Abstract
We report the synthesis of sulfinamides using organometallic reagents, a sulfur dioxide reagent, and nitrogen based-nucleophiles. The addition of an organometallic reagent to the commercially available sulfur dioxide surrogate, DABSO, generates a metal sulfinate which is reacted with thionyl chloride to form a sulfinyl chloride intermediate. Trapping the sulfinyl chlorides in situ with a variety of nitrogen nucleophiles delivers sulfinamides in 32–83% yields. Each stage of the process is performed at room temperature, and the total reaction time is only 1.5 h.
Introduction
Sulfinamides are a broadly useful class of functional groups in organic chemistry. Chiral sulfinamides have been widely used as chiral auxiliaries,1 as ligands in transition-metal catalysis,2 and as organocatalysts.3 They can serve as N-protecting groups, which can be easily cleaved by acidic treatment.4 Sulfinamides are also used as intermediates to prepare sulfonimidamides and sulfoximines, which have recently enjoyed increased interest in the medicinal chemistry community.5 To date, racemic sulfinamides are usually prepared from sulfinic acids or sulfinate salts, most commonly by reaction with oxalyl chloride6 or thionyl chloride6e,7 to give sulfinyl chloride intermediates which then react with amines (Scheme 1a), or by direct coupling with amines using DCC8 or EDCI.9 Alternative methods include the oxidation of disulfides to form sulfinate esters10 that further react with amines11 or lithium amides (Scheme 1b).10b In 2007, the Harmata group reported the synthesis of sulfinamides from aryl sulfonyl chlorides and amines using PPh3 as a reductant.12 More recently, Wei and Sun reported a synthesis based on the activation of tert-butyl sulfoxides with NBS and acetic acid, subsequently quenching the reactions with a selection of nucleophiles including amines.13 Boronic acids and boronate esters have been combined with DAST-type reagents under aerobic conditions to prepare sulfinamides, as reported by the Shi laboratory (Scheme 1c).14 The oxidative copper-catalyzed synthesis of sulfinamides, starting from thiols or sulfenate anions, has also been reported, as has the copper-catalyzed trans-sulfinamidation.15 Despite the success of the methods described above, there remain limitations. For example, sulfinate salts (or their acid form),6−9 odorous thiols,15a,15b disulfides,10a,13,15a and sulfonyl chlorides12 are frequently reacted directly (Scheme 1a) or employed as precursors to the starting materials, and such preinstalled sulfur functional groups can limit commercial availability, especially in complex structural settings. Additionally, nitrogen based-nucleophiles are generally limited to secondary and primary amines.13−15 Ammonia could only be used when sulfinyl chlorides were employed as intermediates.1b,6a,6c,6e,7a Preformed amination reagents have also been used,14,15b,15c which are not commercially available and require additional steps to prepare. The range of sulfinamides accessible is generally limited to aryl and alkyl variants, while the preparation of heteroaryl sulfinamides was less common using these methods. The exceptions to this were the reactions between sulfinyl chlorides and amines6e and the direct reaction of heteroaryl boronic acids and DAST-type reagents.14
Scheme 1. Selected Methods for the Synthesis of Racemic Sulfinamides.
Based on the limitations of the reported methods, we sought a more general sulfinamide synthesis and in particular targeted a method that could provide a range of sulfinyl core structures and also tolerate a variety of nitrogen functional groups. In 2010, our laboratory reported the DABCO·(SO2)2 adduct, DABSO, as an air-stable, and easy-to-handle sulfur dioxide surrogate.16 DABSO now has wide commercial availability. Addition of organometallic reagents to DABSO results in formation of the corresponding sulfinates, which have been converted in situ to a variety of useful sulfonyl functional groups, such as sulfones, sulfonamides, and sulfonyl fluorides.16b,17 We speculated that the metal sulfinates formed in situ could be telescoped with subsequent reactions to prepare sulfinamides, thus avoiding the use of substrates with preinstalled sulfur functionality. Such a transformation, based on the use of organometallic reagents, either obtained from commercial sources or prepared from metal–halogen exchange or direct deprotonation, should allow access to a wide range of complex sulfinamides.
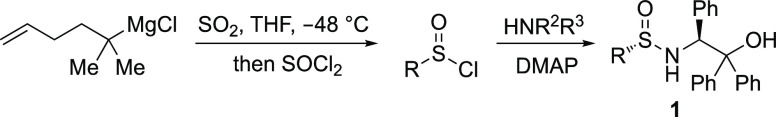
The synthesis of sulfinyl chlorides from the addition of organometallic reagents to sulfur dioxide to form metal sulfinate salts, which were then treated with thionyl chloride, has been reported before;18a for example, the Ellman group employed this method to prepare sulfinamide 1 as a single diastereomer (Scheme 2).18b However, liquid sulfur dioxide used at low temperature, obtained from condensing the gaseous reagent, was employed in this transformation and as such provides an obstacle to use in certain laboratories. We envisioned that using DABSO as a more convenient sulfur dioxide reagent would deliver a streamlined, user-friendly sulfinamide synthesis.
Scheme 2. Stepwise Synthesis of Sulfinamide from Grignard Reagents Using Sulfur Dioxide.
Results and Discussion
Prior work had shown that a range of sulfinyl chlorides, including heteroaryl sulfinyl chlorides, had shown promising reactivity with nitrogen-based nucleophiles,6e and as such, our approach was based on reacting in situ formed metal sulfinates with either thionyl chloride or oxalyl chloride to prepare sulfinyl chloride intermediates. We were pleased to find that the in situ formed p-fluorophenylsulfinate salt, prepared from the addition of p-fluorophenylmagnesium bromide to DABSO, reacted smoothly with 1.1 equiv of thionyl chloride to form the corresponding sulfinyl chloride. This was then combined with 1.5 equiv of morpholine and 1.5 equiv of triethylamine to deliver sulfinamide 2a in 83% yield on a 0.5 mmol scale and 79% yield on a gram scale (Table 1). Each step of this one-pot, three-step sequence was performed at room temperature under a nitrogen atmosphere. Oxalyl chloride could be employed in place of thionyl chloride; however, the reactions with the metal sulfinates were more vigorous and needed to be performed at 0 °C. More significantly, 1,2-diamides formed from oxalyl chloride and amines often coeluted with alkyl sulfinamides in flash column chromatography, resulting in purification difficulties. Accordingly, thionyl chloride was selected for further studies.
Table 1. Scope of the Organometallic Reagenta.
Reaction conditions: RMgX (0.5 mmol, 1 equiv), DABSO (0.25 mmol, 0.5 equiv), THF, rt, 30 min, then SOCl2 (1.1 equiv), rt, 30 min followed by Et3N (1.5 equiv) and morpholine (1.5 equiv), rt, 30 min.
Organolithium reagent was used.
A suspension of DABSO (0.6 equiv) in THF was added to the organolithium reagent at −78 °C then warmed to rt.
5 min for step 2.
With the optimized conditions in hand, we next examined the scope of the reaction with respect to the organometallic reagents, using morpholine as the nucleophile, to prepare the corresponding sulfinamides (Table 1). Generally, a wide range of aryl, alkyl, and heteroaryl organometallic reagents could be effectively converted into sulfinyl chlorides and on to sulfinamides. Aryl Grignard reagents substituted at different positions were well tolerated, delivering para- (2a, 2e), meta- (2b), and ortho-substituted phenyl (2c) and naphthyl (2d) sulfinamides in 70–83% isolated yields. A pharmaceutically relevant organolithium reagent reacted smoothly under the optimized conditions, delivering the arene core of the COX2 inhibitor celecoxib (2e). However, alkenyl Grignard reagent (2f) was less successful, with only 32% of the targeted sulfinamide isolated. Primary (2g), secondary (2h), and tertiary (2i) alkyl Grignard reagents were reacted effectively under the optimized conditions, with 71–82% yields of the products being isolated. It is worth noting that the primary alkyl sulfinamide (2g) was isolated in slightly lower yield than the corresponding secondary and tertiary alkyl systems, possibly due to the instability of the sulfinyl chloride intermediate. We were pleased to find that nitrogen-, oxygen-, and sulfur-containing heteroaromatics provided effective organometallic reagents, delivering pyridine (2j), benzofuran (2k), and thiophene (2l) sulfinamides in 63–76% yield.
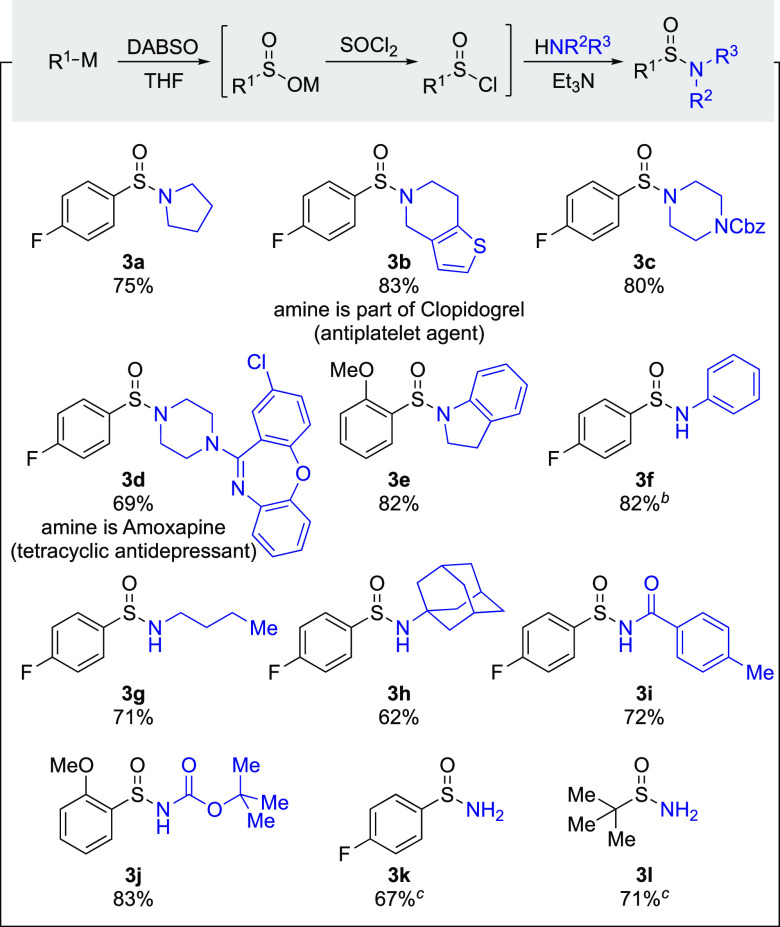
We then explored the scope of nitrogen-based nucleophiles that could be employed, using p-fluorophenylmagnesium bromide as the organometallic reagent (Table 2). Secondary amines, including pyrrolidine (3a), piperidine (3b), piperazine (3c, 3d), and indoline (3e), as well as biologically relevant amines (3b, 3d) reacted smoothly to provide tertiary sulfinamides in 69–83% isolated yields. Aniline (3f) was also a suitable substrate. However, for this example, a significant amount of the double N-substituted sulfinamide was formed as a side product, indicating that the nitrogen in the initially formed sulfinamide was still available for a second nucleophilic attack on the sulfinyl chloride intermediate. We were pleased to find that this could be suppressed by transferring the formed sulfinyl chloride to a solution of aniline, allowing an 82% yield of the targeted sulfinamide to be achieved. Primary amines were also good substrates, with both n-butylamine (3g) and sterically demanding adamantylamine (3h) being employed. Nucleophiles with reduced nucleophilicities, namely amide (3i) and carbamate (3j), were also effective substrates, providing 72% and 83% yields of the corresponding sulfinamides, respectively. These types of amide- and carbamate-substituted sulfinamides are typically synthesized by derivatization of the corresponding primary sulfinamides using strong base (e.g., n-BuLi) in combination with anhydrides6a,19 or dicarbonates (e.g., Boc anhydride),19c,20 and their direct synthesis from sulfinyl chlorides (or sulfinic anhydrides) is rare.21 It is worth noting that, unlike the aniline-substituted sulfinamide, there were no significant amounts of double-substituted sulfinamides formed when primary amines (3g, 3h), amide (3i), and carbamate (3j) nucleophiles were employed. Sulfinamides 3e and 3j feature an alternative arene core, as the corresponding p-fluorophenylmagnesium bromide derived products were difficult to purify using flash column chromatography. Finally, we found that a biphasic mixture of ammonium hydroxide solution and ethyl acetate proved efficient for preparing primary sulfinamides 3k and the racemic form of Ellman’s auxiliary 3l, in 67% and 71% yield, respectively.6c
Table 2. Scope of Nitrogen-Based Nucleophilesa.
Reaction conditions: RMgX (0.5 mmol, 1 equiv), DABSO (0.25 mmol, 0.5 equiv), THF, rt, 30 min, then SOCl2 (1.1 equiv), rt, 30 min followed by Et3N (1.5 equiv) and nucleophile (1.5 equiv), rt, 30 min. Nucleophile was added as a solution in THF if it was a solid.
Sulfinyl chloride was added to a solution of aniline (3.0 equiv) in THF.
Sulfinyl chloride was added to a biphasic mixture of aq NH3/ethyl acetate at 0 °C.
In conclusion, we have developed a high-yielding one-pot synthesis of sulfinamides using organometallic reagents and nitrogen based-nucleophiles, exploiting sulfinyl chlorides as the reactive intermediates. The developed chemistry combines Grignard or organolithium reagents with commercially available DABSO as the source of sulfur dioxide to prepare metal sulfinates which are subsequently treated with thionyl chloride to form sulfinyl chloride intermediates. A broad range of organometallics were compatible with the process, including (hetero)aryl and alkyl systems. Secondary and primary amines, aniline, amide, carbamate, and ammonia all proved suitable N-nucleophiles, allowing access to previously difficult to synthesize sulfinamides. The developed protocol can be performed on a preparative gram scale. Several biologically relevant aryl cores and amines were employed, providing a good demonstration of the suitability of this method for the synthesis of complex sulfinamides. Given the increasing interest in sufinamides, we anticipate wide uptake of the reported method.
Experimental Section
Reactions were performed under inert nitrogen atmosphere with anhydrous solvent unless otherwise stated. Anhydrous tetrahydrofuran (99.5%, Extra dry over molecular sieves, stabilized, AcroSeal) was purchased. DABSO was prepared using a literature method and dried under reduced pressure (<1 mbar) for at least 2 h before use.16a Glassware was oven-dried and allowed to cool to room temperature under nitrogen. Cooling to 0 and −78 °C was achieved using ice–water and dry ice–acetone baths, respectively. Reactions were monitored by HPLC analysis and/or thin-layer chromatography (TLC) using precoated aluminum-backed silica plates (Merck Kieselgel 60 F254). Plates were visualized under ultraviolet light (254 nm) followed by staining with KMnO4. Flash column chromatography was carried out using Geduran Si 60, 40–63 μm silica gel; the compound to be purified was preabsorbed onto silica before loading. Petrol refers to the fraction of light petroleum ether boiling in the range 40–60 °C. 1H, 13C{1H}, and 19F NMR spectra were recorded using 400, 101, and 376 MHz spectrometers, respectively. Chemical shifts (δ) are reported in parts per million (ppm) from the residual solvent peak, and coupling constants (J) are given in hertz (Hz) and rounded to the nearest 0.5 Hz. Low-resolution electrospray ionization (ESI) mass spectra were recorded on a Waters LCT Premier spectrometer. High-resolution mass spectra were recorded on a Brüker MicroTOF spectrometer using ESI conditions by the internal service at the Chemistry Research Laboratory, University of Oxford. Infrared spectra were recorded on a Brüker Tensor 27 FT-IR spectrometer.
General Procedure for the One-Pot Synthesis of Sulfinamides
Predried DABSO (60 mg, 0.25 mmol, 0.5 equiv) was added to an oven-dried 10 mL reaction vial. The vial was then sealed with a rubber septum, evacuated, and filled with N2 (×3). Anhydrous THF (2 mL) was added. The organometallic reagent (0.50 mmol, 1.0 equiv) was added dropwise to the resulting suspension at rt, and the reaction mixture was stirred for 30 min. SOCl2 (40 μL, 0.55 mmol, 1.1 equiv) was then added dropwise, and the mixture was stirred at rt for 30 min. After this, Et3N (110 μL, 0.75 mmol, 1.5 equiv) was added followed by the corresponding amine (0.75 mmol, 1.5 equiv). The mixture was stirred at rt for 30 min, quenched with brine (10 mL), and extracted with EtOAc (3 × 10 mL). A few drops of water were added to dissolve any solid formed during the workup. The combined organic phases were dried (Na2SO4), filtered, and concentrated. Purification by flash column chromatography afforded the product.
4-((4-Fluorophenyl)sulfinyl)morpholine (2a)
Prepared according to the general procedure using 4-fluorophenylmagnesium bromide (0.55 mL, 0.91 M in THF, 0.50 mmol, 1.0 equiv) and morpholine (70 μL, 0.75 mmol, 1.5 equiv). Purification by flash column chromatography (50% EtOAc in petrol) afforded the title product as a white solid (95 mg, 83%). Gram scale synthesis of 2a: the same procedure was followed using 4-fluorophenylmagnesium bromide (6.0 mL, 1.01 M in THF, 6.0 mmol, 1.0 equiv), DABSO (721 mg, 3.0 mmol, 0.5 equiv), SOCl2 (0.48 mL, 6.6 mmol, 1.1 equiv), Et3N (1.25 mL, 9.0 mmol, 1.5 equiv), morpholine (0.79 mL, 9.0 mmol, 1.5 equiv), and THF (24 mL), affording the title product (1.08 g, 79%): Rf (50% EtOAc in petrol) = 0.32; 1H NMR (400 MHz, CDCl3) δ 7.65–7.57 (m, 2H), 7.20–7.11 (m, 2H), 3.71–3.61 (m, 4H), 3.11 (ddd, J = 12.0, 6.0, 3.5 Hz, 2H), 2.90 (ddd, J = 12.0, 6.0, 3.5 Hz, 2H); 13C{1H} NMR (101 MHz, CDCl3) δ 164.5 (d, 1JCF = 251.5 Hz), 138.0 (d, 4JCF = 3.0 Hz), 128.5 (d, 3JCF = 9.0 Hz), 116.2 (d, 2JCF = 22.5 Hz), 66.9, 45.8; 19F NMR (376 MHz, CDCl3) δ −109.0; LRMS (ESI+) m/z 481.7 ([2M + Na]+). Data is consistent with the literature.14
4-(m-Tolylsulfinyl)morpholine (2b)
Prepared according to the general procedure using m-tolylmagnesium chloride (0.65 mL, 0.77 M in THF, 0.50 mmol, 1.0 equiv) and morpholine (70 μL, 0.75 mmol, 1.5 equiv). Purification by flash column chromatography (50% EtOAc in petrol) afforded the title product as a colorless oil (92 mg, 82%): Rf (50% EtOAc in petrol) = 0.27; 1H NMR (400 MHz, CDCl3) 7.48 (s, 1H), 7.43 (d, J = 8.0 Hz, 1H), 7.38 (app t, J = 7.5 Hz, 1H), 7.28 (d, J = 7.0 Hz, 1H), 3.77–3.63 (m, 4H), 3.19–3.10 (m, 2H), 3.01–2.91 (m, 2H), 2.42 (s, 3H); 13C{1H} NMR (101 MHz, CDCl3) δ 142.1, 139.1, 131.9, 128.8, 126.5, 123.2, 66.9, 45.8, 21.4; LRMS (ESI+) m/z 226.0 ([M + H]+), 248.0 ([M + Na]+). Data is consistent with the literature.15b
4-((2-Methoxyphenyl)sulfinyl)morpholine (2c)
Prepared according to the general procedure using 2-methoxyphenylmagnesium bromide solution (0.49 mL, 1.02 M in THF, 0.50 mmol, 1.0 equiv) and morpholine (70 μL, 0.75 mmol, 1.5 equiv). Purification by flash column chromatography (60% EtOAc in petrol) afforded the title product as a pale yellow oil which solidified on standing to an off-white solid (95 mg, 79%): Rf (60% EtOAc in petrol) = 0.27; 1H NMR (400 MHz, CDCl3) δ 7.81 (d, J = 7.5 Hz, 1H), 7.45 (app t, J = 8.0 Hz, 1H), 7.12 (app t, J = 7.5 Hz, 1H), 6.94 (d, J = 8.0 Hz, 1H), 3.88 (s, 3H), 3.72–3.61 (m, 4H), 3.16–3.08 (m, 2H), 2.94–2.86 (m, 2H); 13C{1H} NMR (101 MHz, CDCl3) δ 156.9, 132.9, 129.6, 127.2, 120.9, 111.4, 67.3, 56.0, 45.4; LRMS (ESI+) m/z 264.0 ([M + Na]+). Data is consistent with the literature.14
4-(Naphthalen-2-ylsulfinyl)morpholine (2d)
Prepared according to the general procedure using 2-naphthylmagnesium bromide (0.98 mL, 0.51 M in THF, 0.50 mmol, 1.0 equiv) and morpholine (70 μL, 0.75 mmol, 1.5 equiv). Purification by flash column chromatography (50% EtOAc in petrol) afforded the title product as a pale yellow viscous oil (105 mg, 80%): Rf (50% EtOAc in petrol) = 0.23; 1H NMR (400 MHz, CDCl3) δ 8.27 (s, 1H), 7.99–7.85 (m, 3H), 7.63–7.52 (m, 3H), 3.78–3.63 (m, 4H), 3.20 (ddd, J = 12.0, 6.0, 3.5 Hz, 2H), 2.98 (ddd, J = 12.0, 6.0, 3.5 Hz, 2H); 13C{1H} NMR (101 MHz, CDCl3) δ 139.5, 134.5, 132.8, 129.1, 128.9, 128.03, 127.96, 127.3, 122.0, 67.0, 46.0, 1 × ArC not observed; LRMS (ESI+) m/z 284.0 ([M + Na]+). Data is consistent with the literature.15b
4-((4-(5-(p-Tolyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)phenyl)sulfinyl)morpholine (2e)
1-(4-Bromophenyl)-5-(p-tolyl)-3-(trifluoromethyl)-1H-pyrazole22 (191 mg, 0.50 mmol, 1.0 equiv) and anhydrous THF (5 mL) were added to an oven-dried 25 mL RBF under N2 (balloon) and cooled to −78 °C. n-Butyllithium (0.21 mL, 2.37 M in THF, 0.50 mmol, 1.0 equiv) was added dropwise, and the reaction was stirred at −78 °C for 1 h. A sonicated suspension (prepared in an oven-dried vial under N2) of predried DABSO (72 mg, 0.30 mmol, 0.6 equiv) in anhydrous THF (5 mL) was added slowly before warming to rt for 30 min. SOCl2 (40 μL, 0.55 mmol, 1.1 equiv) was then added dropwise, and the mixture was stirred at rt for 30 min. Et3N (110 μL, 0.75 mmol, 1.5 equiv) and morpholine (70 μL, 0.75 mmol, 1.5 equiv) were added. The mixture was stirred at rt for 30 min, quenched with brine (10 mL), and extracted with EtOAc (3 × 10 mL). A few drops of water were added to dissolve any solid formed during the workup. The combined organic phases were dried (MgSO4), filtered, and concentrated. Purification by flash column chromatography (50% EtOAc in petrol) afforded the title product as a pale yellow viscous oil (154 mg, 70%): Rf (50% EtOAc in petrol) = 0.31; 1H NMR (400 MHz, CDCl3) δ 7.69–7.63 (m, 2H), 7.50–7.44 (m, 2H), 7.16–7.11 (m, 2H), 7.11–7.06 (m, 2H), 6.73 (s, 1H), 3.77–3.62 (m, 4H), 3.20–3.11 (m, 2H), 2.99–2.89 (m, 2H), 2.35 (s, 3H); 13C{1H} NMR (101 MHz, CDCl3) δ 145.3, 143.9 (q, 2JCF = 38.5 Hz), 142.4, 141.6, 139.7, 129.7, 128.8, 127.3, 125.9, 125.8, 121.2 (q, 1JCF = 269.5 Hz), 106.0, 66.9, 46.0, 21.4; 19F NMR (377 MHz, CDCl3) δ −62.4; LRMS (ESI+) m/z 436.0 ([M + H]+); 458.0 ([M + Na]+); HRMS (ESI) m/z [M + H]+ calcd for C21H21O2N3F3S 436.1301, found 436.1296; IR (thin film, νmax/cm–1) 1472, 1373, 1235, 1160, 1133, 1096, 1069, 976, 916, 807, 729.
4-((2-Methylprop-1-en-1-yl)sulfinyl)morpholine (2f)
Prepared according to the general procedure using 2-methyl-1-propenylmagnesium bromide (1.01 mL, 0.49 M in THF, 0.50 mmol, 1.0 equiv), THF (5 mL), and morpholine (70 μL, 0.75 mmol, 1.5 equiv). Purification by flash column chromatography (0–5% MeOH in EtOAc) afforded the title product as a pale yellow oil (30 mg, 32%): Rf (100% EtOAc) = 0.23; 1H NMR (400 MHz, CDCl3) δ 5.93 (app. h, J = 1.5 Hz, 1H), 3.74 (app t, J = 5.0 Hz, 4H), 3.11 (app dd, J = 5.5, 4.0 Hz, 4H), 1.91 (d, J = 1.0 Hz, 3H), 1.87 (d, J = 1.0 Hz, 3H); 13C{1H} NMR (101 MHz, CDCl3) δ 147.9, 129.1, 66.9, 45.4, 25.8, 20.1; LRMS (ESI+) m/z 190.0 ([M + H]+); 212.0 ([M + Na]+); HRMS (ESI) m/z [M + H]+ calcd for C8H16O2NS 190.0896, found 190.0896; IR (thin film, νmax/cm–1) 2855, 1635, 1442, 1258, 1110, 1064, 1037, 914, 795, 695.
4-(Butylsulfinyl)morpholine (2g)
Prepared according to the general procedure using butylmagnesium chloride (0.23 mL, 2.14 M in THF, 0.50 mmol, 1.0 equiv) and morpholine (70 μL, 0.75 mmol, 1.5 equiv). The step 2 was left for 5 min instead. Purification by flash column chromatography (100% EtOAc) afforded the title product as a pale yellow oil (68 mg, 71%): Rf (100% EtOAc) = 0.24; 1H NMR (400 MHz, CDCl3) δ 3.82–3.69 (m, 4H), 3.21–3.06 (m, 4H), 2.83–2.67 (m, 2H), 1.58 (app dq, J = 15.0, 8.0 Hz, 2H), 1.44 (p, J = 7.5 Hz, 2H), 0.93 (t, J = 7.5 Hz, 3H); 13C{1H} NMR (101 MHz, CDCl3) δ 67.0, 51.9, 45.9, 25.6, 22.1, 13.8; LRMS (ESI+) m/z 192.0 ([M + H]+), 214.0 ([M + Na]+); HRMS (ESI) m/z [M + H]+ calcd for C8H18O2NS 192.1053, found 192.1054; IR (thin film, νmax/cm–1) 2980, 1653, 1455, 1382, 1259, 1111, 1068, 1038, 920.
4-(Cyclohexylsulfinyl)morpholine (2h)
Prepared according to the general procedure using cyclohexylmagnesium chloride (0.26 mL, 1.90 M in Et2O, 0.50 mmol, 1.0 equiv) and morpholine (70 μL, 0.75 mmol, 1.5 equiv). Purification by flash column chromatography (0–5% MeOH in EtOAc) afforded the title product as a colorless oil (87 mg, 80%): Rf (5% MeOH in EtOAc) = 0.50; 1H NMR (400 MHz, CDCl3) δ 3.82–3.68 (m, 4H), 3.21–3.04 (m, 4H), 2.75–2.63 (m, 1H), 2.15–2.05 (m, 1H), 1.93–1.60 (m, 4H), 1.46–1.14 (m, 5H); 13C{1H} NMR (101 MHz, CDCl3) δ 67.0, 58.8, 46.3, 27.2, 26.8, 25.6, 25.3; LRMS (ESI+) m/z 218.2 ([M + H]+), 240.0 ([M + Na]+); HRMS (ESI) m/z [M + H]+ calcd for C10H20O2NS 218.1209, found 218.1211; IR (thin film, νmax/cm–1) 2925, 2853, 1450, 1257, 1111, 1066, 917.
4-(tert-Butylsulfinyl)morpholine (2i)
Prepared according to the general procedure using tert-butylmagnesium chloride solution (0.53 mL, 0.94 M in THF, 0.50 mmol, 1.0 equiv) and morpholine (70 μL, 0.75 mmol, 1.5 equiv). Purification by flash column chromatography (100% EtOAc) afforded the title product as a white solid (79 mg, 82%): Rf (100% EtOAc) = 0.35; 1H NMR (400 MHz, CDCl3) δ 3.77–3.69 (m, 4H), 3.21–3.13 (m, 2H), 3.13–3.05 (m, 2H), 1.19 (s, 9H); 13C{1H} NMR (101 MHz, CDCl3) δ 67.3, 58.8, 47.5, 23.2; LRMS (ESI+) m/z 192.3 ([M + H]+), 214.3 ([M + Na]+); HRMS (ESI) m/z [M + H]+ calcd for C8H18O2NS 192.1053, found 192.1055. Data is consistent with enantiomerically pure compound.23
4-((6-Methoxypyridin-3-yl)sulfinyl)morpholine (2j)
5-Bromo-2-methoxypyridine (65 μL, 0.50 mmol, 1.0 equiv) and anhydrous THF (5 mL) were added to an oven-dried 25 mL RBF under N2 (balloon) and cooled to −78 °C. n-Butyllithium (0.21 mL, 2.37 M in THF, 0.50 mmol, 1.0 equiv) was added dropwise and the reaction was stirred at −78 °C for 40 min. A sonicated suspension (prepared in an oven-dried vial under N2) of predried DABSO (72 mg, 0.30 mmol, 0.6 equiv) in anhydrous THF (5 mL) was added slowly before warming to rt for 30 min. SOCl2 (40 μL, 0.55 mmol, 1.1 equiv) was then added dropwise, and the mixture was stirred at rt for 30 min. Et3N (110 μL, 0.75 mmol, 1.5 equiv) and morpholine (70 μL, 0.75 mmol, 1.5 equiv) were added. The mixture was stirred at rt for 30 min, quenched with brine (10 mL), and extracted with EtOAc (3 × 10 mL). A few drops of water were added to dissolve any solid formed during the workup. The combined organic phases were dried (MgSO4), filtered and concentrated. Purification by flash column chromatography (70% EtOAc in petrol) afforded the title product as a pale yellow viscous oil (92 mg, 76%): Rf (70% EtOAc in petrol) = 0.26; 1H NMR (400 MHz, CDCl3) δ 8.35 (dd, J = 2.5, 0.5 Hz, 1H), 7.76 (dd, J = 8.5, 2.5 Hz, 1H), 6.81 (dd, J = 8.5, 0.5 Hz, 1H), 3.95 (s, 3H), 3.75–3.64 (m, 4H), 3.18–3.10 (m, 2H), 3.02–2.93 (m, 2H); 13C{1H} NMR (101 MHz, CDCl3) δ 166.1, 146.4, 136.6, 130.9, 111.5, 66.9, 54.1, 45.8; LRMS (ESI+) m/z 243.0 ([M + H]+); 265.0 ([M + Na]+); HRMS (ESI) m/z [M + H]+ calcd for C10H15O3N2S 243.0798, found 243.0798; IR (thin film, νmax/cm–1) 2854, 1587, 1477, 1366, 1281, 1257, 1095, 1069, 1015, 916, 834, 694.
4-(Benzofuran-2-ylsulfinyl)morpholine (2k)
Benzofuran (55 μL, 0.50 mmol, 1.0 equiv) and anhydrous THF (1.5 mL) were added to an oven-dried 10 mL reaction vial under N2 (balloon) and cooled to 0 °C. n-Butyllithium (0.21 mL, 2.37 M in THF, 0.50 mmol, 1.0 equiv) was added dropwise, and the reaction was stirred at 0 °C for 5 min before reaching to rt for 1 h. The mixture was transferred via a syringe and added dropwise to a stirred suspension of predried DABSO (60 mg, 0.25 mmol, 0.5 equiv) in THF (2.0 mL) in an oven-dried 10 mL reaction vial at rt. The mixture was then stirred at rt for 30 min. SOCl2 (40 μL, 0.55 mmol, 1.1 equiv) was added dropwise, and the mixture was stirred at rt for 30 min. Et3N (110 μL, 0.75 mmol, 1.5 equiv) and morpholine (70 μL, 0.75 mmol, 1.5 equiv) were added. The mixture was stirred at rt for 30 min, quenched with brine (10 mL) and extracted with EtOAc (3 × 10 mL). A few drops of water were added to dissolve any solid formed during the workup. The combined organic phases were dried (MgSO4), filtered, and concentrated. Purification by flash column chromatography (40% EtOAc in petrol) afforded the title product as a yellow oil (83 mg, 66%): Rf (40% EtOAc in petrol) = 0.25; 1H NMR (400 MHz, CDCl3) δ 7.65 (app dt, J = 7.5, 1.0 Hz, 1H), 7.53 (app dq, J = 8.5, 1.0 Hz, 1H), 7.38 (ddd, J = 8.5, 7.5, 1.5 Hz, 1H), 7.34 (d, J = 1.0 Hz, 1H), 7.30 (app td, J = 7.5, 1.0 Hz, 1H), 3.83–3.65 (m, 4H), 3.31 (ddd, J = 12.5, 6.0, 4.0 Hz, 2H), 3.14 (ddd, J = 12.5, 5.5, 3.5 Hz, 2H); 13C{1H} NMR (101 MHz, CDCl3) δ 156.9, 154.4, 126.9, 126.5, 123.9, 122.3, 112.3, 112.1, 67.0, 46.1; LRMS (ESI+) m/z 274.0 ([M + Na]+). Data is consistent with the literature.14
4-(Thiophene-2-ylsulfinyl)morpholine (2l)
Prepared according to the general procedure using 2-thienylmagnesium bromide solution (0.59 mL, 0.85 M in THF, 0.50 mmol, 1.0 equiv) and morpholine (70 μL, 0.75 mmol, 1.5 equiv). Purification by flash column chromatography (50% EtOAc in petrol) afforded the title product as an orange oil (68 mg, 63%): Rf (50% EtOAc in petrol) = 0.31; 1H NMR (400 MHz, CDCl3) δ 7.61 (dd, J = 5.0, 1.5 Hz, 1H), 7.41 (dd, J = 3.5, 1.5 Hz, 1H), 7.14 (dd, J = 5.0, 3.5 Hz, 1H), 3.81–3.70 (m, 4H), 3.25–3.17 (m, 2H), 3.16–3.08 (m, 2H); 13C{1H} NMR (101 MHz, CDCl3) δ 145.3, 131.8, 130.6, 128.1, 67.1, 45.9; LRMS (ESI+) m/z 240.0 ([M + Na]+). Data is consistent with the literature.15c
1-((4-Fluorophenyl)sulfinyl)pyrrolidine (3a)
Prepared according to the general procedure using 4-fluorophenylmagnesium bromide (0.51 mL, 0.98 M in THF, 0.50 mmol, 1.0 equiv) and pyrrolidine (63 μL, 0.75 mmol, 1.5 equiv). Purification by flash column chromatography (30% EtOAc in petrol) afforded the title product as a pale yellow oil (80 mg, 75%): Rf (30% EtOAc in petrol) = 0.24; 1H NMR (400 MHz, CDCl3) δ 7.69–7.62 (m, 2H), 7.20–7.12 (m, 2H), 3.37–3.28 (m, 2H), 3.03–2.92 (m, 2H), 1.90–1.78 (m, 4H); 13C{1H} NMR (101 MHz, CDCl3) δ 164.2 (d, 1JCF = 250.5 Hz), 140.4 (d, 4JCF = 3.0 Hz), 128.2 (d, 3JCF = 9.0 Hz), 116.1 (d, 2JCF = 22.5 Hz), 46.1, 26.1; 19F NMR (377 MHz, CDCl3) δ −110.2; LRMS (ESI+) m/z 236.3 ([M + Na]+); HRMS (ESI) m/z [M + H]+ calcd for C10H13ONFS 214.0696, found 214.0699; IR (thin film, νmax/cm–1) 2967, 2876, 1587, 1488, 1222, 1084, 1062, 969, 836, 814.
5-((4-Fluorophenyl)sulfinyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine (3b)
Prepared according to the general procedure using 4-fluorophenylmagnesium bromide (0.51 mL, 0.98 M in THF, 0.50 mmol, 1.0 equiv) and 4,5,6,7-tetrahydrothieno[3,2-c]pyridine (90 μL, 0.75 mmol, 1.5 equiv). Purification by flash column chromatography (20% EtOAc in petrol) afforded the title product as a pale yellow viscous oil (116 mg, 83%): Rf (20% EtOAc in petrol) = 0.22; 1H NMR (400 MHz, CDCl3) δ 7.72–7.65 (m, 2H), 7.23–7.16 (m, 2H), 7.10 (d, J = 5.0 Hz, 1H), 6.68 (d, J = 5.0 Hz, 1H), 4.26 (d, J = 15.0 Hz, 1H), 3.90 (d, J = 15.0 Hz, 1H), 3.61–3.52 (m, 1H), 3.48–3.39 (m, 1H), 3.04–2.93 (m, 1H), 2.92–2.83 (m, 1H); 13C{1H} NMR (101 MHz, CDCl3) δ 164.5 (d, 1JCF = 252.0 Hz), 138.7 (d, 4JCF = 2.9 Hz), 133.0, 131.8, 128.7 (d, 3JCF = 9.5 Hz), 125.1, 123.5, 116.3 (d, 2JCF = 22.5 Hz), 45.7, 43.9, 26.2; 19F NMR (377 MHz, CDCl3) δ −109.2; LRMS (ESI+) m/z 282.4 ([M + H]+), 304.4 ([M + Na]+); HRMS (ESI) m/z [M + Na]+ calcd for C13H12ONFNaS2 304.0237, found 304.0237; IR (thin film, νmax/cm–1) 2848, 1587, 1488, 1223, 1086, 1064, 906, 836, 704.
Benzyl 4-((4-Fluorophenyl)sulfinyl)piperazine-1-carboxylate (3c)
Prepared according to the general procedure using 4-fluorophenylmagnesium bromide (0.51 mL, 0.98 M in THF, 0.50 mmol, 1.0 equiv) and benzyl piperazine-1-carboxylate (0.15 mL, 0.75 mmol, 1.5 equiv). Purification by flash column chromatography (40% EtOAc in petrol) afforded the title product as a pale yellow viscous oil (145 mg, 80%): Rf (40% EtOAc in petrol) = 0.23; 1H NMR (400 MHz, CDCl3) δ 7.68–7.61 (m, 2H), 7.37–7.27 (m, 5H), 7.23–7.16 (m, 2H), 5.12 (s, 2H), 3.65–3.45 (m, 4H), 3.18–3.08 (m, 2H), 3.02–2.90 (m, 2H); 13C{1H} NMR (101 MHz, CDCl3) δ 164.6 (d, 1JCF = 252.0 Hz), 155.1, 138.2 (d, 4JCF = 3.0 Hz), 136.5, 128.6, 128.5 (d, 3JCF = 9.0 Hz), 128.3, 128.0, 116.3 (d, 2JCF = 22.5 Hz), 67.5, 45.8, 44.2; 19F NMR (377 MHz, CDCl3) δ −108.7; LRMS (ESI+) m/z 385.0 ([M + Na]+); HRMS (ESI) m/z [M + Na]+ calcd for C18H19O3N2FNaS 385.0993, found 385.1000; IR (thin film, νmax/cm–1) 1698, 1587, 1489, 1427, 1240, 1124, 1086, 1067, 914, 837, 697.
2-Chloro-11-(4-((4-fluorophenyl)sulfinyl)piperazin-1-yl)dibenzo[b,f][1,4]oxazepine (3d)
Prepared according to the general procedure using 4-fluorophenylmagnesium bromide (0.51 mL, 0.98 M in THF, 0.50 mmol, 1.0 equiv) and amoxapine (235 mg, 0.75 mmol, 1.5 equiv). Purification by flash column chromatography (20% EtOAc in petrol) afforded the title product as a pale yellow foam (158 mg, 69%): Rf (20% EtOAc in petrol) = 0.23; 1H NMR (400 MHz, CDCl3) δ 7.70–7.63 (m, 2H), 7.34 (dd, J = 8.5, 2.5 Hz, 1H), 7.25 (d, J = 2.5 Hz, 1H), 7.22–7.15 (m, 2H), 7.15–7.09 (m, 2H), 7.09–7.03 (m, 2H), 7.01–6.95 (m, 1H), 3.53 (s, 4H), 3.37–3.20 (m, 2H), 3.20–2.99 (m, 2H); 13C{1H} NMR (101 MHz, CDCl3) δ 164.4 (d, 1JCF = 251.5 Hz), 159.3, 158.6, 151.7, 139.8, 138.2 (d, 4JCF = 3.0 Hz), 132.7, 130.4, 128.8, 128.5 (d, 3JCF = 9.0 Hz), 127.1, 125.8, 124.9, 124.7, 122.7, 120.1, 116.2 (d, 2JCF = 22.5 Hz), 47.7, 45.5; 19F NMR (377 MHz, CDCl3) δ −108.8; LRMS (ESI+) m/z 478.1 ([M + Na]+); HRMS (ESI) m/z [M + H]+ calcd for C23H20O2N335ClFS 456.0943, found 456.0939; IR (thin film, νmax/cm–1) 1561, 1471, 1399, 1238, 1086, 905, 836, 725; mp (CH2Cl2) 108–109 °C.
1-((2-Methoxyphenyl)sulfinyl)indoline (3e)
Prepared according to the general procedure using 2-methoxyphenylmagnesium bromide solution (0.49 mL, 1.02 M in THF, 0.50 mmol, 1.0 equiv) and indoline (85 μL, 0.75 mmol, 1.5 equiv). Purification by flash column chromatography (15–25% EtOAc in petrol) afforded the title product as a brown solid (113 mg, 82%): Rf (15% EtOAc in petrol) = 0.21; 1H NMR (400 MHz, CDCl3) δ 7.94 (dd, J = 7.5, 1.5 Hz, 1H), 7.47 (ddd, J = 8.0, 7.5, 1.5 Hz, 1H), 7.23–7.13 (m, 3H), 7.11 (d, J = 7.5 Hz, 1H), 6.95–6.85 (m, 2H), 4.06–3.93 (m, 1H), 3.76 (s, 3H), 3.13–2.88 (m, 3H); 13C{1H} NMR (101 MHz, CDCl3) δ 156.6, 146.6, 132.8, 130.7, 130.2, 127.5, 126.5, 125.1, 121.9, 120.8, 111.5, 111.2, 56.0, 42.1, 28.4; LRMS (ESI+) m/z 296.0 ([M + Na]+), 569.2 ([2M + Na]+); HRMS (ESI) m/z [M + H]+ calcd for C15H16O2NS 274.0896, found 274.0895; IR (thin film, νmax/cm–1) 1590, 1477, 1274, 1240, 1088, 1050, 1016, 931, 749; mp (CH2Cl2) 116–118 °C.
4-Fluoro-N-phenylbenzenesulfinamide13 (3f)
Predried DABSO (60 mg, 0.25 mmol, 0.5 equiv) was added to an oven-dried 10 mL reaction vial. The vial was then sealed with a rubber septum, evacuated, and filled with N2 (×3). Anhydrous THF (2 mL) was added. 4-Fluorophenylmagnesium bromide (0.50 mL, 1.01 M in THF, 0.50 mmol, 1.0 equiv) was added dropwise to the resulting suspension at rt, and the reaction mixture was stirred for 30 min. SOCl2 (40 μL, 0.55 mmol, 1.1 equiv) was then added dropwise, and the mixture was stirred at rt for 30 min. The reaction mixture was transferred via a syringe and added dropwise to a solution of Et3N (110 μL, 0.75 mmol, 1.5 equiv) and aniline (140 μL, 1.5 mmol, 3 equiv) in THF (2 mL) in a 25 mL oven-dried RBF under N2 at rt. Anhydrous THF (2 mL × 2) was then added to the reaction vial and transferred to the 25 mL RBF to ensure a quantitative transfer of the sulfinyl chloride. After addition, the mixture was stirred at rt for 30 min, quenched with brine (10 mL), and extracted with EtOAc (3 × 10 mL). A few drops of water were added to dissolve any solid formed during the workup. The combined organic phases were dried (MgSO4), filtered, and concentrated. Purification by flash column chromatography (20–25% EtOAc in petrol) afforded the title product as a pale yellow solid (97 mg, 82%): Rf (20% EtOAc in petrol) = 0.21; 1H NMR (400 MHz, CDCl3) δ 7.81–7.71 (m, 2H), 7.31–7.24 (m, 2H), 7.23–7.17 (m, 2H), 7.11–7.04 (m, 3H), 6.30 (s, 1H); 13C{1H} NMR (101 MHz, CDCl3) δ 164.7 (d, 1JCF = 252.0 Hz), 140.4, 140.2 (d, 4JCF = 3.0 Hz), 129.6, 128.1 (d, 3JCF = 9.0 Hz), 124.0, 119.4, 116.4 (d, 2JCF = 23.0 Hz); 19F NMR (377 MHz, CDCl3) δ −108.4; LRMS (ESI+) m/z 258.0 ([M + Na]+); HRMS (ESI) m/z [M + Na]+ calcd for C12H10ONFNaS 258.0359, found 258.0361; IR (thin film, νmax/cm–1) 3174, 1586, 1487, 1224, 1154, 1085, 1055, 882, 816, 745, 688; mp (CH2Cl2): 115–117 °C.
N-Butyl-4-fluorobenzenesulfinamide (3g)
Prepared according to the general procedure using 4-fluorophenylmagnesium bromide (0.51 mL, 0.98 M in THF, 0.50 mmol, 1.0 equiv) and n-butylamine (74 μL, 0.75 mmol, 1.5 equiv). Purification by flash column chromatography (30% EtOAc in petrol) afforded the title product as a pale yellow oil (77 mg, 71%): Rf (30% EtOAc in petrol) = 0.26; 1H NMR (400 MHz, CDCl3) δ 7.68–7.62 (m, 2H), 7.17–7.10 (m, 2H), 4.28 (t, J = 6.0 Hz, 1H), 3.11–3.01 (m, 1H), 2.80–2.69 (m, 1H), 1.49–1.40 (m, 2H), 1.33–1.23 (m, 2H), 0.83 (t, J = 7.3 Hz, 3H); 13C{1H} NMR (101 MHz, CDCl3) δ 164.4 (d, 1JCF = 250.5 Hz), 140.1 (d, 4JCF = 3.0 Hz), 128.4 (d, 3JCF = 9.0 Hz), 116.0 (d, 2JCF = 22.5 Hz), 40.8, 32.6, 20.0, 13.7; 19F NMR (377 MHz, CDCl3) δ −109.8; LRMS (ESI+) m/z 216.0 ([M + H]+), 238.1 ([M + Na]+). Data is consistent with the literature.24
3N-((3s,5s,7s)-Adamantan-1-yl)-4-fluorobenzenesulfinamide (3h)
Prepared according to the general procedure using 4-fluorophenylmagnesium bromide (0.50 mL, 1.01 M in THF, 0.50 mmol, 1.0 equiv) and an emulsion of 1-adamantylamine (114 mg, 0.75 mmol, 1.5 equiv) in THF (3 mL). During aqueous workup, the combined organic phases were dried over MgSO4 instead. Purification by flash column chromatography (25% EtOAc in petrol) afforded the title product as a white solid (91 mg, 62%): Rf (25% EtOAc in petrol) = 0.29; 1H NMR (400 MHz, CDCl3) δ 7.73–7.63 (m, 2H), 7.20–7.11 (m, 2H), 3.83 (s, 1H), 2.19–2.10 (m, 3H), 2.04–1.94 (m, 3H), 1.94–1.86 (m, 3H), 1.75–1.63 (m, 6H); 13C{1H} NMR (101 MHz, CDCl3) δ 164.3 (d, 1JCF = 251.0 Hz), 142.4 (d, 4JCF = 3.0 Hz), 128.2 (d, 3JCF = 9.0 Hz), 115.9 (d, 2JCF = 22.0 Hz), 54.7, 44.8, 36.1, 29.8; 19F NMR (376 MHz, CDCl3) δ −110.2; LRMS (ESI+) m/z 294.1 ([M + H]+); HRMS (ESI) m/z [M + H]+ calcd for C16H21ONFS 294.1322, found 294.1326; IR (thin film, νmax/cm–1) 3090, 2981, 2903, 2849, 1588, 1490, 1452, 1396, 1224, 1159, 1086, 1040, 948, 835, 744; mp (CH2Cl2) 264–266 °C.
N-((4-Fluorophenyl)sulfinyl)-4-methylbenzamide (3i)
Prepared according to the general procedure using 4-fluorophenylmagnesium bromide (0.50 mL, 1.01 M in THF, 0.50 mmol, 1.0 equiv) and a solution of p-toluamide (101 mg, 0.75 mmol, 1.5 equiv) in THF (4.0 mL). During aqueous workup, the combined organic phases were dried over MgSO4 instead. Purification by flash column chromatography (40% EtOAc in petrol) afforded the title product as a white solid (100 mg, 72%): Rf (40% EtOAc in petrol) = 0.38; 1H NMR (400 MHz, CDCl3) δ 8.95 (s, 1H), 7.75–7.66 (m, 4H), 7.21 (d, J = 8.0 Hz, 2H), 7.19–7.13 (m, 2H), 2.38 (s, 3H); 13C{1H} NMR (101 MHz, CDCl3) δ 167.5, 164.9 (d, J = 252.5 Hz), 144.3, 139.5 (d, J = 3.0 Hz), 129.6, 128.7, 128.3, 127.5 (d, J = 9.0 Hz), 116.8 (d, J = 23.0 Hz), 21.7; 19F NMR (377 MHz, CDCl3) δ −107.1; LRMS (ESI+) m/z 300.0 ([M + Na]+); HRMS (ESI) m/z [M + H]+ calcd for C14H13O2NFS 278.0646, found 278.0646; IR (thin film, νmax/cm–1) 3181, 1670, 1587, 1491, 1420, 1395, 1231, 1090, 1060, 888, 833, 750; mp (CH2Cl2) 135–136 °C.
tert-Butyl ((2-Methoxyphenyl)sulfinyl)carbamate (3j)
Prepared according to the general procedure using 2-methoxyphenylmagnesium bromide solution (0.49 mL, 1.02 M in THF, 0.50 mmol, 1.0 equiv) and a solution of tert-butyl carbamate (88 mg, 0.75 mmol, 1.5 equiv) in THF (2.0 mL). Purification by flash column chromatography (30–40% EtOAc in petrol) afforded the title product as a colorless viscous oil (113 mg, 83%): Rf (30% EtOAc in petrol) = 0.15; 1H NMR (400 MHz, CDCl3) δ 7.84 (dd, J = 7.5, 1.5 Hz, 1H), 7.48 (ddd, J = 8.0, 7.5, 1.5 Hz, 1H), 7.11 (app td, J = 7.5, 1.0 Hz, 1H), 6.99 (s, 1H), 6.95 (dd, J = 8.5, 1.0 Hz, 1H), 3.89 (s, 3H), 1.48 (s, 9H); 13C{1H} NMR (101 MHz, CDCl3) δ 156.3, 152.7, 133.6, 131.3, 126.0, 121.2, 111.5, 83.1, 56.1, 28.2.; LRMS (ESI+) m/z 294.0 ([M + Na]+); HRMS (ESI) m/z [M + H]+ calcd for C12H17O4NNaS 294.0771, found 294.0770; IR (thin film, νmax/cm–1) 3156, 2979, 1707, 1590, 1478, 1369, 1276, 1241, 1154, 1087, 1051, 1019, 904, 820, 755, 727.
4-Fluorobenzenesulfinamide (3k)
Predried DABSO (60 mg, 0.25 mmol, 0.5 equiv) was added to an oven-dried 10 mL reaction vial. The vial was then sealed with a rubber septum, evacuated and filled with N2 (×3). Anhydrous THF (2.0 mL) was added. 4-Fluorophenylmagnesium bromide (0.50 mL, 1.01 M in THF, 0.50 mmol, 1.0 equiv) was added dropwise to the resulting suspension at rt, and the reaction mixture was stirred for 30 min. SOCl2 (40 μL, 0.55 mmol, 1.1 equiv) was then added dropwise, and the mixture was stirred at rt for 30 min. The reaction mixture was transferred via a syringe and added dropwise to a stirred solution of 35% aq NH3 (0.5 mL) and EtOAc (1.0 mL) in a 25 mL RBF at 0 °C. Anhydrous THF (2.0 mL × 2) was added to the reaction vial and transferred to the 25 mL RBF to ensure a quantitative transfer of the sulfinyl chloride. After addition, the mixture was stirred at rt for 30 min, quenched with brine (10 mL), and extracted with EtOAc (3 × 10 mL). A few drops of water were added to dissolve any solid formed during the workup. The combined organic phases were dried (MgSO4), filtered, and concentrated. Purification by flash column chromatography (100% EtOAc) afforded the title product as a white solid (53 mg, 67%): Rf (100% EtOAc) = 0.50; 1H NMR (400 MHz, DMSO-d6) δ 7.75–7.64 (m, 2H), 7.41–7.30 (m, 2H), 6.29 (s, 2H); 13C{1H} NMR (101 MHz, DMSO-d6) δ 163.2 (d, 1JCF = 247.0 Hz), 144.2 (d, 4JCF = 2.5 Hz), 127.9 (d, 3JCF = 9.0 Hz), 115.6 (d, 2JCF = 22.0 Hz); 19F NMR (377 MHz, DMSO) δ −111.4; LRMS (ESI+) m/z 341.4 ([2M + Na]+). Data is consistent with the literature.19b
2-Methylpropane-2-sulfinamide (3l)
Predried DABSO (60 mg, 0.25 mmol, 0.5 equiv) was added to an oven-dried 10 mL reaction vial. The vial was then sealed with a rubber septum, evacuated, and filled with N2 (× 3). Anhydrous THF (2.0 mL) was added. tert-Butylmagnesium chloride (0.53 mL, 0.94 M in THF, 0.50 mmol, 1.0 equiv) was added dropwise to the resulting suspension at rt, and the reaction mixture was stirred for 30 min. SOCl2 (40 μL, 0.55 mmol, 1.1 equiv) was then added dropwise, and the mixture was stirred at rt for 30 min. The reaction mixture was transferred via a syringe and added dropwise to a stirred solution of 35% aq NH3 (0.5 mL) and EtOAc (0.5 mL) in a 25 mL RBF at 0 °C. Anhydrous THF (2.0 mL × 2) was added to the reaction vial and transferred to the 25 mL RBF to ensure a quantitative transfer of the sulfinyl chloride. After addition, the mixture was stirred at rt for 30 min, quenched with brine (10 mL), and extracted with EtOAc (3 × 10 mL). A few drops of water were added to dissolve any solid formed during the workup. The combined organic phases were dried (MgSO4), filtered, and concentrated. Purification by flash column chromatography (0–5% MeOH in EtOAc) afforded the title product as a pale yellow viscous oil (43 mg, 71%): Rf (100% EtOAc) = 0.17; 1H NMR (400 MHz, CDCl3) δ 3.72 (s, 2H), 1.22 (s, 9H); 13C{1H} NMR (101 MHz, CDCl3) δ 55.5, 22.3; LRMS (ESI+) m/z 122.0 ([M + H]+); 144.0 ([M + Na]+). Data is consistent with the literature.25
Acknowledgments
We thank the EPSRC (EP/K024205/1) for support of this study. We thank Tony Lo for the cover design.
Supporting Information Available
hThe Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.0c00334.
1H, 13C, and 19F NMR spectra of synthesized compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Ellman J. A.; Owens T. D.; Tang T. P. N-tert-Butanesulfinyl Imines: Versatile Intermediates for the Asymmetric Synthesis of Amines. Acc. Chem. Res. 2002, 35, 984–995. 10.1021/ar020066u. [DOI] [PubMed] [Google Scholar]; b Robak M. T.; Herbage M. A.; Ellman J. A. Synthesis and Applications of tert-Butanesulfinamide. Chem. Rev. 2010, 110, 3600–3740. 10.1021/cr900382t. [DOI] [PubMed] [Google Scholar]
- Otocka S.; Kwiatkowska M.; Madalińska L.; Kiełbasiński P. Chiral Organosulfur Ligands/Catalysts with a Stereogenic Sulfur Atom: Applications in Asymmetric Synthesis. Chem. Rev. 2017, 117, 4147–4181. 10.1021/acs.chemrev.6b00517. [DOI] [PubMed] [Google Scholar]
- Dinér P.; Sadhukhan A.; Blomkvist B. Chiral Sulfinamides as Highly Enantioselective Organocatalysts. ChemCatChem 2014, 6, 3063–3066. 10.1002/cctc.201402558. [DOI] [Google Scholar]
- a Tang T. P.; Volkman S. K.; Ellman J. A. Asymmetric Synthesis of Protected 1,2-Amino Alcohols Using tert-Butanesulfinyl Aldimines and Ketimines. J. Org. Chem. 2001, 66, 8772–8778. 10.1021/jo0156868. [DOI] [PubMed] [Google Scholar]; b Fritz S. P.; Mumtaz A.; Yar M.; McGarrigle E. M.; Aggarwal V. K. Sulfinamides as Highly Effective Amine Protecting Groups and Their Use in the Conversion of Amino Alcohols into Morpholines. Eur. J. Org. Chem. 2011, 2011, 3156–3164. 10.1002/ejoc.201100337. [DOI] [Google Scholar]
- a Nandi G. C.; Arvidsson P. I. Sulfonimidamides: Synthesis and Applications in Preparative Organic Chemistry. Adv. Synth. Catal. 2018, 360, 2976–3001. 10.1002/adsc.201800273. [DOI] [Google Scholar]; b Lucking U. Sulfoximines: A Neglected Opportunity in Medicinal Chemistry. Angew. Chem., Int. Ed. 2013, 52, 9399–9408. 10.1002/anie.201302209. [DOI] [PubMed] [Google Scholar]; c Mendonca Matos P.; Lewis W.; Moore J. C.; Stockman R. A. Sulfonimidates: Useful Synthetic Intermediates for Sulfoximine Synthesis via C-S Bond Formation. Org. Lett. 2018, 20, 3674–3677. 10.1021/acs.orglett.8b01473. [DOI] [PubMed] [Google Scholar]
- a Backes B. J.; Dragoli D. R.; Ellman J. A. Chiral N-Acyl-tert-butanesulfinamides: The “Safety-Catch” Principle Applied to Diastereoselective Enolate Alkylations. J. Org. Chem. 1999, 64, 5472–5478. 10.1021/jo990271w. [DOI] [PubMed] [Google Scholar]; b Coulomb J.; Certal V.; Fensterbank L.; Lacôte E.; Malacria M. Formation of Cyclic Sulfinates and Sulfinamides through Homolytic Substitution at the Sulfur Atom. Angew. Chem., Int. Ed. 2006, 45, 633–637. 10.1002/anie.200503369. [DOI] [PubMed] [Google Scholar]; c Savile C. K.; Kazlauskas R. J. The 3-(3-Pyridine)propionyl Anchor Group for Protease-Catalyzed Resolutions: p-Toluenesulfinamide and Sterically Hindered Secondary Alcohols. Adv. Synth. Catal. 2006, 348, 1183–1192. 10.1002/adsc.200606040. [DOI] [Google Scholar]; d Zhu R.-H.; Shi X.-X. Practical and Highly Stereoselective Method for the Preparation of Several Chiral Arylsulfinamides and Arylsulfinates Based on the Spontaneous Crystallization of Diastereomerically Pure N-Benzyl-N-(1-Phenylethyl)-Arylsulfinamides. Tetrahedron: Asymmetry 2011, 22, 387–393. 10.1016/j.tetasy.2011.01.028. [DOI] [Google Scholar]; e Miller D.; Thom S.; St-Gallay S.; Shannon J.; Leeson P.. Novel Compounds. WO2019068772 (A1), 2019.
- a Kawęcki R. Facile Synthesis of Homochiral Derivatives of 10-Bornane Sulfinates, Sulfinamides and Sulfinimines. Tetrahedron: Asymmetry 1999, 10, 4183–4190. 10.1016/S0957-4166(99)00433-4. [DOI] [Google Scholar]; b Li X.-B.; Xu Z.-F.; Liu L.-J.; Liu J.-T. Synthesis and Identification of Solution-Stable Sulfenic Acids: Perfluoroalkanesulfenic Acids. Eur. J. Org. Chem. 2014, 2014, 1182–1188. 10.1002/ejoc.201301563. [DOI] [Google Scholar]
- Furukawa M.; Okawara T. Convenient Syntheses of Sulfinamide Derivatives. Synthesis 1976, 1976, 339–340. 10.1055/s-1976-24045. [DOI] [Google Scholar]
- Gafur S. H.; Waggoner S. L.; Jacobsen E.; Hamaker C. G.; Hitchcock S. R. Efficient Synthesis of Sulfinate Esters and Sulfinamides via Activated Esters of p-Toluenesulfinic Acid. Synthesis 2018, 50, 4855–4866. 10.1055/s-0037-1610254. [DOI] [Google Scholar]
- a Brownbridge P.; Jowett I. C. ’One-Pot’ Synthesis of Sulphinic Esters from Disulphides. Synthesis 1988, 1988, 252–254. 10.1055/s-1988-27535. [DOI] [Google Scholar]; b García Ruano J. L.; Alemán J.; Belén Cid M.; Parra A. A General Method for the Preparation of N-Sulfonyl Aldimines and Ketimines. Org. Lett. 2005, 7, 179–182. 10.1021/ol048005e. [DOI] [PubMed] [Google Scholar]
- a García Ruano J. L.; Parra A.; Yuste F.; Mastranzo V. M. Mild and General Method for the Synthesis of Sulfonamides. Synthesis 2008, 2008, 311–319. 10.1055/s-2007-1000850. [DOI] [Google Scholar]; b García Ruano J. L.; Parra A.; Marzo L.; Yuste F.; Mastranzo V. M. One-Pot Synthesis of Sulfonamides from Methyl Sulfinates using Ultrasound. Tetrahedron 2011, 67, 2905–2910. 10.1016/j.tet.2011.02.060. [DOI] [Google Scholar]
- Harmata M.; Zheng P.; Huang C.; Gomes M. G.; Ying W.; Ranyanil K.-O.; Balan G.; Calkins N. L. Expedient Synthesis of Sulfinamides from Sulfonyl Chlorides. J. Org. Chem. 2007, 72, 683–685. 10.1021/jo062296i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J.; Sun Z. tert-Butyl Sulfoxide as a Starting Point for the Synthesis of Sulfinyl Containing Compounds. Org. Lett. 2015, 17, 5396–5399. 10.1021/acs.orglett.5b02743. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Tang X.-Y.; Shi M. Metal-Free Cross-Coupling of Arylboronic Acids and Derivatives with DAST-Type Reagents for Direct Access to Diverse Aromatic Sulfinamides and Sulfonamides. Angew. Chem., Int. Ed. 2016, 55, 10811–10815. 10.1002/anie.201605066. [DOI] [PubMed] [Google Scholar]
- a Taniguchi N. Copper-Catalyzed Oxidative Synthesis of Sulfinamides Using Thiols or Disulfides with Amines. Eur. J. Org. Chem. 2016, 2016, 2157–2162. 10.1002/ejoc.201600091. [DOI] [Google Scholar]; b Dai Q.; Zhang J. Direct Synthesis of Sulfinamides by the Copper-Catalyzed Electrophilic Amidation of Sulfenate Anions. Adv. Synth. Catal. 2018, 360, 1123–1127. 10.1002/adsc.201701510. [DOI] [Google Scholar]; c Yu H.; Li Z.; Bolm C. Copper-Catalyzed Transsulfinamidation of Sulfinamides as a Key Step in the Preparation of Sulfonamides and Sulfonimidamides. Angew. Chem., Int. Ed. 2018, 57, 15602–15605. 10.1002/anie.201810548. [DOI] [PubMed] [Google Scholar]
- a Nguyen B.; Emmett E. J.; Willis M. C. Palladium-Catalyzed Aminosulfonylation of Aryl Halides. J. Am. Chem. Soc. 2010, 132, 16372–16373. 10.1021/ja1081124. [DOI] [PubMed] [Google Scholar]; b Woolven H.; González-Rodríguez C.; Marco I.; Thompson A. L.; Willis M. C. DABCO-Bis(sulfur dioxide), DABSO, as a Convenient Source of Sulfur Dioxide for Organic Synthesis: Utility in Sulfonamide and Sulfamide Preparation. Org. Lett. 2011, 13, 4876–4878. 10.1021/ol201957n. [DOI] [PubMed] [Google Scholar]
- a Emmett E. J.; Hayter B. R.; Willis M. C. Palladium-Catalyzed Three-Component Diaryl Sulfone Synthesis Exploiting the Sulfur Dioxide Surrogate DABSO. Angew. Chem., Int. Ed. 2013, 52, 12679–12683. 10.1002/anie.201305369. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Deeming A. S.; Russell C. J.; Hennessy A. J.; Willis M. C. DABSO-Based, Three-Component, One-Pot Sulfone Synthesis. Org. Lett. 2014, 16, 150–153. 10.1021/ol403122a. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Rocke B. N.; Bahnck K. B.; Herr M.; Lavergne S.; Mascitti V.; Perreault C.; Polivkova J.; Shavnya A. Synthesis of Sulfones from Organozinc Reagents, DABSO, and Alkyl Halides. Org. Lett. 2014, 16, 154–157. 10.1021/ol4031233. [DOI] [PubMed] [Google Scholar]; d Lenstra D. C.; Vedovato V.; Ferrer Flegeau E.; Maydom J.; Willis M. C. One-Pot Sulfoxide Synthesis Exploiting a Sulfinyl-Dication Equivalent Generated from a DABSO/Trimethylsilyl Chloride Sequence. Org. Lett. 2016, 18, 2086–2089. 10.1021/acs.orglett.6b00712. [DOI] [PubMed] [Google Scholar]; e Davies A. T.; Curto J. M.; Bagley S. W.; Willis M. C. One-Pot Palladium-Catalyzed Synthesis of Sulfonyl Fluorides from Aryl Bromides. Chem. Sci. 2017, 8, 1233–1237. 10.1039/C6SC03924C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Rosenheim A.; Singer L. Die Darstellung von Alkylsulfinsäuren. Ber. Dtsch. Chem. Ges. 1904, 37, 2152–2154. 10.1002/cber.190403702145. [DOI] [Google Scholar]; b Dragoli D. R.; Burdett M. T.; Ellman J. A. Design, Synthesis, and Utility of a Support-Bound tert-Butanesulfinamide. J. Am. Chem. Soc. 2001, 123, 10127–10128. 10.1021/ja016349j. [DOI] [PubMed] [Google Scholar]
- a Mugford P. F.; Magloire V. P.; Kazlauskas R. J. Unexpected Subtilisin-Catalyzed Hydrolysis of a Sulfinamide Bond in Preference to a Carboxamide Bond in N-Acyl Sulfinamides. J. Am. Chem. Soc. 2005, 127, 6536–6537. 10.1021/ja0506105. [DOI] [PubMed] [Google Scholar]; b Kowalczyk R.; Edmunds A. J. F.; Hall R. G.; Bolm C. Synthesis of CF3-Substituted Sulfoximines from Sulfonimidoyl Fluorides. Org. Lett. 2011, 13, 768–771. 10.1021/ol103030w. [DOI] [PubMed] [Google Scholar]; c Funes Maldonado M.; Sehgelmeble F.; Bjarnemark F.; Svensson M.; Åhman J.; Arvidsson P. I. Synthesis and Arylation of Unprotected Sulfonimidamides. Tetrahedron 2012, 68, 7456–7462. 10.1016/j.tet.2012.06.072. [DOI] [Google Scholar]
- a Xue F.; Wang F.; Liu J.; Di J.; Liao Q.; Lu H.; Zhu M.; He L.; He H.; Zhang D.; Song H.; Liu X.-Y.; Qin Y. A Desulfurative Strategy for the Generation of Alkyl Radicals Enabled by Visible-Light Photoredox Catalysis. Angew. Chem., Int. Ed. 2018, 57, 6667–6671. 10.1002/anie.201802710. [DOI] [PubMed] [Google Scholar]; b Thota N.; Makam P.; Rajbongshi K. K.; Nagiah S.; Abdul N. S.; Chuturgoon A. A.; Kaushik A.; Lamichhane G.; Somboro A. M.; Kruger H. G.; Govender T.; Naicker T.; Arvidsson P. I. N-Trifluoromethylthiolated Sulfonimidamides and Sulfoximines: Anti-microbial, Anti-mycobacterial, and Cytotoxic Activity. ACS Med. Chem. Lett. 2019, 10, 1457–1461. 10.1021/acsmedchemlett.9b00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Pemberton N.; Graden H.; Evertsson E.; Bratt E.; Lepistö M.; Johannesson P.; Svensson P. H. Synthesis and Functionalization of Cyclic Sulfonimidamides: A Novel Chiral Heterocyclic Carboxylic Acid Bioisostere. ACS Med. Chem. Lett. 2012, 3, 574–578. 10.1021/ml3000935. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Jacobsen E.; Chavda M. K.; Zikpi K. M.; Waggoner S. L.; Passini D. J.; Wolfe J. A.; Larson R.; Beckley C.; Hamaker C. G.; Hitchcock S. R. A Mixed Anhydride Approach to the Preparation of Sulfinate Esters and Allylic Sulfones: Trimethylacetic p-Toluenesulfinic Anhydride. Tetrahedron Lett. 2017, 58, 3073–3077. 10.1016/j.tetlet.2017.06.074. [DOI] [Google Scholar]
- Davies T. Q.; Hall A.; Willis M. C. One-Pot, Three-Component Sulfonimidamide Synthesis Exploiting the Sulfinylamine Reagent N-Sulfinyltritylamine, TrNSO. Angew. Chem., Int. Ed. 2017, 56, 14937–14941. 10.1002/anie.201708590. [DOI] [PubMed] [Google Scholar]
- Fulton J. R.; Kamara L. M.; Morton S. C.; Rowlands G. J. The Sulfinyl Moiety in Lewis Base-Promoted Allylations. Tetrahedron 2009, 65, 9134–9141. 10.1016/j.tet.2009.09.042. [DOI] [Google Scholar]
- Meyer A. U.; Wimmer A.; König B. Visible-Light-Accelerated C–H Sulfinylation of Heteroarenes. Angew. Chem., Int. Ed. 2017, 56, 409–412. 10.1002/anie.201610210. [DOI] [PubMed] [Google Scholar]
- Wakayama M.; Ellman J. A. Recycling the tert-Butanesulfinyl Group in the Synthesis of Amines Using tert-Butanesulfinamide. J. Org. Chem. 2009, 74, 2646–2650. 10.1021/jo9001883. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.