Abstract
Background:
Older surgical patients with Alzheimer’s disease (AD) dementia and delirium are at increased risk for accelerated long-term cognitive decline.
Objective:
Investigate associations between a probabilistic marker of preclinical AD, delirium, and long-term cognitive decline.
Methods:
The Successful Aging after Elective Surgery cohort includes older adults (≥70yrs) without dementia who underwent elective surgery. 140 patients underwent preoperative magnetic resonance imaging and had ≥ 6 months cognitive follow-up. Cortical thickness was measured in ‘AD-Signature’ regions. Delirium was evaluated each postoperative day by the Confusion Assessment Method. Cognitive performance was assessed using a detailed neuropsychological battery at baseline; months 1, 2, and 6; and every 6 months thereafter until 36 months. Using either a General Cognitive Performance composite (GCP) or individual test scores as outcomes, we performed linear mixed effects models to examine main effects of AD-signature atrophy and the interaction of AD-signature atrophy and delirium on slopes of cognitive change from post-operative months 2-36.
Results:
Reduced baseline AD-signature cortical thickness was associated with greater 36-month cognitive decline in GCP (standardized beta coefficient, β = −0.030, 95% confidence interval [−0.060, −0.001]). Patients who developed delirium who also had thinner AD signature cortex showed greater decline on a verbal learning test (β = −0.100 [−0.192, −0.007]).
Conclusion:
Patients with the greatest baseline AD-related cortical atrophy who develop delirium after elective surgery appear to experience the greatest long-term cognitive decline. Thus, atrophy suggestive of preclinical AD and the development of delirium may be high-risk indicators for long-term cognitive decline following surgery.
Keywords: preclinical Alzheimer’s disease, AD signature, aging signature, cortical thickness, post-operative, surgery, cognitive decline, delirium
INTRODUCTION
Patients with dementia due to Alzheimer’s disease (AD) who develop delirium are at increased risk for poor post-operative outcomes, including accelerated cognitive decline[1, 2]. Increasing evidence suggests that an interrelationship between delirium and AD occurs even before the onset of dementia[1, 3–5]. The associations between delirium and preclinical or prodromal AD, and implications for cognitive function following surgery, are still being investigated.
Preclinical AD refers to the stage of the disease where pathological changes are present prior to the onset of cognitive or functional impairment; therefore, biomarkers sensitive to early preclinical changes are required for probing this phase of the disease. The AD signature, a specific set of brain regions that show reduction of cortical thickness due to AD, has previously been shown to be associated with cognitive decline and progression to dementia[6–11], as well as with cerebrospinal fluid (CSF) biomarkers of AD[9]. Thus, the AD signature is a good surrogate marker for preclinical AD in the absence of available molecular markers for amyloid-beta (Aβ) and tau, and provides a window into associations between delirium and preclinical AD. Indeed, we have previously shown that patients with thinner cortex in the AD-signature experience delirium with greater severity[3]. This study uses this magnetic resonance imaging (MRI)-based AD biomarker to investigate associations between a preclinical AD risk marker, delirium, and long-term cognitive decline following surgery.
We hypothesized that patients with the thinnest cortex in the AD-signature regions, suggestive of preclinical AD, would have the steepest rates of cognitive decline after surgery. We additionally hypothesized that development of delirium would hasten such decline, such that patients with the most AD-related atrophy and delirium would have the fastest rates of post-operative cognitive decline. To determine if our findings were AD-specific, we also examined associations with the ‘Aging-Only signature,’ which is another set of regions of interest (ROIs) that is associated with reduction of cortical thickness due to aging but is relatively spared in AD[10].
MATERIALS AND METHODS
Participants
Participants were selected from the Successful Aging after Elective Surgery (SAGES) study. The SAGES study design and methods have been described in detail previously.[12, 13] In brief, eligible participants were age 70 years and older, English speaking, scheduled to undergo elective surgery at one of two Harvard-affiliated academic medical centers, with an anticipated length of stay of at least 3 days. Eligible surgical procedures included: total hip or knee replacement; lumbar, cervical, or sacral laminectomy; lower extremity arterial bypass; open abdominal aortic aneurysm repair; and open or laparoscopic colectomy. Exclusion criteria were evidence of dementia, delirium, or hospitalization within 3 months, terminal condition, legal blindness, severe deafness, history of schizophrenia or psychosis, and history of alcohol abuse or withdrawal. A total of 560 patients met all eligibility criteria and were enrolled between June 18, 2010 and August 8, 2013. Written informed consent for study participation was obtained from all participants according to procedures approved by the institutional review boards of Beth Israel Deaconess Medical Center (BIDMC) and Brigham and Women’s Hospital (BWH), the two study hospitals, and Hebrew SeniorLife, the coordinating center. Additional exclusion criteria included contraindications to 3T MRI. Because the primary outcome for this analysis is cognitive decline after surgery, we only included patients (N = 140) with MRI who completed at least 6 months of post-operative follow-up (Supplementary Figure 1).
Neuroimaging protocol and analysis
We analyzed the magnetization-prepared fast gradient-echo (MPRAGE) 3D anatomical T1-weighted images (TR 7.9 ms, TE 3.2 ms, 15° flip angle, 32 kHz bandwidth, 24×19 cm field of view, 0.94 mm in-coronal-plane resolution, 1.4 mm slices, preparation time of 1100 ms with repeated saturation at the beginning of the saturation period, and an adiabatic inversion pulse 500 ms before imaging) collected at the BIDMC Radiology Department on a 3T HDxt MRI (General Electric Medical Systems) scanner using an 8-channel head coil[14].
T1 image volumes were examined quantitatively by FreeSurfer (http://surfer.nmr.mgh.harvard.edu) cortical surface-based reconstruction and analysis of cortical thickness, using a hypothesis-driven approach[3, 6, 7, 11]. Briefly, we followed standard procedures for motion-correction, tissue segmentation, visual inspection for potential errors in reconstruction, and cortical thickness calculation.
Nine bilateral AD-signature ROIs (Supplementary Figure 2) identified from prior analysis were mapped onto the individual participants[6, 7]. For each subject, mean cortical thickness within each ROI was calculated, averaged across the hemispheres, and averaged to obtain a single measure, the “AD signature”, which was used for further statistical analysis as the primary diagnostic biomarker. The AD signature was scaled by a factor of 10 so that the resultant regression coefficients refer to the change in outcome associated with a 0.10 mm (slightly less than 1.5 SD) increase in AD signature cortical thickness. The AD signature was reverse-scored so that smaller values indicate less atrophy (thicker cortex) and larger values indicate greater atrophy (thinner cortex). The term “atrophy” is used throughout this manuscript to refer to lower cortical thickness (i.e. thinner cortex) in a cross-sectional framework and does not refer to longitudinal cortical thinning. We hypothesized greater AD-signature atrophy would be associated with steeper, more negative slopes of cognitive decline.
Our primary models analyzed the AD signature as a continuous variable, but for descriptive or graphical presentation as well as effect size calculation, we created a categorical variable based on tertiles of distribution by AD signature thickness. Tertile 3 refers to those with the thinnest cortex (i.e. most AD-related cortical atrophy), tertile 2 includes those with average thickness, and tertile 1 refers to those with the thickest cortex in these ROIs and serves as the reference group in stratified models.
Additionally, we calculated the ‘Aging-Only signature’ in a similar fashion based on brain regions where atrophy is seen primarily in normal aging with minimal additional effects of AD, and which do not overlap with the AD signature ROIs[10, 15]. Using the same methods as above, we calculated a single summary “Aging-Only signature” measure as the average thickness of the eight bilateral ROIs described in Supplementary Figure 2. Similar to the AD signature, the Aging-Only signature was scaled by a factor of 10 and reverse-scored.
Assessment of delirium and cognitive function
Delirium incidence and severity were assessed on each postoperative day. Cognitive function was assessed at baseline prior to surgery, and at post-operative months 1, 2, 6, and every 6 months thereafter until 36 months.
Delirium Assessment
The Confusion Assessment Method (CAM)[16] diagnostic algorithm, supplemented with a validated chart review method[17], was used to detect delirium presence or absence for each patient. The CAM was rated based on information from patient interviews performed once daily in the late morning or early afternoon at approximately the same time each day; these included a brief cognitive screen (orientation, short-term recall, sustained attention), the questions from the Delirium Symptom Interview, and information related to acute changes in mental status noted by nurses or family members[13]. Both the CAM and the chart-based method have high sensitivity and specificity[17]. The CAM plus chart combined approach is the preferred method for detecting delirium since it maximizes sensitivity; while the CAM detects the majority of delirium cases, the additional chart review increases sensitivity by identifying delirium throughout the 24-hour period[18].
Cognitive function
A complete neuropsychological test battery was completed preoperatively and at postoperative months 1, 2, 6, and every 6 months thereafter until 36 months. The battery included the following tests: Trail Making Tests A and B, Phonemic (F-A-S) Fluency, Category Fluency, Visual Search and Attention Test, Hopkins Verbal Learning Test-Revised (HVLT-R), Digit Span Forward/Backward, Boston Naming Test, and Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) Digit Symbol Substitution. For consistency, Trail Making Tests A and B were reverse-scored so that for all tests, higher scores correspond to better performance and lower scores to worse performance.
From this battery, we created a composite summary measure, the General Cognitive Performance (GCP), which was used as our primary outcome measure of longitudinal cognitive decline. GCP is a weighted composite measure that is calibrated to a nationally representative sample of adults ≥70 years, in order to yield a mean score of 50 and standard deviation (SD) of 10[19, 20] at the U.S. population level. The GCP is sensitive to change, has minimal floor and ceiling effects, and has been utilized in many prior studies to date[14, 21].
We corrected for retest or practice effects using methods previously published [21, 22]. Briefly, this method involves subtracting a correction derived from repeated administrations in an age-matched non-surgical comparison sample that received the same testing protocol. Thus, the cognitive scores are corrected for retest effect before being entered into the statistical models.
Statistical Analysis
Linear mixed effects (LME) models were used to model baseline levels and longitudinal changes in cognitive test scores, and to assess short- and long-term differences based on the degree of cortical atrophy present at the pre-operative baseline MRI. A linear model was selected based on our prior work modeling cognitive trajectories from 2 to 36 months in SAGES [21]. Specifically, we used LME models with maximum likelihood parameter estimation and an unstructured covariance. All conditional models included a random intercept, random time-slope from 2-36 months, fixed time indicator variables for months 1 and 2, and a fixed time-slope from 2-36 months, and fixed effects for covariates (mean-centered age, female sex, and years of education), main effect of atrophy, and interactions between time indicator variables and atrophy (1m × atrophy and 2m × atrophy) and time-slope and atrophy (2-36m slope × atrophy). Data were assessed for outliers, and models assumed that incomplete data were missing at random (Supplementary Table 1). A formal test for a missing data mechanism is not possible; however, we have previously shown through sensitivity analyses (1. imputation of extreme GCP values for those subjects who died or dropped out; 2. exclusion of all the subjects who would later die or drop out; 3. imputation of all GCP observations not observed through 36 months) that the maximum likelihood parameter estimation reported in this study is robust to multiple different assumptions about missing data, and presents a conservative estimate[21; see Supplemental Technical Appendix, “Sensitivity analyses: Missing data” (Page 9) of the cited manuscript for details]. To facilitate comparison across models, GCP score and individual neuropsychological tests scores were transformed into z-scores before being entered into the model and standardized β coefficients are reported.
Model 1 is as described above, with the AD signature modeled as a continuous variable (main effect and interactions with time variables).
Model 2 is the same as Model 1 but additionally adds a main effect of delirium and the interaction of delirium with the AD signature and time variables (i.e. delirium × time; AD signature × time; and delirium × AD signature x time).
Cohen’s d effect sizes were calculated by comparing groups defined using the baseline AD signature measure and/or the presence of delirium with regard to slopes of cognitive outcomes. Slope was calculated as the predicted random slope from a linear mixed effects model with the same parameters as described above, but omitting delirium, the AD signature, and the delirium × AD signature interaction. Predicted slopes used for Cohen’s d analyses are provided in Supplementary Table 2.
For effect size calculations, we divided the sample into tertiles using the baseline AD signature measure. Thus, to estimate effect sizes for Model 1, tertile 3 (greatest atrophy) and tertile 2 were compared to tertile 1. To estimate effect sizes for Model 2, groups were categorized based on both AD signature tertiles and presence/absence of delirium, yielding six groups: 1) AD signature tertile 1, no delirium, 2) AD signature tertile 2, no delirium, 3) AD signature tertile 3, no delirium, 4) AD signature tertile 1, delirium, 5) AD signature tertile 2, delirium, 6) AD signature tertile 3, delirium. The last group—AD signature tertile 3 (greatest AD-related atrophy) who also developed delirium—is hypothesized to be the highest risk group. The first group—AD signature tertile 1 who did not develop delirium—is hypothesized to be the lowest risk group and thus is the reference group against which other groups are compared.
Specificity Analyses
In addition to the primary models (1 and 2 above), we performed the following analyses to probe the specificity of the anatomical findings, and of the cognitive outcomes.
First, we repeated Models 1 and 2, using the Aging-Only signature instead of the AD signature. The purpose of these analyses was to examine the specificity of the effects of atrophy on outcomes, with the hypothesis that baseline cortical thickness in these brain regions would not demonstrate a relationship to long-term cognitive decline. For comparative purposes, we also illustrated GCP slope effects by dividing the sample into tertiles using the baseline Aging-Only signature measure and the presence/absence of delirium.
Based on a priori hypotheses[23] we also examined individual tests of verbal memory (HVLT-R total learning and delayed recall) and executive function (Trails B). For individual tests that showed significantly different Cohen’s d compared to the reference group (AD signature tertile 1 or AD signature tertile 1 and delirium negative), linear mixed effects models were conducted using the methods described above for Model 2 to test for effects of AD signature, delirium, and AD signature × delirium on cognitive decline, with the individual test z-score as the outcome.
All statistical analyses were performed using Stata software (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC) with α = 0.05.
RESULTS
Of the 140 SAGES participants with MRIs and at least six months of cognitive follow-up (mean age 76.1 years, 61% women), thirty participants (21%) developed delirium. Other baseline characteristics are described in Table 1. Sample characteristics by the six AD signature tertile/delirium groups are also available in Supplementary Table 3 and baseline test scores are reported in Supplementary Table 4. Covariate model coefficients for standardized cognitive outcomes (z-scores) are reported in Supplementary Table 6.
Table 1.
Sample characteristics of SAGES neuroimaging sub-cohort
| All | No Delirium | Delirium | |
|---|---|---|---|
| N | 140 | 110 | 30 |
| Female Sex, n (%) | 85 (60.7%) | 64 (58.2%) | 21 (70.0%) |
| Non-White or Hispanic, n (%) | 12 (8.6%) | 10 (9.1%) | 2 (6.7%) |
| Age at Baseline Assessment, years, mean (SD) | 76.1 (4.5) | 75.9 (4.6) | 76.6 (4.4) |
| Education, years, median (IQR) | 14 (13, 18) | 15 (13, 18) | 14 (12, 16) |
| Charlson Comorbidity Index, mean (SD) | 0.85 (1.02) | 0.82 (1.00) | 0.97 (1.13) |
| Baseline GCP, mean (SD) | 58.7 (6.6) | 59.4 (6.7) | 56.2 (6.0) |
| Geriatric Depression Scale, median (IQR) | 2 (.5, 3) | 2 (1, 3) | 2 (0, 3) |
| 3MS Score, median (IQR) | 95 (91, 98) | 95 (92, 98) | 93 (88, 96) |
| Proxy IQCODE, mean (SD) | 3.14 (0.24) | 3.12 (0.19) | 3.19 (0.37) |
| Any ADL Impairment, n (%) | 10 (7.1%) | 7 (6.4%) | 3 (10.0%) |
To compare the delirium group to the no delirium group, chi square tests were used for dichotomous variables, t-tests for continuous variables with normal distributions, and Wilcoxon rank-sum tests for continuous variables with a skewed distribution.
3MS = Modified Mini Mental State Exam (range 0-100, higher scores refer to better performance); ADL = Activities of Daily Living; GCP = General Cognitive Performance; IQCODE = Informant Questionnaire on Cognitive. Decline in the Elderly; SAGES = Successful Aging after Elective Surgery
To facilitate comparison across models, the following results are presented using standardized beta coefficients (β), which refer to the standard deviation change in cognitive score (or slope) per standard deviation increase in AD signature. Because we reverse-scored the AD signature, a negative β indicates that greater atrophy is associated with worse cognitive performance.
Although effect sizes were estimated using cortical signature tertiles, statistical inference was based on analysis of the cortical signatures as continuous variables. Therefore, graphical representations of our models (Figures 1 and 2) visually depict the model estimates from the analyses of the cortical signatures as continuous variables, but are displayed using categorical groups (i.e., tertiles) for descriptive purposes only. Below, effect sizes (Cohen’s d) are reported from the effect size analysis utilizing tertiles and β-coefficients with 95% confidence intervals are reported from the analysis of the cortical signature as a continuous variable.
Figure 1. Group effects of cortical atrophy due to AD (A) or aging (B) and post-operative delirium on short- and long-term change in general cognitive performance after surgery.
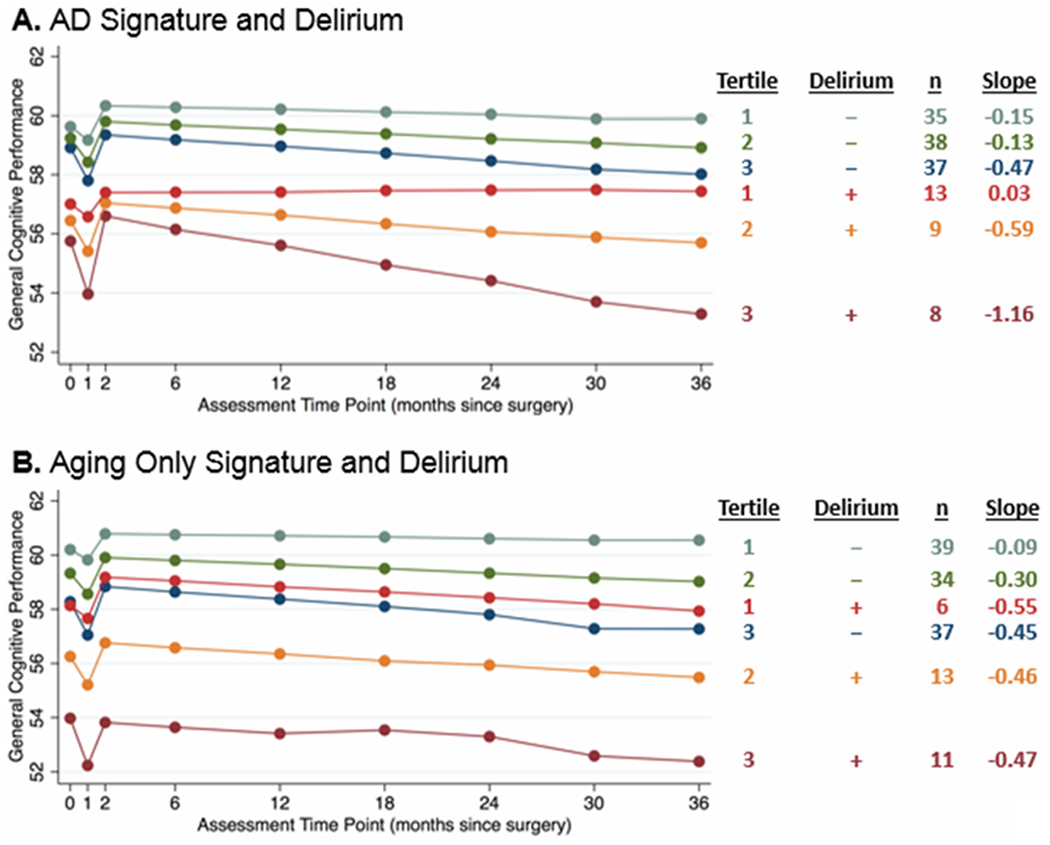
The cortical signatures were analyzed as continuous variables, but for illustration purposes, results are displayed by groups based on cortical signature tertiles (tertile 1 has the thickest cortex, tertile 3 has the thinnest cortex, interpreted as the greatest atrophy) and delirium (present [+] or absent [−]). Tertiles are generated separately for AD signature and for Aging-Only signature measures, leading to slight differences in group size. Estimated slopes are reported based on model coefficients and average cortical thickness in the respective tertile.
Figure 2. Group effects of cortical atrophy due to AD and post-operative delirium on short- and long-term change on selected individual neuropsychological tests.
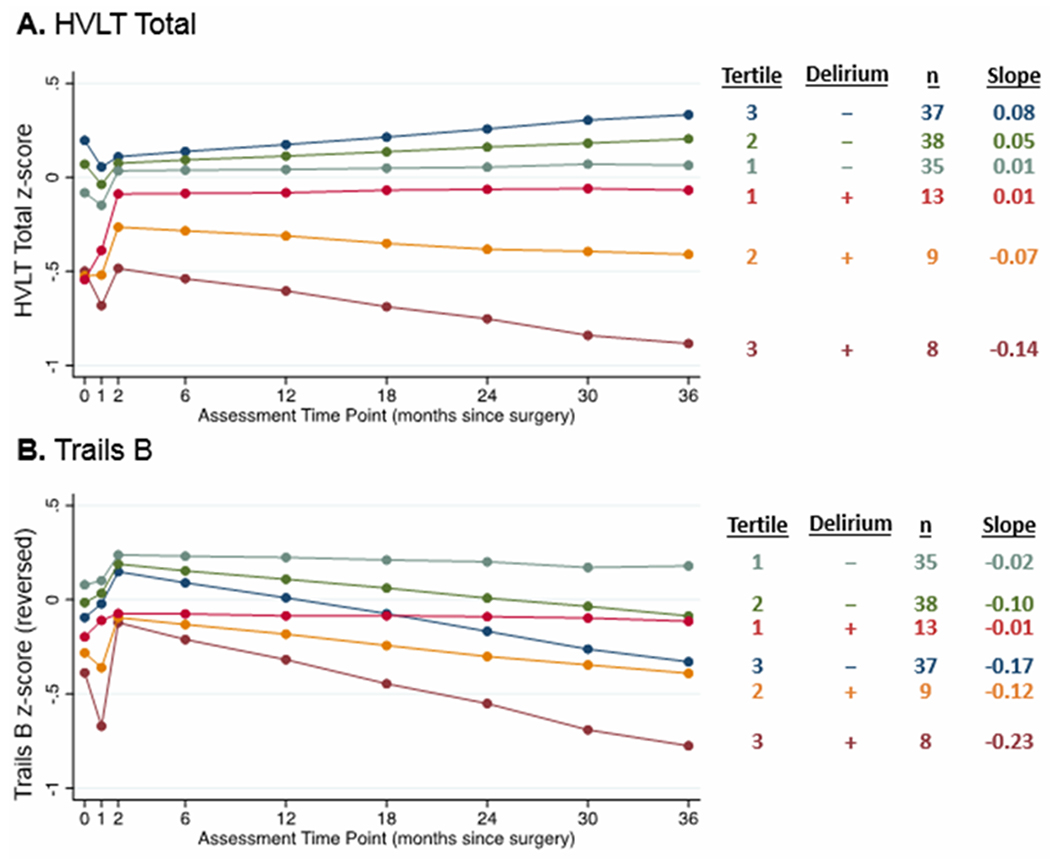
The cortical signatures were analyzed as continuous variables, but for illustration purposes, results are displayed by groups based on cortical signature tertiles (see Figure 1 for details) and delirium (present [+] or absent [−]). Estimated slopes are reported based on model coefficients and average cortical thickness in the respective tertile. (A) HVLT = Hopkins Verbal Learning Test – Revised total score. (B) Trails B = Trail Making Test B (reverse-scored so that lower numbers indicate worse performance). Neuropsychological tests were transformed into z-scores before being entered into the models.
Model 1: Effect of AD signature on cognitive decline
Small to medium effect sizes were observed for the difference between the tertile with the most AD signature atrophy (tertile 3 Cohen’s d = −0.40, Table 3) and with average atrophy (tertile 2 Cohen’s d = −0.26) compared to the tertile with the least atrophy (tertile 1). Greater baseline AD-signature cortical atrophy was associated with a steeper slope of cognitive decline (β = −0.030, 95% confidence interval, CI [−0.060, −0.001]; Table 2). Unstandardized results for our primary analysis with GCP are available in Supplementary Table 5.
Table 3.
Cohen’s d effect sizes for group comparisons of slope from months 2-36 for GCP and selected individual neuropsychological tests
| Effect size: Cohen’s d | ||||
|---|---|---|---|---|
| Group: | HVLT total learning | HVLT delayed recall | Trails B | GCP |
| AD signature Tertiles [reference group: AD signature Tertile 1 (n=45)] | ||||
| AD signature Tertile 2 (n=47) | −0.20 | −0.05 | −0.32 | −0.26 |
| AD signature Tertile 3 (n=48) | −0.13 | −0.32 | −0.66 | −0.40 |
| AD signature Tertiles and Delirium [(reference group: AD signature Tertile 1, no delirium (n=35)] | ||||
| AD signature Tertile 2, no delirium (n=38) | −0.10 | 0.02 | −0.40 | −0.31 |
| AD signature Tertile 3, no delirium (n=37) | 0.11 | −0.23 | −0.66 | −0.25 |
| AD signature Tertile 1, delirium (n=13) | 0.08 | −0.01 | −0.16 | −0.03 |
| AD signature Tertile 2, delirium (n=9) | −0.44 | −0.34 | −0.09 | −0.11 |
| AD signature Tertile 3, delirium (n=8) | −1.13 | −0.83 | −1.16 | −1.10 |
Cohen’s d effect size interpretation: small (d ≥ 0.2), medium (d ≥ 0.5), large (d≥0.8); medium to large effect sizes are bolded.
GCP = General Cognitive Performance; HVLT = Hopkins Verbal Learning Test–Revised; Trails B = Trail Making Test B
Table 2.
Standardized coefficients from linear mixed effects models for associations between AD (or Aging-Only) signature cortical atrophy and/or delirium on post-operative short-term and long-term cognitive change
| Standardized Coefficients (95% Confidence Intervals) | ||||
|---|---|---|---|---|
| Baseline | Month 1 | Month 2 | Months 2-36 slope | |
| GCP (Model 1) | ||||
| AD signature | −0.035 (−0.179, 0.109) | −0.048 (−0.119, 0.024) | 0.041 (−0.020, 0.101) | −0.030 (−0.060, −0.001) |
| GCP (Model 2) | ||||
| AD signature | −0.044 (−0.202, 0.115) | −0.040 (−0.121, 0.040) | 0.024 (−0.044, 0.092) | −0.020 (−0.053, 0.013) |
| Delirium | −0.387 (−0.728, −0.045) | −0.042 (−0.215, 0.132) | 0.048 (−0.100, 0.195) | −0.035 (−0.105, 0.035) |
| AD signature x delirium | −0.033 (−0.381, 0.314) | −0.044 (−0.221, 0.134) | 0.088 (−0.062, 0.239) | −0.053 (−0.123, 0.016) |
| HVLT Total Learning | ||||
| AD signature | 0.127 (−0.033, 0.286) | −0.034 (−0.156, 0.088) | −0.059 (−0.162, 0.044) | 0.032 (−0.013, 0.076) |
| Delirium | −0.582 (−0.927, −0.237) | 0.094 (−0.170, 0.359) | 0.133 (−0.091, 0.357) | −0.110 (−0.203, −0.017) |
| AD signature x delirium | −0.106 (−0.457, 0.245) | −0.120 (−0.391, 0.150) | 0.012 (−0.217, 0.240) | −0.100 (−0.192, −0.007) |
| HVLT Delayed Recall | ||||
| AD signature | 0.118 (−0.050, 0.286) | −0.060 (−0.188, 0.068) | 0.052 (−0.056, 0.161) | −0.027 (−0.077, 0.022) |
| Delirium | −0.475 (−0.838, −0.112) | 0.151 (−0.126, 0.428) | 0.145 (−0.090, 0.380) | −0.117 (−0.222, −0.012) |
| AD signature x delirium | −0.054 (−0.423, 0.316) | −0.113 (−0.397, 0.170) | −0.165 (−0.405, 0.075) | 0.014 (−0.091, 0.118) |
| Trails B | ||||
| AD signature | −0.079 (−0.244, 0.086) | 0.023 (−0.114, 0.161) | 0.015 (−0.101, 0.132) | −0.068 (−0.125, −0.012) |
| Delirium | −0.281 (−0.637, 0.074) | −0.142 (−0.439, 0.155) | 0.134 (−0.119, 0.386) | −0.027 (−0.146, 0.093) |
| AD signature x delirium | −0.008 (−0.370, 0.354) | −0.192 (−0.496, 0.111) | 0.219 (−0.039, 0.476) | −0.031 (−0.150, 0.089) |
| GCP (Model 1) – Aging-Only | ||||
| Aging-Only signature | −0.124 (−0.267, 0.018) | −0.056 (−0.128, 0.016) | 0.042 (−0.019, 0.103) | −0.023 (−0.052, 0.006) |
| GCP (Model 2) – Aging-Only | ||||
| Aging-Only signature | −0.121 (−0.274, 0.032) | −0.053 (−0.133, 0.026) | 0.052 (−0.015, 0.119) | −0.029 (−0.062, 0.004) |
| Delirium | −0.431 (−0.768, −0.095) | −0.043 (−0.218, 0.132) | 0.027 (−0.121, 0.176) | −0.020 (−0.091, 0.051) |
| Aging-Only signature x delirium | −0.139 (−0.508, 0.229) | −0.026 (−0.219, 0.167) | −0.048 (−0.211, 0.116) | 0.028 (−0.050, 0.105) |
All models included mean-centered covariates for age (years), female sex, and years of education
Baseline refers to the pre-operative cognitive assessment and thus provides a reference level of cognitive function prior to the onset of delirium. The coefficients and confidence intervals for the “Baseline” column refer to the cross-sectional association of the AD/Aging Only signature, delirium, or their interaction with baseline cognitive performance. The “Month 1” coefficients and confidence intervals refer to the association of these same predictors and cognitive change from baseline to month 1; “Month 2” coefficients and confidence intervals refer to the association of these predictors and cognitive change from month 1 to month 2; and, finally, “Months 2-36” coefficients and confidence intervals refer to the association of these predictors and cognitive slope from months 2-36.
AD = Alzheimer’s disease; GCP = General Cognitive Performance; HVLT = Hopkins Verbal Learning Test – Revised
Model 2: Effect of AD signature x delirium on cognitive decline
A large effect size (Cohen’s d = 1.10, Table 3) was observed for the difference between the most vulnerable group (AD signature tertile 3, delirium) and the least vulnerable group (AD signature tertile 1, no delirium), as illustrated in Figure 1A. Although the interaction of AD signature x delirium on cognitive outcomes was not statistically significant (β = −0.053, 95% CI [−0.123, 0.016]; Table 2), the direction of the effect indicates that greater cortical atrophy was associated with greater cognitive decline, which was further accelerated by the presence of delirium. However, given the lack of statistical significance of the effect, the observed association may well be due to chance or sampling variation. Additionally, delirium was associated with lower baseline GCP scores (β = −0.387, 95% CI [−0.728, −0.045]).
Specificity Analyses
Aging-Only signature
No statistically significant associations were observed for the Aging-Only signature at baseline, months 1 or 2, or 2-36 month slope in models with or without delirium (Table 2, Figure 1B). Similar to the AD-signature analysis, delirium was associated with lower baseline GCP scores (β = −0.431, 95% CI [−0.768, −0.095]).
Individual cognitive tests
Large effects were observed for HVLT-R total learning score, HVLT-R delayed recall score, and Trail Making Test B (Table 3), and LME models were conducted for all three individual tests (Table 2). Delirium was associated with steeper slopes on HVLT-R delayed recall (β = −0.117, 95% CI [−0.222, −0.012] and AD signature was associated with steeper slopes on Trails B (β = −0.068, 95% CI [−0.125, −0.012], Figure 2B), but there were no significant interactions between AD signature and delirium for either of these outcomes. In contrast, the interaction of AD signature and delirium was associated with greater decline in HVLT-R total learning score (β = −0.100, 95% CI [−0.192, −0.007], Figure 2A). Delirium was associated with lower baseline scores for HVLT-R total learning and delayed recall (Table 2).
DISCUSSION
AD signature cortical atrophy and delirium have previously been shown to independently predict cognitive decline and progression to dementia in people who at baseline are cognitively normal or have mild cognitive impairment[1, 6–8, 11, 21]. This study builds on this prior work by showing that greater baseline AD signature atrophy – an MRI biomarker probabilistically associated with AD-related neurodegeneration[9, 11] – was associated with greater long-term cognitive decline following surgery over the ensuing 3 years. We also found evidence that delirium and AD signature atrophy interact to influence the post-operative cognitive trajectory. Specifically, declining performance in HVLT, a test of learning and memory, was accelerated in patients with greater AD signature atrophy who also developed delirium. These results indicate that older adults with a cortical signature suggestive of preclinical AD who develop delirium during hospitalization for elective surgery may be at higher risk for long-term post-operative cognitive decline. However, these findings are largely hypothesis-generating, and future studies with larger sample sizes are needed in order to make stronger conclusions.
Although the effect size for the interaction of AD signature and delirium on GCP slope was large when examined using group comparisons (Table 3 and Figure 1A), it was not statistically significant in a LME model. By examining two of the most sensitive tests to early AD cognitive changes[23], HVLT-R and Trails B, we were able to gain insight into this apparent discrepancy. For the HVLT-R total learning score, we found consistent effects using both group comparison analyses and the LME model. That is, participants with the greatest baseline AD-signature atrophy who developed delirium showed consistent long-term decline on this measure with a very large Cohen’s d effect size. The HVLT-R is a commonly used clinical neuropsychological test of verbal learning and episodic memory. Our results are consistent with prior studies showing that list learning scores are predictive of dementia[23, 24], decline in preclinical AD [25, 26], and are associated with atrophy in regions that are vulnerable to AD-related neurodegeneration[27] and that are captured by AD-signature ROIs. Interestingly, we observed an association of steeper slopes on HVLT delayed recall with delirium, but not with the AD signature as we would have expected. For Trails B, the AD signature – but neither delirium nor the interaction of delirium and the AD signature – was associated with steeper slopes of decline. These findings suggest domain-specific interactions between AD and delirium, which may not always be captured by global neuropsychological composite scores. However, there are also limitations to using individual tests, including potential floor and ceiling effects, or differing sensitivity to practice effects. Indeed, despite adjusting for retest effects, marginal gains were observed over time on the HVLT-R total learning score (but not on Trails B or GCP) in the lowest risk patients (those without delirium and those with delirium but who had the least AD signature atrophy; Figure 2A). These observations point to the value of examining both general cognitive scores like the GCP, as well as individual tests or domain-specific tests or composites.
Lower baseline cognitive performance was consistently associated with higher risk of delirium across most of our models. Low cognitive ability is a known risk factor for delirium, and in fact has previously been shown to dominate risk in the SAGES cohort [19]. The one exception in the current study was that worse pre-operative performance on Trails B was not a statistically significant predictor of delirium. Although this could be a consequence of the small sample size, it could also indicate some cognitive specificity in terms of delirium risk. Future studies may wish to further examine this finding and its implications for delirium prediction further.
Unlike the AD signature, the Aging-Only signature was not associated with cognitive outcomes and did not interact with delirium. This observation supports the specificity of our findings to likely AD-related neurodegeneration, rather than reflecting an effect that is more diffuse due to global aging effects or pathological processes not due to AD. Thus, the previously reported observation in SAGES that lower baseline performance on certain neuropsychological tests—including HVLT-R total learning and Trails B—is associated with increased risk of delirium[28] is possibly referable to preclinical AD-related neurodegeneration of brain networks subserving learning/memory and executive function, rather than advanced brain aging, in at least a subset of individuals. However, AD-related and aging-related neurodegeneration are not mutually exclusive, and patients may experience both to varying degrees simultaneously. The unique and overlapping contributions of these two brain signatures to cognitive decline is an important area for continued investigation.
Our results align with previous work showing that delirium and neurodegenerative pathologies are independently and synergistically associated with cognitive decline[29], and highlight the need to further investigate the pathophysiologic mechanisms of these complex interactions. Neuroinflammation is the predominant theoretical pathophysiological basis of postoperative delirium and related post-operative cognitive decline[30–33]. There is also extensive evidence that neuroinflammation is involved in AD[34, 35] and that neuroinflammation and AD pathology may interact to accelerate disease worsening[35–38]. Since delirium may be seen as the end result of neuroinflammation, our findings are consistent with the notion that AD pathology and neuroinflammation interact to accelerate cognitive decline. Because the pathophysiology of delirium is still being investigated, it is also possible that the present results are due to interactions between AD pathology and other mechanisms involved in the pathogenesis of delirium, including oxidative stress, neurotransmitter deficiency, blood-brain barrier dysfunction, and network disconnectivity, among others[31, 32]. This an important area for continued research.
This study has several limitations. Although SAGES is one of the largest cohort studies of its kind including detailed pre-operative MRI and cognitive assessment with long-term follow-up, the sample for this study was relatively small since it was limited to only those with both pre-operative MRI and at least six months of cognitive follow-up. Thus, this study was likely underpowered to detect some effects, which is further supported by the lack of statistical significance despite large effect sizes. Statistical inference was based on the mixed effects models which analyzed the cortical signatures as continuous variables; in contrast, the effect size analysis utilized tertiles, which further exacerbated concerns about small sample sizes. We used the tertile approach since the use of ordered categories is a commonly selected methodology in clinical research, which helps to enhance clinical relevance and interpretability. However, due to the small sample size, the effect is estimated with low precision and we cannot rule out chance as a possible explanation for the observed effects. Practice/retest effects are a known challenge in longitudinal cognitive research; although we attempted to control for these effects using a non-surgical cohort, which is a robust, recommended approach [39] and one which we have specifically validated in this cohort [22], we acknowledge that there is no perfect method. To further probe the possible influence of retest correction on our interpretations, we performed a supplementary analysis using retest-uncorrected cognitive scores (Supplementary Figure 3; Supplementary Table 7); although absolute scores showed increases at months 1 and 2 (as expected) the cognitive slopes were identical and the relationships between the cortical signatures and/or delirium at other timepoints were minimally affected. Additionally, we attempted to control for relevant confounding without overfitting. To further probe potential confounding, we performed a sensitivity analysis testing the effect of three health-related variables (vascular comorbidity, medical comorbidity, and depression) as additional covariates; they did not notably change the results (Supplementary Table 8). While the AD signature has been shown to be associated with preclinical AD, molecular markers of amyloid or tau were not collected in SAGES, so the AD signature can only be considered as a probabilistic marker. Finally, generalizability may be limited, since SAGES is largely a white and well-educated sample of adults undergoing select surgical procedures. Indeed, future studies are needed to investigate other populations including but not limited to adults hospitalized for reasons other than surgery (or different surgeries than those studied herein) and adults who develop delirium unrelated to hospitalization or surgery. It will also be important to study these various populations across different geographical, racial, and socioeconomic contexts.
In conclusion, we found that AD-related cortical atrophy was associated with cognitive decline after surgery. Our results also suggest that development of post-operative delirium may further accelerate this decline. However, this latter finding was not statistically significant, and thus must be interpreted with caution as only preliminary evidence for an early association between AD, delirium, and post-operative cognitive decline during the preclinical/prodromal timeframe. Future studies with larger sample sizes are needed to further probe this possible, but as yet unsubstantiated, interaction.
As additional follow-up is collected in the SAGES cohort, we intend to investigate longitudinally measured atrophy as well as progression to mild cognitive impairment and/or dementia, which will provide greater insights into the clinical applicability of these findings. It will be important to replicate the results of the current study and to determine if they generalize to other populations and other causes of delirium outside of the setting of elective surgery. If they do, then it will also be important to consider optimal methods to risk-stratify patients undergoing elective surgery for expensive or invasive pre-operative assessments, such as MRI, and for intensive post-operative monitoring and brain health programs aiming to slow or prevent long-term cognitive decline.
Supplementary Material
ACKNOWLEDGMENTS
The authors gratefully acknowledge the contributions of the patients, family members, nurses, physicians, staff members, and members of the Executive Committee who participated in the Successful Aging after Elective Surgery (SAGES) Study. This work is dedicated to the memory of Joshua Bryan Inouye Helfand and Jane Ann McDowell.
Funding Statement: This work was supported by the National Institutes of Health [P01AG031720 (SKI), K07AG041835 (SKI), R24AG054259 (SKI), R01AG044518 (SKI/RNJ), R01AG051658 (ERM), K24AG035075 (ERM), R21AG057955 (TGF), and T32AG023480(AMR)]. Dr. Inouye holds the Milton and Shirley F. Levy Family Chair. The funding sources had no role in the design, conduct, or reporting of this study.
Abbreviations:
- BIDMC
Beth Israel Deaconess Medical Center
- BWH
Brigham and Women’s Hospital
- HMS
Harvard Medical School
- HSL
Hebrew SeniorLife
- MGH
Massachusetts General Hospital
- PI
principal investigator
- UCONN
University of Connecticut Health Center
Sages Study Group
[Presented in alphabetical order; individuals listed may be part of multiple groups, but are listed only once under major activity, listed in parentheses].
Overall Principal Investigator:
Sharon K. Inouye, MD, MPH (Overall PI, Administrative Core, Project 1; HSL, BIDMC, HMS).
Project and Core Leaders:
David Alsop, PhD (Project 3; BIDMC, HMS); Bradford Dickerson, MD (Project 3; BIDMC, HMS); Richard Jones, ScD (Data Core, Project 4; Brown University); Thomas Travison, PhD (Data Core, HSL, HMS); Edward R. Marcantonio, MD, SM (Overall Co-PI, Epidemiology Core, Project 2; BIDMC, HMS), Towia Libermann, PhD (Project 2, HMS, BIDMC); Alvaro Pascual-Leone, MD, PhD (Project 5, HMS, BIDMC); Mouhsin Shafi, MD, PhD (Project 5, HMS, BIDM).
Executive Committee:
Steven Arnold, MD, (MGH); Michele Cavallari, MD (BWH); Zara Cooper, MD, MSc (HMS, BWH); Tamara Fong, MD, PhD (HMS, HSL, BIDMC); Eran Metzger, MD, (HMS, HSL, BIDMC); Eva M. Schmitt, PhD (Overall Project Director, HSL); Emiliano Santarnecchi, PhD (HMS, BIDM).
Other Co-investigators:
Weiying Dai, PhD (BIDMC); Simon T. Dillon, PhD (HMS, BIDMC); Janet McElhaney, MD (UConn); Charles Guttmann, MD (BWH, HMS); Tammy Hshieh, MD (BWH); George Kuchel, MD, FRCP, (UCONN); Long Ngo, PhD (HMS, BIDMC); Daniel Press, MD (HMS, BIDMC); Annie Racine, PhD, MPA (HSL); Jane Saczynski, PhD, (UMASS); Sarinnapha Vasunilashorn, PhD (BIDMC).
Clinical Consensus Panel:
Margaret O’Connor, PhD (HMS, BIDMC); Eyal Kimchi, MD (MGH), Jason Strauss, MD (Cambridge Health Alliance); Bonnie Wong, PhD (BIDMC).
Surgical and Anesthesia Leaders:
Ayesha Abdeen, MD (HMS, BIDMC); Douglas Ayres, MD (HMS, BIDMC); Brandon Earp, MD (HMS, BWFH); Michael Belkin, MD (HMS, BWH); Mark Callery, MD (HMS, BIDMC); Lisa Kunze, MD (HMS, BIDMC); Jeffrey Lange, MD (HMS, BWH); Frank Pomposelli, MD (HMS, BIDMC); John Wright, MD (HMS, BWH); Marc Schermerhorn, MD (HMS, BIDMC); David Shaff, MD (HMS, BWFH); Kamen Vlassakov, MD (HMS, BWH).
Epidemiology Core:
Tatiana Abrantes (HSL); Asha Albuquerque (HSL); Brett Armstrong (BIDMC); Sylvia Bertrand (HSL); Angelee Butters (BIDMC); Amanda Brown (HSL); Amy Callahan (BIDMC), Madeline D’Aquila, (HSL); Sarah Dowal (HSL); Kristen Erickson (HSL); Meaghan Fox (BIDMC); Jacqueline Gallagher (BIDMC); Rebecca Anna Gersten (BWH); Ariel Hodara (BIDMC); Ben Helfand, (BIDMC); Jennifer Inloes (HSL); Jennifer Kettell (HSL); Aleksandra Kuczmarska (BIDMC); Jacqueline Nee (HSL); Emese Nemeth (HSL); Lisa Ochsner (BWH); Kerry Palihnich (BIDMC); Katelyn Parisi (HSL); Margaret Puelle (HSL); Sarah Rastegar, MA (HSL); Margaret Vella (HSL), Guoquan Xu (HSL).
Data Management and Statistical Analysis Core:
Margaret Bryan (HSL); Yun Gou, MA (HSL); Jamey Guess (BIDMC); Dee Enghorn (HSL); Alden Gross, PhD, MHS (John Hopkins School of Medicine); Yun Gou, MA (HSL); Daniel Habtemariam (HSL); Ilean Isaza, PhD (HSL); Cyrus Kosar, MA (HSL); Christopher Rockett, PhD (HSL); Douglas Tommet, MPH (Brown University).
Fiscal Management Committee:
Sabrina Carretie (HSL), Ted Gruen (HSL); Meg Ross (HSL); Katherine Tasker (Chair, HSL).
Scientific Advisory Board:
James Gee, PhD (University of Pennsylvania); Ann Kolanowski, PhD, RN, FAAN (Pennsylvania State University); Margaret Pisani, MD, MPH (Yale University); Sophia de Rooij, MD, PhD (Academic Medical Center, Amsterdam); Selwyn Rogers, MD, MPH (Temple University), Stephanie Studenski, MD (Chair, NIA); Yaakov Stern, PhD (Columbia University); Anthony Whittemore, MD (BWH, HMS).
Internal Advisory Board:
Gary Gottlieb, MD, MBA (BWH, MGH, HMS); John Orav, PhD (BWH, HMS); Reisa Sperling, MD, MMSc (BWH, HMS).
Footnotes
Present address of author(s), if different from affiliation: Annie Racine, Biogen, Cambridge, MA.
Conflicts of Interest: The authors have no conflict of interest to report.
REFERENCES
- [1].Fong TG, Davis D, Growdon ME, Albuquerque A, Inouye SK (2015) The interface between delirium and dementia in elderly adults. Lancet Neurol 14, 823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gross AL, Jones RN, Habtemariam DA, Fong TG, Tommet D, Quach L, Schmitt E, Yap L, Inouye SK (2012) Delirium and Long-term Cognitive Trajectory Among Persons With Dementia. Arch Intern Med 172, 1324–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Racine AM, Fong TG, Travison TG, Jones RN, Gou Y, Vasunilashorn SM, Marcantonio ER, Alsop DC, Inouye SK, Dickerson BC (2017) Alzheimer’s-related cortical atrophy is associated with postoperative delirium severity in persons without dementia. Neurobiol Aging 59, 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Idland AV, Wyller TB, Stoen R, Eri LM, Frihagen F, Raeder J, Chaudhry FA, Hansson O, Zetterberg H, Blennow K, Bogdanovic N, Braekhus A, Watne LO (2016) Preclinical Amyloid-beta and Axonal Degeneration Pathology in Delirium. J Alzheimers Dis. [DOI] [PubMed] [Google Scholar]
- [5].Racine AM, Fong TG, Gou Y, Travison TG, Tommet D, Erickson K, Jones RN, Dickerson BC, Metzger E, Marcantonio ER, Schmitt EM, Inouye SK (2018) Clinical outcomes in older surgical patients with mild cognitive impairment. Alzheimers Dement 14, 590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bakkour A, Morris JC, Dickerson BC (2009) The cortical signature of prodromal AD: regional thinning predicts mild AD dementia. Neurology 72, 1048–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Grodstein F, Wright CI, Blacker D, Rosas HD, Sperling RA, Atri A, Growdon JH, Hyman BT, Morris JC, Fischl B, Buckner RL (2009) The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex 19, 497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dickerson BC, Stoub TR, Shah RC, Sperling RA, Killiany RJ, Albert MS, Hyman BT, Blacker D, Detoledo-Morrell L (2011) Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology 76, 1395–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dickerson BC, Wolk DA, Alzheimer’s Disease Neuroimaging I (2012) MRI cortical thickness biomarker predicts AD-like CSF and cognitive decline in normal adults. Neurology 78, 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bakkour A, Morris JC, Wolk DA, Dickerson BC (2013) The effects of aging and Alzheimer’s disease on cerebral cortical anatomy: specificity and differential relationships with cognition. Neuroimage 76, 332–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dickerson BC, Wolk DA, Alzheimer’s Disease Neuroimaging I (2013) Biomarker-based prediction of progression in MCI: Comparison of AD signature and hippocampal volume with spinal fluid amyloid-beta and tau. Front Aging Neurosci 5, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Schmitt EM, Marcantonio ER, Alsop DC, Jones RN, Rogers SO Jr., Fong TG, Metzger E, Inouye SK, Group SS (2012) Novel risk markers and long-term outcomes of delirium: the successful aging after elective surgery (SAGES) study design and methods. J Am Med Dir Assoc 13, 818 e811–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Schmitt EM, Saczynski JS, Kosar CM, Jones RN, Alsop DC, Fong TG, Metzger E, Cooper Z, Marcantonio ER, Travison T, Inouye SK, Successful Aging after Elective Surgery Study G (2015) The Successful Aging After Elective Surgery Study: Cohort Description and Data Quality Procedures. J Am Geriatr Soc 63, 2463–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cavallari M, Hshieh TT, Guttmann CR, Ngo LH, Meier DS, Schmitt EM, Marcantonio ER, Jones RN, Kosar CM, Fong TG, Press D, Inouye SK, Alsop DC, Group SS (2015) Brain atrophy and white-matter hyperintensities are not significantly associated with incidence and severity of postoperative delirium in older persons without dementia. Neurobiol Aging 36, 2122–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Racine AM, Brickhouse M, Wolk DA, Dickerson BC, Alzheimer’s Disease Neuroimaging I (2018) The personalized Alzheimer’s disease cortical thickness index predicts likely pathology and clinical progression in mild cognitive impairment. Alzheimers Dement (Amst) 10, 301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wei LA, Fearing MA, Sternberg EJ, Inouye SK (2008) The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc 56, 823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Inouye SK, Leo-Summers L, Zhang Y, Bogardus ST Jr., Leslie DL, Agostini JV (2005) A chart-based method for identification of delirium: validation compared with interviewer ratings using the confusion assessment method. J Am Geriatr Soc 53, 312–318. [DOI] [PubMed] [Google Scholar]
- [18].Saczynski JS, Kosar CM, Xu G, Puelle MR, Schmitt E, Jones RN, Marcantonio ER, Wong B, Isaza I, Inouye SK (2014) A tale of two methods: chart and interview methods for identifying delirium. J Am Geriatr Soc 62, 518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jones RN, Marcantonio ER, Saczynski JS, Tommet D, Gross AL, Travison TG, Alsop DC, Schmitt EM, Fong TG, Cizginer S, Shafi MM, Pascual-Leone A, Inouye SK (2016) Preoperative Cognitive Performance Dominates Risk for Delirium Among Older Adults. J Geriatr Psychiatry Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gross AL, Jones RN, Fong TG, Tommet D, Inouye SK (2014) Calibration and validation of an innovative approach for estimating general cognitive performance. Neuroepidemiology 42, 144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Inouye SK, Marcantonio ER, Kosar CM, Tommet D, Schmitt EM, Travison TG, Saczynski JS, Ngo LH, Alsop DC, Jones RN (2016) The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimers Dement 12, 766–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Racine AM, Gou Y, Fong TG, Marcantonio ER, Schmitt EM, Travison TG, Inouye SK, Jones RN (2018) Correction for retest effects across repeated measures of cognitive functioning: a longitudinal cohort study of postoperative delirium. BMC Med Res Methodol 18, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dickerson BC, Sperling RA, Hyman BT, Albert MS, Blacker D (2007) Clinical prediction of Alzheimer disease dementia across the spectrum of mild cognitive impairment. Arch Gen Psychiatry 64, 1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Albert MS, Moss MB, Tanzi R, Jones K (2001) Preclinical prediction of AD using neuropsychological tests. J Int Neuropsychol Soc 7, 631–639. [DOI] [PubMed] [Google Scholar]
- [25].Baker JE, Lim YY, Jaeger J, Ames D, Lautenschlager NT, Robertson J, Pietrzak RH, Snyder PJ, Villemagne VL, Rowe CC, Masters CL, Maruff P (2018) Episodic Memory and Learning Dysfunction Over an 18-Month Period in Preclinical and Prodromal Alzheimer’s Disease. J Alzheimers Dis 65, 977–988. [DOI] [PubMed] [Google Scholar]
- [26].Racine AM, Koscik RL, Berman SE, Nicholas CR, Clark LR, Okonkwo OC, Rowley HA, Asthana S, Bendlin BB, Blennow K, Zetterberg H, Gleason CE, Carlsson CM, Johnson SC (2016) Biomarker clusters are differentially associated with longitudinal cognitive decline in late midlife. Brain 139, 2261–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wolk DA, Dickerson BC, Alzheimer’s Disease Neuroimaging I (2011) Fractionating verbal episodic memory in Alzheimer’s disease. Neuroimage 54, 1530–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fong TG, Hshieh TT, Wong B, Tommet D, Jones RN, Schmitt EM, Puelle MR, Saczynski JS, Marcantonio ER, Inouye SK (2015) Neuropsychological profiles of an elderly cohort undergoing elective surgery and the relationship between cognitive performance and delirium. J Am Geriatr Soc 63, 977–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Davis DH, Muniz-Terrera G, Keage HA, Stephan BC, Fleming J, Ince PG, Matthews FE, Cunningham C, Ely EW, MacLullich AM, Brayne C, Epidemiological Clinicopathological Studies in Europe Collaborative M (2017) Association of Delirium With Cognitive Decline in Late Life: A Neuropathologic Study of 3 Population-Based Cohort Studies. JAMA Psychiatry 74, 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Marcantonio ER (2012) Postoperative delirium: a 76-year-old woman with delirium following surgery. JAMA 308, 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Maldonado JR (2013) Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry 21, 1190–1222. [DOI] [PubMed] [Google Scholar]
- [32].Berger M, Terrando N, Smith SK, Browndyke JN, Newman MF, Mathew JP (2018) Neurocognitive Function after Cardiac Surgery: From Phenotypes to Mechanisms. Anesthesiology 129, 829–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Cortese GP, Burger C (2017) Neuroinflammatory challenges compromise neuronal function in the aging brain: Postoperative cognitive delirium and Alzheimer’s disease. Behav Brain Res 322, 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, Town T, Morgan D, Shinohara ML, Perry VH, Holmes C, Bazan NG, Brooks DJ, Hunot S, Joseph B, Deigendesch N, Garaschuk O, Boddeke E, Dinarello CA, Breitner JC, Cole GM, Golenbock DT, Kummer MP (2015) Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14, 388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chun H, Marriott I, Lee CJ, Cho H (2018) Elucidating the Interactive Roles of Glia in Alzheimer’s Disease Using Established and Newly Developed Experimental Models. Front Neurol 9, 797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Alcolea D, Vilaplana E, Pegueroles J, Montal V, Sanchez-Juan P, Gonzalez-Suarez A, Pozueta A, Rodriguez-Rodriguez E, Bartres-Faz D, Vidal-Pineiro D, Gonzalez-Ortiz S, Medrano S, Carmona-Iragui M, Sanchez-Saudinos M, Sala I, Anton-Aguirre S, Sampedro F, Morenas-Rodriguez E, Clarimon J, Blesa R, Lleo A, Fortea J (2015) Relationship between cortical thickness and cerebrospinal fluid YKL-40 in predementia stages of Alzheimer’s disease. Neurobiol Aging 36, 2018–2023. [DOI] [PubMed] [Google Scholar]
- [37].Melah KE, Lu SY, Hoscheidt SM, Alexander AL, Adluru N, Destiche DJ, Carlsson CM, Zetterberg H, Blennow K, Okonkwo OC, Gleason CE, Dowling NM, Bratzke LC, Rowley HA, Sager MA, Asthana S, Johnson SC, Bendlin BB (2015) Cerebrospinal Fluid Markers of Alzheimer’s Disease Pathology and Microglial Activation are Associated with Altered White Matter Microstructure in Asymptomatic Adults at Risk for Alzheimer’s Disease. J Alzheimers Dis 50, 873–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Perez-Nievas BG, Stein TD, Tai HC, Dols-Icardo O, Scotton TC, Barroeta-Espar I, Fernandez-Carballo L, de Munain EL, Perez J, Marquie M, Serrano-Pozo A, Frosch MP, Lowe V, Parisi JE, Petersen RC, Ikonomovic MD, Lopez OL, Klunk W, Hyman BT, Gomez-Isla T (2013) Dissecting phenotypic traits linked to human resilience to Alzheimer’s pathology. Brain : a journal of neurology 136, 2510–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Avidan MS, Evers AS (2011) Review of clinical evidence for persistent cognitive decline or incident dementia attributable to surgery or general anesthesia. J Alzheimers Dis 24, 201–216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


