Abstract
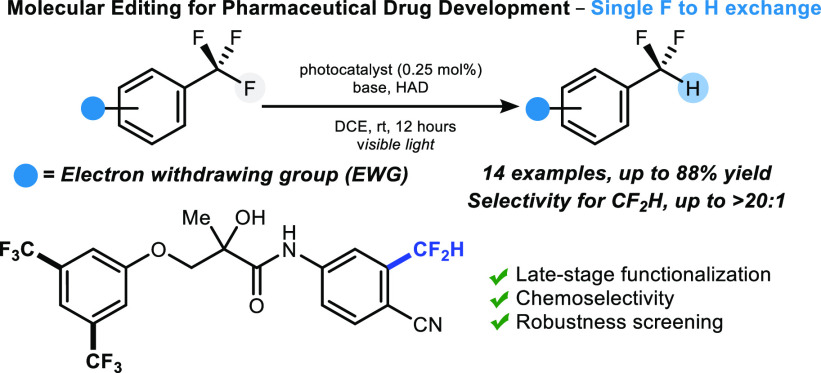
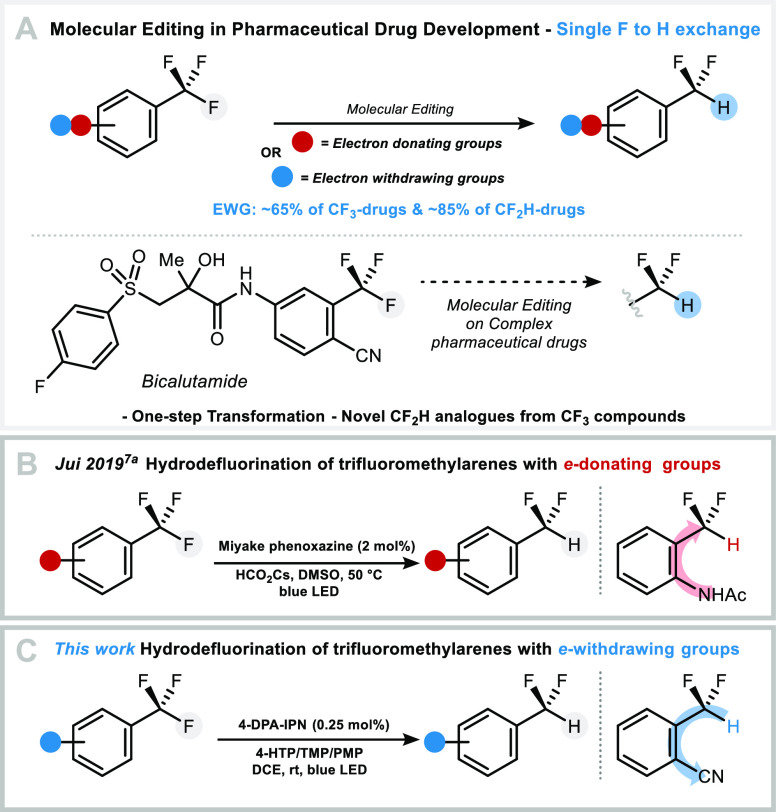
Molecular editing such as insertion, deletion, and single atom exchange in highly functionalized compounds is an aspirational goal for all chemists. Here, we disclose a photoredox protocol for the replacement of a single fluorine atom with hydrogen in electron-deficient trifluoromethylarenes including complex drug molecules. A robustness screening experiment shows that this reductive defluorination tolerates a range of functional groups and heterocycles commonly found in bioactive molecules. Preliminary studies allude to a catalytic cycle whereby the excited state of the organophotocatalyst is reductively quenched by the hydrogen atom donor, and returned in its original oxidation state by the trifluoromethylarene.
Fluorine-containing molecules have found applications in the pharmaceutical and agrochemical sector because of the thermodynamic and kinetic stability of C–F σ-bonds,1 a property offering protection against enzymatic metabolism.1 Today, pharmacophores often bear a trifluoromethyl or difluoromethyl motif in an aromatic system, thereby increasing the demand for methods to install or interchange these groups in a late-stage fashion.2 In this context, molecular editing enabling precision hydrodefluorination (HDF) of trifluoromethylarenes into difluoromethylarenes for applications in drug discovery remains a highly challenging endeavor because the bond dissociation energy (BDE) of C–F bonds decreases as fluorine substitution takes place (Scheme 1A).3,4a Pioneering studies have reported strategies employing boron, silylium or phosphine adducts,5 and (transition) metals, but uncontrolled defluorination could not be avoided for many of these processes.6 Jui and co-workers demonstrated that hydrofluorination is accomplished with cesium formate and the Miyake phenoxazine photocatalyst under blue light activation (Scheme 1B).7a This protocol is applicable to unactivated trifluoromethylarenes adorned with electron-donating groups. Despite these significant advances in the field, HDF of highly activated trifluoromethylarenes featuring electron-withdrawing groups to access difluoromethylarenes has not been accomplished. This is unexpected because most tri- and difluoromethylated drugs feature the CF3 or CF2H group on electron-poor arenes due to their higher resistance to oxidation and defluorination in comparison with electron-rich counterparts.8
Scheme 1. Hydrodefluorination of Trifluoromethylarenes in Drug Discovery.
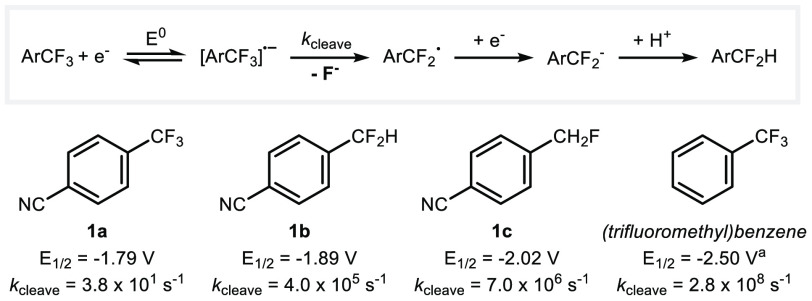
Mechanistic studies reported in 1997 by Savéant and Thiébault provided useful information on the electrochemical reductive cleavage of C–F bonds for 4-cyanofluorotoluenes 1a–c and trifluoromethylbenzene.4a The process involves fluoride expulsion from a radical anion, followed by reduction of the resulting neutral radical, and protonation. As expected, the standard reduction potential for the formation of the radical anion decreases from 1a to 1c, although these are closely spaced (narrow redox window), while the instability of the radical anion toward fluoride mesolytic cleavage increases. Facile exhaustive defluorination is observed experimentally, the challenge at hand for these highly activated substrates (Scheme 2).
Scheme 2. Electrochemical Reductive Cleavage of Trifluoromethylarenes: Standard Reduction Potentials (V vs Standard Calomel Electrode (SCE) in DMF) and Cleavage Rate Constants.
E1/2 (V vs SCE in DMF).4b
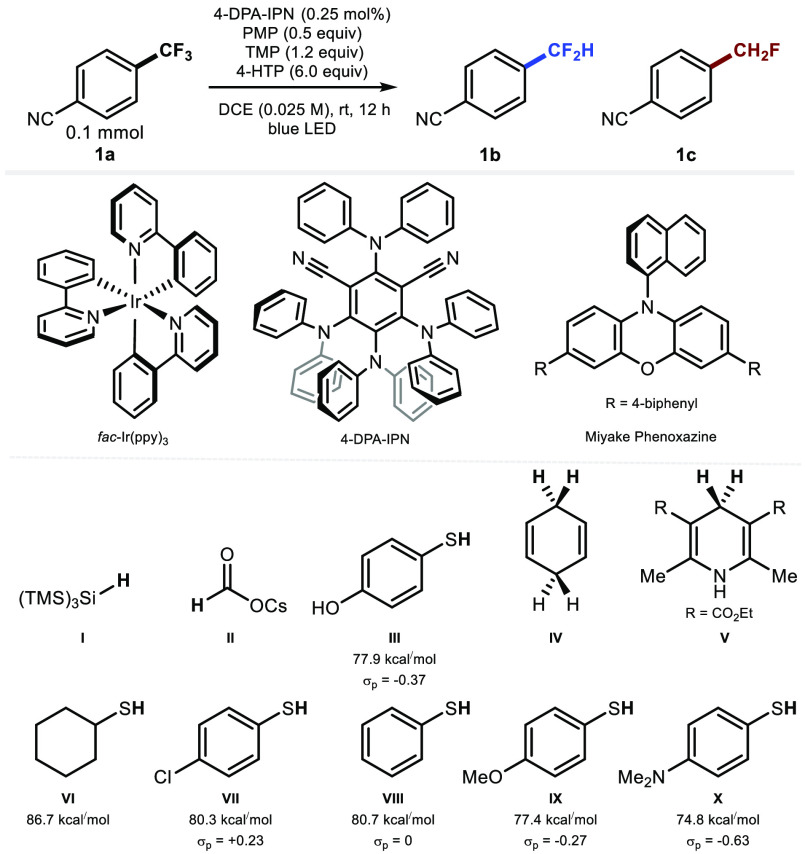
Preliminary experiments with 4-(trifluoromethyl)benzonitrile 1a showed that no product 1b was formed applying the protocols of Prakash or Lalic (Table 1, entries 1 and 2).6 Moreover, the conditions applied for known photoredox defluorination using trifluoromethylarenes led mainly to recovery of starting material (Table 1, entries 3–5).7a−7c The more electrophilic nature of the difluorinated benzylic radical derived from electron-deficient 1a compared to electron-rich substrates encouraged a study examining hydrogen atom donors (HAD) other than cesium formate.7a Gratifyingly, the reaction of 1a in the presence of 2.5 mol % fac-Ir(ppy)3, 4-hydroxythiophenol (4-HTP) and a combination of 2,2,6,6-tetramethylpiperidine (TMP) and 1,2,2,6,6-pentamethylpiperidine (PMP) in 1,2-dichloroethane (DCE) under visible light activation (blue LED) gave 1b in 53% with 5:1 selectivity (1b:1c). The fully reduced 4-methylbenzonitrile product was not detected (Table 1, entry 6). Organophotocatalyst 2,4,5,6-tetrakis(diphenylamino)isophthalonitrile (4-DPA-IPN, 2.5 mol %) was a suitable metal-free replacement for fac-Ir(ppy)3 affording 1b in 62% yield with similar selectivity (Table 1, entry 7). Lowering catalyst loading did not affect the reaction outcome (65% yield, 1b:1c = 5:1) (Table 1, entry 8). Hydrogen atom donors including thiols other than 4-HTP, 1,4-cyclohexadiene, the Hantzsch ester, (Me3Si)3SiH, or CsOCOH were less or not suitable under otherwise similar reaction conditions (Table 1, entries 9–17). The photocatalyst, HAD, base, and blue light are essential components for this transformation to proceed (Table 1, entries 18–23).
Table 1. Experiments for the Hydrodefluorination of 4-(Trifluoromethyl)benzonitrile 1a.
| Entry | Alterations to conditions | Yielda(ratio 1b:1c) |
|---|---|---|
| 1 | Mg0 (30 equiv), H2O/AcOH/DMSO | 0% |
| 2 | Pd(OAc)2 (3 mol %), CuF2 (20 mol %), 2-pyridone (5 mol %), KOSiMe3 (7.0 equiv), DMF, 45 °C, then tBuOH (2.0 equiv), 60 °C | 0% |
| 3b | Miyake Phenoxazine (2 mol %), II (3 equiv), blue LED, DMSO, 50 °C, 24 h | 4% (2:1) |
| 4c | fac-Ir(ppy)3 (1.0 mol %), TMP (2.0 equiv), HBPin (3.0 equiv), blue LED, DCE, rt, 24 h | 0% |
| 5d | PTH (10 mol %), VI (10 mol %), II (3 equiv), blue LED, 5% H2O/DMSO, rt, 24 h | 0% |
| 6 | fac-Ir(ppy)3 (2.5 mol %) | 53% (5:1) |
| 7 | 4-DPA-IPN (2.5 mol %) | 62% (5:1) |
| 8 | No alteration | 65% (5:1) |
| 9 | I (6 equiv) instead of III | trace |
| 10 | II (6 equiv) instead of III | trace |
| 11 | IV (6 equiv) instead of III | 0% |
| 12 | V (6 equiv) instead of III | 15% (5:1) |
| 13 | VI (6 equiv) instead of III | 4% (8:1) |
| 14 | VII (6 equiv) instead of III | 4% (8:1) |
| 15 | VIII (6 equiv) instead of III | 5% (8:1) |
| 16 | IX (6 equiv) instead of III | 22% (7:1) |
| 17 | X (6 equiv) instead of III | 22% (7:1) |
| 18 | no PMP | 51% (5:1) |
| 19 | no TMP | 31% (>20:1) |
| 20 | no TMP and no PMP | 0% |
| 21 | no light | 0% |
| 22 | no 4-HTP | 0% |
| 23 | no photocatalyst | 0% |
Combined yields of 1b and 1c determined by 19F NMR using 4-fluoroanisole as internal standard; the ratio of 1b:1c is given in parentheses.
Reaction carried out on 14a.
Conditions of ref (7c) with no alkene.
With the optimal reaction conditions in hand, we explored the generality of this HDF reaction (Scheme 3). 2-(Trifluoromethyl)benzonitrile gave 2b in 63% yield and >20:1 selectivity favoring CF2H. Additional functionalities on the aromatic ring including fluorine, methoxy, or acetamide were tolerated (3b, 5b, and 7b, 42–88%) with high selectivity for CF2H (>10:1). Methyl and ethyl 4-(trifluoromethyl)benzoate 4a and 8a with a carboxylic ester instead of a cyano group were transformed into difluoromethylated analogues 4b and 8b in moderate yields (30% and 40%) and 3:1 selectivity. When the catalyst loading was increased to 2.5 mol %, the yield was improved (8b, 63%), but the product resulting from double reductive defluorination was formed preferentially (8b:8c = 1:2). Unprotected sulfonamide 6b was within reach with excellent selectivity.
Scheme 3. Scope of HDF.
Yields and CF2H/CH2F ratio determined by quantitative 19F NMR spectroscopy using 4-fluoroanisole as internal standard. Yields of isolated products (RCF2H only) are given in parentheses.
2.5 mol % 4-DPA-IPN.
Solvent is DCE/DMSO (19:1, v/v, c = 0.025 M).
Complex molecules of biological relevance were examined next. Bicalutamide, a drug used to treat prostate cancer, underwent reductive defluorination affording 9b isolated in 43% yield and 10:1 CF2H/CH2F selectivity.10 The doubly trifluoromethylated cannabinoid receptor agonist BAY 59-3074 10a reacted exclusively at the arene. An analogue of Enobosarm featuring three trifluoromethylaryl groups served the purpose to investigate a more complex case of “arene versus arene” chemoselectivity.11 Hydrodefluorination occurred with excellent CF2H/CH2F selectivity (>20:1) at a single site, leaving the 3,5-bis-trifluoromethylarene motif untouched (11b, 53%); this result corroborates a control experiment that demonstrated that 3,5-bis-trifluoromethylbenzene was unreactive under our reaction conditions. Bendroflumethiazide 12a, a drug used for mild heart failure and hypertension via vasodilation,12 was also subjected to C–F bond reduction. Our protocol, slightly modified in order to solubilize the starting material (solvent mixture of DCE/DMSO), gave CF2H-bendroflumethiazide 12b in 56% yield although with decreased selectivity (CF2H/CH2F ratio = 4:1). This result is significant because sulfonamides and/or amines can coordinate some metals, rendering late-stage cross-coupling strategies toward aryl–CF2H bond construction more challenging.13 Enzalutamide, a hormonal therapy drug used to treat prostate cancer, also underwent HDF. This reaction yielded with excellent selectivity the difluoromethylated analogue 13b isolated in 40% yield. The usefulness of this HDF protocol was further illustrated with the synthesis of 14b, a molecule with strong androgen receptor binding affinity in vivo.14 This biologically relevant compound was previously prepared in eight steps.15 With our protocol, 14b was obtained in two steps; the precursor 14a was prepared in one step from commercially available materials,16 and HDF gave 14b isolated in 60% yield. Alternative photoredox HDF protocols were not effective.17
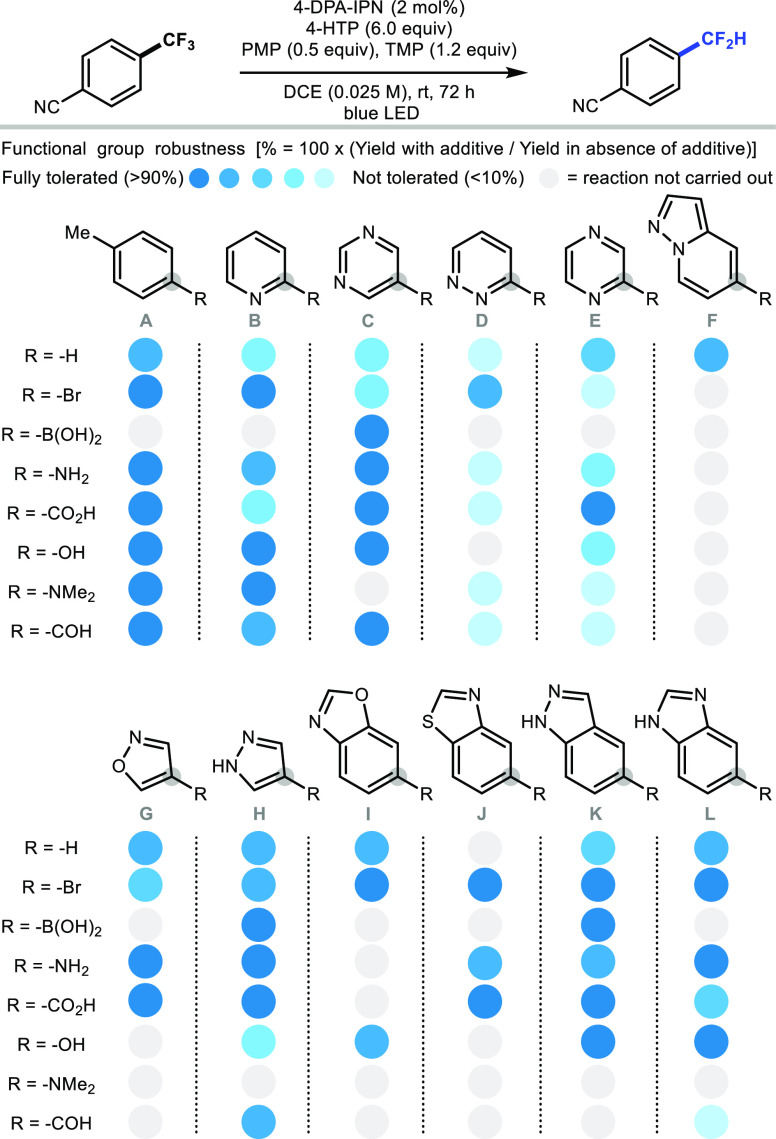
Given the relevance of this novel HDF methodology for drug discovery, we conducted a robustness screening experiment to gain further information on its tolerance to various pharmacophores and functionalities (Scheme 4).18 Common functional groups compatibility was investigated with para-substituted toluenes A, revealing tolerance to bromine, amine, alcohol, carboxylic acid, and aldehyde functionalities. Next, a range of 2-pyridines B, 5-pyrimidines C, 3-pyridazines D, and 2-pyrazines E were subjected to screening. While 2-pyridines and 5-pyrimidines were broadly tolerated, 3-pyridazines and 2-pyrazines more often prevented hydrodefluorination. Isoxazoles G and pyrazoles H with various substitution patterns were well accepted. In addition, fused heteroarenes such as pyrazolopyridines F, benzooxazoles I, benzothiazoles J, indazoles K, and benzimidazoles L did not hamper the HDF of 1a, a benefit considering the frequency of these motifs in modern pharmaceutical drugs.19 Many of the heterocycles investigated in this study could deactivate transition-metal catalysts by coordination,20,21 and the HDF offers an alternative to these methods.
Scheme 4. Additive-Based Screening.
All reactions were performed on 2.5 μmol scale in a 96-well plate suited for photoredox chemistry. Crude mixtures were analyzed by GC-FID/MS.16
Continuous-flow chemistry was considered to scale-up the HDF of 1a (Scheme 5).22 The first reactions were performed in a microflow system made of a perfluoroalkoxyalkane capillary (internal diameter = 0.5 mm; internal volume = 2.0 mL) on a scale similar to batch (0.2 mmol); 1b was obtained in 69% yield with 5:1 selectivity (CF2H/CH2F), within a 15 min residence time (tR) at a flow rate of 0.133 μL/min. Additionally, 13b (0.2 mmol) was isolated in 25% yield with 10:1 selectivity after a 7.5 min tR at a flow rate of 0.266 μL/min. Starting with 2.5 g of 1a (14.6 mmol), 1.4 g of 1b (5:1) was produced in a 15 min tR at a flow rate of 0.133 μL/min.
Scheme 5. Photoredox Hydrodefluorination under Continuous-Flow Conditions.
Yields of isolated products.
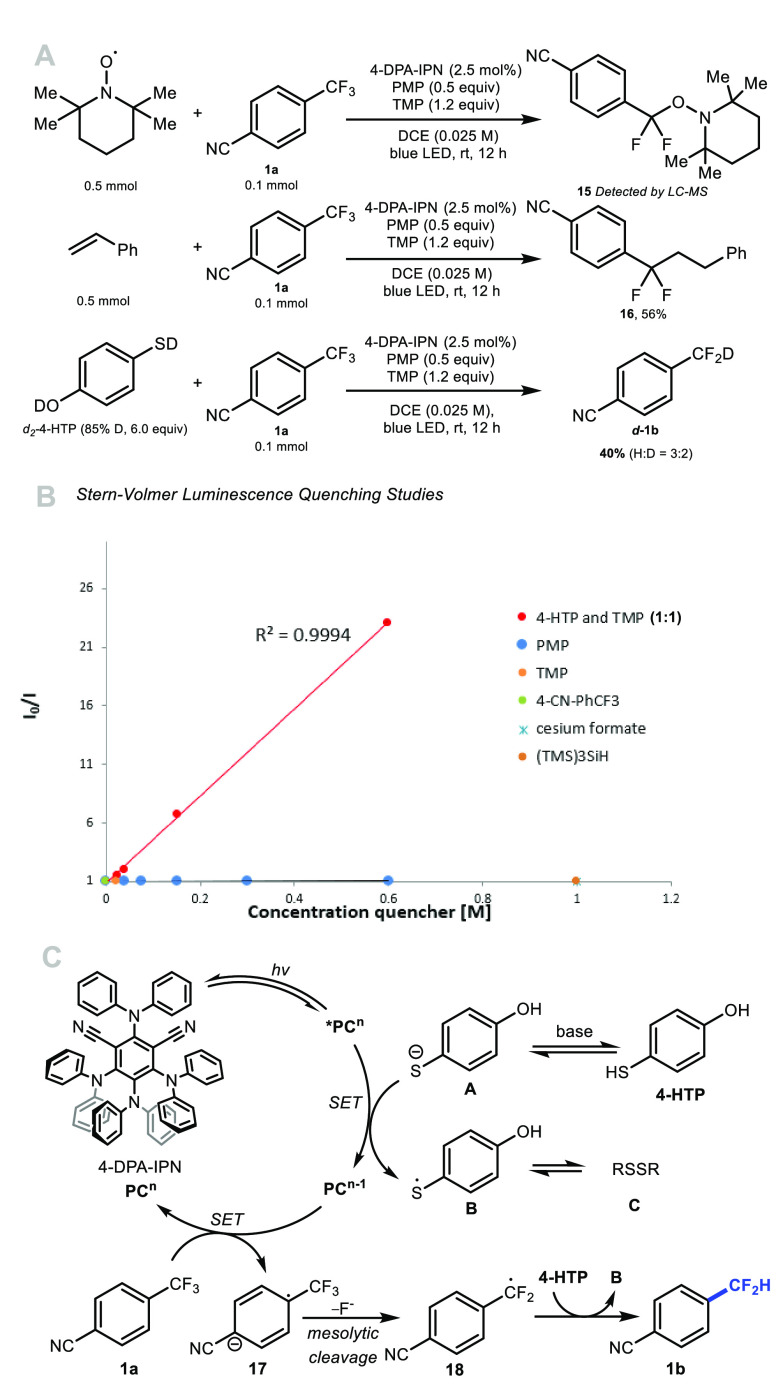
Various experiments were performed to gain preliminary insight on the mechanism of this transformation (Scheme 6).
Scheme 6. (A) Mechanistic Experiments; (B) Stern–Volmer Luminescence Quenching Studies; (C) Proposed Reaction Mechanism.
Yields determined by quantitative 19F NMR using 4-fluoroanisole as internal standard.
A radical scavenger experiment performed with TEMPO led to the formation of adduct 15 (Scheme 6A). When the reaction of 1a was carried out in the presence of styrene and 4-HTP, 16 was obtained in 56% yield (Scheme 6A). These data are consistent with the formation of a benzylic radical species formed by mesolytic C–F bond cleavage of a radical anion. Deuterium was incorporated in the product (40% yield, H/D ratio of 3:2) when d2-4-HTP was used instead of 4-HTP, indicating that 4-HTP is a plausible HAD in this reaction (Scheme 6A). Stern–Volmer luminescence quenching experiments provided additional information (Scheme 6B). We found that the combination of 4-HTP and TMP (1:1) quenches *PCn; this is in contrast to TMP, PMP, CsOCOHor (TMS)3SiH. The inability of 1a to quench the excited state of the photocatalyst *PCn advocates against an oxidative quenching cycle whereby the radical anion 17 would result from SET involving *PCn/PCn+1(−1.28 V).23 These preliminary findings led us to propose the reductive quenching cycle shown in Scheme 6C as a plausible mechanistic pathway. Irradiation with light affords the excited state catalyst *PC. Under basic conditions, deprotonation of 4-HTP leads to a thiolate A capable of reductive quenching of *PC, a process yielding the corresponding thiyl radical B,24 and the reduced photocatalyst (PCn/PCn–1= −1.52 V).23 In this scenario, the substrate acts as the oxidant to return the photocatalyst to its native oxidation state with release of the radical anion species that undergoes mesolytic cleavage of fluoride.25 This latter process leads to the C-centered difluorobenzylic radical 18 which is trapped by 4-HTP affording 1b.
In conclusion, the reductive defluorination of electron-poor trifluoromethylarenes is accomplished under basic conditions with 2,4,5,6-tetrakis(diphenylamino)isophthalonitrile as the organophotocatalyst,26 4-hydroxythiophenol as the HAD, and blue light. This operationally simple protocol tolerates a wide range of functional groups and heteroarenes frequently seen in medicinal chemistry programs, and allows the direct conversion of complex trifluoromethylated drugs into their difluoromethyl analogues. Mechanistic studies allude to a catalytic cycle whereby the photocatalyst is reduced by the HAD and returned in its native oxidation state by the trifluoromethylarene that acts as oxidant.
Acknowledgments
This project has received funding from the Engineering and Physical Sciences Research Council (EP/N509711/1) (J.B.I.S.), European Union’s Horizon 2020 research and innovation program under Marie Skłodowska-Curie Grant Agreement 721902 (C.F.M.) and 840724 (L.B.). Gloria Rosetto (Williams group, Oxford) and Gabriele Laudadio (Eindhoven) are acknowledged for assistance with the deuteration and cyclic voltammetry experiments, respectively.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.0c03881.
Additional optimization, mechanistic data, cyclic voltammetry, experimental procedures and analytical data (1H, 19F, 13C NMR, high resolution mass spectrometry) for all new compounds (PDF)
The authors declare the following competing financial interest(s): T.K. and C.W.A. are employees of Pfizer Inc.; C.F.M. and A.A.T. are employees of Janssen; C.G. is an employee of UCB Biopharma Sprl.
Supplementary Material
References
- a Müller K.; Faeh C.; Diederich F. Fluorine in Pharmaceuticals: Looking Beyond Intuition. Science 2007, 317, 1881–1886. 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]; b Purser S.; Moore P. R.; Swallow S.; Gouverneur V. Fluorine in Medicinal Chemistry. Chem. Soc. Rev. 2008, 37, 320–330. 10.1039/B610213C. [DOI] [PubMed] [Google Scholar]; c Meanwell N. A. Fluorine and Fluorinated Motifs in the Design and Application of Bioisosteres for Drug Design. J. Med. Chem. 2018, 61, 5822–5880. 10.1021/acs.jmedchem.7b01788. [DOI] [PubMed] [Google Scholar]; d Wang J.; Sánchez-Roselló M.; Aceña J. L.; del Pozo C.; Sorochinsky A. E.; Fustero S.; Soloshonok V. A.; Liu H. Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001–2011). Chem. Rev. 2014, 114, 2432–2506. 10.1021/cr4002879. [DOI] [PubMed] [Google Scholar]; e Clayden J. Fluorinated compounds present opportunities for drug discovery. Nature 2019, 573, 37–38. 10.1038/d41586-019-02611-7. [DOI] [PubMed] [Google Scholar]
- a Sessler C. D.; Rahm M.; Becker S.; Goldberg J. M.; Wang F.; Lippard S. J. CF2H, a Hydrogen Bond Donor. J. Am. Chem. Soc. 2017, 139, 9325–9332. 10.1021/jacs.7b04457. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Yerien D. E.; Barata-Vallejo S.; Postigo A. Difluoromethylation reactions of organic compounds. Chem. - Eur. J. 2017, 23, 14676–14701. 10.1002/chem.201702311. [DOI] [PubMed] [Google Scholar]; c Rong J.; Ni C.; Hu J. Metal-Catalyzed Direct Difluoromethylation Reactions. Asian J. Org. Chem. 2017, 6, 139–152. 10.1002/ajoc.201600509. [DOI] [Google Scholar]; d Lu Y.; Liu C.; Chen Q.-Y. Recent Advances in Difluoromethylation Reaction. Curr. Org. Chem. 2015, 19, 1638–1650. 10.2174/1385272819666150615235605. [DOI] [Google Scholar]
- For seminal examples on C(sp3)–F and C(sp2)–F functionalization, see:; a Senaweera S. M.; Weaver J. D. Selective Perfluoro- and Polyfluoroarylation of Meldrum’s Acid. J. Org. Chem. 2014, 79, 10466–10476. 10.1021/jo502075p. [DOI] [PubMed] [Google Scholar]; b Khaled M. B.; El Mokadem R. K.; Weaver J. D. III Hydrogen bond directed photocatalytic hydrodefluorination: overcoming electronic control. J. Am. Chem. Soc. 2017, 139, 13092–13101. 10.1021/jacs.7b06847. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]; c Xu L.; Zhang Q.; Xie Q.; Huang B.; Dai J. J.; Xu J.; Xu H. J. Pd-catalyzed defluorination/arylation of α-trifluoromethyl ketones via consecutive β-F elimination and C-F bond activation. Chem. Commun. 2018, 54, 4406–4409. 10.1039/C8CC01568F. [DOI] [PubMed] [Google Scholar]; d Nishimine T.; Taira H.; Tokunaga E.; Shiro M.; Shibata N. Enantioselective Trichloromethylation of MBH-Fluorides with Chloroform Based on Silicon-assisted C-F Activation and Carbanion Exchange Induced by a Ruppert-Prakash Reagent. Angew. Chem., Int. Ed. 2016, 55, 359–363. 10.1002/anie.201508574. [DOI] [PubMed] [Google Scholar]; e Pigeon X.; Bergeron M.; Barabé F.; Dubé P.; Frost H. N.; Paquin J. F. Activation of Allylic C-F bonds: Palladium-Catalyzed Allylic Amination of 3,3-Difluoropropenes. Angew. Chem., Int. Ed. 2010, 49, 1123–1127. 10.1002/anie.200904747. [DOI] [PubMed] [Google Scholar]; f Hamel J. D.; Paquin J. F. Activation of C-F bonds α to C-C multiple bonds. Chem. Commun. 2018, 54, 10224–10239. 10.1039/C8CC05108A. [DOI] [PubMed] [Google Scholar]; g Paquin J. F. Synthesis of Monofluoroalkenes via the Activation of Allylic C-F Bonds: A Novel Route to β-Aminofluoroalkenes Using Pd-Catalyzed Allylic Amination Reactions of 3,3-Difluoropropenes. Synlett 2011, 2011, 289–293. 10.1055/s-0030-1259333. [DOI] [Google Scholar]; h Haufe G.; Suzuki S.; Yasui H.; Terada C.; Kitayama T.; Shiro M.; Shibata N. C-F Bond Activation of Unactivated Aliphatic Fluorides: Synthesis of Fluoromethyl-3,5-diaryl-2-oxazolidinones by Desymmetrization of 2-Aryl-1,3-difluoropropan-2-ols. Angew. Chem., Int. Ed. 2012, 51, 12275–12279. 10.1002/anie.201207304. [DOI] [PubMed] [Google Scholar]; i Hazari A.; Gouverneur V.; Brown J. M. Palladium-Catalyzed Substitution of Allylic Fluorides. Angew. Chem., Int. Ed. 2009, 48, 1296–1299. 10.1002/anie.200804310. [DOI] [PubMed] [Google Scholar]; j Blessley G.; Holden P.; Walker M.; Brown J. M.; Gouverneur V. Palladium-Catalyzed Substitution and Cross-Coupling of Benzylic Fluorides. Org. Lett. 2012, 14, 2754–2757. 10.1021/ol300977f. [DOI] [PubMed] [Google Scholar]; For selected reviews on C–F bond activation, see:; k Amii H.; Uneyama K. C-F bond activation in organic synthesis. Chem. Rev. 2009, 109, 2119–2183. 10.1021/cr800388c. [DOI] [PubMed] [Google Scholar]; l Ahrens T.; Kohlmann J.; Ahrens M.; Braun T. Functionalization of Fluorinated Molecules by Transition-Metal-Mediated C-F Bond Activation to Access Fluorinated Building Blocks. Chem. Rev. 2015, 115, 931–972. 10.1021/cr500257c. [DOI] [PubMed] [Google Scholar]; m Senaweera S. M.; Singh A.; Weaver J. D. Photocatalytic Hydrodefluorination: Facile Access to Partially Fluorinated Aromatics. J. Am. Chem. Soc. 2014, 136, 3002–3005. 10.1021/ja500031m. [DOI] [PubMed] [Google Scholar]
- a Andrieux C. P.; Combellas C.; Kanoufi F.; Savéant J.; Thiébault A. Dynamics of Bond Breaking in Ion Radicals. Mechanisms and Reactivity in the Reductive Cleavage of Carbon-Fluorine Bonds of Fluoromethylarenes. J. Am. Chem. Soc. 1997, 119, 9527–9540. 10.1021/ja971094o. [DOI] [Google Scholar]; b Clavel P.; Lessene G.; Biran C.; Bordeau M.; Roques N.; Trévin S.; Montauzon D. D. Selective electrochemical synthesis and reactivity of functional benzylic fluorosilylsynthons. J. Fluorine Chem. 2001, 107, 301–310. 10.1016/S0022-1139(00)00373-0. [DOI] [Google Scholar]
- a Ferraris D.; Cox C.; Anand R.; Lectka T. C-F Bond Activation by Aryl Carbocations: Conclusive Intramolecular Fluoride Shifts between Carbon Atoms in Solution and the First Examples of Intermolecular Fluoride Ion Abstractions. J. Am. Chem. Soc. 1997, 119, 4319–4320. 10.1021/ja963090+. [DOI] [Google Scholar]; b Yoshida S.; Shimomori K.; Kim Y.; Hosoya T. Single C-F Bond Cleavage of Trifluoromethylarenes with an ortho-Silyl Group. Angew. Chem., Int. Ed. 2016, 55, 10406–10409. 10.1002/anie.201604776. [DOI] [PubMed] [Google Scholar]; c Mallov I.; Ruddy A. J.; Zhu H.; Grimme S.; Stephan D. W. C-F Bond Activation by Silylium Cation/Phosphine Frustrated Lewis Pairs: Mono-Hydrodefluorination of PhCF3, PhCF2H and Ph2CF2. Chem. - Eur. J. 2017, 23, 17692–17696. 10.1002/chem.201705276. [DOI] [PubMed] [Google Scholar]; d Mandal D.; Gupta R.; Jaiswal A. K.; Young R. D. Frustrated Lewis-Pair-Meditated Selective Single Fluoride Substitution in Trifluoromethyl Groups. J. Am. Chem. Soc. 2020, 142, 2572–2578. 10.1021/jacs.9b12167. [DOI] [PubMed] [Google Scholar]
- a Munoz S. B.; Ni C.; Zhang Z.; Wang F.; Shao N.; Mathew T.; Olah G. A.; Prakash G. K. S. Selective Late-Stage Hydrodefluorination of Trifluoromethylarenes: A Facile Access to Difluoromethylarenes. Eur. J. Org. Chem. 2017, 2017, 2322–2326. 10.1002/ejoc.201700396. [DOI] [Google Scholar]; b Dang H.; Whittaker A. M.; Lalic G. Catalytic activation of a single C-F bond in trifluoromethyl arenes. Chem. Sci. 2016, 7, 505–509. 10.1039/C5SC03415A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Vogt D. B.; Seath C. P.; Wang H.; Jui N. T. Selective C-F Functionalization of Unactivated Trifluoromethylarenes. J. Am. Chem. Soc. 2019, 141, 13203–13211. 10.1021/jacs.9b06004. [DOI] [PMC free article] [PubMed] [Google Scholar]; For seminal examples of photoredox catalyzed hydrodefluoroalkylation, see:; b Wang H.; Jui N. T. Catalytic Defluoroalkylation of Trifluoromethylaromatics with Unactivated Alkenes. J. Am. Chem. Soc. 2018, 140, 163–166. 10.1021/jacs.7b12590. [DOI] [PubMed] [Google Scholar]; c Chen K.; Berg N.; Gschwind R.; König B. Selective Single C(sp3)-F Bond Cleavage in Trifluoromethylarenes: Merging Visible-Light Catalysis with Lewis Acid Activation. J. Am. Chem. Soc. 2017, 139, 18444–18447. 10.1021/jacs.7b10755. [DOI] [PubMed] [Google Scholar]
- a Pan Y. The Dark Side of Fluorine. ACS Med. Chem. Lett. 2019, 10, 1016–1019. 10.1021/acsmedchemlett.9b00235. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Tuan E.; Kirk K. L. Fluorine reactivity in difluoromethylimidazoles. J. Fluorine Chem. 2006, 127, 980–982. 10.1016/j.jfluchem.2006.03.014. [DOI] [Google Scholar]; c Woolridge E. M.; Rokita S. E. Synthesis and reactivity of 6-(fluoromethyl)indole and 6-(difluoromethyl)indole. Tetrahedron Lett. 1989, 30, 6117–6120. 10.1016/S0040-4039(01)93319-2. [DOI] [Google Scholar]
- Fu Y.; Lin B. L.; Song K. S.; Liu L.; Guo Q. X. Substituent effects on the S-H bond dissociation energies of thiophenols. J. Chem. Soc., Perkin Trans. 2 2002, 7, 1223–1230. 10.1039/b201003h. [DOI] [Google Scholar]
- Mohler M. L.; Bohl C. E.; Jones A.; Coss C. C.; Narayanan R.; He Y.; Hwang D. J.; Dalton J. T.; Miller D. D. Nonsteroidal Selective Androgen Receptor Modulators (SARMs): Dissociating the Anabolic and Androgenic Activities of the Androgen Activities of the Androgen Receptor for Therapeutic Benefit. J. Med. Chem. 2009, 52, 3597–3617. 10.1021/jm900280m. [DOI] [PubMed] [Google Scholar]
- Nair V. A.; Mustafa S. M.; Mohler M. L.; Yang J.; Kirkovsky L. I.; Dalton J. T.; Miller D. D. Synthesis of irreversibly binding bicalutamide analogs for imaging studies. Tetrahedron Lett. 2005, 46, 4821–4823. 10.1016/j.tetlet.2005.04.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane T. V.; Pigazzani F.; Flynn R. W. V.; MacDonald T. M. The effect of indapamide vs. Bendroflumethiazide for primary hypertension: a systematic review. Br. J. Clin. Pharmacol. 2019, 85, 285–303. 10.1111/bcp.13787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. T.; Gu Q. S.; Dong X. Y.; Meng X.; Liu X. Y. A Copper Catalyst with a Cinchona-Alkaloid-Based Sulfonamide Ligand for Asymmetric Radical Oxytrifluoromethylation of Alkenyl Oximes. Angew. Chem., Int. Ed. 2018, 57, 7668–7672. 10.1002/anie.201804315. [DOI] [PubMed] [Google Scholar]
- Ito Y.; Sadar M. D. Enzalutamide and blocking androgen receptor in advanced prostate cancer: lessons learnt from the history of drug development of antiandrogens. Res. Rep. Urol. 2018, 10, 23–32. 10.2147/RRU.S157116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozzell J. D.; McCague R.. Selective androgen receptor modulators. US 7205437 B2, April 17, 2007.
- For further details, see Supporting Information.
- Using the optimized conditions by Jui and co-workers (ref (7b)), 14b was obtained in 4% yield and a selectivity of 2:1 (19F NMR). The conditions of Prakash and co-workers (ref (6a)) and Lalic and co-workers (ref (6b)) did not afford 14b. The starting materials were recovered.
- a Collins K. D.; Glorius F. A robustness screen for the rapid assessment of chemical reactions. Nat. Chem. 2013, 5, 597–601. 10.1038/nchem.1669. [DOI] [PubMed] [Google Scholar]; b Collins K. D.; Rühling A.; Glorius F. Application of a robustness screen for the evaluation of synthetic organic methodology. Nat. Protoc. 2014, 9, 1348–1353. 10.1038/nprot.2014.076. [DOI] [PubMed] [Google Scholar]; c Collins K. D.; Rühling A.; Lied F.; Glorius F. Rapid Assessment of Protecting-Group Stability by Using a Robustness Screen. Chem. - Eur. J. 2014, 20, 3800–3805. 10.1002/chem.201304508. [DOI] [PubMed] [Google Scholar]; d Taylor N. J.; Emer E.; Preshlock S.; Schedler M.; Tredwell M.; Verhoog S.; Mercier J.; Genicot C.; Gouverneur V. Derisking the Cu-Mediated 18F-Fluorination of Heterocyclic Positron Emission Tomography Radioligands. J. Am. Chem. Soc. 2017, 139, 8267–8276. 10.1021/jacs.7b03131. [DOI] [PubMed] [Google Scholar]; e Gensch T.; Teders M.; Glorius F. Approach to Comparing the Functional Group Tolerance of Reactions. J. Org. Chem. 2017, 82, 9154–9159. 10.1021/acs.joc.7b01139. [DOI] [PubMed] [Google Scholar]
- Vitaku E.; Smith D. T.; Njardarson J. T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- Crabtree R. H. Deactivation in homogeneous transition metal catalysis: causes, avoidance, and cure. Chem. Rev. 2015, 115, 127–150. 10.1021/cr5004375. [DOI] [PubMed] [Google Scholar]
- a Fier P. S.; Hartwig J. F. Copper-mediated difluoromethylation of aryl and vinyl iodides. J. Am. Chem. Soc. 2012, 134, 5524–5527. 10.1021/ja301013h. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Prakash G. K. S.; Ganesh S. K.; Jones J. P.; Kulkarni A.; Masood K.; Swabeck J. K.; Olah G. A. Copper-Mediated Difluoromethylation of (Hetero) aryl Iodides and ß-Styryl Halides with Tributyl (difluoromethyl) stannane. Angew. Chem., Int. Ed. 2012, 51, 12090–12094. 10.1002/anie.201205850. [DOI] [PubMed] [Google Scholar]; c Matheis C.; Jouvin K.; Goossen L. J. Sandmeyer difluoromethylation of (hetero-)arenediazonium salts. Org. Lett. 2014, 16, 5984–5987. 10.1021/ol5030037. [DOI] [PubMed] [Google Scholar]; d Lu C.; Gu Y.; Wu J.; Gu Y.; Shen Q. Palladium-catalyzed difluoromethylation of heteroaryl chlorides, bromides and iodides. Chem. Sci. 2017, 8, 4848–4852. 10.1039/C7SC00691H. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Bacauanu V.; Cardinal S.; Yamauchi M.; Kondo M.; Fernández D. F.; Remy R.; MacMillan D. W. C. Metallaphotoredox Difluoromethylation of Aryl Bromides. Angew. Chem., Int. Ed. 2018, 57, 12543–12548. 10.1002/anie.201807629. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Fu X. P.; Xiao Y. L.; Zhang X. Nickel-Catalyzed Difluoromethylation of Arylboronic Acids with Bromodifluoromethane. Chin. J. Chem. 2018, 36, 143–146. 10.1002/cjoc.201700624. [DOI] [Google Scholar]; g Sap J. B. I.; Wilson T. C.; Kee C. W.; Straathof N. J. W.; am Ende C. W.; Mukherjee P.; Zhang L.; Genicot C.; Gouverneur V. Synthesis of 18F-difluoromethylarenes using aryl boronic acids, ethyl bromofluoroacetate and [18F]fluoride. Chem. Sci. 2019, 10, 3237–3241. 10.1039/C8SC05096A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Cambié D.; Bottecchia C.; Straathof N. J. W.; Hessel V.; Noël T. Applications of Continuous-Flow Photochemistry in Organic Synthesis, Material Science, and Water Treatment. Chem. Rev. 2016, 116, 10276–10341. 10.1021/acs.chemrev.5b00707. [DOI] [PubMed] [Google Scholar]; b Plutschack M. B.; Correia C. A.; Seeberger P. H.; Gilmore K. Organic Photoredox Chemistry in Flow. Top. Organomet. Chem. 2015, 57, 43–76. 10.1007/3418_2015_155. [DOI] [Google Scholar]; c Rehm T. H. Photochemical Fluorination Reactions - A Promising Research Field for Continuous-Flow Synthesis. Chem. Eng. Technol. 2016, 39, 66–80. 10.1002/ceat.201500195. [DOI] [Google Scholar]; For a personal account on continuous flow photochemistry, see:; d Gilmore K.; Seeberger P. H. Continuous Flow Photochemistry. Chem. Rec. 2014, 14, 410–418. 10.1002/tcr.201402035. [DOI] [PubMed] [Google Scholar]; e Sambiagio C.; Noel T. Flow Chemistry: Shine Some Light on Those Tubes!. Trends Chem. 2020, 2, 92–106. 10.1016/j.trechm.2019.09.003. [DOI] [Google Scholar]
- Luo J.; Zhang J. Donor-Acceptor Fluorophores for Visible-Light-Promoted Organic Synthesis: Photoredox/Ni Dual Catalytic C(sp3)-C(sp2) Cross-Coupling. ACS Catal. 2016, 6, 873–877. 10.1021/acscatal.5b02204. [DOI] [Google Scholar]
- Romero N. A.; Nicewicz D. A. Mechanistic Insight into the Photoredox Catalysis of Anti-Markovnikov Alkene Hydrofunctionalization Reactions. J. Am. Chem. Soc. 2014, 136, 17024–17035. 10.1021/ja506228u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The addition of the fluorophilic additive HBPin was not beneficial for our process.
- This photocatalyst provides just enough ‘under potential’ to control selectivity. For a useful discussion, see:; Lu J.; Khetrapal N. S.; Johnson J. A.; Zeng X. C.; Zhang J. 'π-Hole-π' Interaction Promoted Photocatalytic Hydrodefluorination via Inner-Sphere Electron Transfer. J. Am. Chem. Soc. 2016, 138, 15805–15808. 10.1021/jacs.6b08620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.