Abstract
This Perspective discusses recent experiments that bear on the chiral induced spin selectivity (CISS) mechanism and its manifestation in electronic and magnetic properties of chiral molecules and materials. Although the discussion emphasizes newer experiments, such as the magnetization dependence of chiral molecule interactions with ferromagnetic surfaces, early experiments, which reveal the nonlinear scaling of the spin filtering with applied potential, are described also. In many of the theoretical studies, one has had to invoke unusually large spin–orbit couplings in order to reproduce the large spin filtering observed in experiments. Experiments imply that exchange interactions and Pauli exclusion constraints are an important aspect of CISS. They also demonstrate the spin-dependent charge flow between a ferromagnetic substrate and chiral molecules. With these insights in mind, a simplified model is described in which the chiral molecule’s spin polarization is enhanced by a spin blockade effect to generate large spin filtering.
First reported in 1999,1 the chiral induced spin selectivity (CISS) effect
refers to the preferential transmission (or transfer) of electrons
with one spin orientation over the other through chiral molecules
and materials.2 A wide range of experimental
observations, using various techniques, have examined CISS; however,
consensus on a theoretical description is lacking. Although theoretical
treatments of the phenomenon exist, quantitative comparisons with
experiment remain elusive and no single viewpoint has emerged. The
main discrepancy between the calculations and the experimental results
lies in the magnitude of the spin polarization P,
which may be defined as 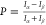 for
which Iα and Iβ are the experimental
measurables (e.g., current, rate constant,
etc.) for spin pointing parallel or antiparallel to the electron’s
velocity. While spin polarization exceeding 80% is known experimentally,3 most models and calculations produce polarizations
of only a few percent, when averaged over the experimental energy
window.
for
which Iα and Iβ are the experimental
measurables (e.g., current, rate constant,
etc.) for spin pointing parallel or antiparallel to the electron’s
velocity. While spin polarization exceeding 80% is known experimentally,3 most models and calculations produce polarizations
of only a few percent, when averaged over the experimental energy
window.
Chiral molecules remain the focus of intensive research in chemistry, mostly driven by pharmaceutical applications. Recent experiments are revealing that CISS, which couples the electron spin direction to the molecular frame, imparts enantiospecificity to chemical reactions4 and to adsorption on ferromagnetic surfaces.5−7 While spin selection rules are well-appreciated in reaction dynamics, and must be considered for molecules with unpaired electrons or electrons that are not paired within a state, the spin direction is not defined in the molecular reference frame. Rather, the direction of the electron’s spin is defined in terms of the relative orientation of the spins of two or more electrons (doublet, triplet, etc.), or by the electron spin alignment relative to an external magnetic field. Thus, spin selection rules are often not explicitly considered in chemical reactions between molecules and between a molecule and a surface (with the notable exception of photochemistry), despite the large energy associated with the relative alignment of two spins, as in singlet–triplet splitting. For chiral molecules, CISS and the consequent coupling of the electron spin to a molecule’s chiral axis generate a preferred electron spin direction in the molecular frame. In this way, spin selection rules can become an important consideration for chemical transformations that involve chiral species. By using magnetized, ferromagnetic substrates, it is possible to generate enantiospecific molecule–substrate interactions and guide reaction outcomes.
A number of recent reviews related to the CISS effect are available.8,9 Most CISS-related experiments have been performed with molecules adsorbed to surfaces,10 which relates the spin direction to the laboratory frame that is defined by the substrate’s surface normal and/or magnetization direction. In this Perspective, we discuss recent experiments that supply a new viewpoint about the electronic and magnetic properties associated with surface-immobilized chiral molecules and their charge and spin transfer (and transport). We also discuss how CISS allows one to distinguish chiral molecules on a ferromagnetic surface, by changing the magnetization of the surface using current or an external magnetic field. We also discuss some recent theoretical approaches, which may explain the large spin polarizations observed in experiments.
Exciting new experimental results involving the CISS effect continue to be reported. For example, experiments with chiral molecules have revealed spin polarizations as large as 85%, a ratio of about 1:12 between the two spins, at room temperature.3 For the first time, the CISS effect has been reported to manifest as an enantiospecific response in solid-state cross-polarization nuclear magnetic resonance experiments11 and in the spin-polarized electrons found in electron paramagnetic resonance (EPR) studies of electrochemical reactions.12 Workers are also demonstrating molecular device concepts; for example, a light-induced molecular configuration change was used to switch electron spin polarizations, realizing a light-triggered, molecular spin valve.13 More chemistry-centric are reports that chemisorption6 of chiral molecules on ferromagnets is enantiospecific and that redox reactions at magnetized electrodes are enantiospecific.4 Rather than providing an analysis of these and other important demonstrations of CISS and its implications, the discussion below focuses on a subset of experiments that provide insights into the mechanism of CISS.
While numerous earlier experiments bear on the CISS mechanism (see discussion in ref (14)), we identify six experiments since 2017 that have important implications for understanding the mechanism of CISS:
STM-break junction experiments15 with peptide molecules and earlier conducting probe measurements with DNA molecules16 demonstrate that spin-selective transport manifests for individual (or a few) chiral molecules; that is, it does not require a monolayer film.
Spin polarization has been reported for long-range electron transport, i.e., through 50 nm chiral perovskite films.17 While it is not known if the spin transfer occurs by tunneling, resonances, or by hopping, it is clear that spin information can be transported over long distances.
The magnitude of the spin polarization correlates with the strength of a material’s chiro-optical response for electron transfer reactions with quantum dots,18 for the catalytic activity in water splitting,19 and for supramolecular fibers,3 suggesting that CD spectra may prove useful as a “predictor” of CISS response.
Kumar et al.20 used Hall effect measurements to show that spin polarization accompanies charge polarization in monolayer films of chiral molecules. Addition of achiral polarizable units on the molecules, or electrical gate, was later shown to enhance the spin polarization in such films.21,22 Thus, the spin polarization can be generated within molecules, and no net electron transfer or transport is required.
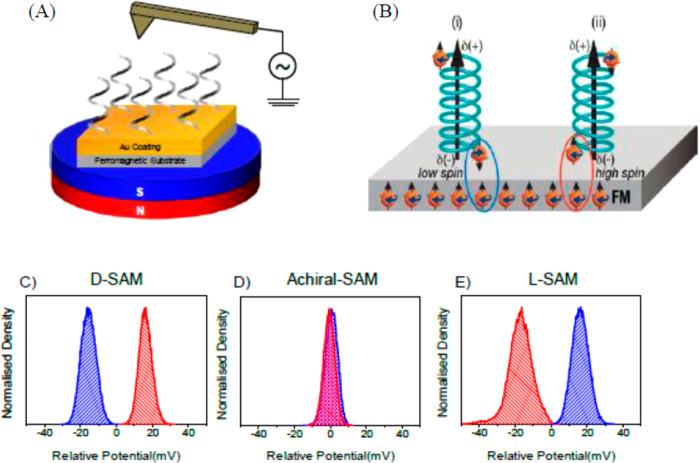
Using Kelvin probe measurements of ferromagnet/chiral molecule interfaces, Ghosh et al.23 showed that the tunneling length of the electron spin wave function into chiral monolayer films is enantiospecific (see Figure 1). In related work, Ziv et al. showed that the interaction force between chiral molecules and a ferromagnetic substrate depends on the spin alignment in the ferromagnet.24 Together these studies indicate that spin exchange interactions and Pauli exclusion are key features for CISS.
Adsorption of chiral molecules on a ferromagnetic substrate is enantiospecific, depending on the enantiomeric form of the molecule and the magnetization state of the substrate. This feature of CISS was demonstrated through switching of the ferromagnetic state of ultrathin magnetic films with chiral molecule chemisorption,25 conversion of superparamagnetic nanoparticle assemblies to ferromagnetic assemblies with chiral monolayer films,26 and chiral molecule separations with magnetized surfaces.6
Figure 1.
(A) Schematic for the Kelvin probe microscopy experiment, in which the distance of an AFM tip from a SAM-coated ferromagnetic surface is varied sinusoidally, and its lateral position is scanned to image the substrate’s potential distribution. The diagram in panel B illustrates the idea that charge polarization is accompanied by spin polarization for chiral molecules. For a given enantiomer the interaction between the magnetized surface and the molecule follows either a low-spin (i) or a high-spin (ii) potential, depending on the direction of magnetization of the substrate. The bottom three panels show the contact potential (CPD) distributions for three different SAM-coated ferromagnetic substrates under two different magnetizations: the D-AL5 peptide (C), an achiral SAM (D) and an L-AL5 peptide (E). The blue color shows the CPD for a south magnetization, and red shows the CPD for a north magnetization. The zero voltage is set by the averaged contact potential difference found in the two measurements. Adapted from ref (23). Copyright 2020 American Chemical Society.
The processes probed in these various experiments are all dynamic; namely, they relate to transient charge (and spin) polarization in the chiral molecules (Figure 1B). Moreover, the decay of the dynamical spin state is expected to be dissipative. In all cases the enantiospecificity manifests at ambient temperatures, which implies that the activation energy and/or tunneling energy differences are ≳kT.
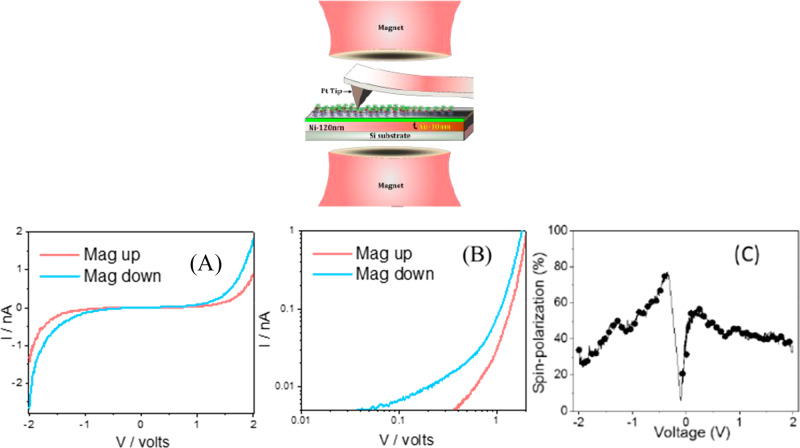
In addition, it is important to note the nonlinear character of the spin polarization measured in current–voltage curves for chiral molecules.27,28 While evident in many of the pre-2017 studies this experimental signature has not been emphasized. Figure 2 presents current versus voltage curves obtained for an oligopeptide using the conducting magnetic AFM configuration.27 Panel A shows the current–voltage data on a linear scale for two different magnetization directions, and panel B plots the same data as a log–log plot. It is important to note that the spin-dependent current starts at a different voltage for the two spins, the current near zero voltage is within the noise of the experimental system, and the slope of the log I vs log V reaches values of up to 3. These data show that the spin-polarization in the current changes very strongly with bias near zero and continues to change, albeit more weakly, at higher applied biases. This rich behavior indicates a need to better describe the substrate–molecule electronic structure and the electronic spin subbands of the metal–molecule interface.
Figure 2.
Top: The contact magnetic AFM setup. (A) Current (I) versus potential (V) applied on a oligopeptide, HSCH2CH2CO-(l-Ala-Aib)4–COOH, using the conductive magnetic AFM setup. (B) Positive component of the curve shown in panel A, but presented on a log–log scale. (C) Absolute spin polarization as a function of the applied potential. Maximum spin polarization of 75% and 55% were observed at −0.4 V and +0.2 V, respectively.
The body of experiments performed by various research groups leads to the following observations about the spin-filtering and spin polarization properties (CISS properties) of chiral molecules:
-
1.
The spin polarization for current flowing through chiral molecules can reach very high values of 85% and more at room temperature.
-
2.
Polarization of the spin current through chiral molecules depends nonlinearly on the voltage, and the spin information can be transported over many tens of nanometers—perhaps even longer.
-
3.
When a potential is applied on chiral molecules, spin polarization accompanies the charge polarization. This polarization can result in strong spin exchange interactions (∼50 meV), with other chiral molecules or a magnetic substrate.24
-
4.
The magnitude and sign of a molecule’s CISS property correlates with the strength of the molecule’s chiro-optical response. It is important to appreciate that the chiral optical response is of course wavelength-dependent; so far the lowest-energy circular dichroism peak or bisignate spectral feature have been used.
In addition to these features, which should be captured by quantitatively accurate models of CISS, the importance of the surface–molecule interface should be included, particularly for ferromagnetic surfaces.
The role of the substrate and its spin–orbit coupling (SOC) in the CISS effect was considered previously in a theoretical treatment;29 however, photoemission experiments with nonmagnetic substrates possessing different SOC strengths display similar spin-filtering efficiencies.30 The experimental findings for chiral molecules on ferromagnetic surfaces, however, imply that the substrate properties are important for accurately describing the behavior. The Kelvin probe and AFM studies on ferromagnetic surfaces point to a spin dependence of the electron density delocalization into chiral molecules from the substrate, even though no net current is flowing.23 More generally, the experiments described above imply that the spin-dependent exchange interaction is important in determining the enantiospecific interaction of chiral molecules with ferromagnetic substrates. This feature of chiral molecule interfaces is similar to recent developments in organic spintronics that assign strong spin polarization (and spin filtering) to the spin-dependent electronic rearrangements of the molecule–ferromagnet interface (spinterface), even without chirality.31 A combination of the spin-filtering that arises from the electronic rearrangements associated with chemisorption on ferromagnetic films, which generate the spin filtering of the spinterface, and the CISS effect in the chiral adsorbed molecules may cooperate to generate large spin filtering ratios.
As early as 1990, the interaction of chiral molecules, in the gas phase, with spin polarized unbound electrons was treated theoretically.32 However, many of the theoretical works that treat spin-filtering of electrons conducted through chiral molecules follow the approach that is used to treat the electrical conductance of molecules. Namely, one calculates the molecular electronic states (with or without including the contacts) and then one calculates the electron transmission through this system for a given electrical potential difference between the two sides of the molecule. Through this approach, useful concepts have been established, and the effects of spin filtering by a chiral potential become manifest;33−36 however, these treatments predict, in most cases, magnitudes for the spin polarization/spin-filtering that are much smaller than what is reported experimentally. This approximation assumes that the electric field falling on the molecule is small compared to the internal electric fields experienced by the valence electrons and that the spin selectivity in the transmission arises from spin–orbit coupling term(s) introduced in the molecular Hamiltonian.
It is well-known that the spin–orbit coupling (SOC) in a hydrocarbon is very small, on the order of microelectronvolts.37 For a curved molecule however, the SOC increases because of the overlap of the p orbitals on the atoms and it reaches values comparable to the SOC in the carbon atom, about 5–10 meV.38,39 Most of the theoretical works overcome the small spin polarizations found in the models by adjusting the magnitude of the SOC.40 However, SOCs on the order of 10 meV generate spin polarizations of only a few percent when considering a wide energy range.
Other approaches to enhance the magnitude of the spin polarization have been tried as well. Early work by Mujica and co-workers34 considered constructive interference from multiple scattering as a mechanism that could enhance spin-filtering through longer helices. More recently, Michaeli and Naaman suggested that the electric field applied on the molecule enhances the spin polarization for tunneling processes occurring through a barrier created by the electric field.33,41 While these models qualitatively predict the experimental spin polarization, their range of applicability is much smaller than what is found in experiment. Thus, some feature(s) of the CISS effect in the molecule is not captured by these treatments and new approaches are needed.
In recent theoretical work,33 one possible solution for the issue of small spin–orbit coupling was provided for a helical chain of atoms. In this treatment, the spin–orbit coupling on each atom in the chiral molecule is given by λLσ, where λ is the spin–orbit coupling observed for carbon atoms (5 meV), L the angular momentum, and σ the Pauli spin matrix. The total spin–orbit coupling of the chiral molecules is then expressed as the vector sum of the atomic terms:
| 1 |
so that the SOC for the chiral chain can be significantly larger (order-of-magnitude) than the atomic value. This SOC, along with accidental degeneracies (curve crossings) that appear in the spectrum of chiral molecules, yields a sizable spin polarization. Although quantitative comparisons are not available, this sort of description may account for the large spin polarization seen in transport and in photoemission experiments. The approaches described above are one-electron (orbital) models, whereas CISS may require a many-body description. Recent treatments along these lines are beginning to appear.42−44
Here we sketch another possible mechanism, which is based on a spin blockade idea. First, consider a process for generating a spin polarization in a chiral molecule. When an electric field (or gradient in electrochemical potential) is applied across a molecule, or a monolayer of molecules, charge polarization occurs. This charge displacement current generates opposite spin polarizations at the negative and the positive electric poles of the molecule; which spin density exists in excess on the pole with electron density excess depends on the molecule’s handedness.20 It is important to appreciate that the total spin of the molecule is still zero because of the total spin conservation. The spin polarization is a transient local property; namely, it means that in the molecule there are sites in which the net spin density is not zero. However, the sum over all those sites results in net zero spin. For chiral molecules with a relatively small SOC of about 5 meV, a spin polarization, ΔP(V), of a few percent accompanies this charge polarization. Please note that the spin polarization will depend on the electric field applied on the molecule (hence on the voltage applied), because it requires charge polarization which is field-dependent.
Now we consider how a molecule’s small spin polarization causes strong spin filtering. As an electron moves from a donor (or negatively biased electrode) into the chiral molecule it can have, in principle, two possible spin orientations with regard to its velocity. Because of the spin polarization at the chiral molecule’s site near the donor/electrode, a difference in energy, ΔeV, is associated with the injection into the molecule of one spin over the other. We make the ansatz that this difference in energy is
| 2 |
in which EST is the energy gap between the singlet and triplet states of the molecule. To motivate this choice, consider a “thought experiment” in which a molecular radical is being reduced by an incident electron. For a chiral molecule, the unpaired electron has a preferred spin direction in the molecular frame. Depending on the relative orientation of the reducing electron’s spin and that of the molecular radical, a molecular anion can form in either a singlet state or a triplet state, as a result of the Pauli exclusion principle. In this way, the spin constraints applied in this thought experiment/reaction result in large energy differences for the two possible reactions (singlet–triplet splitting energy EST). In many spin-filtering measurements, the excess electron is transiting through the molecule and the molecule’s spin polarization is not 100%. In eq 2, the factor ΔP scales the energy splitting to account for the imperfect degree of spin polarization.
Figure 3 presents a scheme of the model. Upon applying an electric field on the chiral molecule, either by two leads or by having contacts with different electrochemical potentials at the two sides (represented by the displaced yellow lines), charge moves in response to the field (excess charge indicated by q+ and q– near the helical coil, which represents the molecule). The charge distribution, following the application of the applied voltage, is presented by the green and purple wave-like curves. Because of the chiral molecule’s SOC, the charge reorganization is spin-dependent. Hence, each curve represents a different spin alignment, spin aligned parallel (green) or antiparallel (purple) relative to the electron displacement direction. The dotted line represents the field inside the molecule, assuming that the molecule has a very low dielectric constant. For a SOC of a few millielectronvolts, the charge reorganization generates a spin polarization, ΔP, of a few percent.
Figure 3.
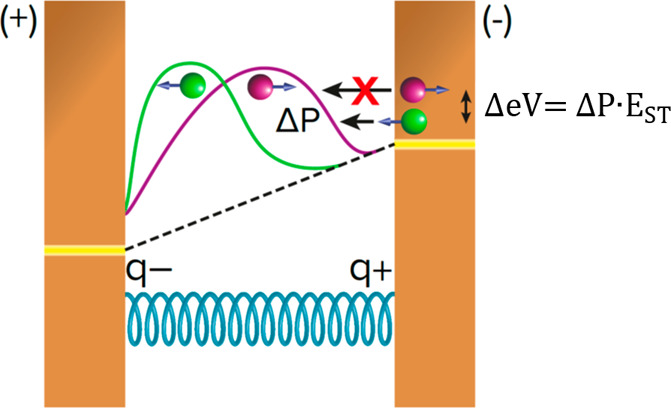
Scheme describing the mechanism of the CISS effect in terms of a small spin polarization, ΔP, that arises from the SOC. This small spin polarization causes a spin blockade, because of the Pauli principle, that is proportional to the singlet–triplet energy gap, EST, in the molecule. The purple and green curves represent the charge distribution occurring upon applying the field across the molecule, for electrons with spin aligned parallel (green) or antiparallel (purple) to their velocities. The molecule is presented schematically as a coil. The yellow lines indicate the Fermi energy at each electrode, and the dotted line shows the electric field across the molecule, assuming a molecule with a very low dielectric constant.
Simultaneously with the charge reorganization and because of the formation of a positively charged pole in the molecule, electron density from the electrode/donor is transferred into the molecule. However, the energy barrier for the electron injection depends on the electron’s spin direction; this difference is given by eq 1. Assuming a barrier for the tunneling of the “favorable spin” through the molecule of Etun, the unfavorable spin will have a barrier of Etun + ΔeV. Given that the typical energy splitting between singlet and triplet states in hydrocarbons is on the order of 1 eV,45 a spin polarization of 3.6% yields an energy splitting of ΔeV = 36 meV. Because ΔeV depends on the applied voltage, so does the tunneling through the barrier, and the current will depend nonlinearly on the applied voltage. If the injection barrier for the unfavorable electron differs by 36 meV, then the ratio between the current of the two spins will be about 1:4, a spin polarization of approximately 60%. If the singlet–triplet energy difference is larger or the initial spin polarization on the formation of the dipole is higher, then a higher spin polarization can be observed in the CISS effect. It is important to appreciate that if the difference in the injection energy threshold (ΔeV) is large, than the spin selectivity will be high when the voltage applied is above the injection energy threshold, but then with increasing voltage the selectivity will decay somewhat because the injection energy is much above the barrier for both spins. This behavior is consistent with the observation in Figure 2. The mechanism proposed here is reminiscent of a “spin blockade”46 that restricts the spin injection from the substrate to the molecule. It may account for the large spin selectivity observed in CISS and the large transient spin-state lifetimes, despite a modest spin–orbit coupling in the chiral molecule.
For chiral molecules, magnetic conducting AFM measurements of various molecules show large spin selectivity with energy gaps between spin states in the range of 50–150 meV.28 The model just presented ascribes this gap to a spin-blockade induced by exchange interactions with the magnetic substrate. It is important to note that the spin blockade is not an equilibrium effect and will decay with time as discussed by Barron.47 These ideas could be tested by dynamic spin transport calculations that introduce the system in a realistic way.
Figure 2 and ref (48) provide examples of experimental conductance data revealing that the spin-dependent conduction through a chiral molecule depends in a nonlinear way on the electric field acting on the molecule. This seems to be a general phenomenon in conduction through organic molecules, because typically the screening length of the field exceeds the size of the molecule, and therefore, the field causes a Stark effect that moves the energy level(s) of the molecule. Even small biases of 1 V across a nanometer generate significant fields, and for ferromagnet–molecule surfaces these effects are spin-dependent. Thus, the electronic state distribution relevant for electron tunneling through chiral molecules is voltage (and spin) dependent. This nonlinearity enables the measurements of spin-dependent transport measurements through molecules, even in two contact configurations.49−51
The experiments show that the CISS effect in a molecule correlates with its optical activity and its electronic polarizability. The explicit relation between polarizability, optical activity, and the magnitude of the CISS effect should be explored theoretically. If well founded, it could provide a way to screen and predict the magnitude of the spin polarization of a molecule based on its chiro-optical response.
The exchange interactions that give rise to the spin-selectivity on a magnetic substrate and between oriented chiral molecules need to be modeled and better understood. Given that experimental results are now available on CISS for transport over tens of nanometers, one should consider that CISS might manifest in the very long-range electron transfer observed in some bacteria and in artificial systems.52 The fact that the effect is transient and dissipation takes place will require dynamic spin transport calculations that introduce the system in a realistic way. Even at this stage, the results obtained for the spinterface properties, resulting from adsorption of chiral molecules on ferromagnets, open the possibility of using them for controlling charge injection into adsorbed chiral molecules, thereby controlling their reactivity.
Finally, the CISS effect has not yet been explored as an important aspect of coherent processes. Because of the small dimensions of chiral molecules and the long lifetime of the electron spin in such systems, chiral molecules and the CISS effect are good candidates for being components of quantum-based devices that utilize the coherent properties of the electron’s spin. This field is still in its infancy, and both theoretical and experimental work will be required to realize its potential.
Acknowledgments
R.N. and D.H.W. acknowledge partial support from DOE (Grant No. ER46430) and D.H.W. acknowledges NSF (CHE 1900078). R.N. and Y.P. acknowledge the support of the Israel Ministry of Science.
The authors declare no competing financial interest.
References
- Ray K.; Ananthavel S. P.; Waldeck D. H.; Naaman R. Asymmetric Scattering of Polarized Electrons by Organized Organic Films Made of Chiral Molecules. Science 1999, 283, 814–816. 10.1126/science.283.5403.814. [DOI] [PubMed] [Google Scholar]
- Naaman R.; Waldeck D. H. The Chiral Induced Spin Selectivity Effect. J. Phys. Chem. Lett. 2012, 3, 2178–2187. 10.1021/jz300793y. [DOI] [PubMed] [Google Scholar]
- Kulkarni C.; Mondal A. K.; Das T. K.; Grinbom G.; Tassinari F.; Mabesoone M. F. J.; Meijer E. W.; Naaman R. Highly efficient and tunable filtering of electrons’ spin by supramolecular chirality of nanofiber-based materials. Adv. Mater. 2020, 32, 1904965. 10.1002/adma.201904965. [DOI] [PubMed] [Google Scholar]
- Metzger T. S.; Mishra S.; Bloom B. P.; Goren N.; Neubauer A.; Shmul G.; Wei J.; Yochelis S.; Tassinari F.; Fontanesi C.; Waldeck D. H.; Paltiel Y.; Naaman R. The Electron Spin as a Chiral Reagent. Angew. Chem., Int. Ed. 2020, 59, 1653–1658. 10.1002/anie.201911400. [DOI] [PubMed] [Google Scholar]
- Tassinari F.; Steidel J.; Paltiel S.; Fontanesi C.; Lahav M.; Paltiel Y.; Naaman R. Enantioseparation by crystallization using magnetic substrates. Chem. Sci. 2019, 10, 5246–5250. 10.1039/C9SC00663J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee-Ghosh K.; Ben Dor O.; Tassinari F.; Capua E.; Yochelis S.; Capua A.; Yang S.-H.; Parkin S. S. P.; Sarkar S.; Kronik L.; Baczewski L. T.; Naaman R.; Paltiel Y. Separation of Enantiomers by Their Enantiospecific Interaction with Achiral Magnetic Substrates. Science 2018, 360, 1331–1334. 10.1126/science.aar4265. [DOI] [PubMed] [Google Scholar]
- Ben Dor O.; Yochelis S.; Radko A.; Vankayala K.; Capua E.; Capua A.; Yang S.-H.; Baczewski L. T.; Parkin S. S. P.; Naaman R.; Paltiel Y. Magnetization switching in ferromagnets by adsorbed chiral molecules without current or external magnetic field. Nat. Commun. 2017, 8, 14567. 10.1038/ncomms14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaeli K.; Varade V.; Naaman R.; Waldeck D. H. A New Approach Towards Spintronics- Spintronics with No Magnets. J. Phys.: Condens. Matter 2017, 29, 103002. 10.1088/1361-648X/aa54a4. [DOI] [PubMed] [Google Scholar]
- Naaman R.; Paltiel Y.; Waldeck D. H. Chiral molecules and the electron’s spin. Nat. Rev. Chem. 2019, 3, 250–260. 10.1038/s41570-019-0087-1. [DOI] [Google Scholar]
- Naaman R.; Waldeck D. H. Spintronics and Chirality: Spin Selectivity in Electron Transport through Chiral Molecules. Annu. Rev. Phys. Chem. 2015, 66, 263–281. 10.1146/annurev-physchem-040214-121554. [DOI] [PubMed] [Google Scholar]
- Santos J. I.; Rivilla I.; Cossío F. P.; Matxain J. M.; Grzelczak M.; Mazinani S. K. S.; Ugalde J. M.; Mujica V. Chirality-Induced Electron Spin Polarization and Enantiospecific Response in Solid-State Cross-Polarization Nuclear Magnetic Resonance. ACS Nano 2018, 12, 11426–11433. 10.1021/acsnano.8b06467. [DOI] [PubMed] [Google Scholar]
- Blumenschein F.; Tamski M.; Roussel C.; Smolinsky E. Z. B.; Tassinari F.; Naaman R.; Ansermet J. P. Spin-dependent charge transfer at chiral electrodes probed by magnetic resonance. Phys. Chem. Chem. Phys. 2020, 22, 997–1002. 10.1039/C9CP04681J. [DOI] [PubMed] [Google Scholar]
- Suda M.; Thathong Y.; Promarak V.; Kojima H.; Nakamura M.; Shiraogawa T.; Ehara M.; Yamamoto H. M. Light-driven molecular switch for reconfigurable spin filters. Nat. Commun. 2019, 10, 2455. 10.1038/s41467-019-10423-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaeli K.; Kantor-Uriel N.; Naaman R.; Waldeck D. H. The Electron’s Spin and Molecular Chirality- How Are They Related and How Do They Affect Life Processes?. Chem. Soc. Rev. 2016, 45, 6478–6487. 10.1039/C6CS00369A. [DOI] [PubMed] [Google Scholar]
- Aragonès A. C.; Medina E.; Ferrer-Huerta M.; Gimeno N.; Teixidó M.; Palma J. L.; Tao N.; Ugalde J. M.; Giralt E. T.; Díez-Pérez I.; Mujica V. Measuring the Spin-Polarization Power of a Single Chiral Molecule. Small 2017, 13, 1602519. 10.1002/smll.201602519. [DOI] [PubMed] [Google Scholar]
- Xie Z.; Markus T. Z.; Cohen S. R.; Vager Z.; Gutierrez R.; Naaman R. Spin Specific Electron Conduction through DNA Oligomers. Nano Lett. 2011, 11, 4652–4655. 10.1021/nl2021637. [DOI] [PubMed] [Google Scholar]
- Lu H.; Wang J.; Xiao C.; Pan X.; Chen X.; Brunecky R.; Berry J. J.; Zhu K.; Beard M. C.; Vardeny Z. V. Spin Dependent Charge Transport through 2D Chiral Hybrid Lead-Iodide Perovskite. Science Adv. 2019, 5, eaay0571. 10.1126/sciadv.aay0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom B. P.; Graff B. M.; Ghosh S.; Beratan D. N.; Waldeck D. H. Chirality Control of Electron Transfer in Quantum Dot Assemblies. J. Am. Chem. Soc. 2017, 139, 9038–9043. 10.1021/jacs.7b04639. [DOI] [PubMed] [Google Scholar]
- Mtangi W.; Tassinari F.; Vankayala K.; Jentzsch A. V.; Adelizzi B.; Palmans A. R. A.; Fontanesi C.; Meijer E. W.; Naaman R. Control of Electrons’ Spin Eliminates Hydrogen Peroxide Formation During Water Splitting. J. Am. Chem. Soc. 2017, 139, 2794–2798. 10.1021/jacs.6b12971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A.; Capua E.; Kesharwani M. K.; Martin J. M. L.; Sitbon E.; Waldeck D. H.; Naaman R. Spin Polarization Accompanies Charge Polarization in Chiral Molecules- Implication for Enantio-selectivity and Bio-recognition. Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 2474–2478. 10.1073/pnas.1611467114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullard G.; Tassinari F.; Ko C.-H.; Mondal A. K.; Wang R.; Mishra S.; Naaman R.; Therien M. J. Low-Resistance Molecular Wires Propagate Spin Polarized Currents. J. Am. Chem. Soc. 2019, 141, 14707–14711. 10.1021/jacs.9b06142. [DOI] [PubMed] [Google Scholar]
- Smolinsky Z. B. E.; Neubauer A.; Kumar A.; Yochelis S.; Capua E.; Carmieli R.; Paltiel Y.; Naaman R.; Michaeli K. Electric field controlled magnetization in GaAs/AlGaAs heterostructures-chiral organic molecules hybrids. J. Phys. Chem. Lett. 2019, 10, 1139–1145. 10.1021/acs.jpclett.9b00092. [DOI] [PubMed] [Google Scholar]
- Ghosh S.; Mishra S.; Avigad E.; Bloom B. P.; Baczewski L. T.; Yochelis S.; Paltiel Y.; Naaman R.; Waldeck D. H. Effect of Chiral Molecules on the Electron’s Spin Wavefunction at Interfaces. J. Phys. Chem. Lett. 2020, 11, 1550–1557. 10.1021/acs.jpclett.9b03487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv A.; Saha A.; Alpern H.; Sukenik N.; Baczewski L. T.; Yochelis S.; Reches M.; Paltiel Y. AFM-Based Spin-Exchange Microscopy Using Chiral Molecules. Adv. Mater. 2019, 31, 1904206. 10.1002/adma.201904206. [DOI] [PubMed] [Google Scholar]
- Ben Dor O.; Yochelis S.; Radko A.; Vankayala K.; Capua E.; Capua A.; Yang S.-H.; Baczewski L. T.; Parkin S. S. P.; Naaman R.; Paltiel Y. Magnetization switching in ferromagnets by adsorbed chiral molecules without current or external magnetic field. Nat. Commun. 2017, 8, 14567. 10.1038/ncomms14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koplovitz G.; Leitus G.; Ghosh S.; Bloom B. P.; Yochelis S.; Rotem D.; Vischio F.; Striccoli M.; Fanizza E.; Naaman R.; Waldeck D. H.; Porath D.; Paltiel Y. Single Domain 10 nm Ferromagnetism Imprinted on Superparamagnetic Nanoparticles Using Chiral Molecules. Small 2019, 15, 1804557. 10.1002/smll.201804557. [DOI] [PubMed] [Google Scholar]
- Kiran V.; Cohen S. R.; Naaman R. Structure Dependent Spin Selectivity in Electron Transport through Oligopeptides. J. Chem. Phys. 2017, 146, 092302 10.1063/1.4966237. [DOI] [Google Scholar]
- Xie Z.; Markus T. Z.; Cohen S. R.; Vager Z.; Gutierrez R.; Naaman R. Spin Specific Electron Conduction Through DNA Oligomers. Nano Lett. 2011, 11, 4652–4655. 10.1021/nl2021637. [DOI] [PubMed] [Google Scholar]
- Gersten J.; Kaasbjerg K.; Nitzan A. Induced spin filtering in electron transmission through chiral molecular layers adsorbed on metals with strong spin-orbit coupling. J. Chem. Phys. 2013, 139, 114111. 10.1063/1.4820907. [DOI] [PubMed] [Google Scholar]
- Kettner M.; Maslyuk V. V.; Nürenberg D.; Seibel J.; Gutierrez R.; Cuniberti G.; Ernst K.-H.; Zacharias H. Chirality-Dependent Electron Spin Filtering by Molecular Monolayers of Helicenes. J. Phys. Chem. Lett. 2018, 9, 2025–2030. 10.1021/acs.jpclett.8b00208. [DOI] [PubMed] [Google Scholar]
- Cinchetti M.; Dediu V. A.; Hueso L. E. Activating the Molecular Spinterface. Nat. Mater. 2017, 16, 507–515. 10.1038/nmat4902. [DOI] [PubMed] [Google Scholar]
- Fandreyer R.; Thompson D.; Blum K. Attenuation of longitudinally polarized electron beams by chiral molecules. J. Phys. B: At., Mol. Opt. Phys. 1990, 23, 3031–3040. 10.1088/0953-4075/23/17/016. [DOI] [Google Scholar]
- Dalum S.; Hedegård P. Theory of Chiral Induced Spin Selectivity. Nano Lett. 2019, 19, 5253–5259. 10.1021/acs.nanolett.9b01707. [DOI] [PubMed] [Google Scholar]
- Yeganeh S.; Ratner M. A.; Medina E.; Mujica V. Chiral electron transport: Scattering through helical potentials. J. Chem. Phys. 2009, 131, 014707 10.1063/1.3167404. [DOI] [PubMed] [Google Scholar]
- Guo A.-M.; Sun Q.-F. Spin-Selective Transport of Electrons in DNA Double Helix. Phys. Rev. Lett. 2012, 108, 218102. 10.1103/PhysRevLett.108.218102. [DOI] [PubMed] [Google Scholar]
- Gutierrez R.; Diaz E.; Gaul C.; Brumme T.; Dominguez-Adame F.; Cuniberti G. Modeling Spin Transport in Helical Fields; Derivation of an Effective Low-Dimensional Hamiltonian. J. Phys. Chem. C 2013, 117, 22276–22284. 10.1021/jp401705x. [DOI] [Google Scholar]
- See for example:Veeman W. S.; van der Waals J. H. Spin-orbit coupling in aromatic molecules. Mol. Phys. 1970, 18, 63–75. 10.1080/00268977000100061. [DOI] [Google Scholar]
- Ando T. Spin-orbit interaction in carbon nanotubes. J. Phys. Soc. Jpn. 2000, 69, 1757–1763. 10.1143/JPSJ.69.1757. [DOI] [Google Scholar]
- Huertas-Hernando D.; Guinea F.; Brataas A. Spin-orbit coupling in curved graphene, fullerenes, nanotubes, and nanotube caps. Phys. Rev. B: Condens. Matter Mater. Phys. 2006, 74, 155426. 10.1103/PhysRevB.74.155426. [DOI] [Google Scholar]
- Gutierrez R.; Diaz E.; Naaman R.; Cuniberti G. Spin Selective Transport Through Helical Molecular Systems. Phys. Rev. B: Condens. Matter Mater. Phys. 2012, 85, 081404 10.1103/PhysRevB.85.081404. [DOI] [Google Scholar]
- Michaeli K.; Naaman R. Origin of Spin Dependent Tunneling Through Chiral Molecules. J. Phys. Chem. C 2019, 123, 17043–17048. 10.1021/acs.jpcc.9b05020. [DOI] [Google Scholar]
- Fransson J. Chirality Induced Spin Selectivity – The Role of Electron Correlations. J. Phys. Chem. Lett. 2019, 10, 7126–7132. 10.1021/acs.jpclett.9b02929. [DOI] [PubMed] [Google Scholar]
- Diaz E.; Contreras A.; Hernandez J.; Dominguez-Adame F. Effective nonlinear model for electron transport in deformable helical molecules. Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top. 2018, 98, 052221 10.1103/PhysRevE.98.052221. [DOI] [Google Scholar]
- Medina E.; Gonzalez-Arraga L. A.; Finkelstein-Shapiro D.; Berche B.; Mujica V. Continuum model for chiral induced spin selectivity in helical molecules. J. Chem. Phys. 2015, 142, 194308. 10.1063/1.4921310. [DOI] [PubMed] [Google Scholar]
- See for example:Kohler A.; Beljonne D. The singlet-triplet exchange energy in conjugated polymers. Adv. Funct. Mater. 2004, 14, 11–18. 10.1002/adfm.200305032. [DOI] [Google Scholar]
- de Bruijckere J.; Gehring P.; Palacios-Corella M.; Clemente-Leon M.; Coronado E.; Paaske J.; Hedegård P.; van der Zant H. S. J. Ground-State Spin Blockade in a Single-Molecule Junction. Phys. Rev. Lett. 2019, 122, 197701. 10.1103/PhysRevLett.122.197701. [DOI] [PubMed] [Google Scholar]
- Barron L. D. False Chirality, Absolute Enantioselection and CP Violation: Pierre Curie’s Legacy. Magnetochemistry 2020, 6, 5. 10.3390/magnetochemistry6010005. [DOI] [Google Scholar]
- Naaman R.; Waldeck D. H. Comment on “Spin-dependent electron transmission model for chiral molecules in mesoscopic devices. Phys. Rev. B: Condens. Matter Mater. Phys. 2020, 101, 026403 10.1103/PhysRevB.101.026403. [DOI] [Google Scholar]
- See for example:Matityahu S.; Aharony A.; Entin-Wohlman O.; Balseiro C. A. Spin filtering in all-electrical three-terminal interferometers. Phys. Rev. B: Condens. Matter Mater. Phys. 2017, 95, 085411 10.1103/PhysRevB.95.085411. [DOI] [Google Scholar]
- Guo A.-M.; Sun Q.-F. Spin-dependent electron transport in protein-like single-helical molecules. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 11658–11662. 10.1073/pnas.1407716111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H.-N.; Zhu Y.-L.; Sun X.; Gong W.-J. Spin polarization and spin separation realized in the double-helical molecules. Phys. E 2015, 74, 156–159. 10.1016/j.physe.2015.07.005. [DOI] [Google Scholar]
- Ing N. L.; El-Naggar M. Y.; Hochbaum A. I. Going the Distance: Long-Range Conductivity in Protein and Peptide Bioelectronic Materials. J. Phys. Chem. B 2018, 122, 10403–10423. 10.1021/acs.jpcb.8b07431. [DOI] [PubMed] [Google Scholar]