Abstract
Patient: Male, 67-year-old
Final Diagnosis: Purpura fulminans • septic shock • Streptococcus pneumoniae bacteremia
Symptoms: Diarhea • nausea • shortness of breath • weakness • rash
Medication: Ceftriaxone
Clinical Procedure: Blood culture
Specialty: Infectious Diseases • Critical Care Medicine
Objective:
Rare co-existance of disease or pathology
Background:
Despite proven efficacy of vaccinations against Streptococcus pneumoniae in preventing infection, only 70% of eligible individuals receive the vaccine in the United States. Pneumococcal bacteremia represents a form of invasive pneumococcal disease and is associated with high mortality, especially in immunocompromised patients and the elderly. Purpura fulminans is a rare complication and manifestation of disseminated intravascular coagulation and sepsis. It is exceedingly rare in the setting of pneumococcal bacteremia, particularly in immunocompetent individuals.
Case Report:
We report a generally healthy 67-year-old male with schizophrenia who refused pneumococcal vaccination. He had an intact and functional spleen with a functional immune system. The patient presented with fever and diarrhea. He subsequently progressed to develop purpura fulminans and septic shock due to S. pneumoniae bacteremia. Despite an extensive search for the primary source of infection, none could not be identified. Due to timely initiation of appropriate antibiotic therapy and aggressive supportive care in an intensive care unit, he recovered despite multi-organ failure that developed throughout his hospitalization.
Conclusions:
We present a rare manifestation of a potentially preventable disease and emphasize the importance of pneumococcal vaccination in order to decrease the risk of developing invasive pneumococcal disease. Furthermore, we discuss etiology, diagnosis, differential diagnosis, and evidence-based management of purpura fulminans and invasive pneumococcal disease with a literature review. Purpura fulminans due to S. pneumoniae is exceedingly rare in immunocompetent patients and an unusual clinical manifestation of pneumococcal bacteremia.
MeSH Keywords: Disseminated Intravascular Coagulation; Pneumococcal Infections; Purpura Fulminans; Sepsis; Shock, Septic; Vaccination
Background
Streptococcus pneumoniae (S. pneumoniae) or pneumococcus is a Gram positive, highly invasive, extracellular pathogen. Typically, this pathogen is a colonizer of human upper respiratory tract mucosa. The main virulence factor of pneumococcus is its polysaccharide capsule which helps it avoid host defenses [1–3]. It spreads from person to person by droplets; colonization of nasopharyngeal cavity is a mandatory prerequisite for disease development. While up to 65% of children carry pneumococcus in their nasopharynx, carrier rates decrease to less than 10% in adults [1]. S. pneumoniae causes 2 distinct types of infections: mild upper respiratory tract infection (URI) (noninvasive) which occurs through local spread of the pathogen (sinusitis, otitis media) or invasive pneumococcal disease (IPD). IPD is defined as isolation of S. pneumoniae from normally sterile sites such as blood, cerebrospinal fluid, and other bodily fluids (peritoneal, synovial, pleural or pericardial) [1–3]. IPD occurs through local spread (meningitis), aspiration (pneumonia), or seeding to bloodstream (bacteremia) either from pneumonia or directly from upper respiratory tract (URT) mucosa [1–3]. The Center for Disease Control and Prevention (CDC) estimates that each year around 5000 cases of pneumococcal bacteremia (without pneumonia) occur in the USA. The overall case-fatality rate for bacteremia is about 20% but can be as high as 60% in the elderly and immuno-compromised [4]. Each year, approximately 2 million people worldwide die of IPD [5,6].
Purpura fulminans (PF) is an uncommon, rapidly progressive and life-threatening thrombotic disorder microscopically characterized by small vessel thrombosis and hemorrhagic skin necrosis. It is an unusual and dramatic skin manifestation of disseminated intravascular coagulation [7–9]. Macroscopically, PF usually starts with well-demarcated macules which rapidly develop central hemorrhagic necrosis and progress to retiform purpura, sometimes with hemorrhagic vesicles or bulla resulting in gangrene [10–12]. In the presence of septic shock, purpura fulminans usually affects the extremities because of poor blood perfusion to distal parts of the body [13]. PF is classified into 3 categories: neonatal PF (due to an autosomal-dominant inherited protein C deficiency), acute infectious PF, and idiopathic PF (usually post- infectious due to transient acquired protein S and/or S deficiency or secondary to medications). The most common form of PF is acute infectious PF, most commonly associated with encapsulated bacterial pathogens (such as Neisseria meningitides, S. pneumoniae, Haemophilus influenza), among others [11–13].
While PF has been described in patients with pneumococcal bacteremia it remains an uncommon entity; it is particularly unusual in immunocompetent patients such as the patient we describe in this report.
Case Report
A 67-year-old African American male with a history of schizophrenia and hypertension was evaluated for complaints of shortness of breath, generalized weakness, nausea, and diarrhea that started abruptly 1 day prior. His surgical history was unremarkable; specifically, there was no history of splenectomy. He was a lifelong nonsmoker and denied use of illicit drugs or heavy alcohol consumption. Due to underlying schizophrenia, he resided in a group home. He denied any sick contacts. The patient refused pneumococcal and influenza vaccination repeatedly due to fear of vaccination side effects. He did not have sickle cell disease, associated with functional asplenia. His vital signs on admission were significant for hypotension with blood pressure of 80/30 mm Hg and a fever of 101.1°F (38.4°C). Heart and respiratory rates were within normal range. The reminder of the physical examination was notable for an ill-appearing male patient in no acute distress. He was alert and oriented; his lungs were clear to auscultation bilaterally. His heart sounds were regular without tachycardia or murmurs. Abdominal examination did not reveal hepatosplenomegaly or peritoneal signs. Neurologic examination was normal. In particular, there were no meningeal signs and he was alert and oriented. He denied headache, cough, and chest pain. He reported watery, non-bloody, and painless diarrhea.
Abnormalities in initial laboratory studies included white blood cell count (WBC) of 42.3/nL with a predominance of neutrophils, hemoglobin 10 g/dL, platelet count of 78/nL, blood urea nitrogen (BUN) of 47 mg/dL, creatinine of 5.5 mg/dL, bicarbonate of 19.2 mEq/L, anion gap of 23, lactic acid of 4.3 mmol/L, alkaline phosphatase of 162 U/L, aspartate aminotransferase (AST) of 210 U/L, total bilirubin of 2 mg/dL, direct bilirubin of 0.4 mg/dL, creatinine kinase (CK) of 5750 U/L, fibrinogen of 170 mg/dL, lactate dehydrogenase (LDH) of 5490 U/L, and D-dimer of >69 000 ng/mL. Peripheral blood smear revealed a markedly elevated WBC with neutrophils and schistocytes consistent with microangiopathic hemolytic anemia. Computer tomography (CT) of the chest, abdomen, and pelvis without contrast was negative for pulmonary infiltrates, colitis, or intraabdominal source of infection. The patient was diagnosed with severe sepsis without an obvious source of infection. He was admitted to intensive care unit (ICU) and treated with fluid resuscitation 30 mL/kg and broad-spectrum antibiotics (including vancomycin and piperacillin-tazobactam) as the etiology of presumed infection remained uncertain. Within the next few hours, the patient developed multiple retiform violaceous patches with central dusky necrosis on the extensor aspects of his upper extremities, thighs, back, lower legs, dorsal feet, and abdomen. These patches progressed to reti-form purpura and rapidly developed ischemic changes of multiple fingers consistent with gangrene (Figures 1–6). Following fluid resuscitation, he remained hypotensive and required norepinephrine infusion. On the second day of hospitalization, blood cultures grew Gram positive cocci in chains and clusters in both bottles; thus, piperacillin-tazobactam was discontinued and ceftriaxone and clindamycin were initiated. Later the same day, the species were identified to be S. pneumoniae; these were pan- sensitive so antibiotics were narrowed to ceftriaxone 2 g intravenously (IV) daily. Further laboratory test results were negative for Legionella and Clostridioides difficile. Due to progressive oliguric renal failure, hemodialysis was initiated. Echocardiogram did not show any valvular vegetations and demonstrated preserved ejection fraction. Repeat blood cultures on the second day remained without bacterial growth. On the third hospital day, he was weaned off norepinephrine. The remainder of the workup was negative, including β-D-Glucan Assay, Strongyloides serology, and autoimmune tests (including antiphospholipid antibodies, anti-cardiolipin antibodies, C-ANCA, P-ANCA, complement C3 and C4, and cryoglobulin). Human immunodeficiency virus (HIV), hepatitis B (HBV), and hepatitis C (HCV) virus serology were negative as well. Over the following week, the patient’s renal and liver function improved. He was weaned off hemodialysis and his rash nearly resolved.
Figure 1.
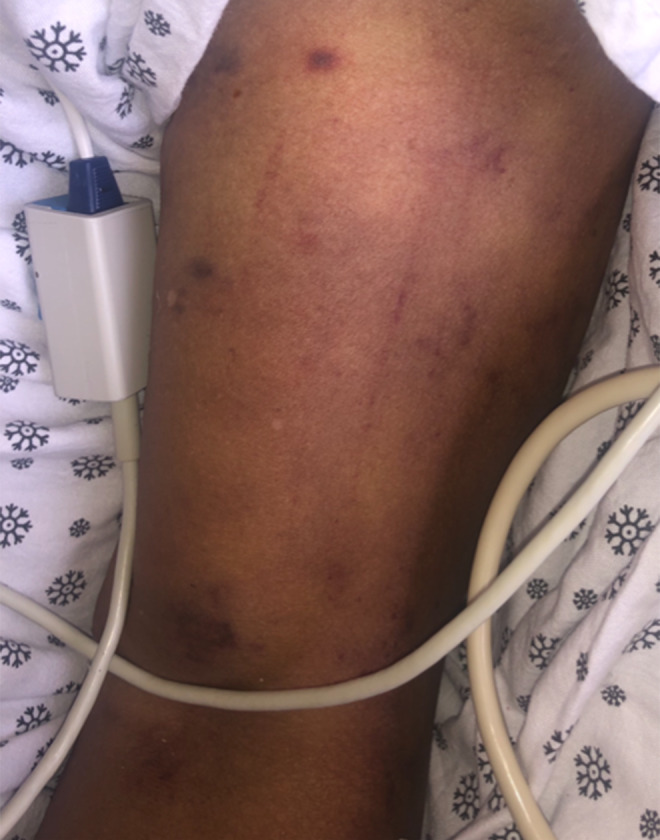
Illustrates initial skin changes in the form of multiple macules with central dusky necrosis located on the anterior aspect of the right upper arm and cubital fossa.
Figure 2.
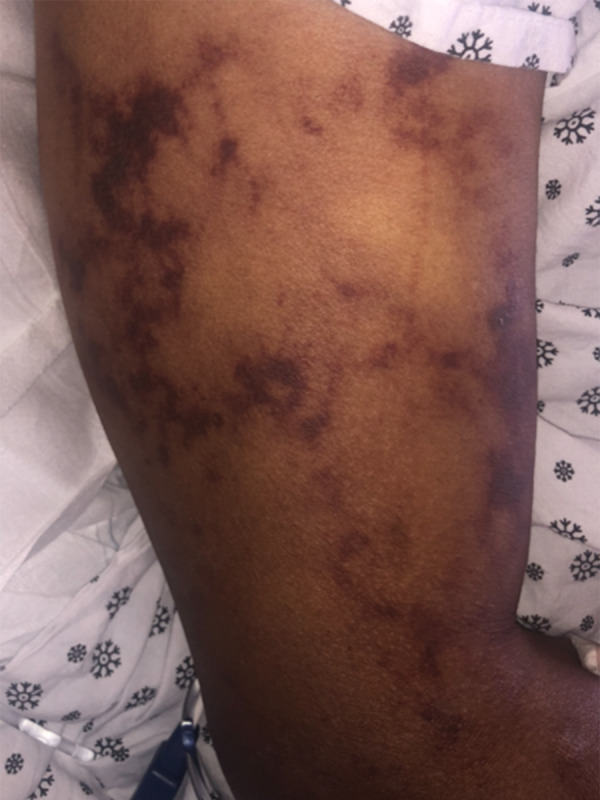
Illustrates disseminated and multiple retiform violaceous patches seen in the external aspect of the right upper arm.
Figure 3.
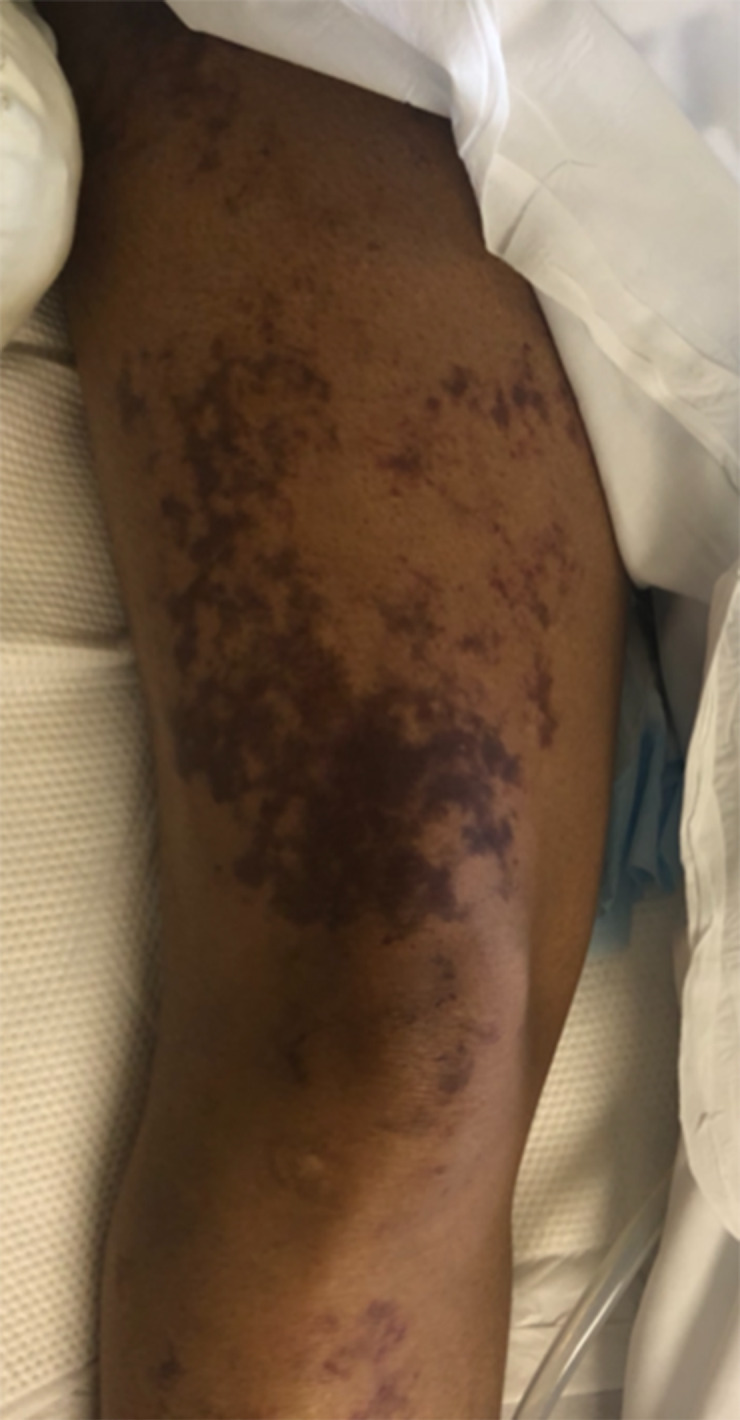
Illustrates ecchymosis and reticular plaques on the right anterior thigh with irregular borders.
Figure 4.
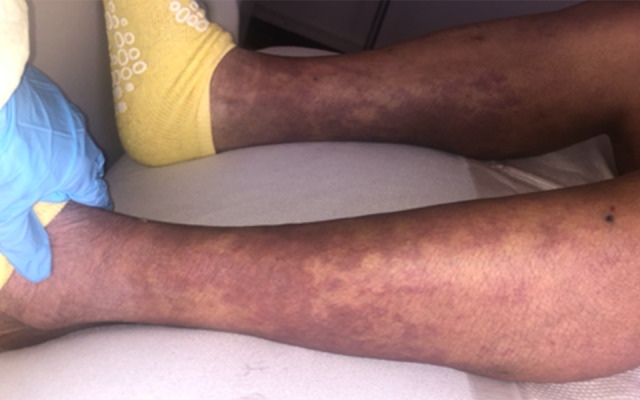
Illustrates symmetric ecchymosis and palpable purpura of both lower extremities with irregular borders.
Figure 5.
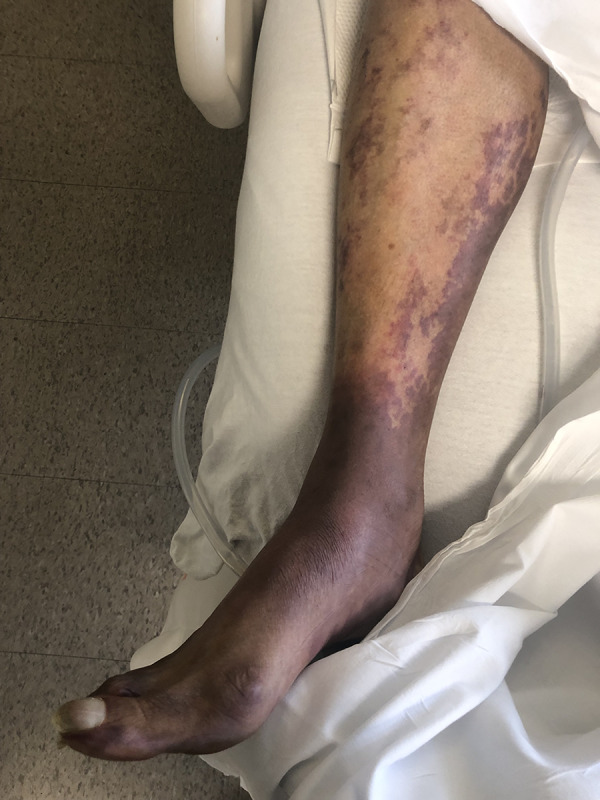
Illustrates further progression of palpable purpura.
Figure 6.
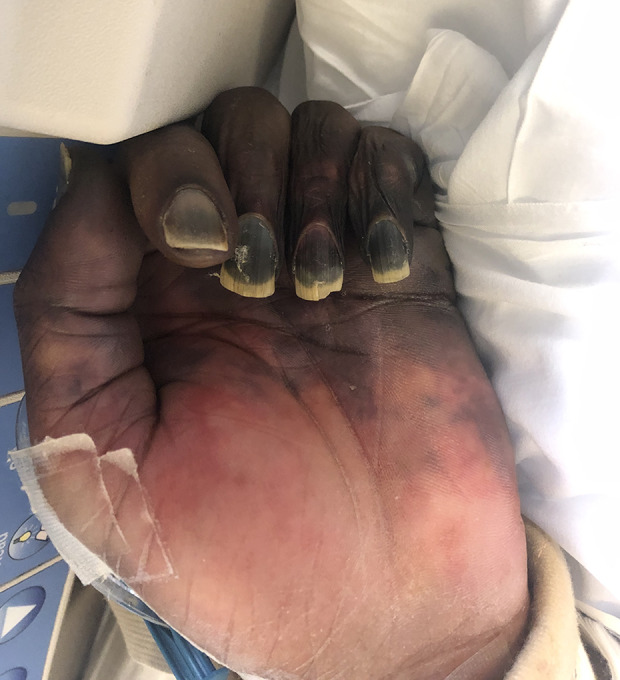
Illustrates digital ischemia from purpura fulminans.
Discussion
Our patient developed a dramatic complication of pneumococcal bacteremia including septic shock, multiorgan failure, disseminated intravascular coagulation, and PF. The source of bacteremia and primary infection remains unclear. Pneumococcal bacteremia most commonly complicates pneumococcal pneumonia or meningitis. However, in some patients the source might not be identified. An unclear source of pneumococcal bacteremia was documented in 3.9% of patients in a study from Norway [5]. Another 2 studies, 1 from Canada and 1 from Spain, documented 12.9% and 29.8%, respectively, of patients with an unclear etiology of pneumococcal bacteremia [3,14].
The risk of IPD can be mitigated and/or prevented by vacci-nation. The Advisory Committee on Immunization Practices (ACIP) currently recommends 23-valent polysaccharide vaccine (PPSV23) for all adults over the age of 65 years, or at an earlier age in immunocompromised individuals who are at increased risk for invasive pneumococcal infections (e.g., asplenic individuals, patients with chronic obstructive pulmonary disease, congestive heart failure, chronic liver disease, smoking, alcohol use disorder, poorly controlled diabetes mellitus, and chronic kidney disease). According to the newest recommendation from ACIP, 13-valent pneumococcal conjugate vaccine (PCV13) administration should be discussed on a case-by-case basis with immunocompetent patients over the age of 65 years and those with cerebrospinal fluid leak, cochlear implant, or those from communities with a low PCV-13 vaccination rate in the pediatric population [15]. Our patient was immunocompetent but was older than 65 years, hence, pneumococcal vaccination was indicated. Unfortunately, due to his underlying schizophrenia, and his fear of vaccination side effects (likely influenced by the “anti-vax” movement), he refused vaccination when initially offered. This emphasizes the importance of patient education regarding the risk of severe diseases, the importance of prevention, and education about misinformation regarding vaccination, and particularly the importance of ongoing discussion and repeated offering to those who refuse vaccination initially. Patients with mental health issues pose a particular challenge as they might not be able to fully grasp the risk associated with vaccine hesitancy.
Pneumococcal vaccination rates among adults over the age of 65 years remain inadequate with only 69% of eligible adults vaccinated in 2017 [16]. For those who are at risk of IPD and aged 19–65 years, the vaccination rate is even lower with a disappointing 24.5% of all eligible adults being vaccinated [16]. As the vaccine is immunogenic in approximately only 65% (due to capsular polysaccharides not being very antigenic) it is not surprising 2 million people worldwide die from IPD [5,6]. Mortality from IPD depends on the severity of disease with 30-day mortality from sepsis estimated at 5.4% in a prospective observational study from Norway. The same study reported mortality of 20.2% for those with severe sepsis with organ failure and in 35% of patients with septic shock [5]. Furthermore, patients older than 80 years had a 3-fold higher risk of death when compared to those 70 years old or younger [5].
While some authors have suggested that pneumococcus sero-type is the most important determinant in regard to disease severity, others have argued that a patient’s characteristics and immune status are equally significant. Most likely, IPD develops as a result of a complex interplay between the host and characteristics of the pathogen. Risk factors associated with development of IPD are male sex, immunodeficiency, comorbidities, and older age [3,5,14]. Additionally, Marrie et al. [3] showed construction workers in Canada, and welders in particular, were at an increased risk of development of IPD. They argued that higher susceptibility to pneumonia was due to lung inflammation associated with welder fumes [3,17].
Interestingly, the authors did not find health workers and teachers to be at increased risk for IPD despite both professions having an increased exposure to pneumococcal infections. The invasive form of the disease is associated with specific serotypes. For example, serotype 3 is more frequently associated with the development of septic shock than serotype 14 [18]. The highly invasive serotypes cause disease in healthy and immunocompetent individuals, unlike less invasive serotypes that are usually implicated as pathogens in the elderly, immunocompromised, and patients with co-morbidities. The latter supports the theory that in this patient population less invasive pneumococcus serotypes might be opportunistic pathogens [19,20]). While our patient was immunocompetent, he was older than 65 years. Unfortunately, at that time, our laboratory did not have the capability to determine the serotype.
Interestingly, pneumococcal bacteremia can lead to a variety of unusual complications, such as pneumococcal induced uveitis. This condition is described as an inflammatory rather than an infectious complication that favorably responds to steroids in addition to targeted antimicrobials [21]. Furthermore, a recent case report described hemophagocytic lymphohistiocytosis related to pneumococcal bacteremia [22].
PF is a dramatic and potentially life-threatening manifestation of disseminated intravascular coagulation. The rarity of this hematologic emergency is best illustrated by the fact that only 306 patients were diagnosed with PF in a multicenter retrospective cohort study from France, which included admissions from 55 ICU over the period of 17 years [23]. Furthermore, only 190 adult patients who required surgical intervention for PF were described in a systematic review and meta-analysis of 79 and 77 studies, respectively [24].
Apart from the most common causes of infectious PF, such as N. meningitides and S. pneumoniae, multiple other pathogens are associated with this dramatic manifestation of disseminated intravascular coagulation. These include Capnocytophaga canimorsus, Staphylococcus aureus, and Haemophilus influenzae, among others [25]. While bacterial pathogens are the most common causes, fungi (such as Fusarium spp, Cryptococcus neoformans, and Aspergillus spp), and viruses (such as West Nile virus and Varicella zoster virus) are documented to cause PF as well [25]. Capnocytophaga canimorsus is an emerging pathogen associated with PF, particularly in dog owners and those with excessive alcohol consumption, and those with immunosuppression, although it has been reported even in those without these underlying conditions [26]. PF can also be seen in cancer patients as a paraneoplastic phenomenon. An example of this was described in a recent case report of a patient with mesothelioma who developed PF during chemotherapy [25]. Rarely, PF may be caused by medications such as fluoroquinolones. In cases of medication-related PF, corticosteroid therapy is recommended [27]. Finally, acquired protein C and S deficiency may put patients at greater risk of developing this condition. One recent case report described an occurrence of PF in a patient following gastric bypass surgery. The authors argued that PF was due to acquired protein C and S deficiency, in addition to pneumococcal bacteremia from gastrointestinal origin at the site of anastomosis [28].
Pathophysiology of PF is complex and the mechanisms leading to its development are not fully understood [29]. It is characterized by dysregulation of pro-coagulant and anticoagulant pathways, which ultimately leads to endothelial damage and widespread thrombosis most pronounced in the small blood vessels of the skin [30,31]. Derangements of these vital homeo-static pathways are likely triggered by bacterial superantigens. These antigens are thought to initiate the cascade of events leading to the release of pro-inflammatory cytokines that ultimately disturb endothelial cells causing thrombosis [7,8,29–33].
One study showed that the pattern of cytokine derangements differs between PF secondary to N. meningitides and PF secondary to S. pneumoniae. While patients with purpura fulminans from N. meningitides had a higher concentration of interleukin (IL)-10, those with PF due to S. pneumoniae had significantly higher plasma levels of interferon gamma [34].
Such a dramatic presentation of sepsis from pneumococcal bacteremia is usually associated with immunosuppression. The majority of published cases in the literature occur in those patients with some form of immunodeficiency. Only a few cases have reported this dramatic of a presentation in otherwise healthy patients [11,35–37]. Asplenia is a significant risk factor, as illustrated in the case of an otherwise healthy 36-year-old mother of 2 who died 12 hours after symptom onset despite timely and appropriate therapy [38]. Patients with PF may also progress to develop bilateral adrenal hemorrhages, known as Waterhouse-Friderichsen syndrome. This phenomenon may lead to adrenergic crisis and contribute to the development of systemic shock. This diagnosis should be considered in any patient presenting with fever refractory shock and purpura [39].
The differential diagnosis of PF is relatively broad and should include the following: thrombotic microangiopathy (TMA), calciphylaxis, warfarin induced skin necrosis, and Henoch-Schonlein purpura (HSP). Our patient was not on warfarin therapy and had previously normal renal function which excluded warfarin induced skin necrosis and calciphylaxis, respectively. HSP usually occurs in children following upper respiratory infection. The degree of skin involvement is less, usually without necrosis. Additionally, the rash in HSP is macular and pete-chial rather than purpura. The most important and challenging diagnosis includes TMA. While patients with PF and disseminated intravascular coagulation have abnormalities in all coagulation parameters (prolonged prothrombin time, prolonged partial thromboplastin time, elevated D-dimer, thrombocytopenia, and decreased fibrinogen levels), patients with TMA usually have normal coagulation parameters except for thrombocytopenia [40–42]. Additionally, disseminated intravascular coagulation is more common than TMA and usually associated with hypotension and shock rather than hypertension, which is commonly seen in TMA [42].
Given the significant morbidity and mortality associated with PF, rapid recognition and timely initiation of treatment is essential for a favorable outcome. Ideally, care should be provided by a medical and surgical multidisciplinary team with experience in treatment of this disease [24,29,43]. The initial management should be focused on the treatment of sepsis, which includes supportive care in an ICU, administration of intravenous fluids, inotropes, antimicrobials, steroids, and mechanical ventilation, if necessary. Our patient was treated with intravenous fluids and antimicrobials directed against S. pneumoniae which included ceftriaxone. Due to timely initiation of appropriate therapy, he made a near complete recovery without the need for amputation or long-term dialysis. Additionally, the fact that he was immunocompetent (unlike the majority of reported cases) most likely contributed to his favorable outcome. The benefit of corticosteroids, anticoagulation, and protein C replacement is controversial and current research is focused on identifying the role of these options in reversing endotoxin-induced multisystem organ failure and disseminated intravascular coagulation [43]. While therapeutic protein C administration is not a recommended therapy in undifferentiated sepsis, as the PROWESS-SHOCK trial and Cochrane database Systematic Review in 2012 failed to demonstrate mortality benefit [44,45], some authors argue that protein C concentrate should be considered in PF treatment due to the benefit observed in a few studies [46].
Patients with extensive wounds due to PF should be treated similarly to burn victims. These patients benefit from earlier transfer to burn units, particularly in cases where all 4 extremities are involved or there is more than 20% of body surface area affected. However, a recent review reported that only 7% of these types of patients were managed in a burn center [24].
Surgical consultation is essential to the management of patients with PF. Treatment will vary by disease severity and may range from a conservative approach with complex wound care to amputations in order to control rapidly spreading infections. Warner et al., in their systemic review, concluded that in some cases fasciotomies, when performed early, appeared to decrease the need for amputation [43]. They postulated that early surgical decompression of involved extremities may be beneficial as aggressive fluid resuscitation and endotoxin-induced fluid shifts can lead to tissue edema, which in turn might result in compartment syndrome, further worsening blood flow to an already compromised extremity [43]. A more recent systemic review and meta-analysis by Klifto et al. [24] reviewed 190 cases in which surgical intervention was performed on patients with PF. Out of the 190 cases reviewed, 71 patients required debridement only (38%), 12 patients had fasciotomies (6%), and 154 patients underwent amputation (81%), with below-the-knee- amputations (BKA) the most common procedure. Patients who survived the initial insult of PF often undergo additional reconstructive procedures such as skin grafts and skin flaps. Management of these patients is complex and expensive, with a reported median hospital stay of 57 days (range from 11 to 292 days) [24].
Conclusions
We report a rare clinical presentation of pneumococcal bacteremia of unknown primary source complicated with septic shock and PF in an immunocompetent host. By reporting this case, our aim is to raise awareness among clinicians of this rare but potentially life-threatening condition. We also emphasize the importance of vaccination in order to minimize the risk and/or prevent occurrence of IPD. Furthermore, early administration of appropriate antimicrobials and supportive care in an ICU might decrease mortality. Progressive purpura, despite appropriate treatment, should prompt an early patient transfer to a higher level of care, ideally to a burn unit.
References:
- 1.Weiser JN, Ferreira DM, Paton JC. Streptococcus pneumoniae: Transmission, colonization and invasion. Nat Rev Microbiol. 2018;16(6):355–67. doi: 10.1038/s41579-018-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henriques-Normark B, Tuomanen EI. The pneumococcus: Epidemiology, microbiology, and pathogenesis. Cold Spring Harb Perspect Med. 2013;3(7):a010215. doi: 10.1101/cshperspect.a010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marrie TJ, Tyrrell GJ, Majumdar SR, Eurich DT. Invasive pneumococcal disease: Still lots to learn and a need for standardized data collection instruments. Can Respir J. 2017;2017:2397429. doi: 10.1155/2017/2397429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pneumococcal Disease, Clinical| Features, CDC. 2020. https://www.cdc.gov/pneumococcal/clinicians/clinical-features.html.
- 5.Askim Å, Mehl A, Paulsen J, et al. Epidemiology and outcome of sepsis in adult patients with Streptococcus pneumoniae infection in a Norwegian county 1993–2011: An observational study. BMC Infect Dis. 2016;16:223. doi: 10.1186/s12879-016-1553-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dockrell DH, Whyte MKB, Mitchell TJ. Pneumococcal pneumonia: Mechanisms of infection and resolution. Chest. 2012;142(2):482–91. doi: 10.1378/chest.12-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gando S, Levi M, Toh C-H. Disseminated intravascular coagulation. Nat Rev Dis Primer. 2016;2:16037. doi: 10.1038/nrdp.2016.37. [DOI] [PubMed] [Google Scholar]
- 8.Levi M, van der Poll T. Disseminated intravascular coagulation: A review for the internist. Intern Emerg Med. 2013;8(1):23–32. doi: 10.1007/s11739-012-0859-9. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez EF, Olarte KE, Ramesh MS. Purpura fulminans secondary to Streptococcus pneumoniae meningitis. Case Rep Infect Dis. 2012;2012:508503. doi: 10.1155/2012/508503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chalmers E, Cooper P, Forman K, et al. Purpura fulminans: Recognition, diagnosis and management. Arch Dis Child. 2011;96(11):1066–71. doi: 10.1136/adc.2010.199919. [DOI] [PubMed] [Google Scholar]
- 11.Teo HG, Wong JY, Ting TLL. Purpura fulminans: A rare presentation of Streptococcus pneumoniae infection. BMJ Case Rep. 2017;2017 doi: 10.1136/bcr-2017-221150. pii: bcr-2017-221150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okuzono S, Ishimura M, Kanno S, et al. Streptococcus pyogenes-purpura fulminans as an invasive form of group A streptococcal infection. Ann Clin Microbiol Antimicrob. 2018;17(1):31. doi: 10.1186/s12941-018-0282-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saraceni C, Schwed-Lustgarten D. Pneumococcal sepsis-induced purpura fulminans in an asplenic adult patient without disseminated intravascular coagulation. Am J Med Sci. 2013;346(6):514–16. doi: 10.1097/MAJ.0b013e31829e02d3. [DOI] [PubMed] [Google Scholar]
- 14.Cobo F, Cabezas-Fernández MT, Cabeza-Barrera MI. Streptococcus pneumoniae bacteremia: Clinical and microbiological epidemiology in a health area of Southern Spain. Infect Dis Rep. 2012;4(2):e29. doi: 10.4081/idr.2012.e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matanock A, Lee G, Gierke R, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: Updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep. 2019;68(46):1069–75. doi: 10.15585/mmwr.mm6846a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaccination coverage among adults in the United States, National Health Interview Survey, 2017. 2019. https://www.cdc.gov/vaccines/imz-managers/coverage/adultvaxview/pubs-resources/NHIS-2017.html.
- 17.Wong A, Marrie TJ, Garg S, et al. SPAT Group Welders are at increased risk for invasive pneumococcal disease. Int J Infect Dis. 2010;14(9):e796–99. doi: 10.1016/j.ijid.2010.02.2268. [DOI] [PubMed] [Google Scholar]
- 18.Ahl J, Littorin N, Forsgren A, et al. High incidence of septic shock caused by Streptococcus pneumoniae serotype 3 – a retrospective epidemiological study. BMC Infect Dis. 2013;13:492. doi: 10.1186/1471-2334-13-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jansen AGSC, Rodenburg GD, van der Ende A, et al. Invasive pneumococcal disease among adults: Associations among serotypes, disease characteristics, and outcome. Clin Infect Dis. 2009;49(2):e23–29. doi: 10.1086/600045. [DOI] [PubMed] [Google Scholar]
- 20.Alanee SRJ, McGee L, Jackson D, et al. Association of serotypes of Streptococcus pneumoniae with disease severity and outcome in adults: An international study. Clin Infect Dis. 2007;45(1):46–51. doi: 10.1086/518538. [DOI] [PubMed] [Google Scholar]
- 21.Collins A, Cortes N, Matthews BN, et al. Reversible blindness: Severe pneumococcal induced uveitis following septicaemia. BMJ Case Rep. 2011;2011 doi: 10.1136/bcr.03.2011.3960. : pii: bcr0320113960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dumancas CY, Reyes HAG, Cosico J, et al. Streptococcus pneumoniae-related hemophagocytic lymphohistiocytosis treated with IVIG and steroids. Am J Case Rep. 2018;19:25–28. doi: 10.12659/AJCR.906590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Contou D, Sonneville R, Canoui-Poitrine F, et al. Clinical spectrum and short-term outcome of adult patients with purpura fulminans: A French multi-center retrospective cohort study. Intensive Care Med. 2018;44(9):1502–11. doi: 10.1007/s00134-018-5341-3. [DOI] [PubMed] [Google Scholar]
- 24.Klifto KM, Gurno CF, Grzelak MJ, et al. Surgical outcomes in adults with purpura fulminans: A systematic review and patient-level meta-synthesis. Burns Trauma. 2019;7:30. doi: 10.1186/s41038-019-0168-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shenoy R, Nanjappa S, Eaton K, et al. Purpura fulminans: A case report and review of all causes. Infect Dis Clin Pract. 2017;25(2):100–4. [Google Scholar]
- 26.Mantovani E, Busani S, Biagioni E, et al. Purpura fulminans and septic shock due to capnocytophaga canimorsus after dog bite: A case report and review of the literature. Case Rep Crit Care. 2018;2018:7090268. doi: 10.1155/2018/7090268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okamura I, Nakamura Y, Katsurada Y, et al. Successful corticosteroid treatment for purpura fulminans associated with Quinolone. Intern Med Tokyo Jpn. 2016;55(20):3047–51. doi: 10.2169/internalmedicine.55.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cone LA, B Waterbor R, Sofonio MV. Purpura fulminans due to Streptococcus pneumoniae sepsis following gastric bypass. Obes Surg. 2004;14(5):690–94. doi: 10.1381/096089204323093507. [DOI] [PubMed] [Google Scholar]
- 29.Asif M, Quiroga L, Lagziel T, et al. A multidisciplinary approach to the management of severe purpura fulminans in a burn center: A case series. Cureus. 2019;11(8):e5478. doi: 10.7759/cureus.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colling ME, Bendapudi PK. Purpura fulminans: Mechanism and management of dysregulated hemostasis. Transfus Med Rev. 2018;32(2):69–76. doi: 10.1016/j.tmrv.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Betrosian AP, Berlet T, Agarwal B. Purpura fulminans in sepsis. Am J Med Sci. 2006;332(6):339–45. doi: 10.1097/00000441-200612000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Salgado-Pabón W, Case-Cook LC, Schlievert PM. Molecular analysis of staphylococcal superantigens. Methods Mol Biol. 2014;1085:169–85. doi: 10.1007/978-1-59745-468-1_9. [DOI] [PubMed] [Google Scholar]
- 33.de Souza AL, Seguro AC. Purpura fulminans secondary to Streptococcus pneumoniae sepsis: Unraveling the pattern of cytokines. Am J Med. 2008;121(3):e5. doi: 10.1016/j.amjmed.2007.10.016. ; author reply e7. [DOI] [PubMed] [Google Scholar]
- 34.Bjerre A, Brusletto B, Høiby EA, et al. Plasma interferon-gamma and interleukin-10 concentrations in systemic meningococcal disease compared with severe systemic Gram-positive septic shock. Crit Care Med. 2004;32(2):433–38. doi: 10.1097/01.CCM.0000104950.52577.97. [DOI] [PubMed] [Google Scholar]
- 35.Murph RC, Matulis WS, Hernandez JE. Rapidly fatal pneumococcal sepsis in a healthy adult. Clin Infect Dis. 1996;22(2):375–76. doi: 10.1093/clinids/22.2.375. [DOI] [PubMed] [Google Scholar]
- 36.Pangonis S, Patamasucon P, Fitzpatrick E. Pneumococcal sepsis complicated by splenic abscesses and purpura fulminans in a 15-month-old child: Case report and review of the literature. J Investig Med High Impact Case Rep. 2016;4(1):2324709616636398. doi: 10.1177/2324709616636398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Intan IH, Rozita AR, Norlijah O. Pneumococcal sepsis presenting as purpura fulminans in a healthy infant. Ann Trop Paediatr. 2009;29(3):235–38. doi: 10.1179/027249309X12467994694139. [DOI] [PubMed] [Google Scholar]
- 38.Hale AJ, LaSalvia M, Kirby JE, et al. Fatal purpura fulminans and Waterhouse-Friderichsen syndrome from fulminant Streptococcus pneumoniae sepsis in an asplenic young adult. IDCases. 2016;6:1–4. doi: 10.1016/j.idcr.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lyon P, Nambi R Faruqi F. Atypical presentation of purpura fulminans following sepsis in an adult. BMJ Case Rep. 2011;2011 doi: 10.1136/bcr.03.2011.3996. pii: bcr0320113996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Myers KA, Marrie TJ. Thrombotic microangiopathy associated with Streptococcus pneumoniae bacteremia: Case report and review. Clin Infect Dis. 1993;17(6):1037–40. doi: 10.1093/clinids/17.6.1037. [DOI] [PubMed] [Google Scholar]
- 41.Wada H, Matsumoto T, Suzuki K, et al. Differences and similarities between disseminated intravascular coagulation and thrombotic microangiopathy. Thromb J. 2018;16:14. doi: 10.1186/s12959-018-0168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warner PM, Kagan RJ, Yakuboff KP, et al. Current management of purpura fulminans: A multicenter study. J Burn Care Rehabil. 2003;24(3):119–26. doi: 10.1097/01.BCR.0000066789.79129.C2. [DOI] [PubMed] [Google Scholar]
- 43.Ranieri VM, Thompson BT, Barie PS, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. 2012;366(22):2055–64. doi: 10.1056/NEJMoa1202290. [DOI] [PubMed] [Google Scholar]
- 44.Martí-Carvajal AJ, Solà I, Gluud C, et al. Human recombinant protein C for severe sepsis and septic shock in adult and paediatric patients. Cochrane Database Syst Rev. 2012;12:CD004388. doi: 10.1002/14651858.CD004388.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Kleijn ED, de Groot R, Hack CE, et al. Activation of protein C following infusion of protein C concentrate in children with severe meningococcal sepsis and purpura fulminans: A randomized, double-blinded, placebo-controlled, dose-finding study. Crit Care Med. 2003;31(6):1839–47. doi: 10.1097/01.CCM.0000072121.61120.D8. [DOI] [PubMed] [Google Scholar]


