Abstract
Objective
This study aimed to investigate the effect of long non-coding TDRG1 on proliferation and migration of osteosarcoma cells through PI3K/AKT signaling pathway.
Materials and Methods
Altogether 87 cases of osteosarcoma tissues and adjacent tissues were collected, and osteosarcoma cells and osteoblasts were purchased. The expression of LncRNA TDRG1 in tissues and cells was detected by RT-PCR. Si-NC, si-TDRG1, and Sh-TDRG1 were transfected into osteosarcoma cells. L740Y-P (activator of PI3K/AKT pathway) and LY294002 (inhibitor of PI3k/AKT pathway) were used to interfere with PI3k/Akt signaling pathway in osteosarcoma cells. qRT-PCR was used to detect the expression of TDRG1 in osteosarcoma tissues and cells. WB was used to detect the expression of p-PI3K, p-AKT, N-cadherin, E-Cadherin, vimentin, Bax, Caspase-3, and Bcl-2 in cells. CCK-8, Transwell and cell scratch tests were used to detect cell proliferation, invasion and migration, and flow cytometry was used to detect cell apoptosis.
Results
TDRG1 was highly expressed in osteosarcoma, and the levels of p-PI3K and p-AKT were also up-regulated. Cell experiments showed that inhibiting the expression of TDRG1 could inhibit the proliferation, invasion, migration and EMT of osteosarcoma cells, promote the apoptosis of cells, and up-regulating the expression of TDRG1 could promote the proliferation, invasion, migration and EMT of osteosarcoma cells and inhibit the apoptosis of cells. The 740Y-P intervention could reverse the inhibition of Si-TDRG1 on osteosarcoma cell proliferation, invasion, migration and EMT and the promotion of cell apoptosis. LY294002 intervention could reverse the promotion of Sh-TDRG1 on osteosarcoma cell proliferation, invasion, migration and EMT and the inhibition of cell apoptosis.
Conclusion
TDRG1 is highly expressed in osteosarcoma tissue. Silencing the expression of osteosarcoma can inhibit the proliferation, invasion, migration and EMT of osteosarcoma cells by inhibiting PI3K/AKT signaling pathway, which may be a new target for diagnosis and treatment of osteosarcoma.
Keywords: LncRNA TDRG1, PI3K/AKT, osteosarcoma cells, proliferation, migration
Introduction
Osteosarcoma, as a primary bone tumor with high incidence in adolescents and children, is also the major cause of cancer-related mortality in children and poses a serious threat to the life and health of children and adolescents worldwide.1,2 At present, the clinical treatment of osteosarcoma is mainly surgical resection and postoperative chemotherapy. Although some progress has been made in the treatment of osteosarcoma with the improvement of medical technology, due to the high metastatic characteristics of osteosarcoma, many patients still have a poor prognosis even after treatment.3,4 At present, the molecular mechanism of osteosarcoma metastasis has not been clarified in detail, but exploring the mechanism of osteosarcoma occurrence and metastasis has important clinical significance for osteosarcoma patients.
Long-chain non-coding RNA (LncRNA) is an RNA with more than 200 nucleotides. In the past, it was thought that it did not have the function of coding protein, so its functions were ignored.5 However, in recent years, more and more studies have found that LncRNA acts on tumor growth and metastasis. For example, previous studies6 pointed out that LncRNA H19 could promote the occurrence and metastasis of endometrial cancer by regulating epithelial–mesenchymal transition (EMT). LncRNA TDRG1 is a newly discovered tumor-related LncRNA in recent years, which was initially considered as a key regulator of sperm motility.7 In recent years it has been developed to acton the proliferation, invasion and migration of malignant cells, such as ovarian cancer8 and gastric cancer.9 However, there is no research on the role of TDRG1 in osteosarcoma and its related mechanism. PI3K/AKT signaling pathway is a classical pathway that acts on tumor occurrence and development. Activated PI3K/AKT is a major effector that regulates the downstream signal transmission of tumor cell response.10 In the past, studies11 also pointed out that the occurrence and development of osteosarcoma were promoted by activating of PI3K/AKT signaling pathway. Therefore, we speculated TDRG1 could affect the occurrence and development of osteosarcoma by regulating PI3K/AKT signaling pathway.
In our research, we detected the expression, function and related mechanism of TDRG1 in osteosarcoma, thus providing more target directions for the diacrisis and cure of osteosarcoma.
Materials and Methods
Clinical Data
Altogether 87 osteosarcoma patients in The Second Xiangya Hospital of Central South University from May 2014 to April 2016 were collected. After obtaining the consent of the patients, 87 osteosarcoma tissues and 87 adjacent tissues were taken for detection during the operation. Inclusion criteria: patients diagnosed as osteosarcoma by pathological diagnosis; patients diagnosed as osteosarcoma for the first time. Exclusion criteria: patients have received radiotherapy and chemotherapy; patients with other malignant tumors, severe liver and kidney dysfunction, and blood system diseases; patients refused to participate in the study. All patients and their families signed an informed consent form. This study conformed to the Declaration of Helsinki and has been approved by the Ethics Committee of The Second Xiangya Hospital of Central South University.
Cell Culture and Transfection
Human osteosarcoma cell lines MG-63, U2OS, OS-732 and osteoblast hFOB1.19 were purchased from ATCC and cultured in DMEM medium including 10%PBS (Gibco, Rockville, MD, USA) at 37°C with 5% CO2. When cell growth reached 85%, a 25% pancreatin was put in for digestion. After that, the cells were placed in the medium to continue culture, complete passage, and the expression of TDRG1 in each cell line was detected. Altogether 1x106MG-63 and OS-732 cells were selected for transfection. Then, 100pmol targeted inhibition of TDRG1 sequence (si-TDRG1) (G150916041833-1-5, Ribobio, Guangzhou, China), 4ug targeted over-expression of TDRG1 plasmid (sh-TDRG1), and negative control RNA (Si-NC) (A06001, GenePharma, Shanghai, China) were transfected into cells by Lipofectamine™ 2000 kit (Invitrogen, CA, US). The operation steps were strictly carried out in accordance with the kit instructions.
Real-Time Quantitative PCR
Firstly, the total RNA in tissues and cells was extracted with Trizol reagent (Invitrogen, USA). The purity, concentration and integrity of the extracted total RNA were detected by UV spectrophotometer and agarose gel electrophoresis. Then, 5μg of total RNA were taken, respectively, to reverse transcribe cDNA according to the instructions of the kit, and the cDNA concentration was diluted to 100ng/ul, and 1μL of synthesized cDNA was taken for amplification. The amplification system was as follows: 1 μ l of cDNA, 0.4μL of upstream and downstream primers, 10 μ l of 2X TransScript® Tip Green qPCR SuperMix, 0.4μL of Passive Reference Dye (50X), with Nuclease-free Water added to make up to 20μL. GAPDH was applied as an internal parameter and 2− ΔCT to explore the data. The primer sequences are shown in Table 1. All primers were designed and synthesized by Sargon Biotech, Shanghai. The experiment was repeated three times.
Table 1.
Primer Sequence Table
| Forward Primer | Reverse Primer | |
|---|---|---|
| TDRG1 | 5ʹ-TCTTCCCTGGCTTGGC-3ʹ | 5ʹ-TGGGCTCTTTCGTGGC-3ʹ |
| GAPDH | 5ʹ-CTCTGCTCCTCCTGTTCGAC-3ʹ | 5ʹ-GCGCCCAATACGACCAAATC-3ʹ |
Cell Proliferation Test
The proliferation ability of MG-63 and OS-732 was evaluated by CCK-8 kit. Cells 48 hours after transfection were collected, diluted to 3×104cell/mL, and inoculated into 96-well plates. Each well was inoculated with 100μL of cells, and cultured in 37°C with 5% CO2. A 10μL CCK8 solution was added to each well at 0h, 24h, 48h and 72h after the cells adhered to the wall. After the reagent was added, it was continuously placed in an incubator at 37°C with 5% CO2 for 2 h. Then, the OD value was measured at 450nm using an enzyme reader to explore the proliferation and visualize the growth curve. The experiment was repeated 3 times.
Cell Migration and Invasion Test
The migration ability was explored by wound healing. For wound healing determination, 200μL aseptic pipette was used to scratch the cells to geta cell-free area, PBS was used to rinse the cells, and a new culture medium was added for culture. At 0h (W0) and 24h (W24) after cell scratch, the cell migration ability was evaluated by a microscope for scratches at three different positions. Cell invasion was detected by transwell assay. Firstly, 200μL of DMEM culture solution containing 1x105 cells was put into the upper chamber and 500mL of DMEM containing 20% FBS to the lower chamber. The substrate and cells that did not pass through the membrane surface in the upper chamber were wiped after 48 hours of culture at 37°C, washed by PBS for 3 times, fixed with paraformaldehyde for 10min, rinsed with double-distilled water for 3 times, stained with 0.1% crystal violet for 10min after it was dried, and cell invasion was observed with a microscope. The experiment was repeated three times.
Detection of Apoptosis by Flow Cytometry
The sample to be tested was prepared into cell suspension, the density was adjusted to 1*106cells/mL, the cells were fixed with 70% ethanol ice-cold solution for 30min at4°C. The ethanol solution was then removed and the cell particles were incubated in Annexin V-FITC/7-AAD mixed solution. FACScan flow cytometer (Becton Dickinson Company, USA) was used to analyze the apoptosis. The experiment was repeated three times.
Western Blot Test
After collecting and culturing each group of cells, RIPA lysate was added into the cells, and total protein was extracted. Then, the protein concentration was detected by BCA method and adjusted to 4μg/μL, 12% SDS-PAGE electrophoresis separation was carried out. After ionization, it was transferred to PVDF membrane, and then the PVDF membrane was sealed with 5% defatted milk powder for 2h. Then, p-PI3K (1:1000), p-AKT (1:1000), N-cadherin (1:500), E-Cadherin (1: 500), vimentin (1: 500), Bax (1:500), Caspase-3 (1:500), Bcl-2 (1:500) and β-Actin (1: 1000) primary antibody (Cell Signaling Technology, Boston, Massachusetts, USA) were added for sealing for a night at 4°C and rinsed to remove the primary antibody. Goat anti-rabbit secondary antibody (1: 1000) (Wuhan Boster Biological Technology Co., Ltd.) was added, incubated at 37°C for 1h, and rinsed with PBS 3 times, each time for 5min. The experiment was repeated three times.
Developing in a darkroom was carried out, excess liquid was absorbed by filter paper, ECL lighting and developing was carried out.
Statistical Method
In this study, SPSS20.0 was used to statistically analyze the collected data, GraphPad 7 to visualize the required pictures, independent t test for group comparison, one-way ANOVA for multi-group comparison, LSD-t test for post-event pairwise comparison, repeated measurement ANOVA for multi-time point expression. Bonferroni was used for backtesting. When P was less than 0.05, there was a statistical difference.
Result
Up-Regulation of TDRG1 in Osteosarcoma
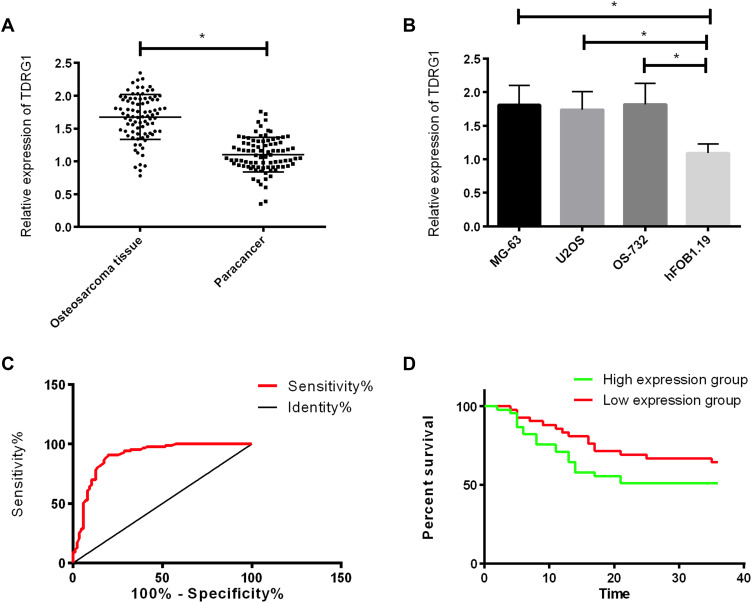
TDRG1 in osteosarcoma tissue was up-regulated compared with normal adjacent tissue. Compared with osteoblast hFOB1.19, TDRG1 expression in osteosarcoma cells was also significantly up-regulated (P<0.05). ROC analysis of the participants found that the area under the TDRG1 curve was 0.899. According to the median expression (1.71) of TDRG1, the patients were grouped into TDRG1 high expression group (45 cases) and low expression group (42 cases). This indicated that the expression of TDRG1 had a relationship with tumor size, pathological stage, differentiation degree and lymph node metastasis of osteosarcoma patients, and the 3-year survival of patients with TDRG1 high expression group was lower than that with low expression group (P<0.05). See Table 2, Figure 1.
Table 2.
Relationship Between TDRG1 and Pathological Data of Osteosarcoma Patients
| Factor | TDRG1 | χ2 | P value | ||
|---|---|---|---|---|---|
| High Expression (n=45) | Low Expression (n=42) | ||||
| Age | 0.011 | 0.918 | |||
| ≥18 years old (n=44) | 23 (51.11) | 21 (50.00) | |||
| < 18 years old (n=43) | 22 (48.89) | 21 (50.00) | |||
| Gender | 0.008 | 0.929 | |||
| Male (n=46) | 24(53.33) | 22(52.38) | |||
| Female (n=41) | 21(46.67) | 20(47.62) | |||
| Tumor size | 21.82 | <0.001 | |||
| ≥2cm (n=39) | 31 (68.89) | 8 (19.05) | |||
| <2cm (n=48) | 14 (31.11) | 34 (80.95) | |||
| TNM staging | 12.63 | <0.001 | |||
| I–II (n=45) | 15 (33.33) | 30 (71.43) | |||
| III (n=42) | 30 (66.67) | 12 (28.57) | |||
| Differentiation | 8.864 | 0.003 | |||
| Low differentiation (n=59) | 37 (82.22) | 22 (52.38) | |||
| High+medium differentiation (n=28) | 8 (17.78) | 20 (47.62) | |||
| Lymphatic metastasis | 4.590 | 0.032 | |||
| Transferred (n=35) | 23 (51.11) | 12 (28.57) | |||
| Non- transferred (n=52) | 22 (48.89) | 30 (71.43) | |||
Figure 1.
Expression and significance of TDRG1 in osteosarcoma. (A) Expression of TDRG1 in osteosarcoma tissue; (B) Expression of TDRG1 in osteosarcoma cells; (C) ROC curve of TDRG1 in the diagnosis of osteosarcoma; (D) Influence of TDRG1 on the prognosis of osteosarcoma patients. *Indicates that P<0.05.
Role of TDRG1 on Proliferation and Apoptosis of Osteosarcoma Cells
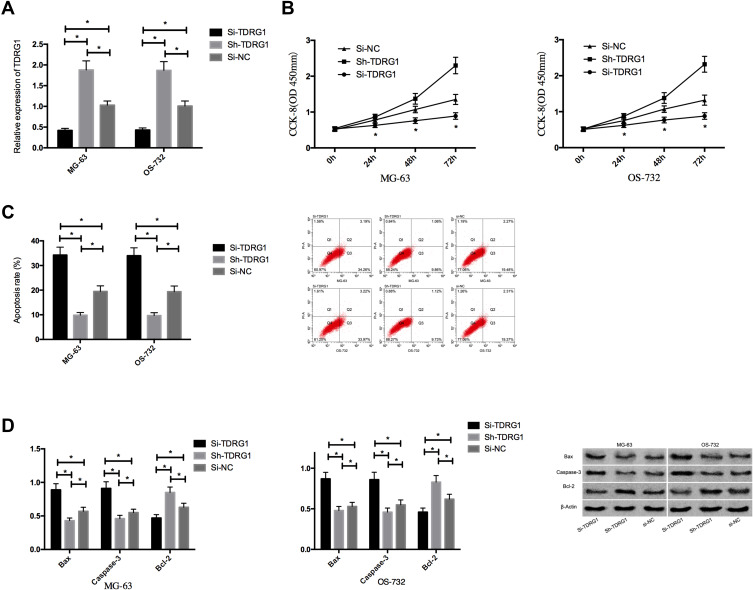
We detected the proliferation and apoptosis after interfering TDRG1 by CCK-8 and flow cytometry. The results showed that after silencing the TDRG1 in osteosarcoma cells, TDRG1 in Si-TDRG1 group was lower than that in Si-NC group. The proliferation ability of MG-63 and OS-732 was hindered, and the apoptosis rate was increased.Anti-apoptosis protein Bcl-2 was reduced, and the expressions of pro-apoptosis-related proteins bax and Caspase-3 were increased. Compared with Si-NC transfected cells, MG-63 and OS-732 were further enhanced after transfection of Sh-TDRG1. The apoptosis rate was significantly reduced, the anti-apoptosis protein Bcl-2 was significantly up-regulated, and the expressions of pro-apoptosis-related proteins bax and Caspase-3 were significantly reduced. See Figure 2.
Figure 2.
Effect of TDRG1 on proliferation and apoptosis of osteosarcoma cells. (A) Expression of TDRG1 in transfected osteosarcoma cells; (B) Effect of TDRG1 on the proliferation of osteosarcoma cells; (C) Effect of TDRG1 on apoptosis rate of osteosarcoma cells; (D) Effect of TDRG1 on apoptosis-related proteins in osteosarcoma cells. *Indicates that P<0.05.
Effect of TDRG1 on Migration, Invasion and EMT of Osteosarcoma Cells
We detected the invasion and migration of osteosarcoma cells after interfering TDRG1 through scratch-healing test and transwell assay and evaluated the EMT of cells through the detection of EMT-related proteins. The results showed that compared with Si-NC group, knocking down the expression of TDRG1 could significantly hinder the invasion and migration of osteosarcoma cells. Moreover, N-cadherin and vimentin in transfected Si-NC cells were down-regulated and the expression of E-cadherin was up-regulated after the transfection of Si-TDRG1, which indicated that EMT was also significantly inhibited. Compared with transfected Si-NC cells, the invasion and migration ability of osteosarcoma cells were significantly up-regulated after transfection of Sh-TDRG1, and N-cadherin and vimentin in cells were obviously up-regulated and the expression of E-cadherin was significantly down-regulated, which indicated that EMT was also significantly enhanced. See Figure 3.
Figure 3.
Effect of TDRG1 on migration, invasion and EMT of osteosarcoma cells. (A) Influence of TDRG1 on the invasion of osteosarcoma cells; (B) Effect of TDRG1 on migration ability of osteosarcoma cells; (C) Effect of TDRG1 on EMT-related proteins in osteosarcoma cells. *Indicates that P<0.05.
Effect of TDRG1 on PI3K/AKT Signal Pathway in Osteosarcoma
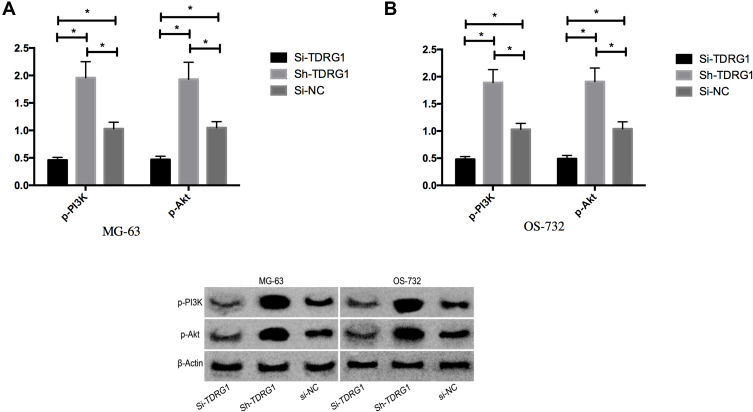
The PI3K/AKT signaling pathway in osteosarcoma cells was determined by Western Blot. The results indicated that the phosphorylation level of PI3K and AKT was significantly reduced by knocking down the expression of TDRG1, but the phosphorylation level of PI3K and AKT was significantly stimulated by over-expression of TDRG1. See Figure 4.
Figure 4.
Effect of TDRG1 on PI3K/AKT signaling pathway in osteosarcoma. (A) Influence of TDRG1 on PI3K/AKT signal pathway in MG-63; (B) Influence of TDRG1 on PI3K/AKT signal pathway in OS-732. *Indicates that P<0.05.
Effect of Stimulating or Inhibiting PI3K/AKT Signal Pathway on Osteosarcoma
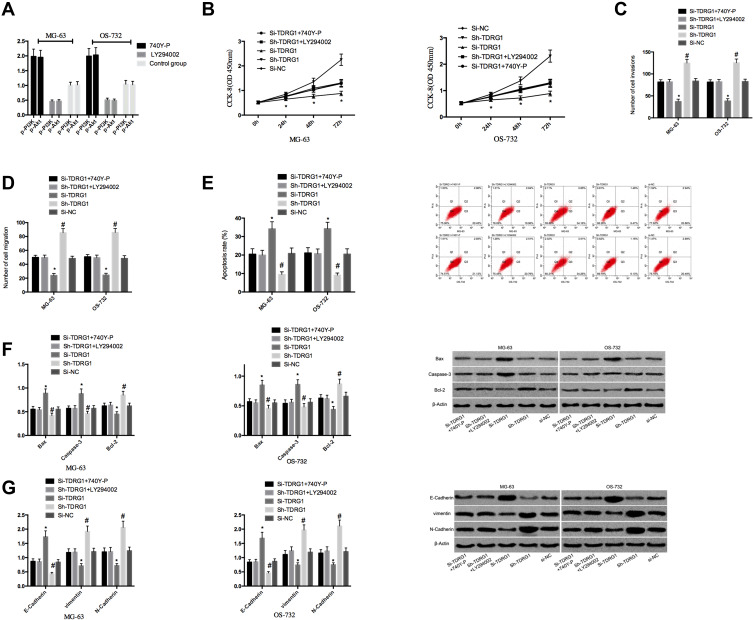
In order to further prove that TDRG1 affected the function of osteosarcoma cells by controlling PI3K/AKT signaling pathway, 5μ L 740 Y-P (activator of PI3K/Akt pathway) and 5μ L LY 294002 (inhibitor of PI3K/Akt pathway) were used to treat MG-63 and OS-732 cells that not transfected for 48h. Then, it was found that the expressions of p-Akt and p-PI3K in MG-63 and OS-732 treated with 740Y-P were obviously up-regulated (P< 0.05), and the expressions of p-Akt and p-PI3K in MG-63 and OS-732 treated with LY294002 were obviously down-regulated (P< 0.05), suggesting that 740Y-P could activate PI3K/Akt signaling pathway while LY294002 could inhibit PI3K/Akt signaling pathway. Subsequently, the cells transfected with Si-TDRG1 were exposed to 5μL 740Y-P, and the cells transfected with Sh-TDRG1 were exposed to 5μL LY294002 for 48h. The results showed that the intervention of 740Y-P could reverse the inhibition of Si-TDRG1 on osteosarcoma cell proliferation, invasion, migration, EMT and the activation of cell apoptosis, while the intervention of LY294002 could reverse the promotion of Sh-TDRG1 on osteosarcoma cell proliferation, invasion, migration and EMT and the inhibition of cell apoptosis. Figure 5.
Figure 5.
Effect of stimulation or inhibition of PI3K/AKT signaling pathway on osteosarcoma. (A) Expression of PI3K/AKT signaling pathway-related proteins after stimulation or inhibition of PI3K/AKT signaling pathway; (B) Effect of stimulation or inhibition of PI3K/AKT signaling pathway on proliferation ability of osteosarcoma cells; *Indicates P<0.05. (C) Effect of stimulation or inhibition of PI3K/AKT signaling pathway on the invasive ability of osteosarcoma cells; (D) Effect of stimulation or inhibition of PI3K/AKT signaling pathway on migration ability of osteosarcoma cells; (E) Effect of stimulation or inhibition of PI3K/AKT signaling pathway on apoptosis rate of osteosarcoma cells; (F) Effect of stimulation or inhibition of PI3K/AKT signaling pathway on apoptosis-related proteins in osteosarcoma cells; (G) Effect of stimulation or inhibition of PI3K/AKT signaling pathway on EMT-related proteins in osteosarcoma cells. *, #Indicates compared with Si-TDRG1+740Y-P, Sh-TDRG1+LY294002 and Si-NC groups, P< 0.05; *,#Indicates comparison P<0.05.
Discussion
In recent years, more and more attention has been paid to the role of LncRNA in the occurrence and promotion of malignant tumors, and most LncRNA is over-expressed in malignant tumors.12 Osteosarcoma is a common primary bone tumor and has also been reported by many studies that there are many ectopic expressions of LncRNA in osteosarcoma. For example, research13 found that LncRNA SND1-IT1 could promote the development of osteosarcoma by acting as a sponge of miR-665. Other studies14 found that silencing LncRNA ANCR could hinder the invasion and migration by activating p38MAPK signaling pathway. All these indicated that LncRNA also had an important regulatory effect in osteosarcoma.
TDRG1, as a specific gene of human testis, was reported to be helpful to promote the occurrence and development of testicular seminoma in the early stage and was later found to play the role of an oncogene in other malignant tumors.9,15 Some studies16 found that TDRG1 can enhance the tumorigenicity of epithelial ovarian cancer by inhibiting miR-93/RhoC pathway. But the mechanism of TDRG1 in osteosarcoma has not been reported yet. In our research, it was firstly indicated that TDRG1 was also highly expressed in osteosarcoma. Analysis of the clinical value found that TDRG1 not only acted on osteosarcoma, but also had a close relationship with the clinical characteristics of osteosarcoma patients, such as pathological staging, tumor size, and metastasis. This also suggested that TDRG1 may also act on the occurrence and promotion of osteosarcoma. In the previous discussion on the clinical value of TDRG1, it17 was also pointed out that TDRG1 could be used as a biomarker for the treatment of early gastric cancer, and it also showed that TDRG1 was correlated with clinicopathological features such as lymph node metastasis. Subsequently, we up-regulated and down-regulated TDRG1 in osteosarcoma cells to explore the mechanism of TDRG1. The above indicated that the down-regulation of TDRG1 obviously hindered the proliferation, invasion, migration and EMT of osteosarcoma cells, and increased the apoptosis rate. However, when we further up-regulated the expression of TDRG1, the proliferation, invasion, migration and EMT were significantly further enhanced, and the apoptosis rate was significantly reduced. This suggested that TDRG1 also played an oncogenic role in osteosarcoma. In the previous reports on TDRG1, most of them also reported that TDRG1 played the role of an oncogene in tumors.18,19 For example, there was also research20 indicated that TDRG1 could regulate the expression of ELK1 through sponging miR-330-5p, thus playing the role of an oncogene in cervical cancer. This was consistent with the results of our research. However, we are still not clear about the relevant mechanism of TDRG1 in osteosarcoma.
PI3K/AKT signaling pathway is often activated during the occurrence and development of tumors and acts on regulating the proliferation and migration of tumor cells.21,22 For example, studies23 have reported that LncRNA FER1L4 can promote apoptosis by inhibiting PI3K/AKT signaling pathway. Another study24 pointed out that LncRNA LIN00628 could inhibit the growth and invasion by regulating PI3K/AKT signaling pathway. All these fully demonstrated that PI3K/AKT signaling pathway played an important role in osteosarcoma. In our research, we also found that the PI3K/AKT signaling pathway was significantly hindered when we inhibited TDRG1. When we further up-regulated the expression of TDRG1, the PI3K/AKT signaling pathway was further activated, suggesting that TDRG1 could affect the occurrence and promotion by controlling the PI3K/AKT signaling pathway. Subsequently, in order to further confirm our conjecture, we conducted rescue experiments. We treated osteosarcoma cells after TDRG1 expression intervention with PI3K/AKT signaling pathway activator or inhibitor. The results showed that 740Y-P intervention could reverse the inhibition of Si-TDRG1 on osteosarcoma cell proliferation, invasion, migration and EMT and the activation of cell apoptosis. LY294002 intervention could reverse the promotion of Sh-TDRG1 on proliferation, invasion, migration and EMT and the inhibition of apoptosis. This suggested that TDRG1 could promote the growth and metastasis by controlling PI3K/AKT signaling pathway. In the past, studies25 indicated that knocking down the expression of TDRG1 could inhibit the occurrence and promotion of endometrial cancer by inhibiting PI3K/AKT signaling pathway. However, studies in testicular seminoma26 also pointed out that the function of TDRG1 was related to the controlling of PI3K/AKT signaling pathway. This could all confirm our conclusion.
To sum up, TDRG1 is highly expressed in osteosarcoma and can promote the occurrence and promotion of osteosarcoma by controlling PI3K/AKT signaling pathway, which may be a new target direction for diacrisis and cure of osteosarcoma. However, there are still some deficiencies in this study. For example, we have not carried out in vivo tumor-forming experiments to prove the mechanism of TDRG1 on tumor growth in vivo. Secondly, it is not clear whether there are other targets of TDRG1 in osteosarcoma. Therefore, we will further carry out basic experiments on this in future studies to provide more data support for our conclusion.
Acknowledgments
This study was financially supported by Scientific Research Plan Project of Health Commission of Hunan Province "A new Wnt/nHAP targeted nanoparticles to improve bone healing", No. 20201466.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Yoshizawa M, Nakamura S, Sugiyama Y, et al. 6-hydroxythiobinupharidine inhibits migration of LM8 osteosarcoma cells by decreasing expression of LIM domain kinase 1. Anticancer Res. 2019;39(12):6507–6513. doi: 10.21873/anticanres.13865 [DOI] [PubMed] [Google Scholar]
- 2.Marko TA, Diessner BJ, Spector LG. Prevalence of metastasis at diagnosis of osteosarcoma: an international comparison. Pediatr Blood Cancer. 2016;63(6):1006–1011. doi: 10.1002/pbc.25963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reed DR, Hayashi M, Wagner L, et al. Treatment pathway of bone sarcoma in children, adolescents, and young adults. Cancer. 2017;123(12):2206–2218. doi: 10.1002/cncr.30589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ogura K, Fujiwara T, Yasunaga H, et al. Development and external validation of nomograms predicting distant metastases and overall survival after neoadjuvant chemotherapy and surgery for patients with nonmetastatic osteosarcoma: a multi-institutional study. Cancer. 2015;121(21):3844–3852. doi: 10.1002/cncr.29575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ponjavic J, Ponting CP, Lunter G. Functionality or transcriptional noise? Evidence for selection within long noncoding RNAs. Genome Res. 2007;17(5):556–565. doi: 10.1101/gr.6036807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao L, Li Z, Chen W, et al. H19 promotes endometrial cancer progression by modulating epithelial-mesenchymal transition. Oncol Lett. 2017;13(1):363–369. doi: 10.3892/ol.2016.5389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmadi Rastegar D, Sharifi Tabar M, Alikhani M, et al. Isoform-level gene expression profiles of human Y chromosome azoospermia factor genes and their x chromosome paralogs in the testicular tissue of non-obstructive azoospermia patients. J Proteome Res. 2015;14(9):3595–3605. doi: 10.1021/acs.jproteome.5b00520 [DOI] [PubMed] [Google Scholar]
- 8.Chen S, Wang LL, Sun KX, et al. LncRNA TDRG1 enhances tumorigenicity in endometrial carcinoma by binding and targeting VEGF-A protein. Biochim Biophys Acta Mol Basis Dis. 2018;1864(9 Pt B):3013–3021. [DOI] [PubMed] [Google Scholar]
- 9.Ma Y, Xu XL, Huang HG, Li YF, Li ZG. LncRNA TDRG1 promotes the aggressiveness of gastric carcinoma through regulating miR-873-5p/HDGF axis. Biomed Pharmacother. 2020;121:109425. doi: 10.1016/j.biopha.2019.109425 [DOI] [PubMed] [Google Scholar]
- 10.Sun Y, Zhao S, Tian H, et al. Depletion of PI3K p85alpha induces cell cycle arrest and apoptosis in colorectal cancer cells. Oncol Rep. 2009;22(6):1435–1441. [PubMed] [Google Scholar]
- 11.Yu C, Zhang B, Li YL, Yu XR. SIX1 reduces the expression of PTEN via activating PI3K/AKT signal to promote cell proliferation and tumorigenesis in osteosarcoma. Biomed Pharmacother. 2018;105:10–17. doi: 10.1016/j.biopha.2018.04.028 [DOI] [PubMed] [Google Scholar]
- 12.Kim J, Piao HL, Kim BJ, et al. Long noncoding RNA MALAT1 suppresses breast cancer metastasis. Nat Genet. 2018;50(12):1705–1715. doi: 10.1038/s41588-018-0252-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin XM, Xu B, Zhang Y, et al. LncRNA SND1-IT1 accelerates the proliferation and migration of osteosarcoma via sponging miRNA-665 to upregulate POU2F1. Eur Rev Med Pharmacol Sci. 2019;23(22):9772–9780. doi: 10.26355/eurrev_201911_19540 [DOI] [PubMed] [Google Scholar]
- 14.Liu B, Zhao H, Zhang L, Shi X. Silencing of long-non-coding RNA ANCR suppresses the migration and invasion of osteosarcoma cells by activating the p38MAPK signalling pathway. BMC Cancer. 2019;19(1):1112. doi: 10.1186/s12885-019-6335-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gan Y, Wang Y, Tan Z, et al. TDRG1 regulates chemosensitivity of seminoma TCam-2 cells to cisplatin via PI3K/Akt/mTOR signaling pathway and mitochondria-mediated apoptotic pathway. Cancer Biol Ther. 2016;17(7):741–750. doi: 10.1080/15384047.2016.1178425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen S, Wang LL, Sun KX, et al. The role of the long non-coding RNA TDRG1 in epithelial ovarian carcinoma tumorigenesis and progression through miR-93/RhoC pathway. Mol Carcinog. 2018;57(2):225–234. doi: 10.1002/mc.22749 [DOI] [PubMed] [Google Scholar]
- 17.Shao Y, Ye M, Jiang X, et al. Gastric juice long noncoding RNA used as a tumor marker for screening gastric cancer. Cancer. 2014;120(21):3320–3328. doi: 10.1002/cncr.28882 [DOI] [PubMed] [Google Scholar]
- 18.Hu X, Mu Y, Wang J, Zhao Y. LncRNA TDRG1 promotes the metastasis of NSCLC cell through regulating miR-873-5p/ZEB1 axis. J Cell Biochem. 2019. doi: 10.1002/jcb.29559 [DOI] [PubMed] [Google Scholar]
- 19.Guo M, Lin B, Li G, Lin J, Jiang X. LncRNA TDRG1 promotes the proliferation, migration, and invasion of cervical cancer cells by sponging miR-214-5p to target SOX4. J Recept Signal Transduct Res. 2020;1–13. [DOI] [PubMed] [Google Scholar]
- 20.Zhao H, Hu GM, Wang WL, Wang ZH, Fang Y, Liu YL. LncRNA TDRG1 functions as an oncogene in cervical cancer through sponging miR-330-5p to modulate ELK1 expression. Eur Rev Med Pharmacol Sci. 2019;23(17):7295–7306. doi: 10.26355/eurrev_201909_18834 [DOI] [PubMed] [Google Scholar]
- 21.Hagihara T, Kondo J, Endo H, Ohue M, Sakai Y, Inoue M. Hydrodynamic stress stimulates growth of cell clusters via the ANXA1/PI3K/AKT axis in colorectal cancer. Sci Rep. 2019;9(1):20027. doi: 10.1038/s41598-019-56739-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Si X, Xu F, Xu F, Wei M, Ge Y, Chenge S. CADM1 inhibits ovarian cancer cell proliferation and migration by potentially regulating the PI3K/Akt/mTOR pathway. Biomed Pharmacother. 2020;123:109717. doi: 10.1016/j.biopha.2019.109717 [DOI] [PubMed] [Google Scholar]
- 23.Ye F, Tian L, Zhou Q, Feng D. LncRNA FER1L4 induces apoptosis and suppresses EMT and the activation of PI3K/AKT pathway in osteosarcoma cells via inhibiting miR-18a-5p to promote SOCS5. Gene. 2019;721:144093. doi: 10.1016/j.gene.2019.144093 [DOI] [PubMed] [Google Scholar]
- 24.He R, Wu JX, Zhang Y, Che H, Yang L. LncRNA LINC00628 overexpression inhibits the growth and invasion through regulating PI3K/Akt signaling pathway in osteosarcoma. Eur Rev Med Pharmacol Sci. 2018;22(18):5857–5866. doi: 10.26355/eurrev_201809_15915 [DOI] [PubMed] [Google Scholar]
- 25.Sun R, Sun X, Liu H, Li P. Knockdown of lncRNA TDRG1 inhibits tumorigenesis in endometrial carcinoma through the PI3K/AKT/mTOR pathway. Onco Targets Ther. 2019;12:10863–10872. doi: 10.2147/OTT.S228168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Gan Y, Tan Z, et al. TDRG1 functions in testicular seminoma are dependent on the PI3K/Akt/mTOR signaling pathway. Onco Targets Ther. 2016;9:409–420. doi: 10.2147/OTT.S97294 [DOI] [PMC free article] [PubMed] [Google Scholar]