by others in regions that overlap with our results, namely the left pars opercularis (Libero et al., 2014) and left temporal regions (Wallace et al., 2010). Also similar to our results, a recent longitudinal investigation in children through young adults found significant age-related cortical thinning in left frontal and bilateral parietal regions in participants with ASD, compared to NT individuals (Zielinski et al., 2014). However, investigating this developmental age range, Zielinski and colleagues (2014) failed to find significant effects in temporal regions. Taken together, adult aging may have unique and robust effect on thinning of the temporal lobe in individuals with ASD, which warrants future longitudinal studies to confirm.
There is contradictory evidence that age-related cortical thinning may be no different or decelerated in individuals with ASD compared to NT. Investigating participants with ASD aged 30 to 75 years, Koolschijn and Geurts (2016) concluded no age-related differences in cortical measures from NT participants. However, when they investigated discrete age groups (younger vs. older adults) they found a significant interaction in the insular cortex with larger age-related differences in the ASD group (Koolschijn and Geurts, 2016). This patter is similar to our results, however, we did not identify the insula as a region that was different between adults with ASD and NT adults. Both Scheel et al. (2011) and Raznahan et al. (2010) found age-related cortical differences to be decreased in individuals with ASD compared to NT. Raznahan and colleagues (2010) included participants under the age of 18, introducing a larger developmental trajectory that could explain the differences from our results and other literature. Again, the collective body of research and our findings identify a need for longitudinal studies to confirm accelerated cortical thinning with age in ASD.
A subset of ABIDE sites reported psychiatric co-morbidities and/or psychotropic medication use. When we removed these participants from the analysis (n=31), results remained largely the same; however, there were no differences between groups in the occipital lobe. This suggests differences in the aging trajectory of the occipital cortex may be influenced by psychiatric co-morbidities and/or medication use. Indeed, others have found thinner occipital cortices (among other regions) in adults with major depressive disorder compared to health controls (Tu et al., 2012). Since ABIDE does not have full psychiatric co-morbidity information, how the interaction between ASD and psychiatric co-morbidities affects cortical thinning in adulthood warrants further investigation with a fully characterized sample.
Behavioral implications
The regions identified in these studies have functional implications for both ASD symptoms and age-related cognitive changes. First, our results show strong effects in areas of the temporal lobe (e.g. superior temporal sulcus) which have been postulated to underlie social cognition deficits in ASD (Zilbovicius et al., 2006). Further, we identified differences in an area of the left frontal lobe (pars opercularis) that is essential for language production, which is a common difficulty in ASD. Others have found reduced volume of the pars opercularis in adults with ASD correlates with social communication difficulties (Yamasaki et al., 2010). Next, we identified differences in the inferior parietal lobe, which is involved in aspects of executive functioning, especially working memory (Alvarez & Emory, 2006). This is concerning for older adults with ASD because existing executive function difficulties (Braden et al., 2017; Davids, 2016; Leeds et al., 2001; Rosenthal et al., 2013) may be exacerbated by aging (Kensinger & Gutchess, 2017; Wallace et al., 2016). In line with these executive function and social communication concerns, we recently found cognitive aspects of social communication difficulties to be greater on older adults with ASD than young adults with ASD, which was related to greater executive function difficulties and hypoconnectivity of the fronto-parietal network (Walsh et al., in press). Finally, contrary to our hypothesis, cortical thickness of the left lateral occipital lobe also showed a stronger, negative relationship with age in adults with ASD. This could have functional implications for aspects of visual attention and working memory that also decline with normal aging (Cabeza et al., 2004). Identifying the behavioral implications age-related gray matter changes in adults with ASD is warranted.
Limitations
In this investigation, we used a large, adult-only dataset to mitigate limitations of previous studies. However, some limitations remained and should be acknowledged. First, the adult age cutoff is not universally agreed upon, and some consider ages 18–20 to be teenagers. We defined adult as over 18 years to allow the most complete comparison to previous literature of brain aging in adults with ASD (e.g. Ecker et al., 2013). However, we acknowledge the variability of adult age cutoff in the larger literature, and possible limitation of grouping “teenagers” with “non-teenagers”. Second, there is an inherent limitation of ABIDE as a multi-site dataset with diverse MRI instruments and acquisition parameters that likely introduces noise to the data. To mitigate this, site was included as a co-variate in all analyses, and we excluded any participants’ data predetermined to have motion artifacts (Turner et al., 2016). Third, ABIDE I limits aging questions to the context of cross-sectional design. Longitudinal designs are the gold-standard for aging research in order to control for individual variability and cohort effects. Cohort effects are of particular concern for ASD research because of the dramatic changes in diagnosis, treatment, and awareness over the past decades. Future longitudinal, single site studies are necessary to delineate age related differences in ASD.
Our sample may not be representative of the entire population of adults with ASD for multiple reasons. First, only about 10% are women. While there is a large gender disparity in ASD diagnosis (~3:1, M:F), there is a greater percentage of females with ASD in the larger population than are represented in this study (U.S. Department of Health and Human Services, 2014). Importantly, ASD and NT groups were similar for distributions of men and women in this study, but we were not powered to investigate sex differences. Further research is warranted to determine how brain aging may differentially affect women and men with ASD. Second, our sample did not include left-handed or ambidextrous participants. We restricted participants to right-handed only because there were no left-handed individuals over the age of 40 in ABIDE I, and ASD and NT groups were significantly different in handedness representation. Others have also demonstrated handedness significantly influences cortical features across a clinical group (e.g. Hamilton et al., 2007). This could explain why our effects were localized to the left hemisphere, which is what others have found in younger ABIDE participants (Khundrakpam et al., 2017). Future research in how aging affects non-right-handed individuals with ASD is warranted. Finally, our sample tended to be of average or above average intellectual functioning based on IQ. To ensure IQ did not confound results, we statistically controlled for IQ as a covariate. But, few participants were in the range of intellectually disabled (ID). Conversely, an estimated 30% of individuals with ASD in the U.S. have IQs in the range of ID (CDC, 2014). Thus, the present results may not be generalizable to brain aging in adults with co-morbid ASD and ID.
Conclusions
Results from this large, cross-sectional study suggests adults with ASD may experience faster age-related cortical thinning than NT adults. Further, the data suggest this potential divergent aging trajectory most strongly affects the left temporal lobe. Findings advance our understanding of how adults with ASD will experience brain aging. These brain aging differences point to behavioral domains to be evaluated in aging adults with ASD, with the goal of maximizing quality of life and longevity.
Supplementary Material
Supp. Fig. 1. Histogram of number of participants with ASD and NT participants in five-year windows from ages 18–64.
Supp. Fig. 3. Left hemisphere areas where the relationship between cortical thickness and age are significantly different between the ASD and NT groups when participants with known psychiatric co-morbidities and/or taking psychotropic medications were excluded.
Supp. Fig. 2. Scatterplots for all significant clusters in Fig. 1a.
Supp. Fig. 4: Areas of age main effects on cortical thickness, p < 0.05, false discovery rate corrected.
Acknowledgements
We are very thankful to the ABIDE I team and participating institutions for access to this valuable data repository. Primary support for the work by Adriana Di Martino was provided by the NIMH (K23MH087770) and the Leon Levy Foundation. Primary support for the work by Michael P. Milham and the INDI team was provided by gifts from Joseph P. Healy and the Stavros Niarchos Foundation to the Child Mind Institute, as well as by an NIMH award to MPM (R03MH096321). The BNI Site from ABIDE II was funded by Department of Defense, Grant Number: AR140105 and State of Arizona (Arizona Alzheimer’s Consortium).
Footnotes
Conflict of Interest
The authors of this study have no financial, personal, or other relationships that may pose a potential conflicts of interest related to this research.
References
- A.M. F. (2010). Structural brain changes in aging: Courses, causes and cognitive consequences. Reviews in the Neurosciences, 21(3), 187–221. 10.1515/REVNEURO.2010.21.3.187 [DOI] [PubMed] [Google Scholar]
- Alvarez JA, & Emory E. (2006). Executive function and the frontal lobes: A meta-analytic review. Neuropsychology Review, 16(1), 17–42. 10.1007/s11065-006-9002-x [DOI] [PubMed] [Google Scholar]
- Barttfeld P, Wicker B, Cukier S, Navarta S, Lew S, Leiguarda R, & Sigman M. (2012). State-dependent changes of connectivity patterns and functional brain network topology in autism spectrum disorder. Neuropsychologia, 50(14), 3653–3662. 10.1016/j.neuropsychologia.2012.09.047 [DOI] [PubMed] [Google Scholar]
- Bonilha L, Cendes F, Rorden C, Eckert M, Dalgalarrondo P, Li LM, & Steiner CE (2008). Gray and white matter imbalance - Typical structural abnormality underlying classic autism? Brain and Development, 30(6), 396–401. 10.1016/j.braindev.2007.11.006 [DOI] [PubMed] [Google Scholar]
- Braden BB, Smith CJ, Thompson A, Glaspy TK, Wood E, Vatsa D, … Baxter LC (2017). Executive function and functional and structural brain differences in middle-age adults with autism spectrum disorder. Autism Research, 1–15. 10.1002/aur.1842 [DOI] [PubMed] [Google Scholar]
- Bressler SL, & Menon V. (2010). Large-scale brain networks in cognition: emerging methods and principles. Trends in Cognitive Sciences, 14(6), 277–290. 10.1016/j.tics.2010.04.004 [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, & Nyberg L. (2004). Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cerebral cortex, 14(4), 364–375. [DOI] [PubMed] [Google Scholar]
- Carper RA, & Courchesne E. (2005). Localized enlargement of the frontal cortex in early autism. Biological Psychiatry, 57(2), 126–133. 10.1016/j.biopsych.2004.11.005 [DOI] [PubMed] [Google Scholar]
- Chen R, Jiao Y, & Herskovits EH (2011). Structural MRI in autism spectrum disorder. Pediatric Research, 69(5), 63R–8R. 10.1203/PDR.0b013e318212c2b3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosman E Jr, Fischl B, Wells III W, & Dale A. (1999). Topology Correction for Cortical Surface Models, 169–170. [Google Scholar]
- Courchesne E, Campbell K, & Solso S. (2011). Brain growth across the life span in autism: Age-specific changes in anatomical pathology. Brain Research, 1380, 138–145. 10.1016/j.brainres.2010.09.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davids RC (2016). Executive functions in older adults with autism spectrum disorder: objective preformance and subjective complaints, 46(Sep), 2016–2017. [DOI] [PubMed] [Google Scholar]
- Di Martino A, O’Connor D, Chen B, Alaerts K, Anderson JS, Assaf M, ... & Blanken LM (2017). Enhancing studies of the connectome in autism using the autism brain imaging data exchange II. Scientific data, 4, 170010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Yan CG, Li Q, Denio E, Castellanos FX, Alaerts K, ... & Deen B. (2014). The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Molecular psychiatry, 19(6), 659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker C, Ginestet C, Feng Y, Johnston P, Lombardo MV, Lai MC, … Murphy DGM (2013). Brain surface anatomy in adults with autism: The relationship between surface area, cortical thickness, and autistic symptoms. Archives of General Psychiatry, 70(1), 59–70. 10.1001/jamapsychiatry.2013.265 [DOI] [PubMed] [Google Scholar]
- Fischl B, & Dale AM (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences, 97(20), 11050–11055. 10.1073/pnas.200033797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi S, Beltramello A, Filippi M, & Frisoni GB (2008). Aging. Neurological Sciences, 29(SUPPL. 3), 296–300. 10.1007/s10072-008-1002-6 [DOI] [PubMed] [Google Scholar]
- Ge Y, Grossman RI, Babb JS, Rabin ML, Mannon LJ, & Kolson DL (2002). Age-related total gray matter and white matter changes in normal adult brain. Part I: volumetric MR imaging analysis. AJNR. American Journal of Neuroradiology, 23(8), 1327–1333. [PMC free article] [PubMed] [Google Scholar]
- Haar S, Berman S, Behrmann M, & Dinstein I. (2016). Anatomical Abnormalities in Autism? Cerebral Cortex, 26(4), 1440–1452. 10.1093/cercor/bhu242 [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, & Tager-Flusberg H. (2006). Anatomical differences in the mirror neuron system and social cognition network in autism. Cerebral Cortex, 16(9), 1276–1282. 10.1093/cercor/bhj069 [DOI] [PubMed] [Google Scholar]
- Hamilton LS, Narr KL, Luders E, Szeszko PR, Thompson PM, Bilder RM, & Toga AW (2007). Asymmetries of cortical thickness: effects of handedness, sex, and schizophrenia. Neuroreport, 18(14), 1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, … Fischl B. (2006). Reliability of MRI-derived measurements of human cerebral cortical thickness: The effects of field strength, scanner upgrade and manufacturer. NeuroImage, 32(1), 180–194. 10.1016/j.neuroimage.2006.02.051 [DOI] [PubMed] [Google Scholar]
- Hyde KL, Samson F, Evans AC, & Mottron L. (2010). Neuroanatomical differences in brain areas implicated in perceptual and other core features of autism revealed by cortical thickness analysis and voxel-based morphometry. Human Brain Mapping, 31(4), 556–566. 10.1002/hbm.20887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Chen R, Ke X, Chu K, Lu Z, & Herskovits EH (2010). Predictive models of autism spectrum disorder based on brain regional cortical thickness. NeuroImage, 50(2), 589–599. 10.1016/j.neuroimage.2009.12.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner L. (1943). Autistic disturbances of affective contact. Nervous Child. Pathology. 10.1105/tpc.11.5.949 [DOI] [PubMed] [Google Scholar]
- Ke X, Hong S, Tang T, Zou B, Li H, Hang Y, … Liu Y. (2008). Voxel-based morphometry study on brain structure in children with high-functioning autism. Neuroreport, 19(9), 921–925. 10.1097/WNR.0b013e328300edf3 [DOI] [PubMed] [Google Scholar]
- Kensinger EA, & Gutchess AH (2017). Cognitive Aging in a Social and Affective Context: Advances Over the Past 50 Years. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences, 72(1), 61–70. 10.1093/geronb/gbw056 [DOI] [PubMed] [Google Scholar]
- Khundrakpam BS, Lewis JD, Kostopoulos P, Carbonell F, & Evans AC (2017). Cortical Thickness Abnormalities in Autism Spectrum Disorders Through Late Childhood, Adolescence, and Adulthood: A Large-Scale MRI Study. Cerebral Cortex, 27(3), 1721–1731. 10.1093/cercor/bhx038 [DOI] [PubMed] [Google Scholar]
- Knecht S. (2000). Handedness and hemispheric language dominance in healthy humans. Brain, 123(12), 2512–2518. 10.1093/brain/123.12.2512 [DOI] [PubMed] [Google Scholar]
- Knickman JR, & Snell EK (2002). The 2030 problem: caring for aging baby boomers. Health Services Research, 37(4), 849–84. 10.1034/j.1600-0560.2002.56.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn PCMP, & Geurts HM (2016). Gray Matter Characteristics in Mid and Old Aged Adults with ASD. Journal of Autism and Developmental Disorders, 46(8), 2666–2678. 10.1007/s10803-016-2810-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeds L, Meara RJ, Woods R, & Hobson JP (2001). A comparison of the new executive functioning domains of the CAMCOG-R with existing tests of executive function in elderly stroke survivors. Age and Ageing, 30(3), 251–254. 10.1093/ageing/30.3.251 [DOI] [PubMed] [Google Scholar]
- Libero LE, DeRamus TP, Deshpande HD, & Kana RK (2014). Surface-based morphometry of the cortical architecture of autism spectrum disorders: Volume, thickness, area, and gyrification. Neuropsychologia, 62(1), 1–10. 10.1016/j.neuropsychologia.2014.07.001 [DOI] [PubMed] [Google Scholar]
- Lord Catherine; Risi Susan, Cook Edwin H. Jr.; Leventhal Bennet L.; DiLavore Pamela C.; Pickles Andrew; Rutter M, & \. (2000). The Autism Diagnostic Observation Schedule— Generic: A Standard Measure of Social and Communication Deficits. CEUR Workshop Proceedings, 1621(June 2000), 36–43. 10.1023/A [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, & Le Couteur A. (1994). Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 10.1007/BF02172145 [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rohlfing T, Rosenbloom MJ, Chu W, Colrain IM, & Sullivan EV (2013). Variation in longitudinal trajectories of regional brain volumes of healthy men and women (ages 10 to 85years) measured with atlas-based parcellation of MRI. NeuroImage, 65, 176–193. 10.1016/j.neuroimage.2012.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piven J, & Rabins P. (2011). Autism spectrum disorders in older adults: Toward defining a research agenda. Journal of the American Geriatrics Society, 59(11), 2151–2155. 10.1111/j.1532-5415.2011.03632.x [DOI] [PubMed] [Google Scholar]
- Raznahan A, Toro R, Daly E, Robertson D, Murphy C, Deeley Q, … Murphy DGM (2010). Cortical anatomy in autism spectrum disorder: An in vivo MRI study on the effect of age. Cerebral Cortex, 20(6), 1332–1340. 10.1093/cercor/bhp198 [DOI] [PubMed] [Google Scholar]
- Reuter M, Tisdall MD, Qureshi A, Buckner RL, van der Kouwe AJW, & Fischl B. (2015). Head motion during MRI acquisition reduces gray matter volume and thickness estimates. NeuroImage, 107, 107–115. 10.1016/j.neuroimage.2014.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas DC, Peterson E, Winterrowd E, Reite ML, Rogers SJ, & Tregellas JR (2006). Regional gray matter volumetric changes in autism associated with social and repetitive behavior symptoms. BMC Psychiatry, 6(1), 56 10.1186/1471-244X-6-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal M, Wallace GL, Lawson R, Wills MC, Yerys BE, & Kenworthy L. (2013). Impairments in Real World Executive Function Increase from Childhood to Adolescence in Autism Spectrum Disorders. Neuropsychology, 27(1), 13–18. 10.1037/a0031299.Impairments [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheel C, Rotarska-Jagiela A, Schilbach L, Lehnhardt FG, Krug B, Vogeley K, & Tepest R. (2011). Imaging derived cortical thickness reduction in high-functioning autism: Key regions and temporal slope. NeuroImage, 58(2), 391–400. 10.1016/j.neuroimage.2011.06.040 [DOI] [PubMed] [Google Scholar]
- Storsve AB, Fjell AM, Tamnes CK, Westlye LT, Overbye K, Aasland HW, & Walhovd KB (2014). Differential Longitudinal Changes in Cortical Thickness, Surface Area and Volume across the Adult Life Span: Regions of Accelerating and Decelerating Change. Journal of Neuroscience, 34(25), 8488–8498. 10.1523/JNEUROSCI.0391-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Uddin LQ, Prater K, Amin H, Greicius MD, & Menon V. (2010). Development of functional and structural connectivity within the default mode network in young children. NeuroImage, 52(1), 290–301. 10.1016/j.neuroimage.2010.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu PC, Chen LF, Hsieh JC, Bai YM, Li CT, & Su TP (2012). Regional cortical thinning in patients with major depressive disorder: A surface-based morphometry study. Psychiatry Research - Neuroimaging, 202(3), 206–213. 10.1016/j.pscychresns.2011.07.011 [DOI] [PubMed] [Google Scholar]
- Turner AH, Greenspan KS, & van Erp TGM (2016). Pallidum and lateral ventricle volume enlargement in autism spectrum disorder. Psychiatry Research - Neuroimaging, 252, 40–45. 10.1016/j.pscychresns.2016.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. (2014). Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2010. Morbidity and Mortality Weekly Report. Surveillance Summaries (Washington, D.C. : 2002), 63(2), 1–21. https://doi.org/24670961 [PubMed] [Google Scholar]
- Uddin LQ (2010a). Saliency, switching, attention and control: a network model of insula function. Brain, 214, 655–667. 10.1007/s00429-010-0262-0.Saliency [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ (2010b). Typical and atypical development of functional human brain networks: insights from resting-state fMRI. Frontiers in Systems Neuroscience, 4(May), 1–12. 10.3389/fnsys.2010.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij D, Anagnostou E, Arango C, Auzias G, Behrmann M, Busatto GF, … Buitelaar JK (2017). Cortical and Subcortical Brain Morphometry Differences Between Patients With Autism Spectrum Disorder and Healthy Individuals Across the Lifespan: Results From the ENIGMA ASD Working Group. American Journal of Psychiatry, (7), appi.ajp.2017.1. 10.1176/appi.ajp.2017.17010100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waiter GD, Williams JHG, Murray AD, Gilchrist A, Perrett DI, & Whiten A. (2005). Structural white matter deficits in high-functioning individuals with autistic spectrum disorder: A voxel-based investigation. NeuroImage, 24(2), 455–461. 10.1016/j.neuroimage.2004.08.049 [DOI] [PubMed] [Google Scholar]
- Walsh MJM, Baxter LC, Smith CJ, & Braden BB (In Press). Age Group Differences in Executive Network Functional Connectivity and Relationships with Social Behavior in Men with Autism Spectrum Disorder. Research in Autism Spectrum Disorder. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace GL, Budgett J, & Charlton RA (2016). Aging and autism spectrum disorder: Evidence from the broad autism phenotype. Autism Research, 9(12), 1294–1303. 10.1002/aur.1620 [DOI] [PubMed] [Google Scholar]
- Wallace GL, Dankner N, Kenworthy L, Giedd JN, & Martin A. (2010). Age-related temporal and parietal cortical thinning in autism spectrum disorders. Brain, 133(12), 3745–3754. 10.1093/brain/awq279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace GL, Eisenberg IW, Robustelli B, Dankner N, Kenworthy L, Giedd JN, & Martin A. (2015). Longitudinal Cortical Development During Adolescence and Young Adulthood in Autism Spectrum Disorders: Increased Cortical Thinning but Comparable Surface Area Changes. Journal of the American Academy of Child & Adolescent Psychiatry, 54(6), 464–9. 10.1016/j.jaac.2015.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub K. (2011). Autism counts. Nature, 479(3), 22–24. 10.1038/479022a [DOI] [PubMed] [Google Scholar]
- Zielinski BA, Prigge MBD, Nielsen JA, Froehlich AL, Abildskov TJ, Anderson JS, … Lainhart JE (2014). Longitudinal changes in cortical thickness in autism and typical development. Brain, 137(6), 1799–1812. 10.1093/brain/awu083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki S, Yamasue H, Abe O, Suga M, Yamada H, Inoue H, ... & Kano Y. (2010). Reduced gray matter volume of pars opercularis is associated with impaired social communication in high-functioning autism spectrum disorders. Biological psychiatry, 68(12), 1141–1147. [DOI] [PubMed] [Google Scholar]
- Zilbovicius M, Meresse I, Chabane N, Brunelle F, Samson Y, & Boddaert N. (2006). Autism, the superior temporal sulcus and social perception. Trends in Neurosciences, 29(7), 359–366. 10.1016/j.tins.2006.06.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supp. Fig. 1. Histogram of number of participants with ASD and NT participants in five-year windows from ages 18–64.
Supp. Fig. 3. Left hemisphere areas where the relationship between cortical thickness and age are significantly different between the ASD and NT groups when participants with known psychiatric co-morbidities and/or taking psychotropic medications were excluded.
Supp. Fig. 2. Scatterplots for all significant clusters in Fig. 1a.
Supp. Fig. 4: Areas of age main effects on cortical thickness, p < 0.05, false discovery rate corrected.