Abstract
Anorexia nervosa (AN) is an eating disorder observed predominantly in women and girls that is characterized by a low body-mass index, hypophagia, and hyperactivity. Activity-based anorexia (ABA), which refers to the weight loss, hypophagia, and hyperactivity exhibited by rodents exposed to both running wheels and scheduled fasting, provides a model for aspects of AN. Increased dopamine D2/D3 receptor binding in the anteroventral striatum has been reported in AN patients. We virally overexpressed D2Rs on nucleus accumbens core (D2R-OENac) neurons that endogenously express D2Rs, and tested mice of both sexes in the open field test, ABA paradigm, and intraperitoneal glucose tolerance test (IGTT). D2R-OENac did not alter baseline body weight, but increased locomotor activity in the open field across both sexes. During constant access to food and running wheels, D2R-OENac mice of both sexes increased food intake and ran more than controls. However, when food was available only 7 hours a day, only female D2R-OENac mice rapidly lost 25% of their initial body weight, reduced food intake, and substantially increased wheel running. Surprisingly, female D2R-OENac mice also rapidly lost 25% of their initial body weight during scheduled fasting without wheel access and showed no changes in food intake. In contrast, male D2R-OENac mice maintained body weight during scheduled fasting. D2R-OENac mice of both sexes also showed glucose intolerance in the IGTT. In conclusion, D2R-OENac alters glucose metabolism in both sexes but drives robust weight loss only in females during scheduled fasting, implicating metabolic mechanisms in this sexually dimorphic effect.
Keywords: compulsive wheel running, eating disorder, food restriction-induced hyperactivity, starvation, energy expenditure
INTRODUCTION
Anorexia nervosa (AN) is a complex illness mostly affecting women and girls1, and is characterized primarily by a low body-mass index2. A recent large-scale genetic analysis found that AN shows significant genetic correlations with both psychiatric disorders and metabolic traits, and should be reconceptualized as a metabo-psychiatric disorder3, 4. AN has the highest mortality rate of all psychiatric disorders, and affects approximately 1% of the population over the lifespan1. Despite the high mortality rate and prevalence in pediatric populations, no approved pharmacological treatments have been developed for AN, and its neurobiological underpinnings remain unknown.
The activity-based anorexia (ABA) phenomenon is observed in normal rodents and other mammals, and consists of several key behaviors that are also observed in AN patients5. In the ABA paradigm, rodents are singly housed with running wheels and exposed to scheduled fasting. Under these conditions, animals develop hypophagia and compulsive wheel running despite extreme weight loss, compared to rodents exposed to either scheduled fasting or running wheels5. Thus, during the development of ABA, underweight animals choose not to feed during times of food availability. If allowed to continue unchecked, ABA results in hypothermia, increased HPA axis activity, and ultimately stomach ulceration and death5–7. Female rodents develop greater hyperactivity and lose body weight more rapidly than males during ABA8–10. These findings in rodents parallel the clinical observation that AN affects approximately one tenth as many males as females1.
The dopaminergic system has been strongly implicated in the neurobiological mechanisms underlying AN. Recovered patients with AN restricting-type were reported to show reduced cerebral spinal fluid homovanillic acid (HVA)11, suggesting that postsynaptic DA receptors might be upregulated in compensation. In line with this finding, recovered AN patients also show increased dopamine D2/D3 receptor binding in the anteroventral striatum as measured by [11C] raclopride binding using positron emission tomography (PET)12. This finding suggests that upregulation of D2/D3 receptors in the anteroventral striatum in recovered AN patients might either predispose to the development of AN, or compensate for illness. However, others have failed to replicate this finding13. Further suggesting a role for dopamine D2 receptors in AN, the partial D2 receptor agonist aripiprazole was associated with weight gain in adolescent AN patients14.
ABA is also modulated by the dopaminergic system, and by dopamine D2 receptors in particular. Chronic systemic treatment with the dopamine D2 receptor (D2R) antagonist L-741,626, or the D2/D3R antagonists, eticlopride or amisulpride, reduced hypophagia and improved survival (the number of mice losing 25% of their initial body weight) in the ABA paradigm15. Although chronic treatment with the D3R antagonist SB277011A also increased survival, this effect was only observed at the high dose that might have also have antagonized D2Rs15. Chronic systemic treatment with the dopamine D1-like receptor antagonist SCH23390 did not alter survival in the ABA paradigm15. Recently, the excitation of VTA-nucleus accumbens (NAc) projections using the excitatory DREADD receptor hM3D(Gq) was shown to reduce the ABA phenotype in rats; however the projections targeted in this study were not specifically dopaminergic, and the effects on reward circuitry remains unclear16. Thus, the role of the dopaminergic system in regulating ABA behavior is complex and circuit-dependent, and requires further study.
Overexpression of D2Rs on NAc core D2R-expressing neurons (D2R-OENac) in mice has been shown to increase locomotion, enhance motivation, and decrease inhibitory transmission to the ventral pallidum17, 18. D2R-expressing neurons in the NAc signal risky choices or punishment and are involved in maintaining behavioral flexibility in mice 19, 20. AN patients might also have altered function of NAc D2R-expressing neurons due to increased levels of D2R in the anteroventral striatum12, an idea supported by the observation that AN patients make more risky choices and exhibit cognitive inflexibility21–25. Here, we sought to determine whether D2R-OENac exacerbates ABA, which would suggest that D2R overexpression in the anteroventral striatum plays a causal rather than compensatory role in AN. We generated D2R-OENac mice of both sexes by infusing a Cre recombinase-dependent adeno-associated virus (AAV) expressing the D2R and/or a fluorophore into the NAc core of Drd2-Cre transgenic mice. Once viral expression was established, we assessed mice in the open field, ABA, and intraperitoneal glucose tolerance test (IGTT).
METHODS
Mice
Adult D2-Cre BAC transgenic mice (ER44 line; GENSAT) backcrossed >10 generations onto a C57BL/6J background were purchased and bred with C57BL/6J mice from Jackson Laboratories. Hemizygous adult mice of both sexes (12-15 weeks) were used for all studies. Mice were housed in a climate-controlled room maintained on a 12:12 light–dark cycle (lights on at 07:00) with food and water available ad libitum unless otherwise stated. All procedures were approved by the Institutional Animal Care and Use Committee at University of California, San Diego.
AAV generation and delivery
The AAV1-hSyn-DIO-D2R(L)-IRES-mVenus virus, described previously17, 18, was packaged by Vector Biolabs (Malvern, PA). The control AAV1-hSyn-DIO-eGFP virus was purchased and packaged from Vector Biolabs (Figure 1a). At 8 weeks of age, hemizygous transgenic mice of both sexes were bilaterally infused with 0.5 μL of D2R or eGFP AAVs to generate D2R-OENac and control mice, respectively. Viral infusions were directed at the stereotaxic coordinates A-P, +1.7 mm, M-L ±1.5 mm, and D-V −4.4 mm, relative to bregma (Figure 1b,c). Virus was infused at a rate of 0.1 μL/min for 5 mins per side, and cannula were left in place for an additional 5 mins to permit diffusion. Experiments began 4 weeks post viral infusion.
Figure 1.
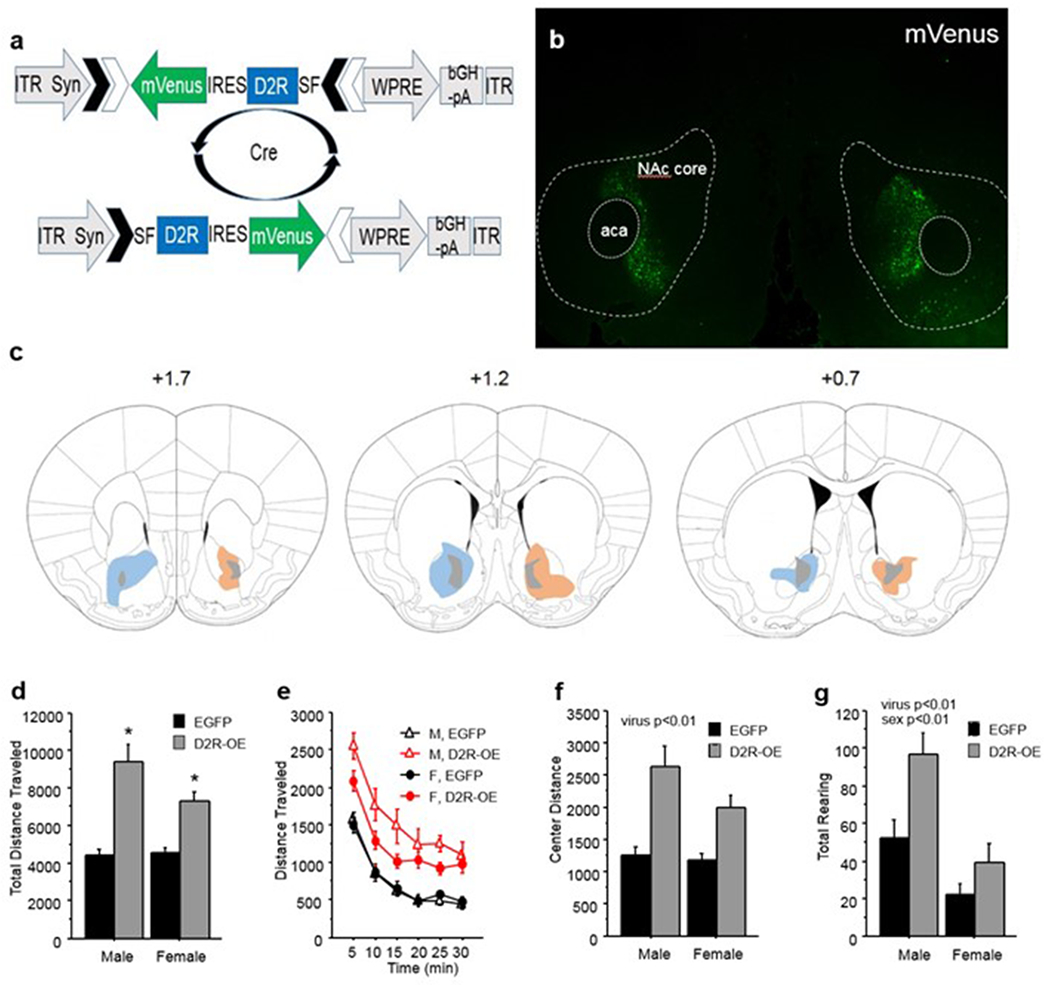
D2R viral overexpression in NAc core increases locomotion. (a) AAV1 vector overexpressing D2R in cre-dependent fashion. (b) mVenus expression in NAc core 6 weeks post infusion at 4x magnification. (c) Diagramatic representation of the maximum (colors) and minimum (grey) spread of D2R-mVenus (orange) and GFP (blue) in the NAc; numbers indicate distance from Bregma; anterior commissure, aca. (d) Total distance traveled in the open field over 30 min by D2R-OENAc and EGFPNAc male and female mice. (e) Distance traveled per 5 min bin in the open field by D2R-OENAc and EGFPNAc male and female mice. (f) Total center distance traveled in the open field over 30 min by D2R-OENAc and EGFPNAc male and female mice. (g) Total number of rearings in the open field over 30 min by D2R-OENAc and EGFPNAc male and female mice. Open field data are pooled for experiments 1 and 2. Mean ± s.e.m, n = 8-12/group, An asterisk indicates a significant effect of virus compared to same sex controls. A pound sign indicates a significant difference between sexes within the same viral condition.
Histology
To verify viral placements, mice were deeply anesthetized and then transcardially perfused with 4% paraformaldehyde in 0.2 M phosphate buffer. Brains were extracted and post-fixed overnight. Brains were then suspended in 30% sucrose cryoprotectant for 2 days and flash frozen in 2-methylbutane (Sigma-Aldrich, St. Louis, MO). Free-floating 30-μm coronal sections were taken using a Leica CM3050-S cryostat. Sections were mounted on slides using Vectashield hard set mounting medium with DAPI (Vector, Burlingame, CA). Digital images of mVenus or eGFP native fluorescence were taken using an Olympus BX51 fluorescent microscope with Infinity Analyze software.
Locomotor Activity
D2R-OENac and control mice were tested in open field boxes to assess locomotor activity, as described previously26. Briefly, mice were placed into one corner of the open field chamber and activity was measured for 30 minutes. Output measures were automatically generated by the Versamax software (Accuscan, Columbus, OH).
Activity-Based Anorexia Paradigm
D2R-OENac and control mice were tested in the ABA paradigm. For ABA studies, home cages (19.56 × 34.70 × 14.41 cm) were equipped with wireless low-profile running wheels (Med Associates, St Albans, VT, USA), which were either locked or unlocked depending on the experiment. Unlocked running wheels transmitted running data every 30 s to a computer with Wheel Manager software 24 h a day. Food (standard chow) was provided in a glass jar (5 cm diameter × 4 cm height) resting on the cage floor, and tap water was always available in standard bottles.
Mice were acclimated for 2 days to single housing with the running wheel and eating from the food jar. Wheels were either locked or unlocked for the duration of the study, depending on the experiment. Then, all mice entered the baseline phase (4 days), during which food was constantly available. Next, all mice entered the restriction phase, during which food was available for 7 h a day beginning at 0900 h. Seven hours of daily food access induces a dropout rate of approximately 7 days, permitting detection of either increases or decreases in survival27. During restriction, mice “drop out” of the paradigm once they lose 25% of their baseline body weight (day 4 of baseline); mice that drop out are removed from the study and immediately sacrificed. Daily body weight, food intake, and wheel running were recorded during baseline and restriction conditions. The experimenter was blind to viral group but not sex of mice.
Intraperitoneal Glucose Tolerance Test
We performed the intraperitoneal glucose tolerance test (IGTT) as described previously 28. Briefly, mice were fasted for 8 h. Then approximately 2 hours into their dark cycle, mice received IP injections of either vehicle or 10 mg/kg of the D2R agonist bromocriptine. Fifteen minutes later, 2 g/kg glucose was injected IP and glucose measurements were obtained 0, 30, 60, and 120 min later using a commercial handheld glucometer (AlphaTRAK, Zoetis). One week later, mice were tested in the same fashion as above, but received the alternate drug treatment. Drug treatments were administered in a counterbalanced fashion and groups were assigned randomly. The experimenter was blind to viral group but not to sex or drug treatments.
Experiment 1: D2R-OENac and EGFPNAc male and female mice underwent open field testing as described above. Three days later, they were tested in the ABA paradigm. All mice had unlocked wheels for the duration of testing.
Experiment 2: D2R-OENac and EGFPNAc male and female mice underwent open field testing as described above. Three days later, they were tested in the ABA paradigm and had locked wheels for the duration of testing.
Experiment 3: Experimentally naïve D2R-OENac and EGFPNAc male and female mice underwent IGTT testing.
Data Analysis
Dependent measures in the open field were analyzed using repeated measures analysis of variance (ANOVA) with time bin as a repeated measure, and virus and sex as between-subjects variables. Since open field testing was identical for Experiments 1 and 2 and mice were experimentally naïve, data were pooled for analysis. For the IGTT, drug was an additional within-subjects variable. Significant interactions were resolved using posthoc ANOVAs for within subjects factors, or Newman Keuls post hoc tests for between subjects factors. Significance was determined at p<0.05, and all tests were two-tailed. No animals were excluded from analyses.
Within the 14 days of the study duration, the time required for mice to meet the criterion for dropout (loss of ≥25% of day 4 baseline body weight) was analyzed using a Cox-Proportionate Hazards Model (survival analysis). This model included factors for sex, virus, and day. Hazard ratios (and 95% confidence limits) were calculated and the effect of each condition determined with z-tests.
When analyzing body weight, food intake, and running, mouse dropout during the food restricted phase of the ABA paradigm creates statistical challenges due to missing values. Thus, general linear mixed models (proc glimmix; SAS v9.2) were used to assess the effects of day, virus, and sex on the percent change in body weight, food intake, and wheel running between baseline and restriction, and on food anticipatory activity (FAA), which consists of wheel running during the 4 hour period before feeding begins. Post hoc t-tests adjusted for multiple comparisons using the false discovery rate method were used to resolve significant interactions.
RESULTS
D2R-OENAc increases activity in the open field
A main effect of virus (F(1,39)=60.12, p<.01) and an interaction of sex and virus (F(1, 39)= 4.74, p<0.05; Figure 1d) were found for total distance traveled. Newman Keuls post-hoc tests revealed that within each sex, D2R-OENAc mice traveled a greater distance than EGFPNAc mice, and male and female mice did not differ from each other within either viral group (Figure 1d,e).
A main effect of virus (F(1, 39)= 36.17, p<0.01; Figure 1f) also showed that D2R-OENAc mice traveled more distance in the center than EGFPNAc controls. No interaction of sex and virus was observed (F(1, 39)= 2.45, p=0.13).
For rearing, a main effect was found for D2R-OENAc mice to rear more often than EGFPNAc mice (F(1, 39)= 10.10, p<0.01; Figure 1g). Therefore, D2R-OENAc increased both horizontal and vertical activity in the open field. Male mice also showed more rearing than female mice across viral groups (F(1, 39)= 22.68, p<0.01; Figure 1g).
D2R-OENAc increases food intake and wheel running during baseline
In the experiment with wheel access, male mice weighed more than female mice across viral treatment and day (F(1, 20)= 112.12, p<0.01; Figure 2a) during baseline. However, no effect of viral treatment on body weight was found. D2R-OENAc increased food intake across sex and day during baseline (F(1, 20)= 12.40, p<0.01; Figure 2b), and males ate more than females overall (F(1, 20)= 23.61, p<0.01; Figure 2b). Finally, an interaction of day and virus was found for wheel running (F(3, 60)= 3.46, p<0.05; Figure 2c). Post hoc analysis indicated that D2R-OENAc mice ran a greater distance than EGFPNAc mice, but only on day 1 of the baseline period (F(1, 20)= 9.00, p<0.01). No effects of virus were found on wheel running on days 2-4.
Figure 2.
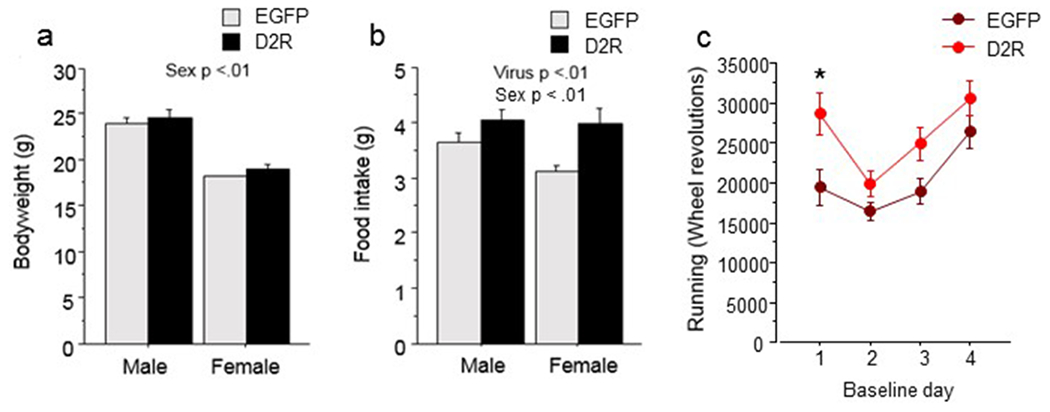
Baseline phase of ABA with wheel access. (a) Body weight in grams of D2R-OENAc and EGFPNAc male and female mice averaged over 4 days. (b) Food intake in grams of D2R-OENAc and EGFPNAc male and female mice averaged over 4 days. (c) Wheel running revolutions of D2R-OENAc and EGFPNAc male and female mice are shown for each day of baseline. Data are mean ± s.e.m, n = 5-8/group. An asterisk indicates a significant effect of virus compared to controls on the same day.
D2R-OENAc reduces survival in female, but not male, mice with wheel access
Cox-Proportionate Hazards Model found a significant interaction between sex and virus (Χ2=4.67, p<0.05) for the time to dropout during food restriction. Hazard Ratios indicated that D2R-OENAc females were substantially more likely to drop out of the study than EGFPNAc females [HR=0.096 (0.021–0.443), z=−3.00, p=0.003] (Figure 3a). Furthermore, D2R-OENAc females were substantially more likely to drop out of the study than D2R-OENAc males [HR=0.088 (0.018-0.445), z=−2.94, p=0.003]. However, the risk of dropout for EGFPNAc males versus females was approximately equal [HR=0.761 (0.249-2.327), z=−0.47, p=0.63]. Likewise, D2R-OENAc males were no more likely to be withdrawn from the study than EGFPNAc males [HR=0.824 (0.239-2.849), z=−0.31, p=0.76].
Figure 3.
Restriction phase of ABA with wheel access. (a) D2R-OENAc (red) reduced the cumulative survival of female (left), but not male (right) mice compared to controls (black). (b) Percent change in body weight relative to baseline was reduced overall by D2R-OENAc in female, but not male, mice compared to EGFPNac mice. (c) Percent change in food intake relative to baseline was reduced overall by D2R-OENAc in female, but not male, mice compared to EGFPNac mice. (d) Percent change in running relative to baseline was increased in female D2R-OENAc mice compared to both EGFPNac females and D2R-OENAc males on day 6, and a trend for the same effects were found on day 4. Furthermore, male D2R-OENAc mice exhibited increased percent of baseline running compared to control males on day 9, and a trend for this same effect was found on days 10 and 11. (f) Food anticipatory activity was increased in female D2R-OENAc mice compared to both same sex EGFPNac mice and male D2R-OENAc mice on day 5. Data in (a) show the number of mice remaining in food restriction each day. Data in (b-e) are mean ± s.e.m, n = 5-8/group. An asterisk indicates a significant effect of virus compared to same sex controls. A pound sign indicates a significant difference between sexes within the same viral condition. Black symbols indicate p<0.05; blue symbols indicate a trend p<.1.
D2R-OENAc reduces body weight and food intake in females with wheel access during restriction
An interaction of sex and virus (F(1, 20)= 6.57, p<0.05; Figure 3b) and post hoc tests indicated that D2R-OENAc decreased body weight during restriction as a proportion of baseline body weight compared to EGFPNAc in female (F(1, 12)= 17.78, p<0.01), but not male, mice.
Similarly, an interaction of sex and virus (F(1, 20)= 4.38, p<0.05; Figure 3c) and post hoc tests revealed that D2R-OENAc females (F(1, 12)= 11.61, p<0.01), but not males, decreased food intake during restriction as a proportion of baseline food intake compared to their EGFPNAc controls.
D2R-OENAc induces larger increases in wheel running in females than males during restriction
A three-way interaction of sex, virus, and day (F(9, 188)= 4.31, p<0.01) was found for wheel running expressed as a percent of baseline running during restriction. Post hoc tests revealed a trend for D2R-OENAc females to run more than EGFPNAc females (p<0.08) and D2R-OENAc males (p<0.06) on day 4 (Figure 4d). Then on day 6, D2R-OENAc females ran more than EGFPNAc females (p<0.01) and D2R-OENAc males (p<0.05). On day 9, one remaining D2R-OENAc female exhibited more wheel running than EGFPNAc females (p<0.05). D2R-OENAc males did not show increased wheel running compared to EGFPNAc males until day 9 of restriction (p<0.05). This effect was also observed on days 10 (p<0.08) and 11 (p<0.06), but only at the trend level.
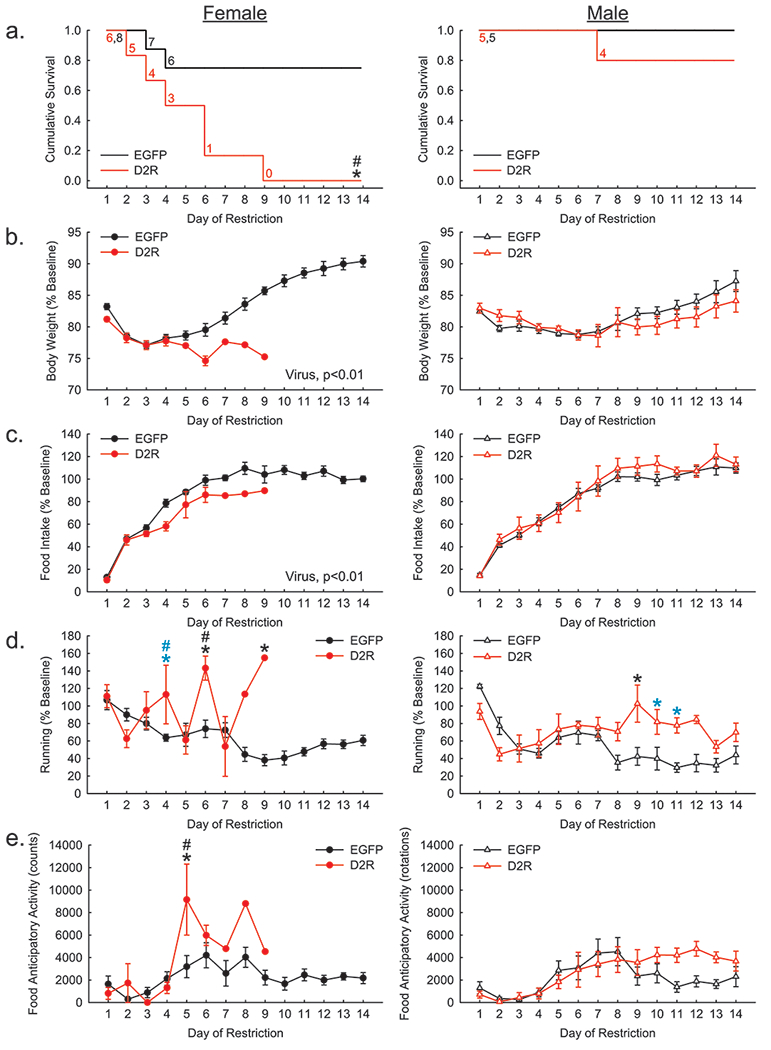
Figure 4.
Restriction phase of ABA without wheel access. (a) D2R-OENAc (red) reduced the cumulative survival of female (left), but not male (right) mice compared to controls (black). (b) Percent change in body weight relative to baseline was unaffected by D2R-OENAc in either sex. (c) Percent change in food intake relative to baseline was unaffected by D2R-OENAc in either sex. Data in (a) show the number of mice remaining in food restriction each day. Data in (b-c) are mean ± s.e.m, n = 5-7/group. An asterisk indicates a significant effect of virus compared to same sex controls. A pound sign indicates a significant difference between sexes within the same viral condition.
In addition, D2R-OENAc increased food anticipatory activity in female, but not male, mice during restriction. Analysis revealed a three-way interaction of sex, virus, and day on food anticipatory activity (F(8, 183)= 2.73, p<0.01). Post hoc tests showed that on day 5, D2R-OENAc female mice had larger food anticipatory activity values than EGFPNAc female (p<0.001) and D2R-OENAc male (p<0.0001) mice (Figure 3e). D2R-OENAc male mice did not show significant increases in food anticipatory activity compared to male controls.
D2R-OENAc increases food intake during baseline without wheel access
During the baseline period of the locked wheel study, male mice weighed more than female mice across viral treatment and day (F(1, 15)= 83.47, p<0.01; Supplemental Figure 1a). No effect of viral treatment on body weight was revealed. An interaction of viral treatment and day (F(3, 45)= 5.80, p<0.01; Supplemental Figure 1b) and Newman Keuls post hoc tests indicated that D2R-OENAc mice ate more than EGFPNAc mice only on day 3 of the baseline period.
D2R-OENAc reduces survival in female mice without wheel access
In the locked wheel study, Cox-Proportionate Hazards Model revealed a significant interaction between sex and virus (Χ2=4.67, p<0.05) for the time to dropout during food restriction. Hazard Ratios indicated that D2R-OENAc females were substantially more likely to drop out of the study than EGFPNAc females [HR=0.096 (0.021-0.443), z=−3.00, p=0.003](Figure 4a). Furthermore, D2R-OENAc females were substantially more likely to drop out of the study than D2R-OENAc males [HR=0.088 (0.018-0.445), z=−2.94, p=0.003]. However, the risk of dropout for EGFPNAc males versus females was approximately equal [HR=0.761 (0.249-2.327), z=−0.47, p=0.63]. Likewise, D2R-OENAc males were no more likely to be withdrawn from the study than EGFPNAc males [HR=0.824 (0.239-2.849), z=−0.31, p=0.76].
D2R-OENAc does not alter body weight or food intake in mice without wheel access
In the locked wheel study, D2R-OENAc did not alter body weight as a proportion of baseline body weight during restriction, and no interaction of virus and sex was observed (F(1, 144)= 2.73, p=0.10)(Figure 4b). Furthermore, D2R-OENAc did not alter food intake as a proportion of baseline food intake during restriction, and no interaction of virus and sex was observed (F(1, 129)= 0.72, p=0.40)(Figure 4c).
D2R-OENAc impairs glucose tolerance
D2R-OENAc mice exhibited glucose intolerance compared to EGFPNAc mice at the 30 minute time point, as indicated by an interaction of virus and time (F (3,33)= 4.01, p<0.05) (Figure 5a). Following treatment with the D2R agonist bromocriptine (Figure 5b), D2R-OENAc and EGFPNAc mice showed comparable blood glucose levels. Although bromocriptine treatment increased blood glucose levels in EGFPNAc mice at timepoints 30 - 120 (Interaction of virus and time: F (3,21)= 8.93, p<0.01)(Figure 5d), bromocriptine only increased blood glucose levels at the trend level in D2R-OENAc mice (F (3,9)= 3.26, p=0.07)(Figure 5c). No interactions of virus and sex were found, indicating that the observed effects on blood glucose levels were comparable in males and females (Supplemental Figure 2).
Figure 5.
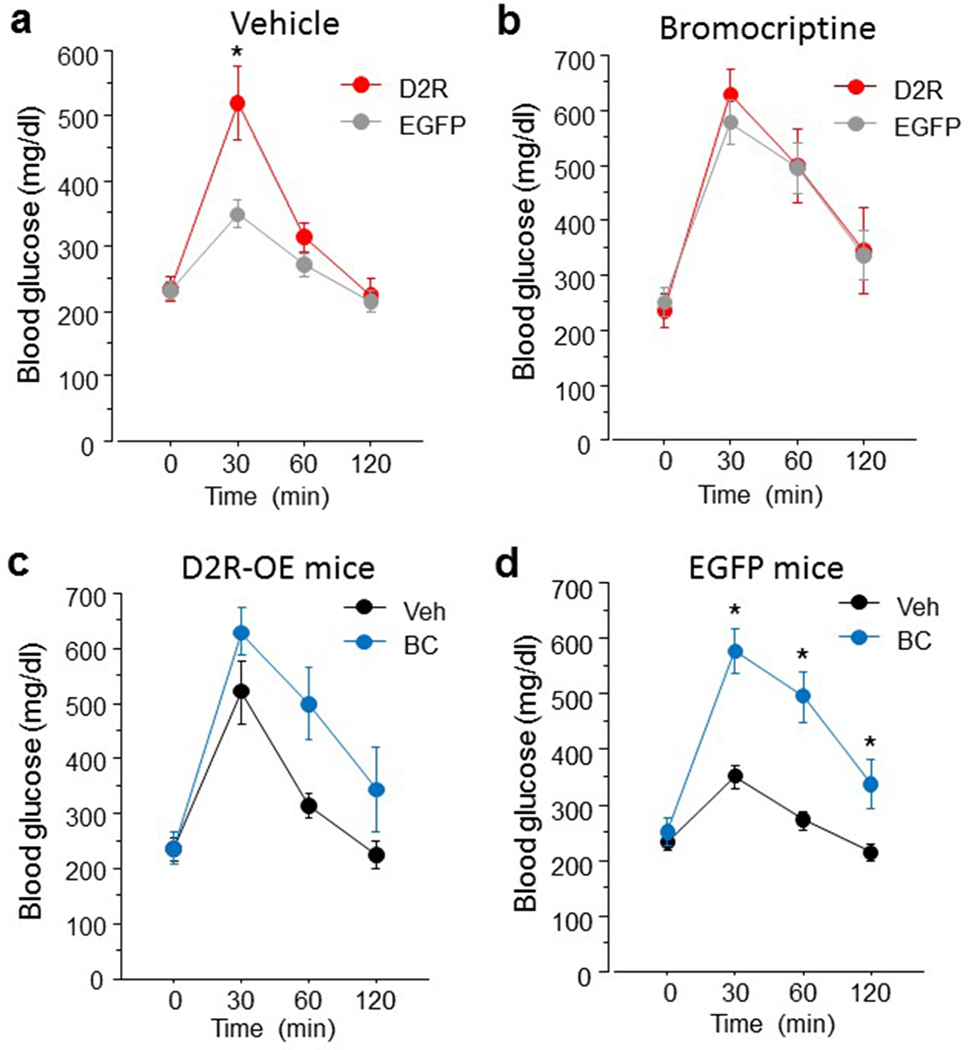
D2R-OENAc mice exhibit glucose intolerance. (a) D2R-OENAc mice show increased blood glucose levels at the 30 min timepoint in the intraperitoneal glucose tolerance test. (b) Blood glucose levels are comparable between D2R-OENAc and EGFPNac mice following 10 mg/kg bromocriptine treatment, and between D2R-OENAc mice receiving vehicle or bromocriptine (c). (d) Bromocriptine treatment induced glucose intolerance in EGFPNac mice compared to vehicle. Data are mean ± s.e.m, n = 6-9/group.
DISCUSSION
Our present findings identify a highly sexually dimorphic effect of D2R-OENAc on survival during scheduled fasting, with female but not male D2R-OENAc mice rapidly reaching the 25% weight loss criterion, either with or without running wheel access. Although female D2R-OENAc mice showed small reductions in food intake and increased wheel running during restriction, they also rapidly dropped out without running wheel access, and without reducing food intake. Therefore, D2R-OENAc reduces survival in females during scheduled fasting without altering behavior. On the other hand, male D2R-OENAc mice maintained body weight during scheduled fasting in either wheel condition. Paradoxically, D2R-OENAc increased food intake during ad libitum feeding in both sexes, revealing the state-dependency of this sexually dimorphic effect. We also found that D2R-OENAc mice of both sexes show glucose intolerance, indicating that D2Rs in the NAc core play a role in glucose homeostasis as well as body weight regulation. In summary, D2R-OENac female, but not male, mice showed reduced survival during scheduled fasting in the absence of running wheels or reduced food intake, suggesting that metabolic mechanisms contribute strongly to this sexually dimorphic effect.
We previously determined that viral D2R overexpression is selective for D2R-containing medium spiny neurons (MSNs) of the NAc core18. Using the same viruses, infusion site, and mouse line as in the present studies, we found that D2Rs and mVenus were coexpressed within cells and enriched in neuropil18. Furthermore, dense labeling of axonal terminals was found in the ventral pallidum, the projection target of NAc D2-MSNs18. We showed that viral expression was selectively targeted to D2-MSNs using Cre co-labeling. Furthermore, only a very small percentage (<5%) of mVenus-positive cells co-expressed tdTomato in D2-Cre x D1-tdTomato mice18. This amount of co-expression was expected based on reports estimating that approximately 3–6% of NAc core neurons co-express D1Rs and D2Rs29, 30. Since striatal cholinergic interneurons are also known to express D2Rs, we examined whether cells expressing the cholinergic marker choline acetyltransferase were transduced by our viral vector. Consistent with previous work using this D2-Cre mouse line31, we found that only 5.6% of choline acetyltransferase-positive cells co-expressed mVenus18. The lack of widespread D2R overexpression in cholinergic interneurons of the NAc is important to the interpretation of our results, because inhibition of these neurons augments cue-motivated behavior, while activation of these neurons prevents cues from invigorating reward-seeking behavior 32. Since activation of D2Rs is inhibitory to cells, D2R overexpression in cholinergic interneurons of the NAc might have increased fasting and wheel running. Finally, using a [3H] N-methyl-spiperone binding assay, we found that viral upregulation of D2R in the NAc led to a threefold increase in binding capacity compared to EGFP-expressing NAc mice18.
We found that D2R-OENAc mice of both sexes showed increased distance traveled in the open field, consistent with an earlier report using males 18. This finding is consistent with the classical basal ganglia model in which D1R-containing direct pathway neurons and D2R-containing indirect striatal output pathway neurons are thought to exert opposing actions on the control of movement31, 33–35. In support of this theory, optogenetic activation of the direct or “go” pathway or inhibition of the indirect or “no go” pathway increases locomotion31, 36,37, 38, while optogenetic activation of the indirect pathway neurons inhibits locomotion 31. Since activation of D2Rs inhibits neuronal activity17, 39, 40, D2R overexpression would be expected to reduce indirect pathway function and lead to increased locomotor activity, as we presently found in D2R-OENAc mice. However, recent studies have questioned the classical pro- versus anti-kinetic model, and suggest that both pathways act in concert to generate movements37, 41, 42. One hypothesis is that the opposing action of both pathways regulate different flexor/extensor muscle pairs whose coordinated action is necessary for coordinated movements35. Another complication is that genetic targeting of D1- versus D2-MSNs was found not to confer specificity for regulating NAc direct versus indirect projections to output nuclei of the basal ganglia, as observed in the dorsal striatum43. Rather, while D2-NAc MSNs densely innervate the VP, so do D1-MSNs although less densely, and both neuron types can inhibit or disinhibit thalamic activity depending on whether they initially project to the ventral mesencephalon or the mediodorsal thalamus44, 45. We also found that D2R-OENAc mice traveled a greater distance in the center of the open field, although this effect was likely driven by increases in locomotion. The increases in locomotor activity induced by D2R-OENAc likely reflect increases in exploration at least in part, since D2R-OENAc mice of both sexes also showed more rearing in the open field.
During the baseline period of ABA, D2R-OENAc mice showed modest increases in food intake. With running wheel access, D2R-OENAc mice also showed increased wheel running on day 1, consistent with observed increases in open field activity. The increase in food intake exhibited by D2R-OENAc mice might compensate for increased activity levels, since no differences in weight were observed between viral groups. D2R-OENAc mice without running wheel access also showed a small increase in food intake, but no difference in body weight compared to EGFPNAc mice. Even without running wheel access, D2R-OENAc mice might spend more energy than EGFPNAc controls, either due to increased energy expenditure and/or increased activity in the home cage. The effects of D2R-OENAc on food intake and wheel running during baseline were comparable in male and female mice.
The present studies revealed a highly sexually dimorphic effect of D2R-OENAc on survival during scheduled fasting, with only female D2R-OENAc mice rapidly reaching the 25% weight loss criterion, either with or without access to running wheels. Under the relatively mild scheduled fasting conditions used (7 hours food availability) EGFPNAc mice of both sexes and D2R-OENAc male mice showed a very low rate of dropout, with only 5 mice within these groups dropping out within the 14 days of restriction. However, all of the D2R-OENAc female mice dropped out within 9 days of scheduled fasting with wheel access, and within 5 days without wheel access. Importantly, the results of the two studies cannot be directly compared since they were obtained separately. Although D2R-OENAc mice of both sexes showed increased wheel running during restriction, this increase was larger in magnitude and began earlier during restriction in females (day 6) than males (day 9). Furthermore, female but not male D2R-OENAc mice developed food anticipatory activity (FAA). The highly erratic levels of wheel running exhibited by D2R-OENAc female mice resulted from the combination of food restriction-induced hyperactivity and the large reductions in wheel running exhibited by mice shortly before dropout. Our finding that D2R-OENAc female mice with running wheel access showed reduced food intake and increased wheel running during the restriction phase might suggest that these behavioral changes underlie the dramatic reduction in survival. Yet, D2R-OENAc female mice also showed robust reductions in survival during restriction without wheel access, without demonstrating any significant changes in food intake. Overall, the rapid weight loss leading to drop out in D2R-OENAc female mice in the absence of hypophagia or wheel running suggests that metabolic alterations contribute strongly to this sexually dimorphic phenotype.
Glucose tolerance refers to the clearing of glucose from the bloodstream after a meal or glucose administration, while glucose intolerance reflects an inability or delay in the clearance of elevated blood glucose28. Our finding that D2R-OENAc mice exhibit glucose intolerance is consistent with recent evidence suggesting that striatal dopamine regulates energy homeostasis28, 46, 47. In particular, Michaelides et al. (2017) recently showed that mice lacking dopamine- and cAMP-regulated neuronal phosphoprotein (DARPP-32) exclusively in D2R-expressing cells exhibit glucose intolerance and upregulation of D2R mRNA selectively in the NAc28, and that direct infusion of the D2R agonist bromocriptine into the NAc of normal mice increases glucose intolerance28. Our present findings demonstrate that overexpression of D2Rs specifically within the NAc core induces glucose intolerance across both sexes (Supplemental Figure 2). Our blood glucose values at time zero are high relative to other published reports; however, the brand of glucometer we used has been reported to yield higher values than four other brands, and our values are consistent with measurements taken using the same glucometer in C57BL/6J mice48. Of interest, AN patients in the ill state exhibit glucose intolerance 49–53 and a low or delayed insulin response to glucose loading 52–54, which are associated with a poor refeeding outcome 49. Further, the anorectic anx/anx mouse model also shows marked glucose intolerance, reduced insulin release after glucose loading, and pancreatic beta cell dysfunction 55. Weight loss and muscle wasting occurs if blood glucose levels becomes too high and excess glucose is removed from the blood by the kidneys and excreted via the urine56. More work will be required to determine whether energy loss or other metabolic changes are responsible for the robust weight loss observed in D2R-OENAc female mice during scheduled fasting.
Many different mechanisms could underlie the observed sex difference in weight loss in D2R-OENac mice during scheduled fasting. Although ovarian steroids could be a culprit, ovariectomy has relatively little effect on the rate of weight loss in female rodents exposed to scheduled fasting57. Sex steroids of gonadal origin are known to exert sexually dimorphic effects on the brain during early development, which might lead to the observed sex difference in D2R-OENac mice subjected to scheduled fasting. Alternatively, differences in gene expression exist between male and female developing brains due to Y chromosome-linked genes, and these differences precede the influences of gonadal hormones on neural development58. Intriguingly, the NAc core sends dense projections to the lateral hypothalamus59–61, which is sexually dimorphic62–64 and controls food intake, metabolism, and energy balance63, 65–67. Thus, the mechanism underlying the sexually dimorphic effect of scheduled fasting in D2R-OENac mice might be mediated at the level of the hypothalamus. Further work will be required to identify the mechanisms underlying the robust sex difference in response to scheduled fasting in D2R-OENac mice.
Our findings are the first to reveal the rapid and robust weight-loss induced by D2R overexpression in the NAc of female mice during scheduled fasting conditions. Our findings contribute to increasing evidence that striatal dopamine plays a role in the regulation of energy homeostasis. For example, deletion of DARPP-32 within D2R-expressing cells regulates glucose homeostasis and glucose-dependent reinforcement learning behaviors28. Within the dorsal striatum, genetically eliminating D2Rs selectively from iMSNs reduces physical activity in lean mice, while restoring Gi signaling in iMSNs increases activity in obese mice68. These manipulations of striatal D2Rs did not alter body weight, although mice were never subjected to fasting conditions 68. Furthermore, positron emission tomography (PET) studies show that the dorsal striatum exhibits robust alterations in a diet-induced obesity (DIO)-susceptible rat strain. DIO-susceptible rats show increased expression of the GTPase accelerating enzyme RGS4 in iMSNs, and knockdown of striatal RGS4 reduces food intake to control levels69. While dorsal striatal D2Rs and iMSNs modulate energy homeostasis in normal, DIO-susceptible, and DIO-resistant strains, their regulation of energy balance during scheduled fasting remains to be determined. Our experiments support the conclusion that increases in metabolism contribute strongly to the dramatic weight loss observed in D2R-OENac females during scheduled fasting.
Our present findings are consistent with the report that anorexic patients show increased dopamine D2/D3 receptor binding in the anteroventral striatum as assessed by positron emission tomography (PET) using [11C] raclopride12. Thus, increased D2Rs in the anteroventral striatum may be a trait marker in AN. Our findings are also in line with a recent genome-wide association study (GWAS) which found that AN shows significant genetic correlations with both psychiatric disorders and metabolic traits, and should be reconceptualized as a metabo-psychiatric disorder3, 4. Thus, our present results provide a potential link between these human studies by suggesting that increased D2R expression in the anteroventral striatum predisposes to AN by substantially increasing energy expenditure during fasting conditions. The remarkable increase in energy expenditure and wheel running triggered by scheduled fasting in the ABA paradigm might reflect a physiological mechanism for fleeing food depleted areas during conditions of famine, and could be upregulated in AN9, 70.
In summary, we found that female, but not male, D2R-OENAc mice develop rapid and robust weight loss during scheduled fasting, as well as hyperactivity when scheduled fasting is combined with running wheel access. Moreover, we found that D2R-OENAc induces glucose intolerance in both sexes. Our findings are consistent with reports that D2/D3R binding is increased in the anteroventral striatum of recovered AN patients, and suggest that increased D2R expression in the NAc core of female AN patients may confer vulnerability to obtaining a low body-mass index during dieting. Future mechanistic studies will examine a potential ventral striatal circuit mediating the effects of intra-NAc D2R expression on ABA behavior.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Walter Kaye for insightful discussions regarding this work.
FUNDING AND DISCLOSURE
The authors declare no conflict of interest. SD was supported by an IMHRO Rising Star Depression Research Award in Memory of George Largay and R21MH115395. AW and JL was supported by R21MH115395 to SD. JJ was supported by R01MH54137 and CK by R01MH093672.
Bibliography
- 1.Attia E Anorexia nervosa: current status and future directions. Annu Rev Med 2010; 61: 425–435. [DOI] [PubMed] [Google Scholar]
- 2.Schaumberg K, Welch E, Breithaupt L, Hubel C, Baker JH, Munn-Chernoff MA et al. The Science Behind the Academy for Eating Disorders’ Nine Truths About Eating Disorders. European Eating Disorders Review 2017; 25(6): 432–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watson HJ, Yilmaz Z, Thornton LM, Hubel C, Coleman JRI, Gaspar HA et al. Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat Genet 2019; 51(8): 1207–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duncan L, Yilmaz Z, Gaspar H, Walters R, Goldstein J, Anttila V et al. Significant Locus and Metabolic Genetic Correlations Revealed in Genome-Wide Association Study of Anorexia Nervosa. Am J Psychiatry 2017; 174(9): 850–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall JF, Hanford PV. Activity as a function of a restricted feeding schedule. J Comp Physiol Psychol 1954; 47(5): 362–363. [DOI] [PubMed] [Google Scholar]
- 6.Routtenberg A, Kuznesof AW. Self-starvation of rats living in activity wheels on a restricted feeding schedule. J Comp Physiol Psychol 1967; 64(3): 414–421. [DOI] [PubMed] [Google Scholar]
- 7.Burden VR, White BD, Dean RG, Martin RJ. Activity of the hypothalamic-pituitary-adrenal axis is elevated in rats with activity-based anorexia. J Nutr 1993; 123(7): 1217–1225. [DOI] [PubMed] [Google Scholar]
- 8.Boakes RA, Mills KJ, Single JP. Sex differences in the relationship between activity and weight loss in the rat. Behav Neurosci 1999; 113(5): 1080–1089. [PubMed] [Google Scholar]
- 9.Klenotich SJ, Dulawa SC. The activity-based anorexia mouse model. Methods Mol Biol 2012; 829: 377–393. [DOI] [PubMed] [Google Scholar]
- 10.Pare WP, Vincent GP, Isom KE, Reeves JM. Sex differences and incidence of activity-stress ulcers in the rat. Psychol Rep 1978; 43(2): 591–594. [DOI] [PubMed] [Google Scholar]
- 11.Kaye WH, Frank GK, McConaha C. Altered dopamine activity after recovery from restricting-type anorexia nervosa. Neuropsychopharmacology 1999; 21(4): 503–506. [DOI] [PubMed] [Google Scholar]
- 12.Frank GK, Bailer UF, Henry SE, Drevets W, Meltzer CC, Price JC et al. Increased dopamine D2/D3 receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11c]raclopride. Biol Psychiatry 2005; 58(11): 908–912. [DOI] [PubMed] [Google Scholar]
- 13.Broft A, Slifstein M, Osborne J, Kothari P, Morim S, Shingleton R et al. Striatal dopamine type 2 receptor availability in anorexia nervosa. Psychiatry Res 2015; 233(3): 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frank GK, Shott ME, Hagman JO, Schiel MA, DeGuzman MC, Rossi B. The partial dopamine D2 receptor agonist aripiprazole is associated with weight gain in adolescent anorexia nervosa. Int J Eat Disord 2017; 50(4): 447–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klenotich SJ, Ho EV, McMurray MS, Server CH, Dulawa SC. Dopamine D2/3 receptor antagonism reduces activity-based anorexia. Transl Psychiatry 2015; 5: e613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foldi CJ, Milton LK, Oldfield BJ. The Role of Mesolimbic Reward Neurocircuitry in Prevention and Rescue of the Activity-Based Anorexia (ABA) Phenotype in Rats. Neuropsychopharmacology 2017; 42(12): 2292–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallo EF, Meszaros J, Sherman JD, Chohan MO, Teboul E, Choi CS et al. Accumbens dopamine D2 receptors increase motivation by decreasing inhibitory transmission to the ventral pallidum. Nat Commun 2018; 9(1): 1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallo EF, Salling MC, Feng B, Moron JA, Harrison NL, Javitch JA et al. Upregulation of dopamine D2 receptors in the nucleus accumbens indirect pathway increases locomotion but does not reduce alcohol consumption. Neuropsychopharmacology 2015; 40(7): 1609–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zalocusky KA, Ramakrishnan C, Lerner TN, Davidson TJ, Knutson B, Deisseroth K. Nucleus accumbens D2R cells signal prior outcomes and control risky decision-making. Nature 2016; 531(7596): 642–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macpherson T, Morita M, Wang Y, Sasaoka T, Sawa A, Hikida T. Nucleus accumbens dopamine D2-receptor expressing neurons control behavioral flexibility in a place discrimination task in the IntelliCage. Learn Mem 2016; 23(7): 359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tchanturia K, Liao PC, Uher R, Lawrence N, Treasure J, Campbell IC. An investigation of decision making in anorexia nervosa using the Iowa Gambling Task and skin conductance measurements. J Int Neuropsychol Soc 2007; 13(4): 635–641. [DOI] [PubMed] [Google Scholar]
- 22.Giannunzio V, Degortes D, Tenconi E, Collantoni E, Solmi M, Santonastaso P et al. Decision-making impairment in anorexia nervosa: New insights into the role of age and decision-making style. Eur Eat Disord Rev 2018; 26(4): 302–314. [DOI] [PubMed] [Google Scholar]
- 23.Tenconi E, Degortes D, Clementi M, Collantoni E, Pinato C, Forzan M et al. Clinical and genetic correlates of decision making in anorexia nervosa. J Clin Exp Neuropsychol 2016; 38(3): 327–337. [DOI] [PubMed] [Google Scholar]
- 24.Friederich HC, Herzog W. Cognitive-behavioral flexibility in anorexia nervosa. Curr Top Behav Neurosci 2011; 6: 111–123. [DOI] [PubMed] [Google Scholar]
- 25.Zastrow A, Kaiser S, Stippich C, Walther S, Herzog W, Tchanturia K et al. Neural correlates of impaired cognitive-behavioral flexibility in anorexia nervosa. Am J Psychiatry 2009; 166(5): 608–616. [DOI] [PubMed] [Google Scholar]
- 26.Jiao J, Opal MD, Dulawa SC. Gestational environment programs adult depression-like behavior through methylation of the calcitonin gene-related peptide gene. Mol Psychiatry 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klenotich SJ, Seiglie MP, McMurray MS, Roitman JD, Le Grange D, Dugad P et al. Olanzapine, but not fluoxetine, treatment increases survival in activity-based anorexia in mice. Neuropsychopharmacology 2012; 37(7): 1620–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michaelides M, Miller ML, DiNieri JA, Gomez JL, Schwartz E, Egervari G et al. Dopamine D2 Receptor Signaling in the Nucleus Accumbens Comprises a Metabolic-Cognitive Brain Interface Regulating Metabolic Components of Glucose Reinforcement. Neuropsychopharmacology 2017; 42(12): 2365–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E et al. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci 2008; 28(22): 5671–5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frederick AL, Yano H, Trifilieff P, Vishwasrao HD, Biezonski D, Meszaros J et al. Evidence against dopamine D1/D2 receptor heteromers. Mol Psychiatry 2015; 20(11): 1373–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 2010; 466(7306): 622–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collins AL, Aitken TJ, Huang IW, Shieh C, Greenfield VY, Monbouquette HG et al. Nucleus Accumbens Cholinergic Interneurons Oppose Cue-Motivated Behavior. Biol Psychiatry 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lobo MK, Covington HE 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science 2010; 330(6002): 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y et al. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci 2011; 14(1): 22–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tecuapetla F, Jin X, Lima SQ, Costa RM. Complementary Contributions of Striatal Projection Pathways to Action Initiation and Execution. Cell 2016; 166(3): 703–715. [DOI] [PubMed] [Google Scholar]
- 36.Cazorla M, de Carvalho FD, Chohan MO, Shegda M, Chuhma N, Rayport S et al. Dopamine D2 receptors regulate the anatomical and functional balance of basal ganglia circuitry. Neuron 2014; 81(1): 153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Durieux PF, Schiffmann SN, de Kerchove d’Exaerde A. Differential regulation of motor control and response to dopaminergic drugs by D1R and D2R neurons in distinct dorsal striatum subregions. EMBO J 2012; 31(3): 640–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carvalho Poyraz F, Holzner E, Bailey MR, Meszaros J, Kenney L, Kheirbek MA et al. Decreasing Striatopallidal Pathway Function Enhances Motivation by Energizing the Initiation of Goal-Directed Action. J Neurosci 2016; 36(22): 5988–6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dobbs LK, Kaplan AR, Lemos JC, Matsui A, Rubinstein M, Alvarez VA. Dopamine Regulation of Lateral Inhibition between Striatal Neurons Gates the Stimulant Actions of Cocaine. Neuron 2016; 90(5): 1100–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dobbs LK, Kaplan AR, Bock R, Phamluong K, Shin JH, Bocarsly ME et al. D1 receptor hypersensitivity in mice with low striatal D2 receptors facilitates select cocaine behaviors. Neuropsychopharmacology 2019; 44(4): 805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM et al. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature 2013; 494(7436): 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yttri EA, Dudman JT. Opponent and bidirectional control of movement velocity in the basal ganglia. Nature 2016; 533(7603): 402–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lobo MK, Nestler EJ. The striatal balancing act in drug addiction: distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat 2011; 5: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kupchik YM, Brown RM, Heinsbroek JA, Lobo MK, Schwartz DJ, Kalivas PW. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat Neurosci 2015; 18(9): 1230–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci 2012; 15(6): 816–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pak K, Shin HK, Kim EJ, Lee JH, Lyoo CH, Son J et al. Weight loss is associated with rapid striatal dopaminergic degeneration in Parkinson’s disease. Parkinsonism Relat Disord 2018; 51: 67–72. [DOI] [PubMed] [Google Scholar]
- 47.Ferrario CR, Labouebe G, Liu S, Nieh EH, Routh VH, Xu S et al. Homeostasis Meets Motivation in the Battle to Control Food Intake. J Neurosci 2016; 36(45): 11469–11481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morley LA, Gomez TH, Goldman JL, Flores R, Robinson MA. Accuracy of 5 Point-of-Care Glucometers in C57BL/6J Mice. J Am Assoc Lab Anim Sci 2018; 57(1): 44–50. [PMC free article] [PubMed] [Google Scholar]
- 49.Yasuhara D, Naruo T, Nagai N, Muranaga T, Nakahara T, Tanaka M et al. Glucose tolerance predicts short-term refeeding outcome in females with anorexia nervosa. Psychosom Med 2005; 67(4): 669–676. [DOI] [PubMed] [Google Scholar]
- 50.Warren MP, Vande Wiele RL. Clinical and metabolic features of anorexia nervosa. Am J Obstet Gynecol 1973; 117(3): 435–449. [DOI] [PubMed] [Google Scholar]
- 51.Drossman DA, Ontjes DA, Heizer WD. Anorexia-Nervosa. Gastroenterology 1979; 77(5): 1115–1131. [PubMed] [Google Scholar]
- 52.Scheen AJ, Castillo M, Lefebvre PJ. Insulin sensitivity in anorexia nervosa: a mirror image of obesity? Diabetes Metab Rev 1988; 4(7): 681–690. [DOI] [PubMed] [Google Scholar]
- 53.Kumai M, Tamai H, Fujii S, Nakagawa T, Aoki TT. Glucagon secretion in anorexia nervosa. Am J Clin Nutr 1988; 47(2): 239–242. [DOI] [PubMed] [Google Scholar]
- 54.Nozaki T, Tamai H, Matsubayashi S, Komaki G, Kobayashi N, Nakagawa T. Insulin response to intravenous glucose in patients with anorexia nervosa showing low insulin response to oral glucose. J Clin Endocrinol Metab 1994; 79(1): 217–222. [DOI] [PubMed] [Google Scholar]
- 55.Lindfors C, Katz A, Selander L, Johansen JE, Marconi G, Schalling M et al. Glucose intolerance and pancreatic beta-cell dysfunction in the anorectic anx/anx mouse. Am J Physiol Endocrinol Metab 2015; 309(4): E418–427. [DOI] [PubMed] [Google Scholar]
- 56.Tahara A, Matsuyama-Yokono A, Nakano R, Someya Y, Shibasaki M. Effects of antidiabetic drugs on glucose tolerance in streptozotocin-nicotinamide-induced mildly diabetic and streptozotocin-induced severely diabetic mice. Horm Metab Res 2008; 40(12): 880–886. [DOI] [PubMed] [Google Scholar]
- 57.Lee TJ, Kinzig KP. Repeated adolescent activity-based anorexia influences central estrogen signaling and adulthood anxiety-like behaviors in rats. Physiol Behav 2017; 171: 199–206. [DOI] [PubMed] [Google Scholar]
- 58.Dewing P, Shi T, Horvath S, Vilain E. Sexually dimorphic gene expression in mouse brain precedes gonadal differentiation. Brain Res Mol Brain Res 2003; 118(1-2): 82–90. [DOI] [PubMed] [Google Scholar]
- 59.Usuda I, Tanaka K, Chiba T. Efferent projections of the nucleus accumbens in the rat with special reference to subdivision of the nucleus: biotinylated dextran amine study. Brain Res 1998; 797(1): 73–93. [DOI] [PubMed] [Google Scholar]
- 60.Zhang JP, Xu Q, Yuan XS, Cherasse Y, Schiffmann SN, de Kerchove d’Exaerde A et al. Projections of nucleus accumbens adenosine A2A receptor neurons in the mouse brain and their implications in mediating sleep-wake regulation. Front Neuroanat 2013; 7: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience 1991; 41(1): 89–125. [DOI] [PubMed] [Google Scholar]
- 62.Adler ES, Hollis JH, Clarke IJ, Grattan DR, Oldfield BJ. Neurochemical characterization and sexual dimorphism of projections from the brain to abdominal and subcutaneous white adipose tissue in the rat. J Neurosci 2012; 32(45): 15913–15921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mogi K, Funabashi T, Mitsushima D, Hagiwara H, Kimura F. Sex difference in the response of melanin-concentrating hormone neurons in the lateral hypothalamic area to glucose, as revealed by the expression of phosphorylated cyclic adenosine 3 ‘,5 ‘-monophosphate response element-binding protein. Endocrinology 2005; 146(8): 3325–3333. [DOI] [PubMed] [Google Scholar]
- 64.Frankfurt M, Fuchs E, Wuttke W. Sex differences in gamma-aminobutyric acid and glutamate concentrations in discrete rat brain nuclei. Neurosci Lett 1984; 50(1–3): 245–250. [DOI] [PubMed] [Google Scholar]
- 65.Berthoud HR, Munzberg H. The lateral hypothalamus as integrator of metabolic and environmental needs: From electrical self-stimulation to opto-genetics. Physiology & Behavior 2011; 104(1): 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stevenson JA, Montemurro DG. Loss of weight and metabolic rate of rats with lesions in the medial and lateral hypothalamus. Nature 1963; 198: 92. [DOI] [PubMed] [Google Scholar]
- 67.Osada T, Suzuki R, Ogawa A, Tanaka M, Hori M, Aoki S et al. Functional subdivisions of the hypothalamus using areal parcellation and their signal changes related to glucose metabolism. Neuroimage 2017; 162: 1–12. [DOI] [PubMed] [Google Scholar]
- 68.Friend DM, Devarakonda K, O’Neal TJ, Skirzewski M, Papazoglou I, Kaplan AR et al. Basal Ganglia Dysfunction Contributes to Physical Inactivity in Obesity. Cell Metab 2017; 25(2): 312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Michaelides M, Miller ML, Egervari G, Primeaux SD, Gomez JL, Ellis RJ et al. Striatal Rgs4 regulates feeding and susceptibility to diet-induced obesity. Mol Psychiatry 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Casper RC, Sullivan EL, Tecott L. Relevance of animal models to human eating disorders and obesity. Psychopharmacology (Berl) 2008; 199(3): 313–329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


