Abstract
Background
While prophylactic human papillomavirus (HPV) vaccines will certainly reduce the incidence of HPV-associated cancers, these malignancies remain a major health issue. PDS0101 is a liposomal-based HPV therapeutic vaccine consisting of the immune activating cationic lipid R-DOTAP and HLA-unrestricted HPV16 peptides that has shown in vivo CD8+ T cell induction and safety in a phase I study. In this report, we have employed the PDS0101 vaccine with two immune modulators previously characterized in preclinical studies and which are currently in phase II clinical trials. Bintrafusp alfa (M7824) is a first-in-class bifunctional fusion protein composed of the extracellular domains of the transforming growth factor-β receptor type II (TGFβRII) fused to a human IgG1 monoclonal antibody blocking programmed cell death protein-1 ligand (PDL1), designed both as a checkpoint inhibitor and to bring the TGFβRII ‘trap’ to the tumor microenvironment (TME). NHS-interleukin-12 (NHS-IL12) is a tumor targeting immunocytokine designed to bring IL-12 to the TME and thus enhance the inflammatory Th1 response.
Methods
We employed TC-1 carcinoma (expressing HPV16 E6 and E7 and devoid of PDL1 expression) in a syngeneic mouse model in monotherapy and combination therapy studies to analyze antitumor effects and changes in immune cell types in the spleen and the TME.
Results
As a monotherapy, the PDS0101 vaccine generated HPV-specific T cells and antitumor activity in mice bearing HPV-expressing mEER oropharyngeal and TC-1 lung carcinomas. When used as a monotherapy in the TC-1 model, NHS-IL12 elicited antitumor effects as well as an increase in CD8+ T cells in the TME. When used as a monotherapy, bintrafusp alfa did not elicit antitumor effects or any increase in T cells in the TME. When all three agents were used in combination, maximum antitumor effects were observed, which correlated with increases in T cells and T-cell clonality in the TME.
Conclusion
These studies provide the rationale for the potential clinical use of combinations of agents that can (1) induce tumor-associated T-cell responses, (2) potentiate immune responses in the TME and (3) reduce immunosuppressive entities in the TME.
Keywords: immunotherapy; genital Neoplasms, female; head and neck neoplasms; therapies, investigational; vaccination
Introduction
Human papillomavirus (HPV) infections are widespread, and a significant cause of cancer worldwide.1 There are over 200 strains of HPV, which are classified into ‘low-risk’ and ‘high-risk’ types.2 Low-risk HPV infections typically result in benign warts that resolve without treatment; however, high-risk HPV infections can lead to cellular dysplasia. While many high-risk papillomavirus infections will resolve on their own within 12–24 months, some long-term infections that continue without resolution will result in epithelial cell dysplasia and can progress to cancer of the cervix, vulva, penis, oropharyngeal cavity and anal cavity.2 The number of cases of HPV-associated malignancies in the USA is 44 000 annually, of which 25 000 are female and 19 000 are male.3 The burden of HPV infection and subsequent malignancy is higher globally, resulting in about 630 000 cases annually.1 The current standard of care for HPV-positive malignancies is surgical resection, chemotherapy and radiation,4 but many carcinomas will recur.
The development of bivalent and quadrivalent prophylactic vaccines against high-risk HPV types 16 and 18 represents an important advance in combating HPV-positive malignancies by reducing the prevalence of HPV infection,5 which has the potential to decrease the HPV-associated cancer burden. Further progress on the 9-valent vaccine, covering low-risk HPV 6 and 11, and high-risk HPV 16, 18, 31, 33, 45, 52 and 58, will likely further reduce the incidence of HPV-associated cancer.6 The prophylactic vaccines provide B-cell and antibody-dependent immunity to the L1 protein; they provide no therapeutic value for individuals who have already been infected with high risk HPV strains. Unvaccinated individuals, in addition, are still at risk for development of HPV-induced cellular dysplasia or carcinoma and invasive cancer. Resolution of established cellular dysplasia resulting from HPV infection requires a robust T-cell response not provided by prophylactic vaccines.7
HPV therapeutic vaccines represent an active area of research, and researchers are investigating a variety of vaccine platforms. Some therapeutic vaccines have entered phase III clinical trials for cervical dysplasia and cervical cancer, including VGX-3100 DNA-based HPV vaccine8 and axalimogene filolisbac–cervical (AXAL-CERV) Listeria-based vaccine.9 Also in clinical studies is the ISA101 vaccine, a synthetic long peptide-based vaccine with overlapping peptides to both HPV16 E6 and E7 proteins.10
Given the limited results of complete remission with monotherapy vaccine treatments for cervical cancer, combination therapy using vaccines and immunotherapy agents may provide more robust immunological responses. The ISA101 vaccine was recently evaluated in a phase II study with an anti-programmed cell death protein-1 (PD1) checkpoint inhibitor, nivolumab, for HPV-positive malignancies.10 The overall response rate was 33%, and the median duration of response was 10.3 months. ISA101 alone showed promise in cervical intraepithelial neoplasia (CIN), but did not induce any responses in patients with advanced cervical cancer. Similarly, nivolumab alone was previously shown to have a response rate of only 20% in a similar patient population.10 These results are some of the first clinical data to support the efficacy of T-cell-based vaccination with checkpoint inhibitors that modulate the tumor microenvironment (TME).
Several other clinical trials are currently underway investigating the use of checkpoint inhibitors and therapeutic vaccines for cervical cancer.11 Another therapeutic vaccine with clinical potential is PDS0101, a lipid nanoparticle (liposome)-based vaccine containing R-DOTAP, the immunologically superior R-enantiomer of the positively charged (cationic) lipid 1,2-dioleoyl-3-trimethylammonium-propane,12 13and human leukocyte antigen (HLA)-unrestricted HPV16 peptides, which has shown safety in a phase I clinical trial (NCT 02065973), and has met the secondary endpoints of regression of cervical dysplasia and increases in antigen-specific CD4+ and CD8+ T cells. In the study reported here, we have investigated in preclinical studies whether antitumor efficacy against HPV-associated cancer could be enhanced if PDS0101 is used in combination with two novel immunomodulatory agents: bintrafusp alfa and NHS-interleukin-12 (NHS-IL12). Bintrafusp alfa (M7824) is a novel bifunctional agent consisting of anti-programmed cell death protein-1 ligand (PDL1) linked to two transforming growth factor-β receptor type II (TGFßRII)14–17to function as a TGFβ ‘trap’. In clinical studies, an ongoing phase I/II clinical trial (NTC02517398) using bintrafusp alfa in HPV-positive malignancies has shown promising early results, including a clinical response rate of 38.9%, which is a higher overall response rate than the 15%–25% seen in previous studies using anti-PD1/PDL1 agents in this patient population.18 The use of bintrafusp alfa has also been shown to increase HPV16-specific T cells in patients with HPV-positive malignancies.18
Another promising novel immunocytokine, NHS-IL12, is composed of two IL12 heterodimers, each fused to the NHS76 antibody,19 which targets tumor necrosis. Preclinically, NHS-IL12 induces antitumor effects due to the longer plasma half-life than recombinant IL-12, and the targeting of IL-12 to the tumor in vivo. NHS-IL12 was evaluated in a phase I clinical trial and was found to be safe, induce interferon-γ (IFNγ), and mediate an influx of lymphocytes into the tumor.20 It is currently being evaluated in combination with avelumab in a phase Ib trial in advanced solid tumors (NCT02994953).
The goal of this research study was to test the hypothesis that based on the mechanisms of action of each of the three immunotherapeutic modalities discussed above, the triple combination should promote a superior induction of immunologically active tumor infiltrating, HPV-specific CD4+ and CD8+ T cells, thus resulting in enhanced antitumor efficacy.
Here, we have employed the lipid-based PDS0101 vaccine, primarily using the TC-1 murine lung carcinoma transformed with HPV16 E6 and E7 oncoproteins, a model commonly used for the evaluation of agents directed against HPV-associated malignancies.21 Moreover, TC-1 tumor cells are essentially devoid of PDL1 and would thus mirror the clinical situation where patients may have a low probability of responding to anti-PD1/PDL1 therapy or may have progressed on such therapy. We have also used the oropharyngeal syngeneic mEER cell line,22 transformed with HPV16 E6 and E7 oncoproteins.
The current study evaluates the PDS0101 vaccine with each of two novel immunomodulatory agents and the combination of both on antitumor effects and immune effects in both the periphery and the TME.
Methods
Experimental reagents
PDS0101 (ImmunoMAPK-RDOTAP/HPV-16 E6 and E7 Peptides) vaccine was obtained from PDS Biotechnology (Princeton, New Jersey, USA) as part of a Collaborative Research and Development Agreement (CRADA) with the National Cancer Institute (NCI), National Institutes of Health (NIH). PDS0101 is a lipid-based vaccine containing six HLA-unrestricted epitopes against HPV16 E6 and E7. The dose used was the murine equivalent of the clinical dose, 300 µg R-DOTAP and 40 µg HPV-peptide mix. Bintrafusp alfa (M7824) consists of two TGFßRII fused to a human IgG1 monoclonal antibody blocking PDL1 and showed safety and de novo generation of antigen-specific responses in a phase I trial in HPV-positive malignancies.18 Bintrafusp alfa was used at a dose of 250 µg. NHS-IL12 is an immunocytokine composed of two IL-12 heterodimers, each fused to the NHS76 antibody, which targets histones in necrotic areas of tumor. For murine studies, the surrogate NHS-muIL12, which is the NHS76 antibody fused to two murine IL-12 heterodimers, was used; this is necessary because human IL-12 lacks bioactivity in the mouse.19 NHS-IL12 was used at a dose of 50 µg. NHS-IL12 has shown safety in a phase I clinical study.20 The bintrafusp alfa and NHS-IL12 agents were obtained from EMD Serono (Rockland, Massachusetts, USA) as part of a CRADA with the NCI, NIH.
Cell lines
The TC-1 cell line (murine lung carcinoma cell line transfected with HPV16 E6 and E7 oncoproteins) was a generous gift from Dr. T.C. Wu (Johns Hopkins University, Baltimore, Maryland, USA), and was tested for mycoplasma and viral contamination according to NIH procedures. TC-1 cells were cultured according to previous studies.13 The mEER cell line (murine oropharyngeal cell line transfected with HPV16 E6 and E7 oncoproteins22) was a generous gift from Dr. Clint Allen (NCI, Bethesda, Maryland, USA), and was tested for mycoplasma and viral contamination according to NIH procedures. mEER cells were cultured according to previous studies.22
Mouse models
Mice were housed in microisolator cages under pathogen-free conditions, in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care guidelines. All animal studies were conducted under approval of the NIH Intramural Animal Care and Use Committee (Protocol #057). C57BL/6J or C57BL/6J-COH mice, from in-house breeding, were shaved on the right flank and instilled with 2×104 TC-1 cells or 5×105 mEER cells in a 1:1 mixture with Matrigel basement matrix (Corning, Corning, New York, USA). TC-1 tumors were allowed to grow for 1 week, or until the tumor was ~5–6 mm on one side. mEER tumors were allowed to grow for 4 days, or until the tumor was ~3–4 mm on one side. PDS0101 was thawed and mixed according to the manufacturer’s instructions (PDS Biotechnology), and 100 µL (300 µg R-DOTAP plus 40 µg HPV peptide mixture) was injected subcutaneously (s.c). Bintrafusp alfa was diluted in sterile 1 x phosphate buffered saline (PBS) to a concentration of 250 µg/100 µL and injected intraperitoneally (i.p.). NHS-IL12 was diluted in sterile 1 x PBS to a concentration of 50 µg/100 µL and injected s.c. Tumor volume was measured biweekly, and mice were euthanized when control (PBS treated) mice reached ethical endpoint.
ELIspot
IFNγ ELIspot was performed overnight according to the manufacturer’s (BD Biosciences, San Jose, California, USA) instructions using 2×105 splenocytes per well. HIV-gag (0.625 µg/mL; American Peptide Company, Sunnyvale, California, USA) was used as a negative control, and PMA/ionomycin (1 µg/mL) cocktail was used as a positive control. Overlapping HPV16 E6 and E7 15-mer peptides (JPT, Berlin, Germany) containing HLA-A2 agonist epitopes from HPV1623 were used as the test antigens (0.625 µg/mL). Results are presented as the number of spots per 5×105 splenocytes after subtracting any spots in the background controls.
Histology
Immunohistochemistry for CD8 and CD4 was performed using the Perkin Elmer Opal IHC kit, according to the manufacturer’s instructions (Perkin Elmer, Waltham, Massachusetts, USA). Slides were imaged on an AxioScan (Zeiss, Oberkochen, Germany).
Flow cytometry
Tumors were excised and homogenized via mechanical dissociation and single cell suspensions were prepared by filtering through a 40 µm nylon cell strainer. Cell suspensions were stained on ice with fluorescently conjugated antibodies diluted in FACS buffer. Dead cells were identified via live/dead fixable stain (ThermoFisher, Waltham, Massachusetts, USA). Antibodies used for flow cytometry were purchased from Biolegend (San Diego, California, USA). Mouse antibodies used were: CD3 (Clone # 500A2), CD4 (Clone # RM4-5), CD8 (Clone # 53–6.7), CD45 (Clone # 30-F11), Ki67 (Clone # B56), CD44 (Clone # IM7), CD62L (Clone # MEL-14), CD25 (Clone # PC61), F4/80 (Clone # BM8), GR1 (Clone # RB6-8C5), FoxP3 (Clone # FJk-16s), CD38 (Clone # 90), PDL1 (Clone # 10F.9G2). Intracellular staining was performed using FoxP3/transcription factor kit (eBioscience, San Diego, California, USA), according to the manufacturer’s instructions. Cells were enumerated using AccuCheck Counting beads (ThermoFisher). Cytometric data were obtained via a four laser Attune Flow Cytometer (ThermoFisher). Data were analyzed via FlowJo (FlowJo, LLC, Ashland, Oregon, USA).
DNA isolation and T-cell receptor diversity
TC-1 tumors were dissociated using Mouse Tumor Dissociation kit (Miltenyi Biotec, Gaithersburg, MD), according to the manufacturer’s directions. Briefly, tumors were cut into 2–4 mm pieces, added to Enzyme D, R and A, and transferred to a gentleMACS C tube. C tubes were attached to the gentleMACS dissociator and run on m_tumor1. Samples were incubated for 30 min at 37°C with continuous rotation. Samples were processed again on the gentleMACS dissociator, filtered, spun down at 300xg for 7 min, then counted and resuspended in isolation buffer (PBS, pH 7.2, 0.5% bovine serum albumin and 2 mM ethylenediaminetetraacetic acid (EDTA)) for tumor infiltrating lymphocyte (TIL) isolation. TILs were isolated using mouse TIL Isolation kit (Miltenyi Biotec). DNA was isolated from TILs using QiaAmp DNA mini kit (Qiagen, Germantown, Maryland, USA), according to the manufacturer’s directions. T-cell receptor (TCR) Vβ CDR3 sequencing (TCRseq) was performed by Adaptive Biotechnologies (Seattle, Washington, USA) using the survey resolution ImmunoSeq platform (Adaptive Biotechnologies); analysis was performed using the ImmunoSeq ANALYZER 3.0 (Adaptive Biotechnologies). Repertoire size, a measure of TCR diversity, was determined by calculating the number of individual clonotypes represented in the top 25th percentile by ranked molecule count after sorting by abundance; this measure is relatively stable to differences in sequencing depth, and not strongly influenced by rare clonotypes.
Statistics
GraphPad Prism V.7 (GraphPad Prism Software, La Jolla, California, USA) was used to perform statistical analyses. Details of the appropriate analysis are found within each figure legend.
Results
PDS0101 monotherapy reduced tumor volume and the combination of PDS0101, bintrafusp alfa and NHS-IL12 resulted in further tumor control
We employed the TC-1 syngeneic mouse model to evaluate the antitumor activity of therapeutic vaccination with PDS0101. Flow cytometry analyzes of CD45NEG cells from transplanted tumors (n=3) showed between 0.1% and 0.4% of cells expressing PDL1 (online supplementary figure 1). R-DOTAP has been reported to enhance dendritic cell uptake and antigen cross-presentation.12 R-DOTAP has also been demonstrated to specifically upregulate type I IFN and associated chemokines primarily within the lymph nodes, thus promoting the recruitment and priming of antigen-specific CD4+ and CD8+ T cells.12
jitc-2020-000612supp001.pdf (351.3KB, pdf)
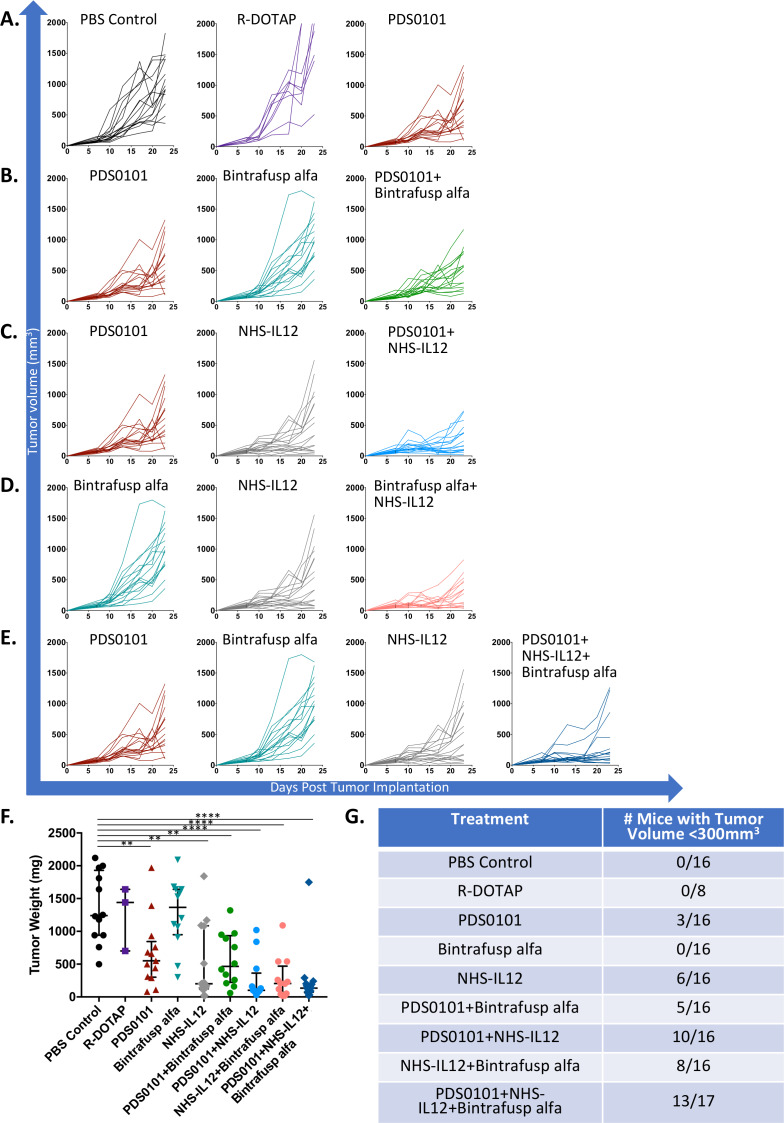
TC-1 is a murine lung carcinoma transformed with HPV16 E6 and E7, and grows aggressively when implanted s.c. in C57BL/6J mice. Treatment was started 7 days postimplantation. First, PBS control vaccination was compared with three weekly injections of the liposomal adjuvant R-DOTAP, to evaluate if the R-DOTAP formulation would mediate any antitumor activity (figure 1A). Tumor volume in the PBS control group compared with R-DOTAP treatment was not significantly different, indicating that in the absence of HPV peptides, s.c. injection of R-DOTAP provided no antitumor efficacy. Next, the PDS0101 vaccine was tested for antitumor activity compared with PBS control or R-DOTAP control. PDS0101 was also injected s.c. once weekly starting on day 7, ipsilateral to the tumor for 3 consecutive weeks. PDS0101 monotherapy resulted in the slowing of tumor growth compared with PBS or R-DOTAP control (figure 1A), and in significantly lower tumor weights compared with PBS control (figure 1F, p<0.01). Tumor weights at the end of study were used as an additional indicator of antitumor activity.
Figure 1.
PDS0101 monotherapy reduced tumor volume in the TC-1 syngeneic tumor model, and the combination of PDS0101, bintrafusp alfa and NHS-IL12 resulted in further tumor control. TC-1 tumor bearing female C57BL/6J mice (n=8–16 per group) were treated with PBS control (100 µL s.c), R-DOTAP control (100 µL s.c), PDS0101 (s.c., 3 weekly doses starting on day 7), bintrafusp alfa (250 µg, i.p., days 7, 9, and 11) and/or NHS-IL12 (50 µg, s.c., day 7). (A) Individual growth curves for PBS control, R-DOTAP, and PDS0101 treated mice. (B) Individual growth curves for PDS0101, bintrafusp alfa, and PDS0101 plus bintrafusp alfa treated mice. (C) Individual growth curves for PDS0101, NHS-IL12, and PDS0101 plus NHS-IL12 treated mice. (D) Individual growth curves for bintrafusp alfa, NHS-IL12, and bintrafusp alfa plus NHS-IL12 treated mice. (E) Individual growth curves for PDS0101, bintrafusp alfa, NHS-IL12, and PDS0101 plus bintrafusp alfa plus NHS-IL12 treated mice. (F) Tumor weights at the end of study. (G) Table of ‘tumor control’, the number of mice with tumors below 300 mm3 at the end of study. A meta-analysis of two independent experiments is shown. **P<0.01, ****P<0.0001. IL12, interleukin-12; i.p. intraperitoneally; s.c., subcutaneously.
Studies were then conducted to determine if the antitumor efficacy of the weekly PDS0101 vaccine could be increased with the addition of bintrafusp alfa, an anti-PDL1/TGFBRII fusion antibody14–18 (figure 1B). Bintrafusp alfa was injected i.p. starting day 7, every 2 days for 6 days (three times total) to avoid mouse−antihuman immune responses to the human IgG present in the antibody.17 Bintrafusp alfa monotherapy treatment was not significantly different from PBS control (figure 1A), for tumor growth rates as well as end of study tumor weight (figure 1F), most likely due to the lack of PDL1 expression on TC-1 tumor cells (online supplementary figure 1). The combination of PDS0101 plus bintrafusp alfa-treated mice showed enhanced antitumor responses compared with mice treated with PDS0101 alone. One possible explanation for this could be the observation that PDS0101 treatment of mice resulted in a slight increase in PDL1 expression (approximately 2%–4.5% of tumor cells) (online supplementary figure 1). The rapid rate of growth in the TC-1 model and no cures in these experiments reflect an aggressive tumor model, and mice with a tumor volume below 300 mm3 appeared to have controlled tumor growth more effectively than mice above that threshold. The combination of PDS0101 and bintrafusp alfa resulted in 5/16 mice with tumor volumes <300 mm3, compared with 0/16 mice in the PBS-treated group (figure 1G).
Studies were also conducted employing treatment with NHS-IL12, an immunocytokine fusion protein consisting of IL-12 and the NHS76 antibody, which targets the immunocytokine to the TME.19 20 24 25 NHS-IL12 was injected s.c. once at the start of treatment on day 7, contralateral to the tumor. NHS-IL12 monotherapy resulted in a slower tumor growth rate compared with PBS or R-DOTAP control treatment (figure 1C), as well as significantly lower end of study tumor weight (figure 1F, p<0.01). The combination of PDS0101 and NHS-IL12 further reduced tumor growth as compared with PBS control (p<0.0001). It also resulted in better tumor control, with 10/16 mice displaying a tumor volume <300 mm3, compared with 0/16 mice in the PBS control group (figure 1G).
The combination of treatment with NHS-IL12 and bintrafusp alfa was also investigated. This resulted in decreased tumor growth rate (figure 1D), as well as significantly decreased tumor weight at the end of study (figure 1F, p<0.001). Additionally, this combination resulted in a greater number of mice with tumor volumes below 300 mm3 than either treatment alone, with 8/16 displaying lower tumor volume from the combination treated, vs 0/16 for bintrafusp alfa and 6/16 for NHS-IL12 (figure 1G).
Finally, the combination of all three agents, PDS0101, bintrafusp alfa and NHS-IL12, was evaluated, employing the same schedules described above. The triple combination most effectively reduced the rate of tumor growth (figure 1E), and compared with all the other treatment groups resulted in the lowest average tumor weight at the end of study (figure 1F, p<0.001 vs PBS control). The combination of the three agents also yielded more mice (13/16) with tumors smaller than 300 mm3 at the end (figure 1G).
Analyses of activated and proliferating CD8+ T cells in TC-1 tumors
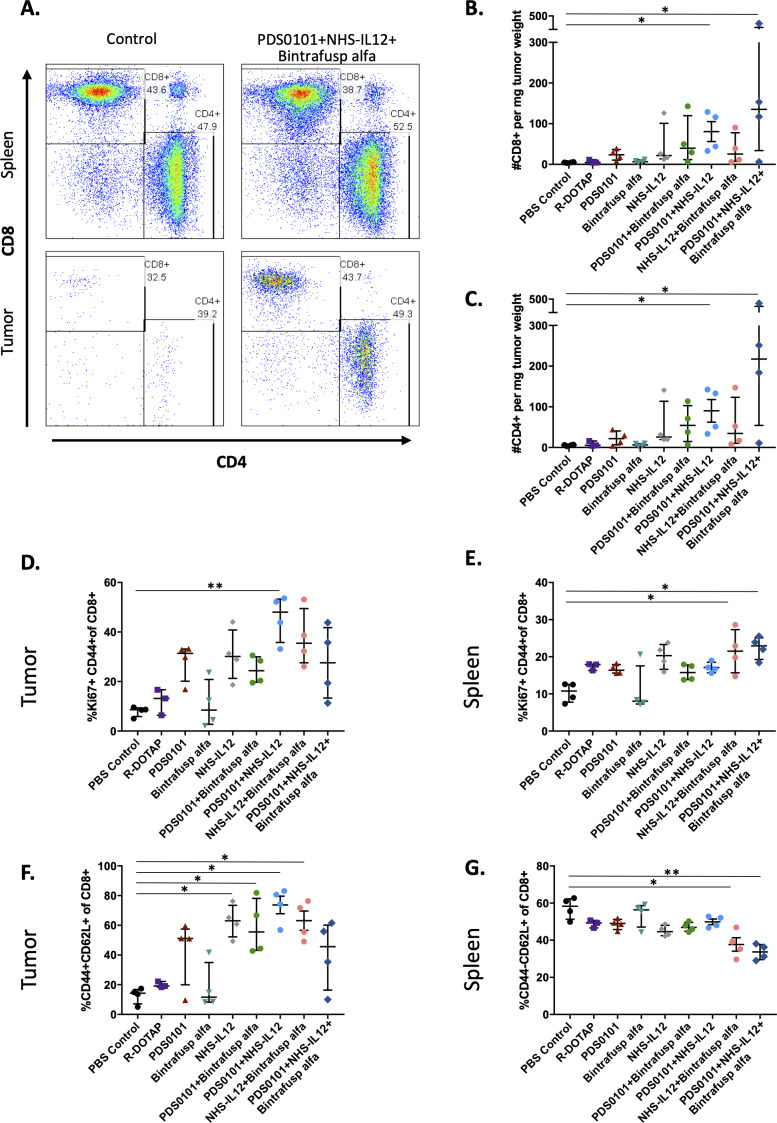
Flow cytometry was performed on both tumors and spleens from mice bearing TC-1 tumors and treated with single agents or the combinations of agents described above. Spleens were used to evaluate the effects of the agents on the peripheral immune cell compartment. Figure 2A shows a representative example of CD8+ and CD4+ T cells in the spleens (upper panels) and tumors (lower panels) of two mice treated with PBS control (left) and the combination of PDS0101, NHS-IL12 and bintrafusp alfa (right). The overall frequencies of T cells did not change in the spleens after treatment, but the combination treatment led to substantial infiltration of T cells into these tumors (lower panels). In agreement with the antitumor data, there were significant increases in CD8+ and CD4+ T cell infiltration per milligram of tumor in the group treated with PDS0101 plus NHS-IL12 (p<0.05) and the group treated with PDS0101, bintrafusp alfa plus NHS-IL12 (p<0.05) (figure 2B). An increase in activated CD8+ T cells with proliferative potential (Ki67+CD44+) was also seen in the tumor of PDS0101 plus NHS-IL12-treated mice, with a trending increase seen in the other treatment groups (figure 2D), as well as in spleens (figure 2E). CD8+ activated effector T cells, defined as CD44+CD62L+CD8+ increased significantly in the tumors of mice treated with NHS-IL12, PDS0101 plus bintrafusp alfa, NHS-IL12 plus bintrafusp alfa, and PDS0101 plus NHS-IL12 compared with controls (figure 2F). In contrast, there were decreases in naïve CD8 T cells (CD44NEGCD62L+) in the spleens of mice treated with NHS-IL12 plus bintrafusp alfa and in the triple combination group (figure 2G).
Figure 2.
CD4 and CD8 T cell infiltration into tumors. TC-1 tumor bearing female C57BL/6J mice (n=4/group) were treated with PBS control (100 µL s.c.), R-DOTAP control (100 µg s.c.), PDS0101 (s.c., 3 weekly doses starting on day 7), bintrafusp alfa (250 µg, i.p., days 7, 9, and 11), and/or NHS-IL12 (50 µg, s.c., day 7). (A) Representative flow cytometry plots of CD4+ and CD8+ T cells in the spleen and tumor of PBS control treated mice, and PDS0101, NHS-IL12 plus bintrafusp alfa combination treated mice. (B) CD8+ T cell infiltration in tumor. (C) CD4+ T cell infiltration in tumor. (D) Activated and proliferating CD8+ T cells in tumor. (E) Activated and proliferating CD8+ T cells in spleen. (F) Activated effector CD8+ T cells in tumor. (G) Naïve CD8+ effector T cells in spleen. *P<0.05, **P<0.01. IL12, interleukin-12; i.p. intraperitoneally; s.c., subcutaneously.
Other immune cell populations, such as natural killer (NK) cells, myeloid derived suppressor cells, and regulatory T cells (Tregs) were not significantly changed in tumor after treatment, whereas the CD38+ macrophages (M1 macrophages) showed a significant increase in the PDS0101 plus NHS-IL12 combination group (online supplementary figure 2).
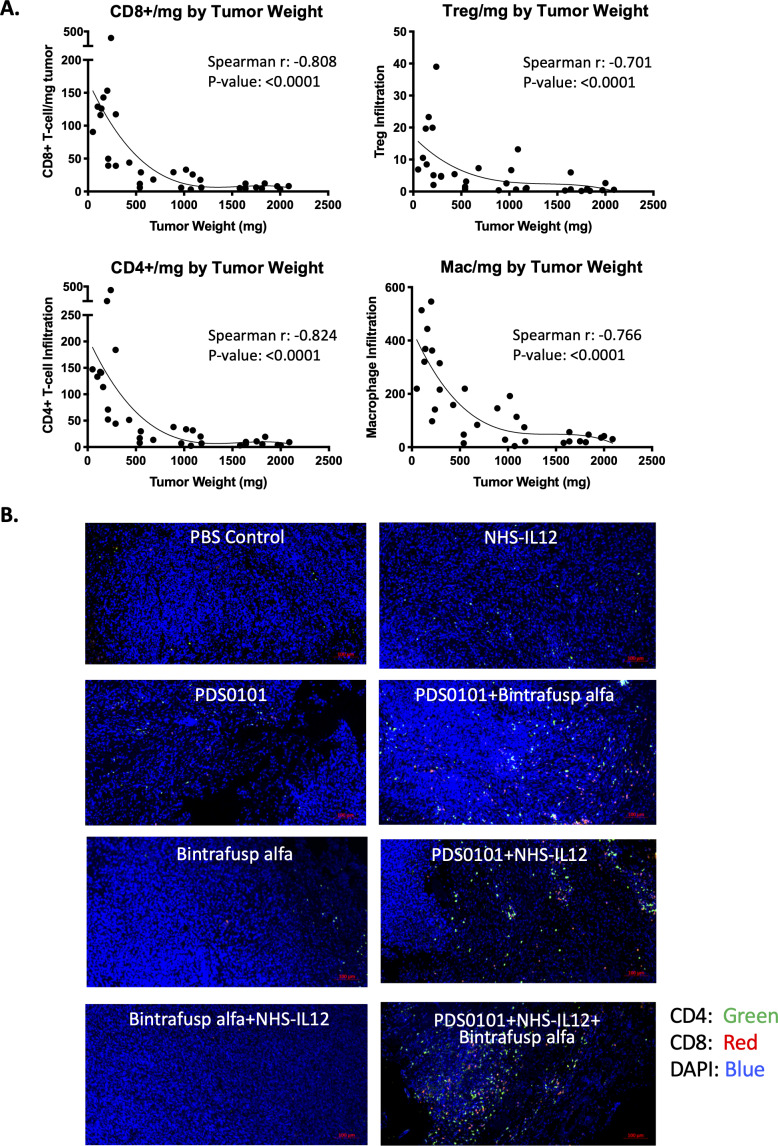
To investigate whether the numbers of immune cells in the tumor were related to the antitumor effects seen in the different groups, that is, tumor size, correlation analyses were performed. CD4+ T cells, CD8+ T cells, Tregs and macrophage infiltration per milligram tumor from all treatment groups inversely correlated to tumor weight, demonstrating that increased infiltration of immune cells was correlated with smaller tumors (figure 3A) with p values of <0.0001 and r values of between −0.701 and −0.824 for the four cell types. Increases in CD4+ (green) and CD8+ T cells (red) can also be seen in the combination groups compared with monotherapy groups in representative immunohistochemistry images (figure 3B). These changes also correspond with the flow cytometry data (figure 2B).
Figure 3.
Correlation between tumor weight and immune cell infiltration per milligram of tumor in the TC-1 syngeneic model. TC-1 tumor bearing female C57BL/6J mice were treated with PBS control (100 µL s.c.), PDS0101 (s.c., 3 weekly doses starting on day 7), bintrafusp alfa (250 µg, i.p., days 7, 9 and 11), and/or NHS-IL12 (50 µg, s.c., day 7). (A) A smaller tumor volume correlated with increased CD4+ and CD8+ T cells, regulatory T cells (Treg), and M1 macrophage infiltration into the tumor. Data are shown for all treatment groups combined. (B) Immunohistochemistry for CD4+ and CD8+ T cells in TC-1 tumors. Tumors were harvested, blocked, sectioned and stained with the Opal Immunology kit. Combination treatment with PDS0101, NHS-IL12, and bintrafusp alfa increased CD8+ and CD4+ T cell infiltration into the tumor compared with monotherapy treatments. IL12, interleukin-12; i.p. intraperitoneally; s.c., subcutaneously.
HPV-specific T-cell responses
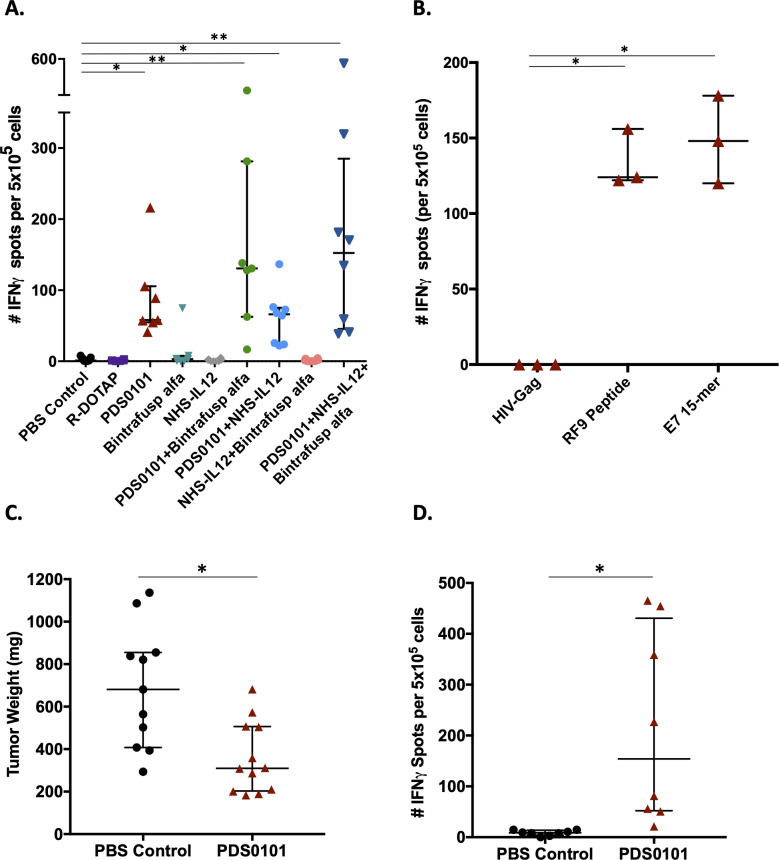
To evaluate T-cell antigen specificity generated by vaccination with PDS0101, splenocytes were isolated from animals from all groups for analysis using IFNγ ELIspot as described in Methods. Overlapping 15-mer peptides from the human HPV16 E7 protein in the PDS0101 vaccine were used as the target antigen. Only mice in the different groups vaccinated with PDS0101 developed significant antigen-specific responses against the HPV16 E7 peptide (figure 4A).
Figure 4.
PDS0101 increased antigen-specific T cells against HPV16 E7 and reduced tumor volume in the syngeneic mEER tumor model. (A, B) TC-1 tumor bearing female C57BL/6J mice were treated with PBS control, R-DOTAP control, PDS0101 (s.c., 3 weekly doses starting on day 7), bintrafusp alfa (250 µg, i.p., days 7, 9 and 11), and/or NHS-IL12 (50 µg, s.c., day 7). (A) ELIspot assay for IFNγ in splenocytes stimulated with overlapping HPV16 E7 15-mers. Kruskall-Wallis analysis was performed. (B) Comparison between RF9 peptide and overlapping HPV16 E7 15-mer peptides as investigative antigens for IFNγ ELIspot. Mann-Whitney unpaired t-test. (C, D) mEER tumor bearing female C57BL/6J mice (n=11–12 per group) were treated with PDS0101 (s.c., 3 weekly doses starting on day 4) or PBS control (100 µL, s.c). (C) Tumor weights at the end of study. (D) IFNγ ELIspot data from splenocytes stimulated with overlapping HPV16 E7 15-mer peptides in PBS control versus PDS0101 treated mice. *P<0.05, **P<0.01. HPV, human papillomavirus; IFNγ, interferon-γ; i.p. intraperitoneally; s.c., subcutaneously.
Because the PDS0101 vaccine also contains a single mouse epitope against E7 (RF9 peptide, part of the human KF18 peptide), assays to evaluate the responses to RF9 were conducted as well. Analyses of splenocytes from PDS0101-vaccinated mice in an IFNγ ELIspot assay showed similar responses using the RF9 peptide compared with HPV16 E7 overlapping 15-mers (figure 4B).
To test the efficacy of the PDS0101 vaccine in another syngeneic HPV-positive tumor model, C57Bl/6J mice bearing mEER tumors were vaccinated with PDS0101. mEER is a syngeneic, oropharyngeal cancer cell line transformed with HPV16 E6 and E7 oncoproteins.22 Three weekly treatments with PDS0101 reduced the tumor burden in individual mice compared with PBS-treated controls (figure 4C, p<0.05). ELIspot analysis using HPV16 E7 overlapping 15-mer peptides for stimulation of splenocytes showed significantly higher IFNγ production in PDS0101-treated versus control-treated mice (figure 4D, p<0.05), confirming that PDS0101 treatment increased antigen-specific T cells in vivo in a second model.
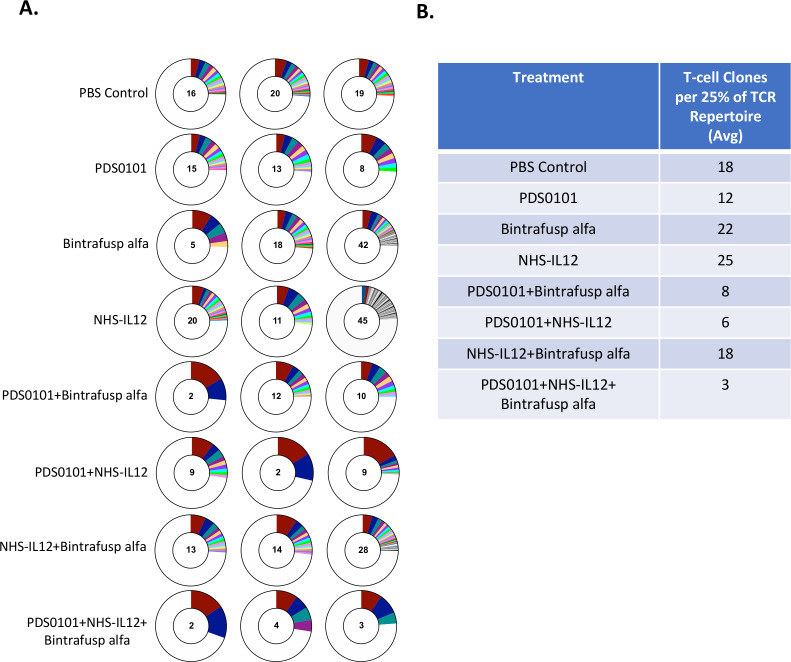
TCR repertoire analyses
To investigate TCR clonality in the tumor, DNA from TILs was isolated from tumors from each treatment group. The PDS0101 monotherapy group displayed a lower number of TCR clones per 25% of the T-cell repertoire, indicating that the vaccine promoted clonality over diversity (figure 5A). The NHS-IL12 monotherapy group showed the highest number of T-cell clones per 25% of the repertoire, which was expected since IL-12 promotes the proliferation of T cells.26 The NHS-IL12 plus bintrafusp alfa group displayed much higher diversity than other combination groups, which is likely due to the absence of the vaccine (figure 5A). The PDS0101 plus NHS-IL12 group and the PDS0101 plus bintrafusp alfa group each displayed a dramatic decrease in diversity (or increase in clonality), potentially due to the antigen-specific response induced by the vaccine and the proliferation. Finally, the triple combination group displayed the largest decrease in diversity/increase in clonality, most likely showing the effects of the T-cell induction of the vaccine, the tumor targeting of bintrafusp alfa, and the proliferation enhancing effects of NHS-IL12 (figure 5A).
Figure 5.
TCR clonality significantly increased in all groups treated with PDS0101. TC-1 tumor bearing female C57BL/6J mice were treated with PBS control (100 µL s.c.), PDS0101 (s.c., 3 weekly doses starting on day 7), bintrafusp alfa (250 µg, i.p., days 7, 9, and 11), and/or NHS-IL12 (50 µg, s.c., day 7). Tumor infiltrating lymphocytes (TILs) were purified from whole tumor. DNA isolated from TILs was analyzed by Adaptive Biotechnology for TCR repertoire. (A) Number of T-cell clones that make up 25% of the TCR repertoire (n=3 mice per group). Red represents the most abundant T-cell clone in the individual mouse tumor; blue is the second most abundant; teal is the third. (B) Table of the average number of clones from three mice per group that make up 25% of the TCR repertoire. IL12, interleukin-12; i.p. intraperitoneally; s.c., subcutaneously; TCR, T-cell receptor.
Discussion
With increasing prophylactic HPV vaccination rates among the general population and the expansion of FDA approval for vaccination of all age groups, the rates of HPV infection and subsequent malignancy are set to drop over the coming decades.3 27 There remains, however, an unmet need for an HPV therapeutic vaccine. Currently, there are over 10 HPV therapeutic vaccines under development, and in various stages of clinical testing, but mostly in phase I or II.11 Vaccines are being developed employing multiple platforms, including DNA, bacterial and viral vectors, peptides and proteins. VGX-3100 is an HPV 16/18 DNA-based vaccine that is administered intramuscularly via electroporation, and is currently being tested in a phase III trial (NCT03721978) for high-grade cervical lesions, CIN2/3, which are a direct precursor to cervical cancer. AXAL-CERV is a Listeria monocytogenes-based vaccine currently in a phase III clinical trial to treat patients with high risk locally advanced cervical cancer (NCT 02853604). Every vaccine platform has its advantages and disadvantages, including parameters such as safety, ease of administration, number of possible administrations, cost and type of immune cells and cytokines activated. The PDS0101 vaccine is not HLA restricted, and its liposomal formulation can be used in immunocompromised patients. A phase I clinical trial using PDS0101 monotherapy in CIN patients showed a significant increase in antigen-specific CD8+ T cells, and regression of cervical dysplasia (NCT02065973).28
Therapeutic vaccines offer antigen-specific education of T cells, but historically vaccines alone have done little to address the immunosuppressive nature of the TME, so combinations with other agents are warranted. Checkpoint inhibitors are being increasingly incorporated into frontline therapy against a variety of different cancers as an effective method of overcoming one of the immunosuppressive elements of the TME. Multiple clinical trials have opened to study the efficacy of including checkpoint therapy in the treatment of HPV-positive malignancies. Results from a recent study using ipilimumab in addition to chemoradiotherapy (CRT) in women with lymph node positive cervical cancer, who often experience recurrence of disease, showed that checkpoint inhibitor therapy was safe in combination with CRT.29 In addition, CRT upregulated PD1 expression on peripheral CD8+ and CD4+ cells, suggesting that T cells are activated and patients may benefit from PD1/PDL1 targeting therapies. Positive results from phase II clinical trials, such as the combination of anti-PD1 nivolumab and ISA101 HPV16 long peptide vaccine,10 provide evidence of the effectiveness of combining two different types of immunotherapy. This study enrolled 24 patients with advanced HPV16 positive malignancy, 22 of whom had oropharyngeal cancer, and followed patients for 1 year after giving repeated doses of ISA101 s.c. and nivolumab intravenously; the combination resulted in a 33% overall response rate, compared with a 20% overall response rate in similar patient populations with nivolumab alone, and no response in advanced head and neck squamous cell carcinoma (HNSCC) patients with ISA101 alone.10 The long peptide-based vaccine, ISA101, is currently being investigated in combination with cemiplimab (anti-PD1, NCT03669718) and utomilumab (anti-4-1BB, NCT03258008), and other studies are investigating the combination of VGX-3100 with anti-PDL1 therapy in a phase Ia/IIb clinical trial (NTC03162224).
There was a defined rationale for the selection of the three immunotherapeutic agents used in these studies: (1) the vaccine was used to induce HPV-specific T cells to target the tumor, (2) bintrafusp alfa was employed to modulate checkpoint inhibition and also sequester TGFβ in the TME to prevent signaling and plasticity changes associated with TGFβ and (3) NHS-IL12 binds to necrotic areas of the tumor and was used to introduce proinflammatory IL-12 and IFNγ to the TME to enhance T-cell and NK-cell proliferation and differentiation. Prior studies have employed the TC-1 model,30 31 and it should be noted that the variant of TC-1 used in the studies presented here was very aggressive and grew rapidly in the C57BL/6J mice.
New classes of checkpoint inhibitors, such as the bifunctional anti-PDL1/TGFβRII bintrafusp alfa, have recently been developed. In the preclinical studies, bintrafusp alfa was shown to decrease plasma TGFβ, bind to PDL1 in the tumor, and decrease TGFβ-related signaling in the TME.16 An ongoing phase I/II clinical trial (NCT02517398) in patients with HPV-associated malignancies including cervical, anal, or HNSCC has shown promising clinical responses (NCT03427411). Of the 36 patients who received bintrafusp alfa, there was an objective clinical response rate of 38.9% with an acceptable safety profile.32 Prior studies with anti-PD1/PDL1 agents have demonstrated 15%–25% response rates in this patient population. Patients who received bintrafusp alfa also generated de novo HPV-specific T-cell responses, evaluated by intracellular cytokine staining.18 In the current study, there were no monotherapy effects of bintrafusp alfa in the TC-1 model; one explanation for this is that TC-1 tumor cells express extremely low levels (less than 0.5%) of PDL1. However, we found a slight increase in tumor PDL1 expression (2%–4.5% of cells) after mice were vaccinated with PDS0101. These results are in accordance with prior studies,31in which TC-1 tumors were shown to have extremely low levels of PDL1 and treatment with a fusion protein targeting dendritic cells slightly enhanced PDL1 expression on tumor cells. This may be due to the increase in T-cell infiltrate in tumors after vaccination and consequent increase in IFNγ levels, which may also explain the augmented antitumor efficacy seen with the combination of PDS0101 and bintrafusp alfa treatment compared with PDS0101 monotherapy.
It is of interest to note that in many clinical studies, cohorts are divided into PDL1-expressors of either less than 1% or greater than 1%. In locally advanced cervical cancer, PDL1 expression ≥1% is seen in 87.9% of patients,33and in HPV-positive oropharyngeal squamous cell carcinoma, PDL1 expression ≥1% is seen in 47% of patients.34 Additionally, a prior study has shown that treatment of tumor bearing mice with bintrafusp alfa reduces the plasma TGFβ levels and decreases TGFβ signaling in the TME.16 This phenomenon could also be a factor in the antitumor effects seen in the combination of vaccine and bintrafusp alfa.
Immunocytokines are also being investigated as potential agents for use in cancer therapy. Currently, the combination of immunocytokine IL-2v and checkpoint inhibitor atezolizumab is being studied for HPV-positive malignancies (NCT0338671). NHS-IL12, a novel tumor-targeting immunocytokine, has shown promising preclinical results in increasing antitumor responses, serum IFNγ, as well as binding to necrotic (DNA/histone) portions of tumors, and thus targeting IL-12 to the TME.19 Preclinical studies have also shown enhanced antitumor effects in combining NHS-IL12 with antiPDL1. A phase I clinical study has shown safety,20 and a phase II study combining NHS-IL12 with anti-PDL1 avelumab is in progress (NCT02994953). Prior studies have shown that locally advanced HPV-positive HNSCC exhibits tumor necrosis35 from hypoxia, which has been associated with an increased resistance to chemotherapy and radiotherapy.36 Tumor necrosis has been correlated with poorer prognosis in many types of cancer.37–39 Incorporating NHS-IL12 in a treatment regimen could potentially augment the efficacy of a vaccine.19
The combination therapy employing the HPV-specific vaccine PDS0101, the bifunctional checkpoint inhibitor bintrafusp alfa, and the immunocytokine NHS-IL12 used in the current study resulted in greater antitumor and immunostimulatory effects than the monotherapies alone. Treatment with the triple combination was shown to increase (1) CD4+ and CD8+ T cell infiltration into tumor, (2) activation and proliferation markers on T cells isolated from tumors, (3) HPV-specific T cells and (4) TCR clonality in groups with better tumor control. While more research is needed to clarify this hypothesis, prior studies have shown that increases in TCR repertoire clonality in the tumor correlate with better antitumor effects.40
In conclusion, the studies reported here help to provide the rationale for further preclinical and clinical studies employing combinations of an HPV therapeutic vaccine with one or more immunomodulatory agents.
Acknowledgments
The authors would like to acknowledge Nicholas Roller and Ariana Sabzevari for their technical assistance and Debra Weingarten for her editorial assistance in the preparation of this manuscript.
Footnotes
CSR and STP contributed equally.
JS and CJ contributed equally.
Contributors: CSR, JS and CJ designed the research studies. CSR, STP and YMM conducted the experiments. CSR, STP and YMM acquired the data. CSR and YMM analyzed and interpreted the data. CSR, JS and CJ wrote the manuscript. All authors read and approved the final manuscript.
Funding: This research was supported in part by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute (NCI), National Institutes of Health, and via Cooperative Research and Development Agreements (CRADAs) between the NCI and EMD Serono and the NCI and PDS Biotechnology.
Competing interests: None declare.
Patient consent for publication: Not required.
Ethics approval: Animal care was in compliance with the Association for Assessment and Accreditation of Laboratory Animal Care guidelines. All animal studies were conducted under approval of the NIH Intramural Animal Care and Use Committee (Protocol #057).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request. The datasets used and/or analyzed during the study are available from the corresponding author (JS; schlomj@mail.nih.gov) on reasonable request.
References
- 1. de Martel C, Plummer M, Vignat J, et al. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer 2017;141:664–70. 10.1002/ijc.30716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boda D, Docea AO, Calina D, et al. Human papilloma virus: apprehending the link with carcinogenesis and unveiling new research avenues (review). Int J Oncol 2018;52:637–55. 10.3892/ijo.2018.4256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. Cancers Associated with Human Papillomavirus, United States—2012–2016 U.S. Cancer Statistics Data Brief. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services, 2019. [Google Scholar]
- 4. Stern PL, van der Burg SH, Hampson IN, et al. Therapy of human papillomavirus-related disease. Vaccine 2012;30 Suppl 5:F71–82. 10.1016/j.vaccine.2012.05.091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Drolet M, Bénard Élodie, Pérez N, et al. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet 2019;394:497–509. 10.1016/S0140-6736(19)30298-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saraiya M, Unger ER, Thompson TD, et al. US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. J Natl Cancer Inst 2015;107:djv086. 10.1093/jnci/djv086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scott M, Nakagawa M, Moscicki AB. Cell-mediated immune response to human papillomavirus infection. Clin Diagn Lab Immunol 2001;8:209–20. 10.1128/CDLI.8.2.209-220.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Trimble CL, Morrow MP, Kraynyak KA, et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2B trial. Lancet 2015;386:2078–88. 10.1016/S0140-6736(15)00239-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cory L, Chu C. ADXS-HPV: a therapeutic Listeria vaccination targeting cervical cancers expressing the HPV E7 antigen. Hum Vaccin Immunother 2014;10:3190–5. 10.4161/hv.34378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Massarelli E, William W, Johnson F, et al. Combining immune checkpoint blockade and tumor-specific vaccine for patients with incurable human papillomavirus 16-related cancer: a phase 2 clinical trial. JAMA Oncol 2019;5:67–73. 10.1001/jamaoncol.2018.4051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chabeda A, Yanez RJR, Lamprecht R, et al. Therapeutic vaccines for high-risk HPV-associated diseases. Papillomavirus Res 2018;5:46–58. 10.1016/j.pvr.2017.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gandhapudi SK, Ward M, Bush JPC, et al. Antigen priming with enantiospecific cationic lipid nanoparticles induces potent antitumor CTL responses through novel induction of a type I IFN response. J Immunol 2019;202:3524–36. 10.4049/jimmunol.1801634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vasievich EA, Chen W, Huang L. Enantiospecific adjuvant activity of cationic lipid DOTAP in cancer vaccine. Cancer Immunol Immunother 2011;60:629–38. 10.1007/s00262-011-0970-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. David JM, Dominguez C, McCampbell KK, et al. A novel bifunctional anti-PD-L1/TGF-β trap fusion protein (M7824) efficiently reverts mesenchymalization of human lung cancer cells. Oncoimmunology 2017;6:e1349589. 10.1080/2162402X.2017.1349589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jochems C, Tritsch SR, Pellom ST, et al. Analyses of functions of an anti-PD-L1/TGFβR2 bispecific fusion protein (M7824). Oncotarget 2017;8:75217–31. 10.18632/oncotarget.20680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Knudson KM, Hicks KC, Luo X, et al. M7824, a novel bifunctional anti-PD-L1/TGFβ trap fusion protein, promotes anti-tumor efficacy as monotherapy and in combination with vaccine. Oncoimmunology 2018;7:e1426519. 10.1080/2162402X.2018.1426519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lan Y, Zhang D, Xu C, et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β. Sci Transl Med 2018;10:eaan5488. 10.1126/scitranslmed.aan5488 [DOI] [PubMed] [Google Scholar]
- 18. Strauss J, Heery CR, Schlom J, et al. Phase I trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGFβ, in advanced solid tumors. Clin Cancer Res 2018;24:1287–95. 10.1158/1078-0432.CCR-17-2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fallon J, Tighe R, Kradjian G, et al. The immunocytokine NHS-IL12 as a potential cancer therapeutic. Oncotarget 2014;5:1869–84. 10.18632/oncotarget.1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Strauss J, Heery CR, Kim JW, et al. First-In-Human phase I trial of a tumor-targeted cytokine (NHS-IL12) in subjects with metastatic solid tumors. Clin Cancer Res 2019;25:99–109. 10.1158/1078-0432.CCR-18-1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lin KY, Guarnieri FG, Staveley-O'Carroll KF, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res 1996;56:21–6. [PubMed] [Google Scholar]
- 22. Vermeer DW, Coppock JD, Zeng E, et al. Metastatic model of HPV+ oropharyngeal squamous cell carcinoma demonstrates heterogeneity in tumor metastasis. Oncotarget 2016;7:24194–207. 10.18632/oncotarget.8254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tsang KY, Fantini M, Fernando RI, et al. Identification and characterization of enhancer agonist human cytotoxic T-cell epitopes of the human papillomavirus type 16 (HPV16) E6/E7. Vaccine 2017;35:2605–11. 10.1016/j.vaccine.2017.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fallon JK, Vandeveer AJ, Schlom J, et al. Enhanced antitumor effects by combining an IL-12/anti-DNA fusion protein with avelumab, an anti-PD-L1 antibody. Oncotarget 2017;8:20558–71. 10.18632/oncotarget.16137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morillon YM, Su Z, Schlom J, et al. Temporal changes within the (bladder) tumor microenvironment that accompany the therapeutic effects of the immunocytokine NHS-IL12. J Immunother Cancer 2019;7:150. 10.1186/s40425-019-0620-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gately MK, Desai BB, Wolitzky AG, et al. Regulation of human lymphocyte proliferation by a heterodimeric cytokine, IL-12 (cytotoxic lymphocyte maturation factor). J Immunol 1991;147:874. [PubMed] [Google Scholar]
- 27. Meites E, Szilagyi PG, Chesson HW, et al. Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on immunization practices. MMWR Morb Mortal Wkly Rep 2019;68:698–702. 10.15585/mmwr.mm6832a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wood LV, Edwards LH, Ferris DG. A novel enantio-specific cationic lipid R-DOTAP + HPV16 E6 & E7 antigens induces potent antigen-specific CD8+ T cell responses in-vivo in subjects with CIN and high-risk human papillomavirus infection (abstr). Society for Immunotherapy of Cancer (SITC) Annual Meeting, Nov. 6-10, 2019. National Harbor, MD 2019.
- 29. Mayadev JS, Enserro D, Lin YG, et al. Sequential ipilimumab after chemoradiotherapy in curative-intent treatment of patients with node-positive cervical cancer. JAMA Oncol 2019. 10.1001/jamaoncol.2019.3857. [Epub ahead of print: 27 Nov 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li J, Sun Y, Garen A. Immunization and immunotherapy for cancers involving infection by a human papillomavirus in a mouse model. Proc Natl Acad Sci U S A 2002;99:16232–6. 10.1073/pnas.192581299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu Z, Zhou H, Wang W, et al. A novel dendritic cell targeting HPV16 E7 synthetic vaccine in combination with PD-L1 blockade elicits therapeutic antitumor immunity in mice. Oncoimmunology 2016;5:e1147641. 10.1080/2162402X.2016.1147641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Strauss J, Gatti-Mays M, Cho B, et al. Phase I evaluation of M7824, a bifunctional fusion protein targeting TGF-β and PD-L1, in patients with human papillomavirus (HPV)-associated malignancies [abstract]. AACR 2019 Annual Meeting, Atlanta, GA (abstract CT075). Cancer Res 2019;79 10.1158/1538-7445.AM2019-CT075 [DOI] [Google Scholar]
- 33. Enwere EK, Kornaga EN, Dean M, et al. Expression of PD-L1 and presence of CD8-positive T cells in pre-treatment specimens of locally advanced cervical cancer. Mod Pathol 2017;30:577–86. 10.1038/modpathol.2016.221 [DOI] [PubMed] [Google Scholar]
- 34. Solomon B, Young RJ, Bressel M, et al. Prognostic Significance of PD-L1+ and CD8+ Immune Cells in HPV+ Oropharyngeal Squamous Cell Carcinoma. Cancer Immunol Res 2018;6:295–304. 10.1158/2326-6066.CIR-17-0299 [DOI] [PubMed] [Google Scholar]
- 35. Ou D, Garberis I, Adam J, et al. Prognostic value of tissue necrosis, hypoxia-related markers and correlation with HPV status in head and neck cancer patients treated with bio- or chemo-radiotherapy. Radiother Oncol 2018;126:116–24. 10.1016/j.radonc.2017.10.007 [DOI] [PubMed] [Google Scholar]
- 36. Visser J, van Baarle D, Hoogeboom BN, et al. Enhancement of human papilloma virus type 16 E7 specific T cell responses by local invasive procedures in patients with (pre)malignant cervical neoplasia. Int J Cancer 2006;118:2529–37. 10.1002/ijc.21673 [DOI] [PubMed] [Google Scholar]
- 37. Katz MD, Serrano MF, Grubb RL, et al. Percent microscopic tumor necrosis and survival after curative surgery for renal cell carcinoma. J Urol 2010;183:909–14. 10.1016/j.juro.2009.11.010 [DOI] [PubMed] [Google Scholar]
- 38. Swinson DEB, Jones JL, Richardson D, et al. Tumour necrosis is an independent prognostic marker in non-small cell lung cancer: correlation with biological variables. Lung Cancer 2002;37:235–40. 10.1016/S0169-5002(02)00172-1 [DOI] [PubMed] [Google Scholar]
- 39. Väyrynen SA, Väyrynen JP, Klintrup K, et al. Clinical impact and network of determinants of tumour necrosis in colorectal cancer. Br J Cancer 2016;114:1334–42. 10.1038/bjc.2016.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cha E, Klinger M, Hou Y, et al. Improved survival with T cell clonotype stability after anti-CTLA-4 treatment in cancer patients. Sci Transl Med 2014;6:238ra70. 10.1126/scitranslmed.3008211 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2020-000612supp001.pdf (351.3KB, pdf)