Abstract
Recent years have seen an advent in population-based studies in children, adolescents, and adults that examine the prevalence, etiology, and developmental trajectories of diverse subclinical psychopathological symptoms that pose a risk for the later development of severe mental illnesses. It is increasingly recognized that most categorically defined psychiatric disorders (i) occur on a spectrum or continuum, (ii) show high heterogeneity and symptom overlap, and (iii) share genetic and environmental risk factors.
Here, we discuss neurodevelopmental underpinnings of psychosis spectrum symptoms and review brain morphometric and functional alterations, as well as genetic liability for psychosis, in individuals experiencing psychotic symptoms (PS) in the general population. With regard to brain structure and function, findings of qualitatively similar alterations in individuals experiencing subthreshold PS and those with overt psychotic disorders support the notion of a psychosis continuum. However, genetic and epidemiological studies have emphasized the overlap of PS and other psychiatric illnesses. In particular, PS during adolescence appear to be a non-specific precursor of different psychopathological outcomes.
Given the evidence presented in this review, we argue that findings from population-based studies are appropriate to guide policy-making to further emphasize public health efforts. Broadly accessible mental health programs are promising to make a difference in the field of adolescent mental health. However, the specific efficacy of these programs warrants further study, and caution is advised to not over-pathologize potentially transient occurrence of mental health problems.
Keywords: psychotic symptoms, youth, risk factors, neuroimaging, psychopathology, adolescence
Recent years have seen an advent in population-based studies that examine the prevalence, etiology, and developmental trajectories of diverse subclinical psychopathological symptoms that pose a risk for the later development of severe mental illnesses. It is increasingly recognized that most categorically defined psychiatric disorders (i) occur on a spectrum or continuum (1) that is not necessarily normally distributed (2), (ii) show high heterogeneity and symptom overlap, and (iii) share genetic and environmental risk factors (3,4). Therefore, population-based studies of psychopathology in youth assess a broad spectrum of symptoms as well as genetic risk, cognitive and general functioning, socioeconomic, and environmental factors to yield a more complete understanding of symptom etiology and development. Pediatric population-based studies with longitudinal study designs may be helpful for defining normative growth charts of diverse disease dimensions that in turn may aid in developing individual risk predictions (5).
Here, we review different aspects of population-based studies with regard to the psychosis spectrum; we discuss neurodevelopmental underpinnings of psychosis spectrum symptoms, brain morphometric and functional alterations in individuals experiencing psychotic symptoms in the general population, and the role of genetic liability for psychosis.
Given the overwhelming evidence offered by this body of recent work that even subclinical psychotic symptoms pose a risk for severe mental illnesses, we highlight promising strategies that facilitate access to mental health services for adolescents, a group highly vulnerable to mental health problems. Even though further research is needed, in particular to understand risk and resilience factors for longitudinal symptom progression, policy-making should take available data into account to further reduce mental health stigma and to invest in prevention and early intervention programs.
The psychosis spectrum and clinical staging models
Despite the longstanding conceptualization of a continuum of psychotic symptoms (PS), this is not reflected in current diagnostic manuals (6-9). Recent findings in both help-seeking individuals experiencing PS, and studies on PS in the general population have further emphasized this notion (10). Presumably, the psychosis continuum is characterized by qualitatively similar PS that vary in levels of conviction and duration, ranging from subclinical schizotypal symptoms to severe psychosis spectrum disorders such as schizophrenia. PS, as typically studied in population-based cohorts, include positive symptoms such as hallucinations and delusions. Sometimes negative symptoms such as flat affect are also considered when establishing subclinical psychosis categories (see (11,12)) for detailed discussion on psychometric issues).
The concept of a psychosis continuum has further facilitated the adoption of a clinical staging model (3,13). Symptoms typically observed in the general population refer to stage 1a, nonspecific general psychopathologies such as depressive and anxiety symptoms alongside subthreshold PS, and stage 1b with more specific PS, i.e., commonly termed clinical- or ultra-high risk state (14). Stage 0 in this model is defined by a genetic risk through a positive family history of severe mental illness and other states are characterized by above-threshold PS (stage 2), persistence of symptoms (stage 3), and severe, non-remitted psychotic disorders (stage 4). Importantly, individuals do not necessarily change stages with time but may remain in their initially assigned stage. Similarly, studies on clinical high-risk (CHR) cohorts specifically examining conversion to full-blown psychosis, i.e., changes of clinical stages, find low transition rates of approximately 20-35% over 2 years (15,16). Furthermore, the clinical staging model is primarily based on retrospective studies and requires further prospective validation.
The fact that the majority of individuals with a first episode of psychosis have not sought help before their ‘psychotic break’ highlights the necessity to broaden the target symptoms and audience of early intervention strategies for psychosis spectrum disorders. Population-based studies have become an important strategy to validate the concept of a psychosis continuum, and may be helpful to tailor future primary prevention strategies to the general population by examining longitudinal trajectories of PS development across childhood and adolescence to detect early predictors (5,17).
Studies on PS in the general population can further be viewed as an alternative to the CHR approach, an enrichment sampling focused on help-seeking individuals fulfilling certain diagnostic criteria (14,18). Studies applying CHR criteria to date have focused primarily on psychosis spectrum outcomes (19). Given that clinical ascertainment is required, CHR cohorts may not reflect the broader population experiencing PS, which may at least partially explain higher pluripotentiality (i.e., risk of developing any kind of psychiatric disorder) of PS observed in population-based studies relative to CHR samples (20,21).
Are psychotic symptoms a public health concern?
Similar to overt psychotic disorders, PS in the general population (prevalence: 5.8% (22)) are often accompanied by cognitive impairments (23), reduced quality of life (24), higher rates of substance use, functional disability, suicidality (25-28), and alterations in brain structure and function (29-34), rendering PS an important public health issue. In accordance with a clinical staging model, PS pose an elevated risk for the later development of overt mental illness; not only severe psychosis spectrum disorders (35) but also depression, anxiety disorder, and bipolar disorder (3,36), amplifying the significance as a public health concern. However, recent epidemiological and genetic findings highlight the complex relationship between PS and severe mental illnesses (36-38): overt psychotic disorders may exhibit diverse psychopathological precursors and similarly, PS in childhood and adolescence do not always foreshadow persistent psychosis and/or schizophrenia later in life. For example, in the Philadelphia Neurodevelopmental Cohort (PNC) a positive predictive value of 0.51 was reported for initial screening of PS (39), but in a small Irish youth sample childhood PS had a positive predictive value of >0.59 for adolescent externalizing and internalizing problems (40).
Population-based longitudinal studies on subclinical/ subthreshold PS in children and adolescents offer promise for identifying disease biomarkers that predict progression to overt mental illness (41). Ultimately, these efforts aim at improving early identification of at-risk youth in order to improve long-term functional outcomes. Risk factors for subthreshold PS and overt psychotic disorders, include genetic risk, both family history (42,43) and high-impact copy number variations (CNVs) such as 22q11.2 deletion syndrome (44,45), exposure to drugs as well as childhood adversities/trauma, obstetric complications, and socioeconomic difficulties, including ethnic minority and immigrant status (46). Importantly, all forms of prevention, i.e., universal, selective, and indicated (47), can be tailored to these risk factors (17). For example, universal prevention targets the general population and could involve destigmatization and anti-bullying campaigns to improve mental well-being overall. Selective preventions for people with increased risk for developing psychiatric disorders (stage 0) could be implemented in clinics by providing services for families of patients with severe mental illnesses, and indicated prevention is aimed at improving outcome in CHR individuals (stages 1a and 1b) (17).
Overall, general population studies allow for larger and unbiased samples, without typical confounders in clinical populations such as medication and illness duration. Such studies can therefore inform and shape policy making for preventive measures of severe mental illnesses. Table 1 provides an overview of population-based studies cited in this review.
Table 1:
Overview of population-based survey studies, sample sizes, and instrument used to assess PS symptoms
| Study | Sample size | Psychopathology instrument relevant to PS |
Longitudinal (y/n) |
|---|---|---|---|
| Avon Longitudinal Study of Parents and Children | 13,988* | PLIKSi | y |
| Dunedin Multidisciplinary Health and Development Study | 1,037 | DISC-C/DIS | y |
| WHO Mental Health Survey | 23,998 | CIDI | n |
| Netherlands Mental Health and Incidence Study | 7,076 | CIDI/SCID-III | y |
| Environmental-Risk Longitudinal Twin Study | 2,232 | SIPS | Y |
| Mental Health in the General Population (WHO) | 38,694 | MINI | n |
| My World Survey – Second Level | 6,062 | APSS | n |
| Twins Early Development Study + Longitudinal Experiences and Perceptions | 5,537 – 5,076 | SPEQ | y |
| The Child and Adolescent Twin Study in Sweden | 17,220 | K-SADS-PL | y |
sample size may vary due to bolstering of the initial sample; ALSPaC and Dunedin will include MRI in subsequent follow-ups.
PLIKSi – Psychosis-like Symptoms interview (45), DISC-C – Diagnostic Interview Schedule for Children (42), CIDI – Composite International Diagnostic Interview, SCID-III – Structured Clinical Interview for DSM-III-R, SIPS – Structured Interview for Prodromal Symptoms (108), MINI – Mini International Neuropsychiatric Interview (109), APSS – Adolescent Psychotic-like Symptom Screener (110), SPEQ – Specific Psychotic Experiences Questionnaire (111), K-SADS-PL – Schedule for Affective Disorders and Schizophrenia for School-age Children – present and lifetime version (112)
Early neurodevelopmental factors associated with psychotic symptoms
Do psychotic symptoms in childhood and adolescence predict psychosis?
A pressing question, requiring longitudinal study, is whether subclinical PS in youth in the general population are in fact associated with the onset of overt psychosis later in life. One such study is the Dunedin Multidisciplinary Health and Development study, a birth cohort study out of New Zealand that followed the initial cohort (N=1,037) over 38 years. A recent follow-up of this cohort reported that PS at age 11 were associated not only with a diagnosis of schizophrenia at age 38 (relative risk (RR) 7.24) but also with diagnoses of Post-Traumatic Stress Disorder (RR 3.03), substance dependence (RR 1.91), depression (RR 1.50), and anxiety (RR 1.47). Higher rates of PS at age 11 further predicted suicide/ suicide attempts at age 38, even when controlling for other psychiatric disorders at age 11 (RR 2.58) (35) [the 15-year follow-up study of the Dunedin cohort at age 26 reported very similar findings (48)]. It is important to note, however, that PS were assessed with the Diagnostic Interview Schedule for Children (DISC-C (49)), an instrument that includes only 5 questions on positive PS.
The Avon Longitudinal Study of Parents and Children (ALSPaC), with over 13,000 study participants, includes a total of 68 assessment points between birth and age 18. Niarchou et al. (50) reported that, similar to results from the Dunedin cohort, PS at age 12 were predictive of a psychotic disorder at age 18 (odds ratio (OR) 12.7). Interestingly, non-specific symptoms such as depersonalization and sub-psychotic unusual experiences were predictive of a psychotic disorder and depression at age 18 (51). Even though, ALSPaC also assessed only positive PS, the semi-structured PLIKSi instrument covers the three major domains of positive PS, i.e., hallucinations, delusions, and bizarre thinking, and therefore reflects a broader spectrum of PS (52).
Overall, in the general population it appears that PS during childhood and adolescence increase the risk of later development of a broad range of psychiatric illnesses (pluripotentiality). PS in help-seeking individuals fulfilling CHR criteria may be more specific in terms of predicting psychosis onset (53) even though rates of co-occurring non-psychotic disorders are also higher in these cohorts relative to the general population (54).
Etiology
Although their etiology is not well understood, PS throughout life are often preceded and accompanied by emotional and behavioral problems, which in turn are often associated with (early) life adversities. Findings from the ALSPaC sample further confirmed previously described risk factors. In particular, early neurodevelopmental problems such as autism spectrum symptoms, asphyxia during birth, lower IQ, and delayed early motor development were specifically associated with PS in adolescence (4). Bolhuis et al. (Generation R study, N=3,984 (55,56)) highlighted emotional and behavioral problems at age 3 and 6 as the earliest significant predictors of PS at age 10. These encompassed depressive symptoms, aggressive behavior, anxiety, sleep difficulties, attention problems, and somatic complaints. Interestingly, emotional and behavioral problems also partially explained the association between previously described risk factors such as autistic traits and childhood adversities and PS, rendering it likely that emotional problems are a core risk factor or precursor for later PS. Further, the authors hypothesize that PS can manifest differently across the lifespan, ranging from emotional problems in early childhood to subclinical PS in late childhood and adolescence, and severe mental illness in adulthood. However, difficulties in validly assessing PS in younger children could lead to a distortion of the true association between childhood emotional problems and PS (55,57,58). A twin study (Twins Early Development Study [TEDS] and Longitudinal Experiences And Perceptions [LEAP], N ~ 5,076) further supports an association between childhood emotional and behavioral problems and adolescent PS by showing a modest genetic overlap across these phenotypes (59). Further, lack of certain personal resources such as low optimism, low self-esteem, and high avoidance, in addition to emotional problems, have been reported as significant predictors of PS during adolescence (60).
Early life stress and childhood adversities are associated with emotional and behavioral problems not only in childhood and adolescence but across the lifespan (61,62). In the largest population-based study to date, the World Health Organization Mental Health Survey (N= 23,998), McGrath and colleagues confirmed that childhood adversities are associated with an at least two-fold increased risk for developing PS, in a dose-response relationship (63). Childhood adversities characterizing ‘maladaptive family functioning’ (parental mental illness and substance abuse, family violence, physical and sexual abuse, neglect) posed a somewhat stronger association with later onset of PS than ‘other childhood adversities’ (parental death, divorce or loss, economic adversity). Interestingly, when adjusting for other mental illnesses with onset prior to PS, the association between childhood adversities and PS onset during adolescence became non-significant. This finding suggests that childhood adversities are not only a risk factor for adolescent-onset PS, but also other psychopathological symptoms with onset prior to adolescence, which in turn may lead to PS.
Finally, an often-discussed risk factor for the consecutive development of PS is cannabis use; longitudinal results from the Netherlands Mental Health and Incidence Study (N=7,076) reported that baseline cannabis use predicted PS at follow-up (OR 2.76) (64). Recent publications conclude that the evidence for this association is sufficient for policy makers to take this risk into consideration when further discussing legalizing cannabis (65,66).
Genetic liability for psychotic symptoms in the general population
Recently, genetic studies have made great progress in elucidating the genetic architecture of severe mental illnesses. In the majority of cases, risk for severe mental illnesses appears to be attributable to the cumulative impact of multiple genes, where each gene individually explains only a small amount of variance, but the sum of risk alleles across all identified variants accounts for up to 18% of variance in schizophrenia diagnosis (67,68). As such, investigation of polygenic risk scores (PRS), based on effect sizes of common variants associated with schizophrenia (68) and other disorders (69) has become increasingly common in population-based studies.
Studies applying PRS (based on adults with established diagnoses) to developmental cohorts have recently emerged. For example, in the ALSPaC cohort schizophrenia PRS was significantly associated with negative symptoms (OR 1.21) and anxiety (OR 1.17) during adolescence, but not with positive symptoms, again suggesting that the genetic basis of PS may present differently across development (70). In line with behavioral studies, Riglin et al. highlighted associations between schizophrenia PRS and diverse problems of childhood development at ages 7 to 9, such as lower IQ (OR 1.13) and poor social (OR 1.08) and language skills (OR 1.10) (71).
A recent study combined three major population-based cohorts [ALSPaC, TEDS (72), and CATSS (Child and Adolescent Twin Study in Sweden (73)], identifying significant associations between schizophrenia PRS and different symptom domains: hallucinations and paranoia (when excluding individuals who scored zero), anhedonia, cognitive disorganization, and parent-rated negative symptoms (74). Interestingly, bipolar disorder PRS was also significantly associated with hallucinations and paranoia, even when including individuals who scored zero on this scale. PRS for major depression was further associated with anhedonia and parent-reported negative symptoms. In a follow-up study taking a multivariate factor analytic approach, Jones et al. found schizophrenia PRS was significantly associated with multiple psychopathology factors (positive symptoms, negative symptoms, depression, and anxiety) (38). However, these specific effects vanished when including a general psychopathology factor, suggesting that psychopathology during adolescence may be explained with one broad factor.
PS during adolescence are rather non-specific and pose risk for a variety of severe mental illnesses. Loohuis and colleagues therefore utilized a novel multi-trait approach (75) including PRS of a broad range of psychiatric disorders, including neurodevelopmental disorders as well as brain and cognitive traits, to assess the association between these genetic risk factors and PS in youth. Interestingly, the ADHD PRS was the only significant predictor of PS in youth of European-American ancestry in the PNC (here N = 7,225), even after removing individuals endorsing any ADHD symptoms to avoid confounds related to phenotypic overlap (75). This finding was replicated in a sample of help-seeking CHR individuals. Further, the association between PS and ADHD PRS was age-dependent, such that the association was strongest in younger children (< 12 years old). It is noteworthy that for individuals < 12 years only collateral information on psychopathology was available, which could affect the results.
In addition to polygenic risk (i.e., common risk variants), recent exome sequencing studies have also found that rare and ultra-rare variants contribute to the genetic risk of schizophrenia (76,77).
Overall, findings from these studies highlight the complex association between genetic risk and PS during adolescence. While such symptoms may be non-specific, and presage later severe mental illnesses, polygenic risk may be indexing global psychopathology as well as risk for specific diagnostic entities. Importantly, because PRS are currently derived from almost entirely European cohorts, their application to non-European ethnic groups is problematic (78); collection of ethnically diverse samples is a research imperative. Further, while PRS are far from clinical utility in the general population, as ever-increasing GWAS size improves the strength of these associations, these risk scores may approach clinical utility in enriched populations in the near future (e.g., (79)).
Alterations in brain structure and function associated with psychotic symptoms
Examples of publicly available population-based datasets in youth that include multimodal imaging and neurocognitive assessments are the PNC (80) and the Adolescent Brain Cognitive Development study (ABCD, (81)). These samples offer unprecedented opportunities for the neuroscience community to study complex brain-behavior interactions during development. In particular, longitudinal data will allow for unique investigations of developmental trajectories. Given the young age of ABCD participants at study baseline (ages 9-10 (81)) it has the potential to capture earliest signs of emotional and behavioral problems associated with subsequent severe mental illnesses. Table 2 summarizes large-scale epidemiological cohorts with multimodal imaging.
Table 2:
Overview of population-based neuroimaging studies
| Study | Sample size | MRI modalities (not exhaustive) |
Longitudinal (y/n) |
Publicly available (y/n) |
|---|---|---|---|---|
| PNC | 1,445+ | Resting BOLD, task fMRI (emotion identification, n-back), structure (MPRAGE T1), DTI | n* | y |
| ABCD | 11,878 | Resting BOLD, task fMRI (MID, stop-signal, emotional n-back), structure (T1, T2), DTI | y** | y |
| HCP-D | 1,350 | Task fMRI (guessing task, go/ no go task, emotion identification), structure (T1, T2), | n | y |
| IMAGEN | ~2,000 | Resting BOLD, task fMRI (face/ emotion processing, MID, stop-signal, global cognition) | y | n |
| GenR | 1,070 | Resting BOLD, structure (T1), DTI | y | n |
| Saguenay Youth Study | 1,029 | Structure (T1, T2) | n | n |
| Nathan Kline Institute-Rockland Sample | >1,000 | Resting BOLD, task fMRI (visual checkerboard, neurofeedback tasks), DTI | y | y |
longitudinal data are currently not publicly available
longitudinal data release anticipated in summer 2020
The PNC neuroimaging sample has a sample size of N = 1,445; however, the entire sample includes 9,421 individuals that completed the neurocognitive and psychopathology assessment
PNC – Philadelphia Neurodevelopmental Cohort, ABCD – Adolescent Brain Cognitive Development, HCP-D – Human Connectome Project Development (113), IMAGEN (114), GenR – Generation R (115), Saguenay Youth Study (116, 117), Nathan Kline Institute-Rockland Sample (NKI-RS (118); MRI – magnetic resonance imaging, MPRAGE – magnetization-prepared rapid acquisition with gradient echo, DTI – Diffusion Tensor Imaging
The PNC has led to a wealth of new findings regarding structural and functional brain alterations in youth experiencing PS; 1,445 youth aged 8 to 21 years were recruited from the greater Philadelphia area and underwent genotyping, multimodal imaging, and neuropsychological testing. This sample was not ascertained for specific neuropsychiatric problems and includes multi-ethnic youth from various socio-economic backgrounds. Exclusion criteria were limited, and included significant medical problems, intellectual disability, neurological and/or endocrine conditions, and general MRI contraindications (80).
Importantly, all studies on PS in the PNC applied the same diagnostic criteria, offering comparability across studies (39,82). Furthermore, neuroimaging data were acquired with a single MRI scanner, reducing artifacts and heterogeneity due to scanner and study site variability.
Brain Morphometry
Gray and white matter morphology have been investigated in detail in the PNC. Reductions in local gray matter volume in youth experiencing PS relative to typically developing youth were observed in bilateral medial temporal lobes, and were also associated with PS severity (32). Further, a significant age by group interaction suggested that these local reductions in gray matter volume only became apparent in mid-adolescence in youth experiencing PS. This pattern of volume reductions in medial temporal regions mirrors a wealth of such findings not only in individuals with chronic schizophrenia, but also in individuals with first-episode psychosis as well as in individuals at clinical high-risk (CHR) for developing psychosis (83,84). Given that the medial temporal lobe in this study (32) included both the amygdala as well as parahippocampal cortex, this finding was followed up with a more detailed parcellation of the temporal lobe: whereas decreased volume of the left amygdala was associated with positive PS, decreased volume of the left entorhinal cortex was correlated with impaired cognition as well as more severe negative and disorganized symptoms (34), suggesting that variation in these brain structures may contribute to distinct symptom domains.
Jalbrzikowski et al. subsequently investigated whole-brain morphology differences in cortical thickness, surface area, and subcortical volume in PS youth in this cohort, relative to both youth with bipolar mood symptoms and typically developing youth (85). This study found thalamic volume reductions that were specific to PS. Again, these findings parallel those observed in individuals with overt psychosis and those at CHR (86), highlighting the role of the thalamus in neural system disruptions in psychosis. In terms of white matter microstructure, youth with PS also exhibited reduced fractional anisotropy in the retrolenticular internal capsule and the superior longitudinal fasciculus (SLF), possibly reflecting altered axonal diameter and/or myelination (87). Development of the SLF was associated with cognitive maturation in typically developing youth, an effect that was absent in youth experiencing PS.
Overall, alterations of brain morphology observed in these non-clinically ascertained cohorts of youth experiencing subthreshold PS can be interpreted as further evidence for a psychosis continuum, given qualitatively similar alterations observed in individuals with overt illness and those at CHR for psychosis.
Functional Brain Alterations
In terms of functional MRI, task-based brain function and resting state functional connectivity have both been investigated in population-based studies of PS. In the PNC, two MRI paradigms have been acquired: an n-back task probing different working memory loads and an emotion identification task. Working memory is viewed as a function of higher cognitive/ executive functioning consistently shown to be impaired in schizophrenia (88). Similarly, a wealth of evidence exists for impaired emotional processing in schizophrenia (89). Wolf et al. found reduced activation in the executive control network in response to increasing working memory demands, concomitant with worse performance, in PS youth relative to typically developing peers (33). Amygdala activation in response to threatening facial expressions was increased in PS youth compared to unaffected youth and was also positively correlated with positive symptom severity (33).
Utilizing data from the IMAGEN study that included longitudinal fMRI and measures of PS at follow-up, Papanstasiou et al. observed increases in right frontal activation during reward anticipation and feedback of win from age 14 to 19 that was associated with PS at age 19; this increase over time was not observed in youth who did not report PS. The authors speculate whether this finding could be a possible compensatory mechanism. However, given that PS were not assessed at age 14, results are to be interpreted with caution.
Resting-state fMRI has become a popular tool to study how distant brain areas are functionally connected. Unlike task-based fMRI, it is less susceptible to performance and vigilance differences between groups, which facilitates interpretation of group differences.
Again, the PNC has allowed large-scale investigation of functional connectivity across development. With regard to static functional connectivity (i.e., average connectivity across the resting-state scan), Satterthwaite et al. showed that PS youth exhibited similar patterns of dysconnectivity to patients with overt psychosis. In particular, they observed hyperconnectivity within the default-mode network (DMN) and reduced functional connectivity within the executive control network (31). However, in one of the largest pediatric population-based samples (ABCD; N = 3,434) Karcher et al. recently reported hypoconnectivity within the DMN and within the executive control networks that is associated with increased PS in 9- to 11-year old children (90). These differences in observed hypo- vs. hyperconnectivity may be attributable to age differences between the two studies. Nevertheless, there has been a similar dissonance in adult cohorts with overt psychosis, where both hypo- and hyperconnectivity of the DMN and executive control networks has been described (91,92).
In an elegant follow-up study that applied multivariate sparse canonical correlation analysis to the PNC resting state data, Xia and colleagues corroborated that in fact the segregation between the DMN and executive control networks is a common feature across multiple psychopathology dimensions, but the psychosis dimension shows the strongest effect (93). Moreover, a recent study of this cohort that investigated dynamic properties of functional connectivity, i.e., time-varying patterns of whole-brain connectivity, found that previously described dysconnectivity between the DMN and executive control networks in youth experiencing PS is time-dependent, and only occurs during certain periods of a resting-state scan, whereas dysconnectivity in visual and sensorimotor areas is much more pervasive (94).
The Human Connectome Project (HCP) is an adult cohort in which resting-state fMRI as well as self-reported PS were acquired. Here, PS were significantly inversely correlated with cognitive abilities, an effect that was partially mediated by global efficiency of the executive control network, a measure of network integration (95). With regard to dynamic functional connectivity in the HCP, it has recently been shown that adults experiencing PS spend more time in a dynamic state, i.e., a distinct time-varying connectivity pattern, characterized by reduced connectivity within the DMN (29); a finding that mirrors previous results in studies on individuals with overt psychosis (96).
Summary
Overall, structural and functional brain imaging results on PS in the general population, in adult as well as youth samples, exhibit high overlap with findings obtained from individuals with overt psychosis and those at CHR for developing psychosis. Studies in CHR samples have shown that neuroanatomical biomarkers can predict conversion to overt psychosis and improve prognostic accuracy (97). Although not yet at the point of clinical utility, in the future such biomarkers may also identify individuals with subthreshold PS and altered brain structure and/ or function that may be at increased risk for progression of PS and onset of overt illness. Presumably, these individuals would benefit the most from prevention strategies such as psychosocial interventions, although this hypothesis has yet to be empirically tested.
Longitudinal imaging studies with comprehensive assessment of broad psychopathology, cognition, and socioeconomic/environmental measures are needed to answer important questions of early precursors, symptom development and progression.
Public health implications
Even though pediatric (including adolescent) population neuroscience is still in its infancy, studies overwhelmingly find that PS in childhood and adolescence pose a risk factor for later development of overt psychiatric illness, and are overall associated with reduced functioning and quality of life. Many early intervention specialty programs offer a coherent multimodal treatment framework for clients, including psychopharmacological treatment, psychotherapy and psychoeducation as well as vocational counseling (‘one-stop-shop’ (98)). Meta-analytic results suggest that multidisciplinary therapies can delay or prevent transition to overt psychosis (99,100). Low risk psychosocial interventions targeting functioning have been shown to be effective in CHR youth; such approaches are likely to be also effective in a broader audience (101-105). These results find consideration in the recently published guidelines of the European Psychiatric Association (106) where a dual treatment consisting of cognitive behavioral therapy and pharmacological treatment yields recommendation grade A (‘meta-analysis, systematic review, or RCTs with very low risk for bias’) for adult CHR individuals. For children and adolescents experiencing PS, as targeted by pediatric population neuroscience, the expert recommendation is specific psychological interventions to improve functioning and close monitoring of PS.
PS are often preceded by non-specific behavioral and emotional problems in childhood related to increased adversity and trauma. Since these precursors in themselves pose a risk for development of diverse psychopathologies, we argue – as others before us (107,108) – that these childhood-onset problems offer another promising target for population-based preventive interventions. However, causal mechanisms from abnormal neurodevelopment to subsequent psychopathology are not yet understood and require further longitudinal research. Since only a minority of individuals with PS access appropriate mental health services, it will be important to implement services appropriate to a broad audience, for example in schools. It will be essential to identify those individuals at highest risk, and to reduce the number of false positives in order to provide cost effective services and to reduce stigma. Individual risk calculators developed and tested in CHR cohorts may not work as well when broadening the target audience. With sufficient longitudinal data, questionnaires such as the Psychosis Questionnaire, Brief Version (109) may be amenable for community samples, and may be used to develop risk calculators for youth in the general population.
Given the evidence presented here and results from the Outreach and Support in South London and Headspace initiatives (110-112), we argue that findings from population-based studies are adequate for guiding policy-making toward further emphasis on public health efforts, although more systematic research is needed in this area. Destigmatization initiatives for mental illness have been shown to be effective in reducing discrimination and stigma (113-115), and broadly accessible mental health programs like Headspace and Jigsaw are promising to make a difference in the field of adolescent mental health (112,116). However, the specific efficacy of these programs warrants further study, and caution is advised to not over-pathologize potentially transient occurrence of mental health problems.
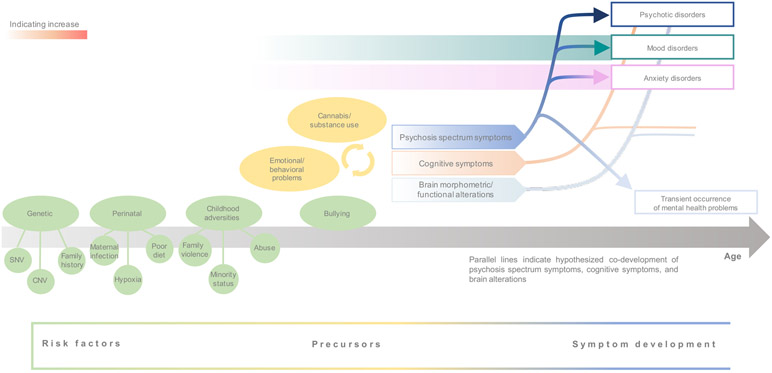
Figure 1:
Schematic of hypothesized development of psychosis spectrum symptoms, associated risk factors and precursors
Acknowledgments
This work was supported in part by grants from the National Institute of Mental Health (U01MH081902, 1R01MH107250) and the Staglin Family Music Festival for Mental Health
Footnotes
Financial disclosure
The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Guloksuz S, van Os J (2018): The slow death of the concept of schizophrenia and the painful birth of the psychosis spectrum. Psychological Medicine 48: 229–244. [DOI] [PubMed] [Google Scholar]
- 2.Curtis D, Derks EM (2018): Letter to the Editor: Schizophrenia does not represent the extreme of a normally distributed trait. Psychological Medicine 48: 521–522. [DOI] [PubMed] [Google Scholar]
- 3.Hartmann JA, Nelson B, Ratheesh A, Treen D, McGorry PD (2019): At-risk studies and clinical antecedents of psychosis, bipolar disorder and depression: a scoping review in the context of clinical staging. Psychological Medicine 49: 177–189. [DOI] [PubMed] [Google Scholar]
- 4.Kounali D, Zammit S, Wiles N, Sullivan S, Cannon M, Stochl J, et al. (2014): Common versus psychopathology-specific risk factors for psychotic experiences and depression during adolescence. Psychological Medicine 44: 2557–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marquand AF, Kia SM, Zabihi M, Wolfers T, Buitelaar JK, Beckmann CF (2019): Conceptualizing mental disorders as deviations from normative functioning. Molecular Psychiatry 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beer MD (1996): The dichotomies: psychosis/neurosis and functional/organic: a historical perspective. Hist Psychiatry 7: 231–255. [DOI] [PubMed] [Google Scholar]
- 7.David AS (2010): Why we need more debate on whether psychotic symptoms lie on a continuum with normality. Psychological Medicine 40: 1935–1942. [DOI] [PubMed] [Google Scholar]
- 8.Strauss JS (1969): Hallucinations and Delusions as Points on Continua Function: Rating Scale Evidence. Arch Gen Psychiatry 21: 581–586. [DOI] [PubMed] [Google Scholar]
- 9.van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L (2009): A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness–persistence–impairment model of psychotic disorder. Psychological Medicine 39: 179–195. [DOI] [PubMed] [Google Scholar]
- 10.Linscott RJ, van Os J (2013): An updated and conservative systematic review and meta-analysis of epidemiological evidence on psychotic experiences in children and adults: on the pathway from proneness to persistence to dimensional expression across mental disorders. Psychological Medicine 43: 1133–1149. [DOI] [PubMed] [Google Scholar]
- 11.Marder SR, Galderisi S (2017): The current conceptualization of negative symptoms in schizophrenia. World Psychiatry 16: 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kotov R, Foti D, Li K, Bromet EJ, Hajcak G, Ruggero CJ (2016): Validating dimensions of psychosis symptomatology: Neural correlates and 20-year outcomes. Journal of Abnormal Psychology 125: 1103–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGorry PD, Hickie IB, Yung AR, Pantelis C, Jackson HJ (2006): Clinical staging of psychiatric disorders: a heuristic framework for choosing earlier, safer and more effective interventions. Australian and New Zealand Journal of Psychiatry 40: 616–622. [DOI] [PubMed] [Google Scholar]
- 14.McGlashan TH, Miller TJ, Woods SW, Hoffman RE, Davidson L (2001): Instrument for the Assessment of Prodromal Symptoms and States In: Miller T, Mednick SA, McGlashan TH, Libiger J, Johannessen JO, editors. Early Intervention in Psychotic Disorders. Springer; Netherlands, pp 135–149. [Google Scholar]
- 15.Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, et al. (2008): Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Archives of general psychiatry 65: 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L, et al. (2012): Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Archives of general psychiatry 69: 220–229. [DOI] [PubMed] [Google Scholar]
- 17.Arango C, Díaz-Caneja CM, McGorry PD, Rapoport J, Sommer IE, Vorstman JA, et al. (2018): Preventive strategies for mental health. The Lancet Psychiatry 5: 591–604. [DOI] [PubMed] [Google Scholar]
- 18.Schultze-Lutter F, Michel C, Schmidt SJ, Schimmelmann BG, Maric NP, Salokangas RKR, et al. (2015): EPA guidance on the early detection of clinical high risk states of psychoses. European Psychiatry 30: 405–416. [DOI] [PubMed] [Google Scholar]
- 19.van Os J, Guloksuz S (2017): A critique of the “ultra-high risk” and “transition” paradigm. World Psychiatry 16: 200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fusar-Poli P, Rutigliano G, Stahl D, Davies C, De Micheli A, Ramella-Cravaro V, et al. (2017): Long-term validity of the At Risk Mental State (ARMS) for predicting psychotic and non-psychotic mental disorders. European Psychiatry 42: 49–54. [DOI] [PubMed] [Google Scholar]
- 21.Woods SW, Powers AR, Taylor JH, Davidson CA, Johannesen JK, Addington J, et al. (2018): Lack of Diagnostic Pluripotentiality in Patients at Clinical High Risk for Psychosis: Specificity of Comorbidity Persistence and Search for Pluripotential Subgroups. Schizophr Bull 44: 254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGrath JJ, Saha S, Al-Hamzawi AO, Alonso J, Andrade L, Borges G, et al. (2016): Age of Onset and Lifetime Projected Risk of Psychotic Experiences: Cross-National Data From the World Mental Health Survey. Schizophr Bull 42: 933–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mollon J, David AS, Morgan C, Frissa S, Glahn D, Pilecka I, et al. (2016): Psychotic Experiences and Neuropsychological Functioning in a Population-based Sample. JAMA Psychiatry 73: 129–138. [DOI] [PubMed] [Google Scholar]
- 24.Alonso J, Saha S, Lim CCW, Aguilar-Gaxiola S, Al-Hamzawi A, Benjet C, et al. (2018): The association between psychotic experiences and health-related quality of life: a cross-national analysis based on World Mental Health Surveys. Schizophrenia Research 201: 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yates K, Lång U, Cederlöf M, Boland F, Taylor P, Cannon M, et al. (2019): Association of Psychotic Experiences With Subsequent Risk of Suicidal Ideation, Suicide Attempts, and Suicide Deaths: A Systematic Review and Meta-analysis of Longitudinal Population Studies. JAMA Psychiatry 76: 180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Degenhardt L, Saha S, Lim CCW, Aguilar-Gaxiola S, Al-Hamzawi A, Alonso J, et al. (2018): The associations between psychotic experiences and substance use and substance use disorders: findings from the World Health Organization World Mental Health surveys. Addiction 113: 924–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Navarro-Mateu F, Alonso J, Lim CCW, Saha S, Aguilar-Gaxiola S, Al-Hamzawi A, et al. (2017): The association between psychotic experiences and disability: results from the WHO World Mental Health Surveys. Acta Psychiatrica Scandinavica 136: 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cornblatt BA, Carrión RE, Addington J, Seidman L, Walker EF, Cannon TD, et al. (2012): Risk Factors for Psychosis: Impaired Social and Role Functioning. Schizophr Bull 38: 1247–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barber AD, Lindquist MA, DeRosse P, Karlsgodt KH (2018): Dynamic Functional Connectivity States Reflecting Psychotic-like Experiences. BPS: CNNI 3: 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeRosse P, Ikuta T, Karlsgodt KH, Peters BD, Gopin CB, Szeszko PR, Malhotra AK (2017): White Matter Abnormalities Associated With Subsyndromal Psychotic-Like Symptoms Predict Later Social Competence in Children and Adolescents. Schizophr Bull 43: 152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Satterthwaite TD, Vandekar SN, Wolf DH, Bassett DS, Ruparel K, Shehzad Z, et al. (2015): Connectome-wide network analysis of youth with Psychosis-Spectrum symptoms. Mol Psychiatry 20: 1508–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Satterthwaite TD, Wolf DH, Calkins ME, Vandekar SN, Erus G, Ruparel K, et al. (2016): Structural Brain Abnormalities in Youth With Psychosis Spectrum Symptoms. JAMA Psychiatry 73: 515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolf DH, Satterthwaite TD, Calkins ME, Ruparel K, Elliott MA, Hopson RD, et al. (2015): Functional Neuroimaging Abnormalities in Youth With Psychosis Spectrum Symptoms. JAMA Psychiatry 72: 456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roalf DR, Quarmley M, Calkins ME, Satterthwaite TD, Ruparel K, Elliott MA, et al. (2017): Temporal Lobe Volume Decrements in Psychosis Spectrum Youths. Schizophr Bull 43: 601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fisher HL, Caspi A, Poulton R, Meier MH, Houts R, Harrington H, et al. (2013): Specificity of childhood psychotic symptoms for predicting schizophrenia by 38 years of age: a birth cohort study. Psychological Medicine 43: 2077–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGrath JJ, Saha S, Al-Hamzawi A, Andrade L, Benjet C, Bromet EJ, et al. (2016): The bidirectional associations between psychotic experiences and DSM-IV mental disorders. Am J Psychiatry 173: 997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nivard MG, Gage SH, Hottenga JJ, van Beijsterveldt CEM, Abdellaoui A, Bartels M, et al. (2017): Genetic Overlap Between Schizophrenia and Developmental Psychopathology: Longitudinal and Multivariate Polygenic Risk Prediction of Common Psychiatric Traits During Development. Schizophr Bull 43: 1197–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones HJ, Heron J, Hammerton G, Stochl J, Jones PB, Cannon M, et al. (2018): Investigating the genetic architecture of general and specific psychopathology in adolescence. Translational Psychiatry 8: 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calkins ME, Moore TM, Satterthwaite TD, Wolf DH, Turetsky BI, Roalf DR, et al. (2017): Persistence of psychosis spectrum symptoms in the Philadelphia Neurodevelopmental Cohort: a prospective two-year follow-up. World Psychiatry 16: 62–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Healy C, Gordon AA, Coughlan H, Clarke M, Kelleher I, Cannon M (n.d.): Do childhood psychotic experiences improve the prediction of adolescent psychopathology? A longitudinal population-based study. Early Intervention in Psychiatry 0 10.1111/eip.12762 [DOI] [PubMed] [Google Scholar]
- 41.White T (2015): Subclinical Psychiatric Symptoms and the Brain: What Can Developmental Population Neuroimaging Bring to the Table? Journal of the American Academy of Child & Adolescent Psychiatry 54: 797–798. [DOI] [PubMed] [Google Scholar]
- 42.Yung AR, Phillips LJ, Yuen HP, McGorry PD (2004): Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophrenia Research 67: 131–142. [DOI] [PubMed] [Google Scholar]
- 43.Rasic D, Hajek T, Alda M, Uher R (2014): Risk of Mental Illness in Offspring of Parents With Schizophrenia, Bipolar Disorder, and Major Depressive Disorder: A Meta-Analysis of Family High-Risk Studies. Schizophr Bull 40: 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Radoeva PD (2017): 22q11.2 Deletion Syndrome: Characterization of Psychosis Spectrum and Future Directions. Biological Psychiatry 82: e5–e7. [DOI] [PubMed] [Google Scholar]
- 45.Tang SX, Moore TM, Calkins ME, Yi JJ, Savitt A, Kohler CG, et al. (2017): The Psychosis Spectrum in 22q11.2 Deletion Syndrome Is Comparable to That of Nondeleted Youths. Biological Psychiatry 82: 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pignon B, Schürhoff F, Szöke A, Geoffroy PA, Jardri R, Roelandt J-L, et al. (2018): Sociodemographic and clinical correlates of psychotic symptoms in the general population: Findings from the MHGP survey. Schizophrenia Research 193: 336–342. [DOI] [PubMed] [Google Scholar]
- 47.World Health Organization (2004): Prevention of Mental Disorders. Geneva: World Health Organization; Retrieved May 17, 2019, from http://public.eblib.com/choice/publicfullrecord.aspx?p=4978589 [Google Scholar]
- 48.Poulton R, Caspi A, Moffitt TE, Cannon M, Murray R, Harrington H (2000): Children’s Self-Reported Psychotic Symptoms and Adult Schizophreniform Disorder: A 15-Year Longitudinal Study. Arch Gen Psychiatry 57: 1053–1058. [DOI] [PubMed] [Google Scholar]
- 49.Costello EJ, Edelbrock CS, Costello AJ (1985): Validity of the NIMH Diagnostic Interview Schedule for Children: A comparison between psychiatric and pediatric referrals. J Abnorm Child Psychol 13: 579–595. [DOI] [PubMed] [Google Scholar]
- 50.Niarchou M, Zammit S, Lewis G (2015): The Avon Longitudinal Study of Parents and Children (ALSPAC) birth cohort as a resource for studying psychopathology in childhood and adolescence: a summary of findings for depression and psychosis. Soc Psychiatry Psychiatr Epidemiol 50: 1017–1027. [DOI] [PubMed] [Google Scholar]
- 51.Sullivan SA, Wiles N, Kounali D, Lewis G, Heron J, Cannon M, et al. (2014): Longitudinal Associations between Adolescent Psychotic Experiences and Depressive Symptoms. PLOS ONE 9: e105758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horwood J, Salvi G, Thomas K, Duffy L, Gunnell D, Hollis C, et al. (2008): IQ and non-clinical psychotic symptoms in 12-year-olds: results from the ALSPAC birth cohort. British Journal of Psychiatry 193: 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cannon TD, Yu C, Addington J, Bearden CE, Cadenhead KS, Cornblatt BA, et al. (2016): An Individualized Risk Calculator for Research in Prodromal Psychosis. AJP 173: 980–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin A, Wood SJ, Nelson B, Beavan A, McGorry P, Yung AR (2015): Outcomes of Nontransitioned Cases in a Sample at Ultra-High Risk for Psychosis. American Journal of Psychiatry 172: 249–258. [DOI] [PubMed] [Google Scholar]
- 55.Bolhuis K, Koopman-Verhoeff ME, Blanken LME, Cibrev D, Jaddoe VWV, Verhulst FC, et al. (2018): Psychotic-like experiences in pre-adolescence: what precedes the antecedent symptoms of severe mental illness? Acta Psychiatrica Scandinavica 138: 15–25. [DOI] [PubMed] [Google Scholar]
- 56.Kooijman MN, Kruithof CJ, van Duijn CM, Duijts L, Franco OH, van IJzendoorn MH, et al. (2016): The Generation R Study: design and cohort update 2017. Eur J Epidemiol 31: 1243–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Edelbrock C, Costello AJ, Dulcan MK, Conover NC, Kala R (1986): Parent-Child Agreement on Child Psychiatric Symptoms Assessed Via Structured Interview*. Journal of Child Psychology and Psychiatry 27: 181–190. [PubMed] [Google Scholar]
- 58.De Los Reyes A, Kazdin AE (2005): Informant Discrepancies in the Assessment of Childhood Psychopathology: A Critical Review, Theoretical Framework, and Recommendations for Further Study. Psychological Bulletin 131: 483–509. [DOI] [PubMed] [Google Scholar]
- 59.Shakoor S, McGuire P, Cardno AG, Freeman D, Ronald A (2018): A twin study exploring the association between childhood emotional and behaviour problems and specific psychotic experiences in a community sample of adolescents. Journal of Child Psychology and Psychiatry 59: 565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dolphin L, Dooley B, Fitzgerald A (2015): Prevalence and correlates of psychotic like experiences in a nationally representative community sample of adolescents in Ireland. Schizophrenia Research 169: 241–247. [DOI] [PubMed] [Google Scholar]
- 61.Nusslock R, Miller GE (2016): Early-Life Adversity and Physical and Emotional Health Across the Lifespan: A Neuroimmune Network Hypothesis. Biological Psychiatry 80: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Callaghan BL, Tottenham N (2016): The Stress Acceleration Hypothesis: effects of early-life adversity on emotion circuits and behavior. Current Opinion in Behavioral Sciences 7: 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McGrath JJ, McLaughlin KA, Saha S, Aguilar-Gaxiola S, Al-Hamzawi A, Alonso J, et al. (2017): The association between childhood adversities and subsequent first onset of psychotic experiences: a cross-national analysis of 23 998 respondents from 17 countries. Psychological Medicine 47: 1230–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Os J, Bak M, Hanssen M, Bijl RV, de Graaf R, Verdoux H (2002): Cannabis Use and Psychosis: A Longitudinal Population-based Study. Am J Epidemiol 156: 319–327. [DOI] [PubMed] [Google Scholar]
- 65.Marconi A, Di Forti M, Lewis CM, Murray RM, Vassos E (2016): Meta-analysis of the Association Between the Level of Cannabis Use and Risk of Psychosis. Schizophr Bull 42: 1262–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gage SH, Hickman M, Zammit S (2016): Association Between Cannabis and Psychosis: Epidemiologic Evidence. Biological Psychiatry 79: 549–556. [DOI] [PubMed] [Google Scholar]
- 67.The International Schizophrenia Consortium (2009): Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 460: 748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schizophrenia Working Group of the Psychiatric Genomics Consortium, Ripke S, Neale BM, Corvin A, Walters JTR, Farh K-H, et al. (2014): Biological insights from 108 schizophrenia-associated genetic loci. Nature 511: 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.The Brainstorm Consortium (2018): Analysis of shared heritability in common disorders of the brain. Science 360 10.1126/science.aap8757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jones HJ, Stergiakouli E, Tansey KE, Hubbard L, Heron J, Cannon M, et al. (2016): Phenotypic Manifestation of Genetic Risk for Schizophrenia During Adolescence in the General Population. JAMA Psychiatry 73: 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Riglin L, Collishaw S, Richards A, Thapar AK, Maughan B, O’Donovan MC, Thapar A (2017): Schizophrenia risk alleles and neurodevelopmental outcomes in childhood: a population-based cohort study. The Lancet Psychiatry 4: 57–62. [DOI] [PubMed] [Google Scholar]
- 72.Haworth CMA, Davis OSP, Plomin R (2013): Twins Early Development Study (TEDS): A Genetically Sensitive Investigation of Cognitive and Behavioral Development From Childhood to Young Adulthood. Twin Research and Human Genetics 16: 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anckarsäter H, Lundström S, Kollberg L, Kerekes N, Palm C, Carlström E, et al. (2011): The Child and Adolescent Twin Study in Sweden (CATSS). Twin Research and Human Genetics 14: 495–508. [DOI] [PubMed] [Google Scholar]
- 74.Pain O, Dudbridge F, Cardno AG, Freeman D, Lu Y, Lundstrom S, et al. (2018): Genome-wide analysis of adolescent psychotic-like experiences shows genetic overlap with psychiatric disorders. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 177: 416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Loohuis LO, Mennigen E, Ori A, Perkins D, Robinson E, Addington J, et al. (2019): Genetic and clinical analyses of psychosis spectrum symptoms in a large multi-ethnic youth cohort reveal significant link with ADHD. bioRxiv 814087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singh T, Walters JTR, Johnstone M, Curtis D, Suvisaari J, Torniainen M, et al. (2017): The contribution of rare variants to risk of schizophrenia in individuals with and without intellectual disability. Nature Genetics 49: 1167–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Genovese G, Fromer M, Stahl EA, Ruderfer DM, Chambert K, Landén M, et al. (2016): Increased burden of ultra-rare protein-altering variants among 4,877 individuals with schizophrenia. Nature Neuroscience 19: 1433–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martin AR, Kanai M, Kamatani Y, Okada Y, Neale BM, Daly MJ (2019): Clinical use of current polygenic risk scores may exacerbate health disparities. Nature Genetics 51: 584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Torkamani A, Wineinger NE, Topol EJ (2018): The personal and clinical utility of polygenic risk scores. Nature Reviews Genetics 19: 581–590. [DOI] [PubMed] [Google Scholar]
- 80.Satterthwaite TD, Elliott MA, Ruparel K, Loughead J, Prabhakaran K, Calkins ME, et al. (2014): Neuroimaging of the Philadelphia Neurodevelopmental Cohort. NeuroImage 86: 544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Volkow ND, Koob GF, Croyle RT, Bianchi DW, Gordon JA, Koroshetz WJ, et al. (2018): The conception of the ABCD study: From substance use to a broad NIH collaboration. Developmental Cognitive Neuroscience 32: 4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Calkins ME, Merikangas KR, Moore TM, Burstein M, Behr MA, Satterthwaite TD, et al. (2015): The Philadelphia Neurodevelopmental Cohort: constructing a deep phenotyping collaborative. J Child Psychol Psychiatry 56: 1356–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nenadic I, Dietzek M, Schönfeld N, Lorenz C, Gussew A, Reichenbach JR, et al. (2015): Brain structure in people at ultra-high risk of psychosis, patients with first-episode schizophrenia, and healthy controls: a VBM study. Schizophrenia Research 161: 169–176. [DOI] [PubMed] [Google Scholar]
- 84.Meisenzahl EM, Koutsouleris N, Bottlender R, Scheuerecker J, Jäger M, Teipel SJ, et al. (2008): Structural brain alterations at different stages of schizophrenia: A voxel-based morphometric study. Schizophrenia Research 104: 44–60. [DOI] [PubMed] [Google Scholar]
- 85.Jalbrzikowski M, Freedman D, Hegarty CE, Mennigen E, Karlsgodt KH, Olde Loohuis LM, et al. (2019): Structural Brain Alterations in Youth With Psychosis and Bipolar Spectrum Symptoms. Journal of the American Academy of Child & Adolescent Psychiatry. 10.1016/j.jaac.2018.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haijma SV, Van Haren N, Cahn W, Koolschijn PCMP, Hulshoff Pol HE, Kahn RS (2013): Brain Volumes in Schizophrenia: A Meta-Analysis in Over 18 000 Subjects. Schizophrenia Bulletin 39: 1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hegarty CE, Jolles DD, Mennigen E, Jalbrzikowski M, Bearden CE, Karlsgodt KH (2018): Disruptions in White Matter Maturation and Mediation of Cognitive Development in Youths on the Psychosis Spectrum. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 10.1016/j.bpsc.2018.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, Velligan DI (2005): Beyond hypofrontality: A quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Human Brain Mapping 25: 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Green MF, Horan WP, Lee J (2015): Social cognition in schizophrenia. Nature Reviews Neuroscience 16: 620–631. [DOI] [PubMed] [Google Scholar]
- 90.Karcher NR, O’Brien KJ, Kandala S, Barch DM (2019): Resting-State Functional Connectivity and Psychotic-like Experiences in Childhood: Results From the Adolescent Brain Cognitive Development Study. Biological Psychiatry. 10.1016/j.biopsych.2019.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, et al. (2009): Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proceedings of the National Academy of Sciences 106: 1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pankow A, Deserno L, Walter M, Fydrich T, Bermpohl F, Schlagenhauf F, Heinz A (2015): Reduced default mode network connectivity in schizophrenia patients. Schizophrenia Research 165: 90–93. [DOI] [PubMed] [Google Scholar]
- 93.Xia CH, Ma Z, Ciric R, Gu S, Betzel RF, Kaczkurkin AN, et al. (2018): Linked dimensions of psychopathology and connectivity in functional brain networks. Nature Communications 9: 3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mennigen E, Jolles DD, Hegarty CE, Gupta M, Jalbrzikowski M, Olde Loohuis LM, et al. (n.d.): State-Dependent Functional Dysconnectivity in Youth With Psychosis Spectrum Symptoms. Schizophr Bull. 10.1093/schbul/sbz052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sheffield JM, Kandala S, Burgess GC, Harms MP, Barch DM (2016): Cingulo-opercular network efficiency mediates the association between psychotic-like experiences and cognitive ability in the general population. Biol Psychiatry Cogn Neurosci Neuroimaging 1: 498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Damaraju E, Allen EA, Belger A, Ford JM, McEwen S, Mathalon DH, et al. (2014): Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. NeuroImage: Clinical 5: 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chung Y, Addington J, Bearden CE, Cadenhead K, Cornblatt B, Mathalon DH, et al. (2018): Use of Machine Learning to Determine Deviance in Neuroanatomical Maturity Associated With Future Psychosis in Youths at Clinically High Risk. JAMA Psychiatry 75: 960–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McGorry P, Trethowan J, Rickwood D (2019): Creating headspace for integrated youth mental health care. World Psychiatry 18: 140–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stafford MR, Jackson H, Mayo-Wilson E, Morrison AP, Kendall T (2013): Early interventions to prevent psychosis: systematic review and meta-analysis. BMJ 346: f185–f185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Millan MJ, Andrieux A, Bartzokis G, Cadenhead K, Dazzan P, Fusar-Poli P, et al. (2016): Altering the course of schizophrenia: progress and perspectives. Nature Reviews Drug Discovery 15: 485–515. [DOI] [PubMed] [Google Scholar]
- 101.Ricciardi A, McAllister V, Dazzan P (2008): Is early intervention in psychosis effective? Epidemiology and Psychiatric Sciences 17: 227–235. [DOI] [PubMed] [Google Scholar]
- 102.McCrone P, Knapp M (2007): Economic evaluation of early intervention services. The British Journal of Psychiatry 191: s19–s22. [DOI] [PubMed] [Google Scholar]
- 103.Mihalopoulos C, Harris M, Henry L, Harrigan S, McGorry P (2009): Is Early Intervention in Psychosis Cost-Effective Over the Long Term? Schizophr Bull 35: 909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Miklowitz DJ, O’Brien MP, Schlosser DA, Addington J, Candan KA, Marshall C, et al. (2014): Family-Focused Treatment for Adolescents and Young Adults at High Risk for Psychosis: Results of a Randomized Trial. Journal of the American Academy of Child & Adolescent Psychiatry 53: 848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hutton P, Taylor PJ (2014): Cognitive behavioural therapy for psychosis prevention: a systematic review and meta-analysis. Psychological Medicine 44: 449–468. [DOI] [PubMed] [Google Scholar]
- 106.Schmidt SJ, Schultze-Lutter F, Schimmelmann BG, Maric NP, Salokangas RKR, Riecher-Rössler A, et al. (2015): EPA guidance on the early intervention in clinical high risk states of psychoses. European Psychiatry 30: 388–404. [DOI] [PubMed] [Google Scholar]
- 107.McLaughlin KA, DeCross SN, Jovanovic T, Tottenham N (2019): Mechanisms linking childhood adversity with psychopathology: Learning as an intervention target. Behaviour Research and Therapy 118: 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Eldreth D, Hardin MG, Pavletic N, Ernst M (2013): Adolescent Transformations of Behavioral and Neural Processes as Potential Targets for Prevention. Prev Sci 14: 257–266. [DOI] [PubMed] [Google Scholar]
- 109.Loewy RL, Pearson R, Vinogradov S, Bearden CE, Cannon TD (2011): Psychosis risk screening with the Prodromal Questionnaire — Brief Version (PQ-B). Schizophrenia Research 129: 42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fusar-Poli P, Byrne M, Badger S, Valmaggia LR, McGuire PK (2013): Outreach and support in South London (OASIS), 2001–2011: Ten years of early diagnosis and treatment for young individuals at high clinical risk for psychosis. European Psychiatry 28: 315–326. [DOI] [PubMed] [Google Scholar]
- 111.Rickwood DJ, Mazzer KR, Telford NR, Parker AG, Tanti CJ, McGorry PD (2015): Changes in psychological distress and psychosocial functioning in young people visiting headspace centres for mental health problems. Medical Journal of Australia 202: 537–542. [DOI] [PubMed] [Google Scholar]
- 112.Bassilios B, Telford N, Rickwood D, Spittal MJ, Pirkis J (2017): Complementary primary mental health programs for young people in Australia: Access to Allied Psychological Services (ATAPS) and headspace. International Journal of Mental Health Systems 11: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sampogna G, Bakolis I, Evans-Lacko S, Robinson E, Thornicroft G, Henderson C (2017): The impact of social marketing campaigns on reducing mental health stigma: Results from the 2009–2014 Time to Change programme. European Psychiatry 40: 116–122. [DOI] [PubMed] [Google Scholar]
- 114.Thornicroft G, Mehta N, Clement S, Evans-Lacko S, Doherty M, Rose D, et al. (2016): Evidence for effective interventions to reduce mental-health-related stigma and discrimination. The Lancet 387: 1123–1132. [DOI] [PubMed] [Google Scholar]
- 115.Robinson EJ, Henderson C (undefined/ed): Public knowledge, attitudes, social distance and reporting contact with people with mental illness 2009–2017. Psychological Medicine 1–10. [DOI] [PubMed] [Google Scholar]
- 116.O’Keeffe L, O’Reilly A, O’Brien G, Buckley R, Illback R (2015): Description and outcome evaluation of Jigsaw: an emergent Irish mental health early intervention programme for young people. Irish Journal of Psychological Medicine 32: 71–77. [DOI] [PubMed] [Google Scholar]