Abstract
Plasmodium ovale can infect humans, causing malaria disease. We aimed to investigate the severity and mortality of severe P. ovale infection to increase the awareness of physicians regarding the prognosis of this severe disease and outcome-related deaths in countries in which this disease is endemic. Articles that were published in the PubMed, Scopus, and ISI Web of Science databases prior to January 5, 2020 and reported the prevalence of severe P. ovale infection were systematically searched and reviewed. Studies that mainly reported severe P. ovale infection according to the 2014 WHO criteria for the treatment of malaria were included. Two reviewers selected, identified, assessed, and extracted data from studies independently. The pooled prevalence of severe P. ovale mono-infections was estimated using the command “metaprop case population, random/fixed”, which yielded the pooled estimate, 95% confidence interval (CI) and the I2 value, indicating the level of heterogeneity. Meta-analyses of the proportions were performed using a random-effects model to explore the different proportions of severity between patients with P. ovale and those with other Plasmodium species infections. Among the eight studies that were included and had a total of 1,365 ovale malaria cases, the pooled prevalence of severe P. ovale was 0.03 (95% CI = 0.03–0.05%, I2 = 54.4%). Jaundice (1.1%), severe anemia (0.88%), and pulmonary impairments (0.59%) were the most common severe complications found in patients infected with P. ovale. The meta-analysis demonstrated that a smaller proportion of patients with P. ovale than of patients with P. falciparum had severe infections (P-value = 0.01, OR = 0.36, 95% CI = 0.16–0.81, I2 = 72%). The mortality rate of severe P. ovale infections was 0.15% (2/1,365 cases). Although severe complications of P. ovale infections in patients are rare, it is very important to increase the awareness of physicians regarding the prognosis of severe P. ovale infections in patients, especially in a high-risk population.
Introduction
Severe malaria results in the dysfunction of one or more vital organs [1]. Plasmodium ovale, which causes tertian malaria, was first reported in 1922 as one of the five Plasmodium species that can infect humans [2]. P. ovale accounts for between 0.5 and 10.5% of all malaria cases, and it is geographically distributed in sub-Saharan Africa, the Western Pacific, Timor, and Indonesia [3]. The highest prevalence of P. ovale has been reported in Papua New Guinea (15%) [4] and Nigeria (15%) [5]. However, the most recent retrospective cohort study conducted in Papua, Indonesia during 2004–2013 demonstrated a low prevalence of P. ovale infections (0.06%) among 68,361 patients [6]. Other recent studies demonstrated that P. ovale infections accounted for 2.5% of malaria cases in Uganda [7] and 2.7% of malaria cases in China [8]. Infections due to P. ovale have been underestimated compared with those of other Plasmodium species, as P. ovale has been demonstrated to lead to low parasitemia and have morphologic similarities with P. vivax and mixed infections [9, 10]. Similar to P. vivax, P. ovale can cause relapsing infection due to the presence of latent parasites (hypnozoites) in the liver long after the first treatment is administered with anti-malarial drugs [3]. P. ovale is detected and identified with the standard microscopic method. Rapid diagnostic tests (RDTs) can also be used in cases where microscopic detection cannot be performed, such as in rural or remote areas. However, the sensitivity of RDTs to detect P. ovale is low (22.2%) [11]. The low sensitivity of RDTs can be attributed to the low parasitemia level [12, 13] or different targeted antigens [14]. Recently, a molecular method with nested polymerase chain reaction (nested PCR) has been used to identify Plasmodium species [15]. Although continuous efforts regarding PCR techniques have been made to develop new diagnostic techniques, such as Plasmodium species-specific PCR-restriction fragment length polymorphism (PCR-RFLP) [16], for identifying malaria parasites, nested PCR techniques with high sensitivity and specificity are needed.
According to the molecular technique, two subspecies of P. ovale, curtisi and P. ovale wallikeri, were identified in 2010 by a nested PCR detection assay of dimorphism in the gene encoding the P. ovale tryptophan-rich antigen (potra) in West Africa [17]. Currently, P. falciparum is still the leading cause of severe malaria [18]. P. ovale is usually associated with low morbidity and mortality. However, P. ovale can cause severe complications and death [19, 20]. Previous studies have reported that the severe complications of P. ovale infections include acute respiratory distress syndrome (ARDS) [21–26], renal impairment [24, 27], jaundice, and hypotension [26, 27]. The most recent study on severe P. ovale malaria in travelers and migrants demonstrated that 5.3% of patients with P. ovale developed severe complications according to the 2015 WHO criteria, including hyperbilirubinemia, pulmonary edema, shock, significant bleeding, and impaired consciousness [28]. There are a limited number of systematic reviews and meta-analyses on severe P. ovale malaria. A previous systematic review of 33 articles published between 1922 and 2015 demonstrated that a total of five out of 22 severe cases of P. ovale malaria were fatal, and two cases of congenital P. ovale malaria were fatal [29]. A more recent systematic review conducted by Yerlikaya et al. in 2018 demonstrated that a RDT had poor performance in detecting P. ovale because P. ovale infections usually occur at very low parasite densities, leading to missed detection by microscopy and RDTs [30]. Nevertheless, studies on the prevalence of severe P. ovale malaria provide more information on this neglected species and are urgently needed. This systematic review and meta-analysis aimed to investigate the severity and mortality rates of severe P. ovale infection to increase the awareness of physicians regarding the prognosis of this severe disease and outcome-related deaths in countries in which this disease is endemic and to identify the differences in the proportions of patients with severe P. ovale manifestations and with other severe Plasmodium spp. infections.
Methods
Study selection
This systematic review was designed on the basis of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (S1 Checklist). Two authors (MK and KUK) searched the Medline, Scopus, and ISI Web of Science databases independently for articles published in English prior to January 5, 2020. Additional articles from other databases were also selected. The search terms, which included the Boolean operators “OR” or “AND”, were as follows: “(severe OR complicated OR complication) AND (Plasmodium ovale)” (S1 Table). EndNote software version X7 (Thomson Reuters, New York, NY) was used to process all references in our study.
Quality of the included studies
The quality of the observational studies was assessed in accordance with the Newcastle-Ottawa Scale (NOS) [31]. A 'star system', in which a study is judged on the basis of the following three broad perspectives was used: the selection of the study groups; the comparability of the groups; and the ascertainment of either the exposure or outcome of interest.
Data extraction
All raw and available data from eight studies were extracted to explore the prevalence of severe complications in patients with P. ovale infections. Two authors (MK and KUK) extracted the data from the selected studies. If there were any discrepancies between the two reviewers, another reviewer (GM) determined whether the study should be included. For the prevalence outcome of severe P. ovale malaria, all prospective cohort, cross-sectional studies, and case-control studies that reported the number of patients with severe complications from P. ovale were included. The following articles were excluded from our study: book and book chapter reviews, conference papers, editorials, letters, correspondences, notes, reviews, animal studies, case reports, drug studies and clinical trials, entomological studies, experimental studies, knowledge assessments, studies written in local languages, studies on mixed infections of P. ovale and other Plasmodium spp., studies of P. ovale during pregnancy, prevalence studies of non-P. ovale infections, and prevalence studies of P. ovale that did not report data on severe complications.
Statistical analysis
The primary outcome of the present study was the pooled prevalence of severe P. ovale infections and mortality rates. The number of severe P. ovale infections and the total number of P. ovale infections were entered into an Excel data sheet (Microsoft Corporation, USA). The pooled prevalence of the severity of P. ovale mono-infections was estimated using the command “metaprop case population, random/fixed” available in STATA software version 15.0 (StataCorp LLC, USA). The results are presented as the pooled estimate and 95% confidence interval (CI). A meta-regression with the median age as a covariate was performed to evaluate whether the age of the participants was a confounder of the pooled prevalence of severe P. ovale.
The secondary outcome of the present study was the different proportions of severity between patients with P. ovale and those with other Plasmodium species. The meta-analyses of the proportions were performed using a random-effects model provided in Review Manager 5.3 software (Cochrane Community). The heterogeneity was assessed with the Mantel-Haenszel method, and the I2 values were also calculated. The I2 was considered low (<25%), moderate (25–50%), or high (>50%). A fixed-effects model was used when I2<50%, whereas a random-effects model was used when I2>50%. The publication bias was also assessed using funnel plots.
Results
Characteristics of included studies
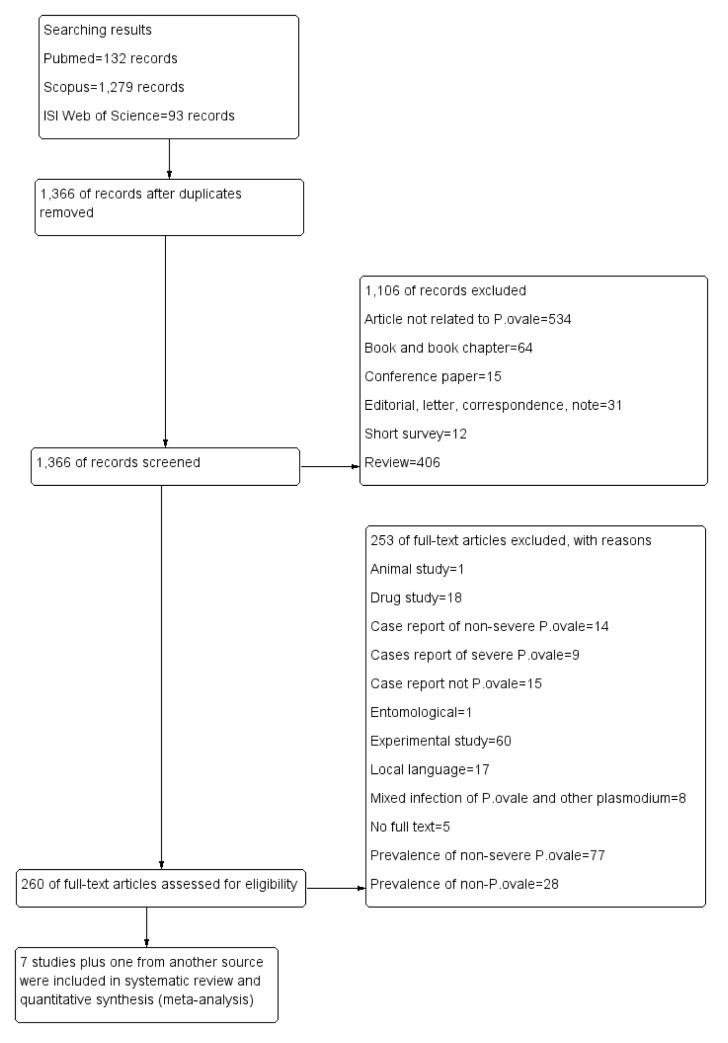
A total of 1,504 articles were retrieved from three databases during the search, and eight of these articles, including retrospective studies, prospective studies, and case series, were included in this study (Fig 1). The characteristics of the eight studies are shown in Table 1. The studies were conducted in Belgium (2000–2005) [32], the Ivory Coast (2007–2008) [33], the US National Malaria Surveillance System (NMSS) (1985–2011) [19], Indonesia (2004–2013) [34], Ethiopia (2013–2014) [35], Italy (2014–2017) [36], Spain (2005–2011) [37], and Sweden (1995–2015) [38]. The ages of the participants were reported in seven of the included studies [32–38], as they were not reported in a study by Hwang et al. [19]. Five studies reported severe imported P. ovale infections in European countries, including Belgium [32], France [33], Italy [36], Spain [37], and Sweden [38], while other studies reported severe P. ovale infections in the United States of America [19], Indonesia [34], and Ethiopia [35]. Most of the included studies (7/8, 87.5%) used microscopy as the gold standard for malaria identification, except for a study conducted by Ramos et al. in 2016 [35]. The combination of microscopy and PCR was used in three of the included studies [19, 36, 37]. Most of the Plasmodium spp. infections that were reported among the included studies were caused by P. falciparum (116,898 cases, 51%), P. vivax (78,282 cases, 34.1%), mixed infection (26,049 cases, 11.4%), P. malariae (6,428 cases, 2.8%), and P. ovale (1,365 cases, 0.6%).
Fig 1. Flow diagram.
Flow chart of the study selection process.
Table 1. Characteristics of the included studies.
| No. | Reference | Study area (years of the survey) | Participants | Age range | Reference method for malaria identification | Plasmodium sp. | Severe malaria | Fetal | Impaired consciousness | Prostration | Severe anemia | Renal impairment | Hyperbilirubinemia | Pulmonary | Significant bleeding | Shock |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Bottieau et al., 2006 | Belgium (2000–2005) | Travelers, expatriates, and foreign Visitors | 35 (11–77) | Microscopy | Pv = 48 | Pv = 8 | Pv = 8 | ||||||||
| Po = 34 | Po = 4 | Po = 4 | ||||||||||||||
| Pm = 16 | Pm = 3 | Pm = 3 | ||||||||||||||
| 2 | de Laval et al., 2010 | France (2007–2008) | French soldiers | 32 (30–36) | Microscopy | Case series | Po = 1 | Po = 1 | ||||||||
| Po = 6 | ||||||||||||||||
| 3. | Hwang et al., 2014 | USA (1985–2011) | Travelers | NA | Microscopy, PCR | Pf = 15,272 | Pf = 1,416 | Pf = 122 | Pf = 514 | Pf = 140 | Pf = 503 | Pf = 176 | ||||
| Pv = 12,152 | Pv = 163 | Pv = 10 | Pv = 37 | Pv = 16 | Pv = 48 | Pv = 35 | ||||||||||
| Po = 903 | Po = 18 | Po = 2 | Po = 4 | Po = 6 | Po = 6 | Po = 4 | ||||||||||
| Pm = 1254 | Pm = 22 | Pm = 2 | Pm = 2 | Pm = 3 | Pm = 7 | Pm = 2 | ||||||||||
| Mixed = 226 | Mixed = NA | |||||||||||||||
| 4. | Langford et al., 2015 | Indonesia (2004–2013) | Patients presenting to the hospital | <1 | Microscopy | Pf = 100,078 | Pf = 4,031 | Pf = 2,521 | Pf = 415 | Pf = 1,095 | ||||||
| 1-<5 | Pv = 65,306 | Pv = 2,118 | Pv = 1,099 | Pv = 81 | Pv = 938 | |||||||||||
| 5-<15 | Po = 0 | Po = 0 | Po = 1 | |||||||||||||
| ≥ 15 | Po = 120 | Po = 1 | Pm = 100 | Pm = 16 | Pm = 44 | |||||||||||
| Pm = 5,097 | Pm = 160 | Mixed = 782 | Mixed = 84 | Mixed = 343 | ||||||||||||
| Mixed = 25,779 | Mixed = 1,209 | |||||||||||||||
| 5. | Ramos et al., 2016 | Ethiopia (2013–2014) | Patients with severe anemia | 15 (0.5–65) | PCR | 111 | Pf = 18 | Pf = 18 | ||||||||
| Pv = 4 | Pv = 4 | |||||||||||||||
| Po = 4 | Po = 4 | |||||||||||||||
| 6. | Rojo-Marcos et al., 2018 | Italy (2014–2017) | Patients with imported P. ovale | 35 (22.2–53) | Microscopy, PCR | 79 | Po = 5 | Po = 1 | Po = 4 | |||||||
| 7. | Rojo-Marcos et al., 2014 | Spain (2005–2011) | Patients with imported P. ovale | 36.5 (11.8–52.7) | Microscopy, PCR | 35 | Po = 3 | Po = 2 | Po = 1 | |||||||
| 8. | Wangdahl et al., 2019 | Sweden (1995–2015) | Travelers and Migrants | All = 32.6 (0.2–83) | Microscopy | Pf = 1,548 | Pf = 146 | Pf = 3 | Pf = 28 | Pf = 9 | Pf = 16 | Pf = 31 | Pf = 66 | Pf = 15 | Pf = 17 | Pf = 33 |
| Pv = 776 | Pv = 60 | Pv = 0 | Pv = 1 | Pv = 1 | Pv = 15 | Pv = 1 | Pv = 28 | Pv = 4 | Pv = 5 | Pv = 8 | ||||||
| Pf = 34.4 (0.2–83) | Po = 188 | Po = 10 | Po = 0 | Po = 1 | Po = 0 | Po = 0 | Po = 0 | Po = 6 | Po = 2 | Po = 1 | Po = 2 | |||||
| Pm = 61 | Pm = 2 | Pm = 0 | Pm = 0 | Pm = 0 | Pm = 1 | Pm = 0 | Pm = 0 | Pm = 0 | Pm = 0 | Pm = 1 | ||||||
| Pv = 29.9 (1–79) | Mixed = 44 | Mixed = 8 | Mixed = 1 | Mixed = 1 | Mixed = 0 | Mixed = 4 | Mixed = 3 | Mixed = 4 | Mixed = 0 | Mixed = 1 | Mixed = 3 | |||||
| Pm = 30.2 (3–65) | ||||||||||||||||
| Po = 30.2 (3–65) | ||||||||||||||||
| Total cases | Pf = 116,898 | Pf = 5,611 | Pf = 125 | Pf = 542 | Pf = 9 | Pf = 395 | Pf = 949 | Pf = 66 | Pf = 1,286 | Pf = 17 | Pf = 33 | |||||
| Pv = 78,282 | Pv = 2,353 | Pv = 10 | Pv = 38 | Pv = 1 | Pv = 1,134 | Pv = 130 | Pv = 36 | Pv = 977 | Pv = 5 | Pv = 8 | ||||||
| Po = 1,365 | Po = 46 | Po = 2 | Po = 5 | Po = 2 | Po = 13 | Po = 6 | Po = 15 | Po = 8 | Po = 1 | Po = 2 | ||||||
| Pm = 6,428 | Pm = 187 | Pm = 2 | Pm = 2 | Pm = 2 | Pm = 104 | Pm = 23 | Pm = 3 | Pm = 46 | Pm = 0 | Pm = 1 | ||||||
| Mixed = 26,049 | Mixed = 1,217 | Mixed = 1 | Mixed = 1 | Mixed = 1 | Mixed = 786 | Mixed = 87 | Mixed = 4 | Mixed = 343 | Mixed = 1 | Mixed = 3 |
Prevalence of severe P. ovale infections
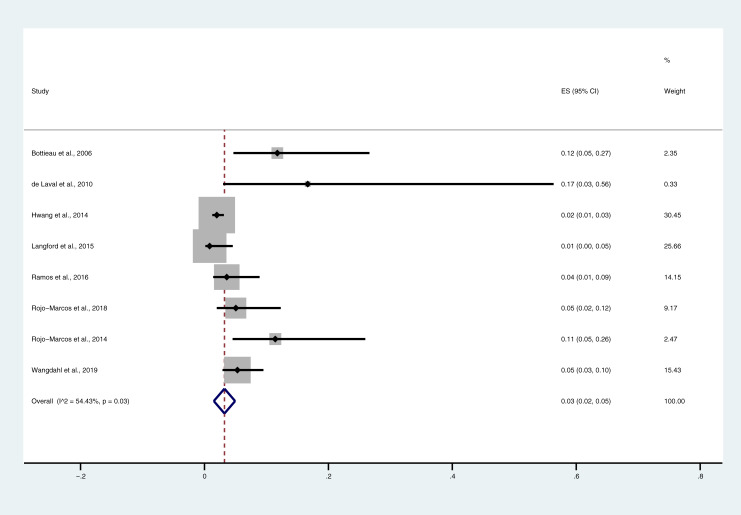
Among the eight included studies, which included a total of 1,365 ovale malaria cases, the pooled prevalence of severe P. ovale was 0.03 (95% CI = 0.03–0.05%, I2 = 54.4%). The highest proportions of severe P. ovale were found in the study by de Laval et al. [33] (OR = 0.17, 95% CI = 0.03–0.56) and the study by Bottieau et al. [32] (OR = 0.12, 95% CI = 0.05–0.27), whereas the lowest proportion was found in the study by Langford et al., 2015 [34] (Fig 2). According to the results of the meta-regression of six included studies, the age of the participants did not confound to the pooled prevalence of severe P. ovale infections (P-value = 0.3, 95% CI = -0.002–0.005). The complications most commonly found in patients with P. ovale infections were jaundice (1.1%), severe anemia (0.88%), and pulmonary impairments (0.59%) (Table 2).
Fig 2. Pooled prevalence of severe P. ovale infections.
Forest plot comparing the proportions of severe P. ovale cases and P. vivax cases.
Table 2. Complications associated with P. ovale.
| Major complication (WHO, 2014) | Total number of patients with a severe case | Proportion of complications (%) among the total number of P. ovale cases (1,365 cases) |
|---|---|---|
| Pulmonary impairment | 8 | 0.59 |
| Cerebral malaria | 5 | 0.37 |
| Renal impairment | 6 | 0.44 |
| Prostration | 2 | 0.15 |
| Hypotension/shock | 2 | 0.15 |
| Jaundice | 15 | 1.10 |
| Severe anemia | 13 | 0.95 |
| Bleeding/DIC | 1 | 0.07 |
Comparison of severity between the P. ovale and Plasmodium spp. infections
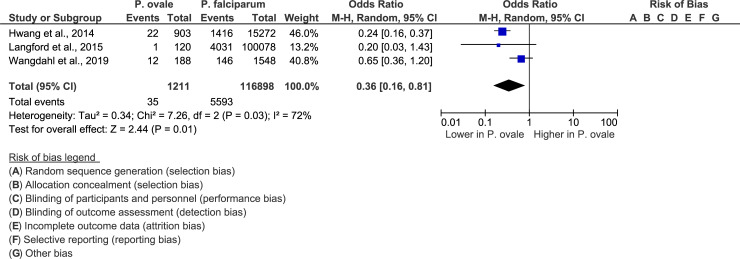
The meta-analysis of three included studies [19, 34, 38] demonstrated that a smaller proportion of patients with P. ovale than of patients with P. falciparum had severe infections (P-value = 0.01, OR = 0.36, 95% CI = 0.16–0.81, I2 = 72%) (Fig 3).
Fig 3. Forest plot comparing severe P. ovale cases and P. falciparum cases.
The forest plot compared the proportions of patients with severe P. ovale infections with that of patients with P. falciparum infections.
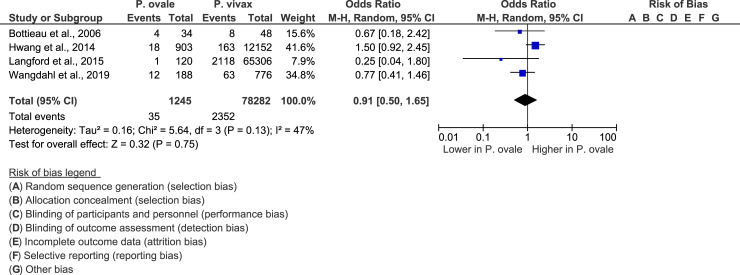
The meta-analysis of four studies [19, 32, 34, 38] revealed there are no significant differences in the proportion of patients with severe P. ovale infections and that of patients with severe P. vivax infections (P-value = 0.75, OR = 0.91, 95% CI = 0.5–1.65, I2 = 47%) (Fig 4).
Fig 4. Forest plot comparing severe P. ovale cases and P. vivax cases.
The forest plot compared the proportions of severe P. ovale cases and P. vivax cases.
The meta-analysis of four studies [19, 32, 34, 38] demonstrated that there was no significant difference between the proportion of patients with severe P. ovale infections and the proportion of patients with severe P. malariae infections (P-value = 0.75, OR = 0.92, 95% CI = 0.56–1.52, I2 = 0%) (Fig 5).
Fig 5. Forest plot comparing severe P. ovale cases and P. malariae cases.
Forest plot comparing the proportion of patients with severe P. ovale cases and the proportion of patients with P. malaria cases.
Mortality rates of P. ovale and Plasmodium spp. infections
There was only one study that reported death cases resulting from P. ovale infections [19].
The mortality rate of severe P. ovale infections in their study was 0.22% (2/903). The mortality rate of severe P. ovale infections among the eight included studies was 0.15% (2/1,365 cases). The mortality rate of other Plasmodium infections reported in the eight included studies was 0.11% for P. falciparum infections, 0.013% for P. vivax infections, 0.03% for P. malariae infections, and 0.004% for mixed infections.
Quality of included studies
Four studies were rated as having 9 stars (good quality), three studies were rated as having 7 stars (adequate quality), and one study was rated as having 6 stars (adequate quality) according to the NOS concerning the selection process for the cases and controls included (Table 3).
Table 3. Quality of the included studies.
| No. | Reference | Selection | Compatibility | Exposure | |||||
|---|---|---|---|---|---|---|---|---|---|
| Is the Case Definition Adequate? | Representativeness of the Cases | Selection of Controls | Definition of Controls | Ascertainment of Exposure | Same method of ascertainment for cases and controls | Non-Response Rate | |||
| 1. | Bottieau et al., 2006 | ✵ | ✵ | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ |
| 2 | de Laval et al., 2010 | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ | ||
| 3. | Hwang et al., 2014 | ✵ | ✵ | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ |
| 4. | Langford et al., 2015 | ✵ | ✵ | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ |
| 5. | Ramos et al., 2016 | ✵ | ✵✵ | ✵ | ✵ | ✵ | |||
| 6. | Rojo-Marcos et al., 2018 | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ | ||
| 7. | Rojo-Marcos et al., 2014 | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ | ||
| 8. | Wangdahl et al., 2019 | ✵ | ✵ | ✵ | ✵ | ✵✵ | ✵ | ✵ | ✵ |
Publication bias
A funnel plot analysis of the included studies could not be performed to assess the publication bias among the included studies, as a minimum of 10 studies were required for the analysis [39].
Discussion
The present systematic review and meta-analysis aimed to explore the prevalence of severe P. ovale infections and to summarize the mortality caused by P. ovale. The results demonstrated that 3% of patients infected with P. ovale developed severe complications according to the WHO 2014 guidelines [1]. Therefore, the term “benign malaria” might not apply to patients tested positive with P. ovale, especially travelers after returning from countries in which P. ovale is endemic. In addition, international and national healthcare providers should be recommending the use of chemoprophylaxis for travelers, as the present study revealed that P. ovale, long-recognized as benign malaria, can cause severe and fatal clinical manifestations. In the present study, a meta-regression with age as a covariate was performed, and the pooled prevalence of severe P. ovale infections was investigated. The results showed that the age of the participants in six of the included studies did not confound the pooled prevalence of severe P. ovale infections. A previous study demonstrated that the highest incidence of P. ovale was observed among children 0–7 years old, who had an 8-fold higher risk of P. ovale infections than did adults; nevertheless, clinical attacks were observed in all age groups [40].
The present study also demonstrated a significant difference between the proportion of patients with severe P. ovale infections and severe P. falciparum infections. A lower proportion of patients with severe P. ovale infections than of patients with P. falciparum infections had severe cases. This finding indicated that although P. ovale can cause severe malaria, the chance of patients developing severe complications is lower with P. ovale than with P. falciparum, which is the Plasmodium species widely known to be the leading cause of severe malaria in humans. This study also demonstrated there are no significant differences in the proportions of patients with severe P. ovale and with P. vivax/P. malariae. This finding demonstrated that there are relatively few severe P. ovale cases, including P. vivax and P. ovale cases. In contrast to P. vivax, which is endemic in Asia, Central America, and South America, P. ovale is only endemic in some African countries [3]. However, the study conducted by Langford et al. in 2015 [34] indicated that there were 120 cases of P. ovale infection in Indonesia, whereas other included studies reported that travelers who returned to their home countries had imported P. ovale malaria cases [19, 32, 35–38].
Although microscopy is still considered to be the gold standard for the identification of Plasmodium species, the morphological characteristics of P. ovale are similar to those of P. vivax and are readily missed by less experienced microscopists [41, 42]. This problem may have caused certain P. ovale cases to have been missed in the included studies conducted by Bottieau et al. [32], de Laval et al. [33], Langford et al. [34], and Wangdahl et al. [38], as only microscopy was used to identify malaria parasites. Missed P. ovale cases have been reported when only microscopy was used [43]. In addition, missed diagnoses of P. ovale infections leading to relapses with a low parasitemia level is a problem related to this Plasmodium species [44]. Previous studies have indicated that the sensitivity of routine microscopy in detecting P. ovale in imported cases is very low and related to low parasitemia levels [32, 45]. Moreover, the rapid diagnosis tests (RDTs) that have been developed and are available lack the sensitivity required to detect low amounts of the circulating antigen of P. ovale [46, 47]. Moreover, a previous study indicated that routine microscopic examinations with thick and thin blood smears should be repeated three times in cases of suspected imported P. ovale malaria [48]. Ideally, molecular detection using polymerase chain reaction (PCR) to detect and confirm P. ovale infections in cases of low parasitemia or negative blood film is needed, although it is not routinely available [32, 43]. Therefore, the limitations of routine microscopic and RDT tests might affect the management/treatment of patients and lead to an increase in the morbidity associated with P. ovale infections [49]. The mortality caused by severe P. ovale infections among travelers who returned to the USA was previously reported by Hwang et al. in 2014 [19]. The authors recommended the use of suppressive prophylaxis and antirelapse treatment with primaquine drugs for patients who returned from ovale-endemic countries [19]. Nevertheless, a previous case report showed that severe complications led to fatality in patients who had received anti-malarial prophylaxis treatment during their trip to Nigeria [20]. This finding may have been caused by the impropriate usage of prophylactic drugs or the survival of hypnozoites during anti-malaria chemoprophylaxis against the P. ovale infection [50].
The mechanism by which P. ovale infections lead to death is still unknown, but acute renal failure and acute respiratory distress syndrome might act as secondary contributing factors to death [51]. People who previously acquired P. falciparum malaria and those staying in malaria-endemic regions are known to show less severe symptoms during subsequent malaria infections than are people who are from non-malaria endemic regions. A previous study investigated two cases of P. ovale in patients, and it was suggested that one patient with a history of malaria was protected against developing severe complications, while the other patient, who was experiencing malaria infection for the first time, suffered severe complications [51]. Therefore, severe P. ovale infections can occur in nonimmune individuals in particular.
This systematic review indicated that jaundice, severe anemia, and pulmonary impairment are the severe complications most commonly found in patients infected with P. ovale. For jaundice, a previous study indicated that mild hyperbilirubinemia can be found in approximately 50% of patients infected with P. ovale [52]. Jaundice concomitant with P. ovale infection might be due to liver dysfunction to conjugate bilirubin in cases of severe red blood cell destruction during malaria infection. Severe anemia concomitant with P. ovale infection might occur in patients with underlying diseases such as hemoglobinopathies, causing the severe destruction of abnormal red blood cells. The mechanism of pulmonary impairment in patients with P. ovale malaria is not clear. In patients with P. falciparum infection, cytoadherence, mechanical obstruction, and inflammation of the microvascular endothelium at the pulmonary vasculature due to infected red blood cells that cytoadhere to the microvascular endothelium causes the mechanical obstruction of pulmonary vasculature; thus, alveolar capillary permeability increases, and intravascular fluid is spread into the lungs, leading to pulmonary failure [53].
Summary of the evidence
The present systematic review and meta-analysis presented cases of severe manifestations and demonstrated the severity and mortality of severe P. ovale infection to increase the awareness of physicians regarding the prognosis of this disease. It is of the utmost importance to test individuals for malaria after they return from malaria-endemic areas, as severe P. ovale complications could occur, especially in nonimmune travelers. Last, the knowledge on appropriate management strategies and the necessary interventions concerning malaria diagnosis affects the severity of malaria manifestations and patient outcomes. Clinicians’ and technicians’ familiarity with the endemicity of malaria in an area or a region therefore plays an important role in the initial stages of laboratory assessments and subsequent diagnoses.
Limitations
A limitation of this systematic review and meta-analysis was that there were a limited number of available publications on severe complications and death related to P. ovale infection. Another limitation was that results from case series and case-control studies were used for prevalence estimations. However, previous studies XXX have suggested and used case reports/series in meta-analyses to facilitate the decision making process when the evidence was limited or a relatively rare or neglected disease was assessed [54–59]. In addition, the most recently published meta-analysis of 44 case series reported the 25-year pooled survival of hip replacements [60]. Considering these limitations, the meta-analysis results and conclusions on the prevalence of severity and mortality associated with P. ovale infection should be considered with caution in combination with the results of newly published reports.
Conclusion
This systematic review demonstrated that although P. ovale infections have long been considered cases of benign malaria, severe complications in patients have been reported on rare occasions. The possible reasons for the small number of reports on the severity of the malaria infections linked or caused directly by P. ovale should be studied further by the scientific community. Clinicians and technicians need to recognize that patients who return from malaria-endemic areas may develop severe P. ovale infections. Additional studies need to consider the potential underreported presence of P. ovale malaria infections in the clinical setting so that appropriate management strategies and interventions can be administered to infected individuals and the knowledge on malaria epidemiology can be expanded further.
Supporting information
PRISMA statement for reporting systematic reviews and meta-analyses.
(DOC)
(DOCX)
Acknowledgments
The authors would like Mr. David C Chang, who helped edit the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was partially supported by the new strategic research (P2P) project, Walailak University, Thailand. The funders had a role in the collection, analysis, and interpretation of the data. There was no additional external funding received for this study.
References
- 1.World Health Organization. Guidelines for the treatment of malaria. 3rd ed. Geneva: WHO; 2015. [Google Scholar]
- 2.Stephens JWW. A new malaria parasite of man. Ann Trop Med Parasitol. 1922;16:383–8. [Google Scholar]
- 3.Collins WE, Jeffery GM. Plasmodium ovale: parasite and disease. Clin Microbiol Rev. 2005;18(3):570–81. 10.1128/CMR.18.3.570-581.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehlotra RK, Lorry K, Kastens W, Miller SM, Alpers MP, Bockarie M, et al. Random distribution of mixed species malaria infections in Papua New Guinea. The American journal of tropical medicine and hygiene. 2000;62(2):225–31. 10.4269/ajtmh.2000.62.225 [DOI] [PubMed] [Google Scholar]
- 5.May J, Mockenhaupt FP, Ademowo OG, Falusi AG, Olumese PE, Bienzle U, et al. High rate of mixed and subpatent malarial infections in southwest Nigeria. The American journal of tropical medicine and hygiene. 1999;61(2):339–43. 10.4269/ajtmh.1999.61.339 [DOI] [PubMed] [Google Scholar]
- 6.Dini S, Douglas NM, Poespoprodjo JR, Kenangalem E, Sugiarto P, Plumb ID, et al. The risk of morbidity and mortality following recurrent malaria in Papua, Indonesia: a retrospective cohort study. BMC Med. 2020;18(1):28 10.1186/s12916-020-1497-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy KJ, Conroy AL, Ddungu H, Shrestha R, Kyeyune-Byabazaire D, Petersen MR, et al. Malaria parasitemia among blood donors in Uganda. Transfusion. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu T, Fu Y, Kong X, Liu X, Yan G, Wang Y. Epidemiological characteristics of imported malaria in Shandong Province, China, from 2012 to 2017. Sci Rep. 2020;10(1):7568 10.1038/s41598-020-64593-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lysenko AJ, Beljaev AE. An analysis of the geographical distribution of Plasmodium ovale. Bull World Health Organ. 1969;40(3):383–94. [PMC free article] [PubMed] [Google Scholar]
- 10.Smith AD, Bradley DJ, Smith V, Blaze M, Behrens RH, Chiodini PL, et al. Imported malaria and high risk groups: observational study using UK surveillance data 1987–2006. BMJ (Clinical research ed). 2008;337:a120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanizaki R, Kato Y, Iwagami M, Kutsuna S, Ujiie M, Takeshita N, et al. Performance of rapid diagnostic tests for Plasmodium ovale malaria in Japanese travellers. Trop Med Health. 2014;42(4):149–53. 10.2149/tmh.2014-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moody A. Rapid diagnostic tests for malaria parasites. Clin Microbiol Rev. 2002;15(1):66–78. 10.1128/cmr.15.1.66-78.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McMorrow ML, Aidoo M, Kachur SP. Malaria rapid diagnostic tests in elimination settings—can they find the last parasite? Clin Microbiol Infect. 2011;17(11):1624–31. 10.1111/j.1469-0691.2011.03639.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mason DP, Kawamoto F, Lin K, Laoboonchai A, Wongsrichanalai C. A comparison of two rapid field immunochromatographic tests to expert microscopy in the diagnosis of malaria. Acta Trop. 2002;82(1):51–9. 10.1016/s0001-706x(02)00031-1 [DOI] [PubMed] [Google Scholar]
- 15.Snounou G, Viriyakosol S, Jarra W, Thaithong S, Brown KN. Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol Biochem Parasitol. 1993;58(2):283–92. 10.1016/0166-6851(93)90050-8 [DOI] [PubMed] [Google Scholar]
- 16.Sharma S, Kaitholia K, Bharti RS, Singh MP, Mishra N. Novel molecular diagnostic technique for detecting the different species of Plasmodium. Infect Genet Evol. 2020;78:104122 10.1016/j.meegid.2019.104122 [DOI] [PubMed] [Google Scholar]
- 17.Sutherland CJ, Tanomsing N, Nolder D, Oguike M, Jennison C, Pukrittayakamee S, et al. Two nonrecombining sympatric forms of the human malaria parasite Plasmodium ovale occur globally. J Infect Dis. 2010;201(10):1544–50. 10.1086/652240 [DOI] [PubMed] [Google Scholar]
- 18.WHO. World malaria report 2018. https://www.who.int/malaria/publications/world-malaria-report-2018/en/
- 19.Hwang J, Cullen KA, Kachur SP, Arguin PM, Baird JK. Severe morbidity and mortality risk from malaria in the United States, 1985–2011. Open Forum Infect Dis. 2014;1(1):ofu034 10.1093/ofid/ofu034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lau YL, Lee WC, Tan LH, Kamarulzaman A, Syed Omar SF, Fong MY, et al. Acute respiratory distress syndrome and acute renal failure from Plasmodium ovale infection with fatal outcome. Malaria journal. 2013;12:389 10.1186/1475-2875-12-389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D'Abramo A, Gebremeskel Tekle S, Iannetta M, Scorzolini L, Oliva A, Paglia MG, et al. Severe Plasmodium ovale malaria complicated by acute respiratory distress syndrome in a young Caucasian man. Malar J. 2018;17(1):139 10.1186/s12936-018-2289-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hachimi MA, Hatim EA, Moudden MK, Elkartouti A, Errami M, Louzi L, et al. The acute respiratory distress syndrome in malaria: Is it always the prerogative of Plasmodium falciparum? Rev Pneumol Clin. 2013;69(5):283–6. 10.1016/j.pneumo.2013.03.001 [DOI] [PubMed] [Google Scholar]
- 23.Haydoura S, Mazboudi O, Charafeddine K, Bouakl I, Baban TA, Taher AT, et al. Transfusion-related Plasmodium ovale malaria complicated by acute respiratory distress syndrome (ARDS) in a non-endemic country. Parasitol Int. 2011;60(1):114–6. 10.1016/j.parint.2010.10.005 [DOI] [PubMed] [Google Scholar]
- 24.Lau YL, Lee WC, Tan LH, Kamarulzaman A, Omar SFS, Fong MY, et al. Acute respiratory distress syndrome and acute renal failure from Plasmodium ovale infection with fatal outcome. Malar J. 2013;12:8 10.1186/1475-2875-12-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rojo-Marcos G, Cuadros-Gonzalez J, Mesa-Latorre JM, Culebras-Lopez AM, de Pablo-Sanchez R. Acute respiratory distress syndrome in a case of Plasmodium ovale malaria. The American journal of tropical medicine and hygiene. 2008;79(3):391–3. [PubMed] [Google Scholar]
- 26.Strydom KA, Ismail F, Frean J. Plasmodium ovale: A case of not-so-benign tertian malaria. Malar J. 2014;13(1):85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomar LR, Giri S, Bauddh NK, Jhamb R. Complicated malaria: A rare presentation of Plasmodium ovale. Trop Doct. 2015;45(2):140–2. 10.1177/0049475515571989 [DOI] [PubMed] [Google Scholar]
- 28.Wangdahl A, Wyss K, Saduddin D, Bottai M, Ydring E, Vikerfors T, et al. Severity of Plasmodium falciparum and non-falciparum malaria in travelers and migrants: A nationwide observational study over 2 decades in Sweden. J Infect Dis. 2019;220(8):1335–45. 10.1093/infdis/jiz292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Groger M, Fischer HS, Veletzky L, Lalremruata A, Ramharter M. A systematic review of the clinical presentation, treatment and relapse characteristics of human Plasmodium ovale malaria. Malaria journal. 2017;16(1):112 10.1186/s12936-017-1759-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yerlikaya S, Campillo A, Gonzalez IJ. A systematic review: Performance of rapid diagnostic tests for the detection of Plasmodium knowlesi, Plasmodium malariae, and Plasmodium ovale monoinfections in human blood. J Infect Dis. 2018;218(2):265–76. 10.1093/infdis/jiy150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol. 2014;14:45 10.1186/1471-2288-14-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bottieau E, Clerinx J, Van Den Enden E, Van Esbroeck M, Colebunders R, Van Gompel A, et al. Imported non-Plasmodium falciparum malaria: A five-year prospective study in a European referral center. Am J Trop Med Hyg. 2006;75(1):133–8. [PubMed] [Google Scholar]
- 33.de Laval F, Oliver M, Rapp C, Pommier de Santi V, Mendibil A, Deparis X, et al. The challenge of diagnosing Plasmodium ovale malaria in travellers: report of six clustered cases in French soldiers returning from West Africa. Malaria journal. 2010;9:358 10.1186/1475-2875-9-358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langford S, Douglas NM, Lampah DA, Simpson JA, Kenangalem E, Sugiarto P, et al. Plasmodium malariae infection associated with a high burden of anemia: A hospital-based surveillance study. PLoS Negl Trop Dis. 2015;9(12):e0004195 10.1371/journal.pntd.0004195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramos JM, Tissiano G, Gosa A, Alegria I, Berzosa P. Study of the prevalence of Plasmodium infections by the polymerase chain reaction (PCR) among patients with severe anaemia treated at a rural hospital in southern Ethiopia. Bol Malariol Salud Ambient. 2016;56(1):75–7. [Google Scholar]
- 36.Rojo-Marcos G, Rubio-Munoz JM, Angheben A, Jaureguiberry S, Garcia-Bujalance S, Tomasoni LR, et al. Prospective comparative multi-centre study on imported Plasmodium ovale wallikeri and Plasmodium ovale curtisi infections. Malar J. 2018;17:11 10.1186/s12936-017-2153-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rojo-Marcos G, Rubio-Muñoz JM, Ramírez-Olivencia G, García-Bujalance S, Elcuaz-Romano R, Díaz-Menéndez M, et al. Comparison of imported Plasmodium ovale curtisi and P. ovale wallikeri infections among patients in Spain, 2005–2011. Emerg Infect Dis. 2014;20(3):409–16. 10.3201/eid2003.130745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wångdahl A, Wyss K, Saduddin D, Bottai M, Ydring E, Vikerfors T, et al. Severity of Plasmodium falciparum and non-falciparum malaria in travelers and migrants: A Nationwide Observational Study over 2 Decades in Sweden. J Infect Dis. 2019;220(8):1335–45. 10.1093/infdis/jiz292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cochrane. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). 2019 [Available from: https://training.cochrane.org/handbook/current.
- 40.Faye FB, Spiegel A, Tall A, Sokhna C, Fontenille D, Rogier C, et al. Diagnostic criteria and risk factors for Plasmodium ovale malaria. J Infect Dis. 2002;186(5):690–5. 10.1086/342395 [DOI] [PubMed] [Google Scholar]
- 41.Mueller I, Zimmerman PA, Reeder JC. Plasmodium malariae and Plasmodium ovale—the "bashful" malaria parasites. Trends Parasitol. 2007;23(6):278–83. 10.1016/j.pt.2007.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Obare P, Ogutu B, Adams M, Odera JS, Lilley K, Dosoo D, et al. Misclassification of Plasmodium infections by conventional microscopy and the impact of remedial training on the proficiency of laboratory technicians in species identification. Malaria journal. 2013;12:113 10.1186/1475-2875-12-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calderaro A, Piccolo G, Perandin F, Gorrini C, Peruzzi S, Zuelli C, et al. Genetic polymorphisms influence Plasmodium ovale PCR detection accuracy. J Clin Microbiol. 2007;45(5):1624–7. 10.1128/JCM.02316-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leder K, Black J, O'Brien D, Greenwood Z, Kain KC, Schwartz E, et al. Malaria in travelers: a review of the GeoSentinel surveillance network. Clin Infect Dis. 2004;39(8):1104–12. 10.1086/424510 [DOI] [PubMed] [Google Scholar]
- 45.Milne LM, Kyi MS, Chiodini PL, Warhurst DC. Accuracy of routine laboratory diagnosis of malaria in the United Kingdom. J Clin Pathol. 1994;47(8):740–2. 10.1136/jcp.47.8.740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bigaillon C, Fontan E, Cavallo JD, Hernandez E, Spiegel A. Ineffectiveness of the Binax NOW malaria test for diagnosis of Plasmodium ovale malaria. J Clin Microbiol. 2005;43(2):1011 10.1128/JCM.43.2.1011.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marx A, Pewsner D, Egger M, Nuesch R, Bucher HC, Genton B, et al. Meta-analysis: accuracy of rapid tests for malaria in travelers returning from endemic areas. Ann Intern Med. 2005;142(10):836–46. 10.7326/0003-4819-142-10-200505170-00009 [DOI] [PubMed] [Google Scholar]
- 48.Brent AJ, Angus BJ. Not all that is malaria is falciparum. Lancet Infect Dis. 2008;8(3):208 10.1016/S1473-3099(08)70044-6 [DOI] [PubMed] [Google Scholar]
- 49.Imbert P, Rapp C, Buffet PA. Pathological rupture of the spleen in malaria: Analysis of 55 cases (1958–2008). Travel Med Infect Dis. 2009;7(3):147–59. 10.1016/j.tmaid.2009.01.002 [DOI] [PubMed] [Google Scholar]
- 50.Nolder D, Oguike MC, Maxwell-Scott H, Niyazi HA, Smith V, Chiodini PL, et al. An observational study of malaria in British travellers: Plasmodium ovale wallikeri and Plasmodium ovale curtisi differ significantly in the duration of latency. BMJ Open. 2013;3(5): e002711 10.1136/bmjopen-2013-002711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lau YL, Lee WC, Tan LH, Kamarulzaman A, Syed Omar SF, Fong MY, et al. Acute respiratory distress syndrome and acute renal failure from Plasmodium ovale infection with fatal outcome. Malar J. 2013;12(1):389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taylor T, Agbenyega T. Malaria. In: Magill AJ, Hill DR, Solomon T, Ryan ET, editors. Hunter's Tropical Medicine and Emerging Infectious Disease. 9th ed. Saunders: Elsevier B.V.; 2012. [Google Scholar]
- 53.Taylor WR, White NJ. Malaria and the lung. Clin Chest Med. 2002;23(2):457–68. 10.1016/s0272-5231(02)00004-7 [DOI] [PubMed] [Google Scholar]
- 54.Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23(2):60–3. 10.1136/bmjebm-2017-110853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nissen T, Wynn R. The clinical case report: a review of its merits and limitations. BMC Res Notes. 2014;7:264 10.1186/1756-0500-7-264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakamura T, Igarashi H, Ito T, Jensen RT. Important of case-reports/series, in rare diseases: Using neuroendocrine tumors as an example. World J Clin Cases. 2014;2(11):608–13. 10.12998/wjcc.v2.i11.608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jackson D, Daly J, Saltman DC. Aggregating case reports: a way for the future of evidence-based health care? Clin Case Rep. 2014;2(2):23–4. 10.1002/ccr3.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sampayo-Cordero M, Miguel-Huguet B, Pardo-Mateos A, Malfettone A, Perez-Garcia J, Llombart-Cussac A, et al. Agreement between results of meta-analyses from case reports and clinical studies, regarding efficacy and safety of idursulfase therapy in patients with mucopolysaccharidosis type II (MPS-II). A new tool for evidence-based medicine in rare diseases. Orphanet J Rare Dis. 2019;14(1):230 10.1186/s13023-019-1202-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chambers D, Rodgers M, Woolacott N. Not only randomized controlled trials, but also case series should be considered in systematic reviews of rapidly developing technologies. J Clin Epidemiol. 2009;62(12):1253–60 e4. 10.1016/j.jclinepi.2008.12.010 [DOI] [PubMed] [Google Scholar]
- 60.Evans JT, Evans JP, Walker RW, Blom AW, Whitehouse MR, Sayers A. How long does a hip replacement last? A systematic review and meta-analysis of case series and national registry reports with more than 15 years of follow-up. Lancet. 2019;393(10172):647–54. 10.1016/S0140-6736(18)31665-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA statement for reporting systematic reviews and meta-analyses.
(DOC)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.