Abstract
Purpose:
To determine the incidence of actionable findings on contrast-enhanced magnetic resonance angiography (MRA) scans performed for the primary diagnosis of pulmonary embolism (PE).
Materials and Methods:
This was a HIPAA-compliant and IRB-approved single center, retrospective study of consecutive series of patients evaluated with contrast-enhanced MRA for PE. The final radiology report of each MRA was reviewed. All technically adequate negative exams were included in the analysis. The findings were divided into three types: those requiring further action (actionable - Type 1) those not requiring follow-up (non-actionable-Type 2) and normal exams. We compared our results with the literature regarding the use of computed tomographic angiography (CTA) in this scenario using Fisher’s exact test.
Results:
580 MRA scans for PE were performed. There were 561/580 (97%) technically adequate exams. Of these, 514/580 (89%) were negative and 47/580 (8%) were positive for PE. In the PE negative group of 514 exams, Type 1 findings were identified in 85/514 (17%), 188/514 (36%) cases were Type 2 and 241/514 (47.0%) were Type 3. There was no significant difference between the incidence of Type 1 and the combination of Type 2 and Type 3 findings on MRA and the reported incidence of actionable findings derived from CTA negative exams for PE (p < 0.5).
Conclusion:
MRA as a first-line test for PE can identify actionable findings in those patients without PE, with an incidence similar to that reported in the literature for CTA.
Keywords: pulmonary embolism, magnetic resonance angiography, non-thrombotic findings, computed tomography angiography, ancillary findings
INTRODUCTION
Acute pulmonary embolism (PE) is reported to occur in 0.1% of the population per year and is associated with considerable morbidity and mortality.[1] The prevalence of pulmonary embolism in those patients with clinical suspicion of PE has been reported to be up to 27%.[2–4] The mortality rate from untreated PE may be as high as 30% [5, 6], but decreases to less than 8% with anticoagulation.[7–9] Early diagnosis and treatment of this disorder are needed for optimal patient outcomes.
The clinical presentation of PE can mimic many other common intrathoracic conditions that also cause chest pain: pneumonia, pericardial effusion, pleural effusion, pericarditis, aortic dissection, non-ST-elevation myocardial infarction (NSTEMI), pneumothorax, cholecystitis, rib fractures, pneumothorax, pneumomediastinum and gastroesophageal reflux. Pulmonary computed tomographic angiography (CTA) is the current gold standard test for patients with symptoms suspicious of PE.[10, 11] If the exam is negative for PE, an advantage of CTA over nuclear medicine ventilation perfusion (V/Q) scanning is its ability to evaluate the many other thoracic pathologies that may be responsible for the patient’s symptoms.[12] CTA is also particularly useful in those patients with pre-existing lung disease (Emphysema./Chronic obstructive pulmonary disease (COPD)), conditions that are known to limit the efficacy of V/Q scanning.[13] Several studies have evaluated the role of CTA in the diagnosis of alternative (non-thromboembolic) etiologies in patients being evaluated for PE.[14–21]
Intravenous contrast-enhanced magnetic resonance angiography (MRA) is increasingly used to identify the presence of PE without exposing patients to ionizing radiation.[22–30] MRA can also be used safely in those patients with an iodinated contrast allergy.[26] Recently Ferumoxytol has been used for pulmonary MRA in patients with renal failure and or renal insufficiency for the diagnosis pulmonary vascular disease.[31] In a single center study, outcomes following primary evaluation with MRA in patients who presented to the emergency department (ED) with symptoms suspicious of PE were found to be similar to those reported following initial evaluation with CTA.[26] However, the diagnostic accuracy of MRA for diagnosis of actionable alternative diagnoses that may explain the patients’ symptoms is unknown.
The purpose of this study was to evaluate those patients with a negative MRA examination for PE and the incidence of alternative diagnoses. Secondarily, we aimed to compare these results with the published data for CTA in this same scenario.
METHODS
Study Design and Setting
This was a HIPAA-compliant, IRB-approved retrospective, observational study of a consecutive series of patients who underwent contrast-enhanced MRA for the primary diagnosis of PE at a single academic center from November 2009 – December 2012. Most of the patients included in this study were referred from the emergency department (ED). All patients who had an MRA performed for the diagnosis of possible PE during the study period were included in this study. These exams were performed because the ordering physician wanted a pulmonary MRA instead of a CTA. Frequently the primary reason for this choice was to avoid medical radiation for younger patients. Patients were identified by querying the radiology picture archiving and communication system (PACS) for all thoracic MRA scans performed during the study period. All MRA exams were originally interpreted and reported by subspecialty trained cardiovascular radiologists with greater than six years’ experience with MRA interpretation. We chose to not analyze any patient for ancillary findings that had a positive examination for pulmonary embolism on MRA. The reason for this is that a patient’s clinical symptoms and pain are presumed to be secondary to the presence of pulmonary embolism/pulmonary infarction, and not related to any ancillary findings. These MRA exams were performed for the primary diagnosis of the patient’s symptoms. CTA exams in these patients were not routinely available for comparison. No attempt was made to retrospectively look for any CTA exams that were performed contemporaneously with the MRA to help find ancillary findings on the CTA that may or may not have been reported on the MRA.
MRA Protocol
The MRA imaging protocol has been previously described.[26] Briefly, this included the following pulse sequences (A) localizer 3-plane single-shot fast spin-echo images; (B) pre-contrast, pulmonary arterial phase, immediate delayed-phase, and a low flip angle delayed-phase contrast-enhanced T1 weighted MRA with near isotropic spatial resolution and full chest coverage with an interpolated voxel size of 0.7×0.7×1.0 mm3; and (C) breath-hold post-contrast fat-saturated T1-weighted spoiled gradient recalled echo images. The gadolinium-based contrast agent used was 0.1 mmol/kg of gadobenate dimeglumine (Multihance™, Bracco Diagnostics, Princeton, NJ), diluted to a total volume of 30 mL with saline and injected at 1.5 mL/s. Total table time for the protocol was approximately 5–6 min.
Data Collection
Our health system’s electronic medical record (EMR) was used as the primary source of information for our data collection. Data abstraction was completed by one of the investigators [JA]. The final radiology report for each of MRA exam that was both technically adequate and negative for PE was reviewed. Any cases that were positive for PE were excluded. All radiological findings in the remaining cases were entered into a deidentified database along with the age and gender of the patient. Specifically all of the ancillary findings tabulated for this study were listed on the original final report that was archived to the EMR. No attempt was made to retrospectively review these cases to add newly observed ancillary findings to the data.
Outcomes
The method used by Richman, et al. [18] (Table 1), was simplified into 3 broad categories: actionable (Type 1), non-actionable (Type 2) and normal (Type 3). This method is similar to the Richman category A- findings requiring immediate follow up and Richman category B- findings requiring follow up to prevent significant morbidity in subsequent weeks or months as subcategories into one new Category – Type 1 and the Richman Category C- findings requiring no immediate action into this study’s Type 2 and Richman Category D- indeterminant findings along with all normal exams into a third group of findings for this study – Type 3. The single most important of the many findings derived from the final report of each case were categorized by consensus of two radiologists (JA and MS) using a very complete rubric derived from the exiting CTA literature for all of the previously listed ancillary findings. (Table 2) [18, 32] The first category (Type 1) includes all radiology report findings that required some form of follow-up by the referring clinician. Ancillary findings and normal variants and not requiring follow up were grouped into the Type 2 category using this rubric. (Table 2) The normal scans were all grouped into the third category (Type 3). For statistical purposes the non-actionable findings and the normals or normal variants were combined. Then the incidence of actionable (non-PE) findings found at MRA using this study’s data was then compared with the largest publication using CTA to study of incidence of ancillary findings in PE negative exams. [18]
Table 1:
Classification used for all contrast enhanced MRA exams that were negative for pulmonary embolism. This scheme is based on the need for further action by the referring clinician after reading the final radiology report. The method used by Richman et al. [18] is also provided for comparison. The findings that were not used by Richman et al. are listed under the category of new. In those cases in which more than one finding was included in the report, the most significant finding was used for classification. Thus each patient was entered only one time based on severity of the need for action by the referring clinician: Type 1 (Actionable) or Type 2 (not actionable).
| Type | Richman Category | Severity and detailed List of Findings |
|---|---|---|
| 1 | A |
Urgent findings which require immediate intervention: New Pneumonia, new acute aortic syndrome, new moderate to large pericardial or pleural effusion, new (mass) cancer, new septic emboli, new aortic aneurysm >3.0 cm, new pneumothorax |
| B |
Non-urgent findings which require further work-up or follow-up: Known cancer or metastatic disease, new lung nodules, lymphadenopathy, new cardiomegaly, enhancing thyroid nodule >1cm, cirrhosis, splenic or renal infarcts, pancreatitis, pericarditis, costochondritis, acute rib fractures, known metastases, gall stones without cholecystitis. |
|
| New for this study | Enhancing breast mass, biliary ductal dilatation, portal venous clots, sarcoidosis, pulmonary artery >3.0 cm suspicious for pulmonary hypertension, Hypersensitivity Radiation pneumonitis, or Drug related organizing pneumonia, invasive Aspergillosis, new or old pulmonary fibrosis, Lymphangitic carcinomatosis, Sternal dehiscence, iron overload of liver and spleen |
|
| 2 | C |
Findings requiring no further action: Simple cysts in the liver or kidney, liver hemangioma, vertebral body hemangioma, normal vascular variant, small pleural effusion, mild atelectasis, known cardiomegaly, old wedge compression fracture, hiatal hernia, artifacts, post-surgical changes, degenerative disease of the spine. |
| D |
Known chronic diseases under current treatment, Anatomic variant or indeterminant findings not requiring follow-up: Atelectasis, post-surgical changes, aberrant right subclavian artery, right aortic arch with tetralogy of Fallot (known), vertebral artery arising from the aorta, Bovine arch, two right renal arteries, liver hemangioma, low right hemidiaphragm, small pleural effusion, median arcuate ligament of celiac artery, liver cyst, bronchiectasis, renal cysts, replaced left hepatic artery, symmetric gynecomastia, azygous continuation of the Inferior vena cava, perfusion defect without pulmonary embolism, hepatomegaly, vertebral body hemangioma, cystic fibrosis with known bronchiectasis, old compression fracture of thoracic spine vertebral body, elevated right hemidiaphragm, thyroid nodule less than 1.0 cm in size, known polycystic kidney disease, extra renal pelvis, mediastinal lipomatosis, known cardiac enlargement, two renal arteries, pectus excavatum, air trapping, hepatic steatosis, cholecystomy clips, lung granulomas, Amplatzer ASD closure device, treated chronic clot in brachiocephalic vein, accessory hepatic vein, hepatomegaly, replaced left hepatic artery, vertebral artery arising from the aortic arch, splenomegaly, known Obliterative bronchiolitis from lung transplant rejection, post mastectomy changes, enlarged pulmonary artery, gastric diverticulum, splenic granulomas, intrathoracic splenosis. |
|
| 3 | New for this study | Normal exam |
Table 2:
Comparison of patient demographics, percentage of exams with pulmonary embolism, Type 1 and Type 2 plus Type 3 findings for the eight separate publications that have reviewed non-thrombotic findings on CTA negative exams for pulmonary embolism. [14–21] The CTA publications in the shaded part of the table can be compared with the current study that performed a similar analysis of MRA for the same indication. Each publication is quite different with respect to the population studied, percentage of positive examinations, age and sex distribution and the percentage of Type 1 and Type 2 plus Type 3 findings that were observed. The publication by Richman et al. [18] is the largest comparison study and is most similar to the present study in its overall study design and its Type 1 and Type 2 plus Type 3 outcome variables.
| First Author’s last name | This study | van Rossum | Kim | Garg | Lombard | Richman | van Strijen | Hall | Stein |
|---|---|---|---|---|---|---|---|---|---|
| Year of Publication | 1998 | 1999 | 1999 | 2003 | 2004 | 2005 | 2009 | 2012 | |
| Modality | MRA | CTA | CTA | CTA | CTA | CTA | CTA | CTA | CTA |
| Prospective (P) / Retrospective (R) | R | R | P | R | R | R | P | R | R |
| Male: Female (% Female) |
100:480 (83%) |
44:79 (64%) |
55:55 (50%) |
72:6 (8%) |
34:28 (45%) |
461:564 (55%) |
202/310 (61%) |
218:371 (63%) |
NR |
| Age range in years (Mean) [SD] |
10–92 (37) [19] |
19–91 (59) |
18–85 (54) |
36–86 (65) |
15–89 (55.9) |
(53) [17] |
18–96 (55) |
(53) [19] |
(61) [18] |
| Total number of patients (N) | 580 | 123 | 110 | 126 | 62 | 1025 | 512 | 589 | 332 |
| # Technically adequate exams | 561 (97%) |
123b | 110b | 126b | 62b | 1025b | 502 (98%) |
589b | 332b |
| # Positive PE exams (% of technically adequate exams) |
47 (8%) |
53 (43%) |
25c
(23%) |
48 (38%) |
11 (18%) |
104 (10%) |
124 (25%) |
55 (9%) |
NR |
| (n) # of patients with technically adequate negative PE exams | 514 | 70b | 85 | 82 (78) | 51 | 921b | 376b | 534b | 332 |
| # of PE(−) Patients with Actionable Type 1 findings |
85 (17%) |
41b (59%) |
57b (67%) |
42b/78 (54%) |
29b (63%) |
160b (17%) |
130b (35%) |
195b (37%) |
166b (50%) |
| # of PE (−) Patients with Non-Actionable or Normal findings Type 2 and Type 3 |
429 (83%) |
29b (41%) |
28b (33%) |
27b/78b (46%) |
22b/51 (37%) |
761b/921b (83%) |
246b/376b (65%) |
339b/534b (63%) |
166b/332 (50%) |
(Abbreviations: PE- pulmonary embolism, N- total number of patients enrolled at the beginning of the study, n- number of technically adequate exams without pulmonary embolism, Type 1- Actionable findings requiring follow-up, Type 2 plus Type 3- Findings not requiring follow-up, NR- not available, not recorded, not inferred from data, b- inferred, c- CTA missed one case of PE based on clinical and imaging findings, d- CTA missed two cases of PE based on clinical and imaging findings.)
Statistical Analysis
Summary descriptive statistics for age and gender were generated. Frequency counts and percentages were obtained to summarize categorical variables; Agresti-Coull modified Wald 95% confidence intervals (CI) for proportions were calculated. Fisher’s exact test was used to compare the incidence of Type 1 (actionable) findings against the reported results of Richman et al. [18]. The statistics program used was R 2.15.1 (R Development Core Team 2012). A p-value of 0.05 was considered statistically significant.
RESULTS
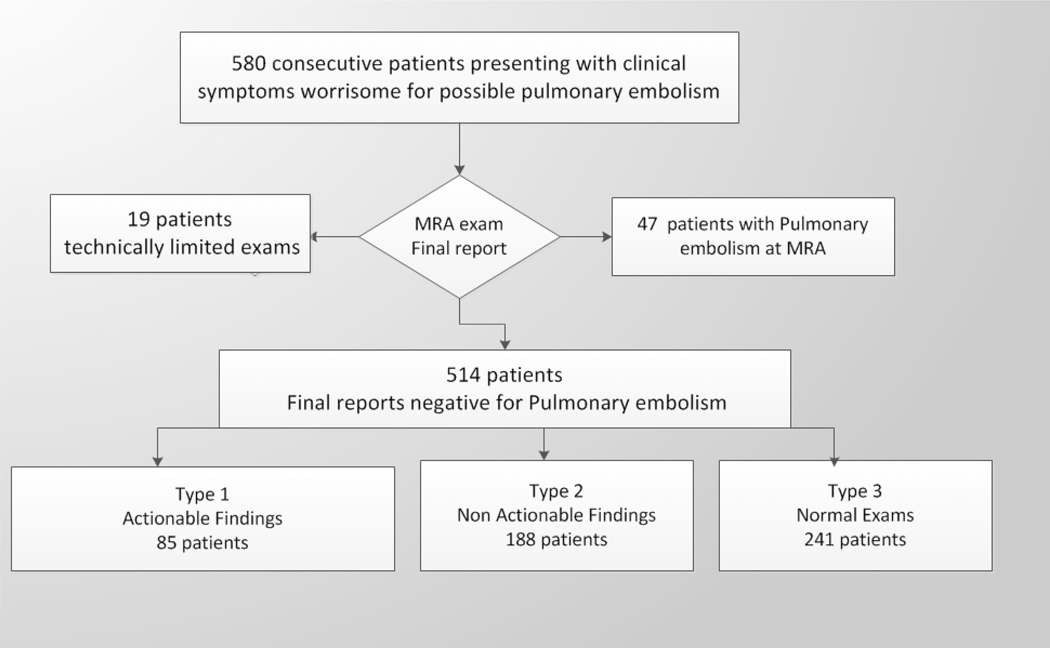
A total of 580 MRA exams were performed on 100 males and 480 females during the study period (Fig. 1). The age range of the entire population was 10–92 (mean=37, median=29, standard deviation=19) (Table 2). Of the 580 MRA exams included in the present study, 19 (3%) were technically inadequate and 47 (8%) were positive for pulmonary embolism. Of the remaining 514 patients, 89 (17%) were male and 425 (83%) were female; age range was 10–92 years (males: 44 ± 16; females: 37 ± 19) (Fig. 1). Comparable studies from the prior CTA literature are summarized in Table 2, the largest of which is the 2004 study performed by Richman et al. in 1025 patients. [14–21]
Fig. 1:
Patient flow chart for this retrospective analysis showing the number of total consecutive patients undergoing MRA for symptoms suspicious for pulmonary embolism. Type 1 findings required some additional action on the part of the ordering physician, while Type 2 findings did not.
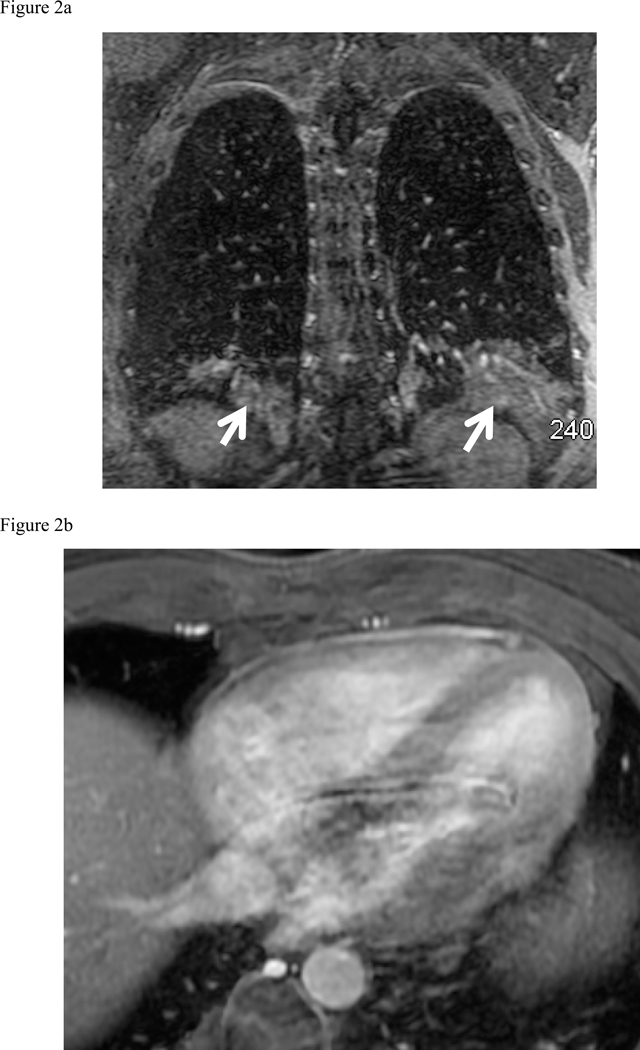
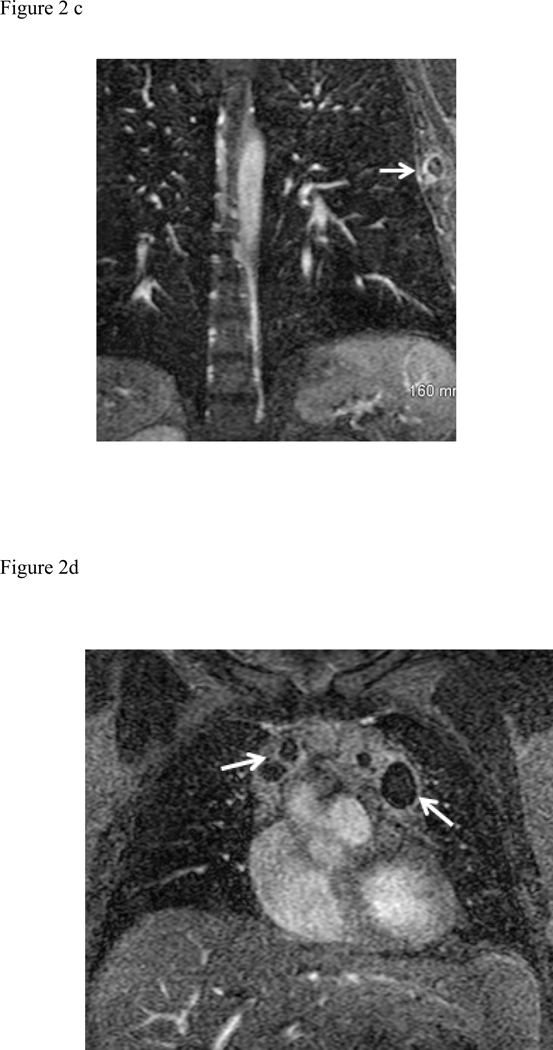
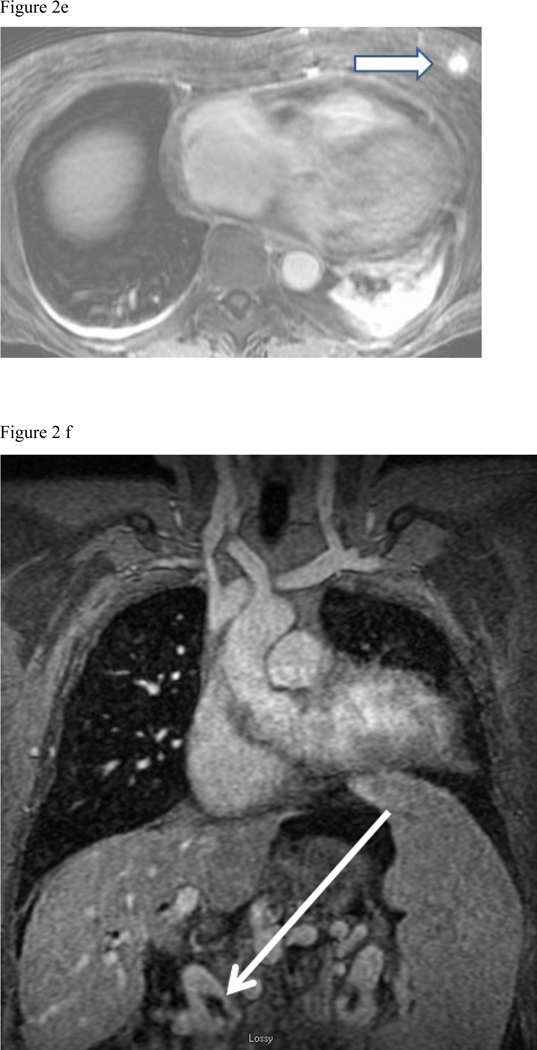
There were 85/514 (17%) cases of Type 1 finding (those requiring some degree of clinical, surgical or imaging follow up), 188/514 (36%) of Type 2 (those findings not requiring follow up) and Type 3– 241/514 (47%) totally normal exams. The following 85 Type 1 findings requiring follow up were found: moderate to large pleural effusions (34 patients), pneumonia (18 patients) (Fig. 2a), malignancy (14 patients) (Fig. 2d), ascending aortic aneurysm or dissection (9 patients), pericardial effusion (Fig. 2b) or heart failure (5 patients), septic emboli or lung abscess (2 patients) (Fig. 2c), trauma (2 patients) (Fig. 2e), and sarcoidosis (1 patient). The most common Type 2 findings were mild dependent atelectasis, small pleural effusion, normal vascular variant, simple cysts in liver or kidney and post-surgical changes.
Fig. 2:
Composite collection of Type 1 actionable findings. (a) Pneumonia- shown on the MRA delayed phase in coronal plane shows consolidation in both lower lobes (white arrows), (b) Pericarditis- enhancing visceral and parietal pericardial tissue (arrow) shown on the MRA delayed phase of contrast enhancement (c) Left lateral 6th rib fracture (arrow) shown on the MRA delayed phase (d) MRA delayed phase in coronal plane shows multiple enlarged necrotic lymph nodes - Hodgkin’s disease (white arrows), (e) Left breast mass at the post contrast T1-weighted images (arrow), (f) Portal vein thrombosis (arrow) shown on the CE-MRA delayed phase of contrast enhancement.
Table 3 shows the rates of Type 1 (actionable) and the combined Type 2 and Type 3 (non-actionable) findings on MRA from the present study versus those reported for CTA by Richman et al. [18] The incidence of Type 1 findings was 17% (CI: 15–20%) on CTA and 17% (95% C.I.: 14–20%) on MRA. This difference was not statistically significant (p = 0.5).
Table 3:
Statistical comparison showing that MRA showed an equivalent incidence of actionable (Type 1) and non-actionable (Type 2+Type 3) findings to CTA.
| CTA (Richman et al. [18]) cases/total %(95% CI) | MRA This study cases/total % (95% CI) | p-value | |
|---|---|---|---|
| Type 1 (Actionable findings) | 160/921 17% (15–20%) |
85/514 17% (14–20%) |
0.5 |
| Types 2 and 3 (Non-actionable findings or normal) |
761/921 83% (80–85%) |
429/514 83% (80–86%) |
0.5 |
DISCUSSION
MRA in the setting of suspected pulmonary embolism is capable of diagnosing actionable alternative diagnoses that require treatment or additional follow-up. The most common acute findings observed were pleural effusion and pneumonia. These results may help to allay concerns that MRA cannot diagnose important possible causes of chest pain in patients in whom PE has been excluded.
Clearly, an advantage of CTA for the emergency physician is its ability to evaluate for alternative diagnosis in addition to its proven ability to diagnose pulmonary embolism.[12, 18–20] Several studies have been published evaluating the role of CTA in determining the incidence of non-thrombotic alternative diagnoses as the most likely cause for chest pain in patients for whom PE is excluded. Unfortunately, we found that there is no common method for categorizing non-thromboembolic/ancillary findings in this clinical setting. Therefore, we built upon the methodology used by Richman et al. [18], the largest study to date in this field, and simplified that categorization scheme into two clinically relevant groups: actionable Type 1 findings requiring follow-up and the combination of non-actionable Type 2 findings requiring no follow-up and exams that were totally normal Type 3 (Table 1). Using these groups to sort the CTA data, we observed a large amount of variability in the incidence of Type 1 (17–67%) and Type 2 + Type 3 findings on CTA (33–83%) extracted from the prior literature (Table 2).[18–21] Surprisingly, however, we found that the percentages of Type 1 and the combined categories of Type 2 and Type 3 findings found at MRA in this study were equivalent to those reported by Richman et al. (Table 3) [18]. Our study’s population was different than Richman’s study population. This study had a smaller total population, was younger, had more females and an MRA was used instead of a CTA. These two studies are therefore not directly comparable.
The primary strengths of MRA in the work up of suspected PE is the lack of ionizing radiation and the lack of a nephrotoxic contrast agent. There has been some recent literature to suggest that gadolinium based contrast agents may accumulate in the body; however, the significance of this is unknown. [33] Another strength of MRA is the ability to perform multiphasic imaging or even reinjection of the gadolinium contrast agent in cases of patient motion.[26] MRA is particularly advantageous for young women, who are often at increased risk for PE because of oral contraception use [34], and also more sensitive to the effects of ionizing radiation. For these patients, PE-MRA serves as a safe and viable alternative test to CTA. For younger individuals undergoing serial follow up of known pulmonary embolism, MRA-PE is a good alternative test to CTA that can help to mitigate the lifetime exposure to medical radiation.
As with any retrospective study of this type there are limitations: (1) Contemporaneous CTA exams were not available for comparison; (2) This study has more women (83%) than men and involves much younger patients (mean age: 37 years). These differences are likely related to selection bias; (3) We did not compare the ancillary findings in this series of patient’s with the 3 month EMR follow up to try to ascertain whether or not the findings on the initial MRI exam likely accounted for the cause of the patient’s chest pain at presentation. We chose not to perform this type of retrospective assignment as there is considerable disagreement about how to determine if a symptom as protean as chest pain was indeed related to an ancillary, non-critical, finding observed at imaging; (4) MRA sequences used were not optimized for the evaluation of lung parenchyma. It is likely that small non-enhancing lung nodules were not seen in these MRA exams. In the future, ultrashort time to echo (TE < 100 μs) MR pulse sequences that are able to image the lung parenchyma [35] and pulmonary vasculature during free breathing could further improve the ability of MR to depict both PE and intrapulmonary pathology in these patients that present with dyspnea.[36] (5) Also, we do not have follow-up on these cases of ancillary findings to determine how they impacted clinical management. We will soon be publishing a retrospective case controlled study that compares an age and sex matched group of MRA and CTA exams for pulmonary embolism and evaluates the all-cause mortality, incidence of venous thromboembolic disease and incidence of major bleeding after six months of follow up. This new study (manuscript in preparation) will help to answer some of the questions regarding the relative safety of using pulmonary MRA as first line test for the diagnosis of pulmonary embolism.
In conclusion, we found that the use of MRA as a primary test for PE can depict clinically important actionable findings in those patients in whom PE is excluded at a rate comparable to the published CTA literature.
Acknowledgements:
GE Healthcare, Bracco Pharmaceuticals, and the Department of Radiology R & D fund
Grant Support: NIH UL1TR000427, NIH KL2TR000428, NIH K24DK102595
References
- [1].Levin D, Seo JB, Kiely DG, Hatabu H, Gefter W, van Beek EJ, Schiebler ML, I.W.f.P.F.I. (IWPFI), Triage for suspected acute Pulmonary Embolism: Think before opening Pandora’s Box, Eur J Radiol, (2015). [DOI] [PubMed] [Google Scholar]
- [2].Sohns C, Amarteifio E, Sossalla S, Heuser M, Obenauer S, 64-Multidetector-row spiral CT in pulmonary embolism with emphasis on incidental findings, Clin Imaging, 32 (2008) 335–341. [DOI] [PubMed] [Google Scholar]
- [3].Winer-Muram HT, Rydberg J, Johnson MS, Tarver RD, Williams MD, Shah H, Namyslowski J, Conces D, Jennings SG, Ying J, Trerotola SO, Kopecky KK, Suspected acute pulmonary embolism: evaluation with multi-detector row CT versus digital subtraction pulmonary arteriography, Radiology, 233 (2004) 806–815. [DOI] [PubMed] [Google Scholar]
- [4].de Monyé W, van Strijen MJ, Huisman MV, Kieft GJ, Pattynama PM, Suspected pulmonary embolism: prevalence and anatomic distribution in 487 consecutive patients. Advances in New Technologies Evaluating the Localisation of Pulmonary Embolism (ANTELOPE) Group, Radiology, 215 (2000) 184–188. [DOI] [PubMed] [Google Scholar]
- [5].Horlander KT, Leeper KV, Troponin levels as a guide to treatment of pulmonary embolism, Curr Opin Pulm Med, 9 (2003) 374–377. [DOI] [PubMed] [Google Scholar]
- [6].Anderson FA, Wheeler HB, Goldberg RJ, Hosmer DW, Patwardhan NA, Jovanovic B, Forcier A, Dalen JE, A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study, Arch Intern Med, 151 (1991) 933–938. [PubMed] [Google Scholar]
- [7].Goldhaber SZ, Visani L, De Rosa M, Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER), Lancet, 353 (1999) 1386–1389. [DOI] [PubMed] [Google Scholar]
- [8].Tapson VF, Acute pulmonary embolism, N Engl J Med, 358 (2008) 1037–1052. [DOI] [PubMed] [Google Scholar]
- [9].Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, Nelson ME, Wells PS, Gould MK, Dentali F, Crowther M, Kahn SR , A.C.o.C. Physicians, Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines, Chest, 141 (2012) e419S–494S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Remy-Jardin M, Remy J, Wattinne L, Giraud F, Central pulmonary thromboembolism: diagnosis with spiral volumetric CT with the single-breath-hold technique--comparison with pulmonary angiography, Radiology, 185 (1992) 381–387. [DOI] [PubMed] [Google Scholar]
- [11].Remy-Jardin M, Pistolesi M, Goodman LR, Gefter WB, Gottschalk A, Mayo JR, Sostman HD, Management of suspected acute pulmonary embolism in the era of CT angiography: a statement from the Fleischner Society, Radiology, 245 (2007) 315–329. [DOI] [PubMed] [Google Scholar]
- [12].Goodman LR, Computed tomography in the emergency department setting, JAMA Intern Med, 173 (2013) 167–168. [DOI] [PubMed] [Google Scholar]
- [13].Moua T, Wood K, COPD and PE: a clinical dilemma, Int J Chron Obstruct Pulmon Dis, 3 (2008) 277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].van Rossum AB, Pattynama PM, Mallens WM, Hermans J, Heijerman HG, Can helical CT replace scintigraphy in the diagnostic process in suspected pulmonary embolism? A retrolective-prolective cohort study focusing on total diagnostic yield, Eur Radiol, 8 (1998) 90–96. [DOI] [PubMed] [Google Scholar]
- [15].Kim KI, Müller NL, Mayo JR, Clinically suspected pulmonary embolism: utility of spiral CT, Radiology, 210 (1999) 693–697. [DOI] [PubMed] [Google Scholar]
- [16].Garg K, Sieler H, Welsh CH, Johnston RJ, Russ PD, Clinical validity of helical CT being interpreted as negative for pulmonary embolism: implications for patient treatment, AJR Am J Roentgenol, 172 (1999) 1627–1631. [DOI] [PubMed] [Google Scholar]
- [17].Lombard J, Bhatia R, Sala E, Spiral computed tomographic pulmonary angiography for investigating suspected pulmonary embolism: clinical outcomes, Can Assoc Radiol J, 54 (2003) 147–151. [PubMed] [Google Scholar]
- [18].Richman PB, Courtney DM, Friese J, Matthews J, Field A, Petri R, Kline JA, Prevalence and significance of nonthromboembolic findings on chest computed tomography angiography performed to rule out pulmonary embolism: a multicenter study of 1,025 emergency department patients, Acad Emerg Med, 11 (2004) 642–647. [PubMed] [Google Scholar]
- [19].van Strijen MJ, Bloem JL, de Monyé W, Kieft GJ, Pattynama PM, van den Berg-Huijsmans A, Huisman MV, Group A-S, Helical computed tomography and alternative diagnosis in patients with excluded pulmonary embolism, J Thromb Haemost, 3 (2005) 2449–2456. [DOI] [PubMed] [Google Scholar]
- [20].Hall WB, Truitt SG, Scheunemann LP, Shah SA, Rivera MP, Parker LA, Carson SS, The prevalence of clinically relevant incidental findings on chest computed tomographic angiograms ordered to diagnose pulmonary embolism, Arch Intern Med, 169 (2009) 1961–1965. [DOI] [PubMed] [Google Scholar]
- [21].Stein PD, Matta F, Sedrick JA, Saleh T, Badshah A, Denier JE, Ancillary findings on CT pulmonary angiograms and abnormalities on chest radiographs in patients in whom pulmonary embolism was excluded, Clin Appl Thromb Hemost, 18 (2012) 201–205. [DOI] [PubMed] [Google Scholar]
- [22].Schiebler ML, Holland GA, Hatabu H, Listerud J, Foo T, Palevsky H, Edmunds H, Gefter WB, Suspected pulmonary embolism: prospective evaluation with pulmonary MR angiography, Radiology, 189 (1993) 125–131. [DOI] [PubMed] [Google Scholar]
- [23].Grist TM, Sostman HD, MacFall JR, Foo TK, Spritzer CE, Witty L, Newman GE, Debatin JF, Tapson V, Saltzman HA, Pulmonary angiography with MR imaging: preliminary clinical experience, Radiology, 189 (1993) 523–530. [DOI] [PubMed] [Google Scholar]
- [24].Meaney JF, Weg JG, Chenevert TL, Stafford-Johnson D, Hamilton BH, Prince MR, Diagnosis of pulmonary embolism with magnetic resonance angiography, N Engl J Med, 336 (1997) 1422–1427. [DOI] [PubMed] [Google Scholar]
- [25].Stein PD, Chenevert TL, Fowler SE, Goodman LR, Gottschalk A, Hales CA, Hull RD, Jablonski KA, Leeper KV Jr., Naidich DP, Sak DJ, Sostman HD, Tapson VF, Weg JG, Woodard PK, Gadolinium-enhanced magnetic resonance angiography for pulmonary embolism: a multicenter prospective study (PIOPED III), Ann Intern Med, 152 (2010) 434–443, W142–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schiebler ML, Nagle SK, Francois CJ, Repplinger MD, Hamedani AG, Vigen KK, Yarlagadda R, Grist TM, Reeder SB, Effectiveness of MR angiography for the primary diagnosis of acute pulmonary embolism: clinical outcomes at 3 months and 1 year, J Magn Reson Imaging, 38 (2013) 914–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sostman HD, Layish DT, Tapson VF, Spritzer CE, DeLong DM, Trotter P, MacFall JR, Patz EF Jr., Goodman PC, Woodard PK, Foo TK, Farber JL, Prospective comparison of helical CT and MR imaging in clinically suspected acute pulmonary embolism, J Magn Reson Imaging, 6 (1996) 275–281. [DOI] [PubMed] [Google Scholar]
- [28].Woodard PK, Sostman HD, MacFall JR, DeLong DM, McDonald JW, Foo TK, Patz EF, Goodman PC, Spritzer CE, Detection of pulmonary embolism: comparison of contrast-enhanced spiral CT and time-of-flight MR techniques, J Thorac Imaging, 10 (1995) 59–72. [PubMed] [Google Scholar]
- [29].Brenner DJ, What we know and what we don’t know about cancer risks associated with radiation doses from radiological imaging, Br J Radiol, 87 (2014) 20130629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Piechowiak EI, Peter JF, Kleb B, Klose KJ, Heverhagen JT, Intravenous Iodinated Contrast Agents Amplify DNA Radiation Damage at CT, Radiology, (2015) 132478. [DOI] [PubMed] [Google Scholar]
- [31].Hope MD, Hope TA, Zhu C, Faraji F, Haraldsson H, Ordovas KG, Saloner D, Vascular imaging with ferumoxytol as a constrast agent, AJR Am. J. Roentgenol. 205 (2015) W366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Stein PD, Matta F, Sedrick JA, Saleh T, Badshah A, Denier JE, Ancillary Findings on CT Pulmonary Angiograms and Abnormalities on Chest Radiographs in Patients in Whom Pulmonary Embolism was Excluded, Clin Appl Thromb Hemost, (2011). [DOI] [PubMed] [Google Scholar]
- [33].Kanda T, Osawa M, Oba H, Toyoda K, Kotoku J, Haruyama T, Takeshita K, Furui S, High signal intensity in dentate nucleus on unenhanced T1-weighted MR images: association with linear versus macrocyclic gadolinium chelate administration, Radiology 275 (2015) 803–809. [DOI] [PubMed] [Google Scholar]
- [34].Parkin L, Sharples K, Hernandez RK, Jick SS, Risk of venous thromboembolism in users of oral contraceptives containing drospirenone or levonorgestrel: nested case-control study based on UK General Practice Research Database, BMJ, 342 (2011) d2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Johnson KM, Fain SB, Schiebler ML, Nagle S, Optimized 3D ultrashort echo time pulmonary MRI, Magn Reson Med, 70 (2013) 1241–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bannas P, Bell LC, Johnson KM, Schiebler ML, François CJ, Motosugi U, Consigny D, Reeder SB, Nagle SK, Pulmonary Embolism Detection with Three-dimensional Ultrashort Echo Time MR Imaging: Experimental Study in Canines, Radiology, 278 (2016) 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]