Abstract
Background:
Anxiety disorders are debilitating conditions that can be treated with cognitive behavioral therapy (CBT). Increased understanding of the neurobiological correlates of CBT may inform treatment improvements and personalization. Prior neuroimaging studies point to treatment-related changes in anterior cingulate, insula, and other prefrontal regions during emotional processing, yet to date the impact of CBT on neural substrates of “top down” emotion regulation remains understudied. We examined the relationship between symptom changes assessed over the course of CBT treatment sessions and pre- to post-treatment neural change during an emotion regulation task.
Method:
In the current study, a sample of 30 participants with panic disorder or generalized anxiety disorder completed a reappraisal-based emotion regulation task while undergoing fMRI before and after completing CBT.
Results:
Reduced activation in the parahippocampal gyrus was observed from pre- to post-treatment during periods of reducing versus maintaining emotion. Parahippocampal activation was associated with change in symptoms over the course of treatment and post-treatment responder status. Results suggest that, from pre- to post-CBT, participants demonstrated downregulation of neural responses during effortful cognitive emotion regulation.
Limitations:
Effects were not observed in frontoparietal systems as would be hypothesized based on prior literature, suggesting that treatment-related change could occur outside of fronto-parietal and limbic regions that are central to most models of neural functioning in anxiety disorders.
Conclusions:
Continued work is needed to better understand how CBT affects cognitive control and memory processes that are hypothesized to support reappraisal as a strategy for emotion regulation.
Keywords: anxiety, cognitive behavioral therapy, fMRI, reappraisal, emotion regulation
Anxiety disorders are among the most common mental health conditions (Kessler et al., 2005) with detrimental effects on quality of life, disability levels, and socioeconomic outcomes (Craske et al., 2017). Cognitive behavioral therapy (CBT) is considered a frontline psychosocial treatment for anxiety disorders with strong empirical support (Carpenter et al., 2018). Despite high average response rates to CBT for anxiety, it is not universally effective, suggesting a need to better understand for whom and how these interventions work (Loerinc et al., 2015). Neuroimaging studies can advance understanding of neurobiological change that occurs over the course of CBT, as well as how observed neural change relates to symptom reduction (Lueken et al., 2016). These insights could in turn inform treatment modifications to maximize gains and treatment personalization by identifying who may benefit most from a particular intervention based on its neural effects.
Abnormalities in neural systems governing the generation and regulation of negative affective states are posited to be key factors underlying anxiety disorders, and potential targets for existing and novel therapeutic intervention (Etkin, 2012; Mathew et al., 2008). Limbic regions associated with fear-based affective reactivity and interoception show relative hyperactivity in individuals with anxiety disorders, while prefrontal regions involved in limbic downregulation and cognitive and emotional control processes demonstrate relative hypoactivation (Ball et al., 2013; Brooks and Stein, 2015; Martin et al., 2010). Recovery from anxiety disorders, including symptomatic improvements through treatment, may occur through changes in functioning in one or both of these sets of brain regions.
Recent meta-analyses evaluating the effect of psychotherapy noted that regions including the anterior cingulate cortex (ACC), insula, and inferior frontal cortex showed decreased activation from pre- to post-treatment in individuals with anxiety or depression (Barsaglini et al., 2014; Marwood et al., 2018). Findings specifically for CBT in studies of patients with anxiety disorders yield similar results, including evidence of associations between treatment response and increased connectivity between frontal and limbic regions (Brooks and Stein, 2015; Porto et al., 2009). These studies have largely probed neural systems involved in emotional responding using paradigms such as fear conditioning and passive affective face viewing. However, CBT for anxiety involves building skills for top-down modulation of this emotional response, and the neural substrates of these skills may be incompletely captured by probes of fear reactivity or responding alone (Diekhof et al., 2011).
The ability to identify and challenge distorted thinking patterns through cognitive reappraisal is one such top-down process considered to be an active component of many CBT interventions (Smits et al., 2012). Reappraisal refers to an emotion regulation strategy wherein an individual generates an alternative appraisal of a situation in order to change its emotional effect (Gross, 2002). Reappraisal processes are associated with increased activation in ventrolateral and dorsolateral prefrontal cortex (vlPFC and dlPFC, respectively), dorsal ACC, supplemental motor area (SMA), and parietal cortex, as well as decreased amygdala and parahippocampal activation during downregulation of emotion (Buhle et al., 2014; Frank et al., 2014; Kohn et al., 2014). Individuals with anxiety disorders demonstrate abnormalities in frontoparietal system functioning (vlPFC, dlPFC, ACC, SMA, parietal cortex) during reappraisal tasks as compared to healthy controls (Ball et al., 2013; Goldin et al., 2009; Zilverstand et al., 2017), leading to the hypothesis that the cognitive and neural correlates of reappraisal may be a transdiagnostic and modifiable treatment target. Reappraisal paradigms thus offer a probe for examining treatment-related changes in neural circuitry during a cognitive process that parallels CBT treatment strategies.
In the current study, participants with panic disorder (PD) or generalized anxiety disorder (GAD) completed a 10-session course of weekly CBT as part of a larger trial examining links between neural functioning and symptom outcomes, and underwent fMRI prior to initiating and after completing treatment. During the scan, participants completed an emotion regulation task which guides participants to either maintain their natural emotions or use reappraisal to downregulate their emotions while viewing negative images. In earlier work with this dataset, our group demonstrated that individuals with either GAD or PD differed from healthy comparison participants (but not from each other) at baseline in a region of the postcentral gyrus extending into the inferior parietal lobule on an emotion regulation task, and magnitude of activation in dorsolateral and dorsomedial regions correlated with anxiety severity (Ball et al., 2013). We sought to extend this earlier work by examining the relationship between symptom changes assessed over the course of treatment sessions and pre- to post-treatment neural change during this emotion regulation task in the subset of participants who also participated in an fMRI session after completing treatment. We anticipated that the magnitude of change in blood oxygen-level dependent (BOLD) signal during the task contrast of interest (reappraisal versus maintain) would increase in prefrontal regions (dlPFC, ACC, SMA) and decrease in limbic regions (amygdala, insula). We further hypothesized that the magnitude of observed neural change would be associated with magnitude of symptom reduction over the course of treatment, such that those with greater neural change would experience greater symptom reduction.
Method
Participants.
Participants were 30 adults drawn from a larger study of CBT who completed a functional neuroimaging scan before and after the treatment protocol. Participants were aged 18 to 55 and were recruited from the broader San Diego community via advertisements and clinical referrals (see Taylor et al., 2017 for description of the parent trial; Clinicaltrials.gov Identifier: NCT00947570). Individuals were required to meet DSM-IV criteria (APA, 2000) for a primary diagnosis of either PD (n = 13) or GAD (n=17); comorbidity with other non-exclusionary disorders (e.g., other anxiety disorders, depression) was permitted. Exclusion criteria included substance dependence (past year), substance abuse (past month), psychotic disorders, bipolar disorder, neurologic and organic disorders, psychopharmacological treatment within the last 6 weeks (2 weeks for benzodiazepines), and inability to safely complete MRI scan (e.g., ferrous metals, pregnancy, unstable medical conditions and claustrophobia; see Ball et al., 2013 for additional description of imaging data and findings reporting baseline performance as a predictor of treatment response). All participants provided written informed consent and this study was approved by the University of California San Diego Human Research Protections Program.
Procedure.
Eligible participants completed a medical examination including drug and pregnancy screenings. Self-report questionnaires were used to collect demographic characteristics and symptom severity. Participants completed an fMRI scan session, followed by 10 1-hour individual CBT sessions completed over up to 12 weeks, with scans typically booked within two weeks of initiation and termination. The CBT treatment sessions followed the protocol adapted from the Coordinated Anxiety Learning and Management program (Craske et al., 2009) and involved a series of modules led by a therapist with computer assistance. Five modules were generic, and able to address concerns that span multiple anxiety disorders (psychoeducation, self-monitoring, breathing retraining, fear hierarchy, and relapse prevention). The three remaining sessions (cognitive restructuring, imaginal and in vivo exposure) were targeted to the primary anxiety disorder. This intervention program was previously found to be efficacious relative to treatment as usual (Roy-Byrne et al., 2010), with similar effect sizes across anxiety disorders that approximate those observed in other active treatments for anxiety disorders (Craske et al., 2011). Earlier work from this sample demonstrated that participants experienced a significant reduction in symptoms over the course of ten sessions (Taylor et al., 2017).
Primary symptom measures.
The Overall Anxiety Severity and Impairment Scale (OASIS Norman et al., 2006) was used as the primary measure of treatment response. The measure was administered at baseline, every other session through treatment, and at the post-treatment follow-up. The OASIS includes 5 items that assess anxiety severity and impairment. The measure possesses good psychometric properties (Norman et al., 2006). The Quick Inventory of Depression Symptomology (QIDS Rush et al., 2003) was given at baseline to characterize depression severity. Effects of treatment on symptom outcomes have been previously reported elsewhere (Taylor et al., 2017).
Reappraisal Task.
Each trial of the emotion regulation task began with a scrambled image presentation for 1–3 seconds (jittered), followed by a baseline emotional rating screen where the participant was asked to indicate their current distress level (1: not at all negative to 4: very negative). After this baseline rating, participants were shown an instruction screen for 3 seconds that indicated the type of trial they were to complete. In maintain trials, participants received instructions to “Keep Up Emotion” during the picture presentation, while in the reappraise trials participants received instructions to “Reduce Emotion” during the picture presentation. Participants then viewed a negative image while employing maintain or reappraise techniques. Images were drawn from the International Affective Picture System (Lang et al., 2008). After 4–6 seconds of image presentation, participants were asked to indicate their emotion level using the same scale (3 seconds). The negative image then resumed until the end of the trial, and each trial was a total of 24 seconds long. Consistent with earlier work, data from the 4–6 second period of either maintain or reappraise was the time of interest for the current analyses (for further task description see Ball et al., 2013; Campbell-Sills et al., 2011). Before the scan participants completed a practice version of the task that contained an administrator-led training on maintain and reappraisal processes to be used during the task, including suggested reappraisal techniques and examples and participant practice, to ensure task comprehension.
Image Acquisition.
One 10-minute BOLD fMRI run was acquired using a Signa EXCITE 3.0 Tesla-GE scanner (T2*-weighted echo planar imaging, TR=2000ms, TE=30ms, FOV=240×240mm3, 64×64 matrix, forty 2.6mm axial slices with a 1.4mm gap, 290 scans, flip angle =90°) collected with ASSET. For anatomical reference, a high resolution T1-weighted image (SPGR, TI=450ms, TR=8ms, TE=3ms, FOV=256×256mm, flip angle=12°, 168 sagittally-acquired 1mm slices, 1mm3 voxels) was obtained during the same session.
Image processing.
All structural and functional image processing was done with the Analysis of Functional NeuroImages (AFNI) software package (Cox, 1996). Image preprocessing steps consisted of despiking of time series outliers, slice-time correction, correction for three-dimensional rotational and translational motion, and spatial smoothing with a Gaussian kernel of 4-mm full-width at half-maximum. Anatomical and echo planar volumes were co-registered using an algorithm that minimizes the amount of image translation and rotation (Saad et al., 2009).
Individual participant time series data were analyzed with AFNI’s 3dDeconvolve program using orthogonal regressors of interest including the Maintain and Reappraise conditions. Regressors of non-interest included the emotion rating periods and post-rating viewing period at the end of each trial. Eight nuisance regressors were also used to account for residual motion (roll, pitch, and yaw; x, y, and z) and to eliminate slow signal drifts (baseline and linear trend). Regressors were convolved with a modified gamma variate function to account for the delay and the dispersion of the hemodynamic response of the BOLD-fMRI signal (AFNI: waver). Post-convolution data were converted to percent signal change by dividing the coefficient by the zero-order regressor within each voxel. Data were aligned to individual anatomical and Talairach templates (Talairach and Tournoux, 1988).
Statistical Analysis
Neuroimaging data.
Group analysis was conducted using AFNI’s R-based 3dLME program with subjects as a random factor. Based on initial pre-treatment data suggesting that the two patient groups demonstrated similar neural response to the task and the relatively small sample size, diagnosis group was not considered as a separate factor (Ball et al., 2013). The contrast of interest was the main effect of time (pre vs. post) on the contrast of reappraise-maintain time. A voxel-wise a priori probability of .005 was determined via simulations using the updated AFNI function 3dClustSim (Cox, 2016), which resulted in a corrected cluster-wise activation probability of .05 using a minimum volume of seventeen connected voxels.
Behavioral data.
Analyses of self-report and behavioral data were performed with SPSS (Version 18.0.0, Chicago IL). Repeated measures analysis of variance (ANOVA) was used to examine main and interaction effects of condition (Reappraise vs. Maintain) and time (pre- vs. post-treatment) on emotional ratings.
Clinical data.
Clinical measures were analyzed using linear mixed effects models to examine change in the total OASIS score, which was the primary outcome measure for the parent trial. This approach modeled the slope of change and intercept for each individual on this measure accounting for the repeated measures of outcomes (Level 1: OASIS measured over 7 time points) nested within individual participants (Level 2). All models used restricted maximum likelihood estimation procedures. Time was treated as a continuous variable representing weeks from baseline, centered such that the intercept represented the post-treatment outcome score.
The first model tested the linear slope of change to examine symptom reduction over the course of treatment. The second model was then used to test the relationship between symptom change and change in neural activation in the task-derived ROI. This model included time, change in neural activation, and an interaction of time and change in neural activation. The interaction term was used to examine if change in neural activation related to improvement trajectory over treatment, and the intercept term was used to examine if change in neural activation related to end-point OASIS severity (i.e., data centered at post-treatment). Change in neural activation was operationalized as percent signal change extracted from the ROI identified in the 3dLME analysis described above, i.e., the difference in percent signal change for the reappraise-maintain contrast from pre- to post-treatment, mean-centered. As a supplemental analysis, we also examined the relationship between change in neural activation and responder status, defined as an OASIS score < 5 at the last treatment session (Roy-Byrne et al., 2010). Logistic regression models were conducted using responder status as the dependent variable and percent signal change for the reappraise-maintain contrast from pre- to post- treatment as the predictor variable. Models were considered significant at p < .05.
Results
Demographic and Behavioral Data.
Table 1 presents demographic and clinical characteristics of the sample. There were no differences between responders and non-responders on age, F(1, 15) = .63, p = .44, education, F(1, 15) = 2.04, p = .17, or anxiety symptom severity, F(1, 15) = 1.34, p = .27, but responders were more likely to be male (χ2= 6.20, p= .01). Behavioral data were analyzed to evaluate change in emotion ratings by reappraisal versus maintain conditions over time. Results of a 2 (Time: pre, post) by 2 (Trial Type: Reappraise, Maintain) ANOVA indicated no significant interaction of time by trial type, but significant main effects of time, F(1, 26) = 9.29, p = .005, ƞ2 = .26, such that ratings were lower post-treatment relative to pre-treatment, and trial type, F(1,26) = 32.93, p < .001, ƞ2 = .56, such that ratings were lower during reappraisal than during maintain trials (see Table 1). Responder status was not significantly associated with change in reappraisal rating (r = .29, p = .14) or maintain rating (r = −.06, p = .78).
Table 1.
Demographic and clinical characteristics
| Variable | Total (N = 30) | Non-responders (n = 10) | Responders (n = 20) | |
|---|---|---|---|---|
| Mean Age (SD) | 36.6 (10.4) | 33.7(9.7) | 32.1(10.9) | |
| Gender (n, (% female)) | 22 (73.3) | 5 (50.0) | 17 (85.0) | |
| Mean Years of Education (SD) | 15.4 (1.9) | 16.0(2.2) | 15.1(1.7) | |
| Race (n) | ||||
| Native American/Alaskan Native | 3 | 1 | 2 | |
| Asian | 2 | 1 | 1 | |
| Hawaiian/Pacific Islander | 0 | 0 | 0 | |
| Black/African American | 0 | 0 | 0 | |
| Caucasian | 23 | 7 | 16 | |
| Unknown | 2 | 1 | 1 | |
| Mean OASIS (SD) | ||||
| Baseline | 8.50 (3.40) | 10.50 (3.54) | 7.50 (2.93) | |
| Session 2 | 8.73 (3.57) | 11.60 (2.41) | 7.30 (3.20) | |
| Session 4 | 7.70 (3.56) | 10.50 (2.72) | 6.30 (3.11) | |
| Session 6 | 6.40 (3.08) | 9.20 (2.15) | 5.00 (2.47) | |
| Session 8 | 5.64 (4.21) | 10.75 (2.49) | 3.60 (2.74) | |
| Session 10 | 5.12 (3.62) | 9.40 (2.55) | 2.98 (1.56) | |
| Post-treatment follow-up | 3.67 (3.38) | 8.40 (2.61) | 1.85 (0.99) | |
| Mean QIDS(SD) | 7.63 (4.63) | 10.90 (3.14) | 6.00 (4.42) | |
| Mean emotional rating during reappraisal trials (SD) | ||||
| Pre-treatment | 2.15 (0.46) | 2.21 (0.48) | 2.13 (0.45) | |
| Post-treatment | 1.82 (0.56) | 1.94 (0.57) | 1.73 (0.55) | |
| Mean emotional rating during maintain trials (SD) | ||||
| Pre-treatment | 2.53 (0.54) | 2.62 (0.50) | 2.51 (0.56) | |
| Post-treatment | 2.25 (0.63) | 2.15 (0.48) | 2.26 (0.72) | |
Note: OASIS = Overall Anxiety Severity and Impairment Scale; QIDS = Quick Inventory of Depression Symptomatology. Due to technical error, 5.6% of behavioral responses were not collected. There was no statistically significant difference between the groups on baseline clinical measures of anxiety, depression, or ratings of emotion during reappraisal or maintain trials (ps > .05).
fMRI Time Effects.
Voxel-wise whole brain analysis of the effect of time on the Reappraise-Maintain contrast revealed a cluster extending through the parahippocampal gyrus and fusiform gyrus. Results revealed a reduction in activation in the reappraise-maintain contrast from pre- to post-treatment (Table 2; Figure 1a).
Table 2.
Significant effect of time on Reappraise-Maintain contrast
| Voxel | x | y | z | Region | BA | t-test |
|---|---|---|---|---|---|---|
| 24 | 26 | −52 | −9 | Right parahippocampal/Fusiform Gyrus | 19 | −3.20 |
Note: Coordinates in Talairach space.
Figure 1.
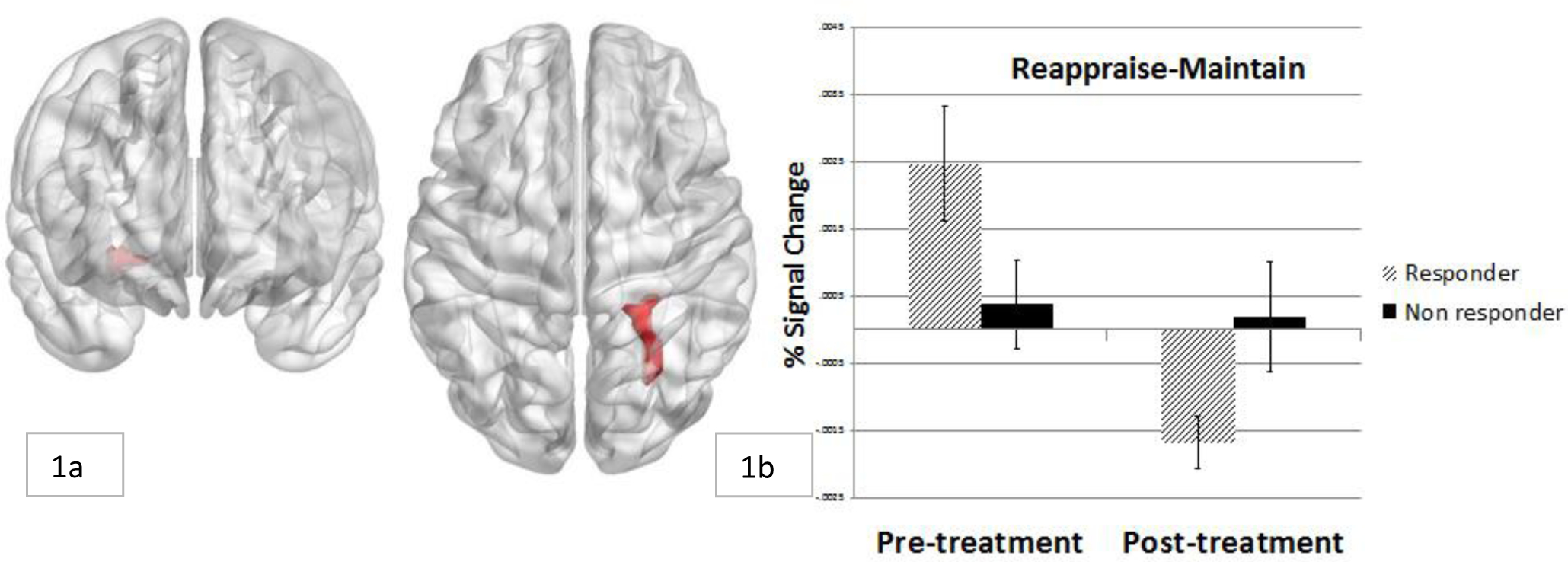
Change in neural activation in right parahippocampal region from pre- to post-treatment in responders and non-responders
Relationship between change in neural activation and clinical symptom change.
OASIS scores reduced significantly over time, B = −0.82, SE = 0.09, p < .001. Results of the longitudinal models predicting outcome improvements from change in parahippocampal activation are presented in Table 3. Results of the model revealed that change in neural activation from pre- to post-treatment predicted the slope of symptom change, B = .53, SE = 0.25, p = .04, and the post-treatment scores on the OASIS outcome measure, B = 4.81, SE = 1.58, p = .004. The relationship between change in symptoms and change in activation in the ROI are presented in Figure 2.
Table 3.
Model predicting symptom improvement by change in neural activation
| Variable | B [SE] | t, p-value |
|---|---|---|
| Time | 0.81 [0.09] | 8.84, p <.001 |
| Neural Change | 4.81 [1.58] | 3.05, p = .004 |
| Neural Change X Time | −0.53 [0.25] | −2.13, p= .040 |
Figure 2.
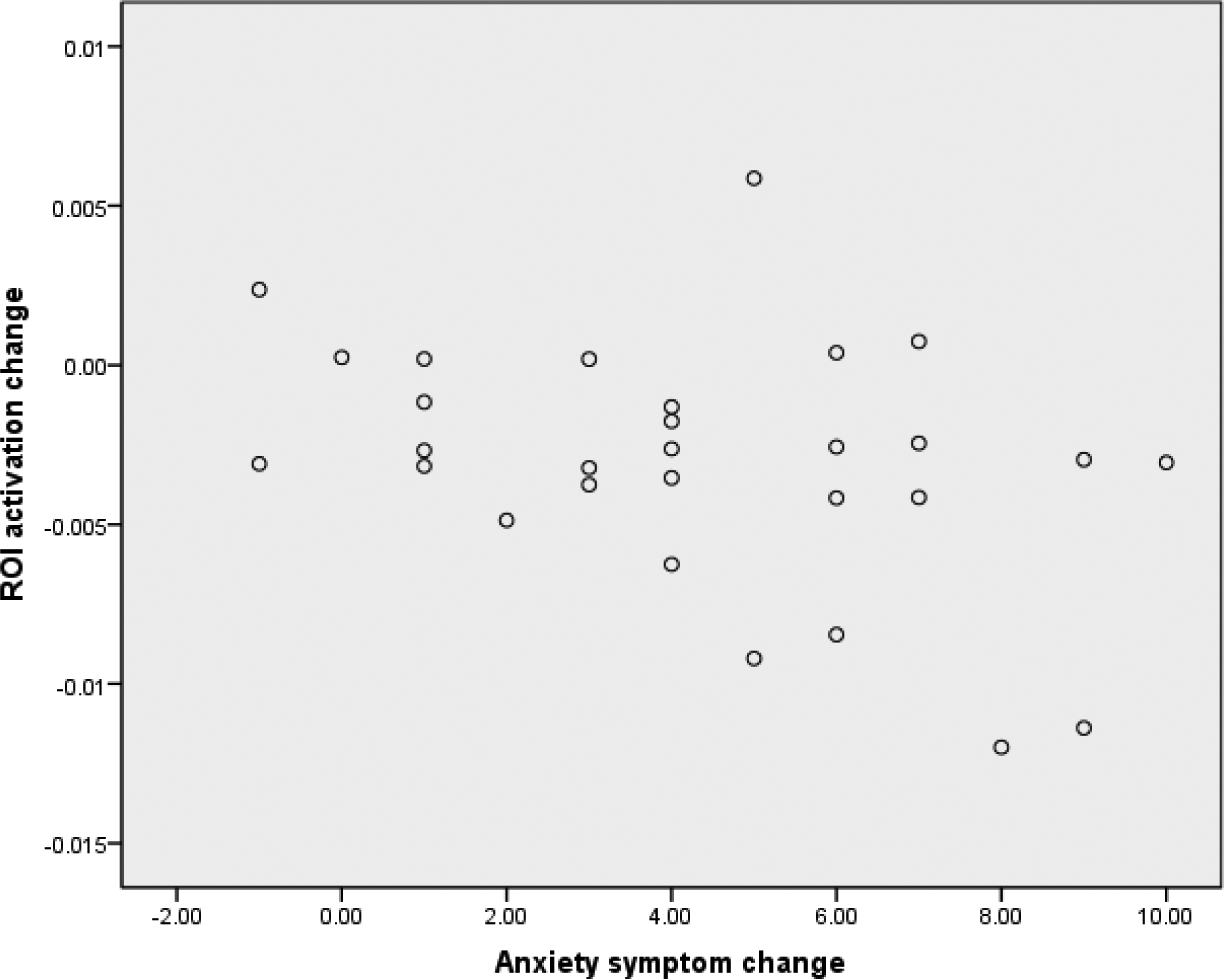
Relationship between change in activation in the parahippocampal region and change in OASIS severity scores from pre- to post-treatment
Overall, 20 participants (66.7%) were considered treatment responders at the last treatment session. In the first step of the model (χ2 (1) = 7.23, p = .007; Cox & Snell R2 = .22), individuals with greater change in neural activation were more likely to be considered responders at the last treatment session (B = −4.08, SE = 1.99, p = .041). Mean activation to each task condition was plotted within responders and non-responders, which revealed a pattern whereby responders showed greater activation during reappraisal versus maintain conditions at pre-treatment1, and the reverse pattern at post-treatment. Non-responders showed similar levels of activation to both reappraise and maintain conditions at each time point (Figure 1b, Figure 3).
Figure 3.
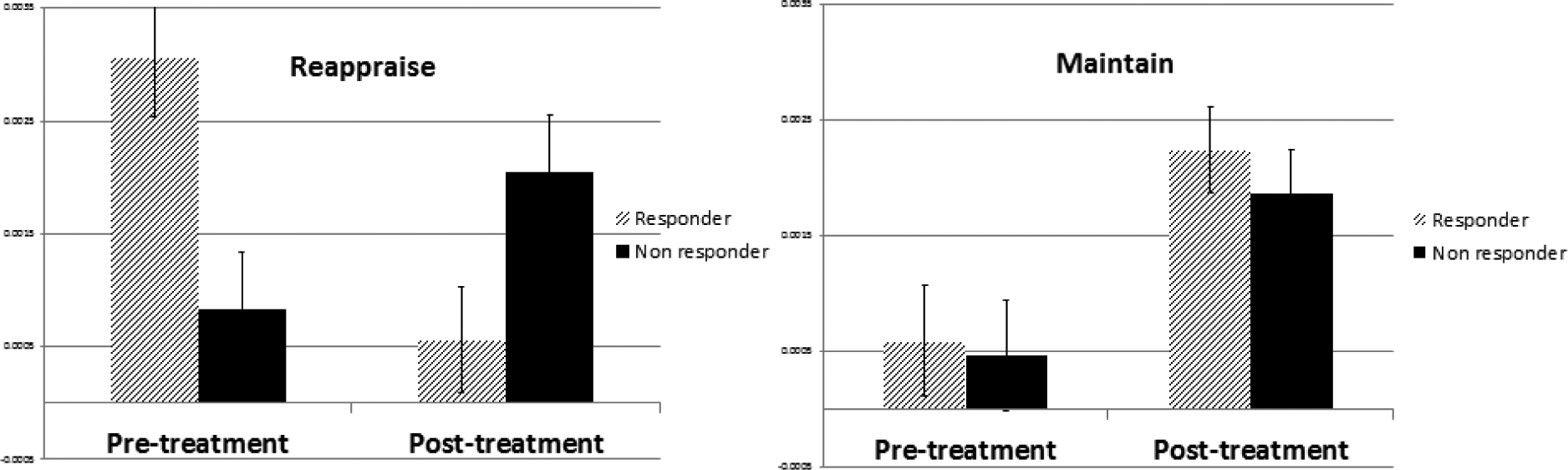
Neural activation during reappraise and maintain (vs. baseline) from pre- to post-treatment
Discussion
The current study sought to identify changes in the neural functioning of individuals with PD or GAD who completed a manualized CBT protocol and underwent fMRI while completing an emotion regulation (reappraisal) task. Over the course of completing CBT treatment, anxiety symptoms reduced significantly, and overall approximately two thirds of participants were considered treatment responders. These findings are consistent with prior work using the full sample from this trial, which found that anxiety significantly reduced over the course of sessions (Taylor et al., 2017). Imaging results revealed changes in neural activation in response to reappraisal attempts versus emotion maintenance from pre- to post-treatment in an area of the parahippocampal gyrus extending into the fusiform gyrus. The extent of change in activation in this region was associated with treatment response. Individuals who were classified as CBT responders showed greater parahippocampal response to reappraise vs. maintain trials at pre-treatment and the reverse pattern at post-treatment, while non-responders showed similar levels of activation to reappraise vs. maintain trials at each time point. The current data extend earlier results (Ball et al., 2013) by linking change in neural activation during a reappraisal task to anxiety symptom outcomes.
Existing models of anxiety disorders propose that aberrant cognitive processing of negative information unfolds over time and is characterized by both initial reactivity (e.g., initial vigilance to and processing of potential threat) and later dysfunctional regulation (e.g., avoidance) following identification of threat (Amir et al., 1998; Williams et al., 1988). Neurobiologically, this vigilance-avoidance model corresponds to greater activation of limbic and contextual processing regions (e.g., amygdala, fusiform gyrus) that onsets rapidly following presentation of emotional cues. Early responding is later modulated with emotion regulation and avoidance behaviors via ongoing interactions with regulatory control processes, including activation in lateral and dorsal PFC regions (Hofmann et al., 2012). Psychotherapy is theorized to catalyze increased prefrontal control over subcortical structures, a proposal supported by meta-analyses evaluating treatment effects for anxiety disorders that find greater activation of prefrontal regions and greater connectivity between prefrontal and limbic structures after treatment (Brooks and Stein, 2015; Etkin et al., 2005; Marwood et al., 2018; Messina et al., 2013; Porto et al., 2009).
In contrast to study predictions, no significant changes in frontoparietal regions were observed in the current data. Several possibilities may account for the lack of observed change in frontal activation from pre- to post-treatment assessments. Given the high cognitive demands required to engage in reappraisal, there may have been insufficient sensitivity to detect change over time in this paradigm. It is possible that reappraisal requires the felt experience of the emotion prior to cognitive processes involved in reappraising the emotion. Although reappraisal is thought to occur temporally downstream from initial threat vigilance, it is possible that observed results reflect changes earlier in the emotion reactivity process, such that individuals may have been less neurally responsive to the negative cues as indexed by lower activity in the parahippocampal/fusiform region. Top-down neural activation might not be observed if the underlying emotional or interoceptive experience differed from pre- to post may have resulted in less “on boarding” of the PFC (Hofmann et al., 2012). Alternatively, It has been proposed that CBT does not necessarily work by overall greater recruitment of PFC, but rather by training adaptive and flexible utilization of the PFC to adaptively engage in emotion regulation (Hofmann et al., 2012). Thus, overall activation in PFC may be similar over time despite differential emotion regulation techniques employed. The current sample consisted of a mixed sample of anxiety patients and did not have a fully representative sample across demographic variables (e.g., gender). These variables each have the potential to contribute variance to overall effects, and thus may have obscured observable group differences. For example, neural responses to reappraisal may vary between men and women (McRae et al., 2008). The analytic choice to examine effects across the whole brain, rather than in select ROIs as in some prior studies, may have influenced our results. In addition, a key difference between the current method and some prior studies was the emotional comparison used, when participants were asked to maintain their emotional response (versus passive viewing). We believe the use of instructions that require active engagement with emotional processing offers advantages. However, the discrepancy in instructions might have contributed to a lower contrast across the two conditions in brain response - particularly if individuals were inadvertently trying to increase emotion, which has been shown to increase activation in top-down control regions (Frank et al., 2014). The impact of task-related parameters has not yet been explored empirically, and will be important to assess in future meta-analytic endeavors.
Successfully using reappraisal requires a multi-step, cognitively-demanding process of identifying one’s initial interpretation of a cue and its associated target emotion, and generating a new interpretation based on prior experiences using the same cue information (McRae et al., 2008; Ochsner and Gross, 2008). The parahippocampal gyrus is associated with diverse cognitive activities spanning episodic memory and visuospatial tasks, and may be implicated in the underlying process of forming contextual associations via relational processing that is common across such activities (Eichenbaum, 2000; Luck et al., 2010; Murty et al., 2010; Rudy, 2009). In the course of repeated exposure to environmental contexts, the parahippocampus is proposed to bind and integrate pieces of information to shape expectations about associations between objects, their locations, associated behaviors, etc. (Aminoff et al., 2013), while the fusiform is critical for object recognition (Grill-Spector and Malach, 2004). It is possible that the observed reduction in neural activity in this region from pre- to post-treatment reflects downregulation of previously entrenched contextual associations (i.e., previous fear-based expectations or negative interpretations of stimuli), particularly related to observed visual cues, that is improved with CBT-related practice. The parahippocampal gyrus and fusiform are also implicated in representations of concrete (vs. abstract) concepts, potentially via engagement of mental imagery processes (D’Argembeau et al., 2014; Hoffman et al., 2015; Wang et al., 2010). Decreased parahippocampal and fusiform activation may also reflect improved efficiency with recall and use of concrete, episodic autobiographical memories from therapy-related learning, such as a previous experience that was consistent with a more benign interpretation. The observed effect in the right hemisphere converges with models proposing lateralization of perception and experience of emotion, particularly negative emotions (Gainotti, 2019). Furthermore, association between reduction of right lateral activation and symptom reduction align with hypothesized role of right hemisphere activation in anxiety (Demaree et al., 2005). However given that findings to date are mixed in regards to the extent that emotions are processed uniquely by hemisphere (Wager et al., 2003) this account should be considered speculative pending further exploration.
The identified regions are not focal points in previously observed neural mechanisms of reappraisal. Prior cross-sectional studies comparing healthy participants to those with anxiety disorders have not typically reported group differences in this region (Zilverstand et al., 2017); although see (Stein et al., 2002) and we did not identify the parahippocampal region as one that differentiated healthy control and anxious participants from each other prior to initiating treatment (Ball et al., 2013). Change in this region identified before and after CBT may thus not reflect a normalization of brain functioning, but could be an alternative compensation-based process that reflects a bottom-up process, rather than top-down regulation as has been previously suggested.
Results should be interpreted in the context of a number of caveats. It is important to note that data were collected as part of an open trial rather than in a randomized controlled trial with a comparator treatment. Thus, one cannot conclude that observed neural change was causally linked to CBT, per se, as opposed to other non-specific factors. For example, it is possible that neural change would correspond to symptom change even in a naturalistic context without intervention. Future work is needed to compare neural change across treatment modalities, to determine if effects are observed specifically in CBT or in anxiety interventions more broadly. In addition, findings should be replicated in a larger sample, which would also facilitate analysis of potential diagnosis-specific effects that could not be completed with adequate power in the current sample. While the two patient groups did not appear significantly different in our prior work using this task or on clinical or behavioral measures, future work should explore potential differences based on disorder presentation (e.g., potentially with idiographic, disorder-relevant stimuli). The task used was designed specifically to elicit effortful downregulation of negative emotions, and cannot address potential neural changes in response to other aspects of emotion regulation that may have changed over the course of treatment (e.g., effortful upregulation of positive emotional experiences; automatic emotion regulation processes).
In sum, the current data indicate that neural change was observed from pre- to post-treatment with CBT in individuals with PD or GAD, and magnitude of neural change was associated with symptom response. Findings suggest that treatment-related change could operate via changes in functioning of structures outside of fronto-parietal and limbic regions that are central to most models of neural functioning in anxiety disorders. Future work is needed to better understand how CBT affects cognitive control and memory processes that are hypothesized to support reappraisal as a strategy for emotion regulation.
Highlights.
Change in neural functioning during reappraisal was assessed before and after CBT
Treatment responders demonstrated decreased parahippocampal activity
Treatment-related neural change may occur outside of top-down regulatory regions
Funding:
This work was supported by the National Institutes of Health [R01MH065413 and K24MH064122, awarded to Dr. Stein and the VA CX001600 awarded to Dr. Bomyea and K23MH113708 awarded to Dr. Ball].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
To explore the hypothesis that baseline severity was driving the results, models were re-analyzed including baseline anxiety and depression as covariates in the models post-baseline through follow up scores. Results were unchanged, demonstrating that neural change remained a significant predictor of symptom change (B = −63.75, p < .04)
Conflict of Interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Conflict of interest: the authors declare no conflicts of interest.
References
- Aminoff EM, Kveraga K, Bar M, 2013. The role of the parahippocampal cortex in cognition. Trends in Cognitive Sciences 17, 379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir N, Foa EB, Coles ME, 1998. Automatic activation and strategic avoidance of threat-relevant information in social phobia. J Abnorm Psychol 107, 285–290. [DOI] [PubMed] [Google Scholar]
- APA, A.P.A., 2000. Diagnostic and statistical manual of mental disorders. American Psychiatric Association, Washington, DC. [Google Scholar]
- Ball TM, Ramsawh HJ, Campbell-Sills L, Paulus MP, Stein MB, 2013. Prefrontal dysfunction during emotion regulation in generalized anxiety and panic disorders. Psychol Med 43, 1475–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsaglini A, Sartori G, Benetti S, Pettersson-Yeo W, Mechelli A, 2014. The effects of psychotherapy on brain function: a systematic and critical review. Progress in Neurobiology 114, 1–14. [DOI] [PubMed] [Google Scholar]
- Brooks SJ, Stein DJ, 2015. A systematic review of the neural bases of psychotherapy for anxiety and related disorders. Dialogues in Clinical Neuroscience 17, 261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, Ochsner KN, 2014. Cognitive Reappraisal of Emotion: A Meta-Analysis of Human Neuroimaging Studies. Cerebral Cortex 24, 2981–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Sills L, Simmons AN, Lovero KL, Rochlin AA, Paulus MP, Stein MB, 2011. Functioning of neural systems supporting emotion regulation in anxiety-prone individuals. Neuroimage 54, 689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter JK, Andrews LA, Witcraft SM, Powers MB, Smits JAJ, Hofmann SG, 2018. Cognitive behavioral therapy for anxiety and related disorders: A meta-analysis of randomized placebo-controlled trials. Depress Anxiety 35, 502–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW, 1996. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research 29, 162–173. [DOI] [PubMed] [Google Scholar]
- Cox RW, Reynolds RC, Taylor PA, 2016. AFNI and clustering: False positive rates redux. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Rose RD, Lang A, Welch SS, Campbell-Sills L, Sullivan G, Sherbourne C, Bystritsky A, Stein MB, Roy-Byrne PP, 2009. Computer-assisted delivery of cognitive behavioral therapy for anxiety disorders in primary-care settings. Depress Anxiety 26, 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Stein MB, Eley TC, Milad MR, Holmes A, Rapee RM, Wittchen HU, 2017. Anxiety disorders. Nature Reviews Disease Primers 3, 17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Stein MB, Sullivan G, Sherbourne C, Bystritsky A, Rose RD, Lang AJ, Welch S, Campbell-Sills L, Golinelli D, Roy-Byrne P, 2011. Disorder-specific impact of coordinated anxiety learning and management treatment for anxiety disorders in primary care. Arch Gen Psychiatry 68, 378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Argembeau A, Cassol H, Phillips C, Balteau E, Salmon E, Van der Linden M, 2014. Brains creating stories of selves: the neural basis of autobiographical reasoning. Social Cognitive and Affective Neuroscience 9, 646–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaree HA, Everhart DE, Youngstrom EA, Harrison DW, 2005. Brain lateralization of emotional processing: historical roots and a future incorporating “dominance”. Behav Cogn Neurosci Rev 4, 3–20. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Geier K, Falkai P, Gruber O, 2011. Fear is only as deep as the mind allows A coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage 58, 275–285. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, 2000. A cortical-hippocampal system for declarative memory. Nature Reviews Neuroscience 1, 41–50. [DOI] [PubMed] [Google Scholar]
- Etkin A, 2012. Neurobiology of anxiety: from neural circuits to novel solutions? Depress Anxiety 29, 355–358. [DOI] [PubMed] [Google Scholar]
- Etkin A, Pittenger C, Polan HJ, Kandel ER, 2005. Toward a neurobiology of psychotherapy: basic science and clinical applications. Journal of Neuropsychiatry and Clinical Neurosciences 17, 145–158. [DOI] [PubMed] [Google Scholar]
- Frank DW, Dewitt M, Hudgens-Haney M, Schaeffer DJ, Ball BH, Schwarz NF, Hussein AA, Smart LM, Sabatinelli D, 2014. Emotion regulation: Quantitative meta-analysis of functional activation and deactivation. Neuroscience and Biobehavioral Reviews 45, 202–211. [DOI] [PubMed] [Google Scholar]
- Gainotti G, 2019. Emotions and the Right Hemisphere: Can New Data Clarify Old Models? Neuroscientist 25, 258–270. [DOI] [PubMed] [Google Scholar]
- Goldin PR, Manber-Ball T, Werner K, Heimberg R, Gross JJ, 2009. Neural mechanisms of cognitive reappraisal of negative self-beliefs in social anxiety disorder. Biological Psychiatry 66, 1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Malach R, 2004. The human visual cortex. Annu Rev Neurosci 27, 649–677. [DOI] [PubMed] [Google Scholar]
- Gross JJ, 2002. Emotion regulation: affective, cognitive, and social consequences. Psychophysiology 39, 281–291. [DOI] [PubMed] [Google Scholar]
- Hoffman P, Binney RJ, Ralph MAL, 2015. Differing contributions of inferior prefrontal and anterior temporal cortex to concrete and abstract conceptual knowledge. Cortex 63, 250–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Ellard KK, Siegle GJ, 2012. Neurobiological correlates of cognitions in fear and anxiety: A cognitive-neurobiological information-processing model. Cognition Emotion 26, 282–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE, 2005. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry 62, 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, Habel U, 2014. Neural network of cognitive emotion regulation--an ALE meta-analysis and MACM analysis. Neuroimage 87, 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN, 2008. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual, Technical Report A-8, in: Florida U.o. (Ed.), Gainesville, FL. [Google Scholar]
- Loerinc AG, Meuret AE, Twohig MP, Rosenfield D, Bluett EJ, Craske MG, 2015. Response rates for CBT for anxiety disorders: Need for standardized criteria. Clinical Psychology Review 42, 72–82. [DOI] [PubMed] [Google Scholar]
- Luck D, Danion JM, Marrer C, Pham BT, Gounot D, Foucher J, 2010. The right parahippocampal gyrus contributes to the formation and maintenance of bound information in working memory. Brain Cogn 72, 255–263. [DOI] [PubMed] [Google Scholar]
- Lueken U, Zierhut KC, Hahn T, Straube B, Kircher T, Reif A, Richter J, Hamm A, Wittchen HU, Domschke K, 2016. Neurobiological markers predicting treatment response in anxiety disorders: A systematic review and implications for clinical application. Neuroscience and Biobehavioral Reviews 66, 143–162. [DOI] [PubMed] [Google Scholar]
- Martin EI, Ressler KJ, Binder E, Nemeroff CB, 2010. The neurobiology of anxiety disorders: brain imaging, genetics, and psychoneuroendocrinology. Clinics in Laboratory Medicine 30, 865–891. [DOI] [PubMed] [Google Scholar]
- Marwood L, Wise T, Perkins AM, Cleare AJ, 2018. Meta-analyses of the neural mechanisms and predictors of response to psychotherapy in depression and anxiety. Neuroscience and Biobehavioral Reviews 95, 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew SJ, Price RB, Charney DS, 2008. Recent advances in the neurobiology of anxiety disorders: implications for novel therapeutics. American Journal of Medical Genetics Part C 148C, 89–98. [DOI] [PubMed] [Google Scholar]
- McRae K, Ochsner KN, Mauss IB, Gabrieli JJD, Gross JJ, 2008. Gender Differences in Emotion Regulation: An fMRI Study of Cognitive Reappraisal. Group Process Intergroup Relat 11, 143–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina I, Sambin M, Palmieri A, Viviani R, 2013. Neural correlates of psychotherapy in anxiety and depression: a meta-analysis. PLoS One 8, e74657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, Ritchey M, Adcock RA, LaBar KS, 2010. fMRI studies of successful emotional memory encoding: A quantitative meta-analysis. Neuropsychologia 48, 3459–3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman SB, Cissell SH, Means-Christensen AJ, Stein MB, 2006. Development and validation of an Overall Anxiety Severity And Impairment Scale (OASIS). Depress Anxiety 23, 245–249. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ, 2008. Cognitive Emotion Regulation: Insights from Social Cognitive and Affective Neuroscience. Curr Dir Psychol Sci 17, 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porto PR, Oliveira L, Mari J, Volchan E, Figueira I, Ventura P, 2009. Does Cognitive Behavioral Therapy Change the Brain? A Systematic Review of Neuroimaging in Anxiety Disorders. Journal of Neuropsychiatry and Clinical Neurosciences 21, 114–125. [DOI] [PubMed] [Google Scholar]
- Roy-Byrne P, Craske MG, Sullivan G, Rose RD, Edlund MJ, Lang AJ, Bystritsky A, Welch SS, Chavira DA, Golinelli D, Campbell-Sills L, Sherbourne CD, Stein MB, 2010. Delivery of evidence-based treatment for multiple anxiety disorders in primary care: a randomized controlled trial. Journal of the American Medical Association 303, 1921–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW, 2009. Context representations, context functions, and the parahippocampal-hippocampal system. Learning & Memory 16, 573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB, 2003. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biological Psychiatry 54, 573–583. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Glen DR, Chen G, Beauchamp MS, Desai R, Cox RW, 2009. A new method for improving functional-to-structural MRI alignment using local Pearson correlation. Neuroimage 44, 839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits JAJ, Julian K, Rosenfield D, Powers MB, 2012. Threat Reappraisal as a Mediator of Symptom Change in Cognitive-Behavioral Treatment of Anxiety Disorders: A Systematic Review. Journal of Consulting and Clinical Psychology 80, 624–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Goldin PR, Sareen J, Zorrilla LTE, Brown GG, 2002. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Archives of General Psychiatry 59, 1027–1034. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P, 1988. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging.
- Taylor CT, Knapp SE, Bomyea JA, Ramsawh HJ, Paulus MP, Stein MB, 2017. What good are positive emotions for treatment? Trait positive emotionality predicts response to Cognitive Behavioral Therapy for anxiety. Behav Res Ther 93, 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF, 2003. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage 19, 513–531. [DOI] [PubMed] [Google Scholar]
- Wang J, Conder JA, Blitzer DN, Shinkareva SV, 2010. Neural representation of abstract and concrete concepts: a meta-analysis of neuroimaging studies. Hum Brain Mapp 31, 1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JMG, Watts FN, MacLeod C, Mathews A, 1988. Cognitive psychology and emotional disorders. John Wiley & Sons. [Google Scholar]
- Zilverstand A, Parvaz MA, Goldstein RZ, 2017. Neuroimaging cognitive reappraisal in clinical populations to define neural targets for enhancing emotion regulation. A systematic review. Neuroimage 151, 105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]


