Abstract
Background
Nivolumab is a human monoclonal antibody against programmed cell death receptor-1 (PD-1) able to rescue quiescent tumor infiltrating cytotoxic T lymphocytes (CTLs) restoring their ability to kill target cells expressing specific tumor antigen-derived epitope peptides bound to homologue human leukocyte antigen (HLA) molecules. Nivolumab is currently an active but expensive therapeutic agent for metastatic non-small cell lung cancer (mNSCLC), producing, in some cases, immune-related adverse events (irAEs). At the present, no reliable biomarkers have been validated to predict either treatment response or adverse events in treated patients.
Methods
We performed a retrospective multi-institutional analysis including 119 patients with mNSCLC who received PD-1 blockade since November 2015 to investigate the predictive role of germinal class I HLA and DRB1 genotype. We investigated the correlation among patients’ outcome and irAEs frequency with specific HLA A, B, C and DRB1 alleles by reverse sequence-specific oligonucleotide (SSO) DNA typing.
Results
A poor outcome in patients negative for the expression of two most frequent HLA-A alleles was detected (HLA: HLA-A*01 and or A*02; progression-free survival (PFS): 7.5 (2.8 to 12.2) vs 15.9 (0 to 39.2) months, p=0.01). In particular, HLA-A*01-positive patients showed a prolonged PFS of 22.6 (10.2 to 35.0) and overall survival (OS) of 30.8 (7.7 to 53.9) months, respectively. We also reported that HLA-A and DRB1 locus heterozygosis (het) were correlated to a worse OS if we considered het in the locus A; in reverse, long survival was correlated to het in DRB1.
Conclusions
This study demonstrate that class I and II HLA allele characterization to define tumor immunogenicity has relevant implications in predicting nivolumab efficacy in mNSCLC and provide the rationale for further prospective trials of cancer immunotherapy.
Keywords: B7-H1 antigen, antigen presentation, lung neoplasms, tumor biomarkers
Background
Non-small cell lung cancer (NSCLC) is the most common malignancy and the leading cause of cancer death. Chemotherapy with platinum doublets±bevacizumab, a monoclonal antibody (mAb) to the vascular endothelial growth factor (reserved for patients with non-squamous histology and low risk of bleeding only), has been for many years the standard treatment for patients in metastatic (m) and advanced stage of disease (stage IIIB-IV), a good performance status (Eastern Cooperative Oncology Group (ECOG) 0–1) and no druggable driver mutations/rearrangements of epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase/echinoderm microtubule-associated protein-like 4 (ALK/EML4) and ROS proto-oncogene 1 (ROS1).1 2
In the last few years, effector immune checkpoint blockade (ICB) with mAbs to programmed cell death receptor-1 (PD-1; nivolumab and pembrolizumab) and PD-1 ligand-1 (PD-L1; atezolizumab, avelumab and durvalumab) alone or in combination with chemotherapy, radiotherapy or bevacizumab has grown as a solid treatment for these patients with remarkable results in terms of clinical benefit, progression-free survival (PFS), and overall survival (OS) which has been observed in more than 30% of the cases.3–5 On the other hand, these immune-oncological treatments may be associated to more or less severe immune-related adverse events (irAEs) and to high costs. It is, therefore, conceivable that reliable biomarkers predictive of response and irAEs are eagerly awaited.6–8
On this basis, we led a real-world multicenter retrospective investigation aimed to evaluate the predictive role of different human leukocyte antigen (HLA) alleles in patients undergone PD-1 blockade with nivolumab by taking in consideration that: (1) these mAbs promote the rescue of cytotoxic T lymphocytes (CTLs) function silenced by the PD-1/PD-L1 or PD-L2 immune checkpoint at tumor sites,8 (2) HLA molecules play a critical role in triggering CTL-mediated tumor cell killing, T cell priming and clonal expansion,9–12 and finally, (3) there is a strong correlation between expression of specific HLA alleles and development of autoimmune disease.13
Here, we correlated the germline expression of the most frequent HLA alleles in the loci A, B, C and DRB1 (reflecting a β subunit of class II HLA-DR) with outcome and risk of iraEs in patients with mNSCLC undergone to salvage therapy with nivolumab treatment.
Methods
Patients sample, treatment and monitoring
This study is part of a retrospective real-world evidence (RWE) multi-institutional study including 119 pretreated patients with mNSCLC who had received salvage therapy with nivolumab at the Medical Oncology Unit, Grand Metropolitan Hospital “Bianchi-Melacrino-Morelli”, Reggio Calabria, Medical and Translational Oncology Unit, Magna Graecia University, Catanzaro, and Section of Radiation Oncology, University of Siena, Siena, between September 2015 and December 2018. This study included patients who had been voluntary screened for A, B, C and DRB1 HLA typing at the Tissue Typing Unit at the Grand Metropolitan Hospital in Reggio Calabria.
All patients gave an informed consent to anonymous use of their examinations for research aim. All patients received PD-1 blockade with intravenous nivolumab at the dosage of 3 mg/kg in 60 min on a biweekly schedule until progression, grade 3–4 adverse events or death. All patients had received at least a previous line of platinum-based doublet±bevacizumab prior PD-1 blockade, presented with ECOG performance status ≤1, have had complete physical examination reports, histological sampling and hematological, biochemical, immune-biological, radiological and instrumental monitoring at baseline. Clinical history, physical examination and record of adverse events were evaluated prior each drug infusion. A CT scan was performed every 3 months or in any case of suspected progressive disease and evaluated according to the immune response evaluation criteria in solid tumors (iRECIST).14 Patients were monitored for blood cell counts and biochemistry prior each treatment course and were also monitored for adrenal hormone profile, adrenocorticotropic hormone (ACTH), thyroid stimulating hormone (TSH), thyroid hormones, antithyroid autoantibodies (AAbs), extractable nuclear antigen antibodies, antinucleus antibodies, antismooth cells antibodies and c/p- antineutrophil cytoplasmic antibodies on monthly bases since the beginning of treatment.
HLA molecular typing of the loci A, B, C, and DRB1 was centralized and carried out in the Tissue Typing Unit at the Grand Metropolitan Hospital in Reggio Calabria by performing low-medium-resolution reverse SSO DNA typing assays (One-Lambda Luminex Technology LABScan 100, HLA Fusion Software) on genomic DNA extracted by whole blood or peripheral blood mononuclear cells according to the kit manufactures. The HLA allele frequency of the studied loci A, B, C and DRB1 in our patients’ cohorts was obtained by comparing the results with our database bank including 3500 healthy bone marrow donors of the Calabrian National Registry.
Survival analysis
PFS and OS were analyzed with the Kaplan-Meier method and significance of survival differences were analyzed by the log-rank test. Median survival and 95% CIs were reported. HRs and their 95% CIs were estimated through the Cox regression proportional model; in the multivariate approach, a forward stepwise procedure was used and enter and remove limit set to 0.05 and 0.10, respectively. Association between irAEs and clinical and biological factors was assessed by the χ2 test. Our statistical analyses were performed by the SPSS software V.23.0.
Results
Patients’ features
Our analysis included a cohort of 119 patients with mNSCLC, 99 males and 20 females with median age of 68 years. Forty-two presented with a squamous and 77 with a non-squamous histology. Ninety-one patients had received a front-line doublet chemotherapy±bevacizumab prior immunotherapy, 27 a platinum-based metronomic chemotherapy and 1 did not receive chemotherapy. Fifty-seven patients had also received palliative radiotherapy (25–30 Gy) on symptomatic single lesions (mediastinum, nodes, soft tissue, bone or brain) prior or at beginning of nivolumab treatment.
Predictive values of clinical parameters
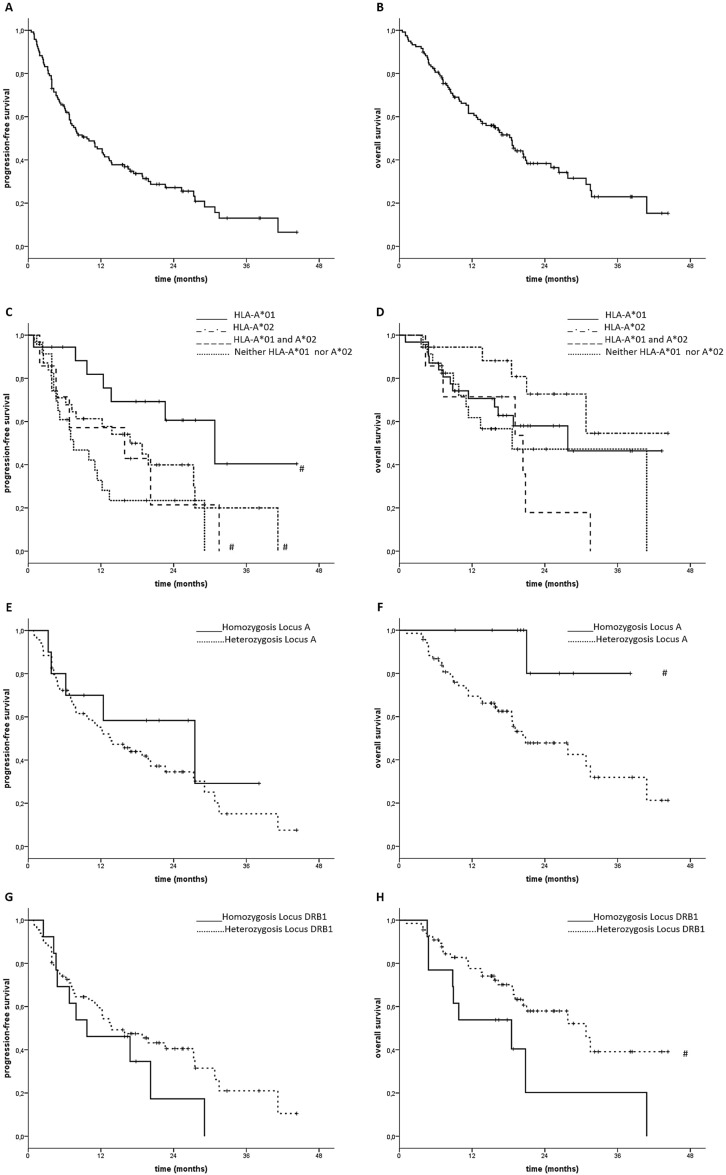
Patients included in the retrospective study presented a median follow-up of 22 months with a median PFS and OS of 9.7 (6.1 to 13.3) and 18.5 (14.6 to 22.4) months, respectively (figure 1A, B).
Figure 1.
Progression-free survival (PFS) and overall survival (OS). (A, B) PSF and OS of patients with metastatic non-small cell lung cancer (mNSCLC) subjected to nivolumab treatment. (C, D) PFS and OS of the same patients with mNSCLC with and without germinal expression of human leukocyte antigen (HLA)-A*01 and or A*02. (E, F) PFS and OS of patients with mNSCLC with and without germinal heterozygosis in class I HLA-A locus. (G, H) PFS and OS of patients with mNSCLC with and without germinal heterozygosis in DRB1 locus. (#): differences statistically significant p<0.05.
IrAEs were reported in 59 patients and mainly consisted in grade 1–2 cutaneous rash, polyarthritis and thyroiditis, generally occurring after 3–4 treatment courses. Autoimmune pneumonitis occurred in five patients among the third and sixth month of treatment and additional three cases of uveitis recorded after 2 and 3 months of treatment.
HLA typing
In this patient population we detected the two most frequent germline alleles for each class I HLA and DRB1 locus and, then, we correlated their expression with both outcome and irAEs frequency. The most frequent alleles resulted A*02 (38 pts) and A*01 (25 pts) for the locus A; B*18 (20 pts) and B*35 (20 pts) for the locus B; C*07 (43 pts) and C*04 (27 pts) for the locus C and DRB1*11 (36 pts) and DRB1*03 (20 pts) for DRB1. In our analysis, we also included allele HLA-B*51 (17 pts) for its known correlation with common autoimmune diseases.15 Allele homozygosis for the loci A, B, C and DRB1 was detected in 10, 4, 11 and 13 patients, respectively. These results were in line with the HLA frequency recorded in healthy bone marrow donors in the Calabrian Regional Registry, representative of the healthy population in our geographical area.
Statistical correlations: patients’ outcome and the most frequent alleles for HLA-A, B, C and DRB1
A poor outcome treatment response was observed in patients not expressing either HLA-A*02 and/or HLA-A*01 alleles (PFS: 7.5 (2.8 to 12.2) vs 15.9 (0 to 39.2) months, p=0.01 with a trend to worse OS of 13.4 (95% CI not evaluable) months vs 20.4 (6.1 to 34.7) and months, p=0.1; figure 1C, D). In this context, HLA-A*01 positive patients showed the best outcome with a PFS and OS of 22.6 (10.2 to 35.0) and 30.8 (7.7 to 53.9) months, respectively. We also found a trend to a worse outcome in patients expressing the HLA-C*07 allele (C*07 positive vs negative; PFS=12.2 (9.3 to 15.1) vs 29.1 (7.2 to 51.0) months p=0.09). Percentage of 1-year PFS rate and 1/2-year survival rate presented in table 1 showed valuable, though not statistically significant, difference among the groups reporting outstanding 1/2-year survivals in patients showing HLA-A*01 (87.5% /55.9%), HLA-B*51 (82.4%/74.1%) and DRB1*03 (83.8%/58.7%) alleles.
Table 1.
Clinical features, 1-year progression-free survival rate and 1/2-year survival rate for each patient cohort expressing the most frequent alleles in class I human leukocyte antigen (HLA) A, B and C and DRB1
| HLA allele | Num (%) | Sex: male/ female |
Histology: squamous/non-squamous | IrAEs frequency | One-year progression-free survival rate | Two-year survival rate |
| A*02 | 39 (41.4%) | 33/6 | 12/27 | (19) 48.7% | 57.6% | 47.7% |
| A*01 | 28 (29.8%) | 24/4 | 10/18 | (12) 42.8% | 74.6% | 55.9% |
| B*18 | 25 (26.5%) | 23/2 | 11/14 | (8) 32.0% | 50.0% | 46.7% |
| B*35 | 24 (25.5%) | 21/3 | 8/16 | (10) 41.6% | 58.4% | 39.0% |
| B*51 | 19 (20.2%) | 14/5 | 5/14 | (10) 52% | 57.0% | 74.1% |
| C*04 | 31 (32.9%) | 26/5 | 9/22 | (13) 41.9% | 55.0% | 46.4% |
| C*07 | 47 (50%) | 39/8 | 15/32 | (22) 46.0% | 61.4% | 53.1% |
| DRB1*03 | 24 (25.5%) | 17/7 | 8/16 | (10) 41.6% | 62.9% | 52.6% |
| DRB1*11 | 43 (45.7%) | 37/6 | 16/27 | (17) 38.8% | 59.1% | 58.7% |
Patients expressing HLA-B*35 showed severe and frequent immune-related adverse events (irAEs) and the worse 2-year survival rate.
Num, numerosity of the patients in each group.
Statistical correlations: patients’ outcome and heterozygosis in HLA-A, B, C and DRB1 loci
Subsequently, we evaluated whether heterozygosis (het) in HLA-A, B, C and DRB1 loci could have a predictive value on patients’ outcome. First, we did not find any correlation between het and PFS (data not shown). Completely different results were instead recorded for what concerns the influence of het of HLA-A and DRB1 genes on patients’ OS. Het in the locus A was indeed correlated to a worse OS (no-het vs het=more than 40 (median not yet reached) vs 20.8 (11.4 to 30.2) months; p=0.03; figure 1E, F) while on the contrary, het in DRB1 was correlated to a prolonged survival (no het vs het=18.5 (4.6 to 32.4) vs 30.8 (20.4 to 41.2) months, p=0.05; figure 1G, H). Finally, patients showing het in the loci B and C showed a trend to prolonged survival that did not achieve statistical significance (data not shown). Due to the small patients’ sample, it was not possible to find any correlation among survival and heterozygosis in specific locus alleles.
We also performed an additional analysis on 375 patients with mNSCLC who received PD-1/PD-L1 blockade, extrapolated by a recent survey published by Chowell et al (NIHMS980063-table S1),16 and those included in our series, correlating their OS with heterozygosis in at least one among class I HLA-A, B or C loci (figure 2A) and those bearing selective heterozygosis in the locus A (figure 2B). In the first case, our analysis failed to demonstrate any statistically significant difference in both patients’ groups (from our study, Italy, ITA and from Chowell’s study, National Institutes of Health; figure 2A; p=0.39 and p=0.12). Similarly, we were unable to demonstrate a significant difference in survival correlated with HLA-A heterozygosis in the patients’ population examined in the Chowell’s study considering that patients not presenting HLA-A heterozygosis (53 out of 375 pts) showed a twice shorter follow-up compared with the other cohort and their median of survival was not achieved yet (figure 2B).
Figure 2.
Survival of 375 patients with metastatic non-small cell lung cancer subjected to programmed cell death receptor-1 (PD-1)/PD-1 ligand-1 blockade included in the database published by Chowell et al (NIHMS980063-table s1; group National Institutes of Health (NIH)).16 (A) Patients presenting homozygosis in at least one class I human leukocyte antigen (HLA) locus (NIH-no-het) versus full heterozygosis (NIH-het). (B) Patients presenting homozygosis versus heterozygosis in HLA-A showing no statistically significant differences (p>0.1). Similar results were observed in our patients’ series (group-IT) for what concerns homozygosis in at least one class I HLA locus (IT-no-het) versus full heterozygosis (IT-het) while significant differences in survival were observed, when the overall survival of patients with homozygosis and heterozygosis in HLA-A locus was compared (p=0.03).
Multivariate analysis: patients’ outcome, gender, irAEs’ frequency, AAb rise, baseline inflammatory status, HLA A, B, C and DRB1 alleles and heterozygosis
Our univariate analysis showed that a prolonged PFS was correlated with occurrence of irAEs, low baseline erythrocyte sedimentation rate values, type of chemotherapy (metronomic chemotherapy±bevacizumab), presence of allele A*01 and, in general, haplotype A*01 and/or A*02. When independent factors among these were investigated, only occurrence of irAEs and expression of A*01 and/or A*02 retained significance (table 2). Differently, a prolonged OS was correlated with the presence of irAEs, low baseline C-reactive protein value, chemotherapy type (metronomic chemotherapy±bevacizumab), no heterozygosis in locus A and heterozygosis in DRB1. When independent factors were investigated, only no heterozygosis in locus A retained significance (table 3).
Table 2.
Univariate and multivariate analyses of progression-free survival (PFS) in relation to immune-related adverse events (irAEs), low baseline erythrocyte sedimentation rate (ESR) values, type of chemotherapy, presence of allele A*01 and haplotype A*01 and/or A*02
| PFS | ||
| Univariate | Multivariate | |
| Gender (M vs F) | 0.63 (0.37 to 1.06) p=0.08 | |
| Histology (squamous vs non-squamous) | 1.07 (0.69 to 1.669) p=0.77 | |
| Age (≥68 vs <68 years) | 0.98 (0.65 to 1.50) p=0.94 | |
| Radiotherapy (yes vs no) | 1.13 (0.74 to 1.72) p=0.57 | |
| TKI (yes vs no) | 0.76 (0.45 to 1.29) p=0.31 | |
| irAEs (yes vs no) | 0.48 (0.32 to 0.74) p=0.001 * | 0.34 (0.15 to 0.78) p=0.01* |
| NLR (≥3 vs <3) | 1.51 (0.96 to 2.37) p=0.07 | |
| CRP (≥7 vs <7) | 1.58 (0.92 to 2.73) p=0.10 | |
| ESR (≥39 vs <39) | 1.88 (1.03 to 3.43) p=0.04* | |
| LDH (≥400 vs <400) | 0.87 (0.54 to 1.41) p=0.58 | |
| Type of chemotherapy (Platinum-based vs other) |
1.73 (1.10 to 2.70) p=0.02* | |
| Allele A01 (yes vs no) | 0.51 (0.27 to 0.96) p=0.04* | |
| A01_A02 haplotype | p=0.02* | p=0.04* |
| (A02 vs no A01 or A02) | 0.57 (0.30 to 1.10) | 0.82 (0.36 to 1.86) |
| (A01 vs no A01 or A02) | 0.25 (0.10 to 0.61) | 0.05 (0.01 to 0.39) |
| (A01 & A02 vs no A01 or A02) | 0.72 (0.28 to 1.83) | 1.29 (0.32 to 5.16) |
HR and its 95% CIs are reported. Data from human leukocyte antigen analysis are considered only if correlated with the endpoint of PFS.
CRP, C-reactive protein; LDH, lactate dehydrogenase; NLR, neutrophil–lymphocyte ratio; TKI, tyrosine kinase inhibitor.
Table 3.
Univariate and multivariate analyses of overall survival (OS) in relation to immune-related adverse events (irAEs), low baseline erythrocyte sedimentation rate (ESR) values, type of chemotherapy, presence of allele A*01 and haplotype A*01 and/or A*02
| OS | ||
| Univariate | Multivariate | |
| Gender (M vs F) |
0.63 (0.36 to 1.12) p=0.12 | |
| Histology (squamous vs non-squamous) |
1.07 (0.66 to 1.74) p=0.79 | |
| Age (≥68 vs <68 years) |
1.11 (0.69 to 1.78) p=0.67 | |
| Radiotherapy (yes vs no) |
1.11 (0.69 to 1.77) p=0.67 | |
| TKI (yes vs no) |
0.92 (0.53 to 1.60) p=0.76 | |
| irAEs (yes vs no) |
0.49 (0.30 to 0.80) p=0.004* | |
| NLR (≥3 vs <3) |
1.59 (0.96 to 2.63) p=0.07 | |
| CRP (≥7 vs <7) |
1.98 (1.08 to 3.64) p=0.03* | |
| ESR (≥39 vs <39) |
1.81 (0.91 to 3.60) p=0.09 | |
| LDH (≥400 vs <400) |
0.76 (0.44 to 1.33) p=0.34 | |
| Type of chemotherapy (Platinum-based vs other) |
1.66 (1.02 to 2.72) p=0.04* | |
| Heterozygosis locus A (yes vs no) |
6.78 (0.93 to 49.6) p=0.06* | 6.78 (0.93 to 49.6) p=0.06* |
| Heterozygosis locus DRB1 (yes vs no) |
0.46 (0.21 to 0.99) p=0.05* | |
HR and its 95% CIs are reported. Data from human leukocyte antigen analysis are considered only if correlated with the endpoint of OS.
CRP, C-reactive protein; LDH, lactate dehydrogenase; NLR, neutrophil–lymphocyte ratio; TKI, tyrosine kinase inhibitor.
Discussion
This retrospective study was carried out in patients with mNSCLC who received a real-world salvage therapy with nivolumab. The results of the study fulfilled the primary endpoint to investigate the possible correlations among patients’ outcome and irAEs frequency with the germinal expression of multiple class I HLA and DRB1 loci. Variability of class I HLA genes has been related to ICB response in a number of malignancies including melanoma and colorectal cancer.16 17 The association between autoimmunity and antitumoral effect induced by immunotherapy is revealed by IRAEs that more strongly emerge after the treatment with anti-PD-1 and anti-PD-L1 than in response to anti-cytotoxic T-lymphocyte protein 4(CTLA-4) antibody.18 Then, it could be useful to study HLA genetic variation in order to define biomarker of response to ICIs in terms of benefit and adverse events. The rationale of the study is based on the evidence that HLA molecules play a critical role in engaging an antigen-specific immune response acting both on primary cross-priming and peripheral T cell effector response. The results of the present study indeed showed that the PFS of these patients was strongly correlated to their germinal expression of HLA-A*01 and/or A*02 alleles, the most frequent class I HLA-A allele in the Caucasian population and Mediterranean geographical area.19 On the contrary, patients expressing less frequent class I A allele and not A*01 and A*02 showed a very poor prognosis. We also showed that the expression of homozygosis in the locus A (mainly represented by A*01 and A*02 alleles) correlated with their OS confirming the critical role for these molecules in promoting an efficient and prolonged anticancer immune response in patients subjected to PD-1 blockade. In our patient population, however, the best survival was observed in those patients presenting germinal heterozygosis in the locus DRB1 and, therefore, class II HLA-DRB1 molecules, which, differently from class I HLA, are not directly involved in the CTL-mediated killing of tumor cells enforced by PD-1 blockade, but rather to cross priming, T and B cell modulation20 and autoimmunity.21 A recent study showed that no significant correlations were observed for HLA class I zygosity and PFS or OS in three independent cohorts, and tumor genomic characteristics, such as EGFR and ALK alterations, should take in consideration to determine benefit from ICB in NSCLC. Moreover, the authors suggest that decreased expression of MHC class I and antigen presentation through MHC class II should be evaluated in NSCLC to predict response to ICB.22 In this context, our analysis confirmed the previous finding that occurrence of irAEs, AAbs rise, a low baseline inflammatory status and the kind of chemotherapy regimen received prior PD-1 blockade were all correlated to patients’ survival.23 Class II HLA molecules alike HLA-DRB1 bind peptides recognized by T cell receptors (TCRs) on CD4+ T cells with helper and/or T regulatory function; thus, they can modulate the differentiation and the proliferation of other lymphocyte subsets including CTLs and B cells.20 We believe that the occurrence of autoimmunity in these patients, in line with the results of other immunological studies, is consequential to the cross priming of antigens released from the tumor site in an immunological context of CTL mediated immune rejection6 24 25 whose cross priming may be facilitated and redirected to the B cell compartment by the germline expression of specific and multiple class II HLA alleles.
All together the results of our study are in line with the knowledge that mAbs to PD-1, such as nivolumab, exert their antitumor effects by reactivating tumor-specific CTLs26 whose existence largely precedes the diagnosis and the immunological treatment and that are consequence of a long-lasting immune response to multiple tumor-associated (TAA) and tumor-specific antigens (TSA).12 27
The ultimate effectors of PD-1 blockade are primed CTLs able to recognize target cells with a specific TCR which, in turn, binds TAA/TSA derived 9–10 mere-peptides (epitopes) bound to homologous class I HLA molecules on their surface. On the other hand, T cells priming and expansion occur within specialized lymphoid organs where T cell precursors use their TCRs to recognize peptide epitopes/class I–II HLA complexes on antigen-presenting cells able to uptake and process antigenic material released in the blood and lymphatic stream by the tumor tissues.9–12 Epitope peptide binding to HLA molecules occurs in the cytoplasm operated by the proteasome system, and it is constrained by definite HLA allele/locus specific amino acid consensus motifs. The latter have been largely described in the literature and specific network algorithms have already been defined to detect epitopic sequences and their potential binding affinity for specific HLA alleles by scanning the amino acid sequence of viral and tumor-derived protein antigens.28–30 A large number of dominant immunogenic epitope peptides from various TAA/TSA and viruses specific for the most frequent class I HLA alleles have already been identified using these specific algorhythms. Many of these peptides have been synthesized and tested in several studies worldwide.31 32 Some of these peptides derived from prostate-specific antigen, carcinoembryonic antigen, human telomerases and thymidilate synthase have also been evaluated in clinical trials of active specific immunotherapy in patients with cancer obtaining contrasting results.31–38 Moreover, the sequence divergence between alleles of HLA-I genotype is related to the increased probability of neoantigen recognition by tumor-infiltrating T cells and subsequently ability of different individual immune system response to checkpoint blockade immunotherapy.39 The main lesson from these studies was that protein antigens present one or more epitope sequence with class I/II HLA allele-specific binding motifs and that both their number and binding affinity for different HLA alleles and TCRs are not uniform. It is consequential that patients with different class I and II HLA germinal haplotypes present divergent immune reactivity to the tumor due to a completely different pool of epitopes available for immune priming, CTL recognition and tumor cell killing even in the presence of the same target antigens. Several studies have already shown that gene and/or upstream downregulation and/or one or more HLA allele and antigen-peptide processing in tumor cells are effective mechanisms of tumor immune escape and resistance to immune checkpoint in patients with cancer.40 41 Another study supports the hypothesis that an allelic variant of killer cell immunoglobulin-like receptor (KIR), KIR3DS1, mainly expressed on natural killer (NK) cells, is associated to resistance to PD-1 blockade in lung cancer.42 The recent results of a large survey by Chowell et al, including a pool of 1535 patients with advanced cancer receiving CTLA-4 and/or PD-1/PD-L1 blockade, showed that germinal homozygosis in at least one of the three class I HLA locus (A, B and C) were predictive of a poor survival.16 These results were in line with our findings concerning heterozygosis in the B, C and DRB1 loci, whereas apparently in contrast with the finding that class I HLA-A locus heterozygosis was correlated to a worse outcome in our series. This difference could be simply explained considering that they do not focus on class I HLA-A and on the other loci separately; additionally, their analysis includes patients with various malignancies with different prognosis and treatment responsiveness and finally, they pool together, patients who received anti-CTLA-4 and anti-PD-1/PD-L1 immune checkpoints inhibitors that act on different phases of the immune response and present a non-homogenous range of activity. Therefore, we performed an additional analysis on the 375 patients with mNSCLC who received PD-1 blockade and included in Chowell’s survey (supplemental NIHMS980063_table_S1)16 finding no outcome differences correlated with class I A, B and C allele heterozygosis. In the same patients’ series, we were unable to extrapolate significant data on the outcome of patients with heterozygosis in the locus A, considering the following: (1) patients with HLA-A locus homozygosis (53 out of 375) showed a twice shorter follow-up; (2) they have not achieved the median survival when the data were collected (before January 2018); (3) the two groups had not received a uniform immunological treatment.
Conclusions
All together these findings suggest that the different response to nivolumab observed in patients with less frequent class I HLA-A alleles might be a consequence of a divergent CTL response, whose entity is dependent on the different amount of high affinity epitope peptides (epitope repertoire) bound to their specific class I HLA profile on the locus A. It is, therefore, possible to speculate that patients with a germline expression of the most frequent class I HLA-A alleles in a specific geographical area (A*02 and/or A*01) also present a more appropriate CTL epitope peptide repertoire aimed to protect them in a specific environment.
If these data should be confirmed on a large series, it is reasonable to propose the combined use of ICB and allele-specific anticancer vaccines in patients who failed to rise a spontaneous tumor antigen-specific immune response. Recent findings concerning the application of PD-1/PD-L1 blockers in combination with front-line chemotherapy in patients with advanced NSCLC compared with the same chemotherapy doublet alone were partially sorrowed by the occurrence of a high risk of early deaths (within the first 60 days of treatment) due to severe immunological disorders. Even though, it is possible to speculate that immune system over-stimulation combining danger signals and massive antigen release consequent to secondary cytotoxic agents (anticancer drugs/radiotherapy/antiangiogenic agents) might be detrimental in patients who already show an inappropriate immune response.43 44 On these bases, we believe that an accurate multimodal definition of tumor immunogenicity at baseline as well as a germline class I and II HLA allele characterization deserves to be studied on a large scale in these patients in a contest of RWE in order to define not only responsive patients but also patients with high risk of lethal irAEs and to propose new treatment modalities combining immune checkpoint inhibitors and class I HLA allele-specific anticancer vaccines.
Acknowledgments
The authors wish to thank patients and their families for their participation to the study and the ARCO patients’ association for the precious fundraising support to the study.
Footnotes
Contributors: PC, DG, MC, PT and PT prepared the manuscript and the figures. PC, RG, RA, AS, AF, PP, PT, PT and PT clinically evaluated patients enrolled for the study. RES, NI, VA and GR performed the HLA typing. DG performed statistical analysis. NS, TDG, DC, VB, VN, PP and PT contributed to the experimental design. MA, AG, AN, EA and LP improved the discussion of the results. AL, MC and PC revised the manuscript. All authors read and approved the final manuscript.
Funding: This work has been supported by the NSP project nos. LO1508 and LO1309, MZ-VES project nos. 16-28637A, 16-2960A, and 17-32285A, and by funds institutional research (TA 29) of UVPS Brno.
Competing interests: None declared.
Patient consent for publication: Obtained.
Ethics approval: All procedures were undertaken in compliance with the ethical statements of the Helsinki Declaration (1964, amended most recently in 2008) of the World Medical Association and respect of their privacy. The protocol and any amendments were reviewed and approved by an Institutional Review Board/Independent Ethics Committee prior to initiation of the study. All patients provided voluntary written informed consent.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1. Pilkington G, Boland A, Brown T, et al. A systematic review of the clinical effectiveness of first-line chemotherapy for adult patients with locally advanced or metastatic non-small cell lung cancer. Thorax 2015;70:359–67. 10.1136/thoraxjnl-2014-205914 [DOI] [PubMed] [Google Scholar]
- 2. Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet 2017;389:299–311. 10.1016/S0140-6736(16)30958-8 [DOI] [PubMed] [Google Scholar]
- 3. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–64. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Horn L, Spigel DR, Vokes EE, et al. Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol 2017;35:3924–33. 10.1200/JCO.2017.74.3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xia L, Liu Y, Wang Y. PD‐1/PD‐L1 blockade therapy in advanced Non‐Small‐Cell lung cancer: current status and future directions. Oncologist 2019;24:31–41. 10.1634/theoncologist.2019-IO-S1-s05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Day D, Hansen AR. Immune-Related adverse events associated with immune checkpoint inhibitors. BioDrugs 2016;30:571–84. 10.1007/s40259-016-0204-3 [DOI] [PubMed] [Google Scholar]
- 7. Weber JS, Yang JC, Atkins MB, et al. Toxicities of immunotherapy for the practitioner. J Clin Oncol 2015;33:2092–9. 10.1200/JCO.2014.60.0379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Topalian SL, Taube JM, Anders RA, et al. Mechanism-Driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 2016;16:275–87. 10.1038/nrc.2016.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wölfel T, Klehmann E, Müller C, et al. Lysis of human melanoma cells by autologous cytolytic T cell clones. Identification of human histocompatibility leukocyte antigen A2 as a restriction element for three different antigens. J Exp Med 1989;170:797–810. 10.1084/jem.170.3.797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hunt DF, Henderson RA, Shabanowitz J, et al. Characterization of peptides bound to the class I MHC molecule HLA-A2.1 by mass spectrometry. Science 1992;255:1261–3. 10.1126/science.1546328 [DOI] [PubMed] [Google Scholar]
- 11. Crowley NJ, Darrow TL, Quinn-Allen MA, et al. MHC-restricted recognition of autologous melanoma by tumor-specific cytotoxic T cells. Evidence for restriction by a dominant HLA-A allele. J Immunol 1991;146:1692–9. [PubMed] [Google Scholar]
- 12. McDonnell AM, Robinson BWS, Currie AJ. Tumor antigen cross-presentation and the dendritic cell: where it all begins? Clin Dev Immunol 2010;2010:1–9. 10.1155/2010/539519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Simmonds MJ, Gough SCL. Genetic insights into disease mechanisms of autoimmunity. Br Med Bull 2004;71:93–113. 10.1093/bmb/ldh032 [DOI] [PubMed] [Google Scholar]
- 14. Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017;18:e143–52. 10.1016/S1470-2045(17)30074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guasp P, Lorente E, Martín-Esteban A, et al. Redundancy and complementarity between ERAP1 and ERAP2 revealed by their effects on the Behcet's disease-associated HLA-B*51 peptidome. Mol Cell Proteomics 2019;18:1491–510. 10.1074/mcp.RA119.001515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chowell D, Morris LGT, Grigg CM, et al. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science 2018;359:582–7. 10.1126/science.aao4572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tran E, Robbins PF, Lu Y-C, et al. T-Cell transfer therapy targeting mutant KRAS in cancer. N Engl J Med 2016;375:2255–62. 10.1056/NEJMoa1609279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Das S, Johnson DB. Immune-Related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer 2019;7:306. 10.1186/s40425-019-0805-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nunes JM, Buhler S, Roessli D, et al. The HLA-net GENE[RATE] pipeline for effective HLA data analysis and its application to 145 population samples from Europe and neighbouring areas. Tissue Antigens 2014;83:307–23. 10.1111/tan.12356 [DOI] [PubMed] [Google Scholar]
- 20. Mangalam AK, Taneja V, David CS. Hla class II molecules influence susceptibility versus protection in inflammatory diseases by determining the cytokine profile. J Immunol 2013;190:513–9. 10.4049/jimmunol.1201891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum 1987;30:1205–13. 10.1002/art.1780301102 [DOI] [PubMed] [Google Scholar]
- 22. Negrao MV, Lam VK, Reuben A, et al. Pd-L1 expression, tumor mutational burden, and cancer gene mutations are stronger predictors of benefit from immune checkpoint blockade than HLA class I genotype in non-small cell lung cancer. J Thorac Oncol 2019;14:1021–31. 10.1016/j.jtho.2019.02.008 [DOI] [PubMed] [Google Scholar]
- 23. Giannicola R, D'Arrigo G, Botta C, et al. Early blood rise in auto-antibodies to nuclear and smooth muscle antigens is predictive of prolonged survival and autoimmunity in metastatic-non-small cell lung cancer patients treated with PD-1 immune-check point blockade by nivolumab. Mol Clin Oncol 2019;11:81–90. 10.3892/mco.2019.1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Attia P, Phan GQ, Maker AV, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol 2005;23:6043–53. 10.1200/JCO.2005.06.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang W, Green M, Rebecca Liu J. CD8+ T Cells in Immunotherapy, Radiotherapy, and Chemotherapy : Zitvogel L, Kroemer G, Oncoimmunology: a practical guide for cancer immunotherapy. Cham: Springer International Publishing, 2018: 23–39. [Google Scholar]
- 26. Kumar P, Bhattacharya P, Prabhakar BS. A comprehensive review on the role of co-signaling receptors and Treg homeostasis in autoimmunity and tumor immunity. J Autoimmun 2018;95:77–99. 10.1016/j.jaut.2018.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gross G, Margalit A. Targeting tumor-associated antigens to the MHC class I presentation pathway. Endocr Metab Immune Disord Drug Targets 2007;7:99–109. 10.2174/187153007780832064 [DOI] [PubMed] [Google Scholar]
- 28. Falk K, Rötzschke O, Stevanović S, et al. Allele-Specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature 1991;351:290–6. 10.1038/351290a0 [DOI] [PubMed] [Google Scholar]
- 29. Parker KC, Bednarek MA, Coligan JE. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J Immunol 1994;152:163–75. [PubMed] [Google Scholar]
- 30. Rammensee H, Bachmann J, Emmerich NP, et al. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics 1999;50:213–9. 10.1007/s002510050595 [DOI] [PubMed] [Google Scholar]
- 31. Parmiani G, Russo V, Maccalli C, et al. Peptide-Based vaccines for cancer therapy. Hum Vaccin Immunother 2014;10:3175–8. 10.4161/hv.29418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Correale P, Botta C, Ciliberto D, et al. Immunotherapy of colorectal cancer: new perspectives after a long path. Immunotherapy 2016;8:1281–92. 10.2217/imt-2016-0089 [DOI] [PubMed] [Google Scholar]
- 33. Correale P, Walmsley K, Nieroda C, et al. In vitro generation of human cytotoxic T lymphocytes specific for peptides derived from prostate-specific antigen. J Natl Cancer Inst 1997;89:293–300. 10.1093/jnci/89.4.293 [DOI] [PubMed] [Google Scholar]
- 34. Tsang KY, Zhu M, Nieroda CA, et al. Phenotypic stability of a cytotoxic T-cell line directed against an immunodominant epitope of human carcinoembryonic antigen. Clin Cancer Res 1997;3:2439–49. [PubMed] [Google Scholar]
- 35. Cusi MG, Botta C, Pastina P, et al. Phase I trial of thymidylate synthase poly-epitope peptide (TSPP) vaccine in advanced cancer patients. Cancer Immunol Immunother 2015;64:1159–73. 10.1007/s00262-015-1711-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Correale P, Botta C, Martino EC, et al. Phase Ib study of poly-epitope peptide vaccination to thymidylate synthase (TSPP) and GOLFIG chemo-immunotherapy for treatment of metastatic colorectal cancer patients. Oncoimmunology 2016;5:e1101205. 10.1080/2162402X.2015.1101205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Correale P, Botta C, Staropoli N, et al. Systemic inflammatory status predict the outcome of K-ras WT metastatic colorectal cancer patients receiving the thymidylate synthase poly-epitope-peptide anticancer vaccine. Oncotarget 2018;9:20539–54. 10.18632/oncotarget.24993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kotsakis A, Vetsika E-K, Christou S, et al. Clinical outcome of patients with various advanced cancer types vaccinated with an optimized cryptic human telomerase reverse transcriptase (TERT) peptide: results of an expanded phase II study. Ann Oncol 2012;23:442–9. 10.1093/annonc/mdr396 [DOI] [PubMed] [Google Scholar]
- 39. Chowell D, Krishna C, Pierini F, et al. Evolutionary divergence of HLA class I genotype impacts efficacy of cancer immunotherapy. Nat Med 2019;25:1715–20. 10.1038/s41591-019-0639-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Seliger B, Ferrone S. Hla class I antigen processing machinery defects in cancer Cells-Frequency, functional significance, and clinical relevance with special emphasis on their role in T cell-based immunotherapy of malignant disease. Methods Mol Biol 2055;2020:325–50. [DOI] [PubMed] [Google Scholar]
- 41. Respa A, Bukur J, Ferrone S, et al. Association of IFN-gamma signal transduction defects with impaired HLA class I antigen processing in melanoma cell lines. Clin Cancer Res 2011;17:2668–78. 10.1158/1078-0432.CCR-10-2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Trefny MP, Rothschild SI, Uhlenbrock F, et al. A variant of a killer cell immunoglobulin-like receptor is associated with resistance to PD-1 blockade in lung cancer. Clin Cancer Res 2019;25:3026–34. 10.1158/1078-0432.CCR-18-3041 [DOI] [PubMed] [Google Scholar]
- 43. Ackermann CJ, Reck M, Paz-Ares L, et al. First-Line immune checkpoint blockade for advanced non-small-cell lung cancer: travelling at the speed of light. Lung Cancer 2019;134:245–53. 10.1016/j.lungcan.2019.06.007 [DOI] [PubMed] [Google Scholar]
- 44. Mulkey F, By K, Theoret MR, et al. Analysis of early mortality in randomized clinical trials evaluating anti-PD-1/PD-L1 antibodies: a systematic analysis by the United States food and drug administration (FDA). JCO 2019;37:2516 10.1200/JCO.2019.37.15_suppl.2516 [DOI] [Google Scholar]