Abstract

Many useful principles of self-assembly have been elucidated through studies of systems where multiple components combine to create a single structure. More complex systems, where multiple product structures self-assemble in parallel from a shared set of precursors, are also of great interest, as biological systems exhibit this behavior. The greater complexity of such systems leads to an increased likelihood that discrete species will not be formed, however. Here we show how the kinetics of self-assembly govern the formation of multiple metal–organic architectures from a mixture of five building blocks, preventing the formation of a discrete structure of intermediate size. By varying ligand symmetry, denticity, and orientation, we explore how five distinct polyhedra—a tetrahedron, an octahedron, a cube, a cuboctahedron, and a triangular prism—assemble in concert around CoII template ions. The underlying rules dictating the organization of assemblies into specific shapes are deciphered, explaining the formation of only three discrete entities when five could form in principle.
Molecules may follow complex pathways during self-assembly processes that generate multiple products. Understanding the self-sorting processes that occur within these pathways may allow us to decipher how simple prebiotic chemicals developed into life,1−3 and also how to design synthetic chemical systems that may be of practical use.4−14
When different molecules self-assemble, one of three outcomes may result: social sorting,15,16 where a statistical distribution of products is observed, narcissistic sorting,17−19 where components self-recognize and generate homoleptic architectures, or integrative self-sorting,20−24 during which all components are assimilated into a single product. Other sorting modes have recently been discovered, including biased sorting regimes25 and those driven by kinetic trapping,26 stereochemical differences,27,28 or template-induced sorting.29
Metal–organic cages have displayed a wealth of sorting behaviors that can be understood in terms of thermodynamic and geometric parameters.30−35 We hypothesized that combinations of ligands with different denticities and symmetries would yield complex, but potentially predictable sorting behavior. We thus explored the sorting characteristics of three- and fourfold symmetric polyamine subcomponents with bidentate and tridentate aldehyde subcomponents, using CoII as the metal ion template during subcomponent self-assembly.
Four polyhedral coordination cages were prepared using CoII as a template ion, as shown in Figure 1, and as described in Supporting Information (SI) Section 2. Tritopic A and tetratopic B thus generated threefold and fourfold symmetry axes, respectively, and 2-formylpyridine P1 and 2-formylphenanthroline P2 created bidentate and tridentate coordination sites upon condensation with these two polyamines, allowing the CoII centers to serve as three- or twofold symmetry axes. The relative orientations of these ligand- and metal-generated symmetry elements thus brought about the geometries of CoII4L4 tetrahedron 1, CoII6L4 octahedron 2, CoII8L6 cube 3, and CoII12L6 cuboctahedron 4.25,29,36,37
Figure 1.
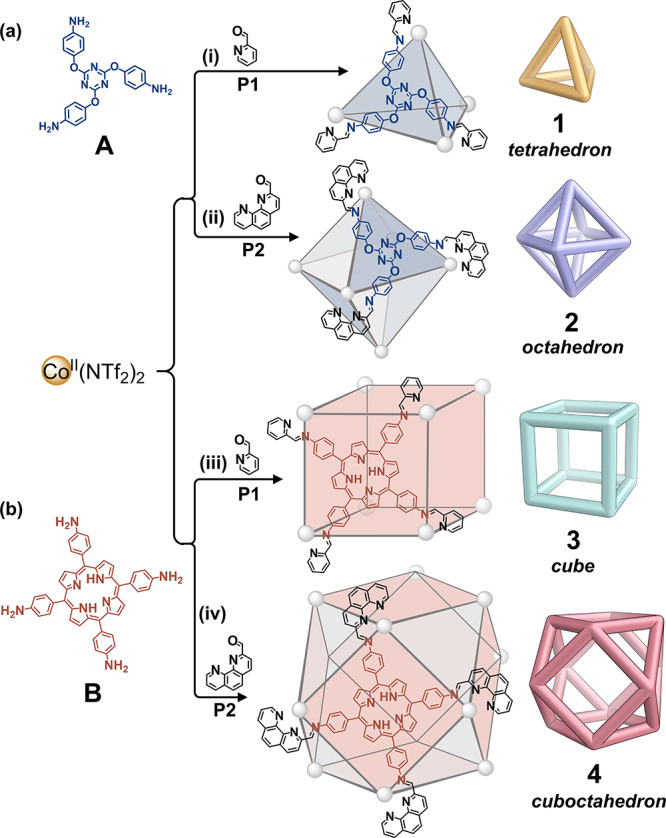
Four different architectures can be synthesized by CoII-templated imine condensation of amine A or B with aldehyde P1 or P2. (a) Threefold symmetric subcomponent A generated (i) CoII4L4 tetrahedron 1 and (ii) CoII6L4 octahedron 2. (b) Fourfold symmetric subcomponent B generated (iii) CoII8L6 cube 3 and (iv) CoII12L6 cuboctahedron 4. Lines connect nearest-neighbor metal ions.
When two different polyamines self-assemble with a single aldehyde, possible outcomes include narcissistic and integrative self-sorting. When tetratopic and tritopic amines B and C reacted with aldehyde P1 and CoII (Figure 2a), both narcissistic and integrative processes were observed to occur in parallel. The narcissistically sorted cube 3 and tetrahedron 5 were thus observed to form in equilibrium with the integrative product 6, a heteroleptic trigonal prism containing two residues of C and three of B (SI Section 3.3.2). The structure of 6 (Figure 2b) is similar to that of a reported analog,21 although the free base porphyrin faces of 6 do not adapt the inward- and outward-facing conformations observed for their NiII-centered congeners.21
Figure 2.
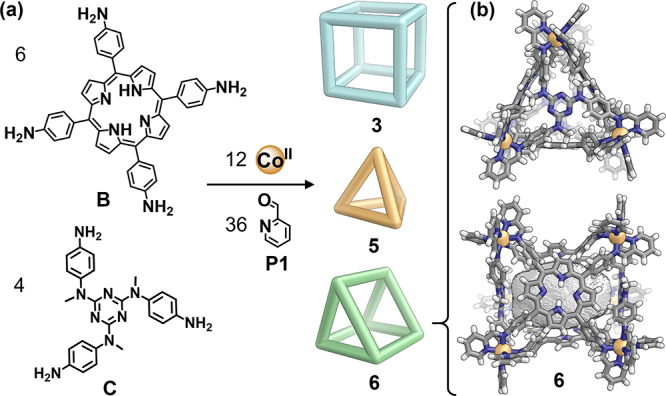
(a) Subcomponents B and C underwent both narcissistic self-sorting to produce a mixture of cube 3 (from B) and tetrahedron 5 (from C), and integrative self-assembly to produce trigonal prism 6, which incorporates both B and C. Product ratios were determined to be 23% cube 3, 51% triangular prism 6, and 26% tetrahedron 5 by 1H NMR integration. (b) X-ray crystal structure of 6, viewed facing the tritopic (top) and tetratopic (bottom) ligands. The void space inside the structure is displayed as a gray solid (Co, orange; C, gray; N, blue; H, white).
When both aldehydes P1 and P2 reacted with CoII and either amine A or B, clean narcissistic self-sorting was observed (Figure 3a, SI Sections 3.1 and 3.2). Tritopic A produced 1 and 2, and tetratopic B produced 3 and 4, with product ratios depending on the amounts of P1 and P2 used initially.38
Figure 3.
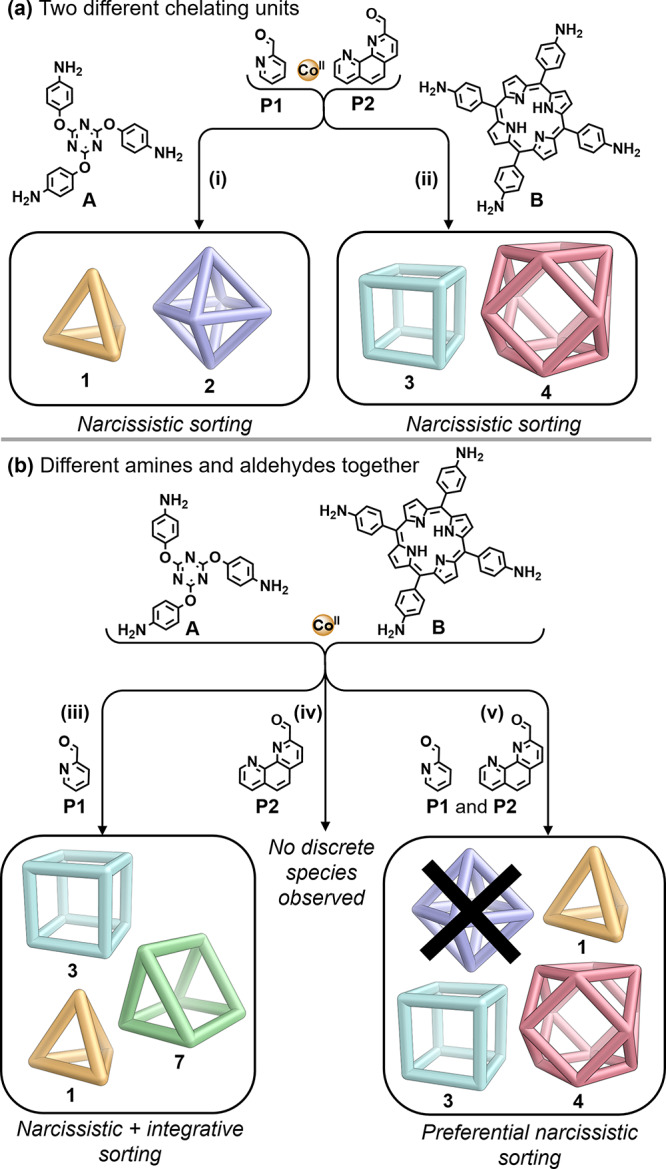
(a) Narcissistic self-sorting was observed when the two aldehyde subcomponents P1 and P2 were both employed with either (i) A or (ii) B during self-assembly. (b) More complex outcomes resulted from the self-assembly of both amines A and B with either or both of the aldehydes. (iii) A, B, and P1 combined to form homoleptic 1 and 3 and heteroleptic 7. (iv) A, B, and P2 yielded no discrete products, and (v) A, B, P1, and P2 gave 1, 3, and 4.
Different behavior was observed when only one of the aldehyde subcomponents P1 or P2 reacted with both amine subcomponents A and B and CoII (Figure 3b). When triamine A and tetramine B were combined with 2-formylpyridine P1 in a 1:1 mixture of DMF/MeCN, only tetrahedron 1 and cube 3 were observed (Figures S14, S15). However, when pure MeCN was used as the reaction solvent, a third product 7 was also observed by ESI-MS (Figure S17). Product 7 incorporates three residues of B and two of A, and we infer it to have a similar trigonal-prismatic framework to structurally characterized 6 (Figure 2b).
The combination of amines A and B with 2-formylphenanthroline P2 (Figure 3b, pathway (iv)) resulted in a mixture of products that gave a complex NMR spectrum (Figure S21) that did not display peaks corresponding to any isolated discrete product. Multiple different products may thus form, integrating both A and B, without a strong thermodynamic preference for any single outcome. The lack of narcissistic sorting of octahedron 2 and cuboctahedron 4 may also contribute to the lack of observation of the former species in the five-component sorting experiment described below.
When all five building blocks (A, B, CoII, P1, and P2) were combined in the correct ratio so as to allow an equimolar mixture of cages 1:2:3:4 to form, octahedron 2 was not observed to form (Figure 3b, pathway (v)). Instead, tetrahedron 1, cube 3, and cuboctahedron 4 were the only species observed by both 1H NMR (Figure 4) and ESI-MS (Figure S24). Monitored over 3 days of heating, 2 was not observed to form at any time (Figure S25).
Figure 4.
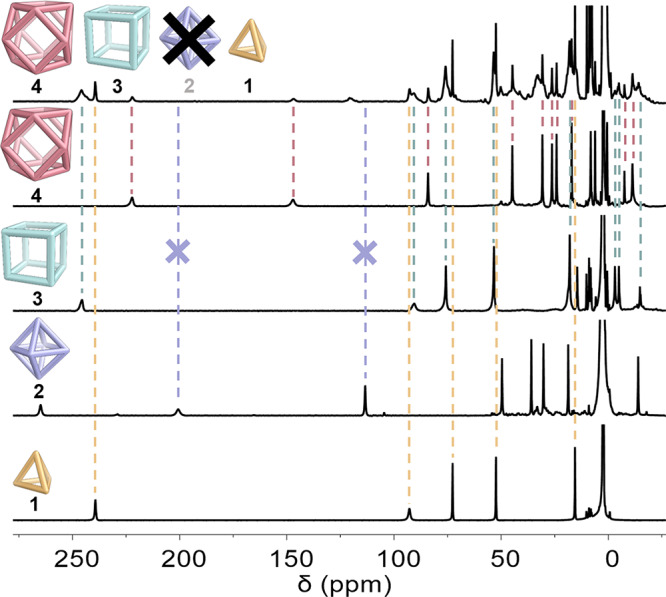
1H NMR spectra (400 MHz, 298 K, CD3CN) of each separate cage (bottom four spectra) compared to the mixture generated (Figure 3b, pathway (v)) when A, B, P1, and P2 were mixed with CoII (topmost spectrum).
In contrast with reported systems,38,39 the mixtures of products obtained in the system of Figure 3b, pathway (v) is not determined uniquely by the stoichiometry of subcomponents employed.38 A subset of only two or three of the four cages (1, 2, 3, 4) will be able to consume all of a balanced set of building blocks, i.e., where the total number of aldehyde groups is equal to the total number of amine groups, and where all CoII is coordinatively saturated. Thus, the selectivity observed when all subcomponents are present together (Figure 3b, pathway (v)) must be a result of further factors acting upon the system.
The rates of formation of the four cages were gauged, as shown in Figure 5. At regular intervals during self-assembly at 60 °C, we extracted aliquots and measured the degree of completion of assembly by UV–vis spectroscopy (SI Section 4). For all cages, we monitored the evolution of MLCT transitions (which often overlapped with ligand π → π* transitions, and porphyrin Soret bands in the cases of 3 and 4) as a function of time. A plateau in the intensity of the absorbance marked complete formation of the cage, which was then verified by 1H NMR spectroscopy. As the assembly kinetics of these structures are complex, we fitted our data to a simple exponential rate equation, enabling a comparison of assembly half-lives between cages.
Figure 5.
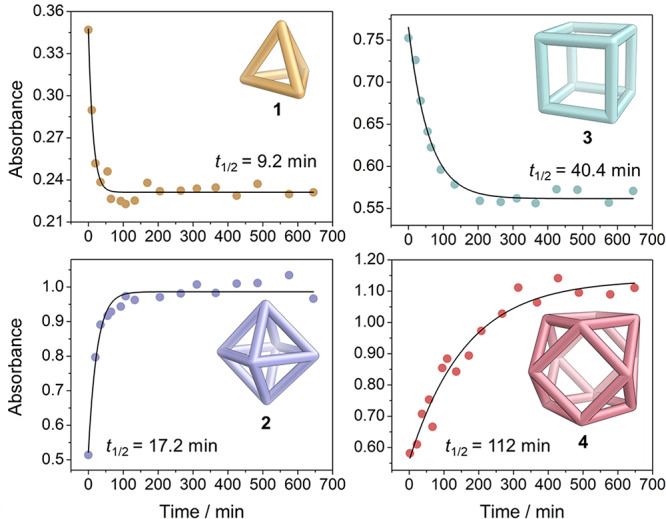
Plots showing the rate of formation of each homoleptic architecture, monitored by UV–vis spectroscopy, following the principal optical bands of 1 (346 nm) and 2 (370 nm), and the MLCT transitions of 3 (430 nm) and 4 (445 nm), which overlapped with porphyrin Soret bands. Black lines represent the best fits to an exponential rate equation (Absorbance = A0 + Aeλt), from which t1/2 = ln 2/λ.
The data of Figure 5 show clear differences between the rates of formation of the four structures. Tetrahedron 1 forms most rapidly, followed by octahedron 2, cube 3, and cuboctahedron 4. This sequence reflects the increasing structural complexity of these assemblies.
These rate differences (Figure 5) shed light upon the selectivity exhibited by the system of Figure 3b, pathway (v), as shown in Figure 6. As tetrahedron 1 forms most rapidly, it consumes all of the A and most of the P1 from the initial mixture. The remaining P1 must react with B to form cube 3, leaving additional B to react with the P2 to form cuboctahedron 4. Structures 3 and 4 may form in either order, as the system becomes deterministic38 following the conversion of all A into 1, with only one fate possible for each of the remaining subcomponents. The relative rates of 3 and 4 formation (Figure 5) suggest that 3 will be formed ahead of 4, however.
Figure 6.

An outline of the process inferred to occur when A, B, P1, P2, and CoII are mixed in the proportions shown. (a) Tetrahedron 1 forms first, sequestering all A. (b) Cuboctahedron 4 may then form, consuming all P2, followed by (c) cube 3, or else (d) cube 3 may form first, consuming all P1, followed by cuboctahedron 4. Both paths lead to an identical final state in which only 1, 3, and 4 are observed.
The first structure to form, tetrahedron 1, thus sets the scene for the system’s subsequent self-assembly by preferentially consuming all of A that octahedron 2 would have otherwise required. This system appears quite sensitive to subtle effects, given the relatively small (less than a factor of 2) difference between the formation times of the competing structures 1 and 2. Notably, 2 was never observed when different reactant stoichiometries were employed during the reaction described in Figure 3b, pathway (v) (Figures S26–28).
Host–guest binding can influence the kinetics of cage formation.40,41 This study thus lays the foundations to direct the self-assembly of systems of cages that share building blocks through the addition of guests, and other “cofactors” whose influence on one component of the system may propagate through its entirety, amplifying certain structures and suppressing others. In systems where cages are serving useful functions, such as catalysis42,43 or cargo transport,44,45 such an understanding may allow the development of these functions to be programmed in a complex way from a simple set of input stimuli.
Acknowledgments
This work was supported by the UK Engineering and Physical Sciences Research Council (EPSRC, EP/P027067/1). F.J.R. thanks Cambridge Australia Scholarships for Ph.D. funding. We thank Diamond Light Source for time on beamline I19 (MT-11397).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.0c02444.
The authors declare no competing financial interest.
Supplementary Material
References
- Altay M.; Altay Y.; Otto S. Parasitic Behavior of Self-Replicating Molecules. Angew. Chem., Int. Ed. 2018, 57, 10564–10568. 10.1002/anie.201804706. [DOI] [PubMed] [Google Scholar]
- Altay Y.; Tezcan M.; Otto S. Emergence of a New Self-Replicator from a Dynamic Combinatorial Library Requires a Specific Pre-Existing Replicator. J. Am. Chem. Soc. 2017, 139, 13612–13615. 10.1021/jacs.7b07346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luisi P. L. Chemistry Constraints on the Origin of Life. Isr. J. Chem. 2015, 55, 906–918. 10.1002/ijch.201400177. [DOI] [Google Scholar]
- Safont-Sempere M. M.; Fernández G.; Würthner F. Self-Sorting Phenomena in Complex Supramolecular Systems. Chem. Rev. 2011, 111, 5784–5814. 10.1021/cr100357h. [DOI] [PubMed] [Google Scholar]
- Lehn J.-M. Toward complex matter: Supramolecular chemistry and self-organization. Proc. Natl. Acad. Sci. U. S. A. 2002, 99, 4763–4768. 10.1073/pnas.072065599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northrop B. H.; Zheng Y.-R.; Chi K.-W.; Stang P. J. Self-Organization in Coordination-Driven Self-Assembly. Acc. Chem. Res. 2009, 42, 1554–1563. 10.1021/ar900077c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C.-W.; Miljanïc O. Š. Adsorption-Driven Self-Sorting of Dynamic Imine Libraries. Angew. Chem. 2015, 127, 2247–2250. 10.1002/ange.201409741. [DOI] [PubMed] [Google Scholar]
- Vantomme G.; Meijer E. W. The construction of supramolecular systems. Science 2019, 363, 1396–1397. 10.1126/science.aav4677. [DOI] [PubMed] [Google Scholar]
- Hou X.; Ke C.; Bruns C. J.; McGonigal P. R.; Pettman R. B.; Stoddart J. F. Tunable solid-state fluorescent materials for supramolecular encryption. Nat. Commun. 2015, 6, 6884. 10.1038/ncomms7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler M.; Dong R. Y.; Michal C. A.; Hamad W. Y.; MacLachlan M. J.; Giese M. Hydrogen-Bonded Liquid Crystals in Confined Spaces - Toward Photonic Hybrid Materials. Adv. Funct. Mater. 2018, 28, 1800207. 10.1002/adfm.201800207. [DOI] [Google Scholar]
- Aβhoff S. J.; Sukas S.; Yamaguchi T.; Hommersom C. A.; Le Gac S.; Katsonis N. Superstructures of chiral nematic microspheres as all-optical switchable distributors of light. Sci. Rep. 2015, 5, 14183. 10.1038/srep14183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg E.; Niazov-Elkan A.; Cohen E.; Tsarfati Y.; Rybtchinski B. Noncovalent Aqua Materials Based on Perylene Diimides. Acc. Chem. Res. 2019, 52, 2634–2646. 10.1021/acs.accounts.9b00188. [DOI] [PubMed] [Google Scholar]
- Karalius A.; Zhang Y.; Kravchenko O.; Elofsson U.; Szabó Z.; Yan M.; Ramström O. Formation and Out-of-Equilibrium, High/Low State Switching of a Nitroaldol Dynamer in Neutral Aqueous Media. Angew. Chem., Int. Ed. 2020, 59, 3434. 10.1002/anie.201911706. [DOI] [PubMed] [Google Scholar]
- Septavaux J.; Tosi C.; Jame P.; Nervi C.; Gobetto R.; Leclaire J. Simultaneous CO2 capture and metal purification from waste streams using triple-level dynamic combinatorial chemistry. Nat. Chem. 2020, 12, 202–212. 10.1038/s41557-019-0388-5. [DOI] [PubMed] [Google Scholar]
- Li J.; Nowak P.; Otto S. Dynamic Combinatorial Libraries: From Exploring Molecular Recognition to Systems Chemistry. J. Am. Chem. Soc. 2013, 135, 9222–9239. 10.1021/ja402586c. [DOI] [PubMed] [Google Scholar]
- Black S. P.; Sanders J. K. M.; Stefankiewicz A. R. Disulfide exchange: exposing supramolecular reactivity through dynamic covalent chemistry. Chem. Soc. Rev. 2014, 43, 1861–1872. 10.1039/C3CS60326A. [DOI] [PubMed] [Google Scholar]
- Beaudoin D.; Rominger F.; Mastalerz M. Chiral Self-Sorting of [2 + 3] Salicylimine Cage Compounds. Angew. Chem., Int. Ed. 2017, 56, 1244–1248. 10.1002/anie.201610782. [DOI] [PubMed] [Google Scholar]
- Zheng K.; Wang H.; Chow H.-F. Organogelating and narcissistic self-sorting behaviour of non-preorganized oligoamides. Chem. Sci. 2019, 10, 4015–4024. 10.1039/C9SC00861F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharyya K.; Mukherjee S.; Mukherjee P. S. Molecular Marriage through Partner Preferences in Covalent Cage Formation and Cage-to-Cage Transformation. J. Am. Chem. Soc. 2013, 135, 554–557. 10.1021/ja310083p. [DOI] [PubMed] [Google Scholar]
- He Z.; Jiang W.; Schalley C. A. Integrative self-sorting: a versatile strategy for the construction of complex supramolecular architecture. Chem. Soc. Rev. 2015, 44, 779–789. 10.1039/C4CS00305E. [DOI] [PubMed] [Google Scholar]
- Rizzuto F. J.; Carpenter J. P.; Nitschke J. R. Multisite Binding of Drugs and Natural Products in an Entropically Favorable, Heteroleptic Receptor. J. Am. Chem. Soc. 2019, 141, 9087–9095. 10.1021/jacs.9b03776. [DOI] [PubMed] [Google Scholar]
- Chen B.; Holstein J. J.; Horiuchi S.; Hiller W. G.; Clever G. H. Pd(II) Coordination Sphere Engineering: Pyridine Cages, Quinoline Bowls, and Heteroleptic Pills Binding One or Two Fullerenes. J. Am. Chem. Soc. 2019, 141, 8907–8913. 10.1021/jacs.9b02207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirulli M.; Kaur A.; Lewis J. E. M.; Zhang Z.; Kitchen J. A.; Goldup S. M.; Roessler M. M. Rotaxane-Based Transition Metal Complexes: Effect of the Mechanical Bond on Structure and Electronic Properties. J. Am. Chem. Soc. 2019, 141, 879–889. 10.1021/jacs.8b09715. [DOI] [PubMed] [Google Scholar]
- Schmittel M.; Saha S. Chapter Three - From Self-Sorting of Dynamic Metal-Ligand Motifs to (Supra)Molecular Machinery in Action. Adv. Inorg. Chem. 2018, 71, 135–175. 10.1016/bs.adioch.2017.11.006. [DOI] [Google Scholar]
- Rizzuto F. J.; Kieffer M.; Nitschke J. R. Quantified structural speciation in self-sorted CoII6L4 cage systems. Chem. Sci. 2018, 9, 1925–1930. 10.1039/C7SC04927G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y.; Huang J.; Tang B. Z. Kinetic trapping – a strategy for directing the self-assembly of unique functional nanostructures. Chem. Commun. 2016, 52, 11870–11884. 10.1039/C6CC03620A. [DOI] [PubMed] [Google Scholar]
- Zhong J.; Zhang L.; August D. P.; Whitehead G. F. S.; Leigh D. A. Self-Sorting Assembly of Molecular Trefoil Knots of Single Handedness. J. Am. Chem. Soc. 2019, 141, 14249–14256. 10.1021/jacs.9b06127. [DOI] [PubMed] [Google Scholar]
- Tateishi T.; Kojima T.; Hiraoka S. Chiral self-sorting process in the self-assembly of homochiral coordination cages from axially chiral ligands. Commun. Chem. 2018, 1, 20. 10.1038/s42004-018-0020-4. [DOI] [Google Scholar]
- Rizzuto F. J.; Nitschke J. R. Stereochemical plasticity modulates cooperative binding in a CoII12L6 cuboctahedron. Nat. Chem. 2017, 9, 903–908. 10.1038/nchem.2758. [DOI] [PubMed] [Google Scholar]
- Holloway L. R.; Bogie P. M.; Hooley R. J. Controlled self-sorting in self-assembled cage complexes. Dalton Trans. 2017, 46, 14719–14723. 10.1039/C7DT03399K. [DOI] [PubMed] [Google Scholar]
- Fujita D.; Ueda Y.; Sato S.; Mizuno N.; Kumasaka T.; Fujita M. Self-assembly of tetravalent Goldberg polyhedra from 144 small components. Nature 2016, 540, 563–566. 10.1038/nature20771. [DOI] [PubMed] [Google Scholar]
- Caulder D. L.; Brückner C.; Powers R. E.; König S.; Parac T. N.; Leary J. A.; Raymond K. N. Design, Formation and Properties of Tetrahedral M4L4 and M4L6 Supramolecular Clusters. J. Am. Chem. Soc. 2001, 123, 8923–8938. 10.1021/ja0104507. [DOI] [PubMed] [Google Scholar]
- Rizzuto F. J.; von Krbek L. K. S.; Nitschke J. R. Strategies for binding multiple guests in metal-organic cages. Nat. Rev. Chem. 2019, 3, 204–222. 10.1038/s41570-019-0085-3. [DOI] [Google Scholar]
- Chakraborty S.; Newkome G. R. Terpyridine-based metallosupramolecular constructs: tailored monomers to precise 2D-motifs and 3D-metallocages. Chem. Soc. Rev. 2018, 47, 3991–4016. 10.1039/C8CS00030A. [DOI] [PubMed] [Google Scholar]
- Frank M.; Krause L.; Herbst-Irmer R.; Stalke D.; Clever G. H. Narcissistic self-sorting vs. statistic ligand shuffling within a series of phenothiazine-based coordination cages. Dalton Trans. 2014, 43, 4587–4592. 10.1039/C3DT53243G. [DOI] [PubMed] [Google Scholar]
- Ferguson A.; Staniland R. W.; Fitchett C. M.; Squire M. A.; Williamson B. E.; Kruger P. E. Variation of guest selectivity within [Fe4L4]8+ tetrahedral cages through subtle modification of the face-capping ligand. Dalton Trans. 2014, 43, 14550–14553. 10.1039/C4DT02337D. [DOI] [PubMed] [Google Scholar]
- Meng W.; Breiner B.; Rissanen K.; Thoburn J. D.; Clegg J. K.; Nitschke J. R. A Self-Assembled M8L6 Cubic Cage that Selectively Encapsulates Large Aromatic Guests. Angew. Chem., Int. Ed. 2011, 50, 3479–3483. 10.1002/anie.201100193. [DOI] [PubMed] [Google Scholar]
- Sarma R. J.; Nitschke J. R. Self-Assembly in Systems of Subcomponents: Simple Rules, Subtle Consequences. Angew. Chem., Int. Ed. 2008, 47, 377–380. 10.1002/anie.200703877. [DOI] [PubMed] [Google Scholar]
- Ayme J.-F.; Lehn J.-M. Self-sorting of two imine-based metal complexes: balancing kinetics and thermodynamics in constitutional dynamic networks. Chem. Sci. 2020, 11, 1114–1121. 10.1039/C9SC04988F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. R.; Manck L. E.; Tidmarsh I. S.; Stephenson A.; Taylor B. F.; Blaikie E. J.; Griend D. A. V.; Ward M. D. Structures, host-guest chemistry and mechanism of stepwise self-assembly of M4L6 tetrahedral cage complexes. Dalton Trans. 2011, 40, 12132–12145. 10.1039/c1dt10781j. [DOI] [PubMed] [Google Scholar]
- Paul R. L.; Bell Z. R.; Jeffery J. C.; McCleverty J. A.; Ward M. D. Anion-templated self-assembly of tetrahedral cage complexes of cobalt(II) with bridging ligands containing two bidentate pyrazolyl-pyridine binding sites. Proc. Natl. Acad. Sci. U. S. A. 2002, 99, 4883–4888. 10.1073/pnas.052575199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y.; Powell J. A.; Li E.; Wang Q.; Perry Z.; Kirchon A.; Yang X.; Xiao Z.; Zhu C.; Zhang L.; Huang F.; Zhou H.-C. Catalytic reactions within the cavity of coordination cages. Chem. Soc. Rev. 2019, 48, 4707–4730. 10.1039/C9CS00091G. [DOI] [PubMed] [Google Scholar]
- Brown C. J.; Toste F. D.; Bergman R. G.; Raymond K. N. Supramolecular Catalysis in Metal-Ligand Cluster Hosts. Chem. Rev. 2015, 115, 3012–3035. 10.1021/cr4001226. [DOI] [PubMed] [Google Scholar]
- Grancha T.; Carné-Sánchez A.; Hernández-López L.; Albalad J.; Imaz I.; Juanhuix J.; Maspoch D. Phase Transfer of Rhodium(II)-Based Metal-Organic Polyhedra Bearing Coordinatively Bound Cargo Enables Molecular Separation. J. Am. Chem. Soc. 2019, 141, 18349–18355. 10.1021/jacs.9b10403. [DOI] [PubMed] [Google Scholar]
- Grommet A. B.; Hoffman J. B.; Percástegui E. G.; Mosquera J.; Howe D. J.; Bolliger J. L.; Nitschke J. R. Anion Exchange Drives Reversible Phase Transfer of Coordination Cages and Their Cargoes. J. Am. Chem. Soc. 2018, 140, 14770–14776. 10.1021/jacs.8b07900. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


