Abstract
Time series of stem diameter variations (SDVs) recorded by dendrometers are composed of two components: (i) irreversible radial stem growth and (ii) reversible stem shrinking and swelling caused by dynamics in water storage in elastic tissues outside the cambium. However, SDVs measured over dead outer bark (periderm) could also be affected by absorption and evaporation of water from remaining dead bark layers after smoothing the stem surface to properly mount dendrometers. Therefore, the focus of this study was to determine the influence of hygroscopicity of a thin dead outer bark layer on the reversible component of dendrometer records of Scots pine (Pinus sylvestris) under field conditions. To accomplish this, SDVs deduced from dendrometers mounted over dead outer bark were compared among living and dead saplings and mature trees. Results revealed that dead trees showed high synchronicity in reversible daily SDVs compared to living trees throughout several growing seasons (mean Pearson correlation coefficient (r) = 0.844 among saplings and r = 0.902 among mature trees, respectively; P<0.001). Furthermore, diurnal and long-term SDVs closely followed changes in relative air humidity (RH) in living and dead trees. A multiple linear regression analysis of environmental influence on SDVs in dead and living trees revealed that the most important predictor of daily SDVs was RH (relative importance 64 %). Hence, results indicate that dendrometers mounted over dead outer bark with a thickness of <4 mm record hygroscopic shrinking and swelling of the bark tissue, which can amplify fluctuations in whole-tree water status. To conclude, hygroscopic processes must be taken into account when extracting intra-annual radial growth, determining environmental drivers of SDVs, and evaluating changes in tree water status from SDVs recorded by dendrometers, which were mounted over even thin dead outer bark layers.
Keywords: Dead outer bark, Dendrometer, Bark hygroscopicity, Relative air humidity, Stem diameter variation, Tree water status
1. Introduction
High-resolution dendrometers are frequently applied to study seasonal dynamics of radial tree growth (Zweifel et al. 2000, Bouriaud et al. 2005, Deslauriers et al. 2007), to monitor tree water status (Zweifel et al. 2005, Drew and Downes 2009, Turcotte et al. 2011, Köcher et al. 2013, De Swaef et al. 2015, Oberhuber et al. 2015) and to determine environmental drivers of stem radial increment and daily stem diameter variations (SDVs; Deslauriers et al. 2003, King et al. 2013, Köcher et al. 2012, Oberhuber et al. 2014, van der Maaten et al. 2018). Time series of SDVs are composed of two components: (i) irreversible radial stem growth due to accumulation of new xylem and bark tissue, and (ii) reversible stem shrinking and swelling as a result of changing water potential gradients within the stem (Zweifel et al. 2001, Sevanto et al. 2011, Pfautsch et al. 2015, Mencuccini et al., 2017). The bark of trees comprises dead outer bark (periderm), and living inner bark, i.e. non-lignified phloem including conducting and non-conducting phloem and parenchyma (Evert 2009), which together with cambium cells form a highly elastic tissue and contribute most to reversible SDVs (Zweifel et al. 2000, Sevanto et al. 2002, Steppe et al. 2006, De Schepper et al. 2012). However, dead outer bark is a highly hygroscopic tissue (Ilek et al. 2016) and experiments under controlled environmental conditions revealed that in 3 to 6-year old Norway spruce seedlings diurnal SDVs closely paralleled changes in relative air humidity (RH), even when all needles were removed (Lövdahl and Odin 1992). Gall et al. (2002) also found that diurnal changes in bark thickness of Norway spruce trees over several days during winter and summer corresponded with changes in RH, but not with changes in tree water status. Therefore, the loose dead outer bark is generally removed to ensure close contact of dendrometers (especially band dendrometers) with the stem and to reduce hygroscopic shrinkage and swelling of the dead outer bark on dendrometer records. Dendrometers put in parallel on xylem and inner bark (living phloem) allow quantification of variation in tree diameter caused by shrinkage/swelling of the rigid xylem or elastic tissues of the inner bark (i.e. cambium and phloem; Sevanto et al. 2011, Mencuccini et al. 2013, Zweifel et al. 2014, Chan et al. 2016, Mencuccini et al., 2017). Results of these studies revealed that the reversible radial water flow from elastic tissues of the inner bark to the xylem is primarily responsible for SDVs, and is induced by predominantly diurnal transpiration-driven changes in xylem water potential and by more gradual changes in osmotic concentration in the inner bark (Sevanto et al. 2003, De Swaef et al. 2013). Although Sevanto et al. (2011) pointed out that SDVs measured over inner bark could be affected by hygroscopicity of the remaining thin layer of dead outer bark under the sensor, the influence of bark hygroscopicity on short- and long-term series of SDVs and meteorological drivers of daily SDVs were not yet determined under field conditions.
The focus of this study therefore was to determine the influence of thin dead outer bark layers on short- (i.e. diurnal) and long-term (i.e. during the growing season) SDVs of Scots pine trees (Pinus sylvestris L.) in a drought-prone environment. The hypothesis was tested that during the growing period daily SDVs of saplings and mature Scots pine trees deduced from dendrometer records mounted over thin dead outer bark are still affected by changes in RH. To accomplish this, daily SDVs of cooccurring living and dead trees were compared during several growing seasons and the relationship of SDVs to climate variables was analysed for both subsets of trees.
2. Material and Methods
The study area is situated in the montane belt (c. 750 m asl) within the inner Alpine dry valley of the Inn River (Tyrol, Austria, 47°13′53″ N, 10°50′51″ E). Mean annual air temperature and total precipitation amount to 7.3°C and 724 mm, respectively (long-term mean during 1911-2017 at Ötz, 812 m asl, 5 km from the study area). A Scots pine stand was selected on a south-west facing xeric plot on a steep slope (30-40°), where light-demanding Pinus sylvestris rejuvenates naturally under open canopy (canopy coverage 30 %). Stem height and diameter of selected saplings (n=7) amounted to 1.5 m and 2.9 cm, respectively. Mature trees (n=4) were c. 4 m in height and stem diameter was 22.5 cm. Age of selected saplings and mature trees was estimated to be c. 25 and c. 150 yr, respectively (Table 1; cf. Oberhuber and Gruber 2010).
Table 1. Tree characteristics of Pinus sylvestris saplings (n=7) and mature trees (n=4) selected for dendrometer measurements. Mean values ± standard deviation are presented (SDM = stem diameter).
| Stem height (m)1 | SDM (cm)1,2 | Bark width (mm)1,3 | |
|---|---|---|---|
| Saplings | 1.5 ± 0.3 | 2.9 ± 0.6 | 1.9 ± 0.2 |
| Mature trees | 3.8 ± 0.2 | 22.5 ± 4.1 | 7.5 ± 2.3 |
Measured at the end of the growing season in October 2018.
Measured at height of dendrometers.
Remaining thickness of dead outer bark (excluding the living phloem) after scraping away loose and up to 2 cm of the bark (periderm) in saplings and mature trees, respectively.
Whereas mortality of a mature tree occurred without interference most likely due to drought stress, tree death of saplings (n = 3) was induced by deploying granular fertilizer in dry form (5 kg 100 m−2 calcium ammonium nitrate containing 27 % N) in early spring 2016 (doy 81), which caused desiccation of root tissues due to osmotic stress. Timing of tree mortality was considered when 100 % needle browning occurred. Thin periderm (<2 mm) of saplings was expected to strongly reduce influence of hygroscopic swelling and shrinkage of the bark on dendrometer records. Stem discs were collected from saplings and micro-cores were taken in each cardinal direction from mature trees to determine remaining width of dead outer bark tissue (periderm) after installation of dendrometers (four measurements per individual).
2.1. Recording stem diameter variations
Temperature compensated electronic diameter dendrometers with resolutions of < 3 μm (DD-S, Ecomatik, Munich, Germany) were installed on saplings (n=7) at c. 15 cm stem height. The temperature coefficient of the sensor was < 0.1 μm/K. The dead outermost loose layers of the bark (periderm) were slightly removed to allow proper mounting of dendrometers and to ensure close contact with the stem. Remaining thickness of dead outer bark amounted to <2 mm (Table 1). Additionally, dead outer bark samples (thickness 3.5 mm) were sampled from living trees and changes in thickness were determined by the same diameter dendrometers (i) at the study plot during one growing season, and (ii) in a climate chamber (SANYO, MLR-350-H, Sanyo Electric Biomedical Co., Ltd, Japan) that allowed controlling of air temperature and RH. Data were recorded every 30 min with analog data loggers (HOBO UX120-006M, ONSET, Bourne, MA, USA). In the climate chamber experiment air temperature was increased/decreased in 10°C steps from 15°C up to 35°C and back again. Temperature remained constant for three hours at each temperature setting and two levels of RH (50 % and 90 %) were used (Supplementary Fig. 1). Applied ranges of temperature and RH were comparable with environmental conditions occurring at the study site during the growing season (Supplementary Fig. 2). This experiment under controlled conditions revealed that changes in temperature by 20°C did not affect dendrometer records. On the other hand, changes in RH caused corresponding changes in thickness of dead outer bark. Hence, results of the climate chamber experiment indicated that a thermal correction of dendrometer readings obtained from diameter dendrometers used in this study was not required.
Additionally, within the same plot electronic circumference dendrometers (DMS dendrometer type D-6 with measuring amplifier t8.MV,UMS, Munich, Germany) were installed on mature Scots pine trees (n = 4) about 1 m above ground in October 2017 at the time when one tree showed 100 % needle browning. Dendrometers consisted of a clip sensor sliding on a Teflon-pad and the measuring band was Invarsteel. Dendrometer records were corrected for temperature sensitivity (4 μm K−1). Up to 2 cm of dead outermost layers of the bark were scraped away around the stem to ensure close contact of the measuring band with the stem and to reduce hygroscopic swelling and shrinking of bark layers (Table 1). Data were recorded with a DT-6 data logger (Delta T Devices, Cambridge, England), which was programmed to record measurements every 30 minutes. Daily SDVs of saplings and mature trees were calculated by averaging all daily measurements (48 values/day), i.e. one value per day was extracted from the time series. The daily mean approach yields time series of daily SDVs, which include both water- and growth-induced diameter changes (Deslauriers et al. 2007). To facilitate the direct comparison of daily SDVs among dead and living trees, time series of daily SDVs were set to zero on January 1st of each year.
Detrended daily SDVs (dSDVs) were then calculated by removing the long-term trend from the data. Hence, irreversible diameter growth and/or drought induced stem shrinkage were subtracted from daily SDVs. To accomplish this, the fast Fourier transform low-pass filter (28 points) included in the Origin software package (OriginLab Corporation, Northampton, MA, USA) was applied, which highlights weekly to monthly variations in SDVs. Hence, if hygroscopicity of dead outer bark is neglected, positive residuals of dSDVs should indicate water replenishment of the expandable tissues, i.e. cambium and living inner bark, when tree water status is high (stem swelling), while negative values should indicate that water stored in these expandable tissues is translocated to the xylem (stem shrinkage). The contribution of reversible shrinkage and swelling in the xylem on total SDVs is regarded to be minor (Irvine and Grace 1997, Steppe et al., 2006, De Schepper et al. 2012). Pearson correlation statistics (r) were calculated to explore the relationship among dSDVs of dead and living trees during the study period 2016–2018 and 2017–2018 for saplings and mature trees, respectively, using Statistica (Version 12, Stat. Soft. Inc. 2014, www.statsoft.com).
2.2. Microclimate records
During the study period, air temperature, relative air humidity (RH), and daily precipitation were collected automatically (ONSET, Pocasset, MA, USA) at 2 m height in a non-vegetated area within the study plot. The measuring intervals for all sensors were 30 min and mean daily air temperature and relative air humidity (RH) were calculated by averaging all measurements (48 values per day). Vapour pressure deficit of the air (VPD) was calculated from the half-hourly records of air temperature and RH using the equation presented in Prenger and Ling (2000). Climate records during the study period (2016–2018) are depicted in Supplementary Figs. 2a-f.
2.3. Environmental influence on stem daily variation of dead and living trees
Statistical analyses were carried out with R version 3.6.2 – “Dark and Stormy Night” (R Core Team 2018). First, a mixed effect model using the lme4 package (Bates et al. 2015) was calculated with tree identity as random slope. A variance close to zero for the random effect indicated that the model is degenerate (Bates 2010) and the random effect was discarded, resulting in a multiple linear regression without random effects. The assumptions for the regression models were investigated according to the protocol in Zuur et al. (2010). The assumption of homoscedasticity was satisfied, the assumption of normality of residuals not. The relative importance of the predictors in the multiple linear regression were calculated using the relaimpo package (Grömping 2006) with the method “lmg” (Lindeman et al. 1980). Regression output tables were created with the stargazer package (Hlavac 2018).
3. Results
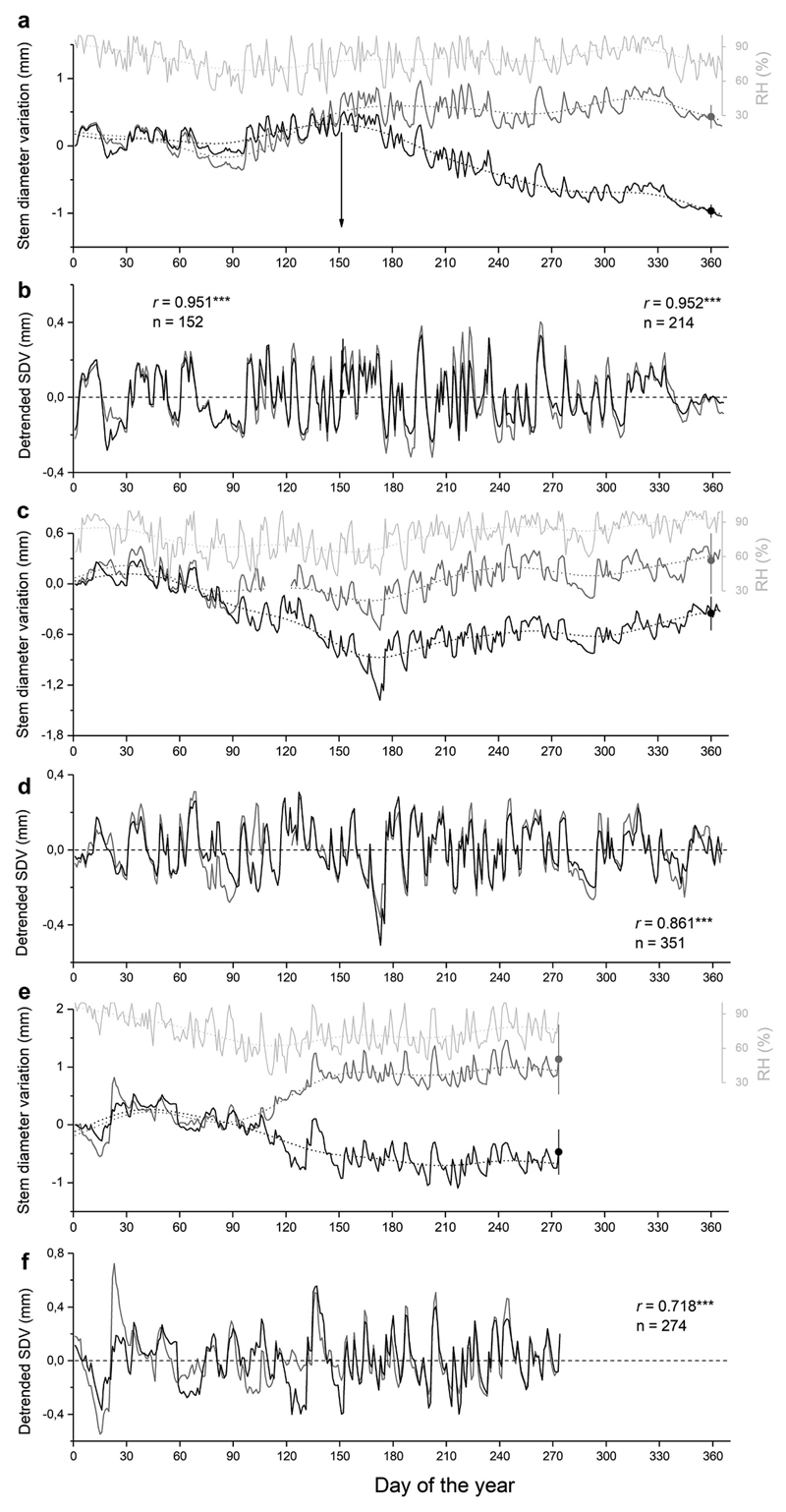
In Fig. 1 SDVs are compared among living and dead saplings during 2016–2018. After death of saplings occurred in late May 2016 stem dehydration caused gradual shrinkage of the stem diameter (Fig. 1a). Daily SDVs in 2017 and 2018 are characterized by shrinkage of the stem diameter during spring followed by increasing diameter primarily in living trees in 2018. Long-term trends in reversible SDV coincided with trend in RH in all study years. Detrended SDV (dSDV) showed high synchronicity among saplings before tree death (r = 0.951, n = 152, P<0.001). After tree death occurred at the end of May 2016 correlations of dSDV among dead and living saplings did not change (r = 0.952, n = 214, P<0.001). In the following years correlation coefficients among dSDVs of living and dead saplings decreased to r = 0.718 in 2018 (n = 274), but were highly significant in all years (Fig. 1).
Fig. 1.
a-f Raw and detrended stem diameter variations (dSDV) in living (grey lines; n=4) and dead (black lines; n=3) saplings and relative air humidity (RH; light grey lines) during 2016 (a, b), 2017 (c, d) and 2018 (e, f). Mean standard deviations (bars) among dendrometer records of living and dead trees (grey and black circle, respectively) are plotted on doy 360 (years 2016 and 2017) and doy 274 (year 2018). Arrows in (a) and (b) indicate timing of tree death deduced from 100 % needle browning. Long-term trends shown for daily SDVs and RH were calculated by fast Fourier transform low-pass filter (28 points). Pearson correlation coefficients (r) among dSDVs of living and dead trees are indicated (*** = P<0.001).
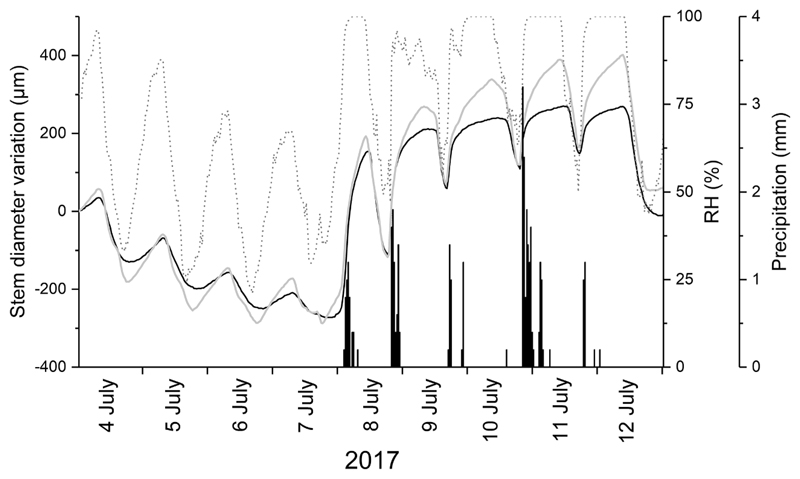
The reversible changes in stem diameter of saplings showed a diurnal fluctuation with shrinkage and swelling during daytime and nighttime, respectively. During drought periods stem diameter continuously decreased in dead and living trees (Fig. 2) and the diurnal cycle of the stem diameter closely followed RH, but SDVs lagged diurnal changes in RH by a few hours. The first precipitation events after a drought period caused abrupt and strong increase in stem diameter of living and dead trees.
Fig. 2.
Diurnal cycles of stem diameter variations of living (solid grey line) and dead (solid black line) saplings during a transition period from dry to wet in early July 2017 compared to relative air humidity (RH; dotted grey line) and precipitation (bars).
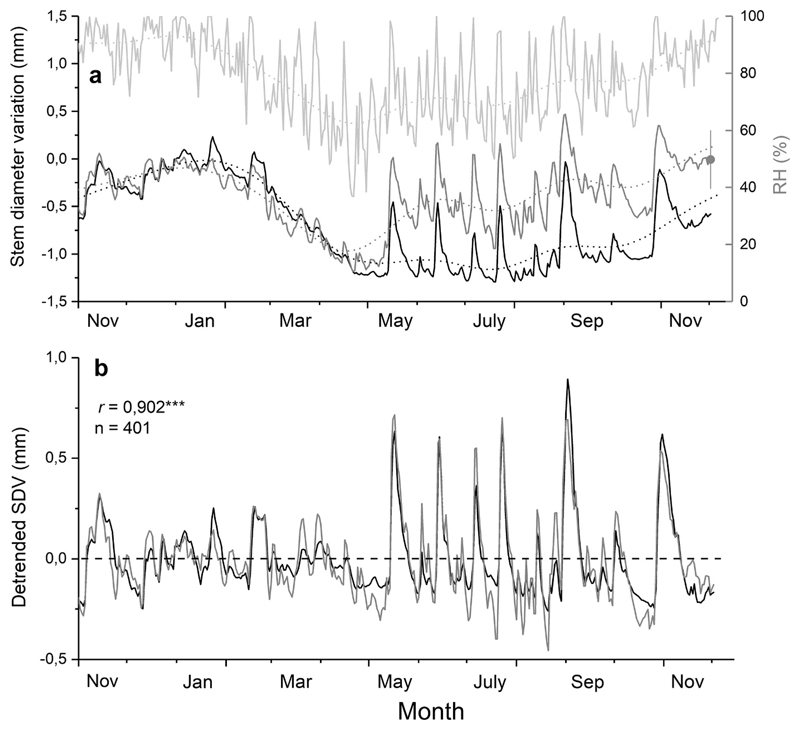
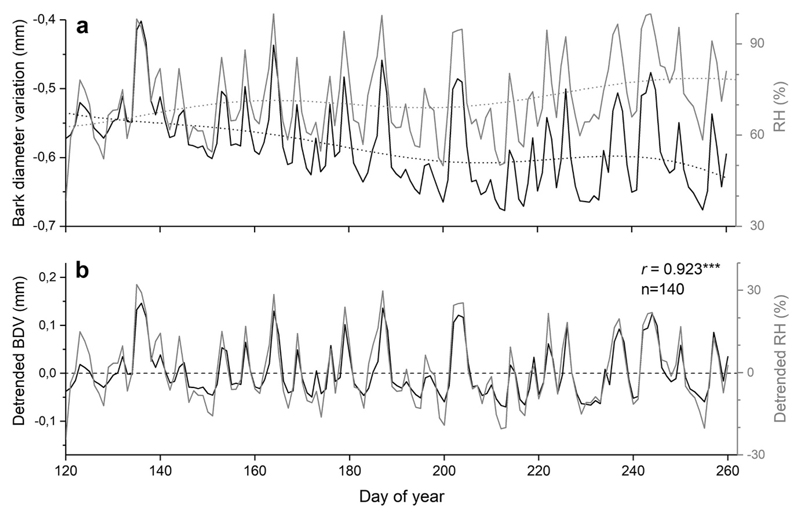
SDVs in mature living trees and a dead tree in comparison with RH and precipitation are depicted in Fig. 3a. From February through April 2018 stem diameter strongly decreased in all trees, which was consistent with a decrease in RH. When precipitation and RH increased in May stem diameter of the dead tree and living trees started to diverge. During the growing season the stem diameter of the dead tree and living trees decreased and increased in accordance with long-term changes in RH. Detrended daily SDVs showed low variation until end of April 2018, when more frequent and intense precipitation events set in (Fig. 3b). Pearson correlation revealed close relationship among dSDVs of the dead tree and living trees (r = 0.902, n = 401, P<0.001). During the growing season diurnal changes in thickness of a dead outer bark sample (thickness 3.5 mm) closely followed changes in RH, and Pearson correlation coefficient between detrended bark diameter variations and RH was highly significant (r=0.923, P<0.001; Fig. 4a-b).
Fig. 3.
a-b Raw (a) and detrended (b) stem diameter variations (SDVs) of living mature trees (n=3; dark grey lines) and one dead mature tree (black lines) during 2018 compared to relative air humidity (RH; grey line) and precipitation (bars). Mean standard deviation (bar) among dendrometer records of living trees (grey circle) is shown. Tree death educed from 100 % needle browning occurred in October 2017. Long-term trends of daily SDVs (dotted lines) were calculated by fast Fourier transform low-pass filter (n=28). Pearson correlation coefficients (r) among detrended SDVs (dSDVs) of living and dead trees are indicated (*** = P<0.001).
Fig. 4.
a-b Raw (a) and detrended (b) bark diameter variations (BDV) and relative air humidity (RH; grey line) from May through mid-September 2018. Thickness of dead outer bark sample was 3.5 mm (n=1). Pearson correlation coefficient (r) among detrended BDV and RH is indicated (*** = P<0.001).
Multiple linear regression results are summarized in Table 2. No multicollinearity was detected (squared generalized variance inflation factors (Fox and Monette 1992) for all predictors were below 5) for the multiple linear regression The whole model had predictive capability (F statistic: P< 0.05). The coefficients for RH, air temperature, precipitation, day of year (DOY) and the year 2018 were highly significant. The model explained 40.19% of the variation in SDV. The relative importance of the predictors for R2, normalized to 100 %, for SDV in the model amounted to 64 % for RH and 21 % for air temperature (Supplementary Fig. 3).
Table 2. Summary of the multiple linear regression (CE = coefficient estimate, SE = standard error). *** = P<0.001; * = P<0.05.
| Predictor | CE | SE |
|---|---|---|
| Relative air humidity | 0.009*** | 0.0002 |
| Air temperature | -0.006*** | 0.001 |
| Precipitation | -0.004*** | 0.001 |
| Day of year | -0.001*** | 0.00005 |
| Year 2017 | 0.011* | 0.006 |
| Year 2018 | 0.057*** | 0.007 |
| Living/dead | 0.003 | 0.005 |
| Age | 0.001 | 0.007 |
| Constant | -0.435*** | 0.023 |
4. Discussion
The focus of this study was to determine the influence of hygroscopic shrinkage and swelling of dead outer bark on the reversible component of dendrometer records of Scots pine saplings and mature trees under field conditions. Dendrometers were mounted on dead outer bark with an effective thickness of 3.8 mm, i.e. twice radial periderm thickness due to usage of diameter dendrometers in saplings, and 7.5 mm in mature trees. Results revealed that SDVs of dead and living trees followed changes in both, the diurnal rhythm and long-term trend of RH. Lövdahl and Odin (1992) already pointed out that RH is the appropriate parameter to describe water exchange between two unsaturated media, i.e. the surface of a tree stem and the surrounding atmosphere. In accordance with several authors (e.g., Zweifel et al. 2001, Steppe et al. 2006) who reported that the contribution of temperature variations to daily SDVs is insignificant, our multiple linear regression model revealed that the proportion of variance in the dependent variable (SDV) that is predictable from RH and air temperature was 64 % and 21 %, respectively, also pointing to considerably less influence of air temperature on daily SDVs. This finding is corroborated by missing correspondence between seasonal fluctuations in mean air temperature amounting to c. 20°C between early spring and summer and long-term trend in SDVs of dead trees during the growing seasons.
Close correlations between atmospheric conditions (RH, VPD, air temperature) and stem water status deduced from SDVs measured over living inner bark (phloem) were frequently interpreted to indicate that transpiration draws upon stem water from elastic tissue reservoirs of the bark, which are rapidly replenished when evaporative demand declines (Zweifel et al. 2000, Čermák et al. 2007, Ehrenberger et al. 2012, Sevanto et al. 2011, Steppe et al. 2015). The existence of high radial conductance to water transport between xylem and living tissues, which is attributable to changes in xylem tension and osmotic concentration of the inner bark, was confirmed by combined measurements of radial changes of xylem and whole stem radius, i.e. point dendrometers were placed in direct contact with xylem and inner bark, respectively (for a review see de Swaef et al. 2015, Chan et al. 2016, Mencuccini et al., 2017). However, highly significant relationships among SDVs of dead and living trees found in this study indicate that reversible SDVs recorded by dendrometers mounted over even thin dead outer bark (thickness < 4 mm in saplings) are caused by hygroscopic swelling and shrinking of the outer bark tissue, although up to 2 cm of dead periderm was removed in mature trees. A highly significant correlation detected among daily changes in RH and bark thickness (all living tissues removed) corroborate this interpretation. These findings were surprising because tree growth is strongly limited by water availability within the study area (e.g. Schuster and Oberhuber 2013). Hence, alternating wet and drought periods during the growing season and concomitant changes in VPD are expected to cause more distinct SDVs in living than in dead trees, because living trees exploit water reservoirs outside the cambium, when high evaporative demand prevails (Zweifel et al. 2005, Sevanto et al. 2005, Pfautsch et al. 2015, Dietrich et al. 2018). Most likely, besides hygroscopicity of dead outer bark, the high content of hydrophilic compounds occurring in cell walls (Rose 2003) cause phloem cells in dead trees to hygroscopically shrink and swell similar to SDVs induced by radial water exchange between phloem and xylem found in living trees. This is supported by Gall et al. (2002), who found that phloem tissue of Norway spruce trees also responds to changes in RH, and decreasing correlations among SDVs of living and dead trees in the course of the study period 2016-2018, which might have been caused by gradual deterioration of the non-lignified cell walls of the phloem. Furthermore, results show that due to pronounced hygroscopic properties of the bark, an increase in stem diameter deduced from dendrometers mounted over dead outer bark might mistakenly indicate radial stem growth (i.e. wood formation) when RH is steadily increasing after an extended drought period during the growing season. Results of this study are corroborated by Stahl et al. (2010), who found that in tropical rain-forest trees seasonal variations in stem circumference are influenced by changes in hygroscopic properties of the bark, and Herrmann et al. (2016), who concluded that over-bark records of stem circumference variations have limited potential to estimate tree water status and can potentially bias stem growth, on daily and weekly time scales, respectively.
In summary, this study revealed that SDVs measured over even thin dead outer bark layers (thickness <4 mm) are strongly affected by evaporation and absorption of water from the bark. Hence, relationships between climate variables and SDVs measured over dead outer bark are strongly affected by the influence of moisture status of the air on bark thickness, i.e. bark hygroscopicity amplifies the influence of atmospheric conditions on transpiration and whole-tree water status. Furthermore, timing of radial tree growth especially of slow-growing trees and at sites, where dry periods alternate with moist conditions during the growing season, is impaired. Because the amount of water that can be absorbed from saturated air by the dead outer bark layer of Scots pine is at the lower end of several coniferous and deciduous tree species analysed by Ilek et al. (2016), results of this study are also of great relevance for dendrometer studies of other tree species. Therefore, to unequivocally (i) relate SDVs to changes in tree water status, (ii) uncover meteorological drivers of SDVs, and (iii) prevent misinterpretation of radial stem growth in periods of increasing RH, total removal of dead outer bark tissue and positioning of the sensor tip of the point dendrometer to the living phloem tissue, which is sealed with silicone grease to prevent evaporative water loss, is a prerequisite (e.g. Steppe et al. 2015, Chan et al. 2016, Mencuccini et al., 2017). Point dendrometers should be applied, because scraping away dead outer bark layers down to the living phloem around the whole stem circumference is a laborious and in large trees with a thick bark an impossible task.
Supplementary Material
Supplementary material associated with this article can be found, in the online version, at 10.1016/j.agrformet.2020.108026.
Acknowledgments
The research was funded by the Austrian Science Fund (FWF): P25643-B16. We thank Brigitta Erschbamer and Vera Margreiter for providing and programming the climate chamber and supervising the experiment.
Footnotes
Author contribution statement
WO conceived the study, analysed and interpreted data, and wrote the paper. FK and MS carried out the statistical analyses in R, FK evaluated the statistics and wrote all corresponding text sections.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Bates D. lme4: Mixed-effects modeling with R. Springer; 2010. [Google Scholar]
- Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- Bouriaud O, Leban J-M, Bert D, Deleuze C. Intra-annual variations in climate influence growth and wood density of Norway spruce. Tree Physiol. 2005;25:651–660. doi: 10.1093/treephys/25.6.651. [DOI] [PubMed] [Google Scholar]
- Čermák J, Kučera J, Bauerle WL, Phillips N, Hinckley TM. Tree water storage and its diurnal dynamics related to sap flow and changes in stem volume in old-growth Douglas-fir trees. Tree Physiol. 2007;27:181–198. doi: 10.1093/treephys/27.2.181. [DOI] [PubMed] [Google Scholar]
- Chan T, Hölttä T, Berninger F, Mäkinen H, Nöjd P, Mencuccini M, Nikinmaa E. Separating water-potential induced swelling and shrinking from measured radial stem variations reveals a cambial growth and osmotic concentration signal. Plant Cell Env. 2016;39:233–244. doi: 10.1111/pce.12541. [DOI] [PubMed] [Google Scholar]
- De Schepper V, van Dusschoten D, Copini P, Jahnke S, Steppe K. MRI links stem water content to stem diameter variations in transpiring trees. J Exp Bot. 2012;63:2645–2653. doi: 10.1093/jxb/err445. [DOI] [PubMed] [Google Scholar]
- Deslauriers A, Morin H, Urbinati C, Carrer M. Daily weather response of balsam fir (Abies balsamea (L.) Mill.) stem radius increment from dendrometer analysis in the boreal forests of Québec (Canada) Trees. 2003;17:477–484. [Google Scholar]
- Deslauriers A, Rossi S, Anfodillo T. Dendrometer and intra-annual tree growth: what kind of information can be inferred? Dendrochronologia. 2007;25:113–124. [Google Scholar]
- De Swaef T, Driever SM, van Meulebroek L, Vanhaecke L, Marcelis LFM, Steppe K. Understanding the effect of carbon status on stem diameter variations. Ann Bot. 2013;111:31–46. doi: 10.1093/aob/mcs233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Swaef T, De Schepper V, Vandegehuchte MW, Steppe K. Stem diameter variations as a versatile research tool in ecophysiology. Tree Physiol. 2015;35:1047–1061. doi: 10.1093/treephys/tpv080. [DOI] [PubMed] [Google Scholar]
- Dietrich L, Zweifel R, Kahmen A. Daily stem diameter variations can predict the canopy water status of mature temperate trees. Tree Physiol. 2018;38:941–952. doi: 10.1093/treephys/tpy023. [DOI] [PubMed] [Google Scholar]
- Drew DM, Downes GM. The use of precision dendrometers in research on daily stem size and wood property variation: A review. Dendrochronologia. 2009;27:159–172. [Google Scholar]
- Ehrenberger W, Rüger S, Fitzke R, Vollenweider P, Günthardt-Goerg M, Kuster T, Zimmermann U, Arend M. Concomitant dendrometer and leaf patch pressure probe measurements reveal the effect of microclimate and soil moisture on diurnal stem water and leaf turgor variations in young oak trees. Funct Plant Biol. 2012;39:297–305. doi: 10.1071/FP11206. [DOI] [PubMed] [Google Scholar]
- Evert RF. Esau's plant anatomy. Meristerms, cells, and tissues of the plant body: their structure, function, and development. 3 rd edition. Wiley & Sons, Inc; Hoboken, New Jersey: 2009. [Google Scholar]
- Fox J, Monette G. Generalized collinearity diagnostics. J Americ Stat Assoc. 1992;87:178–183. [Google Scholar]
- Gall R, Landolt W, Schleppi P, Michellod V, Bucher JB. Water content and bark thickness of Norway spruce (Picea abies) stems: phloem water capacitance and xylem sap flow. Tree Physiol. 2002;22:613–623. doi: 10.1093/treephys/22.9.613. [DOI] [PubMed] [Google Scholar]
- Grömping U. Relative importance for linear regression in R: The Package relaimpo. J Stat Softw. 2006;17:1–27. [Google Scholar]
- Herrmann V, McMahon SM, Detto M, Lutz JA, Davies SJ, Chang-Yang C-H, Anderson-Teixeira KJ. Tree circumference dynamics in four forests characterized using automated dendrometer bands. PloS One. 2016;11(12):e0169020. doi: 10.1371/journal.pone.0169020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlavac M. stargazer: Well-formatted regression and summary statistics tables (Version R package version 5.2.2.) 2018 Retrieved from https://CRAN.R-project.org/package=stargazer.
- Ilek A, Kucza J, Morkisz K. Hygroscopicity of the bark of selected forest tree species. iForest. 2016;10:220–226. doi: 10.3832/ifor1979-009. [DOI] [Google Scholar]
- Irvine J, Grace J. Continuous measurements of water tensions in the xylem of trees based on the elastic properties of wood. Planta. 1997;202:455–461. [Google Scholar]
- King G, Fonti P, Nievergelt D, Büntgen U, Frasnk D. Climatic drivers of hourly to yearly tree radius variations along a 6°C natural warming gradient. Agr For Meteorol. 2013;168:36–46. [Google Scholar]
- Köcher P, Horna V, Leuschner C. Environmental control of daily stem growth patterns in five temperate broad-leaved tree species. Tree Physiol. 2012;32:1021–1032. doi: 10.1093/treephys/tps049. [DOI] [PubMed] [Google Scholar]
- Köcher P, Horna V, Leuschner C. Stem water storage in five coexisting temperate broad-leaved tree species: significance, temporal dynamics and dependence on tree functional traits. Tree Physiol. 2013;33:817–832. doi: 10.1093/treephys/tpt055. [DOI] [PubMed] [Google Scholar]
- Lindeman RH, Merenda PF, Gold RZ. Scott Foresman, Glenview, Illinois. United States; 1980. Introduction to bivariate and multivariate analysis. [Google Scholar]
- Lövdahl L, Odin H. Diurnal changes in the stem diameter of Norway spruce in relation to relative humidity and air temperature. Trees. 1992;6:245–251. [Google Scholar]
- Mencuccini M, Hölttä T, Sevanto S, Nikinmaa E. Concurrent measurements of change in the bark and xylem diameters of trees reveal a phloem-generated turgor signal. New Phytol. 2013;198:1143–1154. doi: 10.1111/nph.12224. [DOI] [PubMed] [Google Scholar]
- Mencuccini M, Salmon Y, Mitchell P, Hölttä T, Choat B, Meir P, O’Grady A, Tissue D, Zweifel R, Sevanto S, Pfautsch S. An empirical method that separates irreversible stem radial growth from bark water content changes in trees: theory and case studies. Plant Cell Environ. 2017;40:290–303. doi: 10.1111/pce.12863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhuber W, Gruber A. Climatic influences on intra-annual stem radial increment of Pinus sylvestris (L.) exposed to drought. Trees. 2010;24:887–898. doi: 10.1007/s00468-010-0458-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhuber W, Gruber A, Kofler W, Swidrak I. Radial stem growth in response to microclimate and soil moisture in a drought-prone mixed coniferous forest at an inner Alpine site. Eur J For Res. 2014;133:467–479. doi: 10.1007/s10342-013-0777-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhuber W, Kofler W, Schuster R, Wieser G. Environmental effects on stem water deficit in co-occurring conifers exposed to soil dryness. Int J Biometeorol. 2015;59:417–426. doi: 10.1007/s00484-014-0853-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfautsch S, Hölttä T, Mencuccini M. Hydraulic functioning of tree stems-fusing ray anatomy, radial transfer and capacitance. Tree Physiol. 2015;35:706–722. doi: 10.1093/treephys/tpv058. [DOI] [PubMed] [Google Scholar]
- Prenger JJ, Ling PP. Fact sheet (Series) AEX-800. Ohio State University Extension; Columbus, OH: 2020. Greenhouse condensation control: Understanding and using vapour pressure deficit (VPD) [Google Scholar]
- R Core Team. R Foundation for statistical computing. Vienna, Austria: 2018. R: A language and environment for statistical computing. Retrieved from: http://www.R-project.org/ [Google Scholar]
- Rose JKC, editor. Annual Plant Reviews 8 Blackwell Publishing Ltd. Oxford; UK: 2003. The plant cell wall. [Google Scholar]
- Schuster R, Oberhuber W. Drought sensitivity of three co-occurring conifers within a dry inner Alpine environment. Trees. 2013;27:61–69. doi: 10.1007/s00468-012-0768-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevanto S, Vesala T, Perämäki M, Nikinmaa E. Time lags for xylem and stem diameter variations in a Scots pine tree. Plant Cell Env. 2002;25:1071–1077. [Google Scholar]
- Sevanto S, Vesala T, Perämäki M, Nikinmaa E. Sugar transport together with environmental conditions controls time lags between xylem and stem diameter changes. Plant Cell Env. 2003;26:1257–1265. [Google Scholar]
- Sevanto S, Hölttä T, Markkanen T, Perämäki M, Nikinmaa E, Vesala T. Relationships between diurnal xylem diameter variation and environmental factors in Scots pine. Boreal Env Res. 2005;10:447–458. [Google Scholar]
- Sevanto S, Hölttä T, Holbrook NM. Effects of hydraulic coupling between xylem and phloem on diurnal phloem diameter variation. Plant Cell Env. 2011;34:690–703. doi: 10.1111/j.1365-3040.2011.02275.x. [DOI] [PubMed] [Google Scholar]
- Stahl C, Burban B, Bompy F, Jolin ZB, Sermage J, Bonal D. Seasonal variation in atmospheric relative humidity contributes to explaining seasonal variation in trunk circumference of tropical rain-forest trees in French Guiana. J Trop Ecol. 2010;26:393–405. [Google Scholar]
- Steppe K, De Pauw DJW, Lemeur R, Vanrolleghem PA. A mathematical model linking tree sap flow dynamics to daily stem diameter fluctuations and radial stem growth. Tree Physiol. 2006;26:257–273. doi: 10.1093/treephys/26.3.257. [DOI] [PubMed] [Google Scholar]
- Steppe K, Sterck F, Deslauriers A. Diel growth dynamics in tree stems: linking anatomy and ecophysiology. Trends Plant Sci. 2015;20:335–343. doi: 10.1016/j.tplants.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Turcotte A, Rossi S, Deslauriers A, Krause C, Morin H. Dynamics of depletion and replenishment of water storage in stem and roots of black spruce measured by dendrometers. Front Plant Sci. 2011;2:21. doi: 10.3389/fpls.2011.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Maaten E, Pape J, van der Maaten-Theunissen M, Scharnweber T, Smiljanic M, Wilmking M. Distinct growth phenology but similar daily stem dynamics in three co-occurring broadleaved tree species. Tree Physiol. 2018;38:1820–1828. doi: 10.1093/treephys/tpy042. [DOI] [PubMed] [Google Scholar]
- Zuur AF, Ieno EN, Elphick CS. A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol. 2010;1:3–14. [Google Scholar]
- Zweifel R, Item H, Häsler R. Stem radius changes and their relation to stored water in the stems of young Norway spruce trees. Trees. 2000;15:50–57. [Google Scholar]
- Zweifel R, Item H, Häsler R. Link between diurnal stem radius changes and tree water relations. Tree Physiol. 2001;21:869–877. doi: 10.1093/treephys/21.12-13.869. [DOI] [PubMed] [Google Scholar]
- Zweifel R, Zimmermann L, Newbery DM. Modeling tree water deficit from microclimate: an approach to quantifying drought stress. Tree Physiol. 2005;25:147–156. doi: 10.1093/treephys/25.2.147. [DOI] [PubMed] [Google Scholar]
- Zweifel R, Drew DM, Schweingruber F, Downes GM. Xylem as the main origin of stem radius changes in Eucalyptus. Funct Plant Biol. 2014;41:520–534. doi: 10.1071/FP13240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.