Abstract
Background
The COVID-19 pandemic is challenging advanced health systems, which are dealing with an overwhelming number of patients in need of intensive care for respiratory failure, often requiring intubation. Prone positioning in intubated patients is known to reduce mortality in moderate-to-severe acute respiratory distress syndrome. We aimed to investigate feasibility and effect on gas exchange of prone positioning in awake, non-intubated patients with COVID-19-related pneumonia.
Methods
In this prospective, feasibility, cohort study, patients aged 18–75 years with a confirmed diagnosis of COVID-19-related pneumonia receiving supplemental oxygen or non-invasive continuous positive airway pressure were recruited from San Gerardo Hospital, Monza, Italy. We collected baseline data on demographics, anthropometrics, arterial blood gas, and ventilation parameters. After baseline data collection, patients were helped into the prone position, which was maintained for a minimum duration of 3 h. Clinical data were re-collected 10 min after prone positioning and 1 h after returning to the supine position. The main study outcome was the variation in oxygenation (partial pressure of oxygen [PaO2]/fractional concentration of oxygen in inspired air [FiO2]) between baseline and resupination, as an index of pulmonary recruitment. This study is registered on ClinicalTrials.gov, NCT04365959, and is now complete.
Findings
Between March 20 and April 9, 2020, we enrolled 56 patients, of whom 44 (79%) were male; the mean age was 57·4 years (SD 7·4) and the mean BMI was 27·5 kg/m2 (3·7). Prone positioning was feasible (ie, maintained for at least 3 h) in 47 patients (83·9% [95% CI 71·7 to 92·4]). Oxygenation substantially improved from supine to prone positioning (PaO2/FiO2 ratio 180·5 mm Hg [SD 76·6] in supine position vs 285·5 mm Hg [112·9] in prone position; p<0·0001). After resupination, improved oxygenation was maintained in 23 patients (50·0% [95% CI 34·9–65·1]; ie, responders); however, this improvement was on average not significant compared with before prone positioning (PaO2/FiO2 ratio 192·9 mm Hg [100·9] 1 h after resupination; p=0·29). Patients who maintained increased oxygenation had increased levels of inflammatory markers (C-reactive protein: 12·7 mg/L [SD 6·9] in responders vs 8·4 mg/L [6·2] in non-responders; and platelets: 241·1 × 103/μL [101·9] vs 319·8 × 103/μL [120·6]) and shorter time between admission to hospital and prone positioning (2·7 days [SD 2·1] in responders vs 4·6 days [3·7] in non-responders) than did those for whom improved oxygenation was not maintained. 13 (28%) of 46 patients were eventually intubated, seven (30%) of 23 responders and six (26%) of 23 non-responders (p=0·74). Five patients died during follow-up due to underlying disease, unrelated to study procedure.
Interpretation
Prone positioning was feasible and effective in rapidly ameliorating blood oxygenation in awake patients with COVID-19-related pneumonia requiring oxygen supplementation. The effect was maintained after resupination in half of the patients. Further studies are warranted to ascertain the potential benefit of this technique in improving final respiratory and global outcomes.
Funding
University of Milan-Bicocca.
Introduction
The COVID-19 pandemic has led to a substantial increase in the number of patients admitted to hospital with respiratory failure.1 Most of these patients require non-invasive ventilatory support; however, the failure rate (ie, worsening of condition or lack of improvement) is extremely high and intubation is often necessary, rapidly saturating resources and the availability of intensive care unit (ICU) beds, potentially leading to increased mortality.2, 3 Acute respiratory distress syndrome (ARDS) is a major complication of COVID-19 that occurs in 20–41% of patients with severe disease.4, 5
Treatment of ARDS requires tracheal intubation and mechanical ventilation, and patients can benefit from prone positioning, which has been shown to improve oxygenation and reduce mortality in non-COVID-19-related ARDS.6 Increase in oxygenation is due to improved ventilation–perfusion matching in the prone position, because the dorsal areas (which anatomically have an increased number of alveolar units7) are no longer compressed by the weight of the abdominal cavity and the mediastinum, and can re-open, leading to recruitment of more gas-exchange-efficient regions.8, 9 The mortality benefit cannot be explained solely by improved oxygenation and has been linked to decreased overdistention and cyclic alveolar recruitment–de-recruitment within tidal breaths, with a decreased risk of ventilator-induced lung injury.10, 11, 12 Prone positioning is also a mainstay of treatment in COVID-19-related ARDS and has been recommended in the Surviving Sepsis Campaign COVID-19 guidelines.13, 14, 15
Research in context.
Evidence before this study
Prone positioning during invasive mechanical ventilation is known to improve oxygenation and reduce mortality in patients with severe acute respiratory distress syndrome (ARDS). Anecdotal evidence and social media interest in the use of prone positioning in spontaneously breathing, non-intubated patients undergoing supplemental oxygen therapy have emerged. We searched PubMed and medRxiv for articles published in English with no date restrictions on April 20, 2020, and we repeated our search on May 27, 2020, about the use of prone positioning in awake patients with ARDS or COVID-19 using combinations of the terms “prone positioning”, “awake”, “spontaneously breathing”, “ARDS”, and “COVID”. We had no restrictions on the study type, including case series. In our first search, we identified five studies (three case series, a retrospective study, and a small prospective trial) and in our second search we identified four additional studies in patients with COVID-19.
Added value of this study
To our knowledge, this is the largest prospective study of the short-term effects of the use of prone positioning in awake, spontaneously breathing patients with COVID-19 undergoing supplemental oxygen therapy. We showed that awake prone positioning is feasible and improves oxygenation in patients with respiratory failure due to COVID-19-related pneumonia; however, this improvement was not maintained when patients were placed back in the supine position.
Implications of all the available evidence
Our study supports previous reports on the benefit of prone positioning in awake patients with respiratory failure due to interstitial confirmed pneumonia. Moreover, it is the first formal demonstration of the feasibility of this technique in patients affected by COVID-19. Further studies should be done to assess the safety and the medium-term and long-term outcomes of awake prone positioning on respiratory parameters, use of critical care resources, and survival.
Some investigators have reported the application of prone positioning in spontaneously breathing, non-intubated patients treated with standard oxygen therapy, continuous positive airway pressure (CPAP), or non-invasive ventilation.16, 17, 18 In this setting, prone positioning seems to improve oxygenation and might decrease respiratory effort, which could be particularly beneficial in patients at increased risk of self-induced lung injury.19 Therefore, this position might postpone or avoid tracheal intubation and its inherent risks (both linked to the procedure itself and to subsequent superinfections). A decrease in the need for intubation, and subsequent admission to ICU, might also prove beneficial in resource-limited scenarios. At the same time, this procedure could carry some risks associated with the change of position (eg, vomiting, thromboembolism) or delayed intubation.
Although prone positioning had been proposed for patients with COVID-19 in personal communications20, 21 and on social media22 at the start of this study, no study had, to our knowledge, systematically addressed the safety, feasibility, and efficacy of prone positioning in awake, non-intubated patients with COVID-19-related pneumonia. During the review process of this Article, the use of awake prone positioning in patients with COVID-19 was reported, suggesting a substantial improvement in oxygenation and a lack of major side-effects when patients were moved into prone position.23, 24, 25, 26
Methods
Study design and participants
In this single-centre, prospective, feasibility study, we enrolled patients over a period of 2 weeks from medical wards, the emergency department, and the respiratory high-dependency unit of our hospital (San Gerardo Hospital, Monza, Italy), which is a large tertiary teaching hospital. Due to the enormous volume of patients admitted to hospital and fulfilling inclusion criteria, consecutive enrolment was impossible.
Patients were eligible for inclusion if they were aged 18–75 years, had been admitted to hospital with a confirmed diagnosis of COVID-19-related pneumonia requiring supplemental oxygen or non-invasive CPAP,27 and gave written or witnessed verbal informed consent. Patients were excluded if they were pregnant, uncollaborative or had an altered mental status, had a New York Heart Association class below II, had increased pro-B-type natriuretic peptide concentrations (more than twice the upper normal limit), had chronic obstructive pulmonary disease requiring home non-invasive ventilation or oxygen therapy, had contraindications (as decided by the attending physician), or had impending intubation (on the basis of clinical judgment, including clinical and physiological parameters).
This study received Ethics Committee approval (ASST Monza, 3345). All patients provided written or verbal (witnessed by health-care staff unrelated to the trial) informed consent at the time of enrolment. The study is reported according to STROBE guidelines.28
Procedures
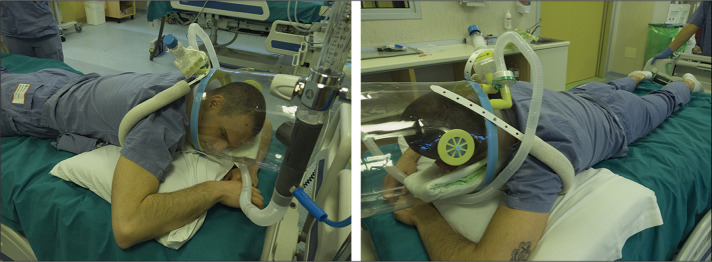
In all patients, a diagnosis of COVID-19 was made with RT-PCR using a nasal swab, if a patient was deemed by their physician to potentially be infected. Once enrolled, baseline data were collected (timepoint SP1), including demographic and anthropometric data, a baseline arterial blood gas measurement (whenever possible, an arterial line was placed, but some patients received multiple arterial stabs), and ventilation parameters including respiratory rate, fractional concentration of oxygen in inspired air (FiO2) and positive end-expiratory pressure (PEEP), use of accessory respiratory muscles, and subjective comfort. Subsequently, each patient was helped into the prone position (figure 1 ; video) and data were collected again after approximately 10 min (timepoint PP1). The patient was then encouraged to maintain the prone position for at least 3 h before being helped back into the supine position. Clinical data were collected again 1 h after resupination (timepoint SP2). Wherever possible, oxygen delivery interface, FiO2, and PEEP were not changed between timepoints. Patients were not kept awake while in the prone position, but allowed to sleep or rest during that period. If patients asked to resume the supine position before the 3-h period was complete, the prone position was considered unfeasible and the reason was reported. At the end of the 3-h period, patients were free to resume the supine position or maintain the prone position at their discretion for up to 8 h in total. Sessions of prone positioning were allowed in the days after the first session according to clinicians' indication and patients' preference. During these additional prone positioning sessions we did not collect data as for the first session, only the number of sessions they had.
Figure 1.
Prone positioning with a helmet interface to enable continuous positive airway pressure
Example demonstrated by volunteer.
Data were prospectively collected via the hospital's electronic health record or with a direct patient visit, as appropriate. Patients were followed-up until hospital discharge for occurrence of intubation, time to intubation, and death.
We did not collect data on any prespecified adverse events. Comfort was assessed by asking the patient how they would evaluate their comfort on a scale of “excellent”, “good”, “acceptable”, or “unacceptable”.
Outcomes
The main study outcome was the change in oxygenation (arterial partial pressure of oxygen [PaO2]/FiO2 ratio) between timepoints SP1 and SP2, as an index of pulmonary recruitment.
Secondary outcomes were the safety and feasibility of prone positioning (prone position lasting at least 3 h), effect of prone positioning on arterial partial pressure of carbon dioxide (PaCO2) and dyspnoea, and predictors of response to the prone position (ie, differences between responders and non-responders). Responders were defined as patients with an increased PaO2/FiO2 ratio from SP1 to SP2 for the main analysis. Incidence and time to tracheal intubation were also recorded.
Statistical analysis
We assumed an improvement in PaO2/FiO2 ratio from 124 mm Hg (SD 50) at SP1 to 140 mm Hg (61) at SP217 with a correlation equal to 0·8. This assumption leads to an SD of paired (individual) differences equal to 37. Thus, we estimated that enrolling 40 patients would provide the study with a two-sided significance level of 0·05 and a power of 80% to detect a difference in PaO2/FiO2 ratio of at least 16 mm Hg. We allowed for the procedure to be unfeasible in 20% of patients, thus we planned to enrol a minimum of 48 patients. Given the fast enrolment rate and the lack of adverse events in our initial sample, we decided to enlarge the sample size by an additional 15%, enrolling 56 patients overall.
We describe continuous data using summary indicators to account for different distribution shapes. We calculated mean (SD) and median (IQR) for continuous variables, based on their distribution. We describe discrete variables using frequencies and percentages. We used data from an independent cohort of patients admitted in the same hospital during the study period (STORM study, Spallanzani Institute approval number 84/2020; NCT04424992) to evaluate differences between enrolled and non-enrolled patients of the hospital. We first describe data for the whole study sample, and estimate the probability of feasibility of the procedure using the 95% CI according to the Clopper–Pearson method.29 We then describe data for the subset of patients for whom the procedure was feasible at the three timepoints SP1, PP1, and SP2 for the variables assessed repeatedly. We compared distributions of continuous variables between the three timepoints using the paired Student's t test on pairs of timepoints, considering SP1 versus PP1 and SP1 versus SP2. We compared proportions of dichotomous variables between several study timepoints using McNemar's test for paired proportions on pairs of timepoints. We estimated the probability of response using 95% CIs according to the Clopper–Pearson method. We compared distributions of continuous variables between subgroups defined by response using the unpaired Student's t test. We validated this comparison using univariate logistic regression models on the binary outcome defined by the response (ie, response vs no response), considering the continuous variable as an explanatory variable. We compared proportions of dichotomous variables between independent groups using the χ2 test. We present the longitudinal trajectory of the response parameters on the three timepoints graphically using profile plots in subgroups defined by the binary response (ie, PaO2/FiO2 ratio difference >0). We investigated the longitudinal trajectory of PaO2/FiO2 for each patient, restricted to the two timepoints SP1 and SP2, using a general linear mixed model with random intercepts and random slopes under an independent correlation matrix. The explanatory factors included in this model in addition to time were the variables associated to the binary response outcome in the univariate analysis. Interactions between time and explanatory factors were also included.
We did several post-hoc sensitivity analyses. We did sensitivity analyses in which we defined responders as patients with a 10% and 20% increase in their PaO2/FiO2 ratio, and repeated the main analyses using these responders. We also did post-hoc sensitivity analyses excluding patients with a baseline PaO2/FiO2 ratio of more than 300 mmHg, and including patients in whom prone positioning was not feasible. We did not apply any corrections for multiple analyses.
From follow-up data, a small additional dataset was compiled post hoc for some additional analyses requested during the review process (SP2 response in patients with unfeasible prone positioning and clinical variables 5 days after enrolment).
We considered p values of less than 0·05 to be significant. We did all analyses using STATA software (version 16.0). This study is registered on ClinicalTrials.gov, NCT04365959.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Between March 20 and April 9, 2020, 667 patients with COVID-19-related pneumonia were admitted to San Gerardo Hospital. Patients who were approached by the investigators' team (AC, GB, DW, MDP, AS, PF, MC, GM, EC, PB, AB, GF; which always included a senior doctor in intensive care medicine) were mainly referred by the medical emergency team (also including an intensivist) or by the attending clinicians. Due to the limited resources secondary to the increase in admissions to hospital and the voluntary participation of only specific wards, 57 patients were approached and provided consent to participate. One patient was later excluded because they met an exclusion criterion that had previously not been considered. Thus, 56 patients were enrolled in the final cohort (figure 2 ). When compared with aggregated data from a simultaneously recorded registry from the same hospital (appendix 2 p 2), patients who were enrolled were significantly younger than those non-enrolled (mean age 67·2 years [SD 10·9] in non-enrolled vs 57·4 [7·4] in enrolled cohort; p<0·0001; difference 9·8 years [95% CI 6·8 to 13·6]), but did not seem to differ in the baseline levels of PaO2/FiO2 ratio (mean ratio 205·8 mm Hg [SD 106·2] in non-enrolled vs 180·5 mm Hg [76·6] in enrolled cohort; p=0·14; difference 24·7 [95% CI −8·4 to 59·1]) and C-reactive protein (11·7 [SD 9·4] in non-enrolled vs 11·1 [7·1] in enrolled cohort; difference 0·6 [–2·4 to 3·7]; p=0·66; difference 0·6 [95% CI −2·4 to 3·7]).
Figure 2.
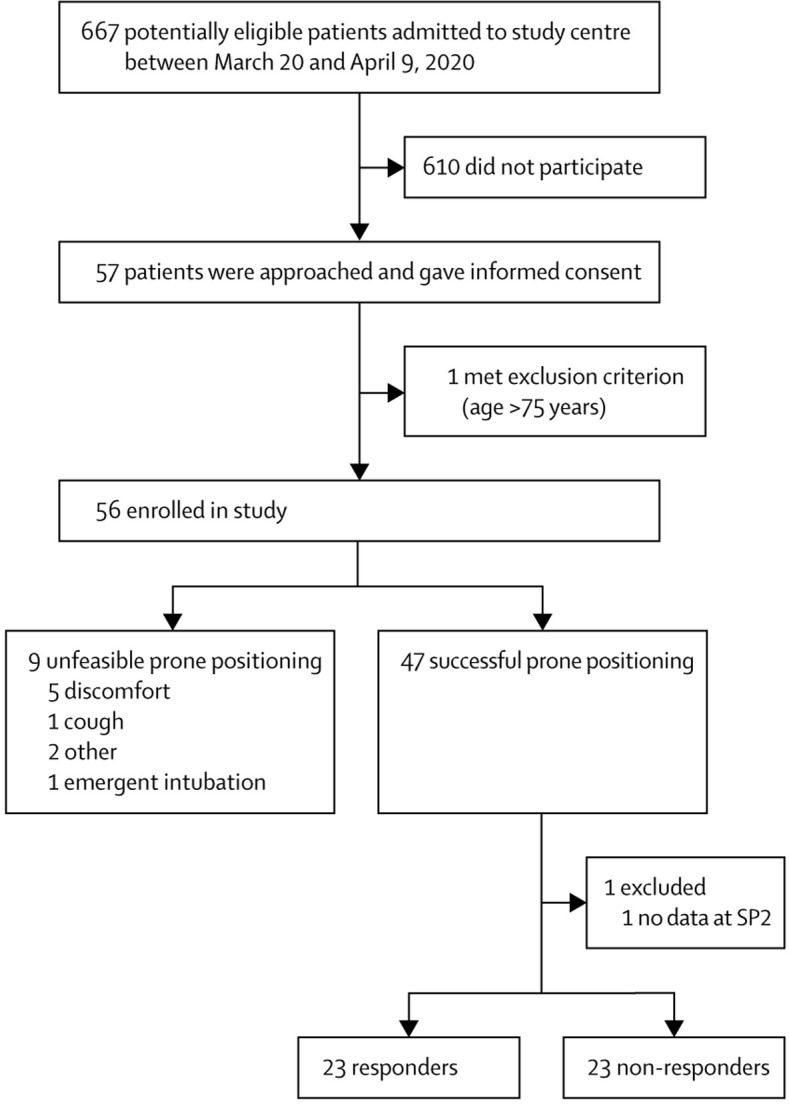
Study profile
Responders were defined as patients with an increased ratio of partial pressure of oxygen to fractional concentration of oxygen in inspired air between SP1 to SP2 for the main analysis. All other patients who were successfully put in the prone position were non-responders. SP1=baseline supine position. SP2=1 h after resuming supine position.
Table 1 shows the characteristics of the study population (further details on parameters collected in the emergency department are in appendix 2 [p 2]). The mean age was 57·4 years (SD 7·4), the mean BMI was 27·5 kg/m2 (3·7), and 44 (78%) patients were male. Common comorbidities included hypertension and diabetes, and most patients were either former smokers or had never smoked. Notably, baseline blood tests showed an activated inflammatory response (mean C-reactive protein concentration of 11·1 mg/dL [SD 7·1], procalcitonin concentration of 0·7 ng/mL [SD 1·6], lactate dehydrogenase concentration of 411·0 U/L [162·2]) and coagulation cascade (mean D-dimer concentration of 640·9 ng/mL [521·5]; all baseline clinical characteristics for the full cohort are in appendix 2 [pp 3–4]). Patients were admitted to hospital a mean of 7·8 days (SD 4·2) after symptom onset, and were put into the prone position a mean of 3·5 days (3·1) after their admission to hospital. 44 (79%) patients were treated with helmet CPAP and 12 (21%) with standard oxygen face mask. Overall, 18 (32%) enrolled patients were eventually intubated.
Table 1.
Baseline demographic and clinical characteristics of analysable population
| Analysable population (n=56) | ||
|---|---|---|
| Age, years | 57·4 (7·4) | |
| Sex | ||
| Female | 12 (21%) | |
| Male | 44 (79%) | |
| BMI, kg/m2 | 27·5 (3·7) | |
| Time between symptom onset and admission to hospital, days | 7·8 (4·2) | |
| Time between admission to hospital and prone positioning, days | 3·5 (3·1) | |
| Comorbidities | ||
| Previous myocardial infarction | 4 (7%) | |
| Congestive heart failure | 0 | |
| Hypertension | 23 (41%) | |
| Vascular disease | 3 (5%) | |
| Chronic bronchopulmonary disease | 2 (4%) | |
| Gastric or liver disease | 3 (5%) | |
| Diabetes | 8 (14%) | |
| Moderate-to-severe chronic kidney disease (eGFR <59 mL/min) | 0 | |
| Solid malignancy | 3 (5%) | |
| Smoking history | ||
| Active smoker | 1 (2%) | |
| Former smoker (<1 year) | 1 (2%) | |
| Former smoker (≥1 year) | 20 (36%) | |
| Never smoked | 29 (52%) | |
| Not declared | 5 (9%) | |
| Oxygen delivery interface | ||
| Helmet CPAP | 44 (79%) | |
| Reservoir mask | 9 (16%) | |
| Venturi mask | 3 (5%) | |
Data are n (%) or mean (SD). BMI=body-mass index. CPAP=continuous positive airway pressure. eGFR=estimated glomerular filtration rate.
Prone positioning was feasible in 47 patients (83·9% [95% CI 71·7 to 92·4]). Prone positioning was unfeasible in nine patients, reasons for which included discomfort during positioning (n=5), coughing (n=1), uncooperativeness of the patient (n=1), and decrease in oxygenation and worsening of respiratory mechanics (n=2, one of whom required emergent intubation). No differences in the main clinical features were found between those in whom prone positioning was feasible and those in whom it was unfeasible, except for increased concentrations of transaminases, lactate dehydrogenase, and urea in those for whom prone positioning was not feasible (appendix 2 pp 5–6). Among patients for whom positioning was feasible, most maintained proning for the initial 3 h period (median 3 h [IQR 3–4]), and 25 patients maintained prone positioning for longer than 3 h. No other relevant side-effects or complications were observed. In the days after prone positioning, 23 (50% [95% CI 36–65]) of the 46 patients in whom proning was initially feasible had further prone positioning sessions (up to seven), outside of the study protocol. In our post-hoc comparison, clinical variables 5 days after enrolment in patients who received only one, two to three, or four or more prone positioning cycles are reported in appendix 2 (p 6). From a descriptive standpoint, these variables did not show relevant differences.
Since the number of participants in whom the procedure was feasible was lower than anticipated, we did a post-hoc power calculation. The detectable effect size (ratio between the difference between means and the SD of paired differences) was equal to 0·49. Considering the observed SD of paired differences to be equal to 78, the corresponding detectable difference between means increased from 18 to 38.
Table 2 shows the arterial blood gas values and ventilation parameters of the 46 patients (data were missing for one patient at SP2) who tolerated prone positioning at the three study timepoints. One patient had a change in interface between study timepoints (underwent prone positioning on CPAP, but at the SP2 timepoint he had nasal cannulae). For all other patients, the interface and settings were not changed between study timepoints. Oxygenation improved on average by more than 50% from SP1 to PP1 (difference in PaO2/FiO2 ratio 104·9 mm Hg [95% CI 70·9 to 134·0]), although this improvement was on average not significant when supine position was resumed (SP1 vs SP2 difference in PaO2/FiO2 ratio 12·3 mm Hg [95% CI −10·9 to 35·5]; table 2, figure 3 ). Improvement in oxygenation was maintained in 23 (50% [95% CI 34·9 to 65·1]) patients, who were categorised as responders. Prone positioning did not significantly decrease accessory muscle use (SP1 vs SP2 difference −8·7% [95% CI −22·7 to 5·2]) or dyspnoea (SP1 vs SP2 difference −10·8% [–23·8 to 2·1]). No difference was observed in PaCO2 or respiratory rate at any timepoint.
Table 2.
Study timepoint analysis among patients who tolerated prone positioning and had available data across the three study timepoints (n=46)
| SP1 | PP1 | SP2 |
SP1 vs PP1 |
SP1 vs SP2 |
||||
|---|---|---|---|---|---|---|---|---|
| Difference (95% CI) | p value | Difference (95% CI) | p value | |||||
| FiO2, % | 68·9 (19·8) | 68·9 (19·8) | 65·9 (20·2) | 0·0 (0·0 to 0·0) | 1·0 | 3·0 (−0·7 to 6·8) | 0·11 | |
| PEEP, cm H2O | 8·3 (2·3) | 8·3 (2·3) | 8·3 (2·3) | 0·1 (−0·1 to 0·2) | 1·0 | 0·2 (−0·1 to 0·2) | 0·32 | |
| Arterial blood gas | ||||||||
| pH | 7·46 (0·03) | 7·46 (0·04) | 7·46 (0·03) | 0·0 (0·0 to 0·0) | 0·50 | 0·0 (0·0 to 0·0) | 0·08 | |
| PaO2, mm Hg | 117·1 (47·4) | 200·4 (110·9) | 121·4 (69·6) | 83·3 (56·1 to 110·4) | <0·0001 | 4·3 (−13·2 to 21·6) | 0·60 | |
| PaO2/FiO2 ratio, mm Hg | 180·5 (76·6) | 285·5 (112·9) | 192·9 (100·9) | 104·9 (70·9 to 134·0) | <0·0001 | 12·3 (−10·9 to 35·5) | 0·29 | |
| PaCO2, mm Hg | 35·3 (4·9) | 35·6 (4·5) | 35·5 (4·4) | 0·4 (−1·3 to 0·6) | 0·48 | 0·3 (−0·9 to 1·4) | 0·64 | |
| SaO2, % | 97·2 (2·0) | 98·4 (1·3) | 97·1 (2·0) | 1·2 (0·8 to 1·7) | <0·0001 | 0·1 (−1·0 to 0·4) | 0·35 | |
| SpO2, % | 97·2 (2·8) | 98·2 (2·2) | 97·1 (1·9) | 1·0 (0·3 to 2·0) | 0·01 | 0·1 (−0·8 to 1·0) | 0·87 | |
| Respiratory rate, breaths per min | 24·5 (5·5) | 24·5 (6·9) | 23·9 (6·3) | 0·1 (−1·0 to 1·5) | 0·71 | −0·6 (−2·0 to 0·8) | 0·40 | |
| Use of accessory respiratory muscles | 9 (20%) | 7 (15%) | 5 (11%) | −4·4% (−15·0 to 6·2) | 0·32 | −8·7% (− 22·7 to 5·3) | 0·16 | |
| Dyspnoea | 7 (15%) | 4 (9%) | 2 (4%) | −6·5% (− 19·8 to 6·8) | 0·26 | −10·9% (− 23·8 to 2·1) | 0·06 | |
| Comfort* | .. | .. | .. | .. | 0·23 | .. | 0·06 | |
| Unacceptable | 0 | 0 | 0 | .. | .. | .. | .. | |
| Acceptable | 10 (22%) | 15 (33%) | 4 (9%) | .. | .. | .. | .. | |
| Good | 23 (50%) | 19 (41%) | 24 (52%) | .. | .. | .. | .. | |
| Excellent | 13 (28%) | 12 (26%) | 17 (37%) | .. | .. | .. | .. | |
Data are mean (SD) or n (%), unless otherwise indicated. p values were calculated using Student's t test for continuous variables and the χ2 test for categorical variables. FiO2=fractional concentration of oxygen in inspired air. PaCO2=arterial partial pressure of carbon dioxide. PaO2=arterial partial pressure of oxygen. PEEP=positive end-expiratory pressure. SaO2=arterial oxygen saturation of haemoglobin. SP1=baseline supine position. PP1=10 min after prone positioning. SP2=1 h after resuming supine position. SpO2=peripheral oxygen saturation of haemoglobin.
A comparative analysis of comfort was done across all categories.
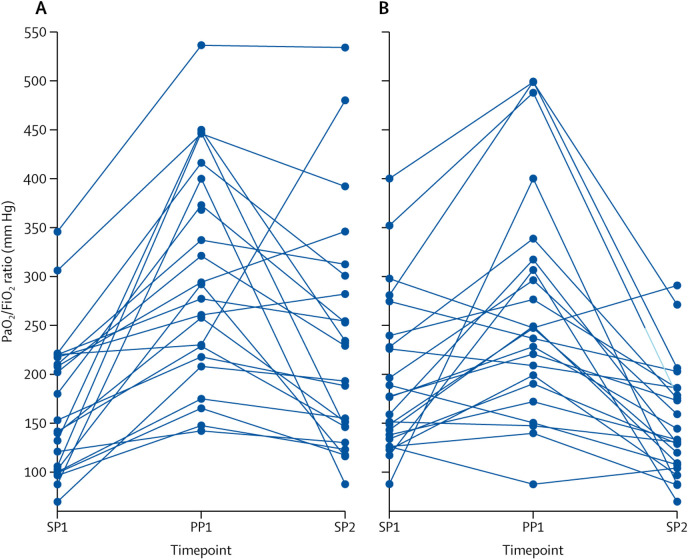
Figure 3.
Per-patient trajectory of PaO2/FiO2 at the three study timepoints, SP1, PP1, and SP2, for responders (A) and non-responders (B)
Each line is the trajectory of one patient, with datapoints showing the PaO2/FiO2 ratio at the three timepoints. Responders were defined as patients with an increased PaO2/FiO2 ratio between SP1 to SP2 for the main analysis. All other patients who were successfully put in the prone position were non-responders. PaO2=partial pressure of oxygen. FiO2=fractional concentration of oxygen in inspired air. SP1=baseline supine position. PP1=10 min after prone positioning. SP2=1 h after resuming supine position.
The results of our main analysis were also supported by a post-hoc sensitivity analysis excluding patients (n=4) with a baseline PaO2/FiO2 ratio of more than 300 mm Hg (appendix 2 pp 7–8) and taking into account patients in whom prone positioning was not feasible (appendix 2 p 9).
A comparison of baseline clinical and demographic data and secondary outcomes for the 23 patients who responded to prone positioning and the 23 patients who did not is shown in table 3 . Outcomes for the overall population are in appendix 2 (pp 3–5). Responders had significantly lower platelets and higher C-reactive protein and lactate dehydrogenase concentrations than did non-responders. Prone positioning was done significantly earlier in patients who responded than in those who did not respond (2·7 days [SD 2·1] vs 4·6 days [3·7] from hospital admission; difference 1·9 days [95% CI 0·1 to 3·7]). Post-hoc sensitivity analyses with more restrictive definitions of responders, and including patients for whom prone positioning was not feasible, were generally in agreement with the main analysis (appendix 2 pp 10–17). These results were further supported in the mixed model analysis considering the association between continuous PaO2/FiO2 values and time (appendix 2 p 18). Stepwise increases in platelet concentrations and time between hospital admission and prone positioning were not associated with trajectory of PaO2/FiO2 ratio between SP1 and SP2. However, when considering the role of C-reactive protein, increasing concentrations were associated with reduced PaO2/FiO2 at SP1, with a significant increase at SP2, further supporting the role of inflammation in the response to prone positioning. A similar pattern was observed for lactate dehydrogenase. The association between the PaO2/FiO2 trajectories and the role of lactate dehydrogenase and C-reactive protein were supported when considered jointly in the mixed model.
Table 3.
Comparison between responders and non-responders
| Responders (n=23) | Non-responders (n=23) | Difference (95% CI) | p value | ||
|---|---|---|---|---|---|
| Age, years | 58·5 (7·5) | 55·9 (7·0) | 2·7 (−7·0 to 1·7) | 0·22 | |
| Sex | .. | .. | .. | 1·0 | |
| Female | 6 (26%) | 6 (26%) | .. | .. | |
| Male | 17 (74%) | 17 (74%) | .. | .. | |
| BMI, kg/m2 | 27·3 (3·5) | 27·4 (3·7) | −0·12 (−2·3 to 2·1) | 0·92 | |
| Time between symptom onset and admission to hospital, days | 8·1 (4·8) | 7·4 (4·3) | −0·7 (−3·4 to 1·9) | 0·58 | |
| Time between admission to hospital and prone positioning, days | 2·7 (2·1) | 4·6 (3·7) | −1·9 (−3·7 to 0·1) | 0·04 | |
| Time between symptom onset and prone positioning, days | 10·8 (4·9) | 12·0 (4·3) | −1·1 (−3·9 to 1·6) | 0·41 | |
| Comorbidities | |||||
| Previous myocardial infarction | 1 (4%) | 1 (4%) | .. | .. | |
| Congestive heart failure | 0 | 0 | .. | .. | |
| Hypertension | 9 (39%) | 9 (39%) | .. | .. | |
| Vascular disease | 0 | 3 (13%) | .. | .. | |
| Chronic bronchopulmonary disease | 1 (4%) | 0 | .. | .. | |
| Gastric or liver disease | 1 (4%) | 1 (4%) | .. | .. | |
| Diabetes | 4 (17%) | 2 (9%) | .. | .. | |
| Moderate-to-severe chronic kidney disease (eGFR <59 mL/min) | 0 | 0 | .. | .. | |
| Solid malignancy | 1 (4%) | 2 (9%) | .. | .. | |
| Smoking history | |||||
| Active smoker | 0 | 1 (4%) | .. | .. | |
| Former smoker (<1 year) | 0 | 1 (4%) | .. | .. | |
| Former smoker (≥1 year) | 9 (39%) | 7 (30%) | .. | .. | |
| Never smoked | 12 (52%) | 13 (57%) | .. | .. | |
| Not declared | 2 (9%) | 1 (4%) | .. | .. | |
| Baseline blood values | |||||
| Sodium, mmol/L | 137·7 (2·6) | 138·78 (3·9) | −1·1 (− 3·1 to 0·9) | 0·27 | |
| Potassium, mmol/L | 3·9 (0·5) | 4·1 (0·6) | −0·2 (−0·5 to 0·1) | 0·21 | |
| Chlorine, mmol/L | 101·8 (3·3) | 100·2 (3·6) | 1·6 (−3·7 to 0·5) | 0·13 | |
| Creatinine, mg/dL | 0·9 (0·2) | 0·8 (0·2) | 0·0 (0·0 to 0·0) | 0·21 | |
| Urea, mg/dL | 31·0 (8·2) | 30·3 (8·0) | 0·7 (−5·8 to 4·4) | 0·78 | |
| White blood cells, 103/μL | 7·7 (3·6) | 7·3 (3·0) | 0·3 (−2·3 to 1·6) | 0·75 | |
| Platelets, 103/μL | 241·1 (101·9) | 319·8 (120·6) | −78·7 (−145·0 to 12·3) | 0·02 | |
| Haemoglobin, g/dL | 12·8 (2·0) | 12·4 (1·3) | 0·4 (−1·4 to 0·6) | 0·40 | |
| Haematocrit, % | 37·5 (5·5) | 36·5 (4·1) | 1·0 (−3·9 to 1·9) | 0·50 | |
| Bilirubin, mg/dL | 0·6 (0·5) | 0·5 (0·3) | 0·0 (−0·3 to 0·1) | 0·50 | |
| C-reactive protein, mg/L | 12·7 (6·9) | 8·4 (6·2) | 4·3 (8·3 to 0·3) | 0·03 | |
| Procalcitonin, ng/mL | 0·6 (1·1) | 0·4 (0·4) | 0·3 (−0·9 to 0·4) | 0·44 | |
| Lactate dehydrogenase, U/L | 449·7 (199·0) | 337·9 (94·1) | 111·8 (15·0 to 208·5) | 0·02 | |
| Aspartate aminotransferase, U/L | 49·7 (26·5) | 48·0 (24·4) | 1·7 (−18·7 to 15·2) | 0·83 | |
| Alanine aminotransferase, U/L | 49·4 (37·5) | 57·7 (37·0) | −8·2 (−31·2 to 14·7) | 0·47 | |
| International normalised ratio | 1·3 (0·6) | 1·1 (0·1) | 0·2 (−0·4 to 0·1) | 0·24 | |
| Activated partial thromboplastin time ratio | 1·1 (0·1) | 1·0 (0·1) | 0·0 (−0·1 to 0·0) | 0·43 | |
| D-dimer, ng/mL | 576·4 (432·2) | 632·9 (431·8) | −56·5 (−342·4 to 229·3) | 0·69 | |
| Arterial blood gas at SP1 | |||||
| pH | 7·46 (0·0) | 7·5 (0·0) | 0·0 (0·0 to 0·0) | 0·79 | |
| PaO2, mm Hg | 114·5 (49·1) | 119·7 (46·7) | −5·2 (−33·6 to 23·3) | 0·72 | |
| PaCO2, mm Hg | 35·1 (5·2) | 35·4 (4·6) | −0·3 (−3·2 to 2·6) | 0·85 | |
| SaO2, % | 97·1 (2·1) | 97·3 (1·9) | −0·2 (−1·4 to 1·0) | 0·73 | |
| Arterial blood gas at PP1 | |||||
| pH | 7·5 (0·0) | 7·5 (0·0) | 0·0 (0·0 to 0·0) | 0·44 | |
| PaO2, mm Hg | 225·3 (112·6) | 175·5 (105·8) | 49·8 (−15·2 to 114·7) | 0·13 | |
| PaCO2, mm Hg | 35·3 (5·4) | 36·0 (3·6) | −0·7 (−3·4 to 2·0) | 0·61 | |
| SaO2, % | 98·5 (1·4) | 98·4 (1·3) | 0·2 (−1·0 to 0·7) | 0·72 | |
| Arterial blood gas at SP2 | |||||
| pH | 7·5 (0·0) | 7·5 (0·0) | 0·0 (0·0 to 0·0) | 0·84 | |
| PaO2, mm Hg | 154·0 (84·9) | 88·7 (21·8) | 65·3 (28·4 to 102·1) | <0·0001 | |
| PaCO2, mm Hg | 35·1 (4·5) | 36·0 (4·3) | −1·0 (−3·6 to 1·7) | 0·47 | |
| SaO2, % | 97·8 (2·1) | 96·4 (1·8) | 1·4 (0·2 to 2·6) | 0·03 | |
| Secondary outcomes | |||||
| Tracheal intubation | 7 (30·4%) | 6 (26·1%) | 4·3 (−30·7 to 21·6) | 0·74 | |
| Time to intubation | 2 (2 to 4) | 2·5 (1·0 to 5·0) | .. | 0·45 | |
| Duration of prone positioning, h | 3·5 (3·0 to 4·0) | 3·5 (3·0 to 4·0) | .. | 0·99 | |
| Prone positioning for >3 h | 12 (52·2%) | 13 (56·5%) | −4·3% (−33·1 to 24·4) | 0·77 | |
| Number of prone positioning cycles | 2 (1 to 3) | 2 (1 to 3) | .. | 0·94 | |
| More than one prone positioning cycle | 12 (52·1%) | 11 (47·8%) | 4·3% (−34·0 to 24·9) | 0·76 | |
Data are mean (SD) or n (%), unless otherwise indicated. Differences are not calculated for data presented as median (range) or for small proportions. p values were calculated using Student's t test for continuous variables and the χ2 test for categorical variables. BMI=body-mass index. CPAP=continuous positive airway pressure. FiO2=fractional concentration of oxygen in inspired air. eGFR=estimated glomerular filtration rate. PaCO2=arterial partial pressure of carbon dioxide. PaO2=arterial partial pressure of oxygen. PEEP=positive end-expiratory pressure. SaO2=arterial oxygen saturation of haemoglobin. SP1=baseline supine position. PP1=10 min after prone positioning. SP2=1 h after resuming supine position. SpO2=peripheral oxygen saturation of haemoglobin.
Finally, incidence of tracheal intubation was not significantly different between responders and non-responders (seven [30%] vs six [26%]; p=0·74), occurring at a median of 2·0 days (IQR 2·0–4·0) after prone positioning among responders and 2·5 days (1·0–5·0) after prone positioning among non-responders (p=0·45), although the study was not adequately powered to detect these differences.
No adverse events related to the procedure were recorded. Overall, five deaths occurred in the whole cohort during follow-up that were not related to the procedure but to the underlying disease (COVID-19).
Discussion
In this observational prospective study, we investigated the feasibility and effect of prone positioning in spontaneously breathing, non-intubated patients with COVID-19-related pneumonia. We found that prone positioning was safe and feasible in most patients, and that it substantially improved physiological measures of oxygenation, although this effect was lost after reverting to the supine position. We found that earlier prone positioning and a more activated inflammatory response were associated with maintenance of improvement in oxygenation after resupination. Finally, we showed that patients who responded to prone positioning had no significant difference in the rate of intubation compared with non-responders.
Use of prone positioning in awake, spontaneously breathing patients has been reported before. In 2003, Valter and colleagues16 reported on four patients in whom awake prone positioning rapidly increased oxygenation and allowed the avoidance of intubation. Feltracco and colleagues30, 31 reported on five recipients of lung transplants who successfully underwent awake prone positioning with non-invasive ventilation, with the resolution of refractory hypoxaemia. However, because these studies were case series, little evidence can be extrapolated.
Scaravilli and colleagues17 did a retrospective study in 2015 on 15 non-intubated patients who overall underwent 43 prone positioning procedures. They found the procedure to be feasible in 95% of all procedures, and reported a significant increase in PaO2 from before prone positioning to after supine repositioning, with PaO2 returning to baseline levels 6 h after repositioning. However, the study by Scaravilli and colleagues is limited by its retrospective nature, the variation of ventilatory interface and settings between procedures, and the small number of patients contributing to a relatively large number of prone positioning procedures. By contrast, our study features a prospective design, in which prone positioning, ventilation interface and parameters, and data collection were standardised. Our study supports the feasibility of prone positioning in spontaneously breathing awake patients. Our results also show the absence of a long-lasting improvement in oxygenation, which is confirmed even when restricting the analysis to patients with a baseline PaO2/FiO2 ratio of less than 300 mm Hg. This finding can be explained by the fact that prone positioning might not determine stable recruitment of the dorsal lung regions, as previously described.32 Further supporting good tolerance among patients who are compliant, prone positioning sessions were repeated in the days after the initial session (outside of the study protocol), possibly indicating the presence of clinical or subjective benefit.
A large study on this topic was a 2020 trial by Ding and colleagues,18 in which the authors assessed the effect of adding prone positioning to use of high-flow nasal cannulae and non-invasive ventilation in 20 patients with moderate-to-severe ARDS. They found that the addition of prone positioning might have contributed to avoidance of intubation in 11 of 20 patients, and that the PaO2/FiO2 ratio was significantly higher in patients who avoided intubation. However, interpretation of these results is limited by the small sample size and the fact that not all patients were managed with only some of the four management strategies. Our results are also in line with those published on patients with COVID-19, which became available after our original submission of this report.23, 24, 25, 26, 33 For example, Caputo and colleagues23 applied prone positioning to patients with COVID-19 in the emergency department, showing a significant improvement in peripheral oxygen saturation.23 Sartini and colleagues25 applied CPAP on medical wards and also found a significant increase in oxygenation. By contrast with these reports, Elharrar and colleagues24 found that oxygenation improved during prone positioning in only six (25%) of 24 participants. The proportion of patients in whom the oxygenation improvement was maintained upon supine repositioning in all these studies varied substantially.
The description of our cohort provides further evidence that prone positioning in awake and spontaneously breathing patients is feasible outside of the ICU environment. Our data suggest that patients are more likely to respond to prone positioning if this procedure is done early after admission to hospital and in patients with increased inflammatory markers (eg, increased lactate dehydrogenase and C-reactive protein concentrations, and decreased platelet counts).34, 35 One of the possible explanations for this finding is the typically higher proportion of potentially recruitable lung in early phases of ARDS compared with later phases.36 Another explanation is persistence of perfusion redistribution, with improved ventilation–perfusion matching. Awake prone positioning did not seem to substantially improve long-term oxygenation in patients with COVID-19; however, it might decrease patients' oxygen requirements and allow the delay or avoidance of tracheal intubation, which might prove particularly valuable in scenarios where ICU bed capacity is reduced. An additional benefit of the reduction in FiO2, allowed by the improved oxygenation, is the decrease in the risk of reabsorption atelectasis. Moreover, even if the study was underpowered to assess the effect of clinical outcomes of prone positioning, we observed a non-significant decrease in dyspnoea, which is consistent with the hypothesis of a reduction in self-induced lung injury allowed by prone positioning and could be investigated as an outcome in larger studies. Finally, prone positioning could be used as an additional non-invasive tool in patients with a do-not-intubate order. Our data, without a control group, do not allow us to investigate the effect of prone positioning on the risk of intubation and, given the great variability in intubation rates reported for patients with COVID-19,37, 38 making a direct comparison is difficult. At the same time, the intubation rate in our study (28%) does not seem worryingly high for a cohort of patients with an average baseline PaO2/FiO2 ratio of 180·5 mm Hg (SD 76·6).
To our knowledge, this study is the largest prospective trial to analyse prone positioning in awake patients, and particularly in those with COVID-19-related pneumonia. This procedure in awake patients with COVID-19 has been unofficially reported in several personal communications among clinicians and on social networks.20, 21, 22 Hence, we believe that a formal assessment of prone positioning effects in this setting was required. The study protocol was clear, simple, and well defined, and we collected high quality, complete data. The external validity of our study is strengthened by the fact that patients were enrolled in various clinical settings within the study centre, each with differently experienced staff and resource availability.
Our study has several limitations. As stated, the lack of a control group (and randomisation) does not allow inference on patient-centred outcomes, such as mortality or need for tracheal intubation and ICU stay. Furthermore, enrolment of non-consecutive patients on the basis of recommendations made by the medical emergency team might have led to selection bias; however, the enrolled cohort seemed to be representative of the whole population of patients admitted to hospital with COVID-19 during the study period, at least for two crucial parameters, PaO2/FiO2 and C-reactive protein. Also, we did not collect data on lung morphology and effects of subsequent cycles of prone positioning that patients might have undergone. We did not prespecify in our protocol the collection of data on specific adverse events because we reasoned that the most likely complications due to prone positioning (eg, vomiting, nausea, device displacement) would have been clinically evident. However, none of these adverse events occurred. Clearly some complications would have required specific monitoring—eg, a doppler scan for venous thromboembolism—and as such might have been missed in our data collection. Additionally, a limitation of our study is that it is a single-centre study, and so might not be generalisable. Most patients were receiving CPAP, which is a standard of care in our institution, while high-flow oxygen is not available and non-invasive ventilation is limited to a few high-dependency units. Finally, the inclusion of patients both on CPAP and on conventional oxygen therapy might have diluted the effects of prone positioning on patients' oxygenation because those on CPAP might have more severe illness, while CPAP might also correct hypoxaemia more than standard oxygen delivery. Further studies exploring the effect of prone positioning on the delay and avoidance of intubation, need for ICU, duration of weaning from oxygen support, duration of hospital stay, and respiratory-related mortality are urgently warranted.
In summary, we found that prone positioning in awake, spontaneously breathing patients is feasible outside of the critical care environment in most patients. We observed improvement in oxygenation during prone positioning, which was maintained upon resupination by half of the patients for at least 1 h, and non-significant decrease in dyspnoea. With minimal patient discomfort, prone positioning was found to be a useful and patient-engaging technique to ameliorate blood gas parameters in the short term in patients with COVID-19-related pneumonia.
Data sharing
Individual participant data that underlie the results reported in this Article will be shared after de-identification (text, tables, figures, and appendices), beginning 6 months and ending 2 years after Article publication, to researchers who provide a methodologically sound proposal (upon approval of an internal commission), to achieve aims in an approved proposal for scientific purposes. Proposals should be directed to bicro@unimib.it. To gain access, data requestors will need to sign a data access agreement.
Acknowledgments
Acknowledgments
We thank Filippo Serra, Beatrice Noé, Luca Bastia, Giuseppe Lapadula, Giulia Gustinetti, Emanuela Rossi, Davide Gaudesi, and all the physicians, nurses, and health-care staff involved in the management of patients with COVID-19 in San Gerardo Hospital ASST Monza.
Contributors
GB, DW, and AS drafted the manuscript. GB, DW, and LA did the data analysis. All authors contributed to study conception and design, revision of the manuscript, data collection, and patient enrolment.
Declaration of interests
GF and GB report grants and personal fees from Dimar and personal fees from Intersurgical during the conduct of the study; and grants and personal fees from Draeger and personal fees from Getinge, Hamilton, and GE Healthcare, outside of the submitted work. GF has a patent pending for a system for non-invasive CPAP. GB has a patent pending for a system for non-invasive CPAP. All other authors declare no competing interests.
Supplementary Materials
Tutorial on prone positioning with helmet continuous positive airway pressure
Video performed with a volunteer. Codec: H.265/HVEC
YouTube URL: https://youtu.be/PYWkHxNwqLA
References
- 1.Grasselli G, Zangrillo A, Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ji Y, Ma Z, Peppelenbosch MP, Pan Q. Potential association between COVID-19 mortality and health-care resource availability. Lancet Glob Health. 2020;8:e480. doi: 10.1016/S2214-109X(20)30068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020;395:1225–1228. doi: 10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D, Hu B, Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu C, Chen X, Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.0994. published online March 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guérin C, Reignier J, Richard JC. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 7.Hopkins SR, Henderson AC, Levin DL. Vertical gradients in regional lung density and perfusion in the supine human lung: the Slinky effect. J Appl Physiol (1985) 2007;103:240–248. doi: 10.1152/japplphysiol.01289.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mure M, Glenny R, Domino K, Hlastala M. Pulmonary gas exchange in pigs improves in the prone position with abdominal distension. Crit Care. 1998;2(suppl 1):122. doi: 10.1164/ajrccm.157.6.9711104. [DOI] [PubMed] [Google Scholar]
- 9.Albert RK, Hubmayr RD. The prone position eliminates compression of the lungs by the heart. Am J Respir Crit Care Med. 2000;161:1660–1665. doi: 10.1164/ajrccm.161.5.9901037. [DOI] [PubMed] [Google Scholar]
- 10.Broccard AF, Shapiro RS, Schmitz LL, Ravenscraft SA, Marini JJ. Influence of prone position on the extent and distribution of lung injury in a high tidal volume oleic acid model of acute respiratory distress syndrome. Crit Care Med. 1997;25:16–27. doi: 10.1097/00003246-199701000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Valenza F, Guglielmi M, Maffioletti M. Prone position delays the progression of ventilator-induced lung injury in rats: does lung strain distribution play a role? Crit Care Med. 2005;33:361–367. doi: 10.1097/01.ccm.0000150660.45376.7c. [DOI] [PubMed] [Google Scholar]
- 12.Cornejo RA, Díaz JC, Tobar EA. Effects of prone positioning on lung protection in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2013;188:440–448. doi: 10.1164/rccm.201207-1279OC. [DOI] [PubMed] [Google Scholar]
- 13.Yang X, Yu Y, Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouadma L, Lescure FX, Lucet JC, Yazdanpanah Y, Timsit JF. Severe SARS-CoV-2 infections: practical considerations and management strategy for intensivists. Intensive Care Med. 2020;46:579–582. doi: 10.1007/s00134-020-05967-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alhazzani W, Møller MH, Arabi YM. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Intensive Care Med. 2020;46:854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valter C, Christensen AM, Tollund C, Schønemann NK. Response to the prone position in spontaneously breathing patients with hypoxemic respiratory failure. Acta Anaesthesiol Scand. 2003;47:416–418. doi: 10.1034/j.1399-6576.2003.00088.x. [DOI] [PubMed] [Google Scholar]
- 17.Scaravilli V, Grasselli G, Castagna L. Prone positioning improves oxygenation in spontaneously breathing nonintubated patients with hypoxemic acute respiratory failure: a retrospective study. J Crit Care. 2015;30:1390–1394. doi: 10.1016/j.jcrc.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Ding L, Wang L, Ma W, He H. Efficacy and safety of early prone positioning combined with HFNC or NIV in moderate to severe ARDS: a multi-center prospective cohort study. Crit Care. 2020;24:28. doi: 10.1186/s13054-020-2738-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195:438–442. doi: 10.1164/rccm.201605-1081CP. [DOI] [PubMed] [Google Scholar]
- 20.Farkas J. PulmCrit - awake proning for COVID-19. EMCrit. May 5, 2020. https://emcrit.org/pulmcrit/awake-prone-covid/
- 21.Farkas J. PulmCrit Wee- proning the non-intubated patient. EMCrit. Sept 21, 2016. https://emcrit.org/pulmcrit/proning-nonintubated/
- 22.@PulmCrit March 26, 2020. https://twitter.com/pulmcrit/status/1243306896804773888?s=21
- 23.Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: a single ED's experience during the COVID-19 pandemic. Acad Emerg Med. 2020;27:375–378. doi: 10.1111/acem.13994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elharrar X, Trigui Y, Dols A-M. Use of prone positioning in nonintubated patients with COVID-19 and hypoxemic acute respiratory failure. JAMA. 2020 doi: 10.1001/jama.2020.8255. published online May 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sartini C, Tresoldi M, Scarpellini P. Respiratory parameters in patients with COVID-19 after using noninvasive ventilation in the prone position outside the intensive care unit. JAMA. 2020 doi: 10.1001/jama.2020.7861. published online May 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Q, Wang T, Qin X, Jie Y, Zha L, Lu W. Early awake prone position combined with high-flow nasal oxygen therapy in severe COVID-19: a case series. Crit Care. 2020;24:250. doi: 10.1186/s13054-020-02991-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellani G, Patroniti N, Greco M, Foti G, Pesenti A. The use of helmets to deliver non-invasive continuous positive airway pressure in hypoxemic acute respiratory failure. Minerva Anestesiol. 2008;74:651–656. [PubMed] [Google Scholar]
- 28.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 29.Fleiss JL, Levin B, Paik MC. 3rd edn. John Wiley & Sons; New York, NY: 2003. Statistical methods for rates and proportions. [Google Scholar]
- 30.Feltracco P, Serra E, Barbieri S. Non-invasive ventilation in prone position for refractory hypoxemia after bilateral lung transplantation. Clin Transplant. 2009;23:748–750. doi: 10.1111/j.1399-0012.2009.01050.x. [DOI] [PubMed] [Google Scholar]
- 31.Feltracco P, Serra E, Barbieri S. Noninvasive high-frequency percussive ventilation in the prone position after lung transplantation. Transplant Proc. 2012;44:2016–2021. doi: 10.1016/j.transproceed.2012.05.062. [DOI] [PubMed] [Google Scholar]
- 32.Gattinoni L, Tognoni G, Pesenti A. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med. 2001;345:568–573. doi: 10.1056/NEJMoa010043. [DOI] [PubMed] [Google Scholar]
- 33.Telias I, Katira BH, Brochard L. Is the prone position helpful during spontaneous breathing in patients with COVID-19? JAMA. 2020 doi: 10.1001/jama.2020.8539. published online May 15. [DOI] [PubMed] [Google Scholar]
- 34.Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020 doi: 10.1515/cclm-2020-0369. published online April 10. [DOI] [PubMed] [Google Scholar]
- 35.Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grasso S, Mascia L, Del Turco M. Effects of recruiting maneuvers in patients with acute respiratory distress syndrome ventilated with protective ventilatory strategy. Anesthesiology. 2002;96:795–802. doi: 10.1097/00000542-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Meng L, Qiu H, Wan L. Intubation and ventilation amid the COVID-19 outbreak: Wuhan's experience. Anesthesiology. 2020;132:1317–1332. doi: 10.1097/ALN.0000000000003296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.CDC COVID-19 Response Team Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tutorial on prone positioning with helmet continuous positive airway pressure
Video performed with a volunteer. Codec: H.265/HVEC
YouTube URL: https://youtu.be/PYWkHxNwqLA
Data Availability Statement
Individual participant data that underlie the results reported in this Article will be shared after de-identification (text, tables, figures, and appendices), beginning 6 months and ending 2 years after Article publication, to researchers who provide a methodologically sound proposal (upon approval of an internal commission), to achieve aims in an approved proposal for scientific purposes. Proposals should be directed to bicro@unimib.it. To gain access, data requestors will need to sign a data access agreement.