Abstract
Particulate matter (PM), an important component of air pollution, induces significant adverse health effects. Many of the observed health effects caused by inhaled PM are associated with oxidative stress and inflammation. This association has been linked in particular to the particles’ chemical components, especially the inorganic/metal and the organic/polycyclic aromatic hydrocarbon (PAH) fractions, and their ability to generate reactive oxygen species in biological systems. The transcription factor NF-E2 nuclear factor erythroid-related factor 2 (Nrf2) is activated by redox imbalance and regulates the expression of phase II detoxifying enzymes. Nrf2 plays a key role in preventing PM-induced toxicity by protecting against oxidative damage and inflammation. This review focuses on specific PM fractions, particularly the dissolved metals and PAH fractions, and their roles in inducing oxidative stress and inflammation in cell and animal models with respect to Nrf2 and mitochondria.
Introduction
Ambient air pollution is a global health risk factor.1−3 It increases mortality and morbidity and has been identified as a leading risk factor for the global burden of disease, contributing to an estimated 4.1 million deaths in 2016,3−5 especially in low-/middle-income countries.6 Air pollution mainly affects the respiratory system,7 and numerous studies have correlated exposure to ambient fine particulate matter (PM) with an aerodynamic diameter smaller than 2.5 μm (PM2.5) with various health-related outcomes, such as premature death, cardiovascular diseases (CVD), lung diseases, stroke, asthma, and chronic obstructive pulmonary disease (COPD).3,4,6−13 Furthermore, the International Agency for Research on Cancer (IARC) has declared outdoor air pollution carcinogenic to humans, as it increases cancer incidence.14
Air pollution is an airborne mixture of substances including gases, particles, and biological components, in the Earth’s atmosphere. PM2.5 in particular has been implicated in adverse health effects. PM2.5 is composed of either primary particles that are emitted directly into the atmosphere or secondary particles produced in situ by atmospheric chemical reactions between precursor gases or between any gas-phase species and primary particles. Primary PM2.5 can originate from both natural sources (dust storms,15 forest fires16) and anthropogenic sources (biomass burning, fossil fuel combustion, consumer products,17,18 cigarette smoke [CS]), resulting in a complex chemical mixture of solids and liquids.19−24 Secondary PM2.5 is generated by chemical reactions and physical interactions that involve sulfuric acid, nitric acid, and volatile organic species from anthropogenic and/or biogenic sources. These components react with ozone, hydroxyl radicals, nitrate radicals, and other reactive agents to form secondary inorganic aerosols (SIAs) and secondary organic aerosols (SOAs).13,25−27 On a global scale, emissions of biogenic volatile organic compounds (VOCs) from surface vegetation, oceans, and soils are greater than anthropogenic emissions.26 On regional and urban scales, anthropogenic VOCs from fuels, industry activities, and consumer products may compose the majority of secondary PM formation.17
The health effects caused by inhaled PM2.5 are associated with the particle sizes and shapes, the chemical composition of the mixture, and the ability of the particles to absorb and retain toxic and carcinogenic compounds.28−30 The toxicity of PM2.5 particles depends on their sizes and masses which influence their ability to penetrate into and deposit in the lungs. PM2.5 particles are particularly important as they account for the majority of the deposited mass in the deeper lung, leading to clear correlation between PM2.5 and epidemiological evidence for health effects. However, it remains unclear whether PM2.5 components of the same mass elicit the same biological effects and whether individual components of PM2.5 cause different health-related effects; furthermore, the main mechanisms of toxicity have not been elucidated.
The chemical composition of PM2.5 may be broadly divided into three groups: (1) minerals from different origins, including common resuspended minerals (e.g., quartz) and minerals that form through chemical and physical reactions;31 (2) dissolved metals (V, Fe, Pb, Zn, Cd, Mn, Co, Cu), which are present in trace concentrations in fossil fuels but are also emitted from biomass burning, combustion processes, vehicle traffic, brake/tire wear and dust, etc.; and (3) organic components generated from primary and secondary sources, including toxicants such as polycyclic aromatic hydrocarbons (PAHs), oxygenated/nitrated PAHs, radicals and persistent organic radicals, phenols, and atmospheric humic-like substances (HULISs). Organic compounds with aromatic rings form through incomplete combustion of coal, petrol, and wood from residential, industrial, and mobile sources.19,20,25,32 The chemical composition of PM is thought to be an important determinant of health outcomes.8,9,30,33−39 However, the specific characteristics or components of PM that are harmful to human health and the actual mechanisms by which they affect health are still not well understood. Recent and continuing advances in atmospheric analytical chemistry and biological research techniques enable researchers to narrow the knowledge gaps and resolve atmospheric pollution compositions,24 so that specific atmospheric components can be connected to the health effects that they cause.
The main mechanisms of PM-induced health effects are thought to involve oxidative stress and inflammation. Various components of PM, including environmentally persistent radicals, peroxides, aromatic compounds, and dissolved metals, can generate reactive oxygen species (ROS), leading to oxidative stress and consequently enhancing various biological processes such as inflammation and cell death.7,40−44 The mitochondria are major locations for ROS production as well as cellular targets of the damaging effects of PM,45−47 suggesting a key role for the mitochondria in PM-induced toxicity. Previous studies have shown that the transcription factor NF-E2 p45-related factor 2 (Nrf2), which regulates the expression of phase II detoxifying enzymes, plays a key role in preventing PM-induced toxicity by protecting against oxidative damage and inflammation. Thus, phase II detoxifying enzymes can influence the courses of cell and tissue responses.39,48−54 This paper provides an overview of how exposure to PM fractions, particularly inorganic and organic fractions (specifically dissolved metals and PAHs), can induce biological responses in vivo (in animals) and in vitro (in cultured cells) with respect to the Nrf2 system and to mitochondria.
PM Induces Redox-Sensitive Nrf2-Dependent Response
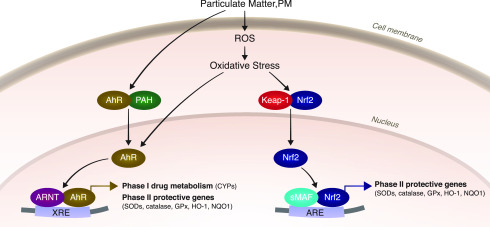
Exposure to PM2.5 is a major global health concern.3,6,10,11 The respiratory system, the major exposure pathway, is inevitably affected; it is exposed to various inhaled toxicants (e.g., environmental gases, PM and radicals that are present in PM2.5).13,51,55 The unique characteristics and anatomy of the lungs generate an oxidative microenvironment that requires constant redox homeostasis. Exposure to environmental pollutants (gases and PM) can disturb the lungs’ redox homeostasis by elevating ROS levels and by reducing antioxidation capacity, thus leading to oxidative stress.43,52 Cells and tissues contain antioxidants that respond to and counteract oxidative stress. These molecules are abundant in the lung epithelial fluid and lung tissue.52 The antioxidant system includes antioxidant enzymes, such as superoxide dismutases (SODs), catalase, and glutathione peroxidase (Gpx), indirect phase II protective genes, such as glutathione-S-transferase isozymes, γ-glutamyl cysteine ligase catalytic and modifier subunits, and NADP(H):quinone oxidoreductase (NQO1), and small antioxidant molecules (GSH). The protective genes are induced at the transcriptional level through a process mediated by a cis-acting element called an antioxidant response element (ARE).56−58 Activation of gene transcription through AREs is mediated by Nrf2.59 In addition, the phase II protective genes are mainly regulated by Nrf2.48,57 Nrf2 belongs to the cap’n’collar family of transcription factors, each member of which has a highly conserved basic-region leucine zipper structure.60 Nrf2 activity is regulated by Kelch-like ECH-associated protein-1 (KEAP-1), an adaptor protein that binds to Nrf2 in the cytoplasm to induce its proteasomal degradation. Activation of Nrf2 in response to stress releases Nrf2 to translocate to the nucleus, where it affects gene transcription.59 Thus, Nrf2 is involved in the oxidative and inflammatory responses induced by exposure to environmental pollution, especially those caused by acute and chronic exposure to PM2.539,48−54,58,61 (Figure 1).
Figure 1.
Activation of Nrf2 and AhR signaling pathways. Inorganic components of PM, such as metals, cross the cell membrane by facilitated diffusion/membrane transport proteins, where they then induce oxidative stress. Organic components such as PAHs are able to span the cells’ membrane due to their lipophilic characteristics and can also act as AhR ligands. Nrf2 is bound to KEAP-1 in the cytosol; a change in redox homeostasis leads to conformational changes, leading to translocation of Nrf2 to the nucleus. Activation of the Nrf2 pathway is mediated by the ARE enhancer sequence in target genes. AhR can be directly activated by PAHs or naturally occurring compounds and endogenous ligands, which bind to AhR and facilitate AhR translocation to the nucleus, where it binds to XRE and activates phase I/II enzyme genes. Activation of the AhR leads to oxidative stress due to metabolism of the ligand and induction of CYP enzymes.
One major question is to what degree specific molecules or particle components (directly or indirectly) drive the oxidative stress response. With regard to particle toxicology, the oxidative stress paradigm includes oxidative effects that originate from the particles or particle components and assumes that the biological reactivity of a particle is due to its oxidative potential (OP) and to secondary ROS production in exposed cells and tissues.8,62,63 ROS derivatives (hydrogen peroxide, organic hydroperoxides, quinones, semiquinones, environmentally persistent radicals) are molecules that can induce oxidative stress.17,19,24,25,34,64−67 Additionally, other molecules can change their oxidation states and promote the formation of ROS, thus exacerbating oxidative stress.39,53,68−71 The two main components of PM2.5 that will be discussed in this review are the metals and the PAHs. We focus on these components because although they are not the most abundant components in PM, they have the greatest potential to generate ROS in biological systems.
Role of Metals in Nrf2 Activation
PM2.5 can contain metals that originate from vehicles (brake and tire wear),70,72 combustion,15 construction, and urban and mineral dust.69 It has been shown that exposure to water-soluble metals present in urban PM can induce the activation and expression of Nrf2/antioxidants/phase II detoxifying enzymes, possibly as part of the protection system against oxidative stress.53,55,70,72−74 Metals present in PM, particularly Fe, Zn ,and Cu,70,72 have been found to activate a Nrf2-dependent antioxidant response that protects exposed human bronchial epithelial cells from apoptosis.68 Removal of these soluble metals by chelation significantly diminishes the pulmonary Nrf2 response.72 A combination of exposure to aqueous extracts of PM containing dissolved metals and feeding of an obesogenic diet has been found to elicit DNA methylation in a tissue-specific manner and to especially affect the expression of catalase and Nrf2.75 Exposure of HL-1 cardiomyocytes to residual oil fly ash particles from oil combustion that also contain trace metals such as V, Al, Si, and Fe76 induces Nrf2 activation and nuclear translocation that lead to a protective antioxidant response.53
Role of the Organic Fraction/PAHs in Nrf2 Activation
A major fraction of the global PM2.5 mass is composed of organic compounds from anthropogenic and biogenic origins. PM2.5 from combustion and biomass burning can contain a high proportion of PAHs and other aromatics such as phenols from lignin pyrolysis and HULIS.17,19,20,25,32 Indeed, it has been shown that PAH-rich particles that form in premixed flames, such as those from vehicle exhaust, can induce oxidative stress and activate Nrf2 in rat lungs.50 Urban pollution particles with high levels of PAHs and polychlorinated biphenyls from Buenos Aires have been found to induce Nrf2 activation in cardiomyocytes.53 Furthermore, positive associations have been observed between traffic-related pollutants (PM2.5 PAHs, black carbon, and NOx) and Nrf2-gene expression in a Los Angeles cohort (elderly subjects with coronary artery disease).54 Exposure of mice to different concentrations of PM from biodiesel burning have been found to activate the Nrf2/heme oxygenase (HO)-1 and inflammation via the nuclear factor kappa-B (NF-κB)/tumor necrosis factor (TNF)-α pathways.49,77 In addition, exposure of endothelial cells residing at the air–liquid interface (ALI) to standard diesel exhaust PM (SRM2975) induced significant activation and nuclear translocation of Nrf2.78
In the atmosphere, PAHs can further react and form oxygenated or nitrated derivatives by atmospheric aging processes.13,19,27,32,79−82 Atmospheric oxidation of PAHs and other organic compounds generates SOAs, which account for a substantial mass fraction of aerosols in the atmosphere.24,25,27,65,80 SOAs can also affect the Nrf2 gene expression pathway. For example, in one study, human airway epithelial cells (BEAS-2B cells) were exposed to isoprene SOAs in an ALI exposure system, and the most affected genes belonged to the Nrf2 pathway.38,83,84 In addition, a study in which BEAS-2B lung cells were exposed to isoprene-derived SOA constituents (from isomeric isoprene epoxydiols, IEPOXs) showed that 33 target gene-miRNA pairings were associated with Nrf2-oxidative stress pathways.36 In another ALI study, Nrf2 levels were found to increase following exposure of lung epithelial cells (A549 cells) to aged naphthalene SOAs.34
Therefore, both trace metals and PAHs in PM2.5 can trigger the Nrf2/antioxidant defense response upon exposure. However, whether Nrf2-mediated induction of antioxidant genes protects against chemical air pollutants and whether organic and inorganic compounds activate Nrf2 via the same mechanism of action are not yet clear.
PM-Induced Nrf2 Redox Signaling: Theory, Dose Response, and Related Mechanisms
Exposure to PM2.5 has been implicated in both oxidative stress and inflammation, which are underlying mechanisms of lung damage and CVD.10−12,42,53−55,72,85 The oxidative stress paradigm suggests that long-term exposure to low levels of environmental ROS or oxidative stressors induces antioxidant production to restore redox homeostasis. When this protection is insufficient,86,87 the increased stress induces other mechanisms, such as inflammation.43,48,88,89 Finally, when all defense systems are overwhelmed, they shut down, leading to cell death.85 These responses depend on pro-/antioxidant balance, which varies from one organism to another.86,87 Under physiological conditions, the basal levels of the transcription factor Nrf2 in the cytosol are low, since Nrf2 is bound to KEAP-1. Elevation in oxidative stress leads to translocation of Nrf2 to the nucleus, where it binds to AREs56−58 and activates phase II protective genes that help to maintain homeostasis (Figure 1).
Chronic exposure to environmental PM affects lung homeostasis, suggesting that Nrf2 activation depends on the PM exposure dose. A recent study compared the impact of a single exposure to PM extract with the impact of repeated exposures to PM extract of the same dose and from the same source in mouse lungs.55 After a single exposure, Nrf2 levels increased, and the lungs remained intact. However, following five repeated exposures, oxidative damage in the lungs and a systemic inflammatory reaction were observed. The lung mRNA levels of Nrf2/antioxidant system phase II detoxifying enzymes decreased following repeated exposures. Disruption of the lung tissue oxidant/antioxidant (inflammatory/defense) balance was evidenced by increased levels of lipid and protein oxidation.55 These results support a phenomenon in which low-dose or single acute exposure to PM induces the expression of Nrf2-related antioxidant genes to counteract the increased ROS levels in tissue. Under high-level exposure conditions or in response to multiple chronic exposures, the antioxidant system may fail to be activated and protect against the oxidative burst, thus leading to lung tissue oxidative damage. Other findings that support this concept were obtained in a study on mice exposed to biodiesel PM. The exposed mice showed increased protein expression levels of Nrf2, p-NF-κB, and HO-1; however, the Nrf2 levels were higher in the low-dose exposure group than in the high-dose exposure group.49 In another study, Nrf2/phase II protective gene expression was directly related to the number of exposures at the lowest PM2.5 dose (2 μg/cm2) but, surprisingly, inversely related to the number of exposures at the highest dose (10 μg/cm2). Again, this response may have been attributable to a compromised capacity to activate the protective Nrf2 tissue defense system under high-dose exposure.90
Other factors can also activate the Nrf2/antioxidant system. These include direct and indirect mechanisms by which PM2.5 exerts its effects. The direct pathways are related to the penetration of PM2.5 particles or their components into the pulmonary system to directly affect lung cells and lung and other tissues.91 The indirect pathways are related to mechanisms by which exposure of the respiratory system to PM causes the release of inflammatory cytokines that circulate through the bloodstream and induce a systemic reaction that can affect remote tissues.7,92,93 In any case, the lungs, as the primary organ that encounter the particles, are exposed to the highest masses/doses of the particles.7 Oxidative stress and inflammation have been detected in the lungs and liver of mice exposed to water and organic extracts of urban PM2.5 collected in Beijing, China. Nrf2/phase II protective enzymes are activated in the liver of these mice but suppressed in the lungs. These findings suggest that toxic components from PM circulate in the bloodstream from the primary organs (lungs) to secondary organs (such as the liver) that can have different susceptibilities to exposure.94 In addition, organic pollution extracts with high PAH content have been found to cause damage to the liver than to the lungs, as PAHs can trigger signaling agents such as inflammatory cytokines or can accumulate in other tissues, especially fatty tissues such as the liver,94 where they induce toxicity. In a similar manner, different basal expression levels and metabolic rates in different tissues may result in organ-specific Nrf2 responses. In one study, the effects of concomitant metal-rich PM2.5 extract exposure and high-fat diet feeding on remote metabolic tissues, such as the liver and white/brown adipose tissues, were investigated. Exposure to the PM2.5 metal extract led to opposite responses (of select genes related to the Nrf2 pathway) in the lung and liver. Compared to a normal diet, the high-fat diet increased Nrf2 and catalase gene expression in the liver.75 Together, the data presented for lungs, liver, and adipose tissues provide general insights into the systemic and tissue-selective impacts of PM exposure and the possible metabolic implications.
Mechanisms of Action of Metals on Nrf2 Signaling
The diverse characteristics of the PM2.5 chemical components may trigger different signaling pathways to activate Nrf2. Initially, metals cross the cell membrane via facilitated diffusion or transport through membrane proteins.95 It has been suggested that the main mechanism of action of metals from PM2.5 involves induction of oxidative stress and ROS formation.35,96,97 Metals can elicit the formation of ROS through Fenton and Haber–Weiss reactions and can subsequently induce oxidative stress. These pro-oxidant factors can change the cellular redox state.44,96
Metals can also evoke an inflammatory response that occurs when tissues or cells are damaged by PM2.5. Immune cells and other molecular mediators are involved in this protective response.48 In humans, exposure to aqueous extracts of PM2.5 containing high concentrations of dissolved metals has been found to cause an inflammatory response, increasing IL-8 and TNF-α levels in the lower respiratory tract and bronchoalveolar lavage fluid. Moreover, PM2.5 can stimulate secondary ROS generation as part of an inflammatory response.48,61,98,99 The studies reviewed so far show the connections between dissolved metals from PM2.5 and the induction of Nrf2, thus supporting the involvement of metals in both oxidative stress and inflammation related to Nrf2 activation. The effects of the metals are likely mediated by enhanced ROS formation or cytokine secretion.
Mechanisms of Action of PAHs on Nrf2 Signaling
The organic component of PM2.5, which is partially made up of PAHs, quinones, peroxides, and radicals, can contribute to oxidative injury in the lungs.13,34,81,82 The mechanisms of the effects of the organic fraction may involve ROS formation and other processes. Due to their lipophilic character, PAHs can cross the cell membrane, but they can also act as ligands for aryl hydrocarbon receptor (AhR).100 AhR is expressed in all tissues but is highly expressed in the liver, adipose tissues, and bronchial epithelial cells.101 Activation of AhR upregulates cytochrome P450 (CYP) metabolizing enzymes that can transform toxicants into less toxic forms (thus providing protection). PAHs are often metabolized into quinones, which can be further metabolized into semiquinones.66 When semiquinones are reduced back into quinones in a process called redox cycling, they can form additional ROS67,96 that further aggravate PM2.5 toxicity.100,102 On the one hand, this dual action of AhR activation may contribute to the amplification of some diseases; on the other hand, it may alleviate others.103 It has been shown that ambient urban dust that contains PAHs induces proinflammatory T cell and dendritic cell responses via AhR in naive CD4+ T cells purified from male/female adult mice.102
PM that contains environmentally persistent free radicals can also induce AhR activation and cytokine secretion in human bronchial epithelial cells.104 Crosstalk between AhR (phase I) and Nrf2 (phase II) protective/metabolizing enzymes98 may exist, because both types of proteins control the expression of xenobiotic-metabolizing enzymes (XRE).56,88 For example, the expression of NQO1 depends on both AhR and Nrf2 (Figure 1). Exposure to PAHs induces Nrf2 activation in the mouse hepatoma cell line 1c1c7, but this effect can be abolished by transfection with a small interfering RNA (siRNA) targeting AhR,105 thus indicating the existence of a link between Nrf2 and AhR. In addition, exposure of rat progenitor cells to PAHs induces the expression of CYP1A1, CYP1B1, and Nrf2 genes in the WB-F344 cell line.106 Another study has revealed that exposure to extracts from PAH-rich PM2.5 collected in Beijing, China, increases Nrf2 and CYP gene (CYP 1a1 and 2e) expression in the liver, but not in the lungs.94 This finding implies that PAHs can exert their toxic effects through two different mechanisms: by increasing ROS formation and thus increasing oxidative stress and by binding to AhR, which may directly or indirectly activate Nrf2. It is yet to be determined whether AhR is activated in correlation with Nrf2 in lung tissue and whether this induction enhances or aggravates protection through Nrf2 signaling
PAHs can also generate strong proinflammatory responses, for example, the release of the proinflammatory cytokines IL-1β, IL-6, and TNF-α from macrophages increases after exposure to PM2.5 organic extracts40,81,107 In addition, exposure to a PM mixture of diesel exhaust together with an urban-like pollutant mixture has been found to produce a significant IL-8 inflammatory response in A549 lung cells.77,108 Acrolein and p-tolualdehyde (representative VOCs) elicit an inflammatory response and cellular damage in A549 cells.109 Furthermore, mouse macrophages exposed to PAHs show induction of IL-1β, Toll-like receptor, and NF-κB, indicating an increased inflammatory response. This response is induced by organic extracts but not water extracts, implying that inflammation is the main mechanism of action of PAHs.110 Whether PAHs function through oxidative stress or inflammatory processes is not clear. It is possible that both mechanisms are activated in response to exposure to PM organic/PAH extracts and that a strong decrease in Nrf2 expression after exposure to PAHs39,40,111 amplifies the inflammatory response.
Mitochondria Are Involved in PM-Induced Toxicity
ROS generation and subsequent increases in oxidative stress have been recognized as major contributors to cell damage, cell death, DNA damage, and inflammation due to PM2.5 exposure.39,41,112,113 As ROS are produced mainly in the mitochondria as byproducts of cellular respiration,113,114 disruption of mitochondrial electron transport (oxidative phosphorylation) can further augment ROS production and amplify oxidative stress.41,114 Mitochondria are highly sensitive to environmental toxicants,47 and PM2.5 has been shown to accumulate within mitochondria112,115 and further disrupt mitochondrial membrane potential,39 damage mitochondrial structure and function,39,45,112 alter mtDNA (through strand breaks and methylation),39,47 and activate mitochondrial programmed apoptosis in pulmonary tissues.41,116 However, studies on the specific connections between PM exposure and mitochondria are limited; thus, these connections require further investigation. Several studies have suggested that metals and PAHs exert their toxicity through different mechanisms involving mitochondria.37,99,112,116−118
Roles of Metals in Mitochondria-Related Toxicity
The specific effects of metals from PM on mitochondria have been shown in several studies. Metals found in oil fly ash, especially V, Fe, and Ni, impair mitochondrial function and increase apoptosis in lung epithelial cells.116 PM-bound metals that penetrate into cells and enter mitochondria can disturb mitochondrial membrane potential, disrupt mitochondrial structure, induce intracellular and mitochondrial ROS production, change calcium homeostasis, and induce apoptosis. All of these processes are also correlated with cancer progression.115 Examination of lung epithelial cells exposed to PM samples collected in Milan containing high concentrations of transition metals (Ni and Zn) and PAHs has shown the occurrence of cell membrane lysis and mitochondrial ultrastructural disruption with increased ROS production, suggesting that metals (but also PAHs) contribute to ROS production and toxicity.117 Pretreatment of alveolar epithelial cells with Fe chelators protects the cells from PM2.5-induced mitochondrial dysfunction, DNA damage, and apoptosis.99 In addition, a recent study has shown that exposure to metals from PM results in increased ROS production in lung epithelial cells and reduced mitochondrial function.39 Collectively, these findings suggest that the cytotoxicity exerted by metals is driven by changes in oxidative status that induce mitochondrial dysfunction and apoptosis.
Roles of PAHs in Mitochondria-Related Toxicity
PAHs toxicity has also been shown to involve mitochondria. It has recently been shown that exposure of bronchial epithelial cells to PM2.5 leads to PAHs penetration into the cells; activation of AhR, which influences mitochondrial membrane potential and apoptosis.37 Another study has demonstrated that ultrafine particles (UFPs), especially organic carbon and PAHs, from PM2.5 collected in Los Angeles induce oxidative stress and mitochondrial damage, suggesting a role of organic agents in generating redox activity.112 Another study has shown that organic extracts containing high concentrations of PAHs increase mortality, reduce ROS production, and reduce mitochondrial function in lung epithelial cells.39
ROS, oxidative stress, and other sources of genotoxic damage45,119 also influence the regulation of mitochondrial copy number (mtDNAcn), which is involved in PAH toxicity. In a study on Polish workers exposed to PAHs, the workers had considerably different mtDNAcns than the control subjects.120 Conversely, decreased mitochondrial DNA content is associated with exposure to PAHs in human TK6 cells,118 and similar findings have been obtained by Pardo et al.,39 who showed that extracts rich in PAH reduce mtDNAcns in lung bronchial cells.
PM-Induced Effects on the Nrf2 Signaling Pathway and Mitochondria
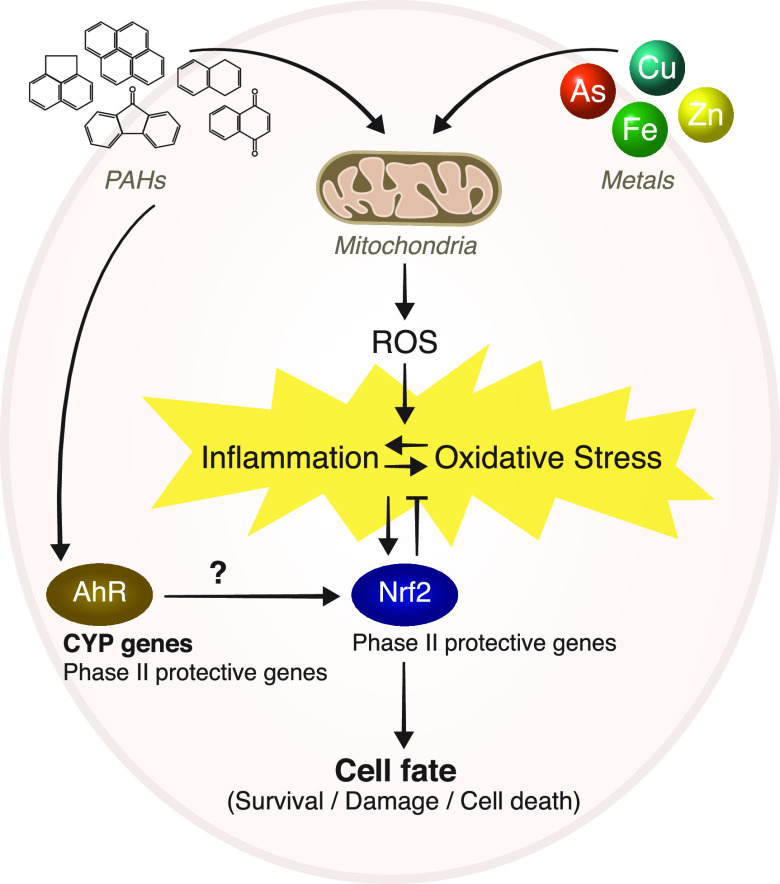
A direct link between PM, mitochondria, and Nrf2 has been found in several recent studies. For example, Leclercq et al.90 observed mitochondrial dysfunction after exposure of human bronchial cells to PM. This dysfunction was accompanied by an increase in Nrf2 (gene expression and binding activity) and a dose-dependent increase in NF-κB. In another study, PM2.5 collected in Los Angeles induced mitochondrial damage and increased HO-1 expression related to the Nrf2 pathway.112 In a study that investigated the mechanism by which Nrf2 exerts its protective effect against PM2.5-induced toxicity in lung cells, Nrf2 was silenced in lung cells. The Nrf2-silenced cells demonstrated increased susceptibility to various PM extracts: Water extracts rich in metals increased mitochondrial ROS production and oxidative stress levels, while organic extracts containing high levels of PAHs increased mortality and reduced ROS production. Changes in mitochondria were also observed in Nrf2-silenced cells that obtained higher basal mitochondrial respiration rates than control cells. Mitochondrial respiration was increased following exposure to the water extracts but not following exposure to the organic extracts. The Nrf2-silenced cells exposed to the organic extracts showed reduced mitochondrial membrane potentials and reduced mtDNAcns.39 These findings suggest that ROS overproduction induces oxidative damage and activates the Nrf2 signaling pathways. However, prolonged and repeated exposure or exposure to PAHs induces an oxidative boost8,67,86,87,102,104 that can partially inactivate the Nrf2 signaling pathway and critically impair mitochondrial redox homeostasis, thereby producing persistent mitochondrial dysfunction and reducing the cell energy supply (Figure 2).
Figure 2.
PM components (inorganic and organics, mostly metals and PAHs, respectively) induce oxidative stress and inflammation. Exposure to PM enhances ROS formation and alters mitochondrial function, which may lead to inflammation. The disturbance of redox homeostasis alters the activation of redox-sensitive signaling pathways such as Nrf2. Additionally, PAHs activate the AhR pathway, contributing further to the physio-pathological inflammatory effects of PM.
Conclusions and Future Perspectives
Despite cumulative data suggesting that there are different mechanisms of action for different PM components, there is still a lack of knowledge regarding the mechanisms that control PM-induced responses. Due to differences in chemical properties, the inorganic and organic fractions, that is, metals and PAHs, of PM seem to exert not only synergistic but also different biological effects. Both inorganic and organic components can enhance ROS generation, and since ROS are generated primarily in the mitochondria, the mitochondria may be the primary organelles affected by changes in redox status. Inorganic compounds, especially dissolved metals, elicit the formation of ROS, causing oxidative stress and consequent inflammation. PAHs may additionally activate the AhR signaling pathway, thus contributing to a pathophysiological inflammatory effect in some cases. PAHs generate a stronger inflammatory response than metals, which could be explained by the activation of several signaling pathways at the same time. We suggest that Nrf2 signaling may coordinate the response linking mitochondrial signaling and cell fate following PM2.5 exposure. We therefore propose that future studies should focus on the crosstalk between mitochondrial signaling and Nrf2 signaling. Additional research is needed to more thoroughly characterize the role of the Nrf2-mediated oxidative stress response in PM-induced toxicity. In addition, other components of PM, such as airborne toxins and biomass burning-related particles, should be studied for their involvement in Nrf2-related PM toxicity.
The doses of toxicants deposited in the lungs are important for the effects of metals and PAHs discussed here. UFPs, which comprise all particles smaller than 100 nm and whose health effects are currently under intense investigation and discussion, do not contribute significantly to the deposited mass of metals and PAHs deep in the lungs. Thus, UFP toxicology might be mediated via other pathways, including particle translocation and particle surface-mediated mechanisms. This is another important topic for future research.
Acknowledgments
This research was partially supported by research grants from the Israeli Ministry of Science (grant no. 3-14010), the Israel Science Foundation (ISF) (#3205/19), and the Weizmann Institute of Science in the framework of the aeroHEALTH Helmholtz International Lab, a German-Israeli project.
Glossary
Abbreviations
- AhR
aryl hydrocarbon receptor
- ARE
antioxidant response element
- COPD
chronic obstructive pulmonary disease
- Gpx-1
glutathione peroxidase 1
- GSH
glutathione
- mtDNAcn
mitochondrial DNA copy number
- NF-κB
nuclear factor kappa B
- PAH
polycyclic aromatic hydrocarbon
- Nrf2
nuclear factor erythroid 2-related factor 2
- PM
particulate matter
- ROS
reactive oxygen species
- SOA
secondary organic aerosols
- SOD
superoxide dismutase
- TNF-α
tumor necrosis factor alpha
- UFP
ultrafine particle
- IL-8
interleukin 8
- XRE
xenobiotic-metabolizing enzymes
Biographies
Michal Pardo has a Ph.D. in Biochemistry from the Hebrew University of Jerusalem, and since 2013 she is a staff scientist at the Weizmann Institute of Science in Rehovot, Israel. She works on health effects of aerosols and environmental particles. Her main research interests are elucidating the molecular mechanisms evoked by atmospheric particulate matter exposure and their relation to adverse health effects. Dr. Pardo is a co-PI in the Weizmann-Hemlholtz International Lab “aeroHEALTH”.
Xinghua Qiu received his B.Sc. degree in applied chemistry and Ph.D. degree in environmental science, both from Peking University, China. After postdoctoral work with Ronald Hites at Indiana University in Bloomington, IN, USA, he joined Peking University as an associate professor at the College of Environmental Sciences and Engineering. His research interests lie in the toxic organic components of particulate matter, in particular, polycyclic aromatic hydrocarbons and the derivatives and their health effects.
Ralf Zimmermann is full professor of Analytical Chemistry at the University of Rostock and head of the department “Comprehensive Molecular Analytics” at the Helmholtz Center Munich. He is particularly interested in the development and application of high-end mass spectrometric and chromatographic methods and technologies for characterization of ambient aerosols and process gases. This includes laser photoionization-, single particle-, and ultrahigh mass resolution-mass spectrometric approaches. In the past decade, he is increasingly focusing on biological and toxicological approaches, targeting health effects of ambient particular matter and air pollution. He is currently spokesperson of the Helmholtz International Laboratory “aeroHEALTH” (www.aerohealth.eu).
Yinon Rudich is a Professor and Dean of Chemistry at the Weizmann Institute, Rehovot, Israel. Prof. Rudich studies the effect of changing chemical composition of aerosols on their optical and toxicological properties. Specifically, he studies aerosols optical properties, ice nucleation by dust and proteins, the microbial communities in transported aerosols, and the health effects of aerosols. Yinon Rudich is a PI in the Weizmann-Helmholtz International Lab aeroHEALTH.
The authors declare no competing financial interest.
References
- Brauer M.; Amann M.; Burnett R. T.; Cohen A.; Dentener F.; Ezzati M.; Henderson S. B.; Krzyzanowski M.; Martin R. V.; Van Dingenen R.; van Donkelaar A.; Thurston G. D. (2012) Exposure Assessment for Estimation of the Global Burden of Disease Attributable to Outdoor Air Pollution. Environ. Sci. Technol. 46, 652–660. 10.1021/es2025752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer M.; Freedman G.; Frostad J.; van Donkelaar A.; Martin R. V.; Dentener F.; Dingenen R. v.; Estep K.; Amini H.; Apte J. S.; Balakrishnan K.; Barregard L.; Broday D.; Feigin V.; Ghosh S.; Hopke P. K.; Knibbs L. D.; Kokubo Y.; Liu Y.; Ma S.; Morawska L.; Sangrador J. L. T.; Shaddick G.; Anderson H. R.; Vos T.; Forouzanfar M. H.; Burnett R. T.; Cohen A. (2016) Ambient Air Pollution Exposure Estimation for the Global Burden of Disease 2013. Environ. Sci. Technol. 50, 79–88. 10.1021/acs.est.5b03709. [DOI] [PubMed] [Google Scholar]
- Forouzanfar M. H.; et al. (2016) Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1659–1724. 10.1016/S0140-6736(16)31679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaddick G.; Thomas M. L.; Amini H.; Broday D.; Cohen A.; Frostad J.; Green A.; Gumy S.; Liu Y.; Martin R. V.; Pruss-Ustun A.; Simpson D.; van Donkelaar A.; Brauer M. (2018) Data Integration for the Assessment of Population Exposure to Ambient Air Pollution for Global Burden of Disease Assessment. Environ. Sci. Technol. 52, 9069–9078. 10.1021/acs.est.8b02864. [DOI] [PubMed] [Google Scholar]
- (2016) Ambient air pollution: a global assessment of exposure and burden of disease. World Health Organization, Geneva.
- Cohen A. J.; Brauer M.; Burnett R.; Anderson H. R.; Frostad J.; Estep K.; Balakrishnan K.; Brunekreef B.; Dandona L.; Dandona R.; Feigin V.; Freedman G.; Hubbell B.; Jobling A.; Kan H.; Knibbs L.; Liu Y.; Martin R.; Morawska L.; Pope C. A.; Shin H.; Straif K.; Shaddick G.; Thomas M.; van Dingenen R.; van Donkelaar A.; Vos T.; Murray C. J. L.; Forouzanfar M. H. (2017) Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet 389, 1907–1918. 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riediker M.; Zink D.; Kreyling W.; Oberdörster G.; Elder A.; Graham U.; Lynch I.; Duschl A.; Ichihara G.; Ichihara S.; Kobayashi T.; Hisanaga N.; Umezawa M.; Cheng T.-J.; Handy R.; Gulumian M.; Tinkle S.; Cassee F. (2019) Particle toxicology and health - where are we?. Part. Fibre Toxicol. 16, 19. 10.1186/s12989-019-0302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovrevik J. (2019) Oxidative Potential Versus Biological Effects: A Review on the Relevance of Cell-Free/Abiotic Assays as Predictors of Toxicity from Airborne Particulate Matter. Int. J. Mol. Sci. 20, 4772. 10.3390/ijms20194772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J. J.; Cohen A.; Dentener F.; Brunekreef B.; Zhu T.; Armstrong B.; Bell M. L.; Brauer M.; Carmichael G.; Costa D. L.; Dockery D. W.; Kleeman M.; Krzyzanowski M.; Kunzli N.; Liousse C.; Lung S. C.; Martin R. V.; Poschl U.; Pope C. A. 3rd; Roberts J. M.; Russell A. G.; Wiedinmyer C. (2016) What We Breathe Impacts Our Health: Improving Understanding of the Link between Air Pollution and Health. Environ. Sci. Technol. 50, 4895–4904. 10.1021/acs.est.5b03827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett R.; Chen H.; Szyszkowicz M.; Fann N.; Hubbell B.; Pope C. A. 3rd; Apte J. S.; Brauer M.; Cohen A.; Weichenthal S.; Coggins J.; Di Q.; Brunekreef B.; Frostad J.; Lim S. S.; Kan H.; Walker K. D.; Thurston G. D.; Hayes R. B.; Lim C. C.; Turner M. C.; Jerrett M.; Krewski D.; Gapstur S. M.; Diver W. R.; Ostro B.; Goldberg D.; Crouse D. L.; Martin R. V.; Peters P.; Pinault L.; Tjepkema M.; van Donkelaar A.; Villeneuve P. J.; Miller A. B.; Yin P.; Zhou M.; Wang L.; Janssen N. A. H.; Marra M.; Atkinson R. W.; Tsang H.; Quoc Thach T.; Cannon J. B.; Allen R. T.; Hart J. E.; Laden F.; Cesaroni G.; Forastiere F.; Weinmayr G.; Jaensch A.; Nagel G.; Concin H.; Spadaro J. V. (2018) Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc. Natl. Acad. Sci. U. S. A. 115, 9592–9597. 10.1073/pnas.1803222115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelieveld J.; Klingmüller K.; Pozzer A.; Pöschl U.; Fnais M.; Daiber A.; Münzel T. (2019) Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions. Eur. Heart J. 40, 1590–1596. 10.1093/eurheartj/ehz135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.-H.; Kabir E.; Kabir S. (2015) A review on the human health impact of airborne particulate matter. Environ. Int. 74, 136–143. 10.1016/j.envint.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Shiraiwa M.; Ueda K.; Pozzer A.; Lammel G.; Kampf C. J.; Fushimi A.; Enami S.; Arangio A. M.; Fröhlich-Nowoisky J.; Fujitani Y.; Furuyama A.; Lakey P. S. J.; Lelieveld J.; Lucas K.; Morino Y.; Pöschl U.; Takahama S.; Takami A.; Tong H.; Weber B.; Yoshino A.; Sato K. (2017) Aerosol Health Effects from Molecular to Global Scales. Environ. Sci. Technol. 51, 13545–13567. 10.1021/acs.est.7b04417. [DOI] [PubMed] [Google Scholar]
- (2013) Air pollution and cancer. The International Agency for Research on Cancer, Lyon, France.
- Philip S.; Martin R. V.; Snider G.; Weagle C. L.; van Donkelaar A.; Brauer M.; Henze D. K.; Klimont Z.; Venkataraman C.; Guttikunda S. K.; Zhang Q. (2017) Anthropogenic fugitive, combustion and industrial dust is a significant, underrepresented fine particulate matter source in global atmospheric models. Environ. Res. Lett. 12, 044018. 10.1088/1748-9326/aa65a4. [DOI] [Google Scholar]
- Ditto J. C.; Joo T.; Khare P.; Sheu R.; Takeuchi M.; Chen Y.; Xu W.; Bui A. A. T.; Sun Y.; Ng N. L. S.; Gentner D. R. (2019) Effects of Molecular-Level Compositional Variability in Organic Aerosol on Phase State and Thermodynamic Mixing Behavior. Environ. Sci. Technol. 53, 13009. 10.1021/acs.est.9b02664. [DOI] [PubMed] [Google Scholar]
- McDonald B. C.; de Gouw J. A.; Gilman J. B.; Jathar S. H.; Akherati A.; Cappa C. D.; Jimenez J. L.; Lee-Taylor J.; Hayes P. L.; McKeen S. A.; Cui Y. Y.; Kim S.-W.; Gentner D. R.; Isaacman-VanWertz G.; Goldstein A. H.; Harley R. A.; Frost G. J.; Roberts J. M.; Ryerson T. B.; Trainer M. (2018) Volatile chemical products emerging as largest petrochemical source of urban organic emissions. Science 359, 760–764. 10.1126/science.aaq0524. [DOI] [PubMed] [Google Scholar]
- Oeder S.; Kanashova T.; Sippula O.; Sapcariu S. C.; Streibel T.; Arteaga-Salas J. M.; Passig J.; Dilger M.; Paur H.-R.; Schlager C.; Mülhopt S.; Diabaté S.; Weiss C.; Stengel B.; Rabe R.; Harndorf H.; Torvela T.; Jokiniemi J. K.; Hirvonen M.-R.; Schmidt-Weber C.; Traidl-Hoffmann C.; BéruBé K. A.; Wlodarczyk A. J.; Prytherch Z.; Michalke B.; Krebs T.; Prévôt A. S. H.; Kelbg M.; Tiggesbäumker J.; Karg E.; Jakobi G.; Scholtes S.; Schnelle-Kreis J.; Lintelmann J.; Matuschek G.; Sklorz M.; Klingbeil S.; Orasche J.; Richthammer P.; Müller L.; Elsasser M.; Reda A.; Gröger T.; Weggler B.; Schwemer T.; Czech H.; Rüger C. P.; Abbaszade G.; Radischat C.; Hiller K.; Buters J. T. M.; Dittmar G.; Zimmermann R. (2015) Particulate matter from both heavy fuel oil and diesel fuel shipping emissions show strong biological effects on human lung cells at realistic and comparable in vitro exposure conditions. PLoS One 10, e0126536–e0126536. 10.1371/journal.pone.0126536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtipalo K.; Yan C.; Dada L.; Bianchi F.; Xiao M.; Wagner R.; Stolzenburg D.; Ahonen L. R.; Amorim A.; Baccarini A.; Bauer P. S.; Baumgartner B.; Bergen A.; Bernhammer A.-K.; Breitenlechner M.; Brilke S.; Buchholz A.; Mazon S. B.; Chen D.; Chen X.; Dias A.; Dommen J.; Draper D. C.; Duplissy J.; Ehn M.; Finkenzeller H.; Fischer L.; Frege C.; Fuchs C.; Garmash O.; Gordon H.; Hakala J.; He X.; Heikkinen L.; Heinritzi M.; Helm J. C.; Hofbauer V.; Hoyle C. R.; Jokinen T.; Kangasluoma J.; Kerminen V.-M.; Kim C.; Kirkby J.; Kontkanen J.; Kürten A.; Lawler M. J.; Mai H.; Mathot S.; Mauldin R. L.; Molteni U.; Nichman L.; Nie W.; Nieminen T.; Ojdanic A.; Onnela A.; Passananti M.; Petäjä T.; Piel F.; Pospisilova V.; Quéléver L. L. J.; Rissanen M. P.; Rose C.; Sarnela N.; Schallhart S.; Schuchmann S.; Sengupta K.; Simon M.; Sipilä M.; Tauber C.; Tomé A.; Tröstl J.; Väisänen O.; Vogel A. L.; Volkamer R.; Wagner A. C.; Wang M.; Weitz L.; Wimmer D.; Ye P.; Ylisirniö A.; Zha Q.; Carslaw K. S.; Curtius J.; Donahue N. M.; Flagan R. C.; Hansel A.; Riipinen I.; Virtanen A.; Winkler P. M.; Baltensperger U.; Kulmala M.; Worsnop D. R. (2018) Multicomponent new particle formation from sulfuric acid, ammonia, and biogenic vapors. Science Advances 4, eaau5363. 10.1126/sciadv.aau5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma V.; Fang T.; Xu L.; Peltier R. E.; Russell A. G.; Ng N. L.; Weber R. J. (2015) Organic Aerosols Associated with the Generation of Reactive Oxygen Species (ROS) by Water-Soluble PM2.5. Environ. Sci. Technol. 49, 4646–4656. 10.1021/es505577w. [DOI] [PubMed] [Google Scholar]
- Chan A. W. H.; Isaacman G.; Wilson K. R.; Worton D. R.; Ruehl C. R.; Nah T.; Gentner D. R.; Dallmann T. R.; Kirchstetter T. W.; Harley R. A.; Gilman J. B.; Kuster W. C.; de Gouw J. A.; Offenberg J. H.; Kleindienst T. E.; Lin Y. H.; Rubitschun C. L.; Surratt J. D.; Hayes P. L.; Jimenez J. L.; Goldstein A. H. (2013) Detailed chemical characterization of unresolved complex mixtures in atmospheric organics: Insights into emission sources, atmospheric processing, and secondary organic aerosol formation. Journal of Geophysical Research: Atmospheres 118, 6783–6796. 10.1002/jgrd.50533. [DOI] [Google Scholar]
- Schlesinger R. B. (2007) The Health Impact of Common Inorganic Components of Fine Particulate Matter (PM2.5) in Ambient Air: A Critical Review. Inhalation Toxicol. 19, 811–832. 10.1080/08958370701402382. [DOI] [PubMed] [Google Scholar]
- Kleeman M. J.; Cass G. R. (1998) Source contributions to the size and composition distribution of urban particulate air pollution. Atmos. Environ. 32, 2803–2816. 10.1016/S1352-2310(98)00001-6. [DOI] [Google Scholar]
- Burkholder J. B.; Abbatt J. P. D.; Barnes I.; Roberts J. M.; Melamed M. L.; Ammann M.; Bertram A. K.; Cappa C. D.; Carlton A. G.; Carpenter L. J.; Crowley J. N.; Dubowski Y.; George C.; Heard D. E.; Herrmann H.; Keutsch F. N.; Kroll J. H.; McNeill V. F.; Ng N. L.; Nizkorodov S. A.; Orlando J. J.; Percival C. J.; Picquet-Varrault B.; Rudich Y.; Seakins P. W.; Surratt J. D.; Tanimoto H.; Thornton J. A.; Tong Z.; Tyndall G. S.; Wahner A.; Weschler C. J.; Wilson K. R.; Ziemann P. J. (2017) The Essential Role for Laboratory Studies in Atmospheric Chemistry. Environ. Sci. Technol. 51, 2519–2528. 10.1021/acs.est.6b04947. [DOI] [PubMed] [Google Scholar]
- Jimenez J. L.; Canagaratna M. R.; Donahue N. M.; Prevot A. S. H.; Zhang Q.; Kroll J. H.; DeCarlo P. F.; Allan J. D.; Coe H.; Ng N. L.; Aiken A. C.; Docherty K. S.; Ulbrich I. M.; Grieshop A. P.; Robinson A. L.; Duplissy J.; Smith J. D.; Wilson K. R.; Lanz V. A.; Hueglin C.; Sun Y. L.; Tian J.; Laaksonen A.; Raatikainen T.; Rautiainen J.; Vaattovaara P.; Ehn M.; Kulmala M.; Tomlinson J. M.; Collins D. R.; Cubison M. J.; Dunlea J.; Huffman J. A.; Onasch T. B.; Alfarra M. R.; Williams P. I.; Bower K.; Kondo Y.; Schneider J.; Drewnick F.; Borrmann S.; Weimer S.; Demerjian K.; Salcedo D.; Cottrell L.; Griffin R.; Takami A.; Miyoshi T.; Hatakeyama S.; Shimono A.; Sun J. Y.; Zhang Y. M.; Dzepina K.; Kimmel J. R.; Sueper D.; Jayne J. T.; Herndon S. C.; Trimborn A. M.; Williams L. R.; Wood E. C.; Middlebrook A. M.; Kolb C. E.; Baltensperger U.; Worsnop D. R. (2009) Evolution of Organic Aerosols in the Atmosphere. Science 326, 1525–1529. 10.1126/science.1180353. [DOI] [PubMed] [Google Scholar]
- Mentel T. F.; Kleist E.; Andres S.; Dal Maso M.; Hohaus T.; Kiendler-Scharr A.; Rudich Y.; Springer M.; Tillmann R.; Uerlings R.; Wahner A.; Wildt J. (2013) Secondary aerosol formation from stress-induced biogenic emissions and possible climate feedbacks. Atmos. Chem. Phys. 13, 8755–8770. 10.5194/acp-13-8755-2013. [DOI] [Google Scholar]
- Shakya K. M.; Griffin R. J. (2010) Secondary Organic Aerosol from Photooxidation of Polycyclic Aromatic Hydrocarbons. Environ. Sci. Technol. 44, 8134–8139. 10.1021/es1019417. [DOI] [PubMed] [Google Scholar]
- Falkovich A. H.; Rudich Y. (2001) Analysis of semivolatile organic compounds in atmospheric aerosols by direct sample introduction thermal desorption GC/MS. Environ. Sci. Technol. 35, 2326–2333. 10.1021/es000280i. [DOI] [PubMed] [Google Scholar]
- Kundu S.; Stone E. A. (2014) Composition and sources of fine particulate matter across urban and rural sites in the Midwestern United States. Environ. Sci. Process Impacts 16, 1360–1370. 10.1039/C3EM00719G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sýkorová B.; Kucbel M.; Raclavský K. (2016) Composition of airborne particulate matter in the industrial area versus mountain area. Perspectives in Science 7, 369–372. 10.1016/j.pisc.2015.12.006. [DOI] [Google Scholar]
- Song X.; Shao L.; Zheng Q.; Yang S. (2014) Mineralogical and geochemical composition of particulate matter (PM10) in coal and non-coal industrial cities of Henan Province, North China. Atmos. Res. 143, 462–472. 10.1016/j.atmosres.2014.03.015. [DOI] [Google Scholar]
- Ravindra K.; Sokhi R.; Van Grieken R. (2008) Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation. Atmos. Environ. 42, 2895–2921. 10.1016/j.atmosenv.2007.12.010. [DOI] [Google Scholar]
- Chan L. K.; Nguyen K. Q.; Karim N.; Yang Y.; Rice R. H.; He G.; Denison M. S.; Nguyen T. B. (2020) Relationship between the molecular composition, visible light absorption, and health-related properties of smoldering woodsmoke aerosols. Atmos. Chem. Phys. 20, 539. 10.5194/acp-20-539-2020. [DOI] [Google Scholar]
- Chowdhury P. H.; He Q.; Lasitza Male T.; Brune W. H.; Rudich Y.; Pardo M. (2018) Exposure of Lung Epithelial Cells to Photochemically Aged Secondary Organic Aerosol Shows Increased Toxic Effects. Environ. Sci. Technol. Lett. 5, 424–430. 10.1021/acs.estlett.8b00256. [DOI] [Google Scholar]
- Dilger M.; Orasche J.; Zimmermann R.; Paur H. R.; Diabate S.; Weiss C. (2016) Toxicity of wood smoke particles in human A549 lung epithelial cells: the role of PAHs, soot and zinc. Arch. Toxicol. 90, 3029–3044. 10.1007/s00204-016-1659-1. [DOI] [PubMed] [Google Scholar]
- Eaves L. A.; Smeester L.; Hartwell H. J.; Lin Y.-H.; Arashiro M.; Zhang Z.; Gold A.; Surratt J. D.; Fry R. C. (2020) Isoprene-derived Secondary Organic Aerosol Induces the Expression of micro RNAs (miRNAs) Associated with Inflammatory/Oxidative Stress Response in Lung Cells. Chem. Res. Toxicol. 33, 381. 10.1021/acs.chemrestox.9b00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferecatu I.; Borot M. C.; Bossard C.; Leroux M.; Boggetto N.; Marano F.; Baeza-Squiban A.; Andreau K. (2010) Polycyclic aromatic hydrocarbon components contribute to the mitochondria-antiapoptotic effect of fine particulate matter on human bronchial epithelial cells via the aryl hydrocarbon receptor. Part. Fibre Toxicol. 7, 18. 10.1186/1743-8977-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.-H.; Arashiro M.; Clapp P. W.; Cui T.; Sexton K. G.; Vizuete W.; Gold A.; Jaspers I.; Fry R. C.; Surratt J. D. (2017) Gene Expression Profiling in Human Lung Cells Exposed to Isoprene-Derived Secondary Organic Aerosol. Environ. Sci. Technol. 51, 8166–8175. 10.1021/acs.est.7b01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo M.; Xu F.; Shemesh M.; Qiu X.; Barak Y.; Zhu T.; Rudich Y. (2019) Nrf2 protects against diverse PM2.5 components-induced mitochondrial oxidative damage in lung cells. Sci. Total Environ. 669, 303–313. 10.1016/j.scitotenv.2019.01.436. [DOI] [PubMed] [Google Scholar]
- Jiang X.; Xu F.; Qiu X.; Shi X.; Pardo M.; Shang Y.; Wang J.; Rudich Y.; Zhu T. (2019) Hydrophobic Organic Components of Ambient Fine Particulate Matter (PM2.5) Associated with Inflammatory Cellular Response. Environ. Sci. Technol. 53, 10479–10486. 10.1021/acs.est.9b02902. [DOI] [PubMed] [Google Scholar]
- Kamdar O.; Le W.; Zhang J.; Ghio A. J.; Rosen G. D.; Upadhyay D. (2008) Air pollution induces enhanced mitochondrial oxidative stress in cystic fibrosis airway epithelium. FEBS Lett. 582, 3601–3606. 10.1016/j.febslet.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodovici M.; Bigagli E. (2011) Oxidative Stress and Air Pollution Exposure. J. Toxicol. 2011, 487074. 10.1155/2011/487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller P.; Danielsen P. H.; Karottki D. G.; Jantzen K.; Roursgaard M.; Klingberg H.; Jensen D. M.; Christophersen D. V.; Hemmingsen J. G.; Cao Y.; Loft S. (2014) Oxidative stress and inflammation generated DNA damage by exposure to air pollution particles. Mutat. Res., Rev. Mutat. Res. 762, 133–166. 10.1016/j.mrrev.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Aztatzi-Aguilar O.; Valdés-Arzate A.; Debray-García Y.; Calderón-Aranda E.; Uribe-Ramirez M.; Acosta-Saavedra L.; Gonsebatt M.; Maciel-Ruiz J.; Petrosyan P.; Mugica-Alvarez V.; Gutiérrez-Ruiz M.; Gómez-Quiroz L.; Osornio-Vargas A.; Froines J.; Kleinman M.; De Vizcaya-Ruiz A. (2018) Exposure to ambient particulate matter induces oxidative stress in lung and aorta in a size- and time-dependent manner in rats. Toxicology Research and Application 2, 2397847318794859. 10.1177/2397847318794859. [DOI] [Google Scholar]
- Jin X.; Xue B.; Zhou Q.; Su R.; Li Z. (2018) Mitochondrial damage mediated by ROS incurs bronchial epithelial cell apoptosis upon ambient PM2.5 exposure. J. Toxicol. Sci. 43, 101–111. 10.2131/jts.43.101. [DOI] [PubMed] [Google Scholar]
- Xia T.; Kovochich M.; Nel A. E. (2007) Impairment of mitochondrial function by particulate matter (PM) and their toxic components: implications for PM-induced cardiovascular and lung disease. Front. Biosci., Landmark Ed. 12, 1238–1246. 10.2741/2142. [DOI] [PubMed] [Google Scholar]
- Fetterman J. L.; Sammy M. J.; Ballinger S. W. (2017) Mitochondrial toxicity of tobacco smoke and air pollution. Toxicology 391, 18–33. 10.1016/j.tox.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S. M. U.; Luo L.; Namani A.; Wang X. J.; Tang X. (2017) Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta, Mol. Basis Dis. 1863, 585–597. 10.1016/j.bbadis.2016.11.005. [DOI] [PubMed] [Google Scholar]
- Cattani-Cavalieri I.; Valenca S. S.; Lanzetti M.; Carvalho G. M. C.; Zin W. A.; Monte-Alto-Costa A.; Porto L. C.; Romana-Souza B. (2019) Acute Exposure to Diesel-Biodiesel Particulate Matter Promotes Murine Lung Oxidative Stress by Nrf2/HO-1 and Inflammation Through the NF-kB/TNF-α Pathways. Inflammation 42, 526–537. 10.1007/s10753-018-0910-8. [DOI] [PubMed] [Google Scholar]
- Chan J. K.; Charrier J. G.; Kodani S. D.; Vogel C. F.; Kado S. Y.; Anderson D. S.; Anastasio C.; Van Winkle L. S. (2013) Combustion-derived flame generated ultrafine soot generates reactive oxygen species and activates Nrf2 antioxidants differently in neonatal and adult rat lungs. Part. Fibre Toxicol. 10, 34. 10.1186/1743-8977-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H. Y.; Kleeberger S. R. (2014) Noblesse oblige: NRF2 functions in the airways. Am. J. Respir. Cell Mol. Biol. 50, 844–847. 10.1165/rcmb.2014-0116PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q.; Gao Y.; Ci X. (2019) Role of Nrf2 and Its Activators in Respiratory Diseases. Oxid. Med. Cell. Longevity 2019, 7090534. 10.1155/2019/7090534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orona N. S.; Astort F.; Maglione G. A.; Yakisich J. S.; Tasat D. R. (2019) Direct and Indirect Effect of Air Particles Exposure Induce Nrf2-Dependent Cardiomyocyte Cellular Response In Vitro. Cardiovasc. Toxicol. 19, 575. 10.1007/s12012-019-09530-z. [DOI] [PubMed] [Google Scholar]
- Wittkopp S.; Staimer N.; Tjoa T.; Stinchcombe T.; Daher N.; Schauer J. J.; Shafer M. M.; Sioutas C.; Gillen D. L.; Delfino R. J. (2016) Nrf2-related gene expression and exposure to traffic-related air pollution in elderly subjects with cardiovascular disease: An exploratory panel study. J. Exposure Sci. Environ. Epidemiol. 26, 141–149. 10.1038/jes.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo M.; Porat Z.; Rudich A.; Schauer J. J.; Rudich Y. (2016) Repeated exposures to roadside particulate matter extracts suppresses pulmonary defense mechanisms, resulting in lipid and protein oxidative damage. Environ. Pollut. 210, 227–237. 10.1016/j.envpol.2015.12.009. [DOI] [PubMed] [Google Scholar]
- Dietrich C. (2016) Antioxidant Functions of the Aryl Hydrocarbon Receptor. Stem Cells Int. 2016, 7943495. 10.1155/2016/7943495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal A. K. (2004) Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radical Biol. Med. 36, 1199–1207. 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- Kaspar J. W.; Niture S. K.; Jaiswal A. K. (2009) Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radical Biol. Med. 47, 1304–1309. 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q. (2013) Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 53, 401–426. 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motohashi H.; O’Connor T.; Katsuoka F.; Engel J. D.; Yamamoto M. (2002) Integration and diversity of the regulatory network composed of Maf and CNC families of transcription factors. Gene 294, 1–12. 10.1016/S0378-1119(02)00788-6. [DOI] [PubMed] [Google Scholar]
- Rubio V.; Valverde M.; Rojas E. (2010) Effects of atmospheric pollutants on the Nrf2 survival pathway. Environ. Sci. Pollut. Res. 17, 369–382. 10.1007/s11356-009-0140-6. [DOI] [PubMed] [Google Scholar]
- Ovrevik J.; Refsnes M.; Lag M.; Holme J. A.; Schwarze P. E. (2015) Activation of Proinflammatory Responses in Cells of the Airway Mucosa by Particulate Matter: Oxidant- and Non-Oxidant-Mediated Triggering Mechanisms. Biomolecules 5, 1399–1440. 10.3390/biom5031399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates J. T.; Fang T.; Verma V.; Zeng L.; Weber R. J.; Tolbert P. E.; Abrams J. Y.; Sarnat S. E.; Klein M.; Mulholland J. A.; Russell A. G. (2019) Review of Acellular Assays of Ambient Particulate Matter Oxidative Potential: Methods and Relationships with Composition, Sources, and Health Effects. Environ. Sci. Technol. 53, 4003–4019. 10.1021/acs.est.8b03430. [DOI] [PubMed] [Google Scholar]
- Lushchak V. I. (2014) Free radicals, reactive oxygen species, oxidative stress and its classification. Chem.-Biol. Interact. 224, 164–175. 10.1016/j.cbi.2014.10.016. [DOI] [PubMed] [Google Scholar]
- Hallquist M.; Wenger J. C.; Baltensperger U.; Rudich Y.; Simpson D.; Claeys M.; Dommen J.; Donahue N. M.; George C.; Goldstein A. H.; Hamilton J. F.; Herrmann H.; Hoffmann T.; Iinuma Y.; Jang M.; Jenkin M. E.; Jimenez J. L.; Kiendler-Scharr A.; Maenhaut W.; McFiggans G.; Mentel T. F.; Monod A.; Prévôt A. S. H.; Seinfeld J. H.; Surratt J. D.; Szmigielski R.; Wildt J. (2009) The formation, properties and impact of secondary organic aerosol: current and emerging issues. Atmos. Chem. Phys. 9, 5155–5236. 10.5194/acp-9-5155-2009. [DOI] [Google Scholar]
- Monks T. J.; Hanzlik R. P.; Cohen G. M.; Ross D.; Graham D. G. (1992) Quinone chemistry and toxicity. Toxicol. Appl. Pharmacol. 112, 2–16. 10.1016/0041-008X(92)90273-U. [DOI] [PubMed] [Google Scholar]
- Penning T. M.; Burczynski M. E.; Hung C. F.; McCoull K. D.; Palackal N. T.; Tsuruda L. S. (1999) Dihydrodiol dehydrogenases and polycyclic aromatic hydrocarbon activation: generation of reactive and redox active o-quinones. Chem. Res. Toxicol. 12, 1–18. 10.1021/tx980143n. [DOI] [PubMed] [Google Scholar]
- Lovera-Leroux M.; Crobeddu B.; Kassis N.; Petit P. X.; Janel N.; Baeza-Squiban A.; Andreau K. (2015) The iron component of particulate matter is antiapoptotic: A clue to the development of lung cancer after exposure to atmospheric pollutants?. Biochimie 118, 195–206. 10.1016/j.biochi.2015.09.030. [DOI] [PubMed] [Google Scholar]
- Entwistle J. A.; Hursthouse A. S.; Marinho Reis P. A.; Stewart A. G. (2019) Metalliferous Mine Dust: Human Health Impacts and the Potential Determinants of Disease in Mining Communities. Current Pollution Reports 5, 67–83. 10.1007/s40726-019-00108-5. [DOI] [Google Scholar]
- Shuster-Meiseles T.; Shafer M. M.; Heo J.; Pardo M.; Antkiewicz D. S.; Schauer J. J.; Rudich A.; Rudich Y. (2016) ROS-generating/ARE-activating capacity of metals in roadway particulate matter deposited in urban environment. Environ. Res. 146, 252–262. 10.1016/j.envres.2016.01.009. [DOI] [PubMed] [Google Scholar]
- Shafer M. M.; Perkins D. A.; Antkiewicz D. S.; Stone E. A.; Quraishi T. A.; Schauer J. J. (2010) Reactive oxygen species activity and chemical speciation of size-fractionated atmospheric particulate matter from Lahore, Pakistan: an important role for transition metals. J. Environ. Monit. 12, 704–715. 10.1039/B915008K. [DOI] [PubMed] [Google Scholar]
- Pardo M.; Shafer M. M.; Rudich A.; Schauer J. J.; Rudich Y. (2015) Single Exposure to near Roadway Particulate Matter Leads to Confined Inflammatory and Defense Responses: Possible Role of Metals. Environ. Sci. Technol. 49, 8777–8785. 10.1021/acs.est.5b01449. [DOI] [PubMed] [Google Scholar]
- Li N.; Wang M.; Barajas B.; Sioutas C.; Williams M. A.; Nel A. E. (2013) Nrf2 deficiency in dendritic cells enhances the adjuvant effect of ambient ultrafine particles on allergic sensitization. J. Innate Immun. 5, 543–554. 10.1159/000347060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.; Liu H.; Davies K. J.; Sioutas C.; Finch C. E.; Morgan T. E.; Forman H. J. (2012) Nrf2-regulated phase II enzymes are induced by chronic ambient nanoparticle exposure in young mice with age-related impairments. Free Radical Biol. Med. 52, 2038–2046. 10.1016/j.freeradbiomed.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo M.; Kuperman Y.; Levin L.; Rudich A.; Haim Y.; Schauer J. J.; Chen A.; Rudich Y. (2018) Exposure to air pollution interacts with obesogenic nutrition to induce tissue-specific response patterns. Environ. Pollut. 239, 532–543. 10.1016/j.envpol.2018.04.048. [DOI] [PubMed] [Google Scholar]
- Dreher K. L.; Jaskot R. H.; Lehmann J. R.; Richards J. H.; McGee J. K.; Ghio A. J.; Costa D. L. (1997) Soluble transition metals mediate residual oil fly ash induced acute lung injury. J. Toxicol. Environ. Health 50, 285–305. 10.1080/009841097160492. [DOI] [PubMed] [Google Scholar]
- Okubo T.; Hosaka M.; Nakae D. (2015) In vitro effects induced by diesel exhaust at an air-liquid interface in a human lung alveolar carcinoma cell line A549. Exp. Toxicol. Pathol. 67, 383–388. 10.1016/j.etp.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Klein S. G.; Cambier S.; Hennen J.; Legay S.; Serchi T.; Nelissen I.; Chary A.; Moschini E.; Krein A.; Blomeke B.; Gutleb A. C. (2017) Endothelial responses of the alveolar barrier in vitro in a dose-controlled exposure to diesel exhaust particulate matter. Part. Fibre Toxicol. 14, 7. 10.1186/s12989-017-0186-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P.; Bluvshtein N.; Rudich Y.; Nizkorodov S. A.; Laskin J.; Laskin A. (2017) Molecular Chemistry of Atmospheric Brown Carbon Inferred from a Nationwide Biomass Burning Event. Environ. Sci. Technol. 51, 11561–11570. 10.1021/acs.est.7b02276. [DOI] [PubMed] [Google Scholar]
- Pai S. J.; Heald C. L.; Pierce J. R.; Farina S. C.; Marais E. A.; Jimenez J. L.; Campuzano-Jost P.; Nault B. A.; Middlebrook A. M.; Coe H.; Shilling J. E.; Bahreini R.; Dingle J. H.; Vu K. (2020) An evaluation of global organic aerosol schemes using airborne observations. Atmos. Chem. Phys. 20, 2637. 10.5194/acp-20-2637-2020. [DOI] [Google Scholar]
- Shang Y.; Zhou Q.; Wang T.; Jiang Y.; Zhong Y.; Qian G.; Zhu T.; Qiu X.; An J. (2017) Airborne nitro-PAHs induce Nrf2/ARE defense system against oxidative stress and promote inflammatory process by activating PI3K/Akt pathway in A549 cells. Toxicol. In Vitro 44, 66–73. 10.1016/j.tiv.2017.06.017. [DOI] [PubMed] [Google Scholar]
- Chowdhury P. H.; He Q.; Carmieli R.; Li C.; Rudich Y.; Pardo M. (2019) Connecting the Oxidative Potential of Secondary Organic Aerosols with Reactive Oxygen Species in Exposed Lung Cells. Environ. Sci. Technol. 53, 13949. 10.1021/acs.est.9b04449. [DOI] [PubMed] [Google Scholar]
- Lin Y.-H.; Arashiro M.; Martin E.; Chen Y.; Zhang Z.; Sexton K.; Gold A.; Jaspers I.; Fry R.; Surratt J. (2016) Isoprene-Derived Secondary Organic Aerosol Induces the Expression of Oxidative Stress Response Genes in Human Lung Cells. Environ. Sci. Technol. Lett. 3, 250. 10.1021/acs.estlett.6b00151. [DOI] [Google Scholar]
- Arashiro M.; Lin Y.-H.; Zhang Z.; Sexton K. G.; Gold A.; Jaspers I.; Fry R. C.; Surratt J. D. (2018) Effect of secondary organic aerosol from isoprene-derived hydroxyhydroperoxides on the expression of oxidative stress response genes in human bronchial epithelial cells. Environmental Science: Processes & Impacts 20, 332–339. 10.1039/C7EM00439G. [DOI] [PubMed] [Google Scholar]
- Andreau K.; Leroux M.; Bouharrour A. (2012) Health and cellular impacts of air pollutants: from cytoprotection to cytotoxicity. Biochem. Res. Int. 2012, 493894. 10.1155/2012/493894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuertes E.; van der Plaat D. A.; Minelli C. (2020) Antioxidant genes and susceptibility to air pollution for respiratory and cardiovascular health. Free Radical Biol. Med. 10.1016/j.freeradbiomed.2020.01.181. [DOI] [PubMed] [Google Scholar]
- Li Y.; Nie J.; Beyea J.; Rudra C. B.; Browne R. W.; Bonner M. R.; Mu L.; Trevisan M.; Freudenheim J. L. (2013) Exposure to traffic emissions: associations with biomarkers of antioxidant status and oxidative damage. Environ. Res. 121, 31–38. 10.1016/j.envres.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D.; Dinkova-Kostova A. T. (2014) The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 39, 199–218. 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Valavanidis A.; Vlachogianni T.; Fiotakis K.; Loridas S. (2013) Pulmonary oxidative stress, inflammation and cancer: respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int. J. Environ. Res. Public Health 10, 3886–3907. 10.3390/ijerph10093886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq B.; Kluza J.; Antherieu S.; Sotty J.; Alleman L. Y.; Perdrix E.; Loyens A.; Coddeville P.; Lo Guidice J. M.; Marchetti P.; Garçon G. (2018) Air pollution-derived PM2.5 impairs mitochondrial function in healthy and chronic obstructive pulmonary diseased human bronchial epithelial cells. Environ. Pollut. 243, 1434–1449. 10.1016/j.envpol.2018.09.062. [DOI] [PubMed] [Google Scholar]
- Nelin T. D.; Joseph A. M.; Gorr M. W.; Wold L. E. (2012) Direct and indirect effects of particulate matter on the cardiovascular system. Toxicol. Lett. 208, 293–299. 10.1016/j.toxlet.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S.; Brook R. D. (2012) Air pollution and type 2 diabetes: mechanistic insights. Diabetes 61, 3037–3045. 10.2337/db12-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao X.; Patel P.; Puett R.; Rajagopalan S. (2015) Air pollution as a risk factor for type 2 diabetes. Toxicol. Sci. 143, 231–241. 10.1093/toxsci/kfu250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo M.; Xu F.; Qiu X.; Zhu T.; Rudich Y. (2018) Seasonal variations in fine particle composition from Beijing prompt oxidative stress response in mouse lung and liver. Sci. Total Environ. 626, 147–155. 10.1016/j.scitotenv.2018.01.017. [DOI] [PubMed] [Google Scholar]
- Lodish H, Berk A, and Zipursky S. L.. et al. (2000) Overview of Membrane Transport Proteins. In Molecular Cell Biology, W. H. Freeman, New York. [Google Scholar]
- Li N.; Xia T.; Nel A. E. (2008) The role of oxidative stress in ambient particulate matter-induced lung diseases and its implications in the toxicity of engineered nanoparticles. Free Radical Biol. Med. 44, 1689–1699. 10.1016/j.freeradbiomed.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghio A. J. (2004) Biological effects of Utah Valley ambient air particles in humans: a review. J. Aerosol Med. 17, 157–164. 10.1089/0894268041457200. [DOI] [PubMed] [Google Scholar]
- Lawal A. O. (2017) Air particulate matter induced oxidative stress and inflammation in cardiovascular disease and atherosclerosis: The role of Nrf2 and AhR-mediated pathways. Toxicol. Lett. 270, 88–95. 10.1016/j.toxlet.2017.01.017. [DOI] [PubMed] [Google Scholar]
- Upadhyay D.; Panduri V.; Ghio A.; Kamp D. W. (2003) Particulate matter induces alveolar epithelial cell DNA damage and apoptosis: role of free radicals and the mitochondria. Am. J. Respir. Cell Mol. Biol. 29, 180–187. 10.1165/rcmb.2002-0269OC. [DOI] [PubMed] [Google Scholar]
- Stevens E. A.; Mezrich J. D.; Bradfield C. A. (2009) The aryl hydrocarbon receptor: a perspective on potential roles in the immune system. Immunology 127, 299–311. 10.1111/j.1365-2567.2009.03054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsay J. J.; Tchou-Wong K.-M.; Greenberg A. K.; Pass H.; Rom W. N. (2013) Aryl Hydrocarbon Receptor and Lung Cancer. Anticancer Res. 33, 1247–1256. [PMC free article] [PubMed] [Google Scholar]
- O’Driscoll C. A.; Gallo M. E.; Hoffmann E. J.; Fechner J. H.; Schauer J. J.; Bradfield C. A.; Mezrich J. D. (2018) Polycyclic aromatic hydrocarbons (PAHs) present in ambient urban dust drive proinflammatory T cell and dendritic cell responses via the aryl hydrocarbon receptor (AHR) in vitro. PLoS One 13, e0209690–e0209690. 10.1371/journal.pone.0209690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neavin D. R.; Liu D.; Ray B.; Weinshilboum R. M. (2018) The Role of the Aryl Hydrocarbon Receptor (AHR) in Immune and Inflammatory Diseases. Int. J. Mol. Sci. 19, 3851. 10.3390/ijms19123851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon A. C.; Hebert V. Y.; Cormier S. A.; Subramanian B.; Reed J. R.; Backes W. L.; Dugas T. R. (2018) Particulate matter containing environmentally persistent free radicals induces AhR-dependent cytokine and reactive oxygen species production in human bronchial epithelial cells. PLoS One 13, e0205412. 10.1371/journal.pone.0205412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao W.; Hu L.; Scrivens P. J.; Batist G. (2005) Transcriptional regulation of NF-E2 p45-related factor (NRF2) expression by the aryl hydrocarbon receptor-xenobiotic response element signaling pathway: direct cross-talk between phase I and II drug-metabolizing enzymes. J. Biol. Chem. 280, 20340–20348. 10.1074/jbc.M412081200. [DOI] [PubMed] [Google Scholar]
- Vondráček J.; Krčmář P.; Procházková J.; Trilecová L.; Gavelová M.; Skálová L.; Szotáková B.; Bunček M.; Radilová H.; Kozubík A.; Machala M. (2009) The role of aryl hydrocarbon receptor in regulation of enzymes involved in metabolic activation of polycyclic aromatic hydrocarbons in a model of rat liver progenitor cells. Chem.-Biol. Interact. 180, 226–237. 10.1016/j.cbi.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Yang L.; Liu G.; Lin Z.; Wang Y.; He H.; Liu T.; Kamp D. W. (2016) Pro-inflammatory response and oxidative stress induced by specific components in ambient particulate matter in human bronchial epithelial cells. Environ. Toxicol. 31, 923–936. 10.1002/tox.22102. [DOI] [PubMed] [Google Scholar]
- Lichtveld K. M.; Ebersviller S. M.; Sexton K. G.; Vizuete W.; Jaspers I.; Jeffries H. E. (2012) In vitro exposures in diesel exhaust atmospheres: resuspension of PM from filters versus direct deposition of PM from air. Environ. Sci. Technol. 46, 9062–9070. 10.1021/es301431s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersviller S.; Lichtveld K.; Sexton K. G.; Zavala J.; Lin Y. H.; Jaspers I.; Jeffries H. E. (2012) Gaseous VOCs rapidly modify particulate matter and its biological effects - Part 1: Simple VOCs and model PM. Atmos. Chem. Phys. Discuss. 12, 5065–5105. 10.5194/acpd-12-5065-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F.; Qiu X.; Hu X.; Shang Y.; Pardo M.; Fang Y.; Wang J.; Rudich Y.; Zhu T. (2018) Effects on IL-1beta signaling activation induced by water and organic extracts of fine particulate matter (PM2.5) in vitro. Environ. Pollut. 237, 592–600. 10.1016/j.envpol.2018.02.086. [DOI] [PubMed] [Google Scholar]
- Li N.; Nel A. E. (2006) Role of the Nrf2-Mediated Signaling Pathway as a Negative Regulator of Inflammation: Implications for the Impact of Particulate Pollutants on Asthma. Antioxid. Redox Signaling 8, 88–98. 10.1089/ars.2006.8.88. [DOI] [PubMed] [Google Scholar]
- Li N.; Sioutas C.; Cho A.; Schmitz D.; Misra C.; Sempf J.; Wang M.; Oberley T.; Froines J.; Nel A. (2003) Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ. Health Perspect. 111, 455–460. 10.1289/ehp.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait S. W.; Green D. R. (2012) Mitochondria and cell signalling. J. Cell Sci. 125, 807–815. 10.1242/jcs.099234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. P. T. (2017) From Physiological Redox Signalling to Oxidant Stress. Adv. Exp. Med. Biol. 967, 335–342. 10.1007/978-3-319-63245-2_21. [DOI] [PubMed] [Google Scholar]
- Zheng L.; Liu S.; Zhuang G.; Xu J.; Liu Q.; Zhang X.; Deng C.; Guo Z.; Zhao W.; Liu T.; Wang Y.; Zhang Y.; Lin J.; Wang Q.; Sui G. (2017) Signal Transductions of BEAS-2B Cells in Response to Carcinogenic PM2.5 Exposure Based on a Microfluidic System. Anal. Chem. 89, 5413–5421. 10.1021/acs.analchem.7b00218. [DOI] [PubMed] [Google Scholar]
- Visalli G.; Baluce B.; Bertuccio M.; Picerno I.; Di Pietro A. (2015) Mitochondrial-mediated apoptosis pathway in alveolar epithelial cells exposed to the metals in combustion-generated particulate matter. J. Toxicol. Environ. Health, Part A 78, 697–709. 10.1080/15287394.2015.1024081. [DOI] [PubMed] [Google Scholar]
- Gualtieri M.; Mantecca P.; Corvaja V.; Longhin E.; Perrone M. G.; Bolzacchini E.; Camatini M. (2009) Winter fine particulate matter from Milan induces morphological and functional alterations in human pulmonary epithelial cells (A549). Toxicol. Lett. 188, 52–62. 10.1016/j.toxlet.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Pieters N.; Koppen G.; Smeets K.; Napierska D.; Plusquin M.; De Prins S.; Van De Weghe H.; Nelen V.; Cox B.; Cuypers A.; Hoet P.; Schoeters G.; Nawrot T. S. (2013) Decreased Mitochondrial DNA Content in Association with Exposure to Polycyclic Aromatic Hydrocarbons in House Dust during Wintertime: From a Population Enquiry to Cell Culture. PLoS One 8, e63208. 10.1371/journal.pone.0063208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L.; Yao X.; Shen Y. (2016) Altered mitochondrial DNA copy number contributes to human cancer risk: evidence from an updated meta-analysis. Sci. Rep. 6, 35859. 10.1038/srep35859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavanello S.; Dioni L.; Hoxha M.; Fedeli U.; Mielzynska-Svach D.; Baccarelli A. A. (2013) Mitochondrial DNA copy number and exposure to polycyclic aromatic hydrocarbons. Cancer Epidemiol., Biomarkers Prev. 22, 1722–1729. 10.1158/1055-9965.EPI-13-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]