Abstract
Asparaginase (ASNase) is an integral part of pediatric induction chemotherapy that has also been shown to improve adult survival rates; however, pegylated (PEG)-ASNase induces severe hepatotoxicity in this population. Recent case reports describe the incorporation of levocarnitine (LC) supplementation into PEG-ASNase-containing induction regimens to prevent or treat hepatotoxicity. Because LC facilitates the metabolism of free fatty acids (FFA), a primary fuel source for ALL cells, LC could potentially interfere with ALL chemotherapy efficacy. To test this, we employed in vitro and in vivo models of ALL. We show in vitro that LC supplementation does not impact cytotoxicity from vincristine, daunorubicin, dexamethasone, or ASNase on human ALL cells nor lead to an increase in ALL cell metabolic rate. In vivo, we demonstrate LC does not impair PEG-ASNase monotherapy in mice with syngeneic ALL. Together, our findings show that LC supplementation is a safe strategy to prevent/reverse ASNase-induced toxicities in preclinical models.
Keywords: Acute lymphoblastic leukemia (ALL), levocarnitine (L-carnitine), PEG-asparaginase, hepatotoxicity
Introduction
Survival for pediatric acute lymphoblastic leukemia (ALL) is excellent, with 5-year overall survival >90% [1,2]. L-asparaginase (ASNase), in its native or pegylated form (PEG-ASNase), is an integral component to pediatric regimens beginning with the induction phase. Severe toxicities associated with PEG-ASNase, including hypersensitivity and hepatotoxicity, led to the limited inclusion of PEG-ASNase into adult ALL chemotherapy regimens for the past several decades. Yet, recent retrospective studies and clinical trials have reported improved remission and survival rates in older adolescents and adults receiving “pediatric-inspired” regimens that include PEG-ASNase/ASNase [3–6]. While promising, hepatotoxicity secondary to PEG-ASNase continues to be a serious dose-limiting complication resulting in reduced and/or delayed administration of hepatic-metabolized chemotherapies [7–9] and possibly contributing to an increased risk of relapse. Since relapse is highly predictive of poor survival for both children and adults with ALL [1,10], it is important to develop strategies to incorporate ASNase into these regimens while minimizing toxicities.
To improve tolerance for PEG-ASNase, investigators have explored clinical interventions to reverse or prevent these adverse effects. Though the exact mechanism of PEG-ASNase-induced hepatotoxicity is not clear, diminished protein synthesis, impaired free-fatty acid (FFA) metabolism, and increased oxidative stress resulting from asparagine and glutamine depletion are suspected of playing significant roles [11–14]. Some clinicians have used levocarnitine (LC), a small amino acid derivative that binds fatty acyl chains and allows them to enter the mitochondria via carnitine palmitoyl transferase, thereby facilitating FFA beta-oxidation. LC supplementation has been shown to reduce ASNase-induced toxicities in ALL patients in case reports and small case series [15–18] as well as in some rodent studies [19,20]. Given the high metabolic rate of cancer cells and their reliance on fatty acid oxidation (FAO) [21,22], LC could potentially increase leukemia cell proliferation rates or chemotherapy resistance, contributing to increased relapse rates and lower patient survival. To our knowledge, this potential adverse interaction of LC with ALL induction chemotherapy has yet to be investigated. Therefore, in the present study, we tested whether LC supplementation would increase ALL cell proliferation or metabolism, or reduce ALL cell chemotherapy sensitivity, using in vitro and in vivo models.
Materials and Methods
Cell Lines
Human cell lines included RS4;11 (pro-B t(4;11), ATCC), SEM (pre-B t(4;11), DSMZ), BV173 (pre-B t(9;22) Ph+, DSMZ), and NALM6 (pre-B Ph−, ATCC). All cells were cultured in 5% CO2 at 37°C in RPMI 1640 media (ThermoFisher) with 10% fetal bovine serum (Omega Scientific, Inc), 2 mM glutamine (ThermoFisher), and 1mM sodium pyruvate (ThermoFisher). Murine GFP+ 8093 ALL cells (previously described [23,24]) were cultured in McCoy’s 5A media (Invitrogen) with 10% FBS, 2 mM Glutamax (ThermoFisher), 1 mM sodium pyruvate, 10 ng/ml IL-3 (Peprotech), and 1:1000 BME/2-Mercaptoethanol (Gibco). Human cell lines were authenticated by the University of Arizona Genetics core in November 2016 and tested negative for mycoplasma.
Cytotoxicity Assays
Cells were seeded into 96-well plates at a density of ~100,000 cells/well in 200 μL media with or without LC (Sigma), made fresh prior to each experiment. Cells were treated with vincristine (VCR, MedChem Express), daunorubicin (DNR, Selleck Chemicals), dexamethasone (DEX, Sigma), or native E. coli ASNase (Abcam ab73439). VCR and DNR were used at their approximate EC90 doses for each cell line, as determined by dose response curves (data not shown). Since ASNase and DEX monotherapy did not reach an EC90, they were used at the lowest doses that reached maximum cytotoxic effects. Viability was assessed after 72 hours by trypan blue exclusion using a Countess II FL Automated Cell Counter (ThermoFisher). Cytotoxicity was also quantified using the alamarBlue assay (Bio-Rad), the readout of which incorporates viability, metabolism, and cellular health [25]. Each experiment was performed in technical duplicates and repeated 4–7 times.
Western Blot
Western blot analysis was performed using total protein lysates extracted from RS4;11. RIPA protein lysis buffer (150 mM NaCl, 1.0% IGEPAL® CA-630, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris, pH 8.0; Sigma) containing Complete Protease Inhibitor Cocktail (Sigma) was used to lyse cells. Protein quality and concentration was assessed using NanoDrop (ThermoFisher Scientific). Equal amounts of protein were separated on a NuPAGE 4–12% Bis-Tris protein gel and transferred to a nitrocellulose membrane using the iBlot 2 Dry Blotting System (Thermo Fisher). Membranes were then probed with anti-caspase 3, anti-cleaved caspase 3, and HRP-linked anti-rabbit secondary (Cell Signaling) using the iBind Western System (Thermo Fisher). Protein bands were detected by chemiluminescence using Pierce ECL Plus Substrate and SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher), and imaged using Sapphire Biomolecular Imager (Azure Biosystems). Densitometric analysis of the protein bands was performed using Fiji software (ImageJ) [26].
Respirometry
Oxygen consumption rates (OCR) of RS4;11 and BV173 cells were measured using Seahorse XF96 Analyzer (Agilent) in the UCLA Mitochondrial and Metabolism Core. ALL cells were suspended at a concentration of 3 × 106 cells/mL in assay media (RPMI with 0.5 mM glucose and 2 mM glutamine), with either LC (200 μM) or vehicle control (PBS), and incubated at 37°C for one hour or 24 hours prior to the assay. Respiration measurements were recorded under basal conditions, after injection of 3 μM oligomycin (inhibits ATP synthase to assess ATP synthesis rate), after each of two injections of 0.3 μM carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone (FCCP; uncoupling agent which collapses the proton gradient and leads to maximal OCR), and after injection of 2 μM antimycin A/rotenone (AA/Rot; shuts down mitochondrial respiration to assess nonmitochondrial respiration). Analysis was performed as previously described [27]. Briefly, rates were corrected for non-mitochondrial respiration using background measurements obtained after injection of AA/Rot. OCR measurements were normalized to cell counts obtained using a plate reader following nuclear staining with Hoestch. A minimum of four technical replicates were included per 96-well plate. Experiments were repeated 3–4 times for each cell line.
Animal Model
High fat diet-induced obese (DIO) C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, MI, USA) and allowed to acclimate for one week. Mice were maintained on a 60% kcal fat diet (Research Diets, D12492) throughout the experiment, and used at 17 weeks of age. On day zero, 1 × 104 syngeneic GPF+ 8093 pre-B-cell ALL cells were implanted via tail vein injection. Mice were randomly assigned to one of four groups: no treatment, PEG-ASNase only, PEG-ASNase with LC pre-treatment, and PEG-ASNase with LC post-treatment. PEG-ASNase (94 IU/mouse given via intraperitoneal injection; Oncaspar; UCLA Pharmacy) was administered to groups 2–4 on day 7. LC was administered via drinking water at a final dose of 250 mg/kg/day to groups 3 and 4, starting three days before (group 3) or after (group 4) the PEG-ASNase treatment. Drinking water was monitored and refreshed 2–3 times/week (Fig 3A). On day 16, mice were sacrificed, and bone marrow from one femur was extracted from each mouse and processed immediately. The use of animals and all experimental procedures were reviewed and approved by the Chancellor’s Animal Research Committee (ARC; IACUC) at the University of California Los Angeles. All animals were handled in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
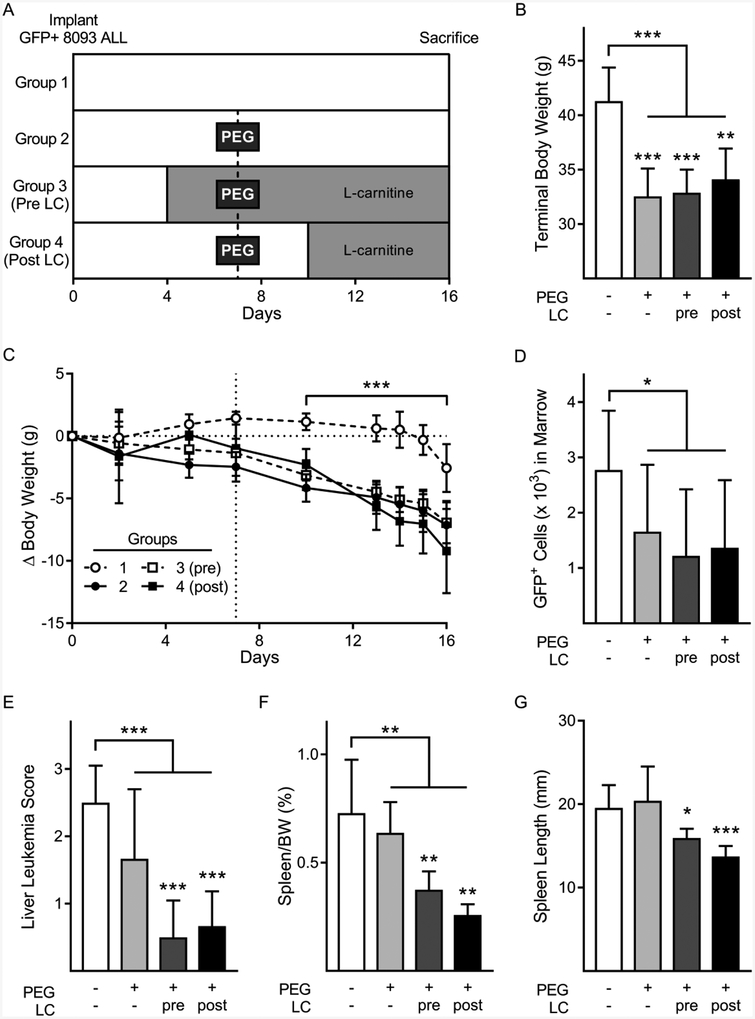
Figure 3:
Leukemia burden in ALL mouse model treated with PEG-ASNase and LC.
A: Schematic of the in vivo mouse study. B: Terminal body weight measurements of mice by treatment group. C: Changes in body weights throughout the experiment. Dotted line denotes the day of PEG-ASNase administration. Weights after PEG-ASNase dosing compared between combined PEG treated groups and no treatment group, by two-way ANOVA D-G: Leukemia burden represented by bone marrow GFP+ 8093 ALL cells (per 1 × 105 cells) measured by flow cytometry (D), liver leukemia score (E), spleen to body weight ratios (F), and spleen lengths (G). Data are shown as mean±SD. Group comparisons in bar graphs by student’s t-test comparing no PEG group vs. all PEG treated groups combined. Asterisks over individual bars represent comparisons to no PEG treatment group, *p < 0.05, **p < 0.01, ***p < 0.001.
Serum Measurements
Serum samples, collected at sacrifice, were stored at −80°C prior to analysis. Metabolite extraction was performed by combining 30 μL of serum with 70 μL water and 400 μL methanol. The solution was vortexed and centrifuged at top speed for 5 minutes. Supernatant was then combined with 300 μL water, 400 μL chloroform, and 10 nmol norvaline (added as an internal control), and again vortexed and centrifuged at top speed for 5 minutes. The aqueous layer was transferred to a glass vial and metabolites dried under vacuum. L-carnitine-(trimethyl-d9) inner salt metabolite (Sigma) was used to determine a standard curve. Metabolites were resuspended in 300 μL 50% acetonitrile (ACN) for mass spectrometry performed on a Vanquish (Thermo Scientific) UHPLC system with mobile phase A (5mM NH4AcO, pH 9.9) and mobile phase B (ACN). Separation was achieved at a 200 uL/min flow rate on a Luna 3mm NH2 100A (150 × 2.0 mm) (Phenomenex) at 40°C. The gradient ran from 15% A to 95% A in 18 minutes followed by an 11-minute isocratic step. The UHPLC was coupled to a Q-Exactive (Thermo Scientific) mass analyzer running in polarity switching mode (−3.5 kV, +3.5 kV) with an MS1 resolution of 70,000. Metabolites were identified using exact mass (MS1) and retention time. Quantification was performed via area under the curve (AUC) integration of MS1 ion chromatograms with an in-house extended version of the MZmine 2 software package.
Flow Cytometry
Bone marrow cells were extracted from femurs by flushing with red blood cell lysis buffer (ThermoFisher Scientific). Cells were tritrated, and then suspensions were centrifuged at 300g for 5 minutes and resuspended in 5% FBS in PBS. Single-cell suspensions were obtained by filtering samples through 40 μm cell strainers. Quantification of GFP+ 8093 ALL cells was acquired using an LSR II analytic flow cytometer (BD Biosciences) in the UCLA Flow Cytometry Core.
Statistics
Data are shown as mean ± SD. Shapiro-Wilk test for normality was completed on data comparing two variables, followed by either an unpaired, two-tailed, Student’s T-test or an unpaired, two-tailed, nonparametric Mann-Whitney test, as appropriate. All data sets comparing three or more variables had equal variance as determined by the Brown-Forsythe test for equal variance. These datasets were analyzed using One-way Analysis of Variance (ANOVA) test followed by Student’s T-test when ANOVA demonstrated significant differences between groups. Grub’s test was used to identify and exclude outliers (α= 0.05). P value of less than 0.05 was used to determine statistical significance.
Results
Effects of LC supplementation on chemotherapy cytotoxicity
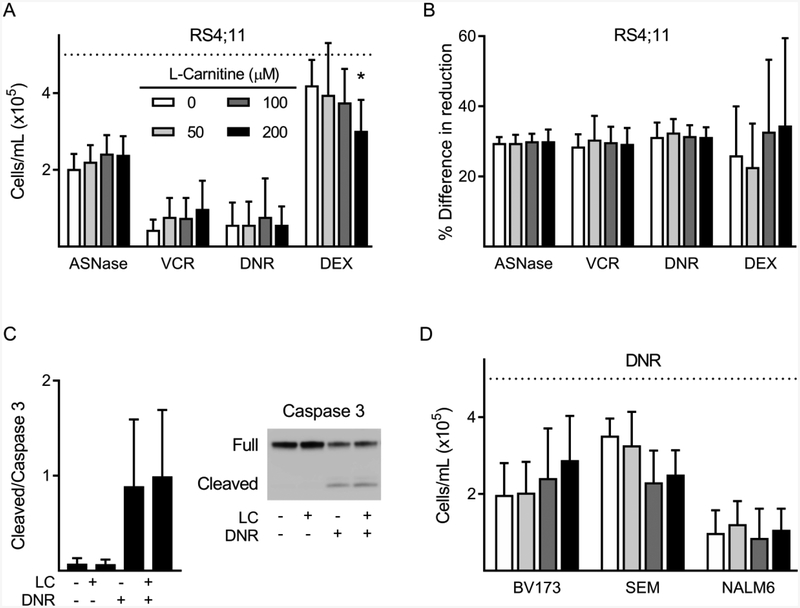
Nearly all international pediatric induction regimens for ALL as well as all protocols used for Children’s Oncology Group (COG) include VCR, DEX (or prednisone), and PEG-ASNase. In addition to these, some protocols also incorporate an anthracycline, such as DNR, depending on the ALL subtype and risk for relapse. To determine whether LC supplementation could impact the cytotoxicity of induction chemotherapy, human ALL cells were exposed to mono-treatments of VCR, DNR, DEX, and L-ASNase, with and without LC supplementation. Viability experiments performed with RS4;11 human ALL cells showed no significant effect of LC to impair the cytotoxicity of any of these chemotherapies (Fig 1A, B). In fact, the highest concentration of LC (200 μM) caused a slight, but statistically significant, increase in DEX-induced cytotoxicity measured by trypan blue exclusion. Additionally, LC did not alter the induction of apoptosis by 24-hour treatment with DNR, as measured by cleaved caspase 3 (Fig 1C). To test the generalizability of these findings, we performed further experiments using additional human ALL cell lines. DNR cytotoxicity was not affected by LC supplementation in BV173, SEM, and NALM6 cell lines, (Fig 1D). There was also no effect of LC on the cytotoxicity of VCR in BV173 cells (data not shown). Thus, LC supplementation did not impair cytotoxicity of induction therapies in multiple human ALL cell lines in vitro.
Figure 1:
Cytotoxicity of chemotherapy agents in the presence of LC.
A-B: RS4;11 ALL cell viability by trypan blue exclusion (A) and AlamarBlue assay (B) after 72-hour incubation with LC in combination with either ASNase (0.1IU/mL), VCR (2 nM), DNR (20 nM), or DEX (100 nM); n=4. C: Cleaved caspase 3 and full-length caspase protein levels in RS4;11 cells after 24-hour treatment with LC (200 μM) and DNR (n=4). D: Viability of BV173, SEM, and NALM6 measured by trypan blue exclusion after 72-hour treatment with DNR (25 nM, 30 nM, 50 nM, respectively), with or without LC (n=4). Dashed line indicates initial cell number. *, p < 0.05.
Effects of LC supplementation on ALL metabolism
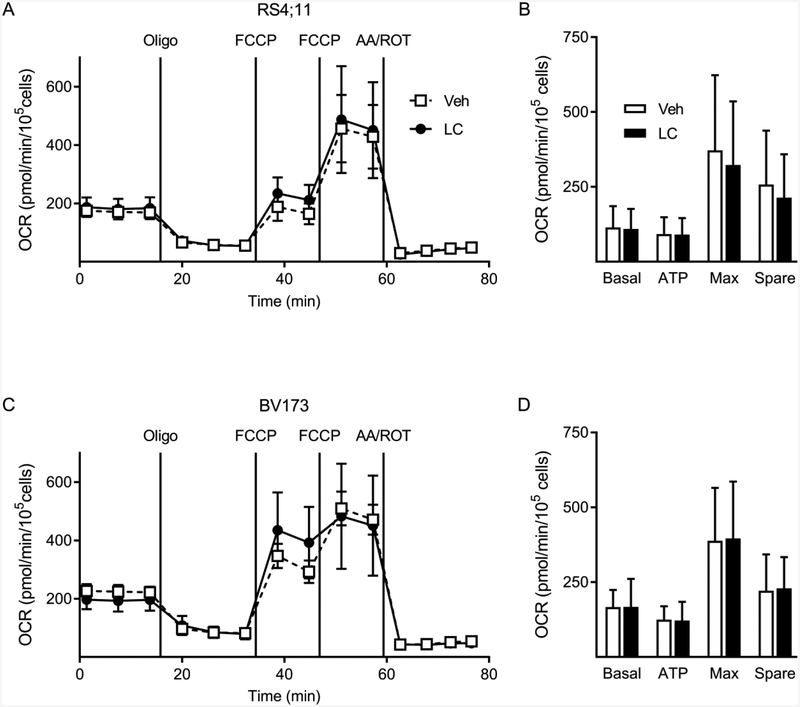
Given the central role of LC in regulating FFA beta-oxidation and energy production, we sought to determine whether LC supplementation would increase metabolic rate in ALL cells, particularly under low glucose conditions (0.5mM) where cells might rely more on FFA oxidation. Addition of LC did not alter basal respiration, ATP-linked respiration, maximal respiration, or spare respiratory capacity of RS4;11 or BV173 ALL cells (Fig 2A–D). Additional experiments done under high glucose (8 mM), or with 24-hour pre-treatment of LC, also did not show an effect of LC on these metabolic parameters (data not shown).
Figure 2:
Oxygen consumption rates during LC supplementation.
A-B: Oxygen consumption rates (OCR) in RS4;11 (A) and BV173 (B) ALL cells after 1-hour incubation without (dashed line) or with (solid line) 200 μM LC supplementation. Graphs show one representative trace of four experimental replicates for each cell line C, D. Average OCR for RS4;11 (C) and BV173 (D) without (white bars) and with (black bars) LC supplementation. Basal: basal respiration rate; ATP: ATP-linked respiration rate; Max: maximal respiration rate; Spare: spare capacity respiration; Oligo: oligomycin; FCCP: carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone; AA/ROT: antimycin A/rotenone.
Effect of LC supplementation on ALL treatment outcome in vivo
Patients who are overweight or obese have a higher risk of developing PEG-ASNase-induced hepatotoxicity [30,31]. Based on this, we utilized a diet-induced obese (DIO) mouse to evaluate for potential effects of LC on ALL treatment (Fig 3A). Although there was no difference in the starting weights between groups, mice treated with PEG-ASNase had significantly lower terminal body weights (Fig 3B, p<0.001), with most weight loss occurring post-PEG-ASNase administration (Fig 3C, p<0.001). As expected, groups which received PEG-ASNase treatment had lower leukemia burden, measured by bone marrow GFP (Fig 3D, p=0.012), liver ALL score (Fig 3E, p=0.002), and spleen weight (Fig 3F, p=0.005), though not spleen length (Fig 3G, p=0.10). Supplementation with LC, either prior to or post PEG-ASNase treatment, did not impair the ability of PEG-ASNase to reduce ALL burden. In fact, the ALL burden by some measures tended to be lower in the LC supplemented groups. For liver leukemia score, spleen length and spleen weight, PEG-ASNase treatment alone did not have significantly lower ALL burden, while PEG-ASNase plus LC supplementation did. Collectively, these results demonstrate that supplementation of LC does not impair PEG-ASNase cytotoxicity against ALL cells in an in vivo model.
Carnitine levels
Because we observed no significant effects of LC on ALL burden, it was important to make sure that the LC was successfully supplemented in this model. Supplemented mice had significantly higher free carnitine concentrations at time of sacrifice than non-supplemented mice (44.3±5.0 vs. 39.7±4.9 μM, p=0.04). Though a modest difference, other studies supplementing mice with similar doses of LC report variability in the effect on serum/plasma carnitine levels, ranging between approximately 0.65 to 20 μM higher [32–35].
Discussion
In the present study, we show that supplementation with LC does not reduce chemotherapy sensitivity of human ALL cells in vitro or in a mouse model. In our hands, LC had no detectible effect on the proliferation rate, metabolic rate, or sensitivity of four human ALL cell lines to four frontline chemotherapies. The cell lines we chose represented a range of B-cell precursor ALL types, and the four chemotherapies represent the backbone of frontline agents used during the initial phase of chemotherapy for childhood ALL. Since many clinicians are already routinely using LC to prevent or reverse L-ASNase induced hepatotoxicity, these results provide support as to the safety of this approach.
PEG-ASNase is a critical component of pediatric induction therapy that has contributed to the increase in survival rates among patients diagnosed with ALL [3,36]. However, severe off-target effects, including hepatotoxicity, continue to be a serious problem, particularly in adolescents and adults [8,30,37]. A retrospective study of adult ALL patients revealed that those who developed PEG-ASNase-induced hepatotoxicity were more likely to miss at least one dose of chemotherapy during induction, and had significantly higher mortality rates [38]. Two cases of death resulting from liver failure secondary to PEG-ASNase administration have been reported [11,13]. Therefore, identifying methods to mitigate hepatotoxicity in these patients is important to increasing the survival rates in ALL patients, particularly in adults with co-morbidities who are most at risk of treatment-associated complications.
The transfer of fatty acids into the mitochondria via carnitine-palmitoyl transferase-1 (CPT-1) is the rate limiting step in fatty acid oxidation (FAO). This shuttle depends on the availability of LC, and LC supplementation has been shown to increase FAO rates in healthy adults and animal models of hepatic injury [15–19]. While this increased FAO is believed to help hepatocytes resist ASNase-induced toxicity, there is a theoretical risk that it could have direct effects on ALL cells, potentially improving their metabolism. Indeed, leukemia cells are notably reliant on FAO [39,40]. Additionally, L-ASNase treatment leads to increased FAO in human ALL cells, which suggests that the ALL cells are utilizing fatty acids as a compensatory mechanism in response to asparagine and glutamine deprivation [41]. Thus, our data provide an important demonstration that LC does not reduce treatment efficacy of ALL in vitro and in a mouse model. Further work needs to be done to confirm this in patients.
One weakness in the present study is that our mouse model did not show significant evidence of ASNase-induced hepatotoxicity (data not shown), and therefore we were not able to address whether LC supplementation would reverse this. High-fat diets routinely induce hepatosteatosis in mice, which was observed in all groups in our study. Lack of hepatotoxicity in our model may have been due to confounding effects of the weight loss caused by PEG-ASNase, or secondary to the relatively low dose used in our study, compared to other animal studies in which PEG-ASNase induced notable toxicity [20,42]. However, others have found that lower doses of native ASNase decreased serum asparagine to undetectable levels [43,44]. Based on these studies and the limited supply of PEG-ASNase available to us during our study, we administered a single dose of 94 IU/mouse (~2466 IU/kg or ~8,032 IU/m2) of PEG-ASNase. Another potential weakness is that the plasma carnitine levels attained in our supplemented mice was only ~5 μM higher than the non-supplemented mice. Though well within the range of other mouse studies, it is not clear how this compares to serum levels in patients supplemented with LC during leukemia treatment, which to our knowledge has not been reported.
Results of our study show that LC supplementation has no impact on the cytotoxicity of four key induction therapies in vitro, which agree with a previous study that found LC to have no effect on ASNase activity against Ramos human Burkitt’s lymphoma cells or Jurkat human acute T-cell leukemia cells [19]. Further, our study also shows that PEG-ASNase treatment of ALL is not affected by LC supplementation in a syngeneic mouse leukemia model. Collectively, these data provide a foundation of in vitro and in vivo evidence that invites future studies to evaluate the clinical impacts and safety of LC supplementation in patients undergoing induction treatment for ALL.
Acknowledgements
We thank the UCLA Mitochondrial and Metabolism Core.
Funding: This study was supported by a grant from the National Institutes of Health National Cancer Institute (CA201444).
Footnotes
Declaration of Interest Statement
The authors have no conflicts of interest to report.
References
- 1.Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. J Clin Oncol 2012;30:1663–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silverman LB, Gelber RD, Dalton VK, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91–01. Blood 2001;97:1211–1218. [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim A, Ali A, Mohammed MM. Outcome of Adolescents with Acute Lymphoblastic Leukemia Treated by Pediatrics versus Adults Protocols. Adv Hematol 2014;2014:697675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huguet F, Leguay T, Raffoux E, et al. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study. J Clin Oncol 2009;27:911–918. [DOI] [PubMed] [Google Scholar]
- 5.Douer D, Yampolsky H, Cohen LJ, et al. Pharmacodynamics and safety of intravenous pegaspargase during remission induction in adults aged 55 years or younger with newly diagnosed acute lymphoblastic leukemia. Blood 2007;109:2744–2750. [DOI] [PubMed] [Google Scholar]
- 6.Stock W, Luger SM, Advani AS, et al. A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10403. Blood 2019;133:1548–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sive JI, Buck G, Fielding A, et al. Outcomes in older adults with acute lymphoblastic leukaemia (ALL): results from the international MRC UKALL XII/ECOG2993 trial. Br J Haematol 2012;157:463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel B, Kirkwood AA, Dey A, et al. Pegylated-asparaginase during induction therapy for adult acute lymphoblastic leukaemia: toxicity data from the UKALL14 trial. Leukemia 2017;31:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke PW, Aldoss I, Lunning MA, et al. Pegaspargase-related high-grade hepatotoxicity in a pediatric-inspired adult acute lymphoblastic leukemia regimen does not predict recurrent hepatotoxicity with subsequent doses. Leuk Res 2018;66:49–56. [DOI] [PubMed] [Google Scholar]
- 10.Berry DA, Zhou S, Higley H, et al. Association of Minimal Residual Disease With Clinical Outcome in Pediatric and Adult Acute Lymphoblastic Leukemia: A Meta-analysis. JAMA Oncol 2017;3:e170580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bodmer M, Sulz M, Stadlmann S, Droll A, Terracciano L, Krahenbuhl S. Fatal liver failure in an adult patient with acute lymphoblastic leukemia following treatment with L-asparaginase. Digestion 2006;74:28–32. [DOI] [PubMed] [Google Scholar]
- 12.Reinert RB, Oberle LM, Wek SA, et al. Role of glutamine depletion in directing tissue-specific nutrient stress responses to L-asparaginase. J Biol Chem 2006;281:31222–31233. [DOI] [PubMed] [Google Scholar]
- 13.Sahoo S, Hart J. Histopathological features of L-asparaginase-induced liver disease. Semin Liver Dis 2003;23:295–299. [DOI] [PubMed] [Google Scholar]
- 14.Villa P, Corada M, Bartosek I. L-asparaginase effects on inhibition of protein synthesis and lowering of the glutamine content in cultured rat hepatocytes. Toxicol Lett 1986;32:235–241. [DOI] [PubMed] [Google Scholar]
- 15.Rausch CR, Paul S, Marx KR, et al. L-carnitine and Vitamin B Complex for the Treatment of Pegasparaginase-induced Hyperbilirubinemia. Clin Lymphoma Myeloma Leuk 2018;18:e191–e195. [DOI] [PubMed] [Google Scholar]
- 16.Alshiekh-Nasany R, Douer D. L-Carnitine for Treatment of Pegasparaginase-Induced Hepatotoxicity. Acta Haematol 2016;135:208–210. [DOI] [PubMed] [Google Scholar]
- 17.Schulte RR, Madiwale MV, Flower A, et al. Levocarnitine for asparaginase-induced hepatic injury: a multi-institutional case series and review of the literature. Leuk Lymphoma 2018;59:2360–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Nawakil C, Willems L, Mauprivez C, et al. Successful treatment of l-asparaginase-induced severe acute hepatotoxicity using mitochondrial cofactors. Leuk Lymphoma 2014;55:1670–1674. [DOI] [PubMed] [Google Scholar]
- 19.Roesmann A, Afify M, Panse J, Eisert A, Steitz J, Tolba RH. L-carnitine ameliorates L-asparaginase-induced acute liver toxicity in steatotic rat livers. Chemotherapy 2013;59:167–175. [DOI] [PubMed] [Google Scholar]
- 20.Kaya I, Citil M, Sozmen M, Karapehlivan M, Cigsar G. Investigation of protective effect of L-carnitine on L-asparaginase-induced acute pancreatic injury in male Balb/c mice. Dig Dis Sci 2015;60:1290–1296. [DOI] [PubMed] [Google Scholar]
- 21.Tung S, Shi Y, Wong K, et al. PPARalpha and fatty acid oxidation mediate glucocorticoid resistance in chronic lymphocytic leukemia. Blood 2013;122:969–980. [DOI] [PubMed] [Google Scholar]
- 22.Boag JM, Beesley AH, Firth MJ, et al. Altered glucose metabolism in childhood pre-B acute lymphoblastic leukaemia. Leukemia 2006;20:1731–1737. [DOI] [PubMed] [Google Scholar]
- 23.Behan JW, Yun JP, Proektor MP, et al. Adipocytes impair leukemia treatment in mice. Cancer Res 2009;69:7867–7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heisterkamp N, Jenster G, ten Hoeve J, Zovich D, Pattengale PK, Groffen J. Acute leukaemia in bcr/abl transgenic mice. Nature 1990;344:251–253. [DOI] [PubMed] [Google Scholar]
- 25.Rampersad SN. Multiple applications of Alamar Blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors (Basel) 2012;12:12347–12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods 2012;9:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Divakaruni AS, Paradyse A, Ferrick DA, Murphy AN, Jastroch M. Analysis and interpretation of microplate-based oxygen consumption and pH data. Methods Enzymol 2014;547:309–354. [DOI] [PubMed] [Google Scholar]
- 28.Liang W, Menke AL, Driessen A, et al. Establishment of a general NAFLD scoring system for rodent models and comparison to human liver pathology. PLoS ONE 2014;9:e115922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eumorphia/Europhenome. Blood chemistry and hematology in 8 inbred strains of mice. MPD:Eumorphia1. Mouse Phenome Database web resource (RRID:SCR_003212), The Jackson Laboratory, Bar Harbor, Maine USA: https://phenome.jax.org [Cited (6/2019)]. [Google Scholar]
- 30.Christ TN, Stock W, Knoebel RW. Incidence of asparaginase-related hepatotoxicity, pancreatitis, and thrombotic events in adults with acute lymphoblastic leukemia treated with a pediatric-inspired regimen. J Oncol Pharm Pract 2018;24:299–308. [DOI] [PubMed] [Google Scholar]
- 31.Denton CC, Rawlins YA, Oberley MJ, Bhojwani D, Orgel E. Predictors of hepatotoxicity and pancreatitis in children and adolescents with acute lymphoblastic leukemia treated according to contemporary regimens. Pediatr Blood Cancer 2018;65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kon K, Ikejima K, Morinaga M, et al. L-carnitine prevents metabolic steatohepatitis in obese diabetic KK-Ay mice. Hepatology Research 2017;47:E44–E54. [DOI] [PubMed] [Google Scholar]
- 33.Morand R, Bouitbir J, Felser A, et al. Effect of carnitine, acetyl-, and propionylcarnitine supplementation on the body carnitine pool, skeletal muscle composition, and physical performance in mice. European Journal of Nutrition 2014;53:1313–1325. [DOI] [PubMed] [Google Scholar]
- 34.Cheema UB, Most E, Eder K, Ringseis R. Effect of lifelong carnitine supplementation on plasma and tissue carnitine status, hepatic lipid metabolism and stress signalling pathways and skeletal muscle transcriptome in mice at advanced age. British Journal of Nutrition:1–28. [DOI] [PubMed] [Google Scholar]
- 35.Zhao Y, Yang N, Gao J, et al. The Effect of Different l-Carnitine Administration Routes on the Development of Atherosclerosis in ApoE Knockout Mice. Molecular Nutrition & Food Research 2018;62:1700299. [DOI] [PubMed] [Google Scholar]
- 36.Abshire TC, Pollock BH, Billett AL, Bradley P, Buchanan GR. Weekly polyethylene glycol conjugated L-asparaginase compared with biweekly dosing produces superior induction remission rates in childhood relapsed acute lymphoblastic leukemia: a Pediatric Oncology Group Study. Blood 2000;96:1709–1715. [PubMed] [Google Scholar]
- 37.Aldoss I, Douer D, Behrendt CE, et al. Toxicity profile of repeated doses of PEG-asparaginase incorporated into a pediatric-type regimen for adult acute lymphoblastic leukemia. Eur J Haematol 2016;96:375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rausch CR, Marini BL, Benitez LL, et al. PEGging down risk factors for peg-asparaginase hepatotoxicity in patients with acute lymphoblastic leukemia (dagger). Leuk Lymphoma 2018;59:617–624. [DOI] [PubMed] [Google Scholar]
- 39.Ricciardi MR, Mirabilii S, Allegretti M, et al. Targeting the leukemia cell metabolism by the CPT1a inhibition: functional preclinical effects in leukemias. Blood 2015;126:1925–1929. [DOI] [PubMed] [Google Scholar]
- 40.Gugiatti E, Tenca C, Ravera S, et al. A reversible carnitine palmitoyltransferase (CPT1) inhibitor offsets the proliferation of chronic lymphocytic leukemia cells. Haematologica 2018;103:e531–e536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hermanova I, Arruabarrena-Aristorena A, Valis K, et al. Pharmacological inhibition of fatty-acid oxidation synergistically enhances the effect of l-asparaginase in childhood ALL cells. Leukemia 2015. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y, Janke LJ, Li L, Relling MV. L-carnitine does not ameliorate asparaginase-associated hepatotoxicity in a C57BL6 mouse model. Leuk Lymphoma 2019:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horvath TD, Chan WK, Pontikos MA, et al. Assessment of l-Asparaginase Pharmacodynamics in Mouse Models of Cancer. Metabolites 2019;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shiromizu S, Kusunose N, Matsunaga N, Koyanagi S, Ohdo S. Optimizing the dosing schedule of l-asparaginase improves its anti-tumor activity in breast tumor-bearing mice. J Pharmacol Sci 2018;136:228–233. [DOI] [PubMed] [Google Scholar]