Abstract

The metal–metal bond in metal-rich chalcogenide is known to exhibit various structures and interesting physical properties. Ta2Se can be obtained by both arc-melting and solid-state pellet methods. Ta2Se crystallizes a layered tetragonal structure with space group P4/nmm (No. 129; Pearson symbol tP6). Each unit cell consists of four layers of body-centered close-packing Ta atoms sandwiched between two square nets of Se atoms, forming the Se–Ta–Ta–Ta–Ta–Se networks. Herein, we present magnetic susceptibility, resistivity, and heat capacity measurements on Ta2Se, which together indicate bulk superconductivity with Tc = 3.8(1) K. According to first-principles calculations, the d orbitals in Ta atoms dominate the Fermi level in Ta2Se. The flat bands at the Γ point in the Brillouin zone yield the van Hove singularities in the density of states around the Fermi level, which is intensified by introducing a spin–orbit coupling effect, and thus could be critical for the superconductivity in Ta2Se. The physical properties, especially superconductivity, are completely different from those of Ta-rich alloys or transition-metal dichalcogenide TaSe2.
Short abstract
The first metal-rich chalcogenide superconductor Ta2Se with extensive metal−metal interactions was reported with Tc ∼ 3.8 K. The electronic structures indicate the importance of d electrons of Ta atoms in the superconductivity and lead to a high probability of discovering more superconductors in metal-rich chalcogenides.
Transition-metal-rich chalcogenides are a fascinating series of solid-state structures in which several-atom-thick slabs of transition-metal atoms are terminated with monolayers of chalcogenides. Moreover, transition-metal-rich chalcogenides usually share a common structural feature in that the transition metal forms an octahedron or trigonal prism with chalcogen elements in the center,1−4 which can be considered to be the antiformat of transition-metal dichalcogenides (TM2).5 The layered transition-metal dichalcogenides have received interest for decades because of their variety in electronic properties in both bulk and surface states.6−12 However, a limited number of studies of transition-metal-rich chalcogenides, mainly with an emphasis on the structures, have been reported.13−15 Most metal-rich chalcogenides occur in the earliest transition metals (Sc, Y, and Ti) and especially the late lanthanides.14−19 Harbrecht discovered the first of these transition-metal-rich chalcogenides, Ta2Se, by a simple arc-melting preparation.2 Ta2Se can be considered to be an insertion of Se into bulk body-centered close-packing Ta metal, with every four layers of Ta intercalated by two layers of Se. However, isoelectronic Nb2Se has a very different structure, which could be described as built up from condensed octahedral clusters. Considering the extensive metal–metal bonding in these examples, we focused on Ta2Se, which may induce interesting exotic physical properties, for example, superconductivity. Moreover, by comparing the crystal structures of Ta2Se and the well-known 2H-TaSe2, one can easily obtain some significant differences. The layered stacking pattern of 2H-TaSe2 can be seen as (TaSe)SeSe(TaSe), while it is (TaSe)TaTa(TaSe) for Ta2Se. The Ta atoms in TaSe2 are three-coordinated but four-coordinated in Ta2Se. Interestingly, when Cu atoms were intercalated into the TaSe2 van der Waals gap, the superconducting transition temperature can be increased from 0.14 K for pure 2H-TaSe2 to Tc,max = 2.7 K CuxTaSe2.20 Thus, for Ta2Se, will the intercalation of Ta atoms induce the superconductivity and yield a high Tc?
The preparation of Ta2Se and the phase determination method are shown in the Supporting Information. An obtained Ta2Se chunk from arc melting was determined to contain a small amount of 1T-TaSe2 (P3̅m1) as the impurity (∼6.5 wt %). The powder X-ray diffraction (XRD) pattern shown in Figure 1b matches the previously reported Ta2Se pattern very well.2 To determine if a charge-density wave exists in Ta2Se at low temperatures, similar to the case in TaSe2, we performed low-temperature powder XRD measurements at 100 and 200 K. No superlattice peaks were observed above 100 K for the Ta2Se phase, as shown in Figure S1. This indicates no evidence for the existence of a charge-density wave above 100 K in Ta2Se. As shown in Figure 1a, two crystallographically different Ta sites, marked as Ta1 and Ta2, and one Se site exist in the Ta2Se binary compound. Specifically, the Ta1 bilayer was sandwiched by two edge-shared Ta2@Se4 layers, and the resulting Se–Ta2–Ta1–Ta1–Ta2–Se layers stack along the c axis to form a layered Ta2Se structure. The Ta1–Ta1 and Ta1–Ta2 bonds are 2.831(2) and 2.895(2) Å, respectively, while the Ta2 atoms are separate with Se with a length of 2.665(3) Å. The long Se–Se distance (∼3.57 Å) indicates that van der Waals force bonds the Se–Ta4–Se layers in Ta2Se. Moreover, the chemical composition was also determined by scanning electron microscopy–energy-dispersive X-ray spectroscopy (SEM–EDS), as shown in Table S1 and Figure S2, which indicates a formula of Ta1.92(6)Se, and excess Se shows that TaSe2 can be a plausible impurity.
Figure 1.
(a) Crystal structure of Ta2Se, where red and cyan balls represent Ta and Se atoms, respectively. (b) Refined powder XRD pattern for Ta2Se. The black line with balls, red line, blue line, green vertical ticks, and orange vertical ticks stand for observed and calculated patterns, difference between the observed and calculated patterns, and Ta2Se and 1T-TaSe2 Bragg peaks. Insets show the magnified versions of powder XRD pattern fitting.
Detailed physical property measurements are described in the Supporting Information. The plot of the volume magnetic susceptibility (χV) versus temperature after diamagnetic correction is shown in Figure 2a. The large diamagnetic signal below 3.8 K indicates the occurrence of superconductivity in this compound. On the basis of the zero-field-cooled (ZFC) signal, the transition is broad, likely because of the relatively large applied field (50 Oe), and does not saturate even at the lowest available temperature. However, 4πχV(2 K) = −1.07, the absolute value of which is larger than that expected for the full Meissner fraction (4πχV = −1). This discrepancy is caused by a demagnetization effect and is dependent on the sample shape and its orientation with respect to the direction of the external magnetic field. The field-cooled (FC) signal is much weaker compared to the ZFC signal, usually resulting from strong flux trapping in Ta2Se and is typically observed in polycrystalline samples. The critical superconducting temperature (Tc) was estimated as the intersection between two lines marked in red in Figure 2a: the first one is the steepest slope line of the superconducting signal, and the second one is an extrapolation of the normal metal state χV to lower temperature.21 The value thus obtained is Tc = 3.85 K, higher than the critical temperature for TaSe2 (Tc = 0.22 K22) and lower than that reported for pure Ta metal (Tc = 4.4 K).23 The width of the transition and the critical temperature decrease with increasing applied magnetic field, and the signal completely vanishes above 3500 Oe. The field dependence of magnetization is shown in Figure S3, which indicates a typical character of type II superconductors.
Figure 2.
(a) Temperature dependence of the ZFC and FC volume magnetic susceptibilities for Ta2Se. The data were collected between 1.8 and 5 K in applied magnetic field μ0H = 5 mT. (b) Electrical resistivity ρ(T) of Ta2Se measured in zero magnetic field. (c) Expanded plot of the low-temperature ρ(T) showing the superconducting transition for different magnetic fields from 0 to 0.5 T. Horizontal lines represent a residual resistivity and half of the transition, respectively. (d) Upper critical field μ0Hc2 versus temperature of Ta2Se determined from the electrical resistivity ρ(T,H) data in panel c. The red curve is a fit obtained using the G–L equation.
Subsequently, the resistivity measurements were carried out in the Physical Property Measurement System Quantum Design Dynacool with a four-probe technique. Figure 2b presents the resistivity as a function of the temperature in the range of 1.8–300 K without application of an external magnetic field. The resistivity undergoes a sudden drop at 3.8 K, which is an indication of superconductivity. In the normal state, the ρ(T) curve exhibits metallic behavior of the Bloch–Grüneisen type. Typical behaviors in the resistivity for polycrystalline metals were observed with a low residual resistivity ratio [RRR = ρ(300 K)/ρ(4 K) = 3]. Figure 2c emphasizes the low-temperature resistivity under various magnetic fields from 0 to 0.5 T. At μ0H = 0 T, an abrupt resistivity drop due to the superconducting transition is clearly observed at Tc = 3.8 K. As can be seen, the superconducting transition temperatures were suppressed with larger fields. Above 1.8 K, the zero-resistance behavior is not observed for μ0H = 0.5 T and the resistivity drop disappears for μ0H > 0.75 T (not shown here). Using the criterion that the point with 50% normal state resistivity suppressed can be considered to be the transition temperature, we determined the upper critical field μ0Hc2(T) for Ta2Se at various temperatures below 3.8 K (Figure 2d). The data are fitted with the following Ginzburg–Landau (G–L) relationship:24
where t = T/Tc, in which Tc is a fitting parameter (transition temperature at zero magnetic field). The G–L relation well describes the experimental data, and it yields μ0Hc2(0) = 0.75(1) T and Tc = 3.86(1) K. The obtained upper critical field does not exceed the Pauli limiting field for the weak-coupling Bardeen–Cooper–Schrieffer (BCS) superconductors25Hc2P(0) = 1.85Tc, which for Tc = 3.8 K gives Hc2(0) = 7 T. The critical field that we obtained for Ta2Se is over 9 times larger than that reported for elemental Ta (∼0.083 T).26,27 Even though the critical temperature of Ta2Se decreases from 4.5 to 3.7 K with the insertion of Se layers into Ta layers, the segregation of Ta atoms increases the ability for Ta2Se to resist a magnetic field, which indicates a stronger electron–phonon coupling.
Heat capacity measurement by measuring the entropy changes during
the superconducting transition is reliable evidence of the presence
of bulk superconductivity. To prove that the superconductivity is
intrinsic to Ta2Se and is not a consequence of the possible
impurity phases in the sample, such as TaSe2 or Ta, specific
heat measurements were conducted on the Ta2Se sample. Superconductivity
can be considered to be a “phase” transition, with a
superconducting phase transition occurring below the critical temperature. Figure 3a depicts a closer
view of the data under zero magnetic field. Bulk superconductivity
was also proven by a significant anomaly at 3.8 K, close to the Tc value obtained from resistivity and magnetic
measurements. Cp jumps at Tc, estimated by using the equal entropy construction (blue
solid lines), are about ΔC/Tc = 16 mJ mol–1 K–2. Figure 3b illustrates
the heat capacity behavior of Ta2Se under an external magnetic
field of 1 T. The data can be fitted by Cp/T = γ + βT2, where γ and β are determined by electronic and phononic
contributions, respectively. The extrapolation gives γ = 12.4(1)
mJ mol–1 K–2 and β = 0.29(1)
mJ mol–1 K–4. Furthermore, the
Debye temperature can be estimated through the relationship 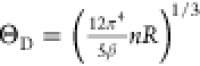 , where R = 8.314 J mol–1 K–1 and n = 3 for Ta2Se.
The obtained Debye temperature is 271(1) K, which is larger than the
value for a pure Ta element (ΘD = 240 K). Using the
Sommerfeld coefficient [γ = 12.4(1) mJ mol–1 K–2] and the previously derived specific heat
jump at Tc, the superconducting parameter
ΔC/γTc =
1.29 can be calculated. The obtained value is slightly lower than
the theoretical value based on the BCS theory (ΔC/γTc ∼ 1.43), likely caused
by the presence of impurity phases, which is consistent with the powder
XRD refinement.
, where R = 8.314 J mol–1 K–1 and n = 3 for Ta2Se.
The obtained Debye temperature is 271(1) K, which is larger than the
value for a pure Ta element (ΘD = 240 K). Using the
Sommerfeld coefficient [γ = 12.4(1) mJ mol–1 K–2] and the previously derived specific heat
jump at Tc, the superconducting parameter
ΔC/γTc =
1.29 can be calculated. The obtained value is slightly lower than
the theoretical value based on the BCS theory (ΔC/γTc ∼ 1.43), likely caused
by the presence of impurity phases, which is consistent with the powder
XRD refinement.
Figure 3.

(a) Specific heat anomaly in a zero magnetic field at low temperatures with Tc = 3.77 K. (b) Cp/T versus T2 plot under a μ0H = 1 T magnetic field.
With the Debye temperature available, the electron–phonon constant λe–p can be obtained through the inverted McMillan equation:28
where μ* is the repulsive screened Coulomb part. The value of μ* is usually set to 0.13 for intermetallic superconductors. Using ΘD = 271(1) K and Tc = 3.8 K (obtained from the specific heat measurements), one obtains λe–p = 0.61, which suggests weak electron–phonon coupling behavior.
The total band structure and projection (as shown by the band thickness) of the d orbitals in Ta atoms and the p orbitals in Se atoms without including the spin–orbit coupling (SOC) effect are calculated, as illustrated in Figure 4. The Fermi levels are dominated by d electrons from both Ta sites both with and without SOC cases, which indicates the critical role of the metal–metal bond in the stability and superconductivity in Ta2Se. The electrons on the p orbitals from Se atoms mainly contribute ∼2.5 eV below the Fermi level to stabilize the structure. The flat bands from the Γ to Z points near the Fermi level, which were mentioned above, are dominated by the d orbitals of Ta atoms and thus lead to a van Hove singularity near the Fermi level in the density of states (DOS). Moreover, after including the SOC effect on Ta atoms, a small band gap (∼100 meV) at the Γ point ∼0.1 eV above the Fermi level can be observed. The DOS at the Fermi level was strengthened by ∼10% the SOC effects marked in the red arrows were included, which ends up forming a sharp peak at EF. Such an intensive DOS at EF can result in some electronic instability and exotic physical properties such as superconductivity. Thus, it is highly possible that more metal-rich chalcogenides can host superconductivity because of the dominant metal–metal interaction. In comparison with the DOS of elemental Ta, as shown in Figure S4, one can find that the d character from Ta is dominant around the Fermi level, similar to Ta2Se. Moreover, a van Hove singularity originating from d electrons can be found ∼100 meV above EF. Note that, in Ta2Se, the d character from the Ta2 atom shows a similar DOS peak above EF. This implies the significance of Ta d electrons in superconductivity in the Ta-rich system.
Figure 4.
Band structure of Ta2Se projected from the d orbitals of Ta atoms and the p orbitals of Se atoms, where the thicker the band, the higher the contribution of the corresponding orbital to the band (left three) and DOS of Ta2Se (right): (a) without consideration of the SOC effect; (b) with consideration of the SOC effect.
In summary, we report the first metal-rich chalcogenide superconductor Ta2Se with extensive metal–metal interactions. Ta2Se was synthesized by the arc-melting method and structurally characterized by powder XRD; the physical properties were measured by magnetism, resistance, and specific heat, and Ta2Se was discovered to be a new weak-coupling BCS superconductor at Tc ∼ 3.8 K. The electronic structures analyzed by the WIEN2k program indicate the importance of d electrons of Ta atoms in superconductivity and lead to a high probability to discover more superconductors in metal-rich chalcogenides.
Acknowledgments
The work at LSU was supported by the Arnold and Mabel Beckman Foundation through a Beckman Young Investigator Award. The work at GUT was supported by the National Science Centre (Poland; Grant UMO-2018/30/M/ST5/00773).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.inorgchem.9b03656.
Experimental details, low-temperature powder XRD for Ta2Se, SEM–EDS results, field dependence of magnetization, and DOS of elemental Ta (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Maggard P. A.; Corbett J. D. Sc2Te: A Novel Example of Condensed Metal Polyhedra in a Metal-Rich but Relatively Electron-Poor Compound. Angew. Chem., Int. Ed. Engl. 1997, 36, 1974–1976. 10.1002/anie.199719741. [DOI] [Google Scholar]
- Harbrecht B. Ta2Se: A Tantalum-Rich Selenide with a New Layer Structure. Angew. Chem., Int. Ed. Engl. 1989, 28, 1660–1662. 10.1002/anie.198916601. [DOI] [Google Scholar]
- Kim S. J.; Nanjundaswamy K. S.; Hughbanks T. Single-Crystal Structure of Tantalum Sulfide (Ta3S2). Structure and Bonding in the Ta6Sn (n = 1,3,4,5?) Pentagonal-Antiprismatic Chain Compounds. Inorg. Chem. 1991, 30, 159–164. 10.1021/ic00002a004. [DOI] [Google Scholar]
- Abdon R. L.; Hughbanks T. Hf3Te2: Ein Neues Tellurid Mit Bemerkenswerter Schichtstruktur. Angew. Chem. 1994, 106, 2414–2416. 10.1002/ange.19941062228. [DOI] [Google Scholar]
- Giester G.; Lengauer C. L.; Tillmanns E.; Zemann J. Tl2S: Re-Determination of Crystal Structure and Stereochemical Discussion. J. Solid State Chem. 2002, 168, 322–330. 10.1006/jssc.2002.9711. [DOI] [Google Scholar]
- Mattheiss L. F. Band Structures of Transition-Metal-Dichalcogenide Layer Compounds. Phys. Rev. B 1973, 8, 3719–3740. 10.1103/PhysRevB.8.3719. [DOI] [Google Scholar]
- Zhu Z. Y.; Cheng Y. C.; Schwingenschlögl U. Giant Spin-Orbit-Induced Spin Splitting in Two-Dimensional Transition-Metal Dichalcogenide Semiconductors. Phys. Rev. B: Condens. Matter Mater. Phys. 2011, 84, 153402. 10.1103/PhysRevB.84.153402. [DOI] [Google Scholar]
- Ayari A.; Cobas E.; Ogundadegbe O.; Fuhrer M. S. Realization and electrical characterization of ultrathin crystals of layered transition-metal dichalcogenides. J. Appl. Phys. 2007, 101, 014507. 10.1063/1.2407388. [DOI] [Google Scholar]
- Dawson W. G.; Bullett D. W. Electronic Structure and Crystallography of MoTe2 and WTe2. J. Phys. C: Solid State Phys. 1987, 20, 6159–6174. 10.1088/0022-3719/20/36/017. [DOI] [Google Scholar]
- Coehoorn R.; Haas C.; Dijkstra J.; Flipse C. J. F.; de Groot R. A.; Wold A. Electronic structure of MoSe2, MoS2, and WSe2. I. Band-structure calculations and photoelectron spectroscopy. Phys. Rev. B: Condens. Matter Mater. Phys. 1987, 35, 6195–6202. 10.1103/PhysRevB.35.6195. [DOI] [PubMed] [Google Scholar]
- Luo H.; Xie W.; Tao J.; Inoue H.; Gyenis A.; Krizan J. W.; Yazdani A.; Zhu Y.; Cava R. J. Polytypism, Polymorphism, and Superconductivity in TaSe2-xTex. Proc. Natl. Acad. Sci. U. S. A. 2015, 112 (11), E1174–E1180. 10.1073/pnas.1502460112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Shao D. F.; Li L. J.; Lu W. J.; Zhu X. D.; Tong P.; Xiao R. C.; Ling L. S.; Xi C. Y.; Pi L.; et al. Nature of Charge Density Waves and Superconductivity in 1T-TaSe2-xTex. Phys. Rev. B: Condens. Matter Mater. Phys. 2016, 94, 045131. 10.1103/PhysRevB.94.045131. [DOI] [Google Scholar]
- Chen L.; Corbett J. D. Remarkable Metal-Rich Ternary Chalcogenides Sc14M3Te8 (M = Ru, Os). J. Am. Chem. Soc. 2003, 125, 1170–1171. 10.1021/ja0291540. [DOI] [PubMed] [Google Scholar]
- Herle P. S.; Corbett J. D. The First Metal-Rich Binary Chalcogenides of the Lanthanides: Dy2Te and Gd2Te. Inorg. Chem. 2001, 40, 1858–1864. 10.1021/ic0012975. [DOI] [PubMed] [Google Scholar]
- Maggard P. A.; Corbett J. D. Sc5Ni2Te2: Synthesis, Structure, and Bonding of a Metal-Metal-Bonded Chain Phase, a Relative of Gd3MnI3. Inorg. Chem. 1999, 38, 1945–1950. 10.1021/ic981073a. [DOI] [PubMed] [Google Scholar]
- Weirich T. E.; Pottgen R.; Simon A. Crystal Structure of Octatitanium Triselenide, Ti8Se3. Z. Kristallogr. - Cryst. Mater. 1996, 211, 929–930. 10.1524/zkri.1996.211.12.929. [DOI] [Google Scholar]
- Castro-Castro L. M.; Chen L.; Corbett J. D. Condensed Rare-Earth Metal-Rich Tellurides. Extension of Layered Sc6PdTe2-Type Compounds to Yttrium and Lutetium Analogues and to Y7Te2, the Limiting Binary Member. J. Solid State Chem. 2007, 180, 3172–3179. 10.1016/j.jssc.2007.09.012. [DOI] [Google Scholar]
- Maggard P. A.; Corbett J. D. Sc9Te2: A Two-Dimensional Distortion Wave in the Scandium-Richest Telluride. J. Am. Chem. Soc. 2000, 122, 838–843. 10.1021/ja993316j. [DOI] [Google Scholar]
- Maggard P. A.; Corbett J. D. The Synthesis, Structure, and Bonding of Sc8Te3 and Y8Te3. Cooperative Matrix and Bonding Effects in the Solid State. Inorg. Chem. 1998, 37, 814–820. 10.1021/ic971107z. [DOI] [Google Scholar]
- Li X. C.; Zhou M. H.; Yang L. H.; Dong C. Significant enhancement of superconductivity in copper-doped 2H-TaSe2. Supercond. Sci. Technol. 2017, 30, 125001. 10.1088/1361-6668/aa9000. [DOI] [Google Scholar]
- Klimczuk T.; Cava R. J. Carbon isotope effect in superconducting MgCNi3. Phys. Rev. B: Condens. Matter Mater. Phys. 2004, 70, 212514. 10.1103/PhysRevB.70.212514. [DOI] [Google Scholar]
- Van Maaren M. H.; Schaeffer G. M. Some new superconducting group Va dichalcogenides. Phys. Lett. A 1967, 24, 645–646. 10.1016/0375-9601(67)91005-5. [DOI] [Google Scholar]
- Worley R. D.; Zemansky M. W.; Boorse H. A. Heat capacities of vanadium and tantalum in the normal and superconducting phases. Phys. Rev. 1955, 99, 447. 10.1103/PhysRev.99.447. [DOI] [Google Scholar]
- Tinkham M.; Emery V. Introduction to Superconductivity. Phys. Today 1996, 49, 74. 10.1063/1.2807811. [DOI] [Google Scholar]
- Clogston A. M. Upper limit for the critical field in hard superconductors. Phys. Rev. Lett. 1962, 9, 266. 10.1103/PhysRevLett.9.266. [DOI] [Google Scholar]
- Shaw R. W.; Mapother D. E.; Hopkins D. C. Critical fields of superconducting tin, indium, and tantalum. Phys. Rev. 1960, 120, 88. 10.1103/PhysRev.120.88. [DOI] [Google Scholar]
- Hinrichs C. H.; Swenson C. A. Superconducting critical field of tantalum as a function of temperature and pressure. Phys. Rev. 1961, 123, 1106. 10.1103/PhysRev.123.1106. [DOI] [Google Scholar]
- McMillan W. L. Transition temperature of strong-coupled superconductors. Phys. Rev. 1968, 167, 331. 10.1103/PhysRev.167.331. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





