Abstract
As the world slowly starts to recover from the coronavirus disease-2019 pandemic, health care systems are now thinking about resuming elective cardiovascular procedures, including procedures in cardiac catheterization laboratories. Rebooting catheterization laboratories will be an arduous process, in part because of limited health care resources, new processes, and fears stemming from the coronavirus disease-2019 pandemic. The authors propose a detailed phased-in approach that considers clinical, patient-centered, and operational strategies to safely and effectively reboot catheterization laboratory programs during these unprecedented times. This model balances the delivery of essential cardiovascular care with reduced exposure and preservation of resources. The guiding principles detailed in this review can be used by catheterization laboratory programs when restarting elective interventional procedures.
Key Words: catheterization laboratory reboot, COVID-19 pandemic, elective interventional procedures, novel care model, operational strategies
Abbreviations and Acronyms: COVID-19, coronavirus disease-2019; CV, cardiovascular; ICU, intensive care unit
Graphical abstract
As the number of new coronavirus disease-2019 (COVID-19) cases stabilizes and some states are lifting stay-at-home orders, hospitals and physicians are planning to restart nonemergent procedures. However, the COVID-19 pandemic has profoundly affected communities, health care professionals, and systems in ways that are not yet fully appreciated, creating new challenges when trying to envision a “new normal.” Rebooting catheterization laboratories that have been functioning with minimal staffing and altered operations for several weeks poses significant challenges. We present a perspective on these issues and propose a strategic plan for a successful catheterization laboratory reboot of elective interventional procedures during the recovery from this pandemic.
Factors Limiting Catheterization Laboratory Reboot
Factors that must be considered include patient issues and personnel and operational concerns. After months of being told to stay home and avoid hospitals unless absolutely necessary, patients often are fearful of hospitals and reluctant to seek care. Canceled visits, canceled procedures, late presentations, and avoidance of care altogether for acute coronary syndrome, stroke, and other acute conditions are evidence of this anxiety (1, 2, 3). Many patients have had changes in their health insurance status, with millions losing employment during this crisis. In the coming months, several patients will not be able to take time off for their diagnostic tests and elective procedures, because of financial insecurities or fear of losing their current jobs. Changing patients’ perceptions will likely require time and effort on the part of health care systems.
Health care facilities in some areas have expanded beyond the limits of normal capacity, and surge areas were created to augment care for patients with COVID-19. Hospitals’ readiness to resume elective procedures will vary depending on their local current COVID-19 caseloads. In some areas, inpatient and intensive care unit (ICU) beds still have significant numbers of patients with COVID-19, also affecting operations for those without COVID-19. Often, essential care team members such as physicians, nurses, and technologists have been temporarily reassigned during the crisis and may not be readily available to return to the catheterization laboratory. Sadly, some also have been infected with COVID-19 and are still recovering from the disease. Furthermore, personal protective equipment remains a limited resource.
Most health care systems have reduced the number of diagnostic studies that lead to catheterization laboratory referrals, including transthoracic echocardiography, transesophageal echocardiography, stress testing, and computed tomographic angiography. Referring physicians, both primary care physicians and cardiologists, have also not been seeing these patients in the office because of restrictions in place during the pandemic. Although telemedicine has expanded, it is possible that many patients are not seeking care through this alternative pathway. In addition, testing for COVID-19 is not yet readily available everywhere and has diagnostic limitations (see later discussion). All these factors will affect the rates of catheterization laboratory procedures done in the early phase of the reboot.
Guiding Principles for Successful Reboot
To safely and effectively reboot catheterization laboratories, health care systems are obligated to comply with federal, state, and local public health recommendations. This mandate includes following guidelines from the Centers for Medicare and Medicaid Services, which recommends that states pass the “gating criteria” prior to restarting nonemergent procedures (4). This means that states should have sustained regional reductions in the rates of new COVID-19 diagnoses and cases for at least 14 days, robust testing programs should be in place, and hospitals should have all the required resources to treat patients without COVID-19. The latter is dependent upon a healthy workforce across all phases of care, adequate personal protective equipment, appropriate numbers of ICU and non-ICU beds, and required supplies to treat all patients without resorting to a crisis standard of care (4, 5, 6).
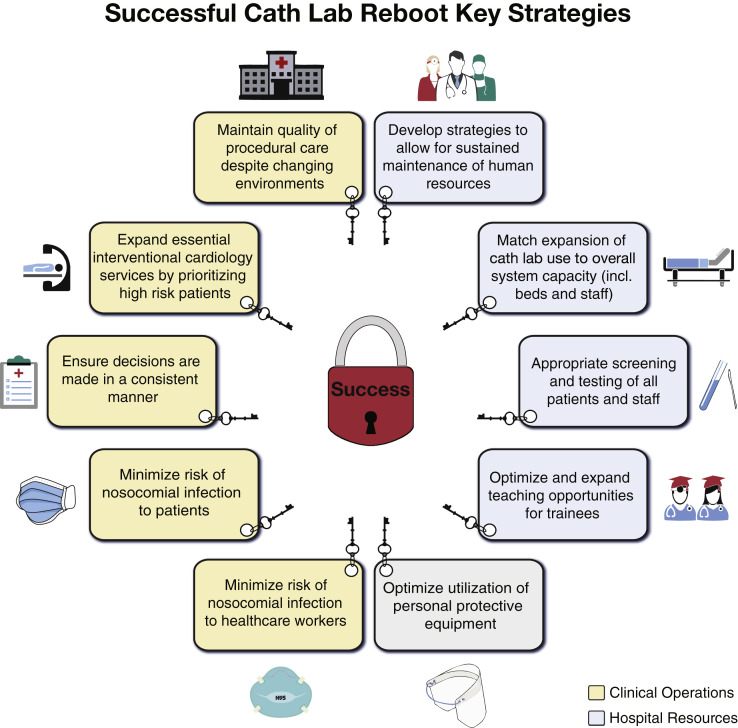
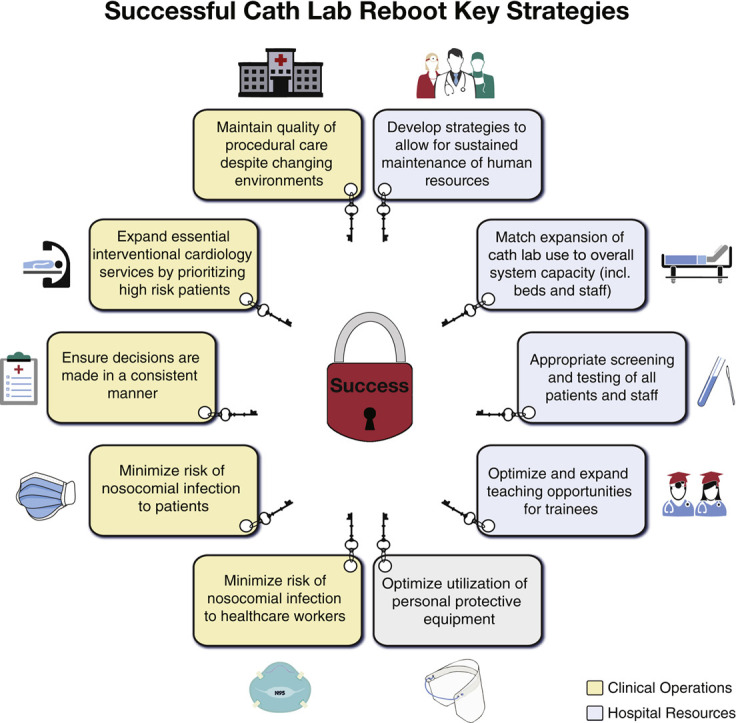
Guiding principles for a successful pathway to rebooting catheterization laboratories are summarized in Figure 1 . Restarting elective cases after the pandemic amplifies and highlights the integrated nature of our health care teams. Diligent coordination with other departments is essential to ensure efficiency and success. Specifically, cardiac anesthesia, surgery, environmental services, infection control, facilities, information technology, and others must be engaged in developing a coherent and sustainable strategy.
Figure 1.
Guiding Principles for Successful Catheterization Laboratory Reboot
Key organizational and resources principles for successful catheterization laboratory reboot are presented.
Gradual Phased-In Model
It should be assumed that all emergent cases such as patients with myocardial infarction, shock, and severe symptomatic valvular heart disease in need of urgent treatment are already being treated according to locally established protocols (7,8). Waiting patients should be prioritized on the basis of severity of symptoms and other established risk factors. They need to be contacted regularly and reclassified as needed while awaiting their procedures. Patients can be classified as category I (urgent, or at high risk for cardiovascular [CV] complications while waiting), category II (semiurgent, or at moderate CV risk), or category III (elective, or at low CV risk) (see Table 1 ) (9,10).
Table 1.
Classification of Interventional Procedures According to Their Indication During the Coronavirus Disease 2019 Pandemic
| Category∗ | Coronary Angiography/PCI | Structural Intervention | Peripheral Angiography/PVI |
|---|---|---|---|
| I |
|
|
|
| II |
|
|
|
| III |
|
|
|
AAA = abdominal aortic aneurysm; AS = aortic stenosis; ASD = atrial septal defect; BP = blood pressure; CTO = chronic total occlusion; DVT = deep vein thrombosis; IVC = inferior vena cava; LAA = left atrial appendage; LVEF = left ventricular ejection fraction; MI = myocardial infarction; NSTEMI = non–ST-segment elevation myocardial infarction; PCI = percutaneous coronary intervention; PDA = patent ductus arteriosus; PFO = patent foramen ovale; PVI = peripheral vascular intervention; TAA = thoracic aortic aneurysm; TAVR = transcatheter aortic valve replacement; TID = transient ischemic dilatation; VSD = ventricular septal defect; VT = ventricular tachycardia.
Category I (urgent procedure): patient at high risk for CV complications while waiting; category II (semiurgent procedure): at moderate CV risk; category III (elective): at low CV risk.
Each program needs to implement a model to increase cardiac care in a deliberate and graded fashion with appropriate safeguards and ability to modify in case of a second wave of infection. Recent general guidance has been published on how to reintroduce elective CV care during the pandemic (5,6). In addition to these recommendations, we propose the following phased-in model (Table 2 ). It should be noted that some resource considerations may not apply to regions that have been less affected by the pandemic, but each program can use these guiding principles to tailor its catheterization laboratory reboot process.
Table 2.
Phased-In Model for Restarting Interventional Elective Procedures During the COVID-19 Pandemic
| Phases | Cases | Dependencies | Tactics |
|---|---|---|---|
| Phase 1: urgent/emergent procedures and those not affecting surge resources 25% usual capacity |
|
|
|
| Phase 2: semiurgent procedures, possibly affecting surge resources 50% usual capacity |
|
As above
|
As above
|
| Phase 3: routine procedures 75% usual capacity |
|
As above
|
As above
|
| Phase 4: 110% of FY20 budgeted procedural cases |
|
As above
|
As above
|
COVID-19 = coronavirus disease 2019; FTE = full-time equivalent; FY20 = fiscal year 2020; ICU = intensive care unit; OR = operating room; TAVR = transcatheter aortic valve replacement.
During phase 1, planned outpatient cases are to be clustered on a few days per week if catheterization laboratory staff members have not returned from repurposing and ICU beds and holding area hours are still limited. The number of cases scheduled should aim to be about 25% of pre-COVID-19 catheterization laboratory volume. Ideally, the first cases to be scheduled will be category I patients (Table 1) at risk for adverse cardiac events or urgent hospitalization but also with low risk for intraprocedural complications. Such a balance may be difficult to strike, but this type of cases would allow prompt discharge and minimize the use of hospital resources, especially ICU resources. Organizational strategies, including extended hours and performing cases on weekends to decompress weekdays and expedite patient discharge, are available as needed after approbation of the catheterization laboratory director. Especially during this phase, when 2 therapeutic options such as percutaneous coronary intervention and coronary artery bypass grafting are available, additional considerations for the heart team over conventional decision making would be length of stay and ICU bed utilization. As such, the heart team may suggest a minimally invasive procedure (e.g., percutaneous coronary intervention over coronary artery bypass grafting) associated with shorter length of stay and less resource utilization (6), with the understanding that there may be an increased risk for downstream events such as repeat revascularization.
This applies to patients requiring coronary or peripheral revascularization as well as aortic valve replacement.
Assuming a successful implementation of phase 1 and with the assent of institutional infectious disease experts, phase 2 can be expected to begin as soon as 1 to 2 weeks later. The volume of outpatients can be expected to reach about 50% of capacity and few elective cases can be done 4 days if not all weekdays. Afternoon start times should be avoided to reduce burdens on holding-area staff members. Flexibility in the schedule and strategies for rapid discharge remain available. Less symptomatic coronary, peripheral, and structural patients can be scheduled at that time. Heart biopsies and noncardiac transplantation evaluation can also be scheduled, assuming that transplantation programs have also rebooted, and adequate isolation measures for this immunocompromised population are in place to reduce the chances of nosocomial transmission.
Structural and peripheral interventions at low risk to require endotracheal intubation and at low risk for ICU care post-procedure can also be scheduled in phase 2. This includes patent foramen ovale and atrial septal defect closure, which can be performed with intracardiac echocardiographic guidance to minimize the risk for aerosolization with transesophageal echocardiography. Transcatheter aortic valve replacement without general anesthesia and peripheral arterial and venous interventions can be considered given the low probability of emergent intubation and cardiopulmonary resuscitation. Other procedural and operational changes to consider adopting for structural heart interventions in the COVID-19 era are listed in Table 3 .
Table 3.
Specific Considerations for Structural Heart Procedures During the COVID-19 Pandemic
| Procedure | Procedural Considerations | Operational Considerations |
|---|---|---|
| TAVR |
|
|
| MitraClip |
|
|
| ASD/PFO closure |
|
|
| LAAO |
|
In phase 3, all elective cases can be scheduled, but volume will be at 75% of capacity until resources allow a transition to phase 4. Cases can be scheduled any day of the week. Elective procedures such as coronary angiography for stable angina, pulmonary hypertension evaluation, alcohol septal ablation, and CardioMEMS implantation can be scheduled. Clinical trial enrollment can be expected to resume in phase 4, when catheterization laboratories are functioning normally, albeit with possible reduced efficiency due to new processes in place.
COVID-19 Testing Prior to Elective Procedures
To protect and instill confidence among health care workers and patients, systematic screening for COVID-19 symptoms (cough, fever, new anosmia or ageusia, dyspnea, diarrhea, or sore throat) and exposure to known cases should be done for all patients prior to hospital admission (6,8,10). All patients should also get tested with a single swab within 24 to 48 h of their elective procedures and told to self-isolate until their procedures to avoid possible new exposure in the interim. Testing on Friday for Monday procedures can be acceptable if testing over the weekend is not possible, with strict self-isolation and repeat screening for COVID-19 symptoms on Monday. Patients with symptoms and/or positive test results should have their elective procedures postponed.
It should be recognized that no pre-procedural testing strategy can eliminate the possibility of bringing a patient to the hospital in an asymptomatic carrier state. The current false-negative rate for COVID-19 polymerase chain reaction–based testing varies from 26% to 100% depending on the timing of testing relative to the disease onset (11). The median false-negative rate on the day of exposure is 100%, 39% on the day of symptom onset, and 26% 3 days later. Factors that can influence the clinical sensitivity of nasopharyngeal swab testing include the timing of specimen collection relative to symptom onset (less sensitive in asymptomatic patients and in those with more advanced disease) and the quality of the sample (11, 12, 13). Of note, several point-of-care testing kits are also available but are currently not recommended for clinical use by the World Health Organization (14). In areas where the prevalence of COVID-19 is high, a second test to increase virus detection may be needed. This decision should be made after conferring with local infectious disease experts. For hospitals without the capacity to perform on-site testing, send-out tests can be done. However, turnover time may be longer, creating logistic challenges for scheduling, particularly if testing windows are dogmatic.
Antibody testing is currently under investigation and is not recommended as the sole screening modality pre-procedure. The presence of antibodies confirms a prior infection but may not correlate with immunity. Despite having positive antibodies, some patients still test positive for COVID-19, suggesting that they may still be contagious (15). Additionally, the absence of antibodies does not exclude an ongoing infection, as some patients may not have yet mounted a detectable immune response or may be unable to.
Other Important Safety Measures
In addition to screening for COVID-19, other important safety measures to reassure patients, health care workers, and referring physicians should be devised for presumed COVID-19-negative patients presenting for elective procedures. Given the limitations of pre-procedural testing, patients and providers should wear surgical masks at all times. Catheterization laboratories should have dedicated “clean” pathways for elective cases with isolated waiting areas, restrooms, pre-procedural and recovery areas away from COVID-19-positive patients, as well as dedicated “COVID-19-negative” catheterization laboratory rooms when feasible. Minimizing observation time post-procedure is prudent. A no-visitation policy should be enforced in the first phases of the reboot, and possibly even longer depending on the local prevalence of COVID-19. Whenever possible, family updates can be done via video apps to replicate a face-to-face encounter and allow them to see their loved ones periprocedurally. Catheterization laboratory teams should avoid congregating in control booths and breakrooms, and assignments should attempt to avoid mixing between COVID-19-positive and COVID-19-negative areas of the catheterization laboratory. Daily symptom screening of staff members is highly recommended. Routine COVID-19 testing of the team is controversial and logistically difficult but can be considered after consultation with local infectious disease experts. The use of telehealth should be maximized for evaluation and follow-up visits of all patients. Recognizing whether a nosocomial infection has occurred is predicated on catheterization laboratory staff members’ checking in with patients after their procedures. If a recrudescence of community COVID-19 or nosocomial infections occurs, stopping most elective procedures should occur again until control is reestablished.
Conclusions
The reboot of elective catheterization laboratory procedures is expected to be a fluid process during the recovery from this pandemic and differ among regions according to their COVID-19 prevalence. Close collaboration with national and local health authorities is essential to a safe and sustained return to a new normal. The success of catheterization laboratory reboots requires complex coordination. It relies on various programs’ collaborating to plan and implement a standardized, detailed, phased-in approach that incorporates clinical, patient-centered, and operational strategies.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Cardiovascular Interventionsauthor instructions page.
References
- 1.Tam C.F., Cheung K.S., Lam S. Impact of coronavirus disease 2019 (COVID-19) outbreak on ST-segment-elevation myocardial infarction care in Hong Kong, China. Circ Cardiovasc Qual Outcomes. 2020;13 doi: 10.1161/CIRCOUTCOMES.120.006631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moroni F., Gramegna M., Ajello S. Collateral damage: medical care avoidance behavior among patients with acute coronary syndrome during the COVID-19 pandemic. J Am Coll Cardiol Case Rep. 2020;2:1620–1624. doi: 10.1016/j.jaccas.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao J., Rudd A., Liu R. Challenges and potential solutions of stroke care during the coronavirus disease 2019 (COVID-19) outbreak. Stroke. 2020;51:1356–1357. doi: 10.1161/STROKEAHA.120.029701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Medicare and Medicaid Services Opening up America again: Centers for Medicare & Medicaid Services (CMS) recommendations: re-opening facilities to provide non-emergent non-COVID-19 healthcare: phase I. https://www.cms.gov/files/document/covid-flexibility-reopen-essential-non-covid-services.pdf Available at:
- 5.American College of Surgeons, American Society of Anesthesiologists Association of periOperative Registered Nurses, American Hospital Association. Joint statement: roadmap for resuming elective surgery after COVID-19 pandemic. https://www.asahq.org/about-asa/newsroom/news-releases/2020/04/joint-statement-on-elective-surgery-after-covid-19-pandemic Available at:
- 6.Wood D.A., Mahmud E., Thourani V.H. Safe reintroduction of cardiovascular services during the COVID-19 pandemic: guidance from North American society leadership. J Am Coll Cardiol. 2020;75:3177–3183. doi: 10.1016/j.jacc.2020.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ranard L.S., Ahmad Y., Masoumi A. Clinical pathway for management of suspected or positive novel coronavirus-19 patients with ST-segment elevation myocardial infarction. Crit Pathw Cardiol. 2020;19:49–54. doi: 10.1097/HPC.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 8.Welt F., Shah P., Aronow H. Catheterization laboratory considerations during the coronavirus (COVID 19) pandemic: a joint statement from the American College of Cardiology (ACC) Interventional Council and the Society of Cardiovascular Angiography and Intervention (SCAI) J Am Coll Cardiol. 2020;75:2372–2375. doi: 10.1016/j.jacc.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung C.J., Nazif T.M., Wolbinski M. The restructuring of structural heart disease practice during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75:2974–2983. doi: 10.1016/j.jacc.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassan A., Arora R.C., Lother S.A. Ramping up the delivery of cardiac surgery during the COVID-19 pandemic: a guidance statement from the Canadian Society of Cardiac Surgeons. Can J Cardiol. 2020;36:1139–1143. doi: 10.1016/j.cjca.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kucirka L., Lauer S., Laeyendecker O., Boon D., Lessler J. Variation in false negative rate of RT-PCR based SARS-CoV-2 tests by time since exposure. medRxiv. https://www.medrxiv.org/content/10.1101/2020.04.07.20051474v1 Available at: [DOI] [PMC free article] [PubMed]
- 12.Cheng M.P., Papenburg J., Desjardins M. Diagnostic testing for severe acute respiratory syndrome–related coronavirus-2: a narrative review. Ann Intern Med. 2020;172:726–734. doi: 10.7326/M20-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo L., Ren L., Yang S. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19). Clin Infect Dis. 2020;71:778–785. doi: 10.1093/cid/ciaa310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization Advice on the use of point-of-care immunodiagnostic tests for COVID-19. https://www.who.int/news-room/commentaries/detail/advice-on-the-use-of-point-of-care-immunodiagnostic-tests-for-covid-19 Available at:
- 15.Abbasi J. The promise and peril of antibody testing for COVID-19. JAMA. 2020;323:1881–1883. doi: 10.1001/jama.2020.6170. [DOI] [PubMed] [Google Scholar]