Abstract
Daily oral exposure to vast numbers (>1013/adult/day) of micron or nano-sized persistent particles has become the norm for many populations. Significant airborne particle exposure is deleterious, so what about ingestion? Titanium dioxide in food grade form (fgTiO2) 1, which is an additive to some foods, capsules, tablets and toothpaste, may provide clues. Certainly, exposed human populations accumulate these particles in specialised intestinal cells at the base of large lymphoid follicles (Peyer’s patches) and it’s likely that a degree of absorption goes beyond this- i.e. lymphatics to blood circulation to tissues. We critically review the evidence and pathways. Regarding potential adverse effects, our primary message, for today’s state-of-art, is that in vivo models have not been good enough and at times woeful. We provide a ‘caveats list’ to improve approaches and experimentation and illustrate why studies on biomarkers of particle uptake, and lower gut/mesenteric lymph nodes as targets, should be prioritized.
Keywords: gastrointestinal, absorption, Peyer’s patch, titanium dioxide, particle, nanoparticle
1. Introduction
One of the current, great human experiments is our continuous oral exposure to large numbers of so- called ‘micro’ and ‘nano’ particles in non-biological and low degradative forms (1013 particles per adult/day for just three common food additive and excipient particles, for example)[1]. Effects, if there are any, are poorly understood but deserve scrutiny given what is now known about population exposures to particulates through another route (airborne) and the positive associations with morbidity and mortality [2]. For the oral route we can learn much from titanium dioxide in food grade form (fgTiO2) which is the exemplar persistent and cell-accessible particle of the modern diet. Its generally unnecessary role in ingested materials is whitening and brightening, and major sources are capsules, tablets, toothpaste and processed foods. For over 20 years its accumulation, as a ‘normal’ occurrence, in human immune cells of the intestine, specifically at the base of large lymphoid follicles (Peyer’s patches), has been well known [3]. And, yet, failure to replicate absorption in experimental animal studies outweighs the published cases that support accumulation from ingestion. Some have interpreted this to mean that orally-dosed fgTiO2 particles do not accumulate in humans. In fact, there are many bear traps in experimentation which frequently snare investigators and mislead interpretation. Here, we consider these as well as how intestinal absorption occurs and what any potential for intestinal toxicity might look like.
2. Particle Uptake by the Gut
The gastrointestinal tract does not recognise the policy makers’ definition of ‘nano’ (e.g. [4]): there is no machinery that enables the uptake of particles of 1-100 nm equitably whilst ignoring larger ones. Instead, the gut has determined its own rules for particle uptake. For example, large ‘nano’ and small ‘micro’ particles (~50-200 nm diameter optimally) get taken up via microfold (M) cells of organised lymphoid follicles (i.e. overlying Peyer’s patches) whilst small ‘nano’ particles (probably < 50 nm but typically a few nm) may access regular epithelial cells [5]. Our group reviewed the pathways for particle uptake in the intestine in 2010 [6] and only a little has changed since then. There is now some evidence that trans-epithelial dendrites of dendritic cells, reaching out from gut tissue into the lumen, can sample non-biological particles [7,8] in the same way that they can sample invasive gut bacteria [9,10]. As yet, however, there is no compelling evidence that this is a constitutive uptake route for regular oral exposure to non-biological particles and the previously described routes remain state-of-art [6].
The typical particle size distribution of fgTiO2 falls within the optimal range of the M cell uptake mechanism which, normally, traffics endogenous calcium phosphate particles into Peyer’s Patch immune cells [11]. In other words, fgTiO2 particles are well suited to hijacking a physiological pathway for particle entry into gut tissue. To demonstrate this, microscopy rather than whole tissue analysis is appropriate because any tiny contamination of gut tissue with luminal contents means that the origin of the analyte signal is not attributable (i.e. how much of the signal is derived from particles in the tissue versus on/out of the tissue?). As such, using appropriate microscopy and microanalysis, Bettini et al. (2017) reported particulate Ti signals in the Peyer’s patches of the small intestine of rats orally exposed to fgTiO2 for 7 days [12]. The most compelling and relevant evidence, however, for M cell uptake of particles in the intestine, is earlier data from the analysis of surgically- and autopsy- resected intestinal tissue, showing that TiO2 and other engineered particles are retained in the human intestinal mucosa, notably at the base of Peyer’s patch lymphoid follicles [3,13] (Figure 1). The level/number of these particles probably correlates with the age of the subject from which the tissue was resected [14] and, in adults, the accumulation can be so remarkable, and the cells so pigmented, that collectively they form microscopic tattoo-like structures at the base of the Peyer’s Patch [13].
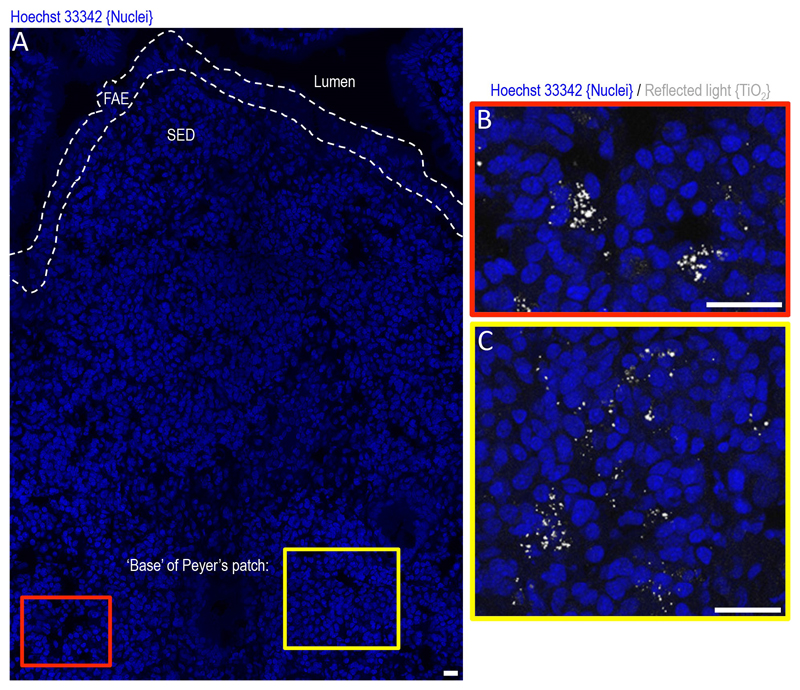
Figure 1. Detection of particle-loaded pigment cells using reflectance confocal microscopy.
(A) Section of human Peyer’s patch tissue (i.e. gut lymphoid follicle) with cell nuclei stained blue by Hoechst 33342 and imaged as a single 0.8 micron-thick optical slice with a confocal microscope. Regions are marked on for the intestinal lumen, follicle associated epithelium (FAE), sub-epithelial dome (SED) and base of the patch. At higher magnification (B and C), the laser reflectance signal reveals particle-loaded pigment cells, residing at the base of the Peyer’s patch. The fgTiO2, which is a major contributor to this pigment [3] is highly reflective and provides a ready bio-marker for detection in tissue using light microscopy. Scale bars = 25 micron. These images are from a study approved by the UK NHS Health Research Authority, North West - Greater Manchester East Research Ethics Committee, REC reference 18/NW/0690.
Fortunately, the cells here appear to be both immunologically and metabolically sluggish and there is no evidence to suggest that these cells are signalling in a pro-inflammatory or other undesirable fashion in spite of their weight of cargo [15]. Notwithstanding, replicating this in murine models and investigating potentially more subtle effects of such exposure would be of value. Moreover, demonstration of basal Peyer’s patch accumulation of fgTiO2 with feeding would be step one in validating a model with human relevance.
3. Particle Absorption beyond the Gut
One question that is rarely broached is how do particles get beyond gut cells and absorbed into more systemic compartments? This is not via capillaries. Although these exhibit small pore (~9nm) and large pore (~50 nm) permeability [16], the latter is rare and inefficient and anyway the ‘reverse’ of the migration/extravasation principles for cells leaving blood vessels is not anticipated. Instead, similar to blood capillaries are lymphatic capillaries and these really are permeable with two specific structural properties. Firstly, endothelial cells, forming the walls of lymphatic capillaries, are not tightly connected. Instead of tight junctions, cells’ edges overlap each other in a relatively loose way that leaves readily opening ‘flaps’ (mini-valves). Secondly, collagen filaments from the surrounding structure serve to anchor endothelial cells such that increases in interstitial fluid volume actually open the flaps, preventing lymphatic capillary collapse. Overall this unique structure of the lymphatic capillaries has been described as ‘a system which is very similar to a bunch of one-way swinging doors’ [17] and this provides a route for the systemic distribution of particles.
Once in the intestinal lymphatic system, particles, potentially alone or carried in cells, will be first drained to and through the local mesenteric lymph nodes. Indeed, Geraets et al. (2014) reported increased levels of Ti in the mesenteric lymph nodes (0.36 ppb vs 0.14 ppb in the control animals) after oral exposure to TiO2 of 2.3 mg per animal per day for 5 days [18]. Difficult as it is for mesenteric lymph nodes to be identified and dissected out in murine models, this is a potential site of persistent particle accumulation that is crying out to be carefully studied (again, ideally by microscopy to make sense of the detail). Lymph, having arrived through the afferent lymphatics, then exits the mesenteric lymph nodes through the efferent lymphatics and, via the cisterna chyli, drains into the thoracic duct joining the blood circulation with subsequent systemic distribution.
To what extent orally-dosed particles actually access the systemic circulation is not clear although three human studies, using TiO2 and similar but not identical trial designs, provide some insights. In the first, Böckmann et al. (2000) investigated sequential blood levels of Ti in six volunteers after the ingestion of gelatine capsules containing 23 or 45 mg of TiO2 [19]. They demonstrated 5 to 10 fold increases above baseline in blood Ti levels, generally peaking at 8-12 h post ingestion. Smaller particles appeared to have greater absorption. In 2015, Pele et al. reported on a modified repeat of this work with 7 volunteers ingesting 100 mg fgTiO2 [20]. The sequential blood samples were analysed for total Ti levels by high resolution ICP-MS, as well as for reflectant bodies (equated to TiO2 particles) by dark field microscopy. The measures correlated and peak absorption, at only ~10 μg/l, was at 6-8 h after ingestion [20], consistent with the Böckmann data, and likely explained by Peyer’s patch absorption of particles which is late compared to the more proximal and rapid absorption of nutrients. In contrast, in the third study, Jones et al. (2015) were not able to demonstrate acute absorption of TiO2 in humans when administering 5 mg/kg body weight [21]. High baseline levels (i.e. apparent signal for Ti) may have masked the detection of absorbed particles in blood whilst sampling intervals were imperfect to detect Peyer’s patch routing [21]. On balance, systemic absorption of ingested fgTiO2 can occur, at low levels, although how the particles are incorporated into the ingested matrix may influence to what extent it does occur, and further research is merited in this respect.
From the bloodstream, particle uptake into tissues is efficient [18]. In the normal population, the liver and spleen are the tissues reported to have measurable Ti due to fgTiO2 exposure. Heringa et al. (2018) suggested these as specific sites for fgTiO2 bioaccumulation in humans [22]. They quantified total Ti and TiO2 particles in 15 post-mortem human liver and spleen samples by high resolution ICP-MS. They reported average total Ti levels of 40 ppb in the liver and 80 ppb in the spleen. They demonstrated the particulate nature of these signals by high resolution single particle ICP-MS and by microanalysis and inferred that the total Ti measured was the result of accumulated TiO2 particles [22]. A few in vivo dosing studies in rodents corroborate the findings in humans, as exemplified by Kreyling and co-workers [23]. They employed a highly sensitive radio-tracer technique (γ-ray spectrometry) to quantify-by-proxy the oral absorption and bio-distribution of 48V radiolabelled TiO2 and found very low but measurable systemic signal. Although the methodology was imperfect [23], the results probably fairly reflect that there is extremely low systemic absorption and tissue loading of TiO2 following ingestion. Despite this, many oral exposure studies with TiO2 have been unable to detect such small increases above the background, probably because of the multiple methodological and analytical challenges that exist, as discussed below and summarised in Figure 2.
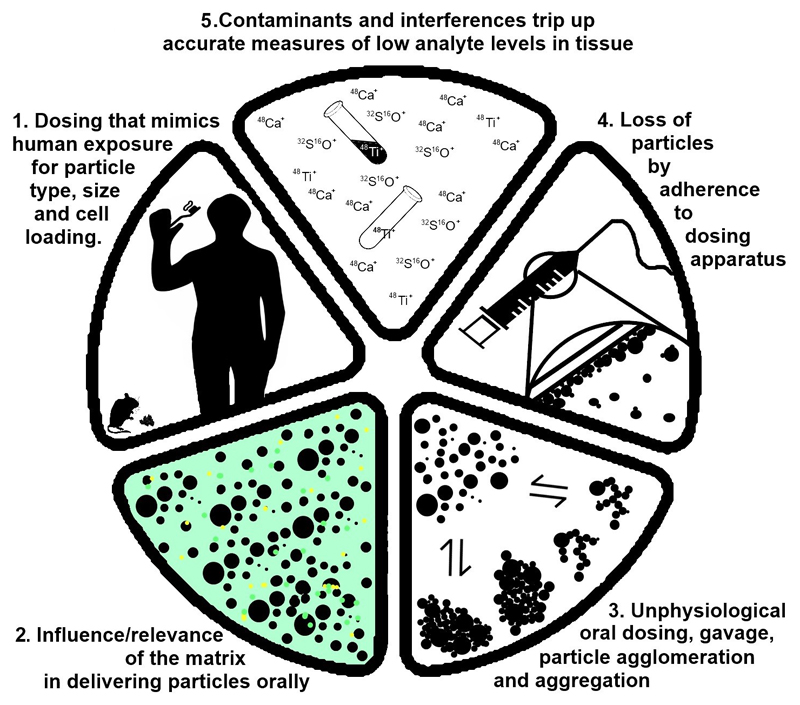
Figure 2. Important considerations and caveats in absorption studies.
Murine models are frequently used to predict effects of oral particle exposure to humans but, mostly, these are flawed. Mimicking particle type is important whilst comparative cell loading (i.e. in the rodent model versus humans) may turn out to be the only reasonable, albeit technically demanding, approach for sensible quantitative dosing (1). If human cell exposure to oral particles is known, as it is for fgTiO2 and some silicates [3], then any dosing matrix needs to allow some gut luminal release of such particles in an animal model (2). Gavage is stressful and its bolus doses are totally unlike general oral exposure to particles for humans. Moreover, such approaches encourage agglomeration and aggregation of particles (3). In addition, particles are sticky and adsorb to such dosing equipment (4). Finally, even if the issues of 1-4 are avoided, then analysis of particles in tissues must be very carefully validated as errors are frequent (5).
4. Challenges and Flaws in Absorption Methodology
The ‘three Rs’, namely replace, reduce and refine, should be at the forefront of any animal experimentation. Is a rodent study absolutely necessary? If the answer is ‘yes’ then it is critical that the approach is thought through very carefully, not only to reduce numbers whilst retaining a properly powered study but also to refine outputs with as much ‘added value’ analysis as possible. To acquire meaningful and relevant results in particle absorption work it is imperative that animal studies (where necessary) reproduce, as far as possible, the exposure picture in man. This entails administering appropriate forms of particles (e.g. fgTiO2) at reasonable doses and in a ‘physiological’ fashion, followed by careful quantitation. Unfortunately, from both informed learning and animal welfare perspectives, most studies to date fall short of this, woefully so at times.
4.1. Particle Dosing. Caveat! Does the exposure approximate the real human situation?
Particle type and exposure must be carefully considered. For example, fgTiO2 consists of nano- and micro- particles of varying sizes, from 15 to 5,000 nanometres in extremis, although, most are 60-300 nm albeit with a nano-fraction of at least 10-15% by number [24]. To dose in a meaningful way it is necessary that fgTiO2, intended for use in foods or as an excipient, is used and not some unrealistic nano-fraction which many studies have employed [25]. Moreover, extrapolation of measured human oral exposure to rodent dosing, on a weight-for-weight basis, is fraught with issues in spite of healthy efforts to do so (e.g. Blanchard and Smoliga, 2015; Nair and Jacob, 2016) [26,27]. The actual oral dose for rodent studies would, in fact, be best adjudged from how cell loading mimics the human situation, despite the undoubted amount of time and resource required for this.
4.2. Dosing Matrix. Caveat! Does the matrix for dosing allow particle accumulation in a way that mimics the human situation?
Oral exposure to nano- and micro- particles in humans is incremental and concomitant with various matrices (e.g. food). The physiochemical nature of the particle-matrix interaction will be one mediator of particle release and dispersion in the alimentary canal. As such, any oral dosing matrix must allow some disperse particle ‘freedom’ in the lumen if absorption is anticipated in humans and the model seeks to recapitulate this. For example, recently, Blevins et al. (2019) reported no real immunological or intestinal effects when administering a diet with fgTiO2 to rats [28]. Whilst the use of a diet rather than gavage is laudable (see 4.3), unfortunately, they fell at the first fence by failing to demonstrate that the diet even rendered TiO2 available- e.g. to intestinal cells in the way that one sees for humans. A validated diet, that generates the TiO2 contribution of intestinal pigment cells [13] or pigment cell precursors, will be of great value.
4.3. Qualitative Exposure. Caveat! Exposure methodology is generally insensible
A number of publications have suggested that grabbing a caged rodent, forcing a tube down its throat and filling its stomach with vast quantities of a test material, on a repeated basis, is a suitable way to predict effects of oral human exposure to particles (e.g. Warheit et al., 2015) [29]. For overt toxicity screening of large numbers of soluble compounds this may be an accepted approach that provides perceived consistency in the measurement of the administered dose. Presumably this is why gavage remains the OECD methodology for acute toxicity testing [30]. But common exposure particles do not, in any way, adhere to this paradigm; nor do all OECD toxicity testing guidelines mandate gavage as the only administration route [31–34]. Particle speciation in complex fluids is highly dynamic and driven by processes such as dissolution and agglomeration that depend upon solution conditions (e.g. particle concentration, ionic strength, the presence or absence of dispersants etc.). Concentrated particle solutions tend to agglomerate, either in the dose solution or in the intestine [35] such that, counter-intuitively, higher dosing may lead to lower exposures. As a more general point, repeated, short-term bolus dosing cannot represent gradual lifelong exposure and the performance of the gut is greatly compromised by the gorging and, especially, the stress, associated with gavage [36,37]. Future work must concentrate on more natural, validated, dietary exposures.
4.4. Quantitative Exposure. Caveat! Intended exposure level may not equal actual exposure level
Gavage is not just unphysiological, it may also be less quantitative than assumed, especially for particles. In essence, one must ensure that what is drawn up into the syringe is representative of the solution that it comes from (i.e. any particle distribution is homogenously dispersed for sampling) and that what is expelled from the syringe/gavage tube combination is what is intended. But particles are often sticky and, for example, Kreyling et al. (2017) showed losses as high as 51% due to adsorption of particles to gavage syringes [23]. Admittedly they were studying very low levels but the point is made that initial suspension homogeneity is as important for representative sampling as measuring what comes out of the tube upon sham gavage [23] – if, indeed, that method of administration really must be used. For diet-based studies, food intake can be measured and particle exposure thus estimated, and whilst this will not be entirely accurate, it will obviate many of the other issues already noted. It would also represent some of the real variance seen for oral particle exposure in the human population [1].
4.5. Analysis. Caveat! Techniques like ICP-MS are extremely sensitive but reported data are still often flawed
Even though (quantitative) microscopy should provide the clearest data in trying to understand particle loading following exposure, the common approach for pragmatic reasons (speed, resource etc.) is generally gross tissue quantification. In the case of TiO2 this is chiefly through the use of elemental Ti as a surrogate marker [38]. Whilst endogenous levels of Ti in mammals are low, one would also expect very little absorption from an oral dose of TiO2, as already described. The accurate quantification of a low signal on top of an existing one is challenging, for reasons of discrimination rather than sensitivity. ICP-MS is the gold standard for quantification of trace and sub-trace levels (ppb/ppt) of elements in biological samples. But it suffers from matrix interference and, especially, interference of mass detection of species in the plasma that might overlap the analyte of interest; for example, 48Ti is closely mass equivalent to a rare isotope (48Ca) of a very abundant bio-element [39]. High resolution ICP-MS, and triple quadrupole ICP-MS (ICP-MS/MS) with its mass-shift mode, help obviate such problems when used properly [38]. A further issue, however, is that even low levels of contamination, picked up during digestion or dilution of samples for example, can increase the background analyte signal masking small differences between samples. Spiking and recovery experiments will not reveal these types of errors and, generally, reference standards have such enhanced levels of ultra-trace elements that they also mask the problem [38]. Caution, therefore, should be exercised in reporting ‘no absorption’; rather it is no discernible absorption with the technique used.
A final concluding note on this subject, to researchers and reviewers alike, is the importance of ‘sanity checking’- i.e. the checks and balances that can be carried out with the data that one has available. As one example from a number, MacNicoll et al. (2015) dosed different forms of TiO2 by gavage at an intended 5 mg/kg body weight, to six groups of 8 week old male rats (348 ± 47 g in weight) [40]. No systemic absorption was detected but faecal Ti levels (μg/g) were highly variable. For example, one group had background Ti levels after 48 h and only about double this at 0-48h, whereas another group had 20-40 fold background Ti levels at 0-48 h. Moreover, in this latter group, with faecal Ti at 373 μg/g over 24-48 hours, and an average intended gavage of 1.74 mg TiO2 (1.04 mg Ti), the entire intended oral dose would be accounted for by just 2.8 g faeces/rat which is about a third of what they should excrete in this period.
5. Consistent Human Exposure - Should we worry?
It could be argued that if only tiny quantities of oral particulates, such as fgTiO2, are absorbed systemically, and the one area where there is known cell targeting (Peyer’s patch base) is immunologically dormant, then why should we care? There are, in fact, a number of potential reasons.
Firstly, gross analysis yields a terrifically ‘diluted’ signal, because all the non-particle containing cells, and extracellular matrix, are analysed alongside the rather specialised ones that do take up particles. Again, pigment cells at the base of gut lymphoid tissue exemplify this [3,13]. The particle concentration in targeted cells may be enormous versus the gross analytical signal, so sub-regions or networks of accumulation could still exist but gross analyses fail to reveal this. Secondly, the idea that tissue-fixed particle-containing macrophages are always dormant is challengeable. Occasional idiosyncratic responses to tattoo ink particles, for example, is reported [41] and latency of effect with particle-causing diseases is well known [42]. Thirdly, recent data suggest that population gene mutations could also affect how cells respond to particles- in this case, responses to fgTiO2 [43]. Finally, other phagocytic cells (such as neutrophils, monocytes and immature dendritic cells) may also scavenge and process particles, potentially with greater activity than tissue fixed macrophages [44–46].
If bio-accumulation of persistent particles does occur in human cells that are not entirely dormant, what consequences should we consider? Two of the authors (REH and JJP) have, in this same Current Opinions series, detailed how common exposure particles might exert (immuno)-toxicity effects [47] and this will not be repeated here. However, it is worth noting that fgTiO2 could adjuvant antigen-driven immune responses and is also a mild inducer of the ‘inflammasome’ and pro-inflammatory signalling- the latter in concert with corona-forming microbial fragments which are so prevalent in the distal intestine environment [48].
Finally, even if the gut does not take up a material following ingestion, the lumen/epithelial cell apical environment still receives full exposure. By potential analogy, it is widely reported that this is why certain soluble iron forms are toxic to the intestine - i.e. they are poorly absorbed as supplements but redox-active in transit through the intestine [49]. There is no direct evidence that the unabsorbed fraction of ingested particulates (i.e. the vast majority) do the same, but it’s worth briefly considering what is known about particles in the colon where, it is theorized that, accumulation of TiO2 occurs in the thick mucus layer whilst the slow colonic transit time allows prolonged particle-epithelial cell contact [50,51]. To date, many colon-related experiments have been carried out in vitro, with the predominant cell lines being CaCo2, HT29-MTX or HT29-MTX/CaCo2 combinations [50–54] and, for which, there is limited read across to the in vivo situation. In vivo, Ruiz et al. (2017) carried out an acute investigation, reporting that mice with both induced-colitis and TiO2 exposure had shorter colon lengths and increased inflammatory cell infiltration versus induced-colitis alone, suggesting that TiO2 might be damaging in pre-existing or concomitant inflammatory conditions [55]. This hypothesis was furthered when Urrutia-Ortega et al. (2016) showed that TiO2 exacerbates tumour progression in an AOM/DSS inflammatory murine model of cancer, with no tumorigenic effect of the particles on control animals [56]. They did, however, induce dysplastic changes in otherwise normal colonic epithelium and decreased goblet cell counts. Further work by this group revealed that TiO2 induced alterations in pathways involved in cell cycle, DNA repair and UCP genes in particle-only-exposed healthy mice, over 2, 7, 14 and 21 days [54,56]. The adverse effect of TiO2 on the colon of control animals was further demonstrated by Bettini et al. (2017) where chronic exposure to 10mg/kg/day of TiO2 in rats led to increases in aberrant crypts in both DMH-cancer-induced animals and controls [12]. Whilst these studies have come in for criticism (e.g. reference [28]), ranging from the choice of particle type (i.e. a nano fraction of TiO2 being used rather than fgTiO2 in some) through to questionable methodology and analytical techniques, careful repeat of such work, respecting the checks and balances laid out in this manuscript, is encouraged. In particular, how the dosed fgTiO2 behaves in the colon is important: darkfield/reflectance microscopy could reveal this readily [13] and, if there is an effect, we should be open to the idea that particle absorption per se may not be necessary to mediate this, as per the supplemental iron paradigm noted above.
Conclusion
We know that large numbers of ingested nano- and micro- particles, persistent enough to survive gastrointestinal processing, are a regular exposure occurrence for many populations. We also know that a fraction is absorbed, and heavily accumulated, into very specific gut cells. Its extremely likely that some also go further- i.e. systemically- and the more careful literature suggests that this is true albeit at extremely low levels. Much better experimental approaches are now required to understand if any of this matters and, if so, to what extent? Some recent patchy data suggests that it just might matter-and with a distal gut focus. Watch that space.
Acknowledgements
WT, REH, RJ and JJP acknowledge the support of the Medical Research Council [grant number MR/R005699/1]. ABdS acknowledges the support of the Brazilian Federal Agency for Support and Evaluation of Graduate Education - CAPES [grant number 88881.127953/2016-01]. JW acknowledges Girton College and the University of Cambridge Herchel-Smith fund for support of his Fellowships.
Footnotes
Here the term ‘fgTiO2’ is used for both food grade and excipient forms of titanium dioxide which can be added to ingested materials. ‘TiO2’ refers to titanium dioxide more generally. ‘Ti’ is used in reference to elemental analysis.
Conflict of Interests statement
The authors Alessandra B. da Silva, Michelle Miniter, William Thom, Rachel E. Hewitt, John Wills, Ravin Jugdaohsingh and Jonathan J. Powell declare they have no competing interests. JJP is a guest editor of this series but he has had no role or input into the editorial handling or independent review of this manuscript.
References
- [1].Lomer MCE, Hutchinson C, Volkert S, Greenfield SM, Catterall A, Thompson RPH, Powell JJ. Dietary sources of inorganic microparticles and their intake in healthy subjects and patients with Crohn’s disease. Br J Nutr. 2004;92:947–955. doi: 10.1079/bjn20041276. [** This paper represents an extensive, quantitative study of normal titanium dioxide and silicate particle intakes in a large number of free-living volunteers and patients.] [DOI] [PubMed] [Google Scholar]
- [2].World Health Organization. World Health Organization; 2016. [accessed January 6, 2020]. Ambient air pollution: a global assessment of exposure and burden of disease. https://apps.who.int/iris/handle/10665/250141. [Google Scholar]
- [3].Powell JJ, Ainley CC, Harvey RS, Mason IM, Kendall MD, Sankey EA, Dhillon AP, Thompson RP. Characterisation of inorganic microparticles in pigment cells of human gut associated lymphoid tissue. Gut. 1996;38:390–395. doi: 10.1136/gut.38.3.390. [** This work built on two prior studies showing Ti, Al and Si signal at the Peyer's patch base, but was the first to identify these signals as coming from intracellular mineral particles of nano- and micro- dimensions.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].European Food Safety Authority. Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: Part 1, human and animal health. [accessed February 10, 2020];2018 doi: 10.2903/j.efsa.2018.5327. https://www.efsa.europa.eu/en/efsajournal/pub/5327. [This document defines the following: “Engineered nanomaterial means ‘any intentionally produced material that has one or more dimensions of the order of 100 nm or less or that is composed of discrete functional parts, either internally or at the surface, many of which have one or more dimensions of the order of 100 nm or less, including structures, agglomerates or aggregates, which may have a size above the order of 100 nm but retain properties that are characteristic of the nanoscale’.”] [DOI] [PMC free article] [PubMed]
- [5].Howe SE, Lickteig DJ, Plunkett KN, Ryerse JS, Konjufca V. The Uptake of Soluble and Particulate Antigens by Epithelial Cells in the Mouse Small Intestine. PLOS ONE. 2014;9:e86656. doi: 10.1371/journal.pone.0086656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Powell JJ, Faria N, Thomas-McKay E, Pele LC. Origin and fate of dietary nanoparticles and microparticles in the gastrointestinal tract. Journal of Autoimmunity. 2010;34:J226–J233. doi: 10.1016/j.jaut.2009.11.006. [* This review is still state-of-art for particle absorption from the gut.] [DOI] [PubMed] [Google Scholar]
- [7].Farache J, Koren I, Milo I, Gurevich I, Kim K-W, Zigmond E, Furtado GC, Lira SA, Shakhar G. Luminal Bacteria Recruit CD103+ Dendritic Cells into the Intestinal Epithelium to Sample Bacterial Antigens for Presentation. Immunity. 2013;38:581–595. doi: 10.1016/j.immuni.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lelouard H, Fallet M, de Bovis B, Méresse S, Gorvel J-P. Peyer’s patch dendritic cells sample antigens by extending dendrites through M cell-specific transcellular pores. Gastroenterology. 2012;142:592–601.e3. doi: 10.1053/j.gastro.2011.11.039. [DOI] [PubMed] [Google Scholar]
- [9].Morita N, Umemoto E, Fujita S, Hayashi A, Kikuta J, Kimura I, Haneda T, Imai T, Inoue A, Mimuro H, Maeda Y, et al. GPR31-dependent dendrite protrusion of intestinal CX3CR1 + cells by bacterial metabolites. Nature. 2019;566:110–114. doi: 10.1038/s41586-019-0884-1. [DOI] [PubMed] [Google Scholar]
- [10].Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl J-P, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- [11].Powell JJ, Thomas-McKay E, Thoree V, Robertson J, Hewitt RE, Skepper JN, Brown A, Hernandez-Garrido JC, Midgley PA, Gomez-Morilla I, Grime GW, et al. An Endogenous Nanomineral Chaperones Luminal Antigen and Peptidoglycan to Intestinal Immune Cells. Nat Nanotechnol. 2015;10:361–369. doi: 10.1038/nnano.2015.19. [* This work provides the reason why the Peyer’s patch transports particles.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bettini S, Boutet-Robinet E, Cartier C, Coméra C, Gaultier E, Dupuy J, Naud N, Taché S, Grysan P, Reguer S. Food-grade TiO 2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Scientific Reports. 2017;7 doi: 10.1038/srep40373. 40373. [* These, potentially, very important findings have stimulated much interest, and some criticism, and deserve careful independent repetition. The work also provides the only real evidence that fgTiO2might access colonic tissue which, again, should be repeated.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Powell JJ, Lomer MC, Thompson RP, Evans S. What are intestinal pigment cells? Inflammatory Bowel Disease Monitor. 1999;1:71–74. [Google Scholar]
- [14].Hummel TZ, Kindermann A, Stokkers PCF, Benninga MA, ten Kate FJW. Exogenous Pigment in Peyer Patches of Children Suspected of Having IBD. Journal of Pediatric Gastroenterology and Nutrition. 2014;58:477. doi: 10.1097/MPG.0000000000000221. [* This work confirms that dietary micro and nano particles, such as titanium dioxide, are not just present in adult gut tissue but start to accumulate in children, and their presence increases with age.] [DOI] [PubMed] [Google Scholar]
- [15].Thoree V, Skepper J, Deere H, Pele LC, Thompson RPH, Powell JJ. Phenotype of exogenous microparticle-containing pigment cells of the human Peyer’s patch in inflamed and normal ileum. Inflammation Research. 2008;57:374–378. doi: 10.1007/s00011-007-7216-x. [DOI] [PubMed] [Google Scholar]
- [16].Clementi F, Palade GE. Intestinal capillaries. I. Permeability to peroxidase and ferritin. J Cell Biol. 1969;41:33–58. doi: 10.1083/jcb.41.1.33. [The interpretation of vascular pores and permeability has been debated and this paper provides a good discussion of the subject in an era when such interests were assiduously considered.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].The Lymphatic System, Anatomy & Physiology. [accessed February 10, 2020];2014 https://anatomyandphysiologyi.com/lymphatic-system/ [* A very good online review of the anatomy and physiology of the lymphatic system with graphical detail of the lymphatic capillaries structure.]
- [18].Geraets L, Oomen AG, Krystek P, Jacobsen NR, Wallin H, Laurentie M, Verharen HW, Brandon EF, de Jong WH. Tissue distribution and elimination after oral and intravenous administration of different titanium dioxide nanoparticles in rats. Particle and Fibre Toxicology. 2014;11:30. doi: 10.1186/1743-8977-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Böckmann HLJ. Blood levels of titanium before and after oral administration of titanium dioxide [Titan-blutspiegel vor und nach belastungsversuchen mit titandioxid] Die Pharmazie. 2000;55:140–3. [PubMed] [Google Scholar]
- [20].Pele LC, Thoree V, Bruggraber SF, Koller D, Thompson RP, Lomer MC, Powell JJ. Pharmaceutical/food grade titanium dioxide particles are absorbed into the bloodstream of human volunteers. Particle and Fibre Toxicology. 2015;12:26. doi: 10.1186/s12989-015-0101-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jones K, Morton J, Smith I, Jurkschat K, Harding A-H, Evans G. Human in vivo and in vitro studies on gastrointestinal absorption of titanium dioxide nanoparticles. Toxicology Letters. 2015;233:95–101. doi: 10.1016/j.toxlet.2014.12.005. [DOI] [PubMed] [Google Scholar]
- [22].Heringa MB, Peters RJB, Bleys RLAW, van der Lee MK, Tromp PC, van Kesteren PCE, van Eijkeren JCH, Undas AK, Oomen AG, Bouwmeester H. Detection of titanium particles in human liver and spleen and possible health implications. Particle and Fibre Toxicology. 2018;15:15. doi: 10.1186/s12989-018-0251-7. [* The authors provide proof-of-principle for the presence of at least some TiO2 particles in human liver and spleen samples by single particle quantification, namely high resolution single particle ICP-MS and microanalysis (EDX) under SEM.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kreyling WG, Holzwarth U, Schleh C, Kozempel J, Wenk A, Haberl N, Haberl S, Schäffler M, Lipka L, Semmler-Behnke M, Gibson N. Quantitative biokinetics of titanium dioxide nanoparticles after oral application in rats: Part 2. Nanotoxicology. 2017;11:443–453. doi: 10.1080/17435390.2017.1306893. [* The minimal radioactivity observed, systemically, in this study underlines the very low absorption of TiO2. However, accurate measures were liable to confounding by the leaching (and subsequent absorption) of soluble 48V from particles in the alimentary tract. Laudably, the authors conducted an auxiliary study with soluble 48V and undertook bio-distribution difference calculations to account for this effect but, numbers were low, there was large inter-individual variability and urinary excretion corrections were calculated relative to administered dose rather than translocated dose.] [DOI] [PubMed] [Google Scholar]
- [24].Peters RJB, van Bemmel G, Herrera-Rivera Z, Helsper HPFG, Marvin HJP, Weigel S, Tromp PC, Oomen AG, Rietveld AG, Rietveld H. Characterization of Titanium Dioxide Nanoparticles in Food Products: Analytical Methods To Define Nanoparticles. Journal of Agricultural and Food Chemistry. 2014;62:6285–6293. doi: 10.1021/jf5011885. [DOI] [PubMed] [Google Scholar]
- [25].Hu H, Guo Q, Wang C, Ma X, He H, Oh Y, Feng Y, Wu Q, Gu N. Titanium dioxide nanoparticles increase plasma glucose via reactive oxygen species-induced insulin resistance in mice. Journal of Applied Toxicology. 2015;35:1122–1132. doi: 10.1002/jat.3150. [Monodisperse titanium dioxide nanoparticles, with an average hydrodynamic size of 49.6 ± 0.4 nm, were used in this work but, in the intestine, their behaviour cannot be expected to replicate the interaction and competition of similar sized particles in a polydisperse (i.e. food grade) material.] [DOI] [PubMed] [Google Scholar]
- [26].Blanchard OL. Translating dosages from animal models to human clinical trials—revisiting body surface area scaling. The FASEB Journal. 2015;29:1629–1624. doi: 10.1096/fj.14-269043. [DOI] [PubMed] [Google Scholar]
- [27].Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27–31. doi: 10.4103/0976-0105.177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Blevins LK, Crawford RB, Bach A, Rizzo MD, Zhou J, Henriquez JE, Khan DMIO, Sermet S, Arnold LL, Pennington KL, Souza NP, et al. Evaluation of immunologic and intestinal effects in rats administered an E 171-containing diet, a food grade titanium dioxide (TiO2) Food and Chemical Toxicology. 2019;133 doi: 10.1016/j.fct.2019.110793. 110793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Warheit DB, Brown SC, Donner EM. Acute and subchronic oral toxicity studies in rats with nanoscale and pigment grade titanium dioxide particles. Food and Chemical Toxicology. 2015;84:208–224. doi: 10.1016/j.fct.2015.08.026. [DOI] [PubMed] [Google Scholar]
- [30].OECD. Test Guideline 425: Acute Oral Toxicity: Up-and-Down Procedure. [accessed January 7, 2020];2008 https://www.oecd-ilibrary.org/environment/test-no-425-acute-oral-toxicity-up-and-down-procedure_9789264071049-en.
- [31].OECD. Test Guideline 407: Repeated Dose 28-day Oral Toxicity Study in Rodents. [accessed January 7, 2020];2008 https://www.oecd-ilibrary.org/environment/test-no-407-repeated-dose-28-day-oral-toxicity-study-in-rodents_9789264070684-en.
- [32].OECD. Test Guideline 408: Repeated Dose 90-Day Oral Toxicity Study in Rodents. [accessed January 7, 2020];2018 https://www.oecd-ilibrary.org/environment/test-no-408-repeated-dose-90-day-oral-toxicity-study-in-rodents_9789264070707-en.
- [33].OECD. Test Guideline 453: Combined Chronic Toxicity/Carcinogenicity Studies. [accessed January 7, 2020];2018 https://www.oecd-ilibrary.org/environment/test-no-453-combined-chronic-toxicity-carcinogenicity-studies_9789264071223-en.
- [34].OECDL. Test Guideline 474: Mammalian Erythrocyte Micronucleus Test. [accessed January7, 2020];2016 https://www.oecd-ilibrary.org/environment/test-no-474-mammalian-erythrocyte-micronucleus-test_9789264264762-en.
- [35].van der Zande M, Vandebriel RJ, Groot MJ, Kramer E, Herrera Rivera ZE, Rasmussen K, Ossenkoppele JS, Tromp P, Gremmer ER, Peters RJ, Hendriksen PJ, et al. Sub-chronic toxicity study in rats orally exposed to nanostructured silica. Particle and Fibre Toxicology. 2014;11:8. doi: 10.1186/1743-8977-11-8. [** This paper exemplifies how particles can aggregate in the gut when dosed at unrealistic levels so actual exposure is lower with higher dosed levels! - a key caveat for stakeholders in (nano) particle research.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Atcha Z, Rourke C, Neo AH, Goh CW, Lim JS, Aw C-C, Browne ER, Pemberton DJ. Alternative Method of Oral Dosing for Rats. Journal of the American Association for Laboratory Animal Science. 2010;49:335–343. [PMC free article] [PubMed] [Google Scholar]
- [37].Brown AP, Dinger N, Levine BS. Stress produced by gavage administration in the rat. Contemp Top Lab Anim Sci. 2000;39:17–21. [PubMed] [Google Scholar]
- [38].Koller D, Bramhall P, Devoy J, Goenaga-Infante H, Harrington CF, Leese E, Morton J, Nuñez S, Rogers J, Sampson B, Powell JJ. Analysis of soluble or titanium dioxide derived titanium levels in human whole blood: consensus from an inter-laboratory comparison. Analyst. 2018;143:5520–5529. doi: 10.1039/C8AN00824H. [* This paper exemplifies how difficult it is to achieve true analytical signal for low level Ti in a biological matrix, and why spiking and recovery experiments or certified reference standards may not solve this or even indicate the issues.] [DOI] [PubMed] [Google Scholar]
- [39].Balcaen L, Bolea-Fernandez E, Resano M, Vanhaecke F. Accurate determination of ultra-trace levels of Ti in blood serum using ICP-MS/MS. Analytica Chimica Acta. 2014;809:1–8. doi: 10.1016/j.aca.2013.10.017. [DOI] [PubMed] [Google Scholar]
- [40].MacNicoll A, Kelly M, Aksoy H, Kramer E, Bouwmeester H, Chaudhry Q. A study of the uptake and biodistribution of nano-titanium dioxide using in vitro and in vivo models of oral intake. Journal of Nanoparticle Research. 2015;17 doi: 10.1007/s11051-015-2862-3. [DOI] [Google Scholar]
- [41].de Cuyper C, Lodewick E, Schreiver I, Hesse B, Seim C, Castillo-Michel H, Laux P, Luch A. Are metals involved in tattoo-related hypersensitivity reactions? A case report. Contact Dermatitis. 2017;77:397–405. doi: 10.1111/cod.12862. [DOI] [PubMed] [Google Scholar]
- [42].Pollard KM. Silica, Silicosis, and Autoimmunity. Front Immunol. 2016;7 doi: 10.3389/fimmu.2016.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Riedle S, Pele LC, Otter DE, Hewitt RE, Singh H, Roy NC, Powell JJ. Pro-inflammatory adjuvant properties of pigment-grade titanium dioxide particles are augmented by a genotype that potentiates interleukin 1β processing. Particle and Fibre Toxicology. 2017;14 doi: 10.1186/s12989-017-0232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Babin K, Antoine F, Gonçalves DM, Girard D. TiO2, CeO2 and ZnO nanoparticles and modulation of the degranulation process in human neutrophils. Toxicology Letters. 2013;221:57–63. doi: 10.1016/j.toxlet.2013.05.010. [DOI] [PubMed] [Google Scholar]
- [45].Gonçalves DM, Girard D. Titanium dioxide (TiO2) nanoparticles induce neutrophil influx and local production of several pro-inflammatory mediators in vivo. International Immunopharmacology. 2011;11:1109–1115. doi: 10.1016/j.intimp.2011.03.007. [DOI] [PubMed] [Google Scholar]
- [46].Hewitt RE, Vis B, Pele LC, Faria N, Powell JJ. Imaging flow cytometry assays for quantifying pigment grade titanium dioxide particle internalization and interactions with immune cells in whole blood. Cytometry Part A. 2017;91:1009–1020. doi: 10.1002/cyto.a.23245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hewitt RE, Chappell H, Powell JJ. Small and dangerous? Potential toxicity mechanisms of common exposure particles and nanoparticles. Curr Opin Toxicol In Press. 2020 doi: 10.1016/j.cotox.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ashwood P, Thompson RPH, Powell JJ. Fine Particles That Adsorb Lipopolysaccharide Via Bridging Calcium Cations May Mimic Bacterial Pathogenicity Towards Cells. Exp Biol Med (Maywood) 2007;232:107–117. doi: 10.3181/00379727-207-2320107. [DOI] [PubMed] [Google Scholar]
- [49].Tolkien Z, Stecher L, Mander AP, Pereira DIA, Powell JJ. Ferrous Sulfate Supplementation Causes Significant Gastrointestinal Side-Effects in Adults: A Systematic Review and Meta-Analysis. PLOS ONE. 2015;10:e0117383. doi: 10.1371/journal.pone.0117383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Brun E, Barreau F, Veronesi G, Fayard B, Sorieul S, Chanéac C, Carapito C, Rabilloud T, Mabondzo A, Herlin-Boime N, Carrière M. Titanium dioxide nanoparticle impact and translocation through ex vivo, in vivo and in vitro gut epithelia. Particle and Fibre Toxicology. 2014;11:13. doi: 10.1186/1743-8977-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Talbot P, Radziwill-Bienkowska JM, Kamphuis JBJ, Steenkeste K, Bettini S, Robert V, Noordine M-L, Mayeur C, Gaultier E, Langella P, Robbe-Masselot C, et al. Food-grade TiO2 is trapped by intestinal mucus in vitro but does not impair mucin O-glycosylation and short-chain fatty acid synthesis in vivo: implications for gut barrier protection. Journal of Nanobiotechnology. 2018;16 doi: 10.1186/s12951-018-0379-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Dorier M, Béal D, Marie-Desvergne C, Dubosson M, Barreau F, Houdeau E, Herlin-Boime N, Carriere M. Continuous in vitro exposure of intestinal epithelial cells to E171 food additive causes oxidative stress, inducing oxidation of DNA bases but no endoplasmic reticulum stress. Nanotoxicology. 2017;11:751–761. doi: 10.1080/17435390.2017.1349203. [DOI] [PubMed] [Google Scholar]
- [53].Jensen DM, Løhr M, Sheykhzade M, Lykkesfeldt J, Wils RS, Loft S, Møller P. Telomere length and genotoxicity in the lung of rats following intragastric exposure to food-grade titanium dioxide and vegetable carbon particles. Mutagenesis. 2019;34:203–214. doi: 10.1093/mutage/gez003. [DOI] [PubMed] [Google Scholar]
- [54].Proquin H, Jetten MJ, Jonkhout MCM, Garduño-Balderas LG, Briedé JJ, de Kok TM, van Loveren H, Chirino YI. Transcriptomics analysis reveals new insights in E171-induced molecular alterations in a mouse model of colon cancer. Sci Rep. 2018;8:1–16. doi: 10.1038/s41598-018-28063-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ruiz PA, Morón B, Becker HM, Lang S, Atrott K, Spalinger MR, Scharl M, Wojtal KA, Fischbeck-Terhalle A, Frey-Wagner I, Hausmann M, et al. Titanium dioxide nanoparticles exacerbate DSS-induced colitis: role of the NLRP3 inflammasome. Gut. 2017;66:1216–1224. doi: 10.1136/gutjnl-2015-310297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Urrutia-Ortega IM, Garduño-Balderas LG, Delgado-Buenrostro NL, Freyre-Fonseca V, Flores-Flores JO, González-Robles A, Pedraza-Chaverri J, Hernández-Pando R, Rodríguez-Sosa M, León-Cabrera S, Terrazas LI, et al. Food-grade titanium dioxide exposure exacerbates tumor formation in colitis associated cancer model. Food and Chemical Toxicology. 2016;93:20–31. doi: 10.1016/j.fct.2016.04.014. [DOI] [PubMed] [Google Scholar]