Abstract
Introduction
Women with twin pregnancies and a short cervix are at increased risk for preterm birth (PTB). Given the burden of prematurity and its attendant risks, the quest for effective interventions in twins has been an area of considerable research. Studies investigating the effectiveness of cervical cerclage, cervical pessary and vaginal progesterone in preventing PTB have yielded conflicting results. The aim of this study is to compare the effectiveness of cervical pessary and cervical cerclage with or without vaginal progesterone to prevent PTB in women with twin pregnancies and a cervical length (CL) ≤ 28 mm.
Methods and analysis
This multicentre, randomised clinical trial will be conducted at My Duc Hospital and My Duc Phu Nhuan Hospital, Vietnam. Asymptomatic women with twin pregnancies and a CL ≤28 mm, measured at 16–22 weeks’ gestation, will be randomised in a 1:1:1:1 ratio to receive a cerclage, pessary, cerclage plus progesterone or pessary plus progesterone. Primary outcome will be PTB <34 weeks. Secondary outcomes will be maternal and neonatal complications. We preplanned a subgroup analysis according to CL from all women after randomisation and divided into four quartiles. Analysis will be conducted on an intention-to-treat basis. The rate of PTB <34 weeks’ gestation in women with twin pregnancies and a cervix ≤28 mm and treated with pessary in our previous study at My Duc Hospital was 24.2%. A sample size of 340 women will be required to show or refute that cervical cerclage decreases the rate of PTB <34 weeks by 50% compared with pessary (from 24.2% to 12.1%, α level 0.05, power 80%, 5% lost to follow-up and protocol deviation). This study is not to be powered to assess interactions between interventions.
Ethics and dissemination
Ethical approval was obtained from the Institutional Ethics Committee of My Duc Hospital and informed patient consent was obtained before study enrolment. Results of the study will be submitted for publication in a peer-reviewed journal.
Trial registration number
NCT03863613 (date of registration: 4 March 2019).
Keywords: maternal medicine, fetal medicine, ultrasonography
Strengths and limitations of this study.
The trial has a randomised, controlled design, which should minimise bias.
A limited number of well-trained, certified staffs will be involved in cervical length measurement, pessary and cerclage placement which increases the validity of the study.
An open design, which is unavoidable due to the nature of the interventions, could introduce bias.
The majority of women with twin pregnancies involving in the trial will conceive from assisted reproductive technology. Therefore, the external validity of the study might be compromised.
Introduction
Preterm birth (PTB) is the most common cause of neonatal morbidity and mortality worldwide1 2 and the second leading cause of death in children under 5 years.3 4 Children who survive may face the risk of significant disability, including cerebral palsy, intellectual impairment, chronic lung disease and vision and hearing loss for a lifetime. They are also at greater risk of developing hypertension, diabetes and development problems later in their lives.5 Therefore, prevention of PTB has been considered a priority worldwide to promote the well-being of mothers and children.6 Due to an increased use of assisted reproductive technologies (ARTs) and an older maternal age, the incidence of twin pregnancies has increased, in the recent three decades, by almost 70% worldwide.7 8
Women with twin pregnancies are at increased risk for PTB. In the USA, data in 2016 showed that more than 60% women with twin pregnancies gave birth before 37 weeks, of whom 21.2% did so before 34 weeks.9 Corresponding figures in women with singleton pregnancies were 8% and 2.1%, respectively.9 In addition, short cervical length (CL) in the second trimester of pregnancy is well known to be an independent risk factor for PTB.10 11 In women with twin pregnancies, the risk of spontaneous PTB also increases with decreasing CL.12 Therefore, women with twin pregnancies and a short cervix are at extremely high risk for PTB.13
Although vaginal progesterone, cervical cerclage and cervical pessary have been used in clinical practice to prevent PTB, evidence regarding the effectiveness of these interventions is still inconclusive.14 A meta-analysis of randomised clinical trials (RCTs) suggested that progesterone potentially reduced PTB and neonatal complications in women with twin pregnancies and a short cervix.15 However, a recent meta-analysis showed that progesterone could only improve some secondary outcomes, regardless of CL.16
For cervical cerclage, a meta-analysis of three trials in 49 women with twin pregnancies and a CL <25 mm could not demonstrate a benefit of cerclage in this population.17 Moreover, cerclage group had higher rates of very low birth weight and of respiratory distress syndrome than control group. In contrast, a recent systematic review and meta-analysis, which includes RCTs and cohort studies, indicate that cerclage placement is beneficial for the reduction of PTB only in twin pregnancies with a CL <15 mm or dilated cervix of >10 mm.18 In this study, authors stated that further high-quality studies are needed to confirm the findings, due to the insufficiency of strong supporting evidence.18
Among types of cervical pessaries, the Arabin is the most common used in the prevention of PTB.19 This pessary has shown encouraging results in women with twin pregnancies. A large open-label RCT was conducted at 40 Dutch centres from 2009 to 2012 among 813 women with a twin pregnancy.20 While there was no significant effect in the overall population, pessary significantly reduced PTB <28 weeks and <32 weeks, median of time to delivery and adverse neonatal outcomes in women with a short cervix (<25% percentile, equal to 38 mm). These findings were further confirmed by another RCT performed by Goya et al.21 However, evidence supporting the futility of cervical pessary in women with twin pregnancies and a short cervix appeared in subgroup analyses of two RCTs, including an early-stopped one.22 23
Trials that directly compare cerclage, progesterone and pessary are limited. We recently compared the effectiveness of cervical pessary and 400 mg progesterone daily in a RCT among women with twin pregnancies and a CL <38 mm.24 In our study, the primary outcome, PTB <34 weeks, was comparable between the two groups. However, other secondary outcomes, including neonatal outcomes, were significantly improved in women treated with pessary. Moreover, in women with a CL ≤28 mm (25th percentile of randomised women), the rate of PTB <34 weeks significantly reduced from 54.5% in the progesterone group to 24.2% in the pessary group.24 A recent cost-effective analysis showed that pessary significantly improves the rate of morbidity-free survival neonate while reducing costs as compared with vaginal progesterone.25 As these data showed that cervical pessary might be superior to vaginal progesterone in the prevention of PTB in women with twin pregnancies and a CL ≤28 mm, the question that arises now is how cervical pessary compares to cervical cerclage. We therefore propose the Pessary versus Cerclage with or without Progesterone in Twins (PCP-Twins) study, in which we will compare the effectiveness of cervical pessary and cervical cerclage with or without vaginal progesterone to prevent PTB in women with twin pregnancies and a CL ≤28 mm.
Methods and analysis
Study design and ethical considerations
The PCP-Twins study is a randomised controlled trial and will be conducted at My Duc Hospital and My Duc Phu Nhuan Hospital, Ho Chi Minh City, Vietnam, according to the principles outlined in the Declaration of Helsinki and its amendments, in accordance with the Medical Research Involving Human Subjects Act (WMO) and using Good Clinical Practice. The trial has been approved by the Institutional Ethics Committee (IEC) of My Duc Hospital (02/2019/MĐ-HĐĐĐ).
It is our daily practice that all pregnant women will undergo urine culture for screening of asymptomatic bacteriuria in the first prenatal visit. If they have asymptomatic bacteriuria, they will be treated with antibiotics.26 At 16–22 weeks of gestation, asymptomatic women with twin pregnancies will undergo CL measurement and digital examination as part of routine clinical care. In case where vaginitis is suspected, vaginal culture could be performed. For women who conceived after ART, gestational age will be determined by the date of embryo transfer or intrauterine insemination. For those who conceived naturally, gestational age will be determined from the menstrual history and confirmed by the fetal crown-rump length of the larger twin at the first-trimester ultrasound examination. CL will be measured transvaginally in each hospital by two ultrasonographers certificated by the Fetal Medicine Foundation. Prior to CL measurement, women will be given a short brochure outlining risk factors and available PTB prevention methods.
A review of participant information will be done prior to enrolment to determine preliminary eligibility according to participant inclusion and exclusion criteria. Eligible participants will be screened by midwives or gynaecologists, then they will be provided a full participant information sheet, consent form and will be invited to a full discussion with investigators about the study. All eligible women will be invited to participate in the study.
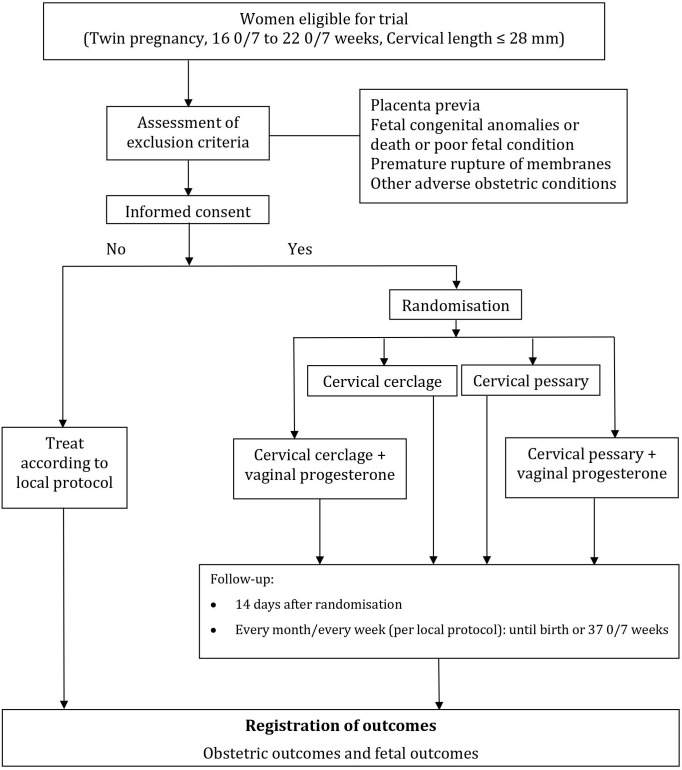
After written informed consent, women will be randomly assigned in a 1:1:1:1 ratio to receive a cerclage, pessary, cerclage plus progesterone or pessary plus progesterone. Assignment to treatment allocation will be done via a web portal hosted by HOPE Research Center, Vietnam. The randomisation schedule will be computer-generated at HOPE Research Center, with a permuted random block size of 4 or 8 (figure 1). Blinding will not be possible due to the nature of interventions. However, neonatologists assessing the children will be unaware of treatment allocation. Apart from randomisation, participants will be followed up and treated according to local protocol. The study is currently recruiting, with first patient in was on 23 March 2019 and an estimated duration of 36 months.
Figure 1.
Flow of participants.
Participation in the study is voluntary. When a participant signs an informed consent, she is considered to be enrolled into the study. Participants can leave the study at any time for any reason, if they wish to do so without any consequences for their treatment. The investigator can decide to withdraw a subject from the study for urgent medical reasons. After randomisation, if a participant wishes to change her assigned protocol, she will be considered as a cross-over subject. All subjects will be remained in the study for analysis based on intention to treat principle.
To maximise retention in the trial, consultation will be available to patients to ensure that they understand the procedures well and to address any questions or complaints that arise during the study.
Patients and public involvement
Patients and/or public were not involved in the study design and study enrolment.
Participants
Women aged ≥18 years, having twin pregnancies and CL ≤28 mm measured at 16–22 weeks’ gestation, will be eligible for the study irrespective of chorionicity. Exclusion criteria: (1) women with uterine anomalies; (2) cervical dilation with visible amniotic membranes or amniotic membranes prolapsed into the vagina; (3) twin-to-twin transfusion syndrome; (4) stillbirth; (5) major congenital abnormalities in any of the fetuses; (6) severe vaginal discharge; (7) acute vaginitis or cervicitis; (8) vaginal bleeding or placenta preavia or vasa preavia; (9) premature rupture of membranes or premature labour with/without ruptured membrane; (10) suspicion of chrorioaminitis; (11) cerclage or pessary in place or unable to undergo cervical cerclage or pessary placement. However, women with acute cervicitis, vaginitis or severe vaginal discharge are eligible once they have been treated and if they have a CL ≤28 mm between 16 and 22 weeks.
Treatment groups
Participants will be allocated to receive a cerclage, pessary, cerclage plus progesterone or pessary plus progesterone. Women allocated to a cervical cerclage will be receiving the intervention according to local protocol, within a week after randomisation. Briefly, two to three senior clinicians at each site who had experienced with cerclage will perform cervical cerclage, using Mc Donald technique, under spinal anaesthesia, in the operation theatre. Cephazolin 1 g will be given intravenously at 1 hour before cerclage as prophylactic antibiotics.27
Pessary, a soft, flexible, silicone pessary, purchased from the manufacturer (Arabin, Dr Arabin GmbH & Co KG, Germany), will be inserted through the vagina, upward around the cervix by four senior clinicians, who had experienced with pessary placement, within 1 week of randomisation. The size of the pessary will be determined at the time of speculum inspection.28
In the cerclage plus progesterone group and pessary plus progesterone group, 400 mg vaginal progesterone, purchased from the manufacturer (Cyclogest 400 mg, Actavis, UK), will be applied once daily at bedtime, within 2 days after cerclage insertion. Participants will be asked to record their drug application in a participant diary sheet for up to 147 days.
In all groups, participants will be reassessed at 14 days post randomisation for any possible adverse events. After that, participants will be seen monthly or weekly per local protocol. CL measurement will not be performed routinely after randomisation, unless for participants’ preference. In case of CL shortens after receiving allocated interventions, patient can be treated with a combination protocol taking additional interventions, for example, adding progesterone or pessary/cerclage, based on the discretion of treating clinician after a thoroughly discussion with the patient. In case of premature rupture of the membranes, active vaginal bleeding, other signs of preterm labour or severe participant discomfort, the vaginal progesterone and pessary or cerclage will be removed. If participants develop (threatened) preterm labour, they will receive treatment per local protocol. Intervention will be stopped at 370/7 weeks of gestation or at delivery if none of the above-stated conditions happen.
At every visit, participant compliance with progesterone therapy will be documented by checking the participant diary and drug purchasing records from the hospital pharmacy. The compliance rate will be calculated by dividing the number of progesterone doses used since the previous visit by the number of progesterone doses that should have been used. Women will be defined as being in compliance when the compliance rate is equal or higher than 80%.
Outcomes
Primary outcome
The primary outcome will be PTB <34 weeks’ gestation for any indication.
Secondary outcomes
Secondary outcomes will be fetal death before 24 weeks of gestation; stillbirth (defined as a fetus born with no signs of life at or after 28 weeks of gestation); delivery before 24, 28, 32 and 37 weeks of gestation; labour induction, delivery mode, live birth; tocolytic drugs, antenatal corticosteroids or MgSO4 for neuroprotection use; admission days for preterm labour; preterm premature rupture of membrane chorioamnionitis; maternal side effects (including vaginal discharge, fever, vaginal infection or pain, pessary repositioning and necrosis or rupture of the cervix); maternal morbidity (including thromboembolic complications, urinary tract infection treated with antibiotics, pneumonia, endometritis, hypertensive disorder, eclampsia, haemolysis, elevated liver enzymes, low platelet count syndrome, death); birth weight; birth weight less than 1500 g and less than 2500 g; congenital anomalies diagnosed after randomisation; 5 min Apgar score; 5 min Apgar score less than 7; perinatal death, neonatal intensive care unit (NICU) admission; days of admission to the NICU; intraventricular haemorrhage; respiratory distress syndrome; necrotising enterocolitis; proven sepsis and a composite of poor perinatal outcomes. Full definitions of these terms are provided in table 1.
Table 1.
Definition of neonatal outcomes
| Secondary endpoint | Definition |
| Respiratory distress syndrome (RDS) | The presence of tachypnoea >60/min, sternal recession and expiratory grunting, need for supplemental oxygen and a radiological picture of diffuse reticulogranular shadowing with an air bronchogram. |
| Intraventricular haemorrhage II B or worse | Repeated neonatal cranial ultrasound by the neonatologist according to the guidelines on neuroimaging described by de Vries et al. |
| Necrotising enterocolitis (NEC) | Will be diagnosed according to Bell. |
| Proven sepsis | Will be diagnosed on the combination of clinical signs and positive blood cultures. |
| Stillbirth | A baby born with no signs of life at or after 28 weeks’ gestation (WHO). |
| Composite of poor perinatal outcomes | Fetal or neonatal death, intraventricular haemorrhage, RDS, NEC or neonatal sepsis. |
Safety
The primary investigator will inform subjects and the reviewing accredited medical research ethics committee if anything occurs that would suggest that the disadvantages of participation may be significantly greater than was foreseen in the research proposal. The study will be suspended pending further review by the accredited medical research ethics committee, except where suspension would jeopardise the participants’ health. The investigator will ensure that all subjects are kept informed.
Adverse events are defined as any undesirable experience occurring to a subject during a clinical trial, whether or not considered related to the intervention. All adverse events reported spontaneously by the subject or observed by the investigator or their staff will be recorded.
A serious adverse event (SAE) is defined as any untoward medical occurrence or effect that results in death, is life threatening (at the time of the event), requires hospitalisation or prolongation of an existing hospitalisation, results in persistent or significant disability or incapacity, is a congenital anomaly or birth defect, is a new event of the trial likely to affect the safety of the subjects such as an unexpected outcome of an adverse reaction. All SAEs will be reported to the accredited ethics committee that approved the protocol, according to the requirements of that committee.
All adverse events will be followed until they have abated or until a stable situation has been reached. Depending on the event, follow-up may require additional tests or medical procedures as indicated and/or referral to a general physician or medical specialist.
Sample size calculation
This study is not to be powered to assess interactions between interventions. The rate of PTB <34 weeks of gestation in women with twin pregnancies and a cervix ≤28 mm and treated with pessary in our previous Arabin versus Progesterone (AP) study at My Duc Hospital was 24.2%.24 In order to show that cervical cerclage decreases the PTB rate by 50% (from 24.2% to 12.1%), we need to randomise 320 women (α level 0.05, power 80%). The rate of PTB <34 weeks of gestation in women with twin pregnancies and a cervix ≤28 mm and treated with progesterone at My Duc Hospital was 54.5%.24 Therefore, the statistical power to study the effect of progesterone by randomising 320 women is 80% (α level 0.05, PTB rate in progesterone group 54.5%, PTB rate in non-progesterone group 39.0%). Considering a 5% lost to follow-up and protocol deviation, we plan to recruit 340 participants (85 per arm).
Confidentiality and ownership of trial data
VQD, LMTN and LNV will have access to the final trial dataset. Any confidential information relating to the trial, including any data and results from the trial, will be the exclusive property of My Duc Hospital. The investigators and any other persons involved in the trial will protect the confidentiality of this proprietary information belonging to My Duc Hospital.
Statistical analysis
Statistical analysis will be conducted according to the intention-to-treat principle, in which all randomised women will be considered in the primary comparison between treatment groups. The per-protocol analysis may be conducted, but these results would be considered exploratory only. All tests will be two tailed and differences with p value <0.05 will be considered statistically significant. In view of the two-by-two factorial design, the analysis will be done separately for cerclage versus pessary and for progesterone versus no progesterone. No adjustments are planned for multiple comparisons.
Baseline characteristics will be described by descriptive analysis (mean and SD for normally distributed variables or median and IQR for skewed variables). For categorical variables, we will present the proportions (%) of the four arms. In addition, we will also report the numbers of recruitment, participants lost to follow-up, protocols violation and other relevant descriptive data.
The primary outcome, PTB <34 weeks’ gestation, will be compared using Pearson’s χ2 test or Fisher’s exact test for unadjusted analysis. We will also compute unadjusted risk ratio (RR) and its 95% CI. In the event of prominent imbalance of potential confounders between arms, we will perform multivariable log–binomial or Poisson regression with robust variance estimate to compute adjusted RR and its 95% CI.
For continuous variables, results will be given as mean (SD) and between-group differences will be assessed using Student’s t-test. For dichotomous endpoints, Relative Risk and 95% CI values will be calculated. Time to delivery will be assessed using a Cox proportional hazard analysis and Kaplan-Meier estimates, where gestational age will be the time scale, birth will be the event and results will be compared with a log-rank test. HR values will be estimated using a Cox proportional hazards model, with a formal test of the proportional hazards assumption. For neonatal outcomes, we will use cluster analysis taking into account the dependency between the twins.29 We plan a prespecified subgroup analysis in: (1) women with different chorionicity and (2) women with a CL <25th percentile and at the 25–50th percentile, 50–75th percentile and >75th percentile. The percentile will be determined based on the CL from all women after randomisation. We will test for interaction between CL and treatment effect on PTB <34 weeks and the composite of poor perinatal outcomes. Interaction between groups of interventions will also be tested due to the 2×2 factorial design of our randomised controlled trial. However, the tests for interactions will be exploratory.
Interim analysis and monitoring
We will establish an independent Data Safety Monitoring Committee (DSMC) to review and interpret data generated from the study and to review revisions of the protocol prior to their implementation. The roles and responsibilities, including the timing of meetings, methods of providing information to and from the DSMC, frequency and format of meetings, statistical issues and relationships with other committees are described in the DSMC charter (online supplementary appendix 1).
bmjopen-2019-036587supp001.pdf (501.5KB, pdf)
All SAEs will be reported to the IEC within 15 working days. Life-threatening SAEs or an event that leads to death will be reported to the IEC immediately. All SAEs will be followed until they have abated, until a stable situation has been reached or the patient is discharged. Due to the nature of interventions, we do not expect to terminate the study prematurely.
We plan one interim analysis. The interim analysis will be performed by an independent statistician who will not directly involve in the study, after completion of data collection of the first 150 randomised participants. At interim analysis, data will be assessed for safety, efficacy and futility. Safety will be assessed in terms of SAEs (perinatal death, maternal mortality or severe maternal morbidity). The interim analysis will be conducted using a two-sided significant test with the Haybittle-Peto spending function and a type I error rate of 5% (final alpha level) with stopping criteria of p<0.001 (Z alpha=3.29). Based on this report, the DSMC will provide guidance on whether to stop or continue the study.
Data handling
Data will be collected using a questionnaire and recorded with Epi Info software (Centers for Disease Control and Prevention). Data of each visit will be documented in the participant’s study profile. When the participant attends for delivery, data on labour, delivery and any complications experienced by participant and the neonates will be collected. For those who do not continue to follow-up at either site, for any reasons, we will contact the participants via telephone/email monthly until birth to collect data. We also ask these participants to scan their profile in every contact.
All data will be entered into the database twice. The first data entry will be made within a day after randomisation. The second will undertake at the termination of the study. Data from the two entries will be checked and adjudicated by independent data monitoring using the original participant medical record. Participant privacy will be ensured by allocation of a five-digit number to each participant, which will be used on all study documentation, with the participant code only available to the local investigator.
Independent study monitoring will be performed monthly at each site by a clinical research associate from HOPE Research Center to ensure adherence to the protocol, International Conference on Harmonisation—Good Clinical Practice, standard operating procedures and applicable regulatory requirements, maintenance of trial-related source records, completeness, accuracy and verifiability of case report form entries compared with source data.
Missing data
For missing values regarding baseline characteristics, we will first perform analysis by excluding missing values; we will then perform multiple imputations to impute missing values and conduct subsequent analysis to estimate the robustness of the findings. For the loss of follow-up and protocol deviation, we will attempt sensitive analyses to explore the effect of these factors on the trial findings.
End of study
We estimate an expected duration of 36 months, with expected final recruitment at March 2022. The principal investigator will notify the IEC of My Duc Hospital of the end of the study within a period of 90 days. The end of the study is defined as the last participant’s last visit. In the event of early study termination, the principal investigator will notify the IEC of My Duc Hospital within 15 days, including the reasons for premature termination.
Ethics and dissemination
Any change to the study protocol will be documented in a protocol amendment. Amendments are submitted for consideration to the approving ethics committee (the IEC of My Duc Hospital). If new information becomes available that may be relevant to a subject’s willingness to continue participation in the trial, a new subject information and informed consent form will be forwarded to the ethics committee. The trial subjects will be informed about this new information and reconsent will be obtained. Changes will be updated on trial registration website clinicaltrials.gov. Changes to the protocol to eliminate immediate hazard(s) to trial subjects may be implemented prior to ethics committee approval or receipt of a favourable opinion.
No specific arrangements will be made between any sponsors and the investigator concerning the public disclosure and publication of the research data. The principle investigator will prepare a manuscript detailing the results of the main study as soon as appropriate and submit this to a peer-reviewed medical journal. Supplementary analyses will be analysed and reported separately.
Discussion
It has been shown that women with twin pregnancies and a short cervix are at extremely high risk for PTB.13 Therefore, prevention of PTB has become priority in healthcare for mothers and children.
In this study, we choose to compare two interventions directly to each other. While we appreciate that when interventions are introduced, they should be compared with expectant management or placebo, this has already been done in a large number of twins, for both cervical pessary20 21 23 and progesterone.15 Our recent trial showed that compared with vaginal progesterone, the use of pessary in women with twin pregnancies and a short cervix improved neonatal outcomes. Moreover, in women with a CL 28 mm (25th percentile of CL distribution), the rate of PTB <34 weeks reduced from 54.5% in the progesterone group to 24.2% in the pessary group.24 In addition, recent small retrospective studies showed that cerclage could lower PTB rates and could improve neonatal outcomes.30–32 These data suggest that any of the two treatments could reduce the risk of PTB and subsequent poor neonatal outcome. The aim of clinical research is not to directly prove in a purely scientific setting whether a treatment works over no treatment, but to show which is the best for patients. In view of the large differences that we found for pessary versus progesterone24 and others found for pessary and progesterone against no treatment,15 20 21 we render it from an ethical point challenging to compare these treatments to expectant management or placebo. This approach can also be found in other ongoing trials.33 34
The cut-off value for CL in twins is still controversial and varies from study to study, with 25 mm being used in some studies.21 31 35 However, the association between CL and the risk of PTB is unlikely to be ‘all or none’ with this cut-off. In this trial, we choose 28 mm as a cut-off based on a preplanned subgroup analysis of our previous RCT that reported that in patients with a CL ≤25th percentile (≤28 mm), the use of pessary was associated with a significant reduction in the risk of PTB <34 weeks, PTB <37 weeks and the risk of poor perinatal outcomes.24 There is scarce evidence regarding the management of women with a CL between 25 mm and 28 mm, who are likely to have an elevated risk of PTB. Only by including women below a cut-off 28 mm, we will be able to understand how pessary compares to cerclage in women with a CL between 25 mm and 28 mm. Meanwhile, we would be able to perform a subgroup analysis confining to those with a CL ≤25 mm.
Strengths of this trial include its randomised, controlled design, which should minimise bias and a multicentre design, which enhance the generalisability of the results. Moreover, there will be a limited number of well trained, certified staffs who will involve in CL measurement, pessary and cerclage placement. This can help to increase the validity of the study. However, an open design, which is unavoidable due to the nature of the interventions, could introduce bias. The external validity of the study might be compromised since the majority of women with twin pregnancies involving in the trial will conceive from ART.
Supplementary Material
Footnotes
Contributors: VQD, WL, BWM and LNV made substantial contributions to the conception and design of the study. VQD and YTNH are responsible for the overall logistical aspects of the trial and general acquisition of data and they also drafted the manuscript. LMTN, TVL and WL provided statistical support regarding future analyses. HNHP, TTTT, TQB, NTV, DTNN and CHL made substantial contributions to acquisition of data. All authors critically revised the manuscript, approved the final version of the manuscript and agreed to be accountable for all aspects of the manuscript ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved.
Funding: This study was funded by My Duc Hospital.
Competing interests: VQD has received grant from Merck Sharpe and Dohme. BWM reports support from a NHMRC Practitioner Fellowship (GNT1082548) and consultancy for ObsEva, Merck and Guerbet. LNV has received speaker and conference fees from Merck, grant, speaker and conference fees from Merck Sharpe and Dohme, and speaker, conference and scientific board fees from Ferring.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008;371:261–9. 10.1016/S0140-6736(08)60136-1 [DOI] [PubMed] [Google Scholar]
- 2.Statistics OfN Gestation-specific infant mortality: 2012, 2014. Available: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/causesofdeath/bulletins/pregnancyandethnicfactorsinfluencingbirthsandinfantmortality/2014-10-15
- 3.Blencowe H, Krasevec J, de Onis M, et al. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: a systematic analysis. Lancet Glob Health 2019;7:e849–60. 10.1016/S2214-109X(18)30565-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2015;385:430–40. 10.1016/S0140-6736(14)61698-6 [DOI] [PubMed] [Google Scholar]
- 5.Allotey J, Zamora J, Cheong-See F, et al. Cognitive, motor, behavioural and academic performances of children born preterm: a meta-analysis and systematic review involving 64 061 children. BJOG 2018;125:16–25. 10.1111/1471-0528.14832 [DOI] [PubMed] [Google Scholar]
- 6.WHO Born to soon: the global action report on preterm birth. WHO, 2012. [Google Scholar]
- 7.Smits J, Monden C. Twinning across the developing world. PLoS One 2011;6:e25239. 10.1371/journal.pone.0025239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ananth CV, Joseph Ks Ks, Smulian JC. Trends in twin neonatal mortality rates in the United States, 1989 through 1999: influence of birth registration and obstetric intervention. Am J Obstet Gynecol 2004;190:1313–21. 10.1016/j.ajog.2003.11.029 [DOI] [PubMed] [Google Scholar]
- 9.Martin JA, Osterman MJK. Describing the increase in preterm births in the United States, 2014-2016. NCHS Data Brief 2018;312:1–8. [PubMed] [Google Scholar]
- 10.Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of child health and human development maternal fetal medicine unit network. N Engl J Med 1996;334:567–72. 10.1056/NEJM199602293340904 [DOI] [PubMed] [Google Scholar]
- 11.Heath VC, Southall TR, Souka AP, et al. Cervical length at 23 weeks of gestation: prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol 1998;12:312–7. 10.1046/j.1469-0705.1998.12050312.x [DOI] [PubMed] [Google Scholar]
- 12.To MS, Fonseca EB, Molina FS, et al. Maternal characteristics and cervical length in the prediction of spontaneous early preterm delivery in twins. Am J Obstet Gynecol 2006;194:1360–5. 10.1016/j.ajog.2005.11.001 [DOI] [PubMed] [Google Scholar]
- 13.Kindinger LM, Poon LC, Cacciatore S, et al. The effect of gestational age and cervical length measurements in the prediction of spontaneous preterm birth in twin pregnancies: an individual patient level meta-analysis. BJOG 2016;123:877–84. 10.1111/1471-0528.13575 [DOI] [PubMed] [Google Scholar]
- 14.Biggio JR. Progesterone, pessary or cerclage for preterm birth prevention in twins: no answers yet. BJOG 2017;124:1175. 10.1111/1471-0528.14551 [DOI] [PubMed] [Google Scholar]
- 15.Schuit E, Stock S, Rode L, et al. Effectiveness of progestogens to improve perinatal outcome in twin pregnancies: an individual participant data meta-analysis. BJOG 2015;122:27–37. 10.1111/1471-0528.13032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarde A, Lutsiv O, Park CK, et al. Preterm birth prevention in twin pregnancies with progesterone, pessary, or cerclage: a systematic review and meta-analysis. BJOG 2017;124:1163–73. 10.1111/1471-0528.14513 [DOI] [PubMed] [Google Scholar]
- 17.Saccone G, Rust O, Althuisius S, et al. Cerclage for short cervix in twin pregnancies: systematic review and meta-analysis of randomized trials using individual patient-level data. Acta Obstet Gynecol Scand 2015;94:352–8. 10.1111/aogs.12600 [DOI] [PubMed] [Google Scholar]
- 18.Li C, Shen J, Hua K. Cerclage for women with twin pregnancies: a systematic review and metaanalysis. Am J Obstet Gynecol 2019;220:543–57. 10.1016/j.ajog.2018.11.1105 [DOI] [PubMed] [Google Scholar]
- 19.Liem SMS, van Pampus MG, Mol BWJ, et al. Cervical pessaries for the prevention of preterm birth: a systematic review. Obstet Gynecol Int 2013;2013:1–10. 10.1155/2013/576723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liem S, Schuit E, Hegeman M, et al. Cervical pessaries for prevention of preterm birth in women with a multiple pregnancy (ProTWIN): a multicentre, open-label randomised controlled trial. Lancet 2013;382:1341–9. 10.1016/S0140-6736(13)61408-7 [DOI] [PubMed] [Google Scholar]
- 21.Goya M, de la Calle M, Pratcorona L, et al. Cervical pessary to prevent preterm birth in women with twin gestation and sonographic short cervix: a multicenter randomized controlled trial (PECEP-Twins). Am J Obstet Gynecol 2016;214:145–52. 10.1016/j.ajog.2015.11.012 [DOI] [PubMed] [Google Scholar]
- 22.Berghella V, Dugoff L, Ludmir J. Prevention of preterm birth with pessary in twins (PoPPT): a randomized controlled trial. Ultrasound Obstet Gynecol 2017;49:567–72. 10.1002/uog.17430 [DOI] [PubMed] [Google Scholar]
- 23.Nicolaides KH, Syngelaki A, Poon LC, et al. Cervical pessary placement for prevention of preterm birth in unselected twin pregnancies: a randomized controlled trial. Am J Obstet Gynecol 2016;214:3.e1–3.e9. 10.1016/j.ajog.2015.08.051 [DOI] [PubMed] [Google Scholar]
- 24.Dang VQ, Nguyen LK, Pham TD, et al. Pessary compared with vaginal progesterone for the prevention of preterm birth in women with twin pregnancies and cervical length less than 38 MM: a randomized controlled trial. Obstet Gynecol 2019;133:459–67. 10.1097/AOG.0000000000003136 [DOI] [PubMed] [Google Scholar]
- 25.Le KD, Nguyen LK, Nguyen LTM, KD L, Nguyen LTM, et al. Cervical pessary vs vaginal progesterone for prevention of preterm birth in women with twin pregnancy and short cervix: economic analysis following randomized controlled trial. Ultrasound Obstet Gynecol 2020;55:339–47. 10.1002/uog.20848 [DOI] [PubMed] [Google Scholar]
- 26.Henderson JT, Webber EM, Bean SI. Screening for asymptomatic bacteriuria in adults: updated evidence report and systematic review for the US preventive services Task force. JAMA 2019;322:1195–205. 10.1001/jama.2019.10060 [DOI] [PubMed] [Google Scholar]
- 27.ACOG practice bulletin No. 195: prevention of infection after gynecologic procedures. Obstet Gynecol 2018;131:e172–89. 10.1097/AOG.0000000000002670 [DOI] [PubMed] [Google Scholar]
- 28.Arabin B, Alfirevic Z. Cervical pessaries for prevention of spontaneous preterm birth: past, present and future. Ultrasound Obstet Gynecol 2013;42:390–9. 10.1002/uog.12540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gates S, Brocklehurst P. How should randomised trials including multiple pregnancies be analysed? BJOG 2004;111:213–9. 10.1111/j.1471-0528.2004.00059.x [DOI] [PubMed] [Google Scholar]
- 30.Adams TM, Rafael TJ, Kunzier NB, et al. Does cervical cerclage decrease preterm birth in twin pregnancies with a short cervix? J Matern Fetal Neonatal Med 2018;31:1092–8. 10.1080/14767058.2017.1309021 [DOI] [PubMed] [Google Scholar]
- 31.Houlihan C, Poon LCY, Ciarlo M, et al. Cervical cerclage for preterm birth prevention in twin gestation with short cervix: a retrospective cohort study. Ultrasound Obstet Gynecol 2016;48:752–6. 10.1002/uog.15918 [DOI] [PubMed] [Google Scholar]
- 32.Fichera A, Prefumo F, Mazzoni G, et al. The use of ultrasound-indicated cerclage or cervical pessary in asymptomatic twin pregnancies with a short cervix at midgestation. Acta Obstet Gynecol Scand 2019;98:487–93. 10.1111/aogs.13521 [DOI] [PubMed] [Google Scholar]
- 33.Nicolaides K. Randomized study of pessary versus standard management in women with increased chance of premature birth U.S. National library of medicine, 2009. Available: https://clinicaltrials.gov/ct2/show/NCT00735137?term=Nicolaides&draw=2&rank=1
- 34.Rahman RA. Randomised controlled trial comparing vaginal pessary and progestogen as an intervention in twin pregnancy with short cervical length to prevent spontaneous preterm birth U.S. National library of medicine, 2020. Available: https://clinicaltrials.gov/ct2/show/NCT04342585?term=twin&recrs=abdf&type=Intr&cond=preterm+birth&draw=2&rank=4
- 35.Di Tommaso M, Seravalli V, Arduino S, et al. Arabin cervical pessary to prevent preterm birth in twin pregnancies with short cervix. J Obstet Gynaecol 2016;36:715–8. 10.3109/01443615.2016.1148127 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-036587supp001.pdf (501.5KB, pdf)