Abstract
Aims/hypothesis
Insufficient sleep is increasingly recognised as a major risk factor for the development of obesity and diabetes, and short-term sleep loss in clinical studies leads to a reduction in insulin sensitivity. Sleep loss-induced metabolic impairments are clinically relevant, since reductions in insulin sensitivity after sleep loss are comparable to insulin sensitivity differences between healthy individuals and those with impaired glucose tolerance. However, the relative effects of sleep loss vs high-fat feeding in the same individual have not been assessed. In addition, to our knowledge no diurnal (active during the daytime) non-human mammalian model of sleep loss-induced metabolic impairment exists, which limits our ability to study links between sleep and metabolism.
Methods
This study examined the effects of one night of total sleep deprivation on insulin sensitivity and beta cell function, as assessed by an IVGTT, before and after 9 months of high-fat feeding in a canine model.
Results
One night of total sleep deprivation in lean dogs impaired insulin sensitivity to a similar degree as a chronic high-fat diet (HFD)(normal sleep: 4.95 ± 0.45 mU−1 l−1 min−1; sleep deprivation: 3.14 ± 0.21 mU−1 l−1 min−1; HFD: 3.74 ± 0.48 mU−1 l−1 min−1; mean ± SEM). Hyperinsulinaemic compensation was induced by the chronic HFD, suggesting adequate beta cell response to high-fat feeding. In contrast, there was no beta cell compensation after one night of sleep deprivation, suggesting that there was metabolic dysregulation with acute sleep loss that, if sustained during chronic sleep loss, could contribute to the risk of type 2 diabetes. After chronic high-fat feeding, acute total sleep deprivation did not cause further impairments in insulin sensitivity (sleep deprivation + chronic HFD: 3.28 mU−1 l−1 min−1).
Conclusions/interpretation
Our findings provide further evidence that sleep is important for metabolic health and establish a diurnal animal model of metabolic disruption during insufficient sleep.
Keywords: Canine, Diabetes, Diet, Dog, Fat, Glucose, Insulin, Obesity, Sleep
Introduction
Rates of obesity in the USA have doubled over the last 30 years, as has the percentage of people with diagnosed type 2 diabetes, and both conditions are rapidly increasing worldwide [1]. Although changes in diet and energy expenditure have played an important role, sleep and circadian disruption have emerged as novel risk factors for the development of metabolic diseases and are often unavoidable in modern, 24 h society [2].
The physiological mechanisms by which insufficient sleep increases the risk for type 2 diabetes and obesity are largely unknown [3]. Findings from experimental studies in human participants demonstrate that insufficient sleep impairs insulin sensitivity [4–27], a primary pathophysiological mechanism underlying type 2 diabetes [28]. The degree of insulin sensitivity impairment induced by insufficient sleep in healthy individuals is comparable to the magnitude of insulin resistance in people with obesity and type 2 diabetes compared with lean controls [28, 29]. Yet, the relative effects of insufficient sleep vs diet-induced insulin resistance, and their combined effects on glucose metabolism in the same individual are unknown. Understanding relative effects of these factors may provide insight into how sleep loss may contribute to metabolic disease progression.
The canine may be a suitable model in which to examine and compare the relative effects of sleep deprivation vs a high-fat diet (HFD). This model has been used extensively to study the impact of high-fat feeding on insulin sensitivity and beta cell compensation [30–34]. Furthermore, the canine is a diurnal mammal, rather than nocturnal, with sleep–wake patterns and electroencephalography (measured by EEG) similar to humans [35, 36].
Therefore, the aim of the present exploratory study was to first evaluate the influence of acute total sleep deprivation on insulin sensitivity and then investigate in a subset of animals the relative effects of one night of sleep deprivation vs a 9 month HFD on glucose metabolism in the same animal. Insulin sensitivity (SI), acute insulin response to glucose (AIRG), and disposition index (DI) were assessed using frequently sampled IVGTTs and were compared between four conditions: normal sleep on a chow diet (NS-chow), sleep deprivation on a chow diet (SD-chow), normal sleep after an HFD (NS-HFD) and sleep deprivation after an HFD (SDHFD).
Methods
Research design
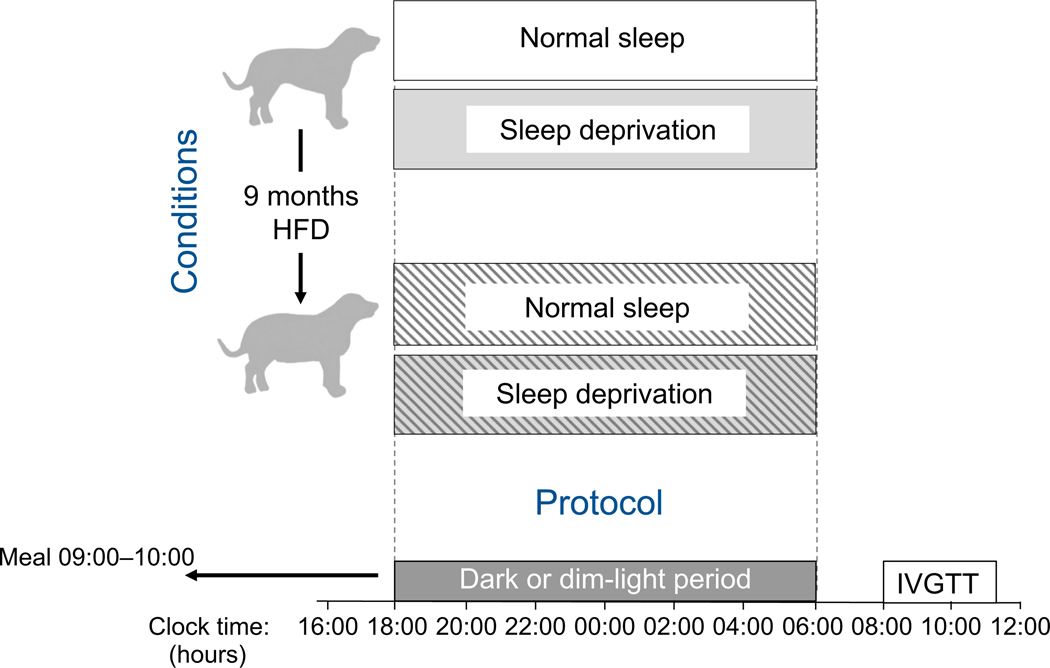
We compared the impact on metabolic outcomes of normal sleep vs sleep deprivation, assigned in randomised order, with effects of a chronic HFD in dogs. Assessments of the effects of one night of total sleep deprivation vs normal sleep were performed during chow feeding, as well as after approximately 9 months on an HFD. See Fig. 1 for a schematic representation of the experimental conditions and protocol. The study was approved by the Cedars-Sinai Medical Center Institutional Animal Care and Use Committee. The effects of high-fat feeding on insulin sensitivity, cardiac function and insulin access to skeletal muscle in some of these animals have been reported previously [30, 33].
Fig. 1.
Schematic representation of experimental conditions and experimental protocol. IVGTTs were conducted following one night of normal sleep (dark period) and one night of total sleep deprivation (dim-light period), assigned in random order in24animals and separated by18 ± 3 days (mean ± SEM). A subset of eight animals then went on to receive an HFD for approximately 9 months, after which IVGTTs were conducted following one night of normal sleep and one night of total sleep deprivation, assigned in random order
Experimental animals
Adult (>1 year of age), male, mixed-breed, normal weight dogs were housed in the Cedars-Sinai Medical Center Vivarium (Los Angeles, CA, USA) under controlled kennel conditions, on a 12:12 h light–dark cycle (lights off from 18:00 to 06:00 hours). The baseline chow diet was provided daily between 09:00 and 10:00 hours and consisted of a combination of moist and dry chow, providing 14,970 kJ/day (3578 kcal), with a breakdown of 39.2% carbohydrate, 32.5% fat and 28.3% protein. Dogs were accepted into the study only if weight was stable and they were judged to be in good health as determined by visual inspection, body temperature and haematocrit.
Interventions
To assess the effects of total sleep deprivation after baseline chow diet and after a chronic HFD, 24 dogs were studied under two sleep conditions—normal sleep and sleep deprivation. Normal sleep involved one night of undisturbed sleep (no researcher present, as this would have aroused dogs and disturbed sleep), whereas sleep deprivation involved constant human attention (petting by and interaction with researchers) for 24 h (06:00–06:00 hours) to prevent sleep. Lights were kept on between 06:00 and 18:00 and were dimmed (<5 lux, during sleep deprivation) or off (during normal sleep) from 18:00 to 06:00. The sleep conditions were separated by at least 4 days (mean >2 weeks) to allow for sufficient recovery sleep and were assigned in random order.
At least 2 weeks following the final experiment on baseline chow diet, a subset of eight animals began an HFD for 9 months (mean 37 weeks [range 31–42 weeks]), consisting of the baseline chow diet supplemented with 160 g of rendered pork fat (lard) to achieve a 52% fat diet providing 21,025 kJ/ day (5025 kcal). Animal allocation to HFD was predetermined based on availability and ongoing projects, thus we did not have control over the selection of the subset that went on to HFD. As mentioned above, effects of high-fat feeding on insulin sensitivity, cardiac function and insulin access to skeletal muscle in these animals have been reported previously [30, 33].
For clinical comparisons, the typical American or European diet contains approximately 36–40% fat, therefore an HFD for humans might contain 50–60% of energy as fat [37]. Thus, a baseline chow diet of approximately 33% fat and a diet comprised of 52% fat are comparable to normal diets and HFDs, respectively, typically consumed by humans.
The baseline chow diet was given on all experimental days, including during HFD conditions, to rule out any acute dietary effects on our outcome measures. All food given on experimental days was therefore matched for energy levels and macronutrient content across conditions.
Outcome measures
Minimal model analyses of frequently sampled IVGTTs were performed to assess whole body SI, AIRG, DI, glucose effectiveness (SG) and glucose tolerance (KG). IVGTTs were performed at 08:00 hours immediately following an overnight fasting period. Animals were mildly restrained in a Pavlov sling during the procedure. An intracatheter was inserted into the saphenous or cephalic vein. Blood samples were drawn every 5 min for 15 min (3 basal samples), after which (time = 0 min) glucose was given as an intravenous bolus (0.3 g/kg body weight). Intravenous insulin (0.02 U/kg body weight) was administered at time = 20 min. Blood samples were collected at 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 19, 22, 23, 24, 25, 30, 40, 50, 60, 70, 90, 100, 120, 140, 160 and 180 min, and glucose and insulin concentrations determined. SI was calculated using the minimal model of glucose kinetics using MINMOD Millennium software (MINMOD 6.02, MinMod, Los Angeles, CA, USA). AIRG was calculated as the AUC of the insulin concentrations above the mean of the basal values from 0 to 10 min. DI was calculated as the product of SI and AIRG. KG was calculated as the negative slope of the natural logarithm of the concentration of glucose from 5 to 19 min. Adipose tissue insulin sensitivity (AdipoIR) was calculated as the product of fasting NEFA and insulin concentrations in plasma [38].
Measures of NEFA, cortisol, noradrenaline (norepinephrine) and adrenaline (epinephrine) were derived from fasting blood samples taken immediately prior to each IVGTT on the mornings immediately following one night of normal sleep or total sleep deprivation. These blood samples were collected every 15 min from 06:30 hours until the start of the IVGTT (6 samples in total; concentrations are mean values).
Blood samples for the analysis of glucose, insulin, cortisol, adrenaline and noradrenaline were collected in tubes that were pre-coated with lithium heparin (Becton Dickinson, Franklin Lakes, NJ, USA), and contained 50 μl EDTA (Sigma Chemicals, St Louis, MO, USA). Tubes for the analysis of NEFA contained only EDTA. Tubes were kept on ice and centrifuged. Plasma was then separated, transferred to storage tubes and placed on ice or at −20°C for the remainder of the experiment. Upon completion of the experiment, samples were transferred to −80°C freezers for storage until further analysis. Glucose was measured using a YSI 2300 or 2700 auto-analyser (Yellow Springs Instruments, Yellow Springs, OH, USA). Insulin was measured with an ELISA originally developed for human serum or plasma (Linco Research, St. Charles, MO, USA) and adapted for dog plasma [39]. Plasma NEFA were measured using the NEFA C kit (Wako Pure Chemical Industries, Richmond, VA, USA). Cortisol was measured using an RIA (Diagnostic Products, Los Angeles, CA, USA). Plasma adrenaline and noradrenaline were measured with an ELISA (Rocky Mountain Diagnostics, Colorado Springs, CO, USA).
Body weight, as well as the size of visceral and subcutaneous fat deposits in the abdominal region, were assessed before and after the HFD. Size of fat depots were assessed under general anaesthesia by MRI imaging using a 3.0-T Siemens Scanner (MAGNETOM Verio, Siemens Healthcare, Erlangen, Germany). Imaging software (SliceOmatic 4.3, Tomovision, Magog, QC, Canada) was used to distinguish fat and non-fat tissues based on pixel intensity from 11 transverse 10 mm thick slices. Physical activity on all experimental days was measured using a collar-bound accelerometer (Actical, Respironics, Murrysville, PA, USA). Food was weighed each day before and after animals were fed to determine exact food intake.
Sample size
On the basis of human studies on the effects of insufficient sleep on insulinsensitivity and previous studies on the effects of a chronic high-fat (lard) diet on insulin sensitivity in dogs, large effect sizes were expected for the effects of these conditions on insulin sensitivity. During the planning of this study, no information was available on the expected differences in insulin sensitivity in response to sleep deprivation vs a chronic HFD, nor on the effect of sleep deprivation after a chronic HFD. Twenty-four dogs took part in the baseline sleep study, which yielded 80% power for detecting medium effect sizes (Cohen’s d of at least 0.60) regarding the effects of total sleep deprivation under baseline conditions (NS-chow vs SD-chow). Eight of these dogs completed an approximate 9 month HFD, which yielded 80% power for detecting a large effect size (partial η2 of at least 0.81) regarding differences among the four conditions (NS-chow vs SD-chow vs NS-HFD vs SD-HFD), assuming no correlation (Pearson’s ρ of 0.0) between repeated measures, as well as large effect sizes (Cohen’s d of at least 1.16) for post hoc comparisons.
Statistical analyses
Differences between baseline and total sleep deprivation conditions before HFD are expressed in mean absolute difference, with SEM, and effect size (Cohen’s d) when relevant. Paired samples t tests were used to compare means between the two sleep conditions (NS-chow vs SD-chow; n = 24; results presented in Table 1). Repeated measures ANOVA with post hoc t tests was used to compare means between the four conditions (NS-chow vs SD-chow vs NS-HFD vs SD-HFD; n = 8; results presented in Table 2). Significance was set at a p value of 0.05. Statistics were performed using SPSS IBM Statistics 22 (Armonk, NY, USA).
Table 1.
Effects of normal sleep vs sleep deprivation
| Measure | Normal sleep, mean (SEM) | Sleep deprivation, mean (SEM) | Mean difference (SEM) | Test statistics |
|---|---|---|---|---|
| Fasting glucose (mmol/l) | 5.34 (0.07) | 5.32 (0.07) | 0.03 (0.07) | t 0.373, p = 0.712 |
| Fasting insulin (pmol/l) | 52.78 (6.25) | 56.25 (5.56) | −3.24 (4.74) | t 0.684, p = 0.501 |
| Fasting, NEFA (mmol/l); n = 8 | 0.53 (0.04) | 0.55 (0.06) | −0.01 (0.06) | t 0.210, p = 0.840 |
| KG (%/min) | 3.7 (0.2) | 3.2 (0.2) | 0.5 (0.2) | t 1.942, p = 0.065 |
| Beta cell response, AIRG (mU/l × min) | 509.7 (32.0) | 504.5 (31.1) | 5.2 (18.4) | t 0.284, p = 0.779 |
| SI (mU−1 min−1) | 6.50 (0.62) | 4.95 (0.51) | 1.55 (0.48) | t 3.243, p = 0.004** |
| DI | 3195 (312) | 2421 (283) | 774 (254) | t 3.049, p = 0.006** |
| SG (%/min) | 0.041 (0.002) | 0.039 (0.002) | 0.002 (0.002) | t 0.926, p = 0.364 |
| Adipo-IR (mmol/l × pmol/l); n = 8 | 31.11 (7.78) | 36.81 (5.76) | −5.72 (8.13) | t − 0.704, p = 0.504 |
| Fasting cortisol (pmol/l); n = 12 | 5.13 (0.68) | 4.00 (0.82) | −1.13 (3.10) | t 1.261, p = 0.233 |
| Fasting adrenaline (pmol/l); n = 12 | 365.8 (67.2) | 322.1 (59.0) | −43.7 (204.2) | t 0.743, p = 0.473 |
| Fasting noradrenaline (pmol/l); n = 12 | 931.0 (251.2) | 834.0 (253.6) | −96.9 (334.0) | t 1.005, p = 0.336 |
| 24 h physical activity (mean counts per h); n = 7 | 273 (52) | 261 (49) | −12 (38) | t 0.326, p = 0.756 |
| Daytime physical activity (mean counts per h); n = 7 | 491 (96) | 481 (102) | −10 (75) | t 0.138, p = 0.895 |
| Night-time physical activity (mean counts per h); n = 7 | 55 (12) | 40 (11) | −15 (22) | t 0.704, p = 0.508 |
Data from all animals after normal sleep and sleep deprivation, after following a chow diet (NS-chow and SD-chow)
n = 24 unless otherwise noted; due to IV issues leading to reduced plasma available for assays, fasting NEFA and Adipo-IR were measured in n = 8 animals, and fasting cortisol, adrenaline and noradrenaline were measured in n = 12 animals
To convert SI values to SI units, multiply by 0.167
Paired samples t test statistic reported with p value;
p < 0.01
Table 2.
Effects of sleep deprivation before and after chronic high-fat feeding
| Measure | Condition, mean (SEM) |
Test statisticsa | Comparisons between conditions: mean difference (SEM), p valueb |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Post chow diet |
Post HFD |
||||||||||
| NS-chow 1 | SD-chow 2 | NS-HFD 3 | SD-HFD 4 | 1 vs 2 | 1 vs 3 | 1 vs 4 | 2 vs 3 | 2 vs 4 | 3 vs 4 | ||
| Fasting glucose (mmol/1) | 5.26 (0.11) | 5.30 (0.13) | 5.23 (0.11) | 5.27 (0.11) | F 0.131, p = 0.940 | 0.04 (0.16), p = 0.824 | −0.03 (0.14), p = 0.817 | 0.01 (0.09), p = 0.891 | −0.07 (0.10), p = 0.506 | −0.02 (0.08), p = 0.772 | 0.04 (0.09), p = 0.640 |
| Fasting insulin (pmol/1) | 56.95(10.42) | 72.23 (12.50) | 88.20(15.28) | 94.66(13.89) | F 6.773, p = 0.002** | 15.21 (6.81), p = 0.062 | 31.46(11.39), p = 0.028* | 37.92 (9.24), p = 0.004** | 16.25 (9.38), p = 0.127 | 22.78 (9.51), p = 0.048* | 6.46(8.61), p = 0.476 |
| Fasting NEFA (mmol/1); p = 6 | 0.52 (0.05) | 0.52 (0.07) | 0.50 (0.03) | 0.47 (0.02) | F 0.282, p = 0.838 | −0.00 (0.08), p = 0.984 | −0.02 (0.05), p = 0.727 | −0.05 (0.07), p = 0.469 | −0.02 (0.05), p= 0.715 | −0.05 (0.09), p = 0.572 | −0.03 (0.05), p = 0.542 |
| KG (%/min) | 2.93 (0.23) | 2.90 (0.18) | 2.89 (0.25) | 2.72 (0.18) | F 0.413, p = 0.745 | −0.03 (0.12), p = 0.823 | −0.04 (0.19), p = 0.824 | −0.21 (0.21), p = 0.353 | −0.02 (0.16), p = 0.929 | −0.18 (0.23), p = 0.458 | −0.16(0.28), p = 0.577 |
| AIRG (mU/1 / min) | 494.9 (70.3) | 509.3 (48.3) | 623.0 (73.0) | 612.1 (49.3) | F 8.239, p = 0.001** | 14.4 (28.4), p = 0.629 | 128.1 (38.2), p = 0.012* | 117.2 (33.7), p = 0.020* | 113.8 (40.0), p = 0.025* | 102.9 (13.3), p<0.001*** | −10.9 (37.0), p = 0.777 |
| SI(nit.” r1 rnin−1) | 4.95 (0.45) | 3.14 (0.21) | 3.74 (0.48) | 3.28 (0.37) | F 8.109, p = 0.001** | −1.81 (0.41), p= 0.001** | −1.21 (0.47), p = 0.038* | −1.67 (0.28), p = 0.001** | 0.60 (0.52), p = 0.290 | 0.14 (0.42), p = 0.751 | −0.46 (0.29), p = 0.154 |
| DI | 2291 (240) | 1570 (141) | 2251 (295) | 1939(162) | F 4.594, p = 0.013* | −721 (200), p = 0.009** | −40 (222), p = 0.863 | −351 (210), p = 0.138 | 681 (219), p = 0.017* | 370 (200), p = 0.107 | −312 (267), p = 0.281 |
| SG (%/rnin) | 0.036 (0.002) | 0.035 (0.003) | 0.032 (0.001) | 0.035 (0.003) | F 0.714, p = 0.554 | −0.001 (0.002), p = 0.733 | −0.004 (0.002), p = 0.107 | −0.001 (0.002), p = 0.815 | −0.003 (0.002), p = 0.179 | −0.000 (0.004), p = 1.000 | 0.003 (0.002), p = 0.274 |
| Adipo-IR (mmol/1 / pmol/1); n = 6 | 32.29 (10.56) | 32.29 (3.26) | 42.99 (9.17) | 45.84 (8.75) | F 1.754, p = 0.199 | −0.07 (7.99), p = 0.995 | 10.70(10.14), p = 0.341 | 13.47 (6.88), p = 0.108 | 10.76 (7.57),p = 0.214 | 13.54 (6.81), p = 0.103 | 2.85 (5.07), p = 0.603 |
| Fasting cortisol (pmol/1); n = l | 4.75(1.01) | 3.95(1.21) | 4.58(1.03) | 5.39 (0.81) | F 0.925, p = 0.449 | −0.80 (1.02), p = 0.465 | −0.17(0.41), p = 0.686 | 0.64 (0.69), p = 0.387 | 0.62 (0.82), p = 0.474 | 1.44(1.33), p = 0.318 | 0.82 (0.66), p = 0.265 |
| Fasting adrenaline (pmol/1); n = l | 280.1 (90.1) | 246.8 (54.6) | 579.2 (111.4) | 636.5 (157.8) | F 25.932, p = 0.002** | −33.3 (91.7), p = 0.731 | 299.2(125.0), p = 0.054 | 355.9 (107.0), p = 0.016* | 332.5 (97.7), p = 0.014* | 389.2 (146.3), p = 0.037* | 56.8 (99.9), p = 0.592 |
| Fasting noradrenaline (pmol/1); n = l | 494.8 (232.3) | 430.3 (201.0) | 1233.6 (220.5) | 871.9 (218.7) | F 16.084, p = 0.007** | −64.4 (79.8), p = 0.449 | 738.3 (224.6), p = 0.017 | 377.1 (139.5), p = 0.036 | 803.3 (179.1), p = 0.004 | 441.6 (167.3), p = 0.038 | −361.8 (238.2), p= 0.180 |
| 24 h physical activity (mean counts per h); n = 4 | 219 (52) | 239 (76) | 77 (20) | 64 (6) | F 5.053, p = 0.025* | 20 (40), p = 0.655 | −143 (54), p = 0.078 | −155 (50), p = 0.054 | −163 (83), p = 0.145 | −175 (76), p = 0.104 | −13 (23), p = 0.618 |
| Daytime physical activity (mean counts per h); n = 4 | 394 (93) | 437 (163) | 135 (40) | 89 (7) | F 4.278, p = 0.039* | 42 (106), p = 0.717 | −259 (97), p = 0.076 | −305 (96), p = 0.050 | −302 (174), p = 0.182 | −348(161), p = 0.120 | −46 (43), p = 0.363 |
| Night-time physical activity (mean counts per h); n = 4 | 43 (16) | 41 (15) | 18(1) | 41(17) | F 0.662, p = 0.596 | −3 (30), p = 0.938 | −25 (16), p = 0.212 | −3 (27), p = 0.931 | −23 (15), p = 0.220 | 0 (13), p = 1.000 | 23 (16), p = 0.264 |
Data from the subset of eight animals that were fed an HFD for 9 months
n = 8 unless otherwise noted; due to technical issues with accelerometers, activity data for all conditions are available from n = 4 animals; due to IV issues leading to reduced plasma available for assays, fasting NEFA and Adipo-IR were measured in n = 6 animals, and fasting cortisol, adrenaline and noradrenaline were measured in n = 7 animals
To convert Si values to SI units, multiply by 0.167
Repeated measures ANOVA test statistic (F) reported with p value
p values calculated by post hoc t test
p<0.05,
p<0.01,
p;<0.001
NS, normal sleep; SD, sleep deprivation
Results
One night of experimental sleep deprivation impairs insulin sensitivity without beta cell compensation
Total sleep deprivation significantly reduced SI by 21 ± 6%; (p = 0.004; data are mean ± SEM; Table 1). AIRG was not elevated in response to sleep deprivation, reflected by a significant reduction in DI (p = 0.006; Table 1). Furthermore, KG was decreased in response to sleep deprivation though this was not statistically significant (p = 0.065; Table 1).
In the subsample of animals that went on to high-fat feeding (n = 8 out of 24), sleep deprivation following baseline chow feeding impaired SI by 33 ± 6% (p = 0.001, Table 2) although fasting insulin concentrations were not different (p = 0.062; Table 2).
No differences were detected between sleep conditions for fasting glucose and NEFA concentrations, SG or Adipo-IR (Tables 1 and 2).
Chronic high-fat feeding induces weight gain, insulin resistance and beta cell compensation
Nine months on an HFD resulted in significant weight gain (4.2 ± 1.0 kg; t 4.084, p = 0.005; mean baseline weight: 31.6 kg), which included a significant increase in visceral fat volume (6.3 ± 2.2 cm3; p < 0.023; mean baseline volume: 11.3 cm3), but not subcutaneous fat volume (2.3 ± 1.0 cm3; p = 0.069; mean baseline weight: 10.9 cm3).
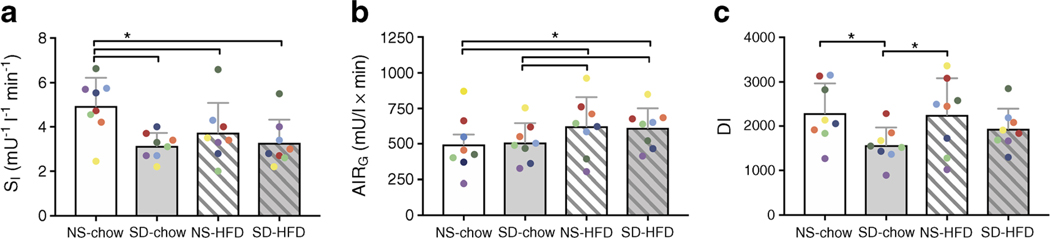
Chronic high-fat feeding resulted in a 21 ± 11% decrease in SI (p = 0.038) with a concomitant increase in AIRG (p = 0.012; Table 2; Fig. 2). As a result, DI remained unchanged (Table 2; Fig. 2). The chronic HFD resulted in significantly higher fasting insulin concentrations (p = 0.028; Table 2). In contrast, no differences were detected in fasting glucose or NEFA concentrations, nor KG, SG, or Adipo-IR (Table 2).
Fig. 2.
Effects of one night of sleep deprivation vs a chronic HFD. IVGTTs were used to assess insulin sensitivity (as SI) (a), beta cell response (as AIRG) (b) and DI (c) in eight animals following one night of normal sleep and one night of total sleep deprivation, assigned in random order, before and after approximately 9 months on an HFD. Data are represented as mean + SEM with individual animals represented by coloured data points. To convert SI values to SI units, multiply by 0.167 *p < 0.05
Impact of sleep deprivation vs chronic high-fat feeding
Reductions in SI were not different when comparing one night of total sleep deprivation and 9 months of chronic high-fat feeding (Table 2; Fig. 2). No differences were detected between conditions for fasting glucose, insulin, NEFA concentrations, nor KG, SG or Adipo-IR (Table 2).
Impact of sleep deprivation in conjunction with a chronic HFD
Total sleep deprivation following 9 months of HFD significantly reduced SI by 32 ± 5% compared with baseline chow and habitual sleep (p = 0.001). However, the combination did not result in a further decrease in SI (p = 0.154; Cohen’s d effect size 0.57), nor any changes to AIRG or DI (Table 2; Fig. 2) when compared with HFD and habitual sleep. Similarly, no differences were detected between these conditions for fasting glucose, insulin, and NEFA concentrations, nor KG, SG or Adipo-IR (Table 2).
Cortisol, adrenaline and noradrenaline
Fasting cortisol concentrations did not differ between any study conditions (Tables 1 and 2). Catecholamines also did not differ between normal sleep and sleep deprivation during baseline chow feeding but were elevated following the chronic HFD (adrenaline p = 0.054, noradrenaline p = 0.017; Tables 1 and 2).
Physical activity and food intake
Physical activity data from accelerometry are shown in Tables 1 and 2. Mean daytime activity counts (06:00–18:00 hours) were higher than night-time activity counts (18:00–06:00 hours) in all conditions. There was a significant effect of diet on 24 h and daytime physical activity (Table 2). No effects of sleep deprivation on physical activity were observed. Food given to animals was identical on all experimental days and actual food intake was not different between study conditions (data not shown).
Discussion
We investigated the effects of one night of total sleep deprivation on metabolism in canines and compared the influence of sleep deprivation and a chronic HFD in a subset of the same individual animals. We found that one night of total sleep deprivation in lean dogs impaired insulin sensitivity to a similar degree as 9 months of chronic high-fat feeding. The DI— an index of beta cell function relative to insulin sensitivity, and an important predictor of type 2 diabetes—was maintained during chronic high-fat feeding, reflecting normal beta cell compensatory mechanisms to insulin resistance. In contrast, sleep deprivation led to a reduction in DI, suggesting that beta cells did not compensate for the reduction in insulin sensitivity. Finally, sleep deprivation following the induction of diet induced insulin resistance did not cause any further impairments to insulin sensitivity or DI.
Our findings are consistent with results from previous studies of insufficient sleep in human participants [4–26] and chronic HFD studies in dogs [30–34], which report impaired insulin sensitivity during both conditions. Consistent with findings from human studies in which food intake was controlled, insufficient sleep in the canine was also accompanied by a reduction in DI due to a lack of compensatory beta cell response [4, 6, 13, 24, 40].
We previously reported that 2–6 weeks of HFD (using the same HFD as in the present study) is required to elicit hyper insulinaemic compensation [32, 41]. Furthermore, results from a clinical study of insufficient sleep in which food intake was allowed ad libitum also reported hyper insulinaemic compensation [25]. Thus, it is possible that hyper insulinaemic compensation would occur if insufficient sleep is sustained chronically.
Our previous work suggests that the specific signal for insulin compensation in response to insulin resistance is elevated nocturnal NEFA [32, 41]. Results from the same canine model showed that nocturnal NEFA are significantly elevated following 6 weeks of high-fat feeding and correlate with insulin secretion, without changes in morning fasting NEFA [32]. In humans, three nights of insufficient sleep resulted in elevated nocturnal NEFA [4]. Since overnight blood sampling in our canine model would have disrupted sleep, it was not possible to investigate the relationship between nocturnal NEFA and insulin secretion in the current study.
Following 9 months of high-fat feeding, one night of sleep deprivation did not result in further reductions in insulin sensitivity or changes in beta cell response. These findings may imply that, once insulin sensitivity is impaired, there could be a ‘floor effect’ such that further reductions are not possible. This possibility is supported by a recent study in human volunteers that demonstrated no additional changes in parameters of fat and glucose metabolism occurred when sleep deprivation took place after 1 week of deliberate overfeeding [42]. Alternatively, a diet high in fat is known to disrupt sleep and circadian rhythms in rodents [43] and the percentage of energy derived from saturated fat intake is associated with reduced slow wave sleep in humans [44]. Sleep in our animals during the HFD, therefore, may already have been disrupted, leading to a reduced impact of our intervention and smaller differences between sleep conditions after chronic high-fat feeding.
Another important consideration is the impact of a long-term HFD combined with chronic insufficient sleep, as is common in modern society. We and others have previously reported that insufficient sleep increases the risk for developing obesity by increasing subjective hunger and appetite, as well as the hunger hormone ghrelin when food intake is controlled [45]. Furthermore, when palatable food is readily available, insufficient sleep leads to a significant increase in energy intake [45, 46]. Since our dogs were not given free access to food during this study, they were unable to increase energy intake during sleep deprivation. However, we hypothesise that, if animals had been maintained on chronic insufficient sleep in conjunction with ad libitum access to the HFD, further reductions in insulin sensitivity would have been observed.
Finally, this study may have been underpowered to detect an impact of sleep deprivation after an HFD (medium-sized effect [Cohen’s d of 0.57]), as it was only powered for the detection of large effect sizes. Studies in larger samples are needed to further elucidate the effects of insufficient sleep in metabolically compromised individuals.
The mechanisms by which sleep loss impairs insulin sensitivity are yet to be unravelled, but roles for sympathetic nervous system and hypothalamic–pituitary–adrenal axis activation, as well as increased inflammation, have been suggested [3]. Studies assessing cortisol, adrenaline and noradrenaline during insufficient sleep have reported mixed findings, with some studies reporting no increases [4, 6, 11, 12, 14, 17, 47] or modest increases [4, 14, 15, 19, 40] in some or all of these factors. In the present study we did not detect changes in cortisol or catecholamines in response to one night of sleep deprivation. Furthermore, physical activity and food intake during the experimental days did not differ between the normal sleep and sleep deprivation conditions, ruling out the possibility that acute differences in either behaviour could have impacted on insulin sensitivity after one night of sleep deprivation. In contrast, fasting noradrenaline was higher after 9 months of high-fat feeding, which may have contributed to impaired insulin sensitivity. However, due to sample-size limitations it was not feasible to test whether effects of the HFD were mediated in part by changes in these variables.
Contrary to studies in human volunteers, we did not observe any indication of adipose tissue insulin resistance in response to sleep deprivation, as assessed by Adipo-IR—the product of fasting NEFA and insulin concentrations in plasma [38]. This may be due in part to protocol differences between studies. For example, the current study assessed Adipo-IR after one night of total sleep deprivation. In contrast, results from human studies suggest chronic partial sleep loss impairs adipose tissue function as indicated by impaired insulin signalling [5], increased nocturnal NEFA [4] and impaired NEFA rebound [27]. Therefore, a longer duration of insufficient sleep may be necessary to induce alterations in Adipo-IR. In contrast, reports from another clinical study found that a single night of sleep deprivation was indeed sufficient to induce changes in adipose tissue assessed from biopsies [48, 49]. Together these results suggest that our method for assessing adipose tissue function using fasting plasma NEFA and insulin was unable to uncover potential alterations in adipose tissue due to one night of insufficient sleep.
In summary, the canine model appears to be a valid large, diurnal animal model for the study of sleep loss-induced insulin resistance. Future studies in dogs could elucidate the pathophysiological mechanisms by which sleep loss impairs insulin sensitivity in ways not possible in human volunteers, for example using physical or pharmacological denervation of specific metabolic tissues to examine whether the impact of insufficient sleep on glucose metabolism is established through altered sympathetic innervation.
However, the results of this study must be interpreted with some limitations in mind. First, insulin resistance induced by a single night of total sleep deprivation is unlikely to truly compare to insulin resistance induced by a hypercaloric HFD, as the impact of sleep loss on insulin sensitivity can be reversed following a period of recovery sleep. For example, reports from experimental sleep restriction studies in humans indicate that two nights of recovery sleep is sufficient to restore insulin sensitivity [23], although some studies report only partial restoration of insulin sensitivity [24, 26]. In contrast, moderate weight loss of 5% or more is typically required to improve insulin sensitivity in people with obesity, which takes weeks or months of intense intervention to achieve [50]. Second, the pilot nature of the protocol resulted in a small all-male sample size, in particular for the subgroup that went on to chronic high-fat feeding, which yielded power for the detection of large effect sizes only. As a consequence, medium or small differences between conditions could not be detected or statistically confirmed by this study. Related to this, the subset of eight animals that went on to an HFD had larger reductions in SI during sleep deprivation than the larger group of 24 animals, and therefore may not be a true representative subgroup. Third, we did not measure sleep directly, for example by using EEG, and therefore we cannot provide information on the duration or quality of sleep throughout the protocol. Previous research in experimental dogs suggests a sleep–wake pattern similar to humans, with a third of the time sleeping, and most of sleep occurring overnight [35, 36]. Fourth, the sleep study conditions differed, not only based on sleep duration, but also based on the absence (normal sleep) or presence (sleep deprivation) of the researcher during the experimental night. We do not expect this to have influenced our findings, however, as the dogs were considerably habituated to investigators prior to study, and no differences were detected in fasting cortisol, adrenaline and noradrenaline concentrations between conditions.
In conclusion, our findings indicate that one night of total sleep deprivation is as detrimental to insulin sensitivity as 9 months of an HFD. These results emphasise the importance of sleep for glucometabolic health. Furthermore, the canine may represent a new model to study mechanisms by which sleep loss causes acute metabolic dysfunction.
Research in context.
What is already known about this subject?
Insufficient sleep is a risk factor for the development of obesity and diabetes
The relative effects of insufficient sleep and unhealthy lifestyle are not known
What is the key question?
How does insufficient sleep compare with an unhealthy diet in the same individual canine model?
What are the new findings?
One night of total sleep deprivation in lean dogs impaired insulin sensitivity to a similar degree as chronic high-fat feeding
Acute total sleep deprivation after chronic high-fat feeding did not lead to further impairments in insulin sensitivity
The canine is a valid non-human diurnal animal model for the study of sleep loss-induced insulin resistance
How might this impact on clinical practice in the foreseeable future?
Sleep loss has significant clinical relevance as one night of sleep deprivation can impair next day insulin sensitivity to a similar degree as a long-term high-fat diet
Acknowledgements
The authors thank R. Thomas, Biomedical Sciences Department, Cedars-Sinai Medical Center, Los Angeles, CA, USA, for performing the insulin, cortisol and catecholamine assays and the Comparative Medicine staff, Cedars-Sinai Medical Center, for their assistance with and care for our animals. We thank K.P. Wright, Jr., University of Colorado Boulder, CO, USA, for comments on the manuscript.
Funding This work was supported by an EMGO Institute for Health and Care Research Travel Grant 2017 (to AB), as well as NIH grants R01DK29867 and R01DK27619 (to RNB), and the Society in Science Branco Weiss Fellowship, administered by the ETH Zurich (to JLB).
Abbreviations
- Adipo-IR
Adipose tissue insulin sensitivity
- AIRG
Acute insulin response to glucose
- DI
Disposition index
- HFD
High-fat diet
- KG
Glucose tolerance
- NS-chow
Normal sleep on a chow diet
- NS-HFD
Normal sleep on a high-fat diet
- SD-chow
Sleep deprivation on a chow diet
- SD-HFD
Sleep deprivation on a high-fat diet
- SG
Glucose effectiveness
- SI
Insulin sensitivity (index)
Footnotes
Data availability The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Duality of interest The authors declare that there is no duality of interest associated with this manuscript.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP (2001) The continuing epidemics of obesity and diabetes in the United States. JAMA 286(10):1195–1200. 10.1001/jama.286.10.1195 [DOI] [PubMed] [Google Scholar]
- 2.Schmid SM, Hallschmid M, Schultes B (2015) The metabolic burden of sleep loss. Lancet Diabetes Endocrinol 3(1):52–62. 10.1016/S2213-8587(14)70012-9 [DOI] [PubMed] [Google Scholar]
- 3.Reutrakul S, Van Cauter E (2018) Sleep influences on obesity, insulin resistance, and risk of type 2 diabetes. Metab Clin Exp 84: 56–66. 10.1016/j.metabol.2018.02.010 [DOI] [PubMed] [Google Scholar]
- 4.Broussard JL, Chapotot F, Abraham V et al. (2015) Sleep restriction increases free fatty acids in healthy men. Diabetologia 58(4):791–798. 10.1007/s00125-015-3500-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broussard JL, Ehrmann DA, Van Cauter E, Tasali E, Brady MJ (2012) Impaired insulin signaling in human adipocytes after experimental sleep restriction: a randomized, crossover study. Ann Intern Med 157(8):549–557. 10.7326/0003-4819-157-8201210160-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK (2010)Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes 59(9):2126–2133. 10.2337/db09-0699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cedernaes J, Lampol aL, Axelsson EK et al. (2016) A single night of partial sleep loss impairs fasting insulin sensitivity but does not affect cephalic phase insulin release in young men. J Sleep Res 25(1):5–10. 10.1111/jsr.12340 [DOI] [PubMed] [Google Scholar]
- 8.de Souza JFT, Dattilo M, de Mello MT, Tufik S, Antunes HKM (2017) High-intensity interval training attenuates insulin resistance induced by sleep deprivation in healthy males. Front Physiol 8:992 10.3389/fphys.2017.00992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donga E, van Dijk M, van Dijk JG et al. (2010) Partial sleep restriction decreases insulin sensitivity in type 1 diabetes. Diabetes Care 33(7):1573–1577. 10.2337/dc09-2317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donga E, van Dijk M, van Dijk JG et al. (2010) A single night of partial sleep deprivation induces insulin resistance in multiple metabolic pathways in healthy subjects. J Clin Endocrinol Metab 95(6): 2963–2968. 10.1210/jc.2009-2430 [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez-Ortiz M, Martinez-Abundis E, Balcazar-Munoz BR, Pascoe-Gonzalez S (2000) Effect of sleep deprivation on insulin sensitivity and cortisol concentration in healthy subjects. Diabetes Nutr Metab 13:80–83 [PubMed] [Google Scholar]
- 12.Klingenberg L, Chaput JP, Holmback U et al. (2013) Acute sleep restriction reduces insulin sensitivity in adolescent boys. Sleep 36(7):1085–1090. 10.5665/sleep.2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nedeltcheva AV, Imperial JG, Penev PD (2012) Effects of sleep restriction on glucose control and insulin secretion during diet induced weight loss. Obesity 20(7):1379–1386. 10.1038/oby.2012.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nedeltcheva AV, Kessler L, Imperial J, Penev PD (2009) Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J Clin Endocrinol Metab 94(9):3242–3250. 10.1210/jc.2009-0483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rao MN, Neylan TC, Grunfeld C, Mulligan K, Schambelan M, Schwarz JM (2015) Subchronic sleep restriction causes tissuespecific insulin resistance. J Clin Endocrinol Metab 100(4):1664–1671. 10.1210/jc.2014-3911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robertson MD, Russell-Jones D, Umpleby AM, Dijk DJ (2013) Effects of three weeks of mild sleep restriction implemented in the home environment on multiple metabolic and endocrine markers in healthy young men. Metab Clin Exp 62(2):204–211. 10.1016/j.metabol.2012.07.016 [DOI] [PubMed] [Google Scholar]
- 17.Schmid SM, Hallschmid M, Jauch-Chara K et al. (2011) Disturbed glucoregulatory response to foodintakeafter moderate sleep restriction. Sleep 34(3):371–377. 10.1093/sleep/34.3.371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sweeney EL, Jeromson S, Hamilton DL, Brooks NE, Walshe IH (2017) Skeletal muscle insulin signaling and whole-body glucose metabolism following acute sleep restriction in healthy males. Phys Rep 5(23):e13498. 10.14814/phy2.13498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tajiri E, Yoshimura E, Hatamoto Y, Tanaka H, Shimoda S (2018) Effect of sleep curtailment on dietary behavior and physical activity: A randomized crossover trial. Physiol Behav 184:60–67. 10.1016/j.physbeh.2017.11.008 [DOI] [PubMed] [Google Scholar]
- 20.van Leeuwen WM, Hublin C, Sallinen M, Harma M, Hirvonen A, Porkka-Heiskanen T (2010) Prolonged sleep restriction affects glucose metabolism in healthy young men. Int J Endocrinol 2010: 108641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.VanHelder T, Symons JD, Radomski MW (1993) Effects of sleep deprivation and exercise on glucose tolerance. Aviat Space Environ Med 64:487–492 [PubMed] [Google Scholar]
- 22.Wang X, Greer J, Porter RR, Kaur K, Young stedt SD (2016) Short-term moderate sleep restriction decreases insulin sensitivity in young healthy adults. Sleep Health 2(1):63–68. 10.1016/j.sleh.2015.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Broussard JL, Wroblewski K, Kilkus JM, Tasali E (2016) Two nights of recovery sleep reverses the effects of short-term sleep restriction on diabetes risk. Diabetes Care 39(3):e40–e41. 10.2337/dc15-2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ness KM, Strayer SM, Nahmod NG, Chang AM, Buxton OM, Shearer GC (2019) Two nights of recovery sleep restores the dynamic lipemic response, but not the reduction of insulin sensitivity, induced by five nights of sleep restriction. Am J Physiol Regul Integr Comp Physiol 316(6):R697–R703. 10.1152/ajpregu.00336.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eckel RH, Depner CM, Perreault L et al. (2015) Morning circadian misalignment during short sleep duration impacts insulin sensitivity. Curr Biol 25(22):3004–3010. 10.1016/j.cub.2015.10.011 [DOI] [PubMed] [Google Scholar]
- 26.Depner CM, Melanson EL, Eckel RH et al. (2019) Ad libitum weekend recovery sleep fails to prevent metabolic dysregulation during a repeating pattern of insufficient sleep and weekend recovery sleep. Curr Biol 29(6):957–967. 10.1016/j.cub.2019.01.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ness KM, Strayer SM, Nahmod NG et al. (2019) Four nights of sleep restriction suppress the postprandial lipemic response and decrease satiety. J Lipid Res 60(11):1935–1945. 10.1194/jlr.P094375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor R (2012) Insulin resistance and type 2 diabetes. Diabetes 61(4):778–779. 10.2337/db12-0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conte C, Fabbrini E, Kars M, Mittendorfer B, Patterson BW, Klein S (2012) Multiorgan insulin sensitivity in lean and obese subjects. Diabetes Care 35(6):1316–1321. 10.2337/dc111951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Broussard JL, Bergman RN, Bediako IA, Paszkiewicz RL, Iyer MS, Kolka CM (2018) Insulin access to skeletal muscle is preserved in obesity induced by polyunsaturated diet. Obesity 26(1):119–125. 10.1002/oby.22057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Broussard JL, Castro AV, Iyer M et al. (2016) Insulin access to skeletal muscle is impaired during the early stages of diet-induced obesity. Obesity 24(9):1922–1928. 10.1002/oby.21562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Broussard JL, Kolka CM, Castro AV et al. (2015) Elevated nocturnal NEFA are an early signal for hyperisulinaemic compensation during diet-induced insulin resistance in dogs. Diabetologia 58(11):2663–2670. 10.1007/s00125-015-3721-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Broussard JL, Nelson MD, Kolka CM et al. (2016) Rapid development of cardiac dysfunction in a canine model of insulin resistance and moderate obesity. Diabetologia 59(1):197–207. 10.1007/s00125-015-3767-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ionut V, Liu H, Mooradian V et al. (2010) Novel canine models of obese prediabetes and mild type 2 diabetes. Am J Physiol Endocrinol Metab 298(1):E38–E48. 10.1152/ajpendo.00466.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lucas EA, Powell EW, Murphree OD (1977) Baseline sleep-wake patterns in the pointer dog. Physiol Behav 19(2):285–291. 10.1016/0031-9384(77)90340-7 [DOI] [PubMed] [Google Scholar]
- 36.Kis A, Szakadat S, Kovacs E et al. (2014) Development of a noninvasive polysomnography technique for dogs (Canis familiaris). Physiol Behav 130:149–156. 10.1016/j.physbeh.2014.04.004 [DOI] [PubMed] [Google Scholar]
- 37.Speakman JR (2019) Use of high-fat diets to studyrodent obesity as a model of human obesity. Int J Obes 43(8):1491–1492. 10.1038/s41366-019-0363-7 [DOI] [PubMed] [Google Scholar]
- 38.Sondergaard E, Espinosa De Ycaza AE, Morgan-Bathke M, Jensen MD (2017)How to measure adipose tissue insulinsensitivity. J Clin Endocrinol Metab 102(4):1193–1199. 10.1210/jc.2017-00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steil GM, Ader M, Moore DM, Rebrin K, Bergman RN (1996) Trans endothelial insulin transport is not saturable in vivo. No evidence for a receptor-mediated process. J Clin Invest 97(6): 1497–1503. 10.1172/JCI118572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spiegel K, Leproult R, Van Cauter E (1999) Impact of sleep debt on metabolic and endocrine function. Lancet 354(9188):1435–1439. 10.1016/S0140-6736(99)01376-8 [DOI] [PubMed] [Google Scholar]
- 41.Kim SP, Catalano KJ, Hsu IR, Chiu JD, Richey JM, Bergman RN (2007) Nocturnal free fatty acids are uniquely elevated in the longitudinal development of diet-induced insulin resistance and hyperinsulinemia. Am J Physiol Endocrinol Metab 292(6): E1590–E1598. 10.1152/ajpendo.00669.2006 [DOI] [PubMed] [Google Scholar]
- 42.Cros J, Pianezzi E, Rosset R et al. (2019) Impact of sleep restriction on metabolic outcomes induced by overfeeding: a randomized controlled trial in healthy individuals. Am J Clin Nutr 109(1):17–28. 10.1093/ajcn/nqy215 [DOI] [PubMed] [Google Scholar]
- 43.Mavanji V, Billington CJ, Kotz CM, Teske JA (2012) Sleep and obesity: a focus on animal models. Neurosci Biobehav Rev 36(3): 1015–1029. 10.1016/j.neubiorev.2012.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.St-Onge MP, Roberts A, Shechter A, Choudhury AR (2016) Fiber and saturated fat are associated with sleep arousals and slow wave sleep. J Clin Sleep Med 12(1):19–24. 10.5664/jcsm.5384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Broussard JL, Kilkus JM, Delebecque F et al. (2016) Elevated ghrelin predicts food intake during experimental sleep restriction. Obesity 24(1):132–138. 10.1002/oby.21321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.St-Onge MP, Roberts AL, Chen J et al. (2011) Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr 94(2):410–416. 10.3945/ajcn.111.013904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmid SM, Hallschmid M, Jauch-Chara K, Bandorf N, Born J, Schultes B (2007) Sleep loss alters basal metabolic hormone secretion and modulates the dynamic counterregulatory response to hypoglycemia. J Clin Endocrinol Metab 92(8):3044–3051. 10.1210/jc.2006-2788 [DOI] [PubMed] [Google Scholar]
- 48.Cedernaes J, Osler ME, Voisin S et al. (2015) Acute sleep loss induces tissue-specific epigenetic and transcriptional alterations to circadian clock genes in men. J Clin Endocrinol Metab 100(9): E1255–E1261. 10.1210/JC.2015-2284 [DOI] [PubMed] [Google Scholar]
- 49.Cedernaes J, Schonke M, Westholm JO et al. (2018) Acute sleep loss results in tissue-specific alterations in genome-wide DNA methylation state and metabolic fuel utilization in humans. Sci Adv 4(8):eaar8590. 10.1126/sciadv.aar8590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Magkos F, Fraterrigo G, Yoshino J et al. (2016) Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab 23(4): 591–601. 10.1016/j.cmet.2016.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]