Abstract
Background:
The integrity and connectivity of the frontal lobe, which subserves fluency, may be compromised by both ASD and aging. Alternate networks often integrate to help compensate for compromised functions during aging. We used network analyses to study how compensation may overcome age-related compromised in individuals with ASD.
Method:
Participants consisted of middle-aged (40–60; n=24) or young (18–25; n=18) right-handed males who have a diagnosis of ASD, and age- and IQ-matched control participants (n=20, 14, respectively). All performed tests of language and executive functioning and a fluency functional MRI task. We first used group individual component analysis (ICA) for each of the 4 groups to determine whether different networks were engaged. An SPM analysis was used to compare activity detected in the network nodes from the ICA analyses.
Results:
The individuals with ASD performed more slowly on two cognitive tasks (Stroop word reading and Trailmaking Part A). The 4 groups engaged different networks during the fluency fMRI task despite equivalent performance. Comparisons of specific regions within these networks indicated younger individuals had greater engagement of the thalamus and supplementary speech area, while older adults engaged the superior temporal gyrus. Individuals with ASD did not disengage from the Default Mode Network during word generation.
Conclusion:
Interactions between diagnosis and aging were not found in this study of young and middle-aged men, but evidence for differential engagement of compensatory networks was observed.
Introduction
Individuals diagnosed with Autism Spectrum Disorder (ASD) often experience impairments in language functioning, ranging from non-verbalism to difficulty with semantic comprehension. Impairments in the language domain result in marked difficulty with communication and social interaction. Problems with verbal fluency may be part of a constellation of executive functions linked to other common symptoms of ASD, such as repetitive, restricted behaviors and interests (RRBI;(Turner, 1999). Studies of verbal fluency in ASD have shown mixed results, with some studies reporting impairments (Ambery, Russell, Perry, Morris, & Murphy, 2006; Bramham et al., 2009; Spek, Schatorjé, Scholte, & van Berckelaer-Onnes, 2009) while others have not (Brady et al., 2017). Geurts and Vissers (2012) compared verbal fluency in older adults with ASD and older NT adults, and found only marginal between-group differences, while verbal fluency in only the NT group showed a strong negative relationship with age. As with other studies, their study focused on tests emphasizing executive functioning because this cognitive domain shows more prominent age- and ASD-related declines, and the frontal lobe subserving executive functioning is affected by both aging and ASD. To date, few studies have studied older individuals with ASD (Alaerts et al., 2015; Braden et al., 2017; Davids et al, 2016; Farrant & Uddin, 2016; Geurts & Vissers, 2012; Lever & Guerts, 2016, Koolschijn, Caan, Teeuw, Olabarriaga, & Geurts, 2017, Powell et al, 2017), but the extensive literature regarding aging effects on cognition in normal aging points to a general negative effect that is most prominent for executive functioning, including fluency, cognitive flexibility, and processing speed (Park et al., 2002) even after risk for dementia is controlled for (Barnes et al., 2003).
Magnetic resonance imaging (MRI) studies of regional changes in brain volume indicate that a steady decline associated with normal aging, especially in the frontal and parietal lobes and the hippocampal region (Raz et al., 2005). White matter decline follows a similar pattern and can be observed as early as the 5th decade (Sexton et al., 2014). Studies of brain structural differences in individuals with ASD have implicated changes in frontal lobe structure, long-range connections, and frontal lobe connectivity (Catani et al., 2008; Di Martino et al., 2014; Hadjikhani, Joseph, Snyder, & Tager-Flusberg, 2006). Although individuals with ASD may have frontal lobe vulnerabilities different from those associated with aging, it is possible that aging may exacerbate these ASD-related vulnerabilities, resulting in more noticeable differences in cognitive functioning, as well as greater difficulties compensating for age-related changes (Braden et al., 2017; Park & Reuter-Lorenz, 2009)
Fluency is dependent upon an area in the left inferior frontal gyrus (LIFG), also referred to as “Broca’s area” or Brodmann area 44. Word generation tasks are often used to investigate frontal lobe integrity in many conditions that affect the frontal lobe, including ASD (Kenworthy et al., 2013). In a study of adolescent and young adults, Kenworthy et al. (2013) investigated the neural correlates of automatic speech, several fluency, and switching tasks in well-matched groups of ASD and NT individuals. A complex pattern of group differences in brain activity was observed, such that the individuals with ASD showed decreased blood-oxygen-level dependent (BOLD) contrast compared to the NT group in several frontal cortical regions, including the LIFG and middle frontal gyri as well as bilateral thalamus and putamen, and cerebellar regions during fluency tasks. However, in a different region of the LIFG, they found an increase in brain activity for individuals with ASD (Kenworthy et al., 2013). These and other findings suggest that word generation, which requires flexible and effortful processing, differentially recruits neural networks that are integrating functions to perform the task. We sought to investigate the effects of age and ASD on networks activated during word generation by comparing activation patterns during a word generation task in young and middle-aged adults with ASD with age- and IQ-matched NTs. We utilized a phonemic fluency fMRI task, rather than a semantic fluency task, because phonemic fluency relies on frontal systems while semantic memory tasks engage the temporal lobe (Biesbroek et al., 2016). We hypothesized that older adults with ASD would show greater decline in frontal lobe activity than their age-matched counterparts, due to the additive effects of ASD and aging.
Similar to the findings described above, compensatory increases in neural activity in ASD (Kenworthy et al., 2013), there is evidence for compensatory increases during some tasks in the elderly. This finding has been reported primarily for verbal working memory tasks, and is characterized by a change from more focal left prefrontal activation in younger adults to increased bilateral engagement in older adults when performance is maintained (Cabeza, Anderson, Locantore, & McIntosh, 2002; Rosen et al., 2002; Stebbins et al., 2002). In order to capture the effects of ASD or aging on neural network engagement during word generation, we first used group independent component analysis (ICA) separately on the young and middle-aged ASDs and their matched NT groups. Given our prediction that compensation based on age or diagnostic group will result in group differences, separate group ICA helps minimize heterogeneity of variance and will help to capture any network that may be represented in just one of the groups. After identifying all of the networks significantly involved in performing the task, the brain activity within these circuits was then statistically assessed for interactions between age and diagnosis. By assessing both cognitive and fMRI brain activation patterns in younger and older adults, we extend the literature regarding the effects of aging and possible ways this “double jeopardy” (Geurts & Vissers, 2012) predicts long term outcome for individuals with ASD.
Methods
Participants
We assessed behavioral and neuroimaging data from 4 groups: middle-aged (40–60 year old) and young (18–25) male adults with a diagnosis of ASD and age- and IQ-matched controls. Our sample consisted of 24 middle-aged individuals with ASD (M = 53, SD = 8) and 20 NT (M = 50, SD = 7) men and 18 young men with ASD (M = 21, SD = 3) and 14 NT (M = 21, SD = 3). Participants with ASD were recruited primarily from the Southwest Autism Research and Resource Center (SARRC) as well as from community organizations. Recruitment of age- and IQ-matched NT participants was done via the local community. All ASD participants met DSM 5 criteria for the disorder (American Psychiatric Association, 2000, 2013) either by history or current presentation. Symptoms were confirmed with the Autism Diagnostic Observation Schedule-2, module 4 [ADOS-2, (Lord, DiLavore, & Gotham, 2012)] which was administered by a research reliable rater. The average age of diagnosis was reported for most participants. Diagnosis age for middle-aged men was 38 years (SD = 17 years; range: 3–60, n = 19) and for the young adults was 14 years (SD = 7 years; range 3 – 23; n = 12). The NT group participants had no first degree relative with ASD and a negative screen for ASD symptoms using the Social Responsiveness Scale [SRS-2; (Constantino & Gruber, 2012)]. IQ was estimated with the Kaufman Brief Intelligence Test 2nd edition (Kaufman & Kaufman, 2004). To screen for cognitive disorder prior to full neuropsychological testing, we required a score 26 on the Mini Mental State Exam (Folstein, Folstein, & McHugh, 1975), and no reported history of neurological illness such as stroke, traumatic brain injury, or loss of consciousness. A small percentage of the ASD participants had experienced a single childhood seizure (4%), but none continued to experience seizures in adulthood nor took seizure medication. While NT participants were excluded if they had a history of psychiatric disorders like depression or anxiety, we did not exclude presence of history of depression or anxiety in ASD participants due to the common comorbidity of ASD and these disorders. Anxiety or depression medications were used by 31% of the ASD participants. Current symptoms of depression and anxiety were identified through self-report Beck Depression Inventory-II and State-Trait Anxiety Inventory, respectively (Beck, Steer, & Brown, 1996; Spielberger, 2010). Presence of depression/anxiety medication was assessed to determine if there were any differences in performance in the ASD group, and none were observed (all p’s >.30). No participants had current or past history of other major psychiatric diagnoses such as schizophrenia or bipolar disorder. All participants provided informed consent approved by the Institutional Review Boards of both institutions (Barrow Neurological Institute and Southwest Autism Resource and Research Center). All work was carried out in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Declaration of Helsinki as revised in 2000.
Cognitive Tests
Language and other cognitive abilities were assessed via commonly used neuropsychological tests on the same day as the MRI. The Controlled Oral Word Association Test (Benton, Hamsher, & Sivan, 1994) was given to assess phonemic fluency. The COWAT measures the number of words generated that begin with a specified letter within a one minute time frame and a total from three letter trials (C, F, and L) is computed. We assessed semantic fluency with Animal Naming (number of animals in one minute). We estimated basic verbal processing speed using the word reading trial of the Stroop task which requires the participant to read the words “red”, “green” or “blue” presented randomly in columns as quickly as they can in 45 seconds. Another nonverbal processing task was the Trail-making task, Part A, in which participants drew a line along a consecutive series of numbers that are scattered on the page. General verbal knowledge was estimated using the Vocabulary subtest from the Wechsler Adult Intelligence Scale- III (Wechsler, 1997).
MRI parameters
All participants were scanned on the same 3-Tesla Philips Ingenia MRI scanner. We acclimated participants to the MRI environment if necessary to minimize anxiety-related motion. The scans used in this analysis were collected as part of a larger battery of functional and structural scans. FMRI scans were collected in a counterbalanced order. We collected a T1-weighted anatomical scan (3D magnetization prepared rapid acquisition gradient echo [MPRAGE], 256 × 256 in-plane resolution, 240 mm field of view (FOV); 170 sagittal slices, 1.2 mm thick). Gradient-echo EPIs were collected to map blood oxygen-level dependent signal for the fluency fMRI task with TE = 25ms, TR = 3,000 ms, flip angle = 80, 24 mm FOV, 64 × 64 in-plane resolution, with 3 mm-thick axial slices covering the entire brain.
The fluency fMRI task was created based on the COWAT fluency test as a covert word generation to letter task. Unlike the COWAT that requires generation of words to a letter for one minute to determine a maximal ability, the fMRI task uses the blood oxygenation level-dependent (BOLD) technique that requires brief periods of behavior alternating with a “control” condition. As with other studies, (Kenworthy et al., 2013), task performance in fMRI does not need to be different between groups in order to see differences in neural engagement during the task. Specifically, the participants were instructed to silently think of as many words as they could that begin with a specified letter that is presented on the screen for 6 seconds. The fMRI task consisted of 5 word generation blocks that showed 2 letters per block for 12 second blocks (4 TRs) alternating with baseline blocks in which the word “Relax” was presented for 18 seconds, resulting in a total of 60 volumes, for a total task time of 3 minutes. Participants performed the task twice, with different letter prompts per task. To measure number of words generated and ensure task engagement, participants were instructed to press a button on a response box for each word generated during the fluency blocks. Responses were recorded via button press using a fiber optic response device (Current Designs, Philadelphia, PA). Goggles (Nordic Neurolab, Bergen, Norway) were used to present visual prompts. Padding and headphones were used to stabilize the head for motion control and noise minimization. The goggles, which are attached to the head coil and required a steady gaze to maintain visualization of the stimuli, also helped minimize movement.
MRI data processing
The fMRI images were preprocessed using Statistical Parametric Mapping 12 (SPM12; Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK) in Matlab (MathWorks, Natick, MA). Images were realigned to correct for motion, using a least squares approach and a six parameter (rigid body) spatial transformation to the mean image and stereotactically normalized to the Montreal Neurological Institute (MNI) template by minimizing the sum of squares difference between the linear combination of the original and template images. Images were smoothed to 8 mm3 full-width half-maximum Gaussian kernel. As previously reported (Braden et al., 2017), using goggles and prepping of the participants minimizes motion; SPM realign motion parameters for all participants confirmed less than one voxel or one degree of movement in any direction for each fMRI scan, thus no further motion-scrubbing steps during ICA steps were taken. Additionally, each participants’ scans were visually inspected to confirm no susceptibility distortions and no need for field map correction (Braden et al., 2017).
Data analysis to determine networks contributing to fluency
We performed a two-step data analysis. Since our main interest was to identify how age and ASD may affect what networks are engaged to perform a fluency task, we wished to ensure that we did not obscure any network engagement that would be present in any individual group. Thus, we first used independent component analysis (ICA) on each of the four groups separately to visually identify brain regions that contributed to any network engaged in this phonemic fluency task. ICA is a data-driven analysis that can identify temporally coherent networks underlying fMRI activity (V. Calhoun, Adali, Pearlson, & Pekar, 2001) and has been shown to work well in small samples (Celone et al., 2006); (Romero-Rebollar, Jiménez-Ángeles, Dragustinovis-Ruiz, & Medina-Bañuelos, 2016). Group ICA was performed in Matlab using the GIFT toolbox (Group ICA fMRI Toolbox; GIFT; http://icatb.sourceforge.net). The procedure is described in Braden et al. (2017). Briefly, spatial maps were averaged across both runs of the task. Each voxel from the spatial maps was calibrated with a z-score denoting how much the time course contributed to the average time course of the component (Beckmann, DeLuca, Devlin, & Smith, 2005). Components were first spatially sorted to standard white matter and cerebral spinal fluid masks and disregarded from analysis if r2>.02 or r2>.05, respectively (Kim et al., 2009). As previously described (Jung et al., 1998), ICA is capable of determining motion components, which are not included as final components for analysis (V. D. Calhoun & de Lacy, 2017) As per Pignat et al. (2013), we did not apply low- and high-pass filters in order to capture potential frequency information that can contribute to component decomposition (Pignat et al., 2013). We employed the traditional band-pass filter free ICA approach (Kim et al., 2009). We removed all motion-related components and the component associated with finger tapping from assessment. Since each resulting component is thought to represent a brain network engaged in the task, we will refer to components as “networks”.
Assessing group differences in specific brain regions
As expected, the 4 groups engaged both unique and common networks (see Results and Fig 1). We performed statistical analyses to compare BOLD signal differences in each of the network nodes identified in the ICA analyses using SPM. We first performed individual first-level time series analyses to generate images representing the contrast of word generation > baseline. These contrast images were entered into a second order, random effects analysis. Region of interest data from each of the identified nodes from all of the significant ICA networks (see Table 2) were extracted from the SPM analysis using Marsbar (Brett, Anton, Valabregue, & Poline, 2002), using a 5 mm sphere from the peak of the activation in the group analysis. Statistical group differences from the mean beta value were determined using SPSS version 19.
Figure 1.
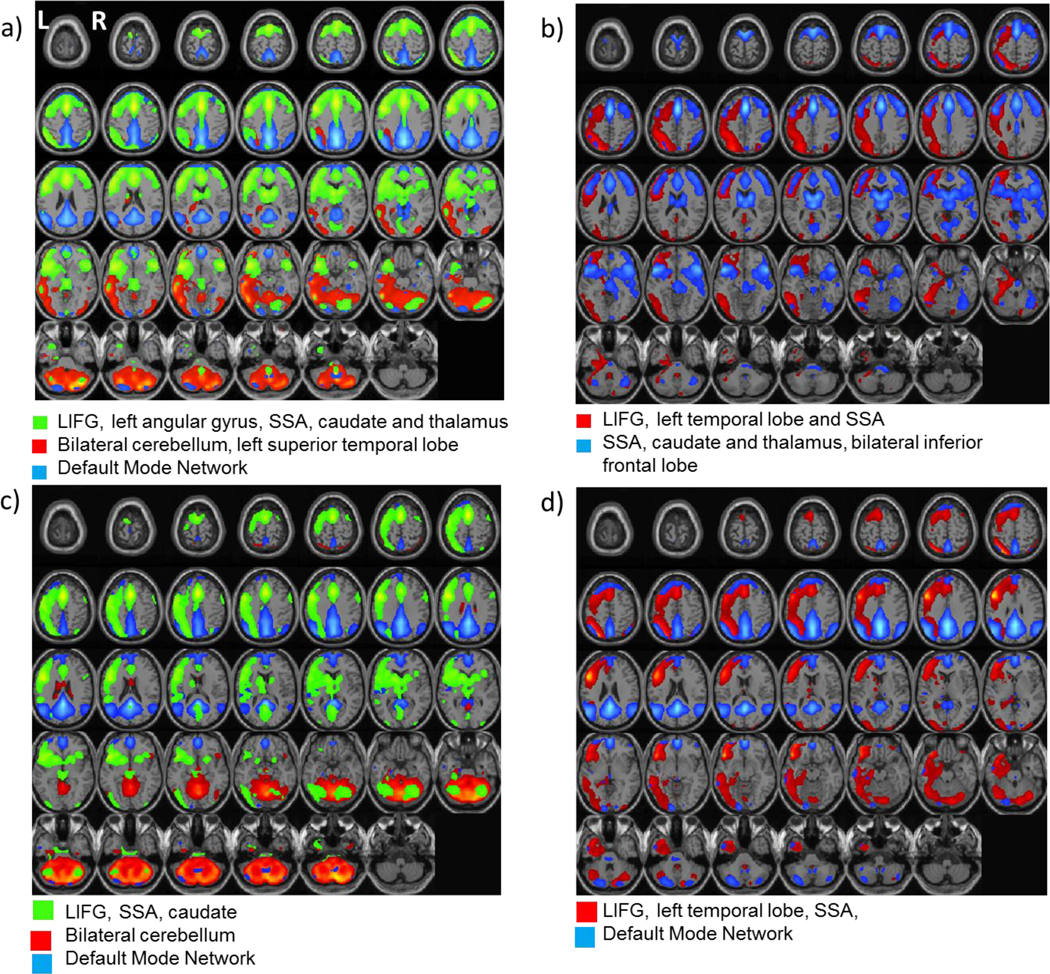
Fluency neural networks in aging and ASD. Group independent components analyses associated for word generation produced common and unique networks. All groups produced a network including left inferior frontal gyrus. Some but not all groups showed a network involving the SSA and subcortical structures, the Default Mode Network, and bilateral cerebellar network. a: young NT, b:young ASD, c: middle-aged NT, d: middle-aged ASD. LIFG: left inferior frontal gyrus; SSA: supplemental speech area.
Table 2.
Networks Recruited During Word Generation vs Rest
| Group | Network | Condition (Fluency or Rest) | M | SD | F | p* |
|---|---|---|---|---|---|---|
| NT Young | ||||||
| LIFG | Fluency | 0.44 | 0.56 | 7.21 | .01 | |
| Cerebellar | Fluency | 0.52 | 0.48 | 6.61 | .02 | |
| DMN | Rest | −0.029 | 0.70 | 5.47 | .02 | |
| ASD Young | ||||||
| Language | Fluency | 0.37 | 0.53 | 8.24 | .007 | |
| SSA/subcortical | Fluency | 0.41 | 0.50 | 7.17 | .01 | |
| NT Middle-aged | ||||||
| LIFG | Fluency | 0.33 | 0.37 | 27.07 | < .001 | |
| Cerebellum | Fluency | 0.41 | 0.27 | 19.83 | < .001 | |
| DMN | Fluency | −0.31 | 0.42 | 15.84 | < .001 | |
| ASD Middle-aged | ||||||
| LIFG | Fluency | 0.31 | 0.45 | 9.53 | .003 | |
| DMN | Rest | 0.21 | 0.37 | 32.07 | < .001 |
Family-wise error corrected. NT: Neurotypical; ASD: Autism Spectrum Disorder; LIFG: left inferior frontal gyrus: DMN: Default Mode Network; SSA: supplementary speech area
Results
Groups were well-matched and did not differ in estimated IQ across Group or Age, p = .41 (see Table 1). An independent samples t-test showed that the mean age of young adults with ASD did not differ from young NTs, t (30) = 0.40, p =.69, Cohen’s d = 0.14, and the mean age of middle-aged adults with ASD did not significantly differ from middle-aged NTs, t (42) = −1.19, p = .24, Cohen’s d = 0.36. There was a significant Age effect for education, where older individuals (M = 15.50, SD = 2.49) had more years of education than young (M = 13.47, SD = 1.65) individuals, F (1, 68) = 15.91, p < .001, partial eta squared (η2) = 0.96. There was also a Group effect for education, where NT (M = 15.29, SD = 2.39) individuals had more years of education than adults with ASD (M = 14.12, SD 2.27) individuals, F (1, 68) = 5.68, p .02, ƞ2 = 0.50. Using education as a covariate in the fMRI analyses did not alter the results; the results presented below are without the covariate.
Table 1.
Demographic and Cognitive Task Performance Across Age Cohorts and Groups
| NT | ASD | F | |||||
|---|---|---|---|---|---|---|---|
| Young N = 14 M (SD) | Middle-aged N = 20 M (SD) | Young N = 18 M (SD) | Middle-aged N = 24 M (SD) | Age (ES; p) | Group (ES; p) | Age* Group (partial η2; p) | |
| IQ | 107.21 (12.68) | 112.50 (12.79) | 105.33 (13.50) | 106.00 (17.93) | 0.74 (0.19; .39) | 1.47 (0.32; .23) | 0.45 (0.01; .51) |
| Educationa,b (years) | 14.29 (1.86) | 16 (2.51) | 12.83 (1.15) | 15.08 (2.45) | 15.91** (0.96; <.001) | 5.68* (0.50; .02) | 0.29 (.004; .59) |
| COWATc (words) | 41.07 (12.73) | 40.35 (13.50) | 31.06 (9.03) | 41.04 (13.08) | 2.60 (0.42; .11) | 2.63 (0.31; .11) | 3.47 (.05; .07) |
| Animalsd (words) | 24.29 (5.37) | 21.85 (3.82) | 20.83 (4.83) | 20.17 (6.36) | 1.60 (0.27; .20) | 4.39* (0.46; .04) | 0.52 (0.01; .47) |
| WAIS-III Vocabularye (raw score) | 47.93 (10.08) | 50.95 (12.19) | 46.11 (9.81) | 52.33 (7.83) | 3.91* (0.48; .05) | 0.01 (0.003; .92) | 0.47 (0.006; .49) |
| Stroopf (words) | 92.57 (29.38) | 88.80 (15.14) | 79.94 (12.00) | 77.04 (21.42) | 0.51 (0.15; .48) | 6.85* (0.60; .01) | 0.01 (0.001; .93) |
| Trail Making Test Part Ag (secs) | 22.79 (6.48) | 26.6 (8.61) | 31.56 (7.08) | 39.50 (25.08) | 2.64 (0.38; .11) | 8.95* (0.74; .004) | 0.33 (0.004; .57) |
| fMRI Fluency (words via button response) | 54.46 (13.30) | 50.10 (10.47) | 48.79 (12.85) | 47.08 (9.73) | 1.27 (0.24; .26) | 2.59 (0.36; .11) | 0.24 (0.003; .62) |
Young < Old
NT > ASD
ASD Young < All
NT > ASD * p values uncorrected for multiple comparisons
Young < Old
NT > ASD
NT < ASD
p ≤ .05
p < .001
To test for effects of Age and Group on performance of the neuropsychological tests, a series of 2 (Age) by 2 (Group) analysis of variance tests were conducted. On the COWAT, there were no main effects for Age, F (1, 68) = 2.60, p = .11, η2 = 0.42, or Group F (1,68) = 2.63, p = .11, η2 = 0.17. Only a marginally significant interaction effect emerged, F (1, 68) = 3.47, p = .07, ƞ2 = 0.05, indicating that the young adults with ASD had a lower mean COWAT score than all other groups. There was a main effect of Group on the animals category task, F (1, 68) = 4.39, p = .04, ƞ2 = 0.46, where NT individuals (M = 22.85, SD = 4.61) generated significantly more animals than adults with ASD (M = 20.45, SD = 5.69). There was no main effect of Age, F (1, 68) = 1.60, p = .21, ƞ2= 0.26, and no significant interaction (F = 0.52, p = .47, ƞ2= 0.01). There was no effect of Group for the WAIS-III Vocabulary subtest, F (1, 68) = .009, p = .93, ƞ2= 0.001, however a main effect of Age was found, F (1, 68) = 3.91, p = .05, ƞ2= 0.49, indicating that older individuals (M = 51.7, SD = 9.95) defined more words than younger individuals (M = 46.91, SD = 9.81). The corresponding interaction was not significant (F =0.47, p = 0.49). A main effect of Group was found for the Stroop word reading task, F (1, 68) = 6.85, p = .01, ƞ2= 0.61, where the NT group (M = 90.35, SD = 21.81) read significantly more words in the given amount of time than adults with ASD (M = 78.30, SD = 17.87). A Group effect was also found for Trailmaking Part A test, F (1,68) = 2.64, p = .004, ƞ2= 0.74, where NT participants (M = 25.02, SD = 7.02) were significantly faster than the adults with ASD (M = 36.09, SD = 19.73). (Please see Table 1 for all statistical effects on behavioral measures).
fMRI group analyses
Network connectivity
Four individuals were omitted from the fMRI analyses either due to missing data or compromised integrity of the data collected, therefore a total of 72 individuals (NT: 14 young, 20 middle-aged; ASD: 17 young, 24 middle-aged) were included in the analyses for functional connectivity and region activation during the fluency task. The behavioral performance during the fMRI word generation task was not significantly different across Age, F (1, 68) = 1.27, p = .26, ƞ2= 0.25, or Group, F (1, 68) = .46, p = .70, ƞ2= 0.35.
Using ICA via GIFT, we first identified the networks that were uniquely recruited for each of the 4 groups during the word generation blocks of the fMRI fluency task (Fig 1). All four groups produced a network that included the left inferior frontal cortex/pars opercularis (LIFG) which is a central region involved in expressive language. While all four groups had a network with LIFG as a main contributor, the other nodes within this network varied somewhat across groups (see Fig 1 and as described below).
The young NT group (Fig 1a) showed two networks significantly correlated with word generation. The network that included LIFG (BA 44) also included the supplementary speech area (SSA; BA 6), the left angular gyrus and subcortical structures of caudate and thalamus. There was also a network that included bilateral cerebellum (right greater than left) and left superior temporal lobe (BA 22). Posterior regions associated with the Default Mode Network (DMN) comprised a third network that was significantly related to the baseline (rest) condition. Group ICA for young adults with ASD (Fig 1b) produced a network dominated by the LIFG, with some contribution from the left temporal lobe and right cerebellum. This group also showed a significant network dominated by the SSA and also included bilateral inferior frontal lobe and caudate. For the group of middle-aged NT adults (Fig 1c), the network involving the LIFG was also linked to the supplementary speech area and to a lesser extent, the left caudate. Another network significantly related to word generation was specific for bilateral lateral cerebellum. The posterior regions of the DMN were also found in a third network for the baseline condition. ICA for the group of older adults with ASD (Fig1d) produced a network for the LIFG that also included the SSA, left temporal lobe and bilateral lateral cerebellum. The middle-aged adults with ASD also produced a significant network during the rest condition representing the default network, including posterior cingulate, bilateral posterior temporal lobe, frontal pole and cerebellum.
Network Nodes
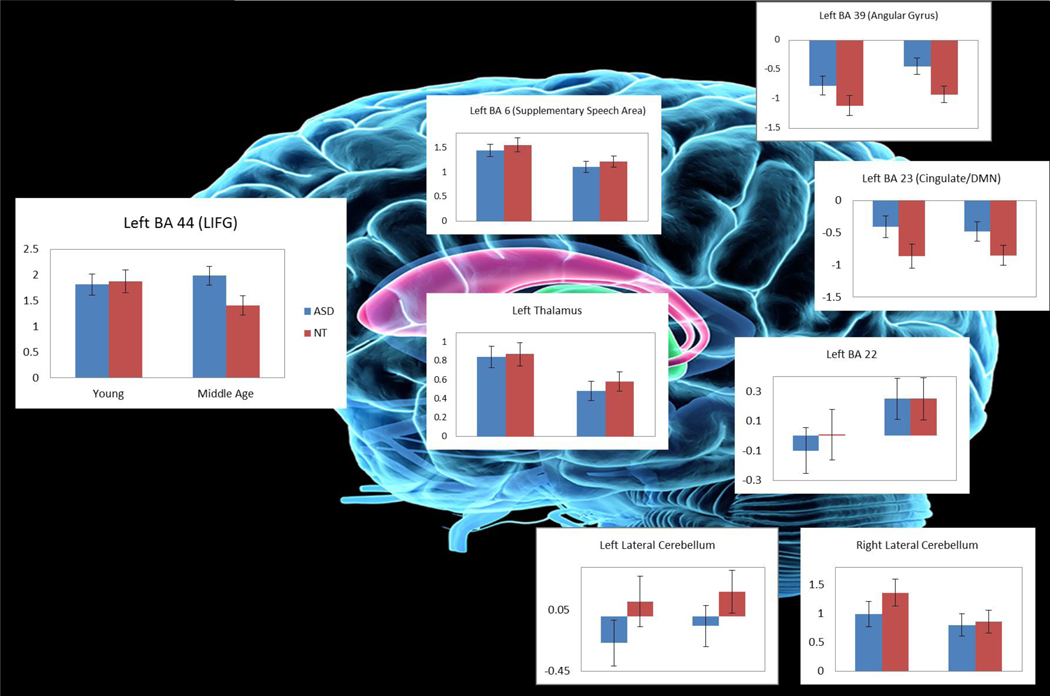
A second analysis combined all participants’ fMRI data in a one-way ANOVA analysis using SPM12 in order to statistically determine how network nodes from the ICA results were differentially contributing to word generation. Mean region-of-interest (ROI) beta values were extracted from the random-effects SPM analysis. The ROIs were selected based on their inclusion in GIFT analyses and were also regions that were significant in the SPM one-way analysis at p < .001, family-wise error corrected. Regions meeting these criteria were primarily in the left hemisphere and are illustrated in Fig 2: Left BA 44 (LIFG), left BA 6 (supplemental speech area), left and right lateral cerebellum, left thalamus, left BA 22 (superior temporal gyrus. We also included key regions associated with the DMN in the left BA 23 (posterior cingulate) and BA 39 (angular gyrus).
Figure 2.
Age and group differences in activation associated with word generation. Two way analyses of variance for extracted regions of interest demonstrated that the ASD groups had less engagement of the Default Mode Network. Older adults had greater activity in the SSA and superior temporal gyrus while younger adults showed greater activation in the left thalamus. No age by group interaction was significant. BA: Brodmann area; LIFG: left inferior frontal gyrus; SSA: supplemental speech area
Two way ANOVAs were conducted to test for age by group interactions on activation of these statistically significant, extracted ROIs. Because some differences in cognitive performance were observed, we analyzed our data with and without the performance data and education as covariates; however, no differences in the findings were found. Despite all groups showing similar task performance, not all groups equally utilized all regions to perform the task (Table 3 and Fig 2). Main effects for Age and Group were found. A Group effect was found where adults with ASD showed significantly less activation in the posterior cingulate (BA 23) (F = (1,68) = 5.99, p = .01, ƞ2= 0.60) and left angular gyrus (BA 39) (F (1, 68) = 7.10, p < .05, ƞ2= 0.64), although the latter difference does not survive multiple comparison correction. However, Age effects were non-significant for BA 39, F (1, 68) = 2.94, p = .09, ƞ2= 0.38 and BA 23, F (1, 68) = 0.03, p = .87, ƞ2= 0.06.
Table 3.
ROI Beta Values Across Age, Group, and Age by Group Interactions During fMRI Fluency Task
| ROI | MNI Coordinates | NT | ASD | F | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Young N = 14 M (SD) | Middle-aged N = 20 M (SD) | Young N = 17 M (SD) | Middle-aged N = 24 M (SD) | Age (ES; p) | Group (ES; p) | Age* Group ( partial η2; p) | ||||
| LIFG: BA 44 | −52 | 14 | 28 | 1.88 (0.93) | 1.41 (0.79) | 1.83 (0.90) | 1.99 (0.80) | 0.54 (0.16; .46) | 1.77 (0.38; .19) | 2.50 (0.04; .12) |
| L. SSA: BA 6a | −8 | 12 | 51 | 1.39 (0.59) | 1.03 (0.53) | 1.49 (0.95) | 1.18 (0.55) | 4.26* (0.67; .008) | 0.56 (0.15; .41) | 0.02 (<0.001; .97) |
| L. Cerebellum | −20 | −76 | −50 | 0.12 (0.86) | 0.20 (0.55) | −0.22 (1.05) | −.09 (0.63) | 0.34 (0.15; .56) | 2.95 (0.42; .09) | 0.02 (<0.001; .89) |
| R. Cerebellum | 20 | −76 | −50 | 1.36 (0.79) | 0.88 (0.79) | 0.99 (1.14) | 0.81 (0.81) | 2.53 (0.36; .17) | 1.02 (0.20; .32) | 0.53 (0.008; .47) |
| L. Thalamusb | −16 | 0 | 10 | 0.32 (0.50) | 0.10 (0.33) | 0.41 (0.71) | 0.06 (0.49) | 5.33* (0.69; .005) | 0.05 (0.12; .55) | 0.23 (0.002; .74) |
|
L. Superior temporal gyrus: BA 22 c |
−52 | −42 | 14 | 0.01 (0.41) | 0.25 (0.62) | −0.10 (0.54) | 0.25 (0.72) | 3.78* (0.49; .05) | 0.12 (0.08; .73) | 0.12 (0.002; .73) |
| L. Angular Gyrus: BA 39d |
−46 | −66 | 36 | −1.11 (0.70) | −0.92 (0.49) | −0.77 (0.65) | −0.45 (0.71) | 2.94 (0.08; .09) | 7.10* (0.63; .01) | 0.19 (.003; .67) |
| L Posterior cingulate (DMN): BA 23 e | −7 | −52 | 22 | −0.86 (0.52) | −0.85 (0.66) | −0.42 (0.66) | −0.48 (0.83) | 0.03 (0.63; .87) | 5.99* (0.59; .01) | 0.05 (0.001; .82) |
Young < Middle-age
Young > Middle-age
Young < Middle-age
TD < ASD
TD > ASD
p values uncorrected for multiple comparisons
A*G: Age by Group interaction; L.: Left; R.: Right; BA: Brodmann Area; LIFG: Left Inferior Frontal Gyrus (Pars Opercularis) SSA: Supplementary Speech Area; DMN: Default Mode Network
Younger individuals showed greater activation in the left thalamus than older individuals, F (1, 68) = 8.42, p = .005, ƞ2= 0.69. Conversely, the older adults showed greater activity in the SSA (BA 6), F (1, 68) = 7.37, p = .008, ƞ2= 0.66. The superior temporal gyrus (BA 22), F (1, 68) = 3.78, p = .05, ƞ2= 0.49 also shows somewhat greater activity in the older adults compared to younger adults, although this comparison does not survive correction for multiple comparisons.
While main effects of Group and Age were observed, no significant Age by Group interactions were observed (see Table 3 for F, p, and effect sizes).
Discussion
Using a network approach, we found evidence for both age and diagnostic group differences in the neural networks that are engaged during fluency. The effect size was very small for the age-group interactions predicted in this study, suggesting the statistical significance would not have emerged even with a much larger sample size. Other similar studies have found mixed results investigating cross-sectional differences in aging in ASD. Since this is a cross-sectional design, we may benefit from studying this question of aging using a within subject longitudinal test that would serve as a better model of the effects of group diagnosis on brain and cognitive aging.
The fMRI fluency task performance was very similar for all of the groups, reducing the likelihood that these group differences in brain activation were due to differences in either task engagement or difficulty and there was a strong correlation (r(74)= 0.55, p <.001) between performance on the COWAT and the fMRI task. The letter fluency fMRI task is easier than the fluency tasks given as part of the cognitive tests. The COWAT and animal fluency cognitive tasks require the participant to generate words from one category for a longer period of time than the fMRI fluency task, likely requiring a more extensive search of vocabulary and need to sustain a cognitive set. The young and older adults with ASD performance was generally weaker on cognitive tests. Both groups of individuals with ASD read fewer words and generated less animals than the NTs, and they were also slower on a test of simple visuomotor processing (Trails Part A). On the COWAT, group differences were nonsignificant, although the young adults with ASD produced about 10 less words within a minute than all of the other groups. Other studies of aging and ASD have also failed to find age-related changes in fluency (e.g., Davids et al, 2016; Lever and Guerts, 2016; Powell et al, 2017), and Powell et al. (2017) also raised the notion that neural compensation may explain the relatively sustained performance. Most of the baseline differences in performance were not statistically significant, and we found that using any of these cognitive variables as covariates in brain activation analyses did not change the results. This raises the question of whether contribution of any of the defined network nodes was not entirely due to processing speed or extent of vocabulary knowledge. Since cohort effects are not completely removed by statistical methods,we plan to further evaluate any age-related changes using longitudinal analyses of change over time.
We found that both young and middle-aged adults with ASD showed less “deactivation” during the word generation condition compared to the NT groups. There is considerable interest in gaining a better understanding of how individuals with ASD transition between fMRI conditions because activation patterns comparing the transition from a low-level, or baseline condition to one requiring more effort is often more telling than the more demanding condition (Braden et al., 2017; Gilbert, Bird, Brindley, Frith, & Burgess, 2008; Kennedy, Redcay, & Courchesne, 2006). Whether these findings represent enhanced activity or failure to deactivate during the low-level condition remains an area of debate. The posterior cingulate region has been implicated in both dynamic attentional focus (Hahn et al., 2007) and inefficiencies in sustaining or attention (Weissman, Roberts, Visscher, & Woldorff, 2006), consistent with findings of impaired attention in individuals with ASD (Goldstein, Johnson, & Minshew, 2001). Since the posterior cingulate, and related regions of the default mode network show less suppression in aging (Grady, Springer, Hongwanishkul, McIntosh, & Winocur, 2006; Reuter-Lorenz & Cappell, 2008) and are an early area of dysfunction in dementia (Greicius, Krasnow, Reiss, & Menon, 2003), the default mode network may be a region of greater vulnerability in older adults with ASD. This may be related to persistent cognitive inflexibility in older adults with ASD (Braden et al., 2017).
Our findings are in alignment with studies of both ASD and aging effects on brain and cognition. The major contribution of the LIFG, supplementary speech area (pre-supplementary motor area), and posterior temporoparietal regions have been reported by others (Birn et al., 2010; Gourovitch et al., 2000). Kenworthy et al. (2013) found a similar pattern of abnormal frontal-subcortical-cerebellar neural responsivity during a more complex fluency task. Large, age-related decreases in subcortical structures with a focus in the left thalamus were observed in our study, suggesting possible weakening in frontal-subcortical connectivity in older adults, or differences in processing of internally directed task demands (Crosson et al., 2001). Interestingly, a small aging effect in the superior temporal lobe showed increased engagement during word generation, suggesting that the older individuals may have relied more heavily on lexical processing than younger adults (Binder & Price, 2001; Graves, Grabowski, Mehta, & Gupta, 2008). This differential engagement of brain regions beyond the LIFG was observed in light of similar performance in all groups, suggesting that the challenges associated with aging and possibly ASD results in recruitment of neural networks to support effective performance. These findings are in alignment with the model of the “adaptive brain” described by (Park & Reuter-Lorenz, 2009). Based primarily on working memory and executive functioning compensatory mechanisms, they hypothesize that maintaining behaviors at a higher level in older adults (and other conditions that involve neural challenges, like ASD) are related to the brain’s ability to use compensatory scaffolding, or “recruitment of additional circuitry that shores up” for neural changes that can negatively affect functioning (Park & Reuter-Lorenz, 2009). While our findings align with changes in circuitry due to aging and ASD vulnerabilities, we did not see interactions in these compensatory functions. Other cross-sectional behavioral studies fail to see age-diagnosis interactions, but it is possible that our longitudinal analyses of these changes over time, especially in the older groups, will provide clarification of the interactions between ASD and aging.
Limitations
Although we used a word generation task that is commonly used in research, having participants covertly generate words is a limitation because it does not strictly provide a direct measure of behavioral performance on the task. We included a motor response as a proxy for direct observation, and the equivalence in behavior between groups indicates that this was likely successful. Furthermore, there was a strong correlation between COWAT and fMRI fluency performance. We carefully matched our groups for both age and estimated IQ; however, the younger groups performed worse on tests of vocabulary knowledge, and the younger group of individuals with ASD had fewer years of education. Given the dramatic changes in diagnosis of and treatment options for individuals with ASD over the past decades, it is likely that cohort differences exist between our middle-aged and younger ASD adults. Whether the selection bias results in a group of older adults with ASD, who were diagnosed at a later age and who had less advantages in educational support and interventions and had less cognitive difficulties early on, or conversely, the young group benefitted because of greater or earlier intervention is unclear. There is some evidence that a more general age-related bias may be observed, since both of the younger groups have lower mean scores on the Vocabulary test than the older groups. Correlations of covariates of education and Vocabulary scores to the dependent variables were non-significant (Supplemental Table 1), and because the nature of the fMRI task was to keep equivalence in group performance and this was achieved, this suggests that differences in brain activity cannot be solely accounted for by these cohort differences. Although we attempted to control for the group differences in education and vocabulary abilities through equivalent performance and covarying these variables in our analyses, there are limitations in interpreting statistical approaches when real cohort effects are present. As in most studies, our older cohort consists of individuals were diagnosed much later than the younger individuals, and therefore they may have had quite different interventions available to them, and may be functioning differently than younger individuals who have similar ASD profiles; thus there is a limitation to how well comparisons can be made between the groups. Both education and vocabulary knowledge have been shown to impact fluency ability, (Constantinidou, Christodoulou & Prokopiou, 2012; Tombaugh, Kozak & Rees, 1999). Thus, it is possible that the lack of interaction between age and diagnosis was tempered by these basic group differences in history, and less detectable in this cross-sectional study. We continue to follow these participants and will investigate longitudinal changes in performance, which will provide more definitive conclusions regarding a possible exacerbation of brain related changes in our older cohort of individuals with ASD. Our findings are limited to understanding fluency in men, and given that men and women may use different strategies during fluency tasks (Weiss et al., 2006) and sex differences in the functional organization of language have been reported (Baxter et al., 2003; Shaywitz et al., 1995), this may mean that different patterns would be seen for women. We are currently acquiring data from women matched to the criteria of this study, and will be addressing this interesting question of how this added influence of organizational sex differences alters the effects observed in this study. Finally, this study focused on individuals with ASD who have average to high average general cognitive skills, and thus does not address how aging affects language functioning in all individuals with ASD.
Implications
Our study of phonemic fluency networks suggests that multiple networks are engaged to help maintain fluency as a result of aging as well as having a diagnosis of ASD. Using this network approach may help us to form an even clearer picture of how language is altered by autism and aging. Better understanding of the brain networks engaged to produce similar levels of cognitive performance also may have implications for the ways in which these compensatory mechanisms may be engaged in other cognitive areas.
Supplementary Material
Acknowledgements
This work was funded by the Department of Defense (AR140105) and the Arizona Alzheimer’s Consortium (State of Arizona). Neither sponsor was involved in study design; the collection, analysis or interpretation of data; the writing of the report; and the decision to submit the article for publication. We would like to express our heartfelt thanks for our participants who shared their time and enthusiasm for our work. We would also like to thank Sharmeen Maze, whose MRI scanning expertise helped make this research possible.
Footnotes
Conflict of Interest
The authors of this study have no financial, personal, or other relationships that may pose potential conflicts of interest related to this research.
References
- Alaerts K, Nayar K, Kelly C, Raithel J, Milham MP, & Di Martino A (2015). Age-related changes in intrinsic function of the superior temporal sulcus in autism spectrum disorders. Social cognitive and affective neuroscience, 10(10), 1413–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambery FZ, Russell AJ, Perry K, Morris R, & Murphy DG (2006). Neuropsychological functioning in adults with Asperger syndrome. Autism, 10(6), 551–564. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2000). Diagnostic and statistical manual-text revision. Washington, DC: American Psychiatric Association, 256. [Google Scholar]
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders (DSM-5®): American Psychiatric Pub. [Google Scholar]
- Barnes L, Wilson R, Schneider J, Bienias J, Evans D, & Bennett D (2003). Gender, cognitive decline, and risk of AD in older persons. Neurology, 60(11), 1777–1781. [DOI] [PubMed] [Google Scholar]
- Baxter LC, Saykin AJ, Flashman LA, Johnson SC, Guerin SJ, Babcock D, & Wishart HA (2003). Sex differences in semantic language processing: a functional MRI study. Brain and language, 84(2), 264–272. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, & Brown GK (1996). Beck depression inventory-II. San Antonio, 78(2), 490–498. [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, & Smith SM (2005). Investigations into resting-state connectivity using independent component analysis. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 360(1457), 1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton L, Hamsher K, & Sivan A (1994). Controlled oral word association test. Multilingual aphasia examination. [Google Scholar]
- Biesbroek JM, van Zandvoort MJ, Kappelle LJ, Velthuis BK, Biessels GJ, & Postma A (2016). Shared and distinct anatomical correlates of semantic and phonemic fluency revealed by lesion-symptom mapping in patients with ischemic stroke. Brain Structure and Function, 221(4), 2123–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J, & Price CJ (2001). Functional neuroimaging of language. Paper presented at the THIS EXCERPT FROM HANDBOOK OF FUNCTIONAL NEUROIMAGING OF COGNITION. [Google Scholar]
- Birn RM, Kenworthy L, Case L, Caravella R, Jones TB, Bandettini PA, & Martin A (2010). Neural systems supporting lexical search guided by letter and semantic category cues: a self-paced overt response fMRI study of verbal fluency. Neuroimage, 49(1), 1099–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braden BB, Smith CJ, Thompson A, Glaspy TK, Wood E, Vatsa D, . . . Baxter LC. (2017). Executive function and functional and structural brain differences in middle-age adults with autism spectrum disorder. Autism Research. [DOI] [PubMed] [Google Scholar]
- Brady DI, Saklofske DH, Schwean VL, Montgomery JM, Thorne KJ, & McCrimmon AW (2017). Executive Functions in Young Adults With Autism Spectrum Disorder. Focus on Autism and Other Developmental Disabilities, 32(1), 31–43. doi: 10.1177/1088357615609306 [DOI] [Google Scholar]
- Bramham J, Ambery F, Young S, Morris R, Russell A, Xenitidis K, . . . Murphy D (2009). Executive functioning differences between adults with attention deficit hyperactivity disorder and autistic spectrum disorder in initiation, planning and strategy formation. Autism, 13(3), 245–264. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, & Poline J-B (2002). Region of interest analysis using the MarsBar toolbox for SPM 99. Neuroimage, 16(2), S497. [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, & McIntosh AR (2002). Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage, 17(3), 1394–1402. [DOI] [PubMed] [Google Scholar]
- Calhoun V, Adali T, Pearlson G, & Pekar J (2001). Group ICA of functional MRI data: separability, stationarity, and inference. Paper presented at the Proc. Int. Conf. on ICA and BSS San Diego, CA. [Google Scholar]
- Calhoun VD, & de Lacy N (2017). Ten key observations on the analysis of resting-state functional MR imaging data using independent component analysis. Neuroimaging Clinics, 27(4), 561–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Jones DK, Daly E, Embiricos N, Deeley Q, Pugliese L, . . . Murphy DG. (2008). Altered cerebellar feedback projections in Asperger syndrome. Neuroimage, 41(4), 1184–1191. doi: 10.1016/j.neuroimage.2008.03.041 [DOI] [PubMed] [Google Scholar]
- Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL, . . . Blacker D. (2006). Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: an independent component analysis. Journal of Neuroscience, 26(40), 10222–10231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinidou F, Christodoulou M, Prokopiou J (2012). The effects of age and education on executive functioning and oral naming performance in Greek Cypriot adults: The Neurocognitive Study for the Aging. Folia Phoniatrica et Logopaedica, 64, 187–198. doi: 10.1159/00340015. [DOI] [PubMed] [Google Scholar]
- Constantino JN, & Gruber CP (2012). Social responsiveness scale (SRS): Western Psychological Services; Torrance, CA. [Google Scholar]
- Crosson B, Sadek JR, Maron L, Gökçay D, Mohr CM, Auerbach EJ, . . . Briggs RW. (2001). Relative shift in activity from medial to lateral frontal cortex during internally versus externally guided word generation. Journal of cognitive neuroscience, 13(2), 272–283. [DOI] [PubMed] [Google Scholar]
- Davids Ro C. D. , Groen Y, Berg IJ, Tucha OM, van Balkom IDC (2016). Executive functions in older adults with Autism Spectrum Disorder: Objective performance and subjective complaints. Journal of Autism and Developmental Disorders, 46:2859–2873. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Yan CG, Li Q, Denio E, Castellanos FX, Alaerts K, . . . Milham MP (2014). The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol Psychiatry, 19(6), 659–667. doi: 10.1038/mp.2013.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant K, & Uddin LQ (2016). Atypical developmental of dorsal and ventral attention networks in autism. Developmental science, 19(4), 550–563. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research, 12(3), 189–198. [DOI] [PubMed] [Google Scholar]
- Geurts HM, & Vissers ME (2012). Elderly with autism: Executive functions and memory. Journal of autism and developmental disorders, 42(5), 665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SJ, Bird G, Brindley R, Frith CD, & Burgess PW (2008). Atypical recruitment of medial prefrontal cortex in autism spectrum disorders: An fMRI study of two executive function tasks. Neuropsychologia, 46(9), 2281–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein G, Johnson CR, & Minshew NJ (2001). Attentional processes in autism. Journal of autism and developmental disorders, 31(4), 433–440. [DOI] [PubMed] [Google Scholar]
- Gourovitch ML, Kirkby BS, Goldberg TE, Weinberger DR, Gold JM, Esposito G, . . . Berman KF. (2000). A comparison of rCBF patterns during letter and semantic fluency. Neuropsychology, 14(3), 353. [DOI] [PubMed] [Google Scholar]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, & Winocur G (2006). Age-related changes in brain activity across the adult lifespan. Journal of cognitive neuroscience, 18(2), 227–241. [DOI] [PubMed] [Google Scholar]
- Graves WW, Grabowski TJ, Mehta S, & Gupta P (2008). The left posterior superior temporal gyrus participates specifically in accessing lexical phonology. Journal of cognitive neuroscience, 20(9), 1698–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, & Menon V (2003). Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences, 100(1), 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, & Tager-Flusberg H (2006). Anatomical differences in the mirror neuron system and social cognition network in autism. Cereb Cortex, 16(9), 1276–1282. doi: 10.1093/cercor/bhj069 [DOI] [PubMed] [Google Scholar]
- Hahn B, Ross TJ, Yang Y, Kim I, Huestis MA, & Stein EA (2007). Nicotine enhances visuospatial attention by deactivating areas of the resting brain default network. Journal of Neuroscience, 27(13), 3477–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T-P, Humphries C, Lee T-W, Makeig S, McKeown MJ, Iragui V, & Sejnowski TJ (1998). Extended ICA removes artifacts from electroencephalographic recordings. Paper presented at the Advances in neural information processing systems. [Google Scholar]
- Kaufman AS, & Kaufman NL (2004). Kaufman brief intelligence test: Wiley Online Library. [Google Scholar]
- Kennedy DP, Redcay E, & Courchesne E (2006). Failing to deactivate: resting functional abnormalities in autism. Proceedings of the National Academy of Sciences, 103(21), 8275–8280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenworthy L, Wallace GL, Birn R, Milleville SC, Case LK, Bandettini PA, & Martin A (2013). Aberrant neural mediation of verbal fluency in autism spectrum disorders. Brain and cognition, 83(2), 218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DI, Mathalon D, Ford J, Mannell M, Turner J, Brown G, . . . Wible C. (2009). Auditory oddball deficits in schizophrenia: an independent component analysis of the fMRI multisite function BIRN study. Schizophrenia bulletin, 35(1), 67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn PCM, Caan MW, Teeuw J, Olabarriaga SD, & Geurts HM (2017). Age-related differences in autism: The case of white matter microstructure. Human brain mapping, 38(1), 82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever AG & Geurts HM (2016). Age-related differences in cognition across the adult lifespan in Autism Spectrum Disorder. Autism Research, 9, 666–676. [DOI] [PubMed] [Google Scholar]
- Lord C, DiLavore PC, & Gotham K (2012). Autism diagnostic observation schedule: Western Psychological Services; Torrance, CA. [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, & Smith PK (2002). Models of visuospatial and verbal memory across the adult life span. Psychology and aging, 17(2), 299. [PubMed] [Google Scholar]
- Park DC, & Reuter-Lorenz P (2009). The adaptive brain: aging and neurocognitive scaffolding. Annual review of psychology, 60, 173–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignat JM, Koval O, Van De Ville D, Voloshynovskiy S, Michel C, & Pun T (2013). The impact of denoising on independent component analysis of functional magnetic resonance imaging data. Journal of neuroscience methods, 213(1), 105–122. [DOI] [PubMed] [Google Scholar]
- Powell PS, Klinger LG, & Klinger MR (2017). Patterns of age-related cognitive differences in adults with Autism Spectrum Disorder. Journal of Autism Developmental Disorders, 47,3204–3219. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, . . . Acker JD. (2005). Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cerebral cortex, 15(11), 1676–1689. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, & Cappell KA (2008). Neurocognitive aging and the compensation hypothesis. Current directions in psychological science, 17(3), 177–182. [Google Scholar]
- Romero-Rebollar C, Jiménez-Ángeles L, Dragustinovis-Ruiz EA, & Medina-Bañuelos V (2016). Neural Modulation in Aversive Emotion Processing: An Independent Component Analysis Study. Computational and mathematical methods in medicine, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen AC, Prull MW, O’hara R, Race EA, Desmond JE, Glover GH, . . . Gabrieli JD. (2002). Variable effects of aging on frontal lobe contributions to memory. Neuroreport, 13(18), 2425–2428. [DOI] [PubMed] [Google Scholar]
- Sexton CE, Walhovd KB, Storsve AB, Tamnes CK, Westlye LT, Johansen-Berg H, & Fjell AM (2014). Accelerated changes in white matter microstructure during aging: a longitudinal diffusion tensor imaging study. Journal of Neuroscience, 34(46), 15425–15436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywltz SE, Pugh KR, Constable RT, Skudlarski P, Fulbright RK, . . . Katz L. (1995). Sex differences in the functional organization of the brain for language. Nature, 373(6515), 607. [DOI] [PubMed] [Google Scholar]
- Spek A, Schatorjé T, Scholte E, & van Berckelaer-Onnes I (2009). Verbal fluency in adults with high functioning autism or Asperger syndrome. Neuropsychologia, 47(3), 652–656. [DOI] [PubMed] [Google Scholar]
- Spielberger CD (2010). State-Trait anxiety inventory: Wiley Online Library. [Google Scholar]
- Stebbins GT, Carrillo MC, Dorfman J, Dirksen C, Desmond JE, Turner DA, . . . Gabrieli JD. (2002). Aging effects on memory encoding in the frontal lobes. Psychology and aging, 17(1), 44. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, Kozak J, Rees L (1999). Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Archives of Clinical Neuropsychology, 14(2), 167–177. [PubMed] [Google Scholar]
- Turner MA (1999). Generating Novel Ideas: Fluency Performance in High-functioning and Learning Disabled Individuals with Autism. Journal of Child Psychology and Psychiatry, 40(2), 189–201. [PubMed] [Google Scholar]
- Wechsler D (1997). Adult intelligence scale. New York, 21. [Google Scholar]
- Weiss EM, Ragland JD, Brensinger CM, Bilker WB, Deisenhammer EA, & Delazer M (2006). Sex differences in clustering and switching in verbal fluency tasks. Journal of the International Neuropsychological Society, 12(4), 502–509. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts K, Visscher K, & Woldorff M (2006). The neural bases of momentary lapses in attention. Nature neuroscience, 9(7), 971–978. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.