Abstract
Previous reports suggest that diabetes may differentially affect the vascular beds of females and males. However, there is insufficient evidence to establish the timeline of the vascular dysfunction in diabetes, specifically in relation to sex. Here, we determined whether mesenteric arterial function is altered in UC Davis Type-2 Diabetes Mellitus (UCD-T2DM) rats and if this occurs as early as the pre-diabetic stage of the disease. Specifically, we investigated whether vascular dysfunction differs between pre-diabetic or diabetic status and if this varies by sex. We measured the responses to endothelium-dependent and -independent vasorelaxant as well as vasoconstrictor agents and explored the potential mechanisms involved in sex-specific development of arterial dysfunction in UCD-T2DM rats. In addition, indices of insulin sensitivity were assessed. We report the reduced insulin sensitivity in pre-diabetic males and diabetic females. Vascular relaxation to acetylcholine was impaired to a greater extent in mesenteric artery from males in the pre-diabetic stage than in their female counterparts. In contrast, the arteries from females with diabetes exhibited a greater impairment to acetylcholine compared with diabetic males. Additionally, the sensitivity of mesenteric artery to contractile agents in females, but not in males, after the onset of diabetes was increased. Our data suggest that the reduced insulin sensitivity through Akt may predispose vessels to injury in the pre-diabetic stage in males. On the other hand, reduced insulin sensitivity as well as enhanced responsiveness to contractile agents may predispose arteries to injury in the diabetic stage in females.
Keywords: endothelial dysfunction, type-2 diabetes, pre-diabetes, insulin sensitivity, sex differences, mesenteric artery
1. Introduction
Cardiovascular diseases (CVD) are the leading cause of mortality and morbidity in diabetic patients (Brunner et al., 2005; Kannel and McGee, 1979; Morrish et al., 2001). CVD is less common in premenopausal women compared with men, but this difference disappears in the postmenopausal years (Barrett-Connor, 1994). Epidemiological studies indicate that diabetic premenopausal women not only lose this sex-based protection, they experience a higher CVD risk compared to diabetic men (Brandenburg et al., 2002; Di Carli et al., 2002; Kalyani et al., 2014; Steinberg et al., 2000), suggesting that diabetes abrogates the beneficial vascular effects of sex steroids. We previously reported sex differences in the endothelium-dependent vascular relaxation in type 1 diabetic rats (Han et al., 2016; Zhang et al., 2012). However, the pathophysiology of endothelial dysfunction in type 2 diabetes is likely to differ from that in type 1 diabetes, and more importantly, the prevalence of type 2 diabetes is rapidly increasing worldwide.
To better study the pathogenesis and potential treatment strategies, a proper animal model is required. This study was performed on mesenteric arteries obtained from a polygenic rat model of type 2 diabetes developed at the University of California (UC) Davis. The UC Davis type 2 diabetes mellitus (UCD-T2DM) rat has been validated in more than 20 published studies (Cummings et al., 2013; Cummings et al., 2011; Cummings et al., 2012; Cummings et al., 2008; Cummings et al., 2010; Kleinert et al., 2018). This model more closely replicates the pathophysiology of type 2 diabetes in humans than other rodent models of the disease and exhibits the key features of the disease in humans such as polygenic adult-onset obesity, insulin resistance, intact leptin signaling, and eventual β-cell decompensation with preserved fertility in both sexes (Cummings et al., 2008). Based on these similarities, the UCD-T2DM model can provide us with much needed data on pathophysiological mechanisms of diabetes observed in humans.
Diabetes is associated with vascular complications (Bakker et al., 2009; Sachidanandam et al., 2006; Schach et al., 2014). A recent study noted the exaggerated responses in sympathetic activity and blood pressure in UCD-T2DM model (Grotle et al., 2019). However, there is no data on vascular reactivity changes in this model. Endothelial dysfunction represents an early step in the vascular complications of diabetes. Nonetheless, there is insufficient evidence to establish the timeline of the endothelial dysfunction in diabetes, specifically in relation to sex. Thus, we determined whether endothelium-dependent vascular relaxation is impaired in UCD-T2DM rats and if this is apparent during the pre-diabetic stage of disease. We investigated whether vascular dysfunction differs between pre-diabetic or diabetic status, and if this may be different between sexes. Here, we measured the responses to vasorelaxant as well as vasoconstrictor agents and explored the potential mechanisms involved in sex-specific development of vascular dysfunction in UCD-T2DM rats.
Although nitric oxide (NO) is the primary mediator of vascular relaxation, endothelium-derived hyperpolarizing factor (EDHF) is also an important regulator of vascular tone, especially in small vessels (Zhang et al., 2012). We also determined the influence of diabetes in modulating the contribution of NO and EDHF to vascular relaxation.
This is a first report demonstrating mesenteric arterial function is impaired as early as the pre-diabetic stage in both sexes in the UCD-T2DM rat. It also reveals that sex differences exist in vascular dysfunction in the pre-diabetic and diabetic stages. Our data suggest that the reduced insulin sensitivity through Akt, in addition to a loss of EDHF-mediated vasorelaxation, may predispose vessels to injury in the pre-diabetic stage in males. However, reduced insulin sensitivity and enhanced responsiveness to contractile agents may predispose arteries to injury in the diabetic stage in females.
2. Materials and Methods
All chemicals were purchased from Sigma Chemical Co. (St. Louis, MO, USA), and dissolved in water unless otherwise stated.
2.1. Experimental animals
In the present study, female and male UCD-T2DM rats in the pre-diabetic or diabetic stage were studied. The UCD-T2DM rats were generated by breeding obese Sprague Dawley (SD) rats with Zucker Diabetic Fatty (ZDF) lean rats that were homozygous wild-type for the leptin receptor and had inherent ß-cell defects (Cummings et al., 2008).
Overall, the development of diabetes in UCD rats is variable and dependent on body weight. For the purposes of this study, we selected 19–20-week-old UCD rats that had developed diabetes (diabetic group) and 19–20-week-old UCD rats that had not yet developed diabetes (prediabetic group) but had higher weight than controls. The age of disease onset in the diabetic group was 14 weeks for females and 11 weeks for males. At the time of killing, the females had diabetes for a duration of 47 ± 3.4 days and the males for 60 ± 5 days.
Additionally, age-matched (~19–20-week-old) female and male non-obese and non-diabetic SD rats (Simonsen Laboratories, Gilroy, CA) were employed as controls for UCD-T2DM rats. When compared to controls, the pre-diabetic rats had significantly higher body weight, but their non-fasting plasma glucose level or HBA1C was not high enough to be diagnosed as diabetes. The diabetic rats exhibited a non-fasting blood glucose level above 300 mg/dl, and expressed three features of hyperglycemia: polyuria, polydipsia, and polyphagia.
UCD-T2DM rats were bred and housed in the animal facility in the Department of Nutrition at UC Davis, before being transferred to the University of the Pacific. The UCD and control animals were maintained with ad libitum water and standard rodent chow food (Mazuri Rodent food) at constant humidity and temperature, with a light/dark cycle of 12 h at the University of the Pacific (Unlike female ZDF rats, female UCD-T2DM rats develop diabetes without the need for supplementation with high fat/high sugar diets). After acclimation for one week at the University of Pacific, animals were euthanized using CO2. The euthanization process followed recommendations from the 2013 AVMA Guidelines on Euthanasia and the NIH Guidelines for the Care and Use of Laboratory Animals. All animal protocols were approved by the Institutional Animal Care and Use Committee of the University of the Pacific (protocol#18R11) and UC Davis (protocol#19986) and complied with the Guide for the Care and Use of Laboratory Animals: Eighth Edition (US National Institutes of Health 2011) and with ARRIVE guidelines.
2.2. Blood and plasma analysis
Blood samples were obtained by an intracardiac puncture and collected in tubes containing heparin or sodium citrate. Plasma was obtained by centrifugation at 10,000×g for 5 min at 4°C and stored at −80°C for later analysis. Glucose and triglycerides were measured in 12-h-fasted rats using an Accutrend Plus System (hand-held point-of-care device) and specific test strips (Roche Farma, Barcelona, Spain) with a drop of blood collected from the tail vein. Insulin and leptin levels were also assessed by using ELISA kits (Mercodia Ab, Uppsala, Sweden) according to the manufacturer’s instructions. Insulin sensitivity index (ISI) was determined from fasting plasma glucose and insulin using the following formula as previously described (Sanguesa et al., 2016; Shaligram et al., 2018): ISI = [2/ (blood insulin (nM) x blood glucose (μM) + 1].
Blood collected after killing of the animals was also used to assess the level of glycated hemoglobin (HbA1c). The HbA1c was measured using the Bayer A1cNow test kit, according to manufacturer’s instructions. Animals with HbA1c level greater than 6.5 percent on two consecutive measurements are considered to have developed overt diabetes.
2.3. Measurement of mesenteric arterial tension
The branches of mesenteric arteries of the second and third order were separated from veins and cleared of fatty and adhering tissues and cut into 2 mm rings with an internal diameter ranging from 250–350 μm. Each 2-mm segment was mounted between two jaws with the use of tungsten wire (40 μm diameter) in an organ bath of myograph (model 610M; Danish Myo Technology, Denmark). The organ bath contained Krebs solution of (in mM) 119 NaCl, 4.7 KCl, 1.6 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 25 NaHCO3, 0.023 EDTA, and 6 glucose at 37°C, bubbled with 95% O2-5% CO2. The arterial tension was monitored with a computer-based data acquisition system (Chart5, Powerlab; ADInstruments, Colorado Springs, CO). The rings were normalized to a resting tension of 13.3 kPa and equilibrated for 30 min to obtain a basal tone. Arterial segments were then stimulated with 80 mM KCl solution twice. To test the viability of the endothelium, acetylcholine (10 μM)-induced relaxation was recorded in phenylephrine (PE, 2 μM) pre-contracted vessels.
2.4. Relaxation responses to acetylcholine
Arterial rings were contracted with PE (2 μM), which produced about 80% of the maximal contraction. The concentration-response curve (CRC) was obtained by the addition of increasing concentrations of acetylcholine (10−8 to 10−5 M).
The vascular relaxation to acetylcholine (10−8 to 10−5 M) in rat mesenteric arterial rings were then obtained before and after pretreatment with indomethacin (Indo, 10 μM, a blocker of the cyclooxygenase (COX), followed by addition of L-NAME (200 μM, NO synthase (NOS) blocker) and then a combination of barium chloride (100 μM, KIR channel blocker) and ouabain (10 μM, Na+-K+-ATPase blocker).
2.5. Relaxation responses to sodium nitroprusside (SNP)
The CRC to SNP (10−9 to 10−5 M), a NO donor, was generated in mesenteric arterial rings pre-contracted with PE (2 μM).
2.6. Constrictor responses to PE or endothelin-1 (ET-1)
The CRC to PE or ET-1 was obtained by the addition of increasing concentrations of PE (10−7 to 3×10−5 M) or ET-1 (10−10 to 3×10−8 M).
The concentration of drugs used to generate relaxation or contraction curves were based on the standard protocol used by our group (Han et al., 2016; Han et al., 2014; Shaligram et al., 2018; Zhang et al., 2012).
2.7. Real-time polymerase chain reaction (PCR)
The whole mesenteric artery bed was submerged in RNAlater (Life Technologies, Carlsbad, CA) shortly after dissection. All second- to third-order branches of mesenteric arteries were excised and cleared from veins, fat, and adhesive tissues. They were placed in a 1.5 ml centrifuge tube containing RNAlater, kept at 4°C overnight and stored at −80°C for later analysis.
Total RNA was extracted from Arteries using RNeasy Mini Kit with on-column DNase treatment (Qiagen, Valencia, CA). Omniscript RT Kit (Qiagen, Valencia, CA) and random primers (Life Technologies, Carlsbad, CA) were used to synthesize the cDNA, according to the manufacturer’s instruction. cDNA was used for specific mRNA expression quantified with real-time PCR (MyiQ Single-Color Real-Time PCR Detection System; Bio-Rad). Internal variations were normalized to rat β-actin. The following primers were used for detection of gene expression rat KCa2.3: 5′-ACTTCAACACCCGATTCGTC-3′ (forward) and 5′-GGAAAGGAACGTGATGGAGA-3′ (reverse); rat NADPH oxidase (Nox4): 5′-CCA GAA TGA GGA TCC CAG AA-3′ (forward) and 5′-AGC AGC AGC AGC ATG TAG AA-3′ (reverse); rat Nox2: 5′-ACC CTT TCA CCC TGA CCT CT-3′(forward) and 5′-TCC CAG CTC CCA CTA ACA TC-3′ (reverse); rat β-actin: 5′-CTGGGTATGGAATCCTGTGG (forward) and 5′-TCATCGTACTCCTGCTTG CTG (reverse).
2.8. Western-blot analysis
Arterial tissue was assessed for Akt, phosphorylated (pAkt) and the suppressor of cytokine signaling-3 (SOCS3) proteins via standard Western blotting procedures performed as described previously by us (Sanguesa et al., 2016; Shaligram et al., 2018). Briefly, mesenteric arterial samples were micronized through freezing with liquid nitrogen and ground with a mortar as previously described (Baena et al. 2015). Liver samples were homogenized with a Dounce homogenizer. For total protein extraction, lysis buffer with proteases, phosphatases and acetylases inhibitors (50 mM Tris− HCl pH=8, 150 mM NaCl, 1% Igepal, 10mM NaF, 1 mM EDTA, 1mM EGTA, 2 mM Nappi, 1mM PMSF, 2 μg/mL leupeptin, 2 μg/mL aprotinin, 1 mM Na3VO4, 10mM NaM, 1μM TSA) was used. Samples were homogenized for 1.5 h at 4°C, centrifuged at 15,000×g for 15 min at 4°C and supernatants were collected. The homogenates were kept on ice for 10 min and centrifuged at 1000xg for 10 min at 4°C. Lysis buffer was added to the pellet obtained and samples were incubated for 1.5 h at 4°C, centrifuged at 25,000×g for 30 min at 4°C and supernatants were collected. Protein concentrations were determined by the Bradford method (Bradford 1976).
Primary antibodies for total and pAkt (Ser-473) were supplied by Cell Signaling (Danvers, MA, USA), and the antibody against SOCS3 was from Santa Cruz Biotechnologies (Dallas, TX, USA). To confirm the uniformity of protein loading, blots were incubated with β-actin antibodies (Sigma-Aldrich, St. Louis, MO, USA), normalized for β-actin levels and expressed as arbitrary units (a.u.).
2.9. Statistical analysis
The relaxation induced by acetylcholine and SNP was represented as a percentage of relaxation from maximum PE contraction at each concentration. EC50, the concentration of the agonist, which produced half of the maximum response (Emax), was calculated by a sigmoidal dose-response model (for variable slope) using GraphPad Prism 7.0 (GraphPad Software, San Diego, CA). The sensitivity of the agonists was expressed as pD2 values (−log [EC50]), which was normally distributed. One-way ANOVA test was used for comparisons of means among experimental groups (e.g., EC50, Emax, and metabolic data). When the one-way ANOVA test returned (P < 0.05), post hoc analysis using Bonferroni’s test was performed. Comparison of CRC between two groups was made using two-way ANOVA followed by Tukey’s post hoc test. Comparison of CRC in a pre/posttest format within a group was made using two-way ANOVA with repeated measures followed by Bonferroni’s post hoc test. Statistical analysis for protein expression was performed by one-way ANOVA test, followed by post hoc analysis using Šidák’s test.
3. Results
3.1. Pre-diabetes and diabetes alter metabolic parameters
We used age-matched non-diabetic, pre-diabetic, and diabetic animals (19–20-week-old). The UCD male and female animals were different with respect to the age of onset of diabetes (female: 14 weeks; male: 11 weeks). As shown in Table 1, the body weights of female and male UCD rats in both pre-diabetic and diabetic stages were significantly higher than those in nondiabetic controls. Similarly, the weight of intra-abdominal adipose tissue (visceral white adipose tissue located around the digestive organs such as mesenteric and omental) was significantly higher in pre-diabetic and diabetic rats than those in non-diabetic controls in both sexes. When compared to pre-diabetic or diabetic females, the male counterparts had a higher body weight. The ratio of intra-abdominal adipose tissue to body weight was significantly higher in pre-diabetic and diabetic female rats than in males. Furthermore, when compared with pre-diabetic and diabetic male rats, pre-diabetic and diabetic females had higher circulating triglyceride levels (although it did not reach statistical significance in pre-diabetic females). As expected, fasting blood glucose and HbA1c levels in female and male rats in the diabetic stage were significantly higher than those in their respective pre-diabetic animals and non-diabetic control animals (Table 1).
Table 1.
Body weight and adipose weight, blood glucose levels, HbA1c, and other metabolic/endocrine parameters of control, pre-diabetic, and diabetic rats.
| Control female | Pre-diabetic female | Diabetic female | Control male | Pre-diabetic male | Diabetic male | |
|---|---|---|---|---|---|---|
| Final body weight (g) | 210±6c | 337±16ac | 405±8abc | 340±19 | 550±8a | 515±18ab |
| Adipose tissue (g) | 1.2±0.3 | 10.3±1. 5a | 15. 4± 1. 4abc | 1.5±0.2 | 9.5±0.8a | 10.2±1. 5a |
| Adipose tissue/body weight (g) | 0.006±0.0 | 0.029±0.0ac | 0.038±0.0abc | 0.005±0.0 | 0.017±0.0 | 0.019±0.0 |
| Blood Glucose (mg/dl) | 145±22 | 152±42 | 310±25ab | 140±34 | 145±46 | 305±92ab |
| HbA1c | 4.3±0.2 | 5.0±0.1a | 11.6±0.7ab | 4.4±0.1 | 5.2±0.1a | 9.4±0.3ab |
| Insulin (ng/ml) | 1.4 ±0.5 | 1.0±0.2 | 10.0±2.8abc | 1.2±0.5 | 3.8±0.8 | 2.0±0.9 |
| ISI | 1.0±0.2 | 1±0.1c | 0.2±0.1abc | 1.1±0.2 | 0.5±0.1a | 0.7±0.1 |
| Triglycerides (mmol/L) | 1.2±0.1 | 2.5±0.4 | 3.5±0.4ac | 1.1±0.1 | 1.6±0.2 | 1.7±0.2 |
| Leptin (ng/ml) | 1.0±0.2 | 1.8±0.4 | 4.1±0.5abc | 0.7±0.3 | 3.3±0.4a | 1.3±0.3b |
Data are expressed as mean ± S.E.M.; n=6–10.
(vs. Control, same sex),
(vs. Pre-diabetic, same sex),
(vs. Male, respective group), P<0.05, analyzed using one-way ANOVA followed by Bonferroni’s post hoc test.
Insulin sensitivity index (ISI) = [2/ (blood insulin (nM) x blood glucose (μM) + 1]. HbA1c= glycated hemoglobin (A1c).
3.2. Pre-diabetes and diabetes alter insulin sensitivity
Fasting plasma insulin concentration was significantly higher in only diabetic female rats compared with pre-diabetic and non-diabetic female controls (Table 1). Only pre-diabetic male rats tended to have higher circulating insulin levels than non-diabetic control male rats, potentially due to ß-cell decompensation in diabetic male rats (Table 1). Furthermore, the ISI was significantly lower (half-fold change, P<0.05) in pre-diabetic males than in nondiabetic controls or pre-diabetic females. The ISI was lower for both sexes in the diabetic stage compared with their respective non-diabetic controls, although in diabetic males the difference was not statistically significant.
Similar to the results for plasma insulin levels, pre-diabetic males but not pre-diabetic females exhibited elevated circulating leptin levels. However, circulating leptin concentrations were significantly higher in diabetic female rats compared with those in pre-diabetic females, non-diabetic control, and diabetic males. Circulating leptin concentrations in pre-diabetic males and diabetic females may reflect adiposity and leptin resistance in these groups. Thus, this observation prompted us to examine the hepatic expression of SOCS3, which is involved in suppression of the leptin signaling pathway (Lubis et al., 2008). However, Western blot analysis revealed that hepatic SOCS3 expression was not significantly different between pre-diabetic or diabetic groups and their respective control groups in both sexes, suggesting that the leptin signaling pathway is likely to be intact in the liver in the UCD-T2DM rat model (Table 1, Supplemental Fig. 1).
3.3. Pre-diabetes impairs relaxation responses to acetylcholine more intensely in males, and diabetes impairs it more intensely in females
The CRC to acetylcholine was markedly different in the pre-diabetic and diabetic stages for both sexes compared with non-diabetic controls (Fig. 1A and 1B). Moreover, there was a shift in the CRC of diabetic animals compared with pre-diabetic animals, regardless of sex. Both Emax and pD2 to acetylcholine were significantly reduced in arteries from diabetic rats compared with those in their respective non-diabetic control animals, regardless of sex (Table 2). Interestingly, in the pre-diabetic stage, the extent of impairment was higher in males compared with that of female rats, as shown by a significant decrease in the Emax (Table 2). On the other hand, the effect of diabetes in blunting acetylcholine-mediated relaxation in females was significantly greater than that in males (Table 2). Both sensitivity and Emax to acetylcholine in arteries of diabetic females were significantly lower than those in diabetic males (Table 2).
Fig. 1.
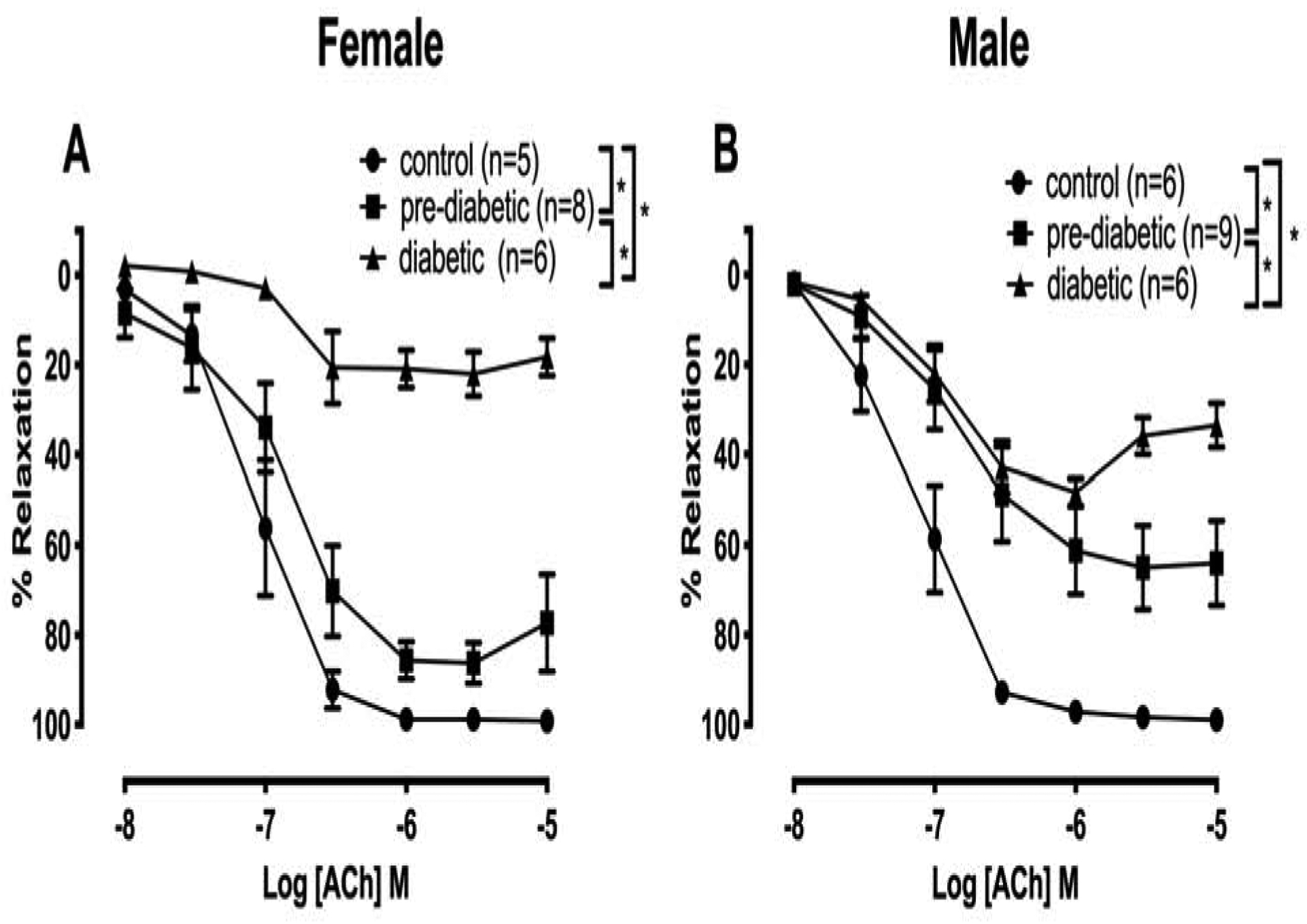
Relaxation response to cumulative concentrations of acetylcholine (ACh) in intact mesenteric arterial rings precontracted with phenylephrine (PE, 2 μM) from control, pre-diabetic, and diabetic female (A) and male (B) rats. Data are expressed as mean ± S.E.M. *P<0.05 between two groups analyzed using two-way ANOVA followed by Tukey’s post hoc test.
Table 2.
Sensitivity (pD2: −logEC50) and maximum response (Emax) to acetylcholine (ACh) in mesenteric arteries from control, pre-diabetic, and diabetic rats.
| Groups | N | pD2 | Emax (%) |
|---|---|---|---|
| Control female | 5 | 7.06±0.1 | 99.20±0.4 |
| Pre-diabetic female | 8 | 6.61±0.3 | 88.25±3.3c |
| Diabetic female | 6 | 2.69±0.8abc | 28.67±5.8abc |
| Control male | 6 | 7.13±0.1 | 99±0.4 |
| Pre-diabetic male | 9 | 6.03±0.5 | 65.89±9a |
| Diabetic male | 6 | 4.42±0.5ab | 51.67±3.7a |
Data are expressed as mean ± S.E.M.
(vs. Control, same sex),
(vs. Pre-diabetic, same sex),
(vs. Male, respective group), P<0.05, analyzed using one-way ANOVA followed by Bonferroni’s post hoc test.
3.4. Pre-diabetes and diabetes alter the relative contributions of NO and EDHF to relaxation responses to acetylcholine
The relative contributions of prostacyclin (PGI2), NO, and EDHF to acetylcholine-induced relaxation responses were determined by sequentially blocking COX, NOS, and a combination of KIR channel and Na+-K+-ATPase pump. The administration of Indo to block COX activity increased acetylcholine responses in diabetic models, but the effects on Emax to acetylcholine did not reach significance in either sex (Fig. 2 and 3, Table 3). The addition of L-NAME resulted in a further reduction in acetylcholine-induced relaxation of arteries from control rats, however the effect was more prominent in pre-diabetic or diabetic animals of both sexes, suggesting the contribution of NO-mediated relaxation was enhanced (or EDHF-type relaxation was diminished in arteries of these groups) (Fig. 2 and 3). In the pre-diabetic stage, the effect of L-NAME was much greater in males compared with females (Fig. 3B vs. 2B: pre-diabetic male vs. pre-diabetic female, respectively, two way ANOVA with repeated measures, P<0.05, between third curve vs. fourth curve). The pretreatment of arteries with barium and ouabain in the presence of Indo and L-NAME almost completely abolished the remaining relaxation responses to acetylcholine in both sexes of control and UCD rats regardless of their disease status (Fig. 2 and 3, Table 3).
Fig. 2.

Effects of inhibiting cyclooxygenase, nitric oxide synthase, KIR channel, and Na+-K+-ATPase on acetylcholine (ACh)-induced vasorelaxation in mesenteric arteries taken from control (A), pre-diabetic (B), and diabetic (C) female rats. Acetylcholine relaxation was measured in the presence of indomethacin (Indo; 10 μM), followed by addition of Nɯ-nitro-L-arginine methyl ester (L-NAME; 200 μM), and then with a combination of barium chloride (100 μM) and ouabain (10 μM). Data are expressed as mean ± S.E.M. *P<0.05 vs. no drug; # P< 0.05 vs. indo; ^ P<0.05 vs. indo+L-NAME, analyzed using 2-way ANOVA with repeated measures followed by Bonferroni post hoc test (n=5–8 per group). Dark grey shaded area: Contribution of endothelium-derived hyperpolarizing factor (EDHF) to vascular relaxation and light grey shaded area: Contribution of nitric oxide (NO) to vascular relaxation.
Fig. 3.

Effects of inhibiting cyclooxygenase, nitric oxide synthase, KIR channel, and Na+-K+-ATPase on acetylcholine (ACh)-induced vasorelaxation in mesenteric arteries taken from control (A), pre-diabetic (B), and diabetic (C) male rats. Acetylcholine relaxation was measured in the presence of indomethacin (Indo; 10 μM), followed by addition of Nɯ-nitro-L-arginine methyl ester (L-NAME; 200 μM), and then with a combination of barium chloride (100 μM) and ouabain (10 μM). Data are expressed as mean ± S.E.M. *P<0.05 vs. no drug; # P< 0.05 vs. indo; ^ P<0.05 vs. indo+L-NAME, analyzed using 2-way ANOVA with repeated measures followed by Bonferroni post hoc test (n=6–9 per group). Dark grey shaded area: Contribution of endothelium-derived hyperpolarizing factor (EDHF) to vasorelaxation and light grey shaded area: Contribution of nitric oxide (NO) to vasorelaxation.
Table 3.
Sensitivity (pD2: −logEC50) and maximum tension (Emax) to acetylcholine (ACh) in mesenteric arteries from control, pre-diabetic, and diabetic rats.
| Groups | No drug | Indo | Indo+L-N AME | Indo+L-NAME +barium+ouabain | |||
|---|---|---|---|---|---|---|---|
| pD2 | Emax (%) | pD2 | Emax (%) | pD2 | Emax (%) | pD2 Emax (%) | |
| Control female | 7.06±0.1 | 99.20±0.4 | 7.22±0.2 | 99±0.4 | ND | 90.25±4.9 | ND 12.75±4.8def |
| Pre-diabetic female | 6.61±0.3 | 88.25±3.3c | 6.52±0.5 | 74.50±15.2 | ND | 38.75±12.8de | ND 11.50±3.8de |
| Diabetic female | 2.69±0.8abc | 28.67±5.8abc | 3.95±0.6 | 31.16±7.7 | ND | 4.66±3.5de | ND 2.33±1.8de |
| Control male | 7.13±0.1 | 99±0.4 | 6.91±0.2 | 98.66±0.6 | ND | 85.16±5.78 | ND 10.83±3def |
| Pre-diabetic male | 6.03±0.5 | 65.89±9a | 5.90±0.5 | 72.55±15.7 | ND | 18.66±10.3de | ND 4.88±1.7de |
| Diabetic male | 4.42±0.5ab | 51.67±3.7a | 5.20±0.4 | 57.50±3.5 | ND | 15 ±3.8de | ND 5.66±2.1de |
A comparison of the sensitivity (pD2) and maximum response (Emax) to acetylcholine in the absence (no drug) or in the presence of indo, indo+L-NAME, and indo+ L-NAME +barium+ouabain in mesenteric arteries from control, pre-diabetic and diabetic female and male rats. Data are expressed as mean ± S.E.M.
(vs. Control, same sex),
(vs. Pre-diabetic, same sex),
(vs. Male in respective group), P<0.05, one-way ANOVA;
(vs. no drug within each group),
(vs. indo within each group),
(vs. indo+L-NAME within each group), P<0.05, Student’s paired t-test. ND, not determined.
3.5. Pre-diabetes and diabetes alter mRNA expression of small-conductance calcium activated potassium channels (SKCa)
It is well known now that smooth muscle hyperpolarization results indirectly from the opening of endothelial small- and intermediate-conductance calcium activated potassium channels (SKCa and IKCa) (Feletou, 2016; McNeish et al., 2006; Weston et al., 2010). Therefore, a defective EDHF-mediated relaxation would also be expected to result from any significant loss of these hyperpolarizing KCa channels on the endothelium (Garland, 2010; Gillham et al., 2007; Kong et al., 2015). To study whether the loss of EDHF-type relaxation was associated with the changes in SKCa, the mRNA expression levels of KCa2.3 were measured (Fig. 4). Real-time PCR analysis revealed that the expression of KCa2.3 was significantly downregulated in pre-diabetic males, but not in females. Although KCa2.3 mRNA expression tended to be lower in pre-diabetic females than in control females, the difference was not statistically significant. The levels of KCa2.3 mRNA expression were significantly reduced in arteries taken from diabetic animals in both sexes compared with those in their respective non-diabetic control animals (Fig. 4).
Fig. 4.

Real-time PCR analysis of SKCa (KCa 2.3) mRNA expression in mesenteric arteries from control, pre-diabetic, and diabetic female and male rats. Data are expressed as mean ± S.E.M. Capped lines indicate significant differences between two groups (P<0.05), as analyzed by one-way ANOVA test followed by Bonferroni’s post hoc test (n=6).
3.6. Pre-diabetes and diabetes impair relaxation responses to SNP
The sensitivity of arteries to NO was investigated by performing CRC to SNP (10−9 to 10−5 M). There was a significant rightward shift in the CRC to SNP in diabetic animals, regardless of sex. Both diabetic female and male rats showed lower sensitivity, as assessed by pD2 values, and lower Emax compared with their respective non-diabetic controls (Fig. 5A and 5B, Table 4). However, when compared to their respective pre-diabetic groups, arteries from diabetic females exhibited a more pronounced shift of SNP CRC, as indicated by lower pD2 values and Emax (Table 4).
Fig. 5.

Relaxation response to cumulative concentrations of sodium nitroprusside (SNP) in intact mesenteric arterial rings precontracted with phenylephrine (PE, 2 μM) from control, pre-diabetic, and diabetic female (A) and male (B) rats. Data are expressed as mean ± S.E.M. *P<0.05 between two groups analyzed using two-way ANOVA followed by Tukey’s post hoc test.
Table 4.
Sensitivity (pD2: −logEC50) and maximum tension (Emax) to sodium nitroprusside (SNP) in mesenteric arteries from control, pre-diabetic, and diabetic rats.
| Groups | N | pD2 | Emax (%) |
|---|---|---|---|
| Control female | 5 | 7.32±0.2 | 91±3.3 |
| Pre-diabetic female | 9 | 5.77±0.3a | 61.78±7.0a |
| Diabetic female | 6 | 3.78±0.5ab | 28.67±7.5ab |
| Control male | 6 | 7.01±0.3 | 86.60±5.1 |
| Pre-diabetic male | 8 | 5.81±0.3 | 64.50±7.3 |
| Diabetic male | 6 | 5.05±0.2a | 47.50±4.5a |
Data are expressed as mean ± S.E.M.
(vs. Control, same sex),
(vs. Pre-diabetic, same sex), P<0.05, analyzed using one-way ANOVA followed by Bonferroni’s post hoc test.
3.7. Diabetes enhances sensitivity to contractile agents in females, but not in males
To examine the sensitivity of arteries to contractile agents, CRC to PE (an α-adrenoceptor agonist, 10−7 to 3×10−5 M) or ET-1 (10−10 to 3×10−8 M) were generated (Fig. 6 and 7). The Tensionmax to PE or ET-1 was not different between sexes in pre-diabetic and diabetic groups. In the diabetic stage, females, but not males, showed a higher sensitivity (pD2 value) to PE (Table 5) or ET-1 (Table 6) compared with their respective non-diabetic control or pre-diabetics. When compared with diabetic males, the sensitivity to PE in arteries of diabetic females was significantly higher (Table 5).
Fig. 6.

Concentration-response curves to phenylephrine (PE) in mesenteric arteries of control, pre-diabetic, and diabetic female (A) and male (B) rats. Data are expressed as mean ± S.E.M. *P<0.05 between two groups analyzed using two-way ANOVA followed by Tukey’s post hoc test.
Fig. 7.

Concentration-response curves to endothelin-1 (ET-1) in mesenteric arteries of control, pre-diabetic, and diabetic female (A) and male (B) rats. Data are expressed as mean ± S.E.M. *P<0.05 between two groups analyzed using two-way ANOVA followed by Tukey’s post hoc test.
Table 5.
Sensitivity (pD2: −logEC50) and maximum tension (Tensionmax) to phenylep hrine (PE) in mesenteri c arteries from control, pre-diabetic, and diabetic rats.
| Groups | N | pD2 | Tensionmax |
|---|---|---|---|
| Control female | 5 | 6.26±0.1 | 23.99±1.9 |
| Pre-diabetic female | 9 | 6.48±0.1 | 24.97±1.6 |
| Diabetic female | 6 | 7±0.1abc | 20.62±1.9 |
| Control male | 7 | 6.36±0.4 | 23.12±1.9 |
| Pre-diabetic male | 9 | 6.39±0.1 | 27.28±3.1 |
| Diabetic male | 6 | 6.46±0.2 | 23.76±1.7 |
Data are expressed as mean ± S.E.M.
(vs. Control, same sex),
(vs. Pre-diabetic, same sex),
(vs. Male in respective group), P<0.05, analyzed using one-way ANOVA followed by Bonferroni’s post hoc test.
Table 6.
Sensitivity (pD2: −logEC50) and maximum tension (Tensionmax) to endothelin-1 (ET-1) in mesenteric arteries from control, pre-diabetic, and diabetic rats.
| Groups | N | pD2 | Tensionmax |
|---|---|---|---|
| Control female | 5 | 8.84±0.1 | 21.2±2.0 |
| Pre-diabetic female | 9 | 9.06±0.2 | 18.43±1.2 |
| Diabetic female | 7 | 9.91±0.2ab | 17.59±2.0 |
| Control male | 6 | 8.8±0.2 | 21.78±2.0 |
| Pre-diabetic male | 9 | 8.76±0.2 | 21.72±1.9 |
| Diabetic male | 6 | 9.35±0.1 | 21.09±1.6 |
Data are expressed as mean ± S.E.M.
(vs. Control, same sex),
(vs. Pre-diabetic, same sex), P<0.05, analyzed using one-way ANOVA followed by Bonferroni’s post hoc test.
3.8. Sex and diabetic stage differentially alter intracellular pathways related to vascular function
We further investigated the possible mechanisms underlying the impairment of the acetylcholine responses as well as the elevated responses to contractile agents in this model. Since vascular dysfunction could be related to insulin resistance, and our results suggested that insulin signaling could be impaired in pre-diabetic and diabetic rats, we analyzed the expression of Akt, one of the main transducers of insulin signaling, using Western blot analysis. As shown in Fig. 8, the amount of p-Akt (Ser-473) was significantly reduced in arteries from both pre-diabetic and diabetic males, while total Akt protein increased, suggesting impaired insulin signaling. The p-Akt expression also tended to be lower in diabetic females than in nondiabetic controls, although the difference was not statistically significant.
Fig. 8.

Western blot analysis of phosphorylated and total Akt protein expression in mesenteric arteries from control, pre-diabetic, and diabetic female and male rats. Data are expressed as mean ± S.E.M. Capped lines indicate significant differences between two groups (P<0.05), as analyzed by one-way ANOVA test followed by Šidák’s test (n=4–5).
We also measured the mRNA expression of NADPH oxidase (Nox) subunit, Nox4, major sources of superoxide in the small vessel wall arteries (Touyz et al., 2002). Real-time PCR analysis revealed that the level of Nox4 mRNA expression was significantly higher in arteries from pre-diabetic males, but not females, and diabetic rats of both sexes compared with their respective non-diabetic controls (Fig. 9). Furthermore, there was a sex difference in the Nox4 mRNA expression in the pre-diabetic stage. The mRNA expression for Nox4 was significantly higher in pre-diabetic males than in the pre-diabetic females (Fig. 9). Furthermore, we measured the level of mRNA expression for Nox2. Except for pre-diabetic females compared with their respective controls, there were no differences in Nox2 mRNA expression in arteries taken from the experimental groups. The levels of Nox2 mRNA expression was lower in the mesenteric artery of pre-diabetic females than in controls (P< 0.05, One way ANOVA, 6 per group) (Supplemental Fig. 2).
Fig. 9.

Real-time PCR analysis of NADPH oxidase (Nox4) mRNA expression in mesenteric arteries from control, pre-diabetic, and diabetic female and male rats. Data are expressed as mean ± S.E.M. Capped lines indicate significant differences between two groups (P<0.05), as analyzed by one-way ANOVA test followed by Bonferroni’s post hoc test (n=6).
4. Discussion
We provide the first measures of vascular function in a validated polygenic rat model of type 2 diabetes. Our study demonstrates the impaired mesenteric arterial function as early as the pre-diabetic stage in both sexes in the UCD-T2DM rat model. It also reveals that sex differences exist in the development of abnormal vascular relaxation in the pre-diabetic and diabetic stages of this model.
Here, the pre-diabetic males showed a trend of higher insulin levels compared with pre-diabetic females and non-diabetic control. However, at the diabetic stage, only females exhibited hyperinsulinemia. Given the males had earlier onset of disease than the females, their lower insulin levels could represent ß-cell decompensation. Ohta et al. also observed an elevated blood insulin level in spontaneously diabetic torii (SDT) female rats compared with SDT male rats (Ohta et al., 2014). In the study of Ohta et al., the diabetes progression, a rise in blood glucose, and a decline in blood insulin level (suggestive of ß-cell decompensation), was observed earlier in male rats than in females.
Consistent with the increases in circulating insulin levels, plasma leptin levels were also elevated in pre-diabetic males and diabetic females. These rats appear to have intact leptin signaling, as shown by moderately increased levels of leptin and no significant differences in hepatic SOCS3 expression level between the pre-diabetic or diabetic groups and their respective controls in both sexes. Several studies have shown SOCS3 protein expression can be elevated by the increased circulating level of leptin and is associated with leptin resistance (Bjorbaek et al., 1998; Howard et al., 2004). The unaltered SOCS3 protein level suggests intact leptin signaling in this model, as previously reported by Dr. Havel’s group (Cummings et al., 2008).
Endothelial dysfunction represents an early step in the development of vascular complications in diabetes (Bakker et al., 2009; De Vriese et al., 2000; Ding et al., 2005; McVeigh et al., 1992; Oltman et al., 2006; Takenouchi et al., 2009). Here, we demonstrated that acetylcholine-induced vascular relaxation was impaired in the pre-diabetic and diabetic stage in UCD animals in both sexes. An intriguing observation of this study was that, in the pre-diabetic stage, arteries of males exhibited a greater impairment in vascular relaxation compared to females. Moreover, the pre-diabetic males, but not pre-diabetic females, demonstrated significantly lower ISI compared with their respective control or pre-diabetic females. A positive correlation has been reported between insulin sensitivity and flow-mediated dilation in hypertensive patients (Grassi et al., 2008).
At the diabetic stage, however, the arteries from females demonstrated a greater suppression of acetylcholine-induced relaxation than those observed in arteries from males. Sex-based differences in vascular function are well studied (Miller, 2010), and there are also reports on the effects of sex hormones on the vascular endothelial function in diabetic animals (Martinez-Nieves and Dunbar, 2001; Matsumoto et al., 2008; Park et al., 2011; Zhang et al., 2012). However, to the best of our knowledge, this study is the first to characterize vascular function as early as the pre-diabetic stage with respect to sex. The greater impairment of relaxation responses to acetylcholine in pre-diabetic males than pre-diabetic female arteries suggests that female sex hormones may protect pre-diabetic animals against more severe endothelial dysfunction. However, when females become diabetic, they exhibit a greater endothelial dysfunction than males. Additional studies are needed to establish the specific role of sex hormones in the progression of vascular dysfunction. Nevertheless, these results are, in part, in agreement with the findings of prospective population-based studies on humans suggesting an association of diabetes with increased risk of CVD mortality in both sexes, but a greater degree of association in women than men (Hu, 2003).
The reduction of acetylcholine responses after incubation of arteries with L-NAME represents the effect of NO, and the remaining relaxation is considered as EDHF-mediated response (Feletou and Vanhoutte, 1988; Komori et al., 1988). The altered EDHF activity in the arteries of diabetic animals has been reported (Fukao et al., 1997; Shi et al., 2006). Furthermore, a decrease in EDHF-mediated response has also been reported in the mesenteric arteries from young female rats during the oestrus cycle and after ovariectomy. (Liu et al., 2001; Liu et al., 2002). However, the specific roles for the NO and EDHF pathways in the pre-diabetic or diabetic stage in relation to sex have not been shown. In this study, the importance of NO to the regulation of vascular reactivity was enhanced, while EDHF-type relaxation was diminished in both pre-diabetic and diabetic animals, regardless of sex. Despite the higher relative importance of NO, vascular relaxation was significantly impaired in the pre-diabetic and diabetic models. It is important to note that we used a nonselective inhibitor of NOS, which can also inhibit the uncoupled action of NOS leading to superoxide production. Therefore, the elevated L-NAME responses in the pre-diabetic and diabetic animals may be associated with uncoupled NOS, a major source of superoxide in vasculature in diabetes (Cai and Harrison, 2000). In this study, we did not measure superoxide production. However, we observed an elevated expression of Nox4 mRNA in pre-diabetic males (but not pre-diabetic females) and diabetic models of both sexes. Elevated expression of Nox4 has been reported in many CVD such as atherosclerosis and diabetic vasculopathies (Chen et al., 2012). In blood vessels, Nox4 is by far the most abundant Nox isoform (>1000 fold copy number over that of Nox2) (Matsuno et al., 2005). Here, no difference in Nox2 mRNA expression was found in arteries of diabetic animals or pre-diabetic males. However, the mRNA expression of Nox2 was lower in arteries of pre-diabetic females than in controls. Although the functional consequences of lower expression of Nox2 in pre-diabetic female arteries are unclear, these data may further suggest a possible protective effect of female sex hormones in pre-diabetic animals against more severe vascular dysfunction. Another point to consider is that endothelium-dependent relaxation in the smaller arteries is mediated by both NO and EDHF (McCulloch and Randall, 1998). Therefore, the impairment of relaxation responses to acetylcholine observed in the pre-diabetic and diabetic models may be due to reduced EDHF-mediated relaxation, which is the major vasodilatory mediator in arteries in these groups. This theory is supported by observation of a greater extent of impairment of vasorelaxation in pre-diabetic males than that in pre-diabetic females, possibly due to a larger (or complete) loss of EDHF-type relaxation in pre-diabetic males (Fig. 3B vs. 2B). It should be noted that diabetic females exhibited a greater impairment of acetylcholine relaxation responses than diabetic males despite there being no difference in the diminished contribution of EDHF between these two diabetic groups (both male and female diabetic groups exhibited the loss of EDHF-mediated relaxation). This may suggest that, in the setting of the loss of EDHF, other factors are contributing to the greater impairment of acetylcholine responses observed in diabetic females.
A defective EDHF-mediated relaxation observed here could result from a significant loss of hyperpolarizing KCa channels on the endothelium (Garland, 2010; Gillham et al., 2007; Kong et al., 2015). It has been shown that SKCa channels activity was down-regulated in mesenteric arteries of spontaneously hypertensive rats (Weston et al., 2010). In the current study, we observed a significant decrease in mRNA expression of KCa2.3 in pre-diabetic males and diabetic rats of both sexes. Burnham et al (Burnham et al., 2006) observed a reduced EDHF in mesenteric arteries of ZDF rats accompanied with the impaired functional role of SKCa. Thus, the loss of EDHF-type relaxation observed in pre-diabetic males and diabetic models of both sexes could in part, be attributed to the downregulation of SKCa expression.
Other factors that may contribute to vascular dysfunction are a reduced sensitivity to NO or an increased responsiveness to contractile agents. In the present study, there was a rightward shift in CRC to SNP in diabetic animals of both sexes compared with their respective controls, suggesting that decreased sensitivity of smooth muscle to NO may, in part, contribute to the decreased acetylcholine-induced vasorelaxation in those groups. Nevertheless, there was no sex difference in the SNP responses in diabetic rats, suggesting that decreased responsiveness to NO may not play a role in the sex differences observed in impairment of acetylcholine relaxation responses in diabetic vasculature. On the other hand, we showed sex differences in the contractile responses to the vasoconstrictor agents in mesenteric arteries of UCD-T2DM rats. Specifically, the sensitivity to PE was significantly enhanced in arteries of diabetic females, but not in males. Interestingly, this is in accordance with our previous report on the sex disparity in responses to PE in type 1 diabetes model (Zhang et al., 2012). Previous studies have demonstrated elevated plasma levels of the ET-1 along with the enhanced expression ET-1 receptors in diet-induced metabolic syndrome and ZDF rats (Juan et al., 1998; Wu et al., 2000). However, there is little data on the sex-specific augmentation of contractile responses in type 2 diabetes models. Similar to the findings for PE, we observed increased sensitivity to ET-1 in diabetic females, but not males. Accordingly, Matsumoto et al. (Matsumoto et al., 2008) showed enhanced ET-1 responses in mesenteric artery in the chronic stage of type 1 diabetic female mice.
Insulin resistance is an important characteristic in the pathogenesis of type 2 diabetes. In the current study, we observed a comparable trend between vascular dysfunction and decreased insulin sensitivity, as ISI was significantly reduced in males during the pre-diabetic stage but was not reduced in females until the diabetic stage. This prompted us to investigate whether a selective impairment in insulin signaling could accompany the vascular dysfunction in those groups. Accordingly, we observed a significant decrease in p-Akt (Ser-473) in pre-diabetic males, but not in pre-diabetic females, suggesting that preserved insulin signaling in female arteries in the early stage (pre-diabetes) may protect them from a more severe vascular injury. In diabetic groups, both sexes exhibited a decrease in the p-Akt level (although the difference was not statistically significant in females). This suggests that other factors may contribute to a greater impairment of vascular relaxation observed in diabetic females. Insulin resistance is associated with endothelial dysfunction through several mechanisms, such as increased production of the vasoconstrictor ET-1 (Marasciulo et al., 2006; Schneider et al., 2000). Previous studies on experimental models of insulin resistance revealed impaired Akt-dependent signaling in the vasculature, whereas ERK1/2 pathways were preserved or elevated (Jiang et al., 1999; Potenza et al., 2005; Symons et al., 2009). Insulin-mediated activation of ERK1/2 led to ET-1 production and subsequent vasoconstriction (Hopfner et al., 1998; Wu et al., 2000). Accordingly, it has been shown that insulin induces ERK1/2 activation in human microvascular endothelial cells (Meng et al., 2012). Although, we did not measure the ERK1/2 or ET-1 levels in diabetic females, they exhibited significant hyperinsulinemia along with the elevated responsiveness to ET-1, suggesting that activation of the ERK1/2-ET-1 axis due to high insulin may underlie vascular dysfunction in this group.
5. Conclusions
This is a first report showing that mesenteric arterial function is impaired as early as the pre-diabetic stage in both sexes in the UCD-T2DM rat. This study also reveals a sex disparity in the development of arterial dysfunction in this model. It suggests that reduced insulin sensitivity through Akt in addition to the loss of EDHF-mediated relaxation may predispose male arteries to injury in the pre-diabetic stage. However, the predisposition of female arteries to injury in the diabetic stage may be due to the reduced insulin sensitivity and elevated responsiveness to contractile agents such as ET-1 in this group.
Supplementary Material
Supplemental Fig. 1. Western blot analysis of cytokine signaling-3 (SOCS3) protein expression in mesenteric arteries from control, pre-diabetic, and diabetic female and male rats. Data are expressed as mean ± S.E.M. Capped lines indicate significant differences between two groups (P<0.05), as analyzed by one-way ANOVA test followed by Šidák’s test (n=4–5).
Supplemental Fig. 2. Real-time PCR analysis of NADPH oxidase (Nox2) mRNA expression in mesenteric arteries from control, pre-diabetic, and diabetic female and male rats. Data are expressed as mean ± S.E.M. Capped lines indicate significant differences between two groups (P<0.05), as analyzed by one-way ANOVA test followed by Bonferroni’s post hoc test (n=6).
Highlights:
This study represents the first report demonstrating impaired mesenteric arterial function as early as the pre-diabetic stage in UC Davis Type-2 Diabetes Mellitus (UCD-T2DM) model of both sexes. We observed a sex difference in pre-diabetic rats with a predilection for greater impairment in mesenteric arterial function in male compared to female animals. This suggests female sex hormones may protect pre-diabetic animals against more severe vascular dysfunction. However, when females become diabetic, they exhibit greater vascular dysfunction than males. This study also explored the potential mechanisms involved in sex-specific development of mesenteric arterial dysfunction in the pre-diabetic and diabetic stages in UCD-T2DM rats.
Acknowledgment
This work was supported by the National Heart, Lung and Blood Institute grant R15HL128988 to R. Rahimian and the University of the Pacific. P. Havel’s laboratory received support during the project period from National Institutes of Health Grants HL121324, DK095960, and U24 DK092993, and a multi-campus grant from the University of California Office of the President (Award #142691).
We thank Mujibullah Karimi and Grayson Dillon (undergraduate students) for their help with preparation for experiments. We also thank Dr. Gemma Sangüesa for her input.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bakker W, Eringa EC, Sipkema P, van Hinsbergh VW, 2009. Endothelial dysfunction and diabetes: roles of hyperglycemia, impaired insulin signaling and obesity. Cell Tissue Res 335, 165–189. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, 1994. Heart disease in women. Fertil Steril 62, 127S–132S. [PubMed] [Google Scholar]
- Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS, 1998. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell 1, 619–625. [DOI] [PubMed] [Google Scholar]
- Brandenburg SL, Reusch JE, Felder KK, Nelson-Wong E, Lindenfeld J, Manco-Johnson M, Regensteiner JG, 2002. Impaired fibrinolysis in premenopausal women and age-matched men with Type 2 diabetes mellitus: a pilot study. J Investig Med 50, 110–115. [DOI] [PubMed] [Google Scholar]
- Brunner H, Cockcroft JR, Deanfield J, Donald A, Ferrannini E, Halcox J, Kiowski W, Luscher TF, Mancia G, Natali A, Oliver JJ, Pessina AC, Rizzoni D, Rossi GP, Salvetti A, Spieker LE, Taddei S, Webb DJ, Working Group on, E., Endothelial Factors of the European Society of, H., 2005. Endothelial function and dysfunction. Part II: Association with cardiovascular risk factors and diseases. A statement by the Working Group on Endothelins and Endothelial Factors of the European Society of Hypertension. Journal of hypertension 23, 233–246. [DOI] [PubMed] [Google Scholar]
- Burnham MP, Johnson IT, Weston AH, 2006. Impaired small-conductance Ca2+-activated K+ channel-dependent EDHF responses in Type II diabetic ZDF rats. British journal of pharmacology 148, 434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Harrison DG, 2000. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circulation research 87, 840–844. [DOI] [PubMed] [Google Scholar]
- Chen F, Haigh S, Barman S, Fulton DJ, 2012. From form to function: the role of Nox4 in the cardiovascular system. Front Physiol 3, 412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings BP, Bettaieb A, Graham JL, Kim J, Ma F, Shibata N, Stanhope KL, Giulivi C, Hansen F, Jelsing J, Vrang N, Kowala M, Chouinard ML, Haj FG, Havel PJ, 2013. Bile-acid-mediated decrease in endoplasmic reticulum stress: a potential contributor to the metabolic benefits of ileal interposition surgery in UCD-T2DM rats. Dis Model Mech 6, 443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings BP, Bettaieb A, Graham JL, Stanhope KL, Dill R, Morton GJ, Haj FG, Havel PJ, 2011. Subcutaneous administration of leptin normalizes fasting plasma glucose in obese type 2 diabetic UCD-T2DM rats. Proc Natl Acad Sci U S A 108, 14670–14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings BP, Bettaieb A, Graham JL, Stanhope KL, Kowala M, Haj FG, Chouinard ML, Havel PJ, 2012. Vertical sleeve gastrectomy improves glucose and lipid metabolism and delays diabetes onset in UCD-T2DM rats. Endocrinology 153, 3620–3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings BP, Digitale EK, Stanhope KL, Graham JL, Baskin DG, Reed BJ, Sweet IR, Griffen SC, Havel PJ, 2008. Development and characterization of a novel rat model of type 2 diabetes mellitus: the UC Davis type 2 diabetes mellitus UCD-T2DM rat. American journal of physiology. Regulatory, integrative and comparative physiology 295, R1782–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings BP, Stanhope KL, Graham JL, Baskin DG, Griffen SC, Nilsson C, Sams A, Knudsen LB, Raun K, Havel PJ, 2010. Chronic administration of the glucagon-like peptide-1 analog, liraglutide, delays the onset of diabetes and lowers triglycerides in UCD-T2DM rats. Diabetes 59, 2653–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM, 2000. Endothelial dysfunction in diabetes. Br J Pharmacol 130, 963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Carli MF, Afonso L, Campisi R, Ramappa P, Bianco-Batlles D, Grunberger G, Schelbert HR, 2002. Coronary vascular dysfunction in premenopausal women with diabetes mellitus. Am Heart J 144, 711–718. [PubMed] [Google Scholar]
- Ding H, Hashem M, Wiehler WB, Lau W, Martin J, Reid J, Triggle C, 2005. Endothelial dysfunction in the streptozotocin-induced diabetic apoE-deficient mouse. British journal of pharmacology 146, 1110–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feletou M, 2016. Endothelium-Dependent Hyperpolarization and Endothelial Dysfunction. J Cardiovasc Pharmacol 67, 373–387. [DOI] [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM, 1988. Endothelium-dependent hyperpolarization of canine coronary smooth muscle. British journal of pharmacology 93, 515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukao M, Hattori Y, Kanno M, Sakuma I, Kitabatake A, 1997. Alterations in endothelium-dependent hyperpolarization and relaxation in mesenteric arteries from streptozotocin-induced diabetic rats. Br J Pharmacol 121, 1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland CJ, 2010. Compromised vascular endothelial cell SK(Ca) activity: a fundamental aspect of hypertension? Br J Pharmacol 160, 833–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillham JC, Myers JE, Baker PN, Taggart MJ, 2007. Regulation of endothelial-dependent relaxation in human systemic arteries by SKCa and IKCa channels. Reprod Sci 14, 43–50. [DOI] [PubMed] [Google Scholar]
- Grassi D, Desideri G, Necozione S, Lippi C, Casale R, Properzi G, Blumberg JB, Ferri C, 2008. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J Nutr 138, 1671–1676. [DOI] [PubMed] [Google Scholar]
- Grotle AK, Crawford CK, Huo Y, Ybarbo KM, Harrison ML, Graham J, Stanhope KL, Havel PJ, Fadel PJ, Stone AJ, 2019. Exaggerated cardiovascular responses to muscle contraction and tendon stretch in UCD type-2 diabetes mellitus rats. American journal of physiology. Heart and circulatory physiology 317, H479–H486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Shaligram S, Zhang R, Anderson L, Rahimian R, 2016. Sex-specific vascular responses of the rat aorta: effects of moderate term (intermediate stage) streptozotocin-induced diabetes. Canadian journal of physiology and pharmacology 94, 408–415. [DOI] [PubMed] [Google Scholar]
- Han X, Zhang R, Anderson L, Rahimian R, 2014. Sexual dimorphism in rat aortic endothelial function of streptozotocin-induced diabetes: possible involvement of superoxide and nitric oxide production. European journal of pharmacology 723, 442–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfner RL, Hasnadka RV, Wilson TW, McNeill JR, Gopalakrishnan V, 1998. Insulin increases endothelin-1-evoked intracellular free calcium responses by increased ET(A) receptor expression in rat aortic smooth muscle cells. Diabetes 47, 937–944. [DOI] [PubMed] [Google Scholar]
- Howard JK, Cave BJ, Oksanen LJ, Tzameli I, Bjorbaek C, Flier JS, 2004. Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat Med 10, 734–738. [DOI] [PubMed] [Google Scholar]
- Hu G, 2003. Gender difference in all-cause and cardiovascular mortality related to hyperglycaemia and newly-diagnosed diabetes. Diabetologia 46, 608–617. [DOI] [PubMed] [Google Scholar]
- Jiang ZY, Lin YW, Clemont A, Feener EP, Hein KD, Igarashi M, Yamauchi T, White MF, King GL, 1999. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest 104, 447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan CC, Fang VS, Hsu YP, Huang YJ, Hsia DB, Yu PC, Kwok CF, Ho LT, 1998. Overexpression of vascular endothelin-1 and endothelin-A receptors in a fructose-induced hypertensive rat model. Journal of hypertension 16, 1775–1782. [DOI] [PubMed] [Google Scholar]
- Kalyani RR, Lazo M, Ouyang P, Turkbey E, Chevalier K, Brancati F, Becker D, Vaidya D, 2014. Sex differences in diabetes and risk of incident coronary artery disease in healthy young and middle-aged adults. Diabetes Care 37, 830–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannel WB, McGee DL, 1979. Diabetes and cardiovascular disease. The Framingham study. JAMA 241, 2035–2038. [DOI] [PubMed] [Google Scholar]
- Kleinert M, Clemmensen C, Hofmann SM, Moore MC, Renner S, Woods SC, Huypens P, Beckers J, de Angelis MH, Schurmann A, Bakhti M, Klingenspor M, Heiman M, Cherrington AD, Ristow M, Lickert H, Wolf E, Havel PJ, Muller TD, Tschop MH, 2018. Animal models of obesity and diabetes mellitus. Nat Rev Endocrinol 14, 140–162. [DOI] [PubMed] [Google Scholar]
- Komori K, Lorenz RR, Vanhoutte PM, 1988. Nitric oxide, ACh, and electrical and mechanical properties of canine arterial smooth muscle. The American journal of physiology 255, H207–212. [DOI] [PubMed] [Google Scholar]
- Kong BWC, Man RYK, Gao Y, Vanhoutte PM, Leung SWS, 2015. Reduced activity of SKCaand Na-K ATPase underlies the accelerated impairment of EDH-type relaxations in mesenteric arteries of aging spontaneously hypertensive rats. Pharmacology Research & Perspectives 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MY, Hattori Y, Fukao M, Sato A, Sakuma I, Kanno M, 2001. Alterations in EDHF-mediated hyperpolarization and relaxation in mesenteric arteries of female rats in long-term deficiency of oestrogen and during oestrus cycle. Br J Pharmacol 132, 1035–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MY, Hattori Y, Sato A, Ichikawa R, Zhang XH, Sakuma I, 2002. Ovariectomy attenuates hyperpolarization and relaxation mediated by endothelium-derived hyperpolarizing factor in female rat mesenteric artery: a concomitant decrease in connexin-43 expression. J Cardiovasc Pharmacol 40, 938–948. [DOI] [PubMed] [Google Scholar]
- Lubis AR, Widia F, Soegondo S, Setiawati A, 2008. The role of SOCS-3 protein in leptin resistance and obesity. Acta Med Indones 40, 89–95. [PubMed] [Google Scholar]
- Marasciulo FL, Montagnani M, Potenza MA, 2006. Endothelin-1: the yin and yang on vascular function. Curr Med Chem 13, 1655–1665. [DOI] [PubMed] [Google Scholar]
- Martinez-Nieves B, Dunbar JC, 2001. The effect of diabetes and sex on nitric oxide-mediated cardiovascular dynamics. Exp Biol Med (Maywood) 226, 37–42. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Kakami M, Kobayashi T, Kamata K, 2008. Gender differences in vascular reactivity to endothelin-1 (1–31) in mesenteric arteries from diabetic mice. Peptides 29, 1338–1346. [DOI] [PubMed] [Google Scholar]
- Matsuno K, Yamada H, Iwata K, Jin D, Katsuyama M, Matsuki M, Takai S, Yamanishi K, Miyazaki M, Matsubara H, Yabe-Nishimura C, 2005. Nox1 is involved in angiotensin IImediated hypertension: a study in Nox1-deficient mice. Circulation 112, 2677–2685. [DOI] [PubMed] [Google Scholar]
- McCulloch AI, Randall MD, 1998. Sex differences in the relative contributions of nitric oxide and EDHF to agonist-stimulated endothelium-dependent relaxations in the rat isolated mesenteric arterial bed. Br J Pharmacol 123, 1700–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeish AJ, Sandow SL, Neylon CB, Chen MX, Dora KA, Garland CJ, 2006. Evidence for involvement of both IKCa and SKCa channels in hyperpolarizing responses of the rat middle cerebral artery. Stroke; a journal of cerebral circulation 37, 1277–1282. [DOI] [PubMed] [Google Scholar]
- McVeigh GE, Brennan GM, Johnston GD, McDermott BJ, McGrath LT, Henry WR, Andrews JW, Hayes JR, 1992. Impaired endothelium-dependent and independent vasodilation in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 35, 771–776. [DOI] [PubMed] [Google Scholar]
- Meng D, Mei A, Liu J, Kang X, Shi X, Qian R, Chen S, 2012. NADPH oxidase 4 mediates insulin-stimulated HIF-1alpha and VEGF expression, and angiogenesis in vitro. PLoS One 7, e48393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller VM, 2010. Sex-based differences in vascular function. Womens Health (Lond) 6, 737–752. [DOI] [PubMed] [Google Scholar]
- Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H, 2001. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia 44 Suppl 2, S14–21. [DOI] [PubMed] [Google Scholar]
- Ohta T, Katsuda Y, Miyajima K, Sasase T, Kimura S, Tong B, Yamada T, 2014. Gender differences in metabolic disorders and related diseases in Spontaneously Diabetic Torii-Lepr(fa) rats. J Diabetes Res 2014, 841957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltman CL, Richou LL, Davidson EP, Coppey LJ, Lund DD, Yorek MA, 2006. Progression of coronary and mesenteric vascular dysfunction in Zucker obese and Zucker diabetic fatty rats. American journal of physiology. Heart and circulatory physiology 291, H1780–1787. [DOI] [PubMed] [Google Scholar]
- Park SH, Bahk JH, Oh AY, Gil NS, Huh J, Lee JH, 2011. Gender difference and change of alpha(1)-adrenoceptors in the distal mesenteric arteries of streptozotocin-induced diabetic rats. Korean J Anesthesiol 61, 419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MA, Marasciulo FL, Chieppa DM, Brigiani GS, Formoso G, Quon MJ, Montagnani M, 2005. Insulin resistance in spontaneously hypertensive rats is associated with endothelial dysfunction characterized by imbalance between NO and ET-1 production. Am J Physiol Heart Circ Physiol 289, H813–822. [DOI] [PubMed] [Google Scholar]
- Sachidanandam K, Harris A, Hutchinson J, Ergul A, 2006. Microvascular versus macrovascular dysfunction in type 2 diabetes: differences in contractile responses to endothelin-1. Exp Biol Med (Maywood) 231, 1016–1021. [PubMed] [Google Scholar]
- Sanguesa G, Shaligram S, Akhter F, Roglans N, Laguna JC, Rahimian R, Alegret M, 2016. Type of Supplemented Simple Sugar, Not Merely Calorie Intake, Determines Adverse Effects on Metabolism and Aortic Function in Female Rats. American journal of physiology. Heart and circulatory physiology, ajpheart 00339 02016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schach C, Resch M, Schmid PM, Riegger GA, Endemann DH, 2014. Type 2 diabetes: increased expression and contribution of IKCa channels to vasodilation in small mesenteric arteries of ZDF rats. American journal of physiology. Heart and circulatory physiology 307, H1093–1102. [DOI] [PubMed] [Google Scholar]
- Schneider MP, Hilgers KF, Klingbeil AU, John S, Veelken R, Schmieder RE, 2000. Plasma endothelin is increased in early essential hypertension. American journal of hypertension 13, 579–585. [DOI] [PubMed] [Google Scholar]
- Shaligram S, Sanguesa G, Akther F, Alegret M, Laguna JC, Rahimian R, 2018. Differential effects of high consumption of fructose or glucose on mesenteric arterial function in female rats. The Journal of nutritional biochemistry 57, 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Ku DD, Man RY, Vanhoutte PM, 2006. Augmented endothelium-derived hyperpolarizing factor-mediated relaxations attenuate endothelial dysfunction in femoral and mesenteric, but not in carotid arteries from type I diabetic rats. The Journal of pharmacology and experimental therapeutics 318, 276–281. [DOI] [PubMed] [Google Scholar]
- Steinberg HO, Paradisi G, Cronin J, Crowde K, Hempfling A, Hook G, Baron AD, 2000. Type II diabetes abrogates sex differences in endothelial function in premenopausal women. Circulation 101, 2040–2046. [DOI] [PubMed] [Google Scholar]
- Symons JD, McMillin SL, Riehle C, Tanner J, Palionyte M, Hillas E, Jones D, Cooksey RC, Birnbaum MJ, McClain DA, Zhang QJ, Gale D, Wilson LJ, Abel ED, 2009. Contribution of insulin and Akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circulation research 104, 1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenouchi Y, Kobayashi T, Taguchi K, Matsumoto T, Kamata K, 2009. Gender differences in endothelial function in aortas from type 2 diabetic model mice. Journal of pharmacological sciences 111, 91–99. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, Pagano PJ, Schiffrin EL, 2002. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circulation research 90, 1205–1213. [DOI] [PubMed] [Google Scholar]
- Weston AH, Porter EL, Harno E, Edwards G, 2010. Impairment of endothelial SK(Ca) channels and of downstream hyperpolarizing pathways in mesenteric arteries from spontaneously hypertensive rats. British journal of pharmacology 160, 836–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SQ, Hopfner RL, McNeill JR, Wilson TW, Gopalakrishnan V, 2000. Altered paracrine effect of endothelin in blood vessels of the hyperinsulinemic, insulin resistant obese Zucker rat. Cardiovascular research 45, 994–1000. [DOI] [PubMed] [Google Scholar]
- Zhang R, Thor D, Han X, Anderson L, Rahimian R, 2012. Sex differences in mesenteric endothelial function of streptozotocin-induced diabetic rats: a shift in the relative importance of EDRFs. Am J Physiol Heart Circ Physiol 303, H1183–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. 1. Western blot analysis of cytokine signaling-3 (SOCS3) protein expression in mesenteric arteries from control, pre-diabetic, and diabetic female and male rats. Data are expressed as mean ± S.E.M. Capped lines indicate significant differences between two groups (P<0.05), as analyzed by one-way ANOVA test followed by Šidák’s test (n=4–5).
Supplemental Fig. 2. Real-time PCR analysis of NADPH oxidase (Nox2) mRNA expression in mesenteric arteries from control, pre-diabetic, and diabetic female and male rats. Data are expressed as mean ± S.E.M. Capped lines indicate significant differences between two groups (P<0.05), as analyzed by one-way ANOVA test followed by Bonferroni’s post hoc test (n=6).


